Significance
Anesthesia-resistant memory (ARM) has been puzzling because unlike long-term memory (LTM), it is translation independent in Drosophila. Although the two forms of consolidated memory are housed within the mushroom body neurons, they seem to employ distinct molecular pathways, with those that underlie ARM largely unknown. Elucidation of these pathways is essential to understanding ARM, how it differs from LTM, and what underlies their apparent inverse relationship. We reveal a signaling pathway that underlies ARM. Collectively, our results and already published results lead us to propose that a molecular hallmark of ARM formation is activity-dependent localized structural and functional changes in the neuronal actin cytoskeleton that alter synaptic strength or properties stable enough to last at least 24 h.
Keywords: ARM, Drk, Drok, memory, Drosophila
Abstract
Anesthesia-resistant memory (ARM) was described decades ago, but the mechanisms that underlie this protein synthesis-independent form of consolidated memory in Drosophila remain poorly understood. Whether the several signaling molecules, receptors, and synaptic proteins currently implicated in ARM operate in one or more pathways and how they function in the process remain unclear. We present evidence that Drk, the Drosophila ortholog of the adaptor protein Grb2, is essential for ARM within adult mushroom body neurons. Significantly, Drk signals engage the Rho kinase Drok, implicating dynamic cytoskeletal changes in ARM, and this is supported by reduced F-actin in the mutants and after pharmacological inhibition of Drok. Interestingly, Drk–Drok signaling appears independent of the function of Radish (Rsh), a protein long implicated in ARM, suggesting that the process involves at least two distinct molecular pathways. Based on these results, we propose that signaling pathways involved in structural plasticity likely underlie this form of translation-independent memory.
Temporal coincidence of an odor (conditioned stimulus, CS) with electric footshocks (unconditioned stimulus, US) elicits different types of aversive short- (STM), intermediate- (ITM), and long-term (LTM) memories in Drosophila (1). Multiple forms of coincident memories contribute to posttraining selective avoidance of the CS. ITM, for example, which is measured 3 h posttraining with 12 US/CS pairings, has been dissected into a labile component, sensitive to cold-induced amnestic treatment called anesthesia-sensitive memory (ASM) and an anesthesia-resistant (ARM) form. ARM lasting at least 24 h can also be induced by 5–10 consecutive sessions of 12 US/CS pairings (massed training) (1–3). ARM, unlike LTM, does not depend on protein synthesis but may involve modifications of preexisting proteins (1, 4) and is thought to be antagonistic to LTM (2, 4).
In contrast to ASM, the molecular pathways underlying ARM formation, storage, and recall remain poorly understood. Proteins with demonstrated roles in ARM formation include Radish (Rsh) (5); the constitutively active atypical PKC, PKM (6); the calcium channel Bruchpilot (Brp) (7); the d5HT1A serotonin and Oct2β2 octopamine receptors (8, 9); the Dop1R1, Dop2R dopamine receptors (10, 11); and Protein Kinase A (12). However, whether these molecules operate in one or more ARM-mediating signaling cascades is presently unclear.
ARM requires functional mushroom bodies (MBs), neurons essential for learning and memory in insects (13, 14). The MBs are bilateral neuronal clusters in the dorsal posterior brain extending dendrites ventrally to their somata and fasciculated axons projecting anteriorly and bifurcating to form the medial lobes (β/β′, γ) and dorsally to comprise the α/α′ lobes (15). Inhibition of synaptic output from α/β neurons impaired ARM (2), and this is consistent with the distribution of most proteins with known roles in this form of memory (5, 8), except for Oct2β2, which is required in α′ß′ (9). ARM formation appears to require octopaminergic input to the MB α′ß′ lobes from the Anterior Paired Lateral (APL) neurons (9, 16), whereas retrieval requires serotonergic input to the αβ lobes from the Dorsal Paired Medial (DPM) neurons. It appears then that at least two circuits and parallel molecular pathways contribute to ARM (16): an Oct2β2 receptor-mediated Rsh-independent in the α′ß′ lobes (9) and an Rsh-dependent, d5HT1A serotonin receptor-mediated in the αβ (8), which also receives Dop2R-mediated signals (11). Finally, Dop1R1 and Dop2R activities in the γ lobes have been suggested to contribute to ARM (10, 11). Drk, an SH2–SH3 domain adaptor protein orthologous to the mammalian Grb2, is also expressed preferentially in α/β neurons. Drk-mediated signaling to Ras and Raf is required for normal aversive learning signaling, whereas its role in ITM is independent of Ras activation (17). Because ITM comprises ASM and ARM (18), we investigated which of the two forms of memory is affected in drk mutant heterozygotes and revealed a specific role for the protein in ARM, mediated via the Drosophila homolog of Rho kinase, Drok, and apparently independent of the Rsh protein.
Results
Drk Reduction Selectively Affects ARM.
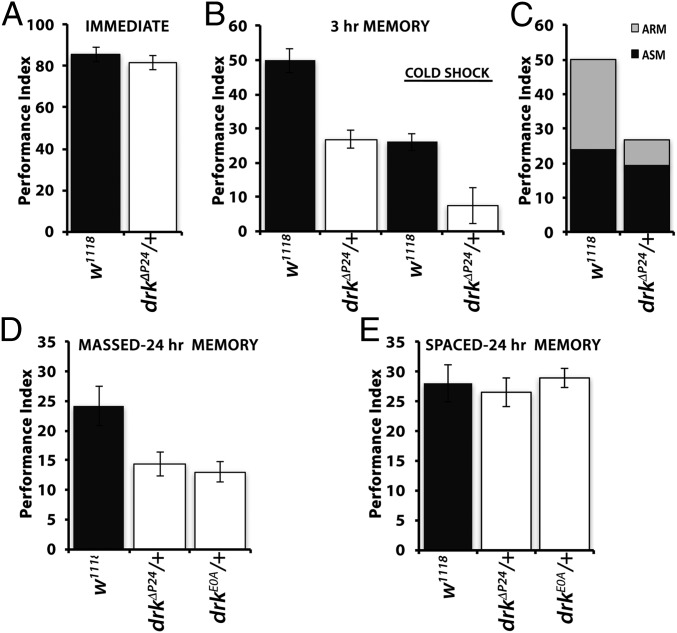
Heterozygotes for loss-of-function alleles of drk learn at a slower rate, a deficit reversible by Ras1 or Raf activation (17). However, memory of the association was significantly reduced even if these mutant heterozygotes were trained equivalently to controls and their memory deficit was independent of Ras1 and Raf activities (17). Because 3-h memory consists of ASM and ARM, we sought to identify which memory form is affected in the mutants. We capitalized on the fact that conditioning the mutants with 12 US/CS pairings results in immediate memory (learning) equivalent to that of controls (Fig. 1A). To differentiate between the two types of memory (1), animals trained with one round of 12 US/CS pairings were subjected to cold shock anesthesia 2 h posttraining, and their performance was assessed 1 h later along with similarly conditioned non-cold shocked flies. As expected, 3-h memory post-cold shock was significantly reduced in control animals (compare filled bars), indicating abrogation of the labile ASM but persistence of ARM (Fig. 1B). Notably, however, ARM appeared nearly absent in drkΔP24 heterozygotes (Fig. 1B, open bars), indicating that Drk reduction may selectively affect this form of aversive olfactory memory. This was better illustrated after fragmentation of 3-h memory to its components by subtracting the performance after cold shock of controls and mutants from the respective scores of untreated animals (7, 11). This verified that 3-h memory comprised nearly equal parts of ASM and ARM for controls (2), and whereas ASM seemed largely unaffected, ARM was severely attenuated for drkΔP24/+ (Fig. 1C). Therefore, 3-h memory in drk mutant heterozygotes consists nearly exclusively of ASM.
Fig. 1.
ARM deficits in drk mutant heterozygotes. Performances of mutants are indicated by open bars and controls by black bars. The mean ± SEM are shown. Following ANOVA, potential differences among controls and mutants were assessed for significance with least square means contrast analyses. (A) Three-minute memory of drkΔP24/+ animals after 12 US/CS conditioning once was not significantly different (P = 0.23, n = 8 each) than that of controls. (B) Three-hour memory (left side of the graph) of the null heterozygotes of single 12 US/CS session conditioning was significantly different from controls (P < 0.001, n = 7). Significant differences (P < 0.0013, n = 7) in 3-h ARM were also revealed following cold shock (right side of graph as indicated). (C) Partitioning the 3-h memory of drkΔP24 heterozygotes and controls into ARM and ASM by subtracting from normal 3-h memory that after cold shock. ASM (black) is nearly identical, but ARM is highly reduced in drkΔP24 heterozygotes. (D) ARM induced after 5× massed training and assessed 24 h later was significantly different in drkΔP24/+ (P < 0.007, n = 9) and drkE0A/+ (P < 0.005, n = 7) than controls. (E) In contrast, 24-h LTM induced by 5× spaced training was not affected (P = 0.9, n = 14 for drkΔP24/+; P = 0.78, n = 12 for drkE0A/+).
To independently verify this conclusion, we elicited ARM using a different conditioning protocol, massed training (1, 2), consisting of five consecutive cycles of 12 US/CS. Again, drkΔP24/+ and heterozygotes for an additional mutant allele, drkE0A, presented deficient ARM (Fig. 1D). However, when conditioned with five spaced training cycles, which yield protein synthesis-dependent LTM (1, 2), the performance of both drkΔP24/+ and drkE0A/+ was indistinguishable from that of controls. These results strongly suggest that Drk is specifically required for normal ARM. Both these consolidated memory forms reside within the MBs (3, 8, 9) and have been hypothesized to be mutually exclusive and to engage distinct signaling pathways (2, 4). Although various signaling cascades and molecular pathways have been implicated in LTM formation, storage, and recall (19), such mechanisms remain largely elusive for ARM.
Drk Is Required in the α/β Lobes of the MBs for Normal ARM.
Drk is detected in many adult brain structures, including the antennal lobe (AL), ellipsoid body, and prominently the α, β, and γ but not the α′ß′ lobes of the MBs (17), the anatomical site where the Rsh-dependent ARM trace is reported to reside (2, 5, 7). Therefore, we sought to determine whether Drk is required for ARM within the MBs or other adult brain structures. MB-specific Drk abrogation was achieved with transgenes (drkR-1.2), shown to effectively knock down its levels (17, 20) via RNA-mediated interference (RNAi).
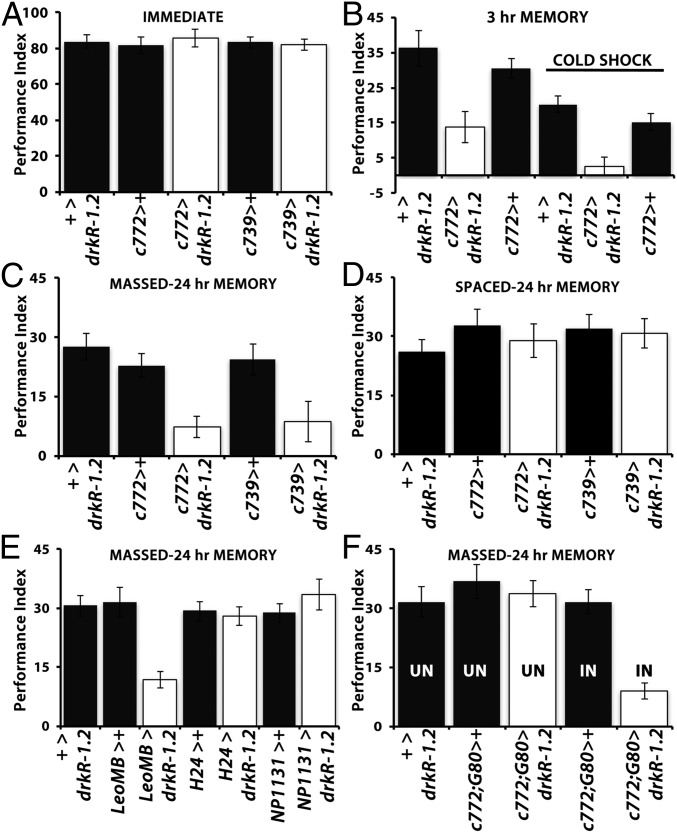
Initially, we confirmed that 12 US/CS elicited normal immediate (3-min) memory in animals expressing drkR-1.2 under two different MB αβ lobe-preferential Gal4 drivers (Fig. 2A), c772-Gal4 and c739-Gal4 (21). In contrast, expression of drkR-1.2 under these drivers recapitulated the 3-h memory deficit of drk mutant heterozygotes (Fig. 2B and Fig. S1A), whereas cold shock appeared to abolish their 3-h memory (Fig. 2B), in accord with the ARM deficit of the mutants (Fig. 1C). The cold shock-induced ARM defect was further confirmed with the massed training protocol, which yielded highly compromised 24-h memory in drkR-1.2–expressing animals under both c772-Gal4 and c739-Gal4 (Fig. 2C). In agreement with the results for drkΔP24/+ (Fig. 1D), LTM was unaffected in drkR-1.2–expressing animals (Fig. 2D), in strong support of the notion that Drk is required specifically for ARM. Because our data indicate that both conditioning protocols yield nearly identical results, henceforth we used the more robust mass training method unless otherwise specified.
Fig. 2.
Drk is required within the MB α/β lobes for normal ARM. The mean performance ± SEM is shown at the indicated times postconditioning. Controls are represented by black bars and animals with attenuated Drk via UASdrkR1.2 (drkR1.2) expression by open bars. + denotes the presence of a w1118-derived chromosome indicating either the lack of driver (+>) and heterozygosity for the transgene or heterozygosity for the Gal4 driver (i.e., c772>+). Potential differences were assessed with ANOVA and least square means contrast analyses. (A) Performance immediately after 12 US/CS conditioning of controls and animals with abrogated Drk in the indicated MB neurons. Loss of Drk in the MBs did not affect the performance relative to that of controls (P = 0.634, n > 9 per genotype). (B) Three-hour memory without and with cold shock of animals with abrogated Drk in 772 Gal4-marked neurons. The performance of drkR1.2-expressing animals was significantly different from that of both (+> drkR1.2 and c772>+) controls without (P < 0.0011, n > 7) and with cold shock (P < 0.002, n > 8). (C) Abrogation of Drk within α/β neurons precipitated deficits in 24-h ARM either under the c772 Gal4 (P < 0.003, n > 6) or the c739 Gal4 driver (P < 0.001, n > 8). (D) LTM induced with spaced conditioning was not affected in animals with abrogated Drk in α/β lobes relative to controls (P = 0.82, n > 7). (E) Abrogation of Drk in all MB neurons under LeoGal4 resulted in a highly significant (P < 0.001, n > 14) deficit in 24-h ARM, while abrogation in the AL and γ-neurons (H24) Gal4 or γ-neurons (NP1131) only, did not yield significant differences from their respective controls (P = 0.894 and P = 0.900, respectively; n > 10). (F) Adult-specific abrogation of Drk in the MBs. Experimental flies (c772> drkR1.2) held under Gal80ts-mediated suppression of Drk abrogation (UN) did not exhibit behavioral deficits compared with controls (P = 0.899, n > 9), whereas transgene induction (IN) precipitated deficient 24-h ARM (P < 0.007, n = 7).
Fig. S1.
(A) Cold shock-dependent 3-h memory is deficient upon drk abrogation within c739-marked MB neurons. The mean performance ± SEM is shown 3 h postconditioning for the indicated genotypes. All genotypes were given a 2-min cold shock 2 h posttraining. + denotes the presence of a w1118-derived chromosome indicating either the lack of driver (+>) and heterozygosity for the transgene or, conversely, heterozygosity for the Gal4 driver. The performance of drkR1.2-expressing animals was significantly different from that of both (+> drkR1.2 and c739>+) controls after cold shock (P < 0.001, n > 6). (B) Adult-specific abrogation of Drk throughout the MBs precipitated deficits in 24-h ARM. Expression of the Drk-attenuating transgene under LeoGal4 was suppressed under Gal80ts-mediated suppression by raising these flies at 18 °C along with the two controls. The abrogating transgene was induced by incubation of experimental flies and controls at 30 °C for 24 h. The performance of LeoGal4:Gal80ts > drkR-1.2 animals was significantly different from both controls (P < 0.001, n > 6). (C) Conditional expression (IN-induced) of DrokCAT in c772-marked MB neurons rescues the 3-h memory deficit of drkΔP24 heterozygotes after cold shock, whereas the significant ARM deficit (P < 0.002, n = 6) of drkΔP24 heterozygotes is apparent (open bar). (D) The Drok activity attenuator Fasudil was administered to control w1118 animals within 30–45 min after five cycles of massed training but did not yield detectable defects 24 h later (ANOVA P = 0.5243, n > 5). (E) Twenty-four–hour ARM of control (black bars) males was significantly different from that of rsh1/Y; c739 Gal4,G80ts/+ (P < 0.0001) and from that of rsh1/Y; c739 Gal4,G80ts/UASDrokCAT animals (P < 0.0001). In addition, the performance of rsh1/Y; c739 Gal4,G80ts/+ was not significantly different from that of flies of the same genotype but expressing DrokCAT (P = 0.1382, n > 11). (F) Representative agarose electrophoresis of PCR products following Reverse Transcription (+RT) of RNAs isolated from heads of drkΔP24/hs rsh animals after a 30-min heat shock (+HS). The PCR product was absent if RT was performed on RNA isolated from such animals not heat shocked to induce the transgene (−HS) or if RNA isolated from heat-shocked animals was treated with RNase A before RT (+RNase, +RT).
Furthermore, because the expression pattern of c772-Gal4 and c739-Gal4 overlaps within the α/β MB lobes (21), these results strongly indicate that Drk is required specifically within these neurons for normal ARM. Because both of these Gal4 drivers are also expressed in the AL, which has been implicated in ARM (22), we used H24-Gal4 and NP1131-Gal4 to specifically abrogate Drk in the AL and γ lobes. We utilized Leo-Gal4, which is an MB-specific driver expressed in all neurons (23), as the positive control. Clearly, whereas Drk abrogation within γ neurons and the AL did not precipitate deficits, ARM was compromised under Leo-Gal4 (Fig. 2E), which is also expressed in the α′β′ neurons. However, as Drk is not expressed in α′β′ neurons, RNAi-mediated Drk abrogation in all MB neurons (Fig. 2E) appeared quantitatively similar to that limited to αβ lobes (Fig. 2C). Therefore, Drk is required for ARM within αβ neurons and appears to function independently of the octopaminergic signaling to the α′ß′ lobes.
Finally, to establish that deficient ARM does not result from developmental defects in the αβ lobes due to reduced Drk, we used TARGET (24, 25) to conditionally express drkR-1.2 in adult MBs under c772-Gal4 (Fig. 2F) or Leo-Gal4 (Fig. S1B). Deficient 24-h ARM was observed only upon adult-specific induction of the Drk abrogating transgene, indicating that the ARM deficit does not originate from and underlie developmental deficits within MBs. Hence, Drk is specifically required within the postdevelopmental αβ MB neurons for normal ARM.
Rho Kinase Activation Restores ARM in drk Mutants.
Since Drk does not signal to Raf1 for its function in memory (17), we searched for potential involvement of alternative signaling cascades. We were guided by its vertebrate ortholog GRB2, which engages a Rho kinase to maintain fear memory in rodents (26) to investigate the possibility that a similar pathway is involved in Drk-mediated ARM. Drosophila possesses a single Rho kinase ortholog, Drok, which like Raf is a serine/threonine kinase activated by the GTPase Rho1 (27).
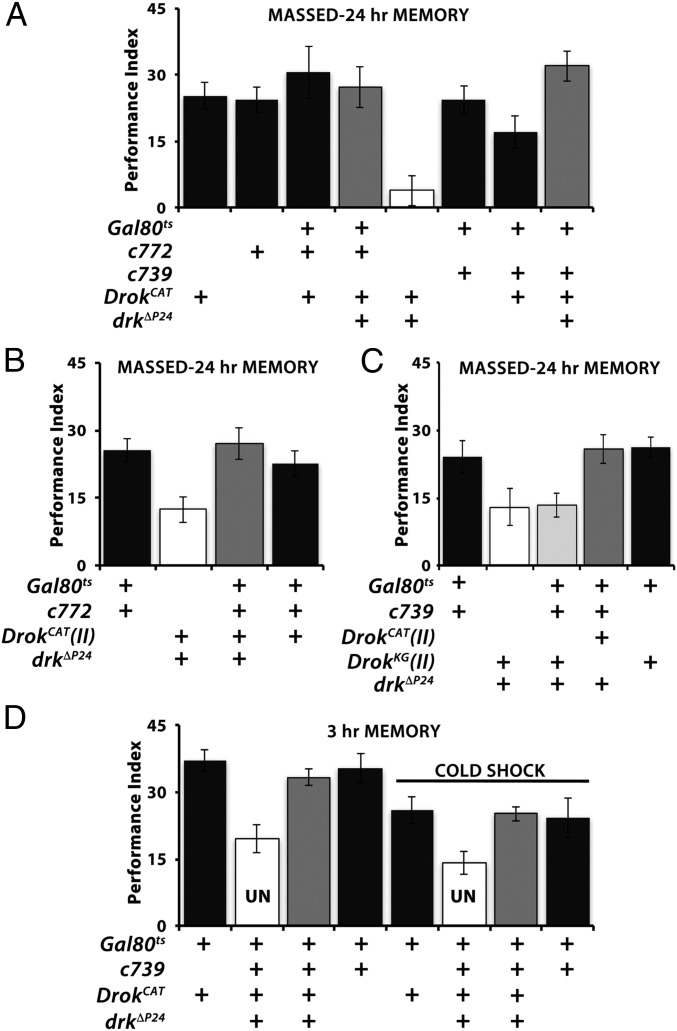
If Drk signals engage Drok to mediate normal ARM, then a transgenic constitutively active form of the kinase, DrokCAT (28), may rescue the ARM deficit of drkΔP24/+. To avoid complications because of the reported aberrant MB development precipitated by continuous DrokCAT expression (27–29), we expressed it conditionally and exclusively within adult drkΔP24/+ αβ neurons (25). Significantly, DrokCAT expression in the αβ neurons fully restored 24-h ARM in drk heterozygotes (Fig. 3A). Full rescue was also achieved with an independent DrokCAT transgene on a different chromosome (Figs. 3B and 4C). In contrast, expression of the catalytically inactive transgenic protein DrokCAT-KG (28) in the same MB neurons did not rescue the deficient ARM of drkΔP24 heterozygotes (Fig. 3C). Conditional expression of either DrokCAT or DrokCAT-KG in wild-type adult MBs did not suppress or enhance ARM (Fig. 3 A–C), indicating that rescue did not result because of nonspecific effects of transgene overexpression. In addition, acute DrokCAT expression in αβ neurons under the TARGET system also restored 3-h cold shock-dependent ARM to levels exhibited by control animals (Fig. 3D), verifying that rescue was adult MB-specific. Similar results were obtained with the c772 driver (Fig. S1C).
Fig. 3.
Catalytically active Drok rescues the ARM deficits of drk mutant heterozygotes. Mean performances ± SEM are shown. Controls are represented by black bars, mutants by open bars, and mutant animals with potential behavioral rescue by gray bars. Following ANOVA, potential differences were assessed for significance with planned comparisons (least square means contrast analyses) as necessary. (A) Adult-specific expression of the constitutively active UASDrokCAT within α/β neurons rescued (gray bars) the ARM deficit of drkΔP24/+ flies (open bar). The entire data shown are from animals induced in parallel. Whereas drkΔP24/+; DrokCAT/Gal80ts flies were significantly (P < 0.0001) deficient in ARM compared with any of the controls (black bars), drkΔP24/c772; DrokCAT/Gal80ts were not (P = 0.9967). Similarly, drkΔP24/c739; DrokCAT/Gal80ts flies performed equally well with controls (P = 0.688) but significantly different (P < 0.0001) from drkΔP24/+; DrokCAT/Gal80ts animals. n > 7 for all. (B) Rescue of the ARM deficit of drkΔP24/+; DrokCAT/Gal80ts animals (open bar) by induction of an independent DrokCAT transgene on chromosome II (gray bar). Whereas the performance of drkΔP24, DrokCAT/c772; +/Gal80ts was not different from that of controls (P = 0.829), it was significantly different from that of drkΔP24, DrokCAT/+; +/Gal80ts (P < 0.0007). (C) The catalytically inactive DrokKG transgene does not rescue (light gray bar) the ARM deficit of drkΔP24, DrokKG/+; +/Gal80ts (P = 0.774) under the c739 driver, which recues (P = 0.731) the drkΔP24 deficit with the DrokCAT transgene (drkΔP24, DrokCAT/c739; +/Gal80ts). n > 7. (D) Conditional expression of DrokCAT in α/β neurons rescues (gray bars) the 3-h memory deficit of drkΔP24 heterozygotes after cold shock. Uninduced (UN) drkΔP24/c739; DrokCAT/Gal80ts animals were used as negative controls (open bars), and they performed significantly different from controls (P < 0.0002), while after induction they did not (gray bars, P = 0. 849). Similarly, ARM after cold shock was significantly different in UN (open bars) drkΔP24/c739; DrokCAT/Gal80ts from controls (+/+; DrokCAT/Gal80ts, or +/c739; +/Gal80ts; P < 0.003 for both) and from the same flies after transgene induction (gray bar; P < 0.0015). n > 7.
Fig. 4.
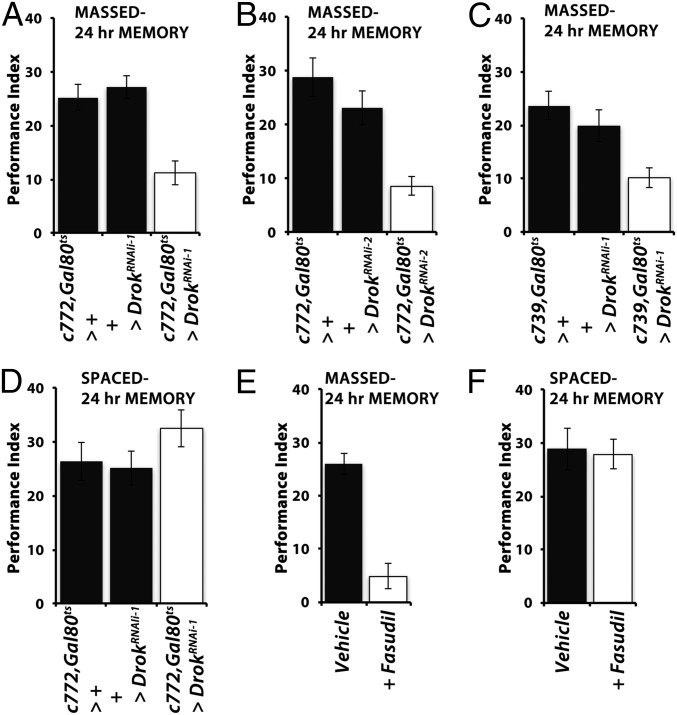
Drok activity is required for ARM. Mean performances ± SEM are shown. Controls are represented by black bars and Drok attenuated flies by open bars. Following ANOVA, differences were assessed for significance with planned comparisons (least square means contrast analyses) as necessary. (A) Adult-specific attenuation of Drok in c772Gal4-marked neurons precipitated a significant deficit in 24-h ARM after massed conditioning relative to both controls (P < 0.0001, n > 10). (B) Adult-specific attenuation of Drok in c772Gal4-marked neurons with an independent abrogating transgene (UASDrokRNAi-2) precipitated a significant deficit in 24-h ARM relative to both controls (P < 0.0002, n > 10). (C) A similar ARM deficit was observed upon Drok abrogation under the c739Gal4 driver (P < 0.0007, n > 12). (D) Adult-specific Drok attenuation in αβ neurons did not affect 24-h LTM (P = 0.2631, n > 8). (E) Adult-specific Drok activity attenuation in control w1118 animals with Fasudil precipitated a highly significant 24-h ARM deficit (ANOVA P < 0.0001, n = 12 each). (F) Spaced conditioning-induced LTM was not affected by Fasudil treatment in w1118 animals (ANOVA P = 0.7541, n > 10).
Collectively, the results support a genetic Drk–Drok interaction within αβ neurons acutely required for normal ARM revealed after massed training or after cold shock.
Rho Kinase Activity Is Required for Normal ARM.
Drok activity can be specifically required for Drk-mediated ARM, or it could function in both forms of consolidated memory. To differentiate between these possibilities, we conditionally abrogated the kinase within adult αβ neurons by Drok RNAi-mediating transgenes. Adult-specific attenuation of Drok within 772 Gal4-marked neurons did not affect 3-min memory (71.6 ± 2.46 for c772 Gal4, Gal80ts>+; 74.2 ± 1.16 for UAS-DrokRNAi-1>+; and 77.8 ± 3.08 for c772-Gal4, Gal80ts > UAS-DrokRNAi-1; ANOVA P = 0.2676). However, it yielded significant deficits in ARM (Fig. 4 A and B) with two distinct RNAi-mediating transgenes. A similar deficit was also observed upon Drok abrogation within 739 Gal4-marked neurons (Fig. 4C). In contrast, LTM was not affected by Drok attenuation therein (Fig. 4D), strongly suggesting that the kinase plays a role specifically on the ARM form of memory.
In addition to its kinase activity, Drok contains a Rho GTPase binding site and a Pleckstrin domain (30), suggesting multiple ways that the protein could be involved in ARM. Because only constitutively active Drok rescued the deficit of drk mutants (Fig. 3), we hypothesized that ARM requires its kinase activity and not its Pleckstrin or GTPase domains. To differentiate between these possibilities and further validate the results with the kinase-dead transgene, we sought to inhibit the kinase activity without altering the levels of the protein itself and hence the dosage of these conserved domains. Because Rok family proteins are kinases of medical importance implicated in cancer, pulmonary hypertension, and neurodegenerative diseases (31), specific inhibitors are commercially available. We opted for the potent selective Rok inhibitor Fasudil hydrochloride (HA-1077), because it had been used on Drosophila before without apparent ill effects (32). Adult 2–3-d-old w1118 flies were fed the inhibitor (200 μM) for 16 h before conditioning. As illustrated in Fig. 4E, flies treated with the inhibitor presented little 24-h ARM after massed conditioning, but the drug did not affect LTM. Exposing the flies to the inhibitor only after conditioning did not affect ARM (Fig. S1D). Collectively then, the kinase activity of Drok is required for Drk-mediated ARM formation within αβ MB neurons.
Rho Kinase Activation Does Not Restore ARM in rsh Mutants.
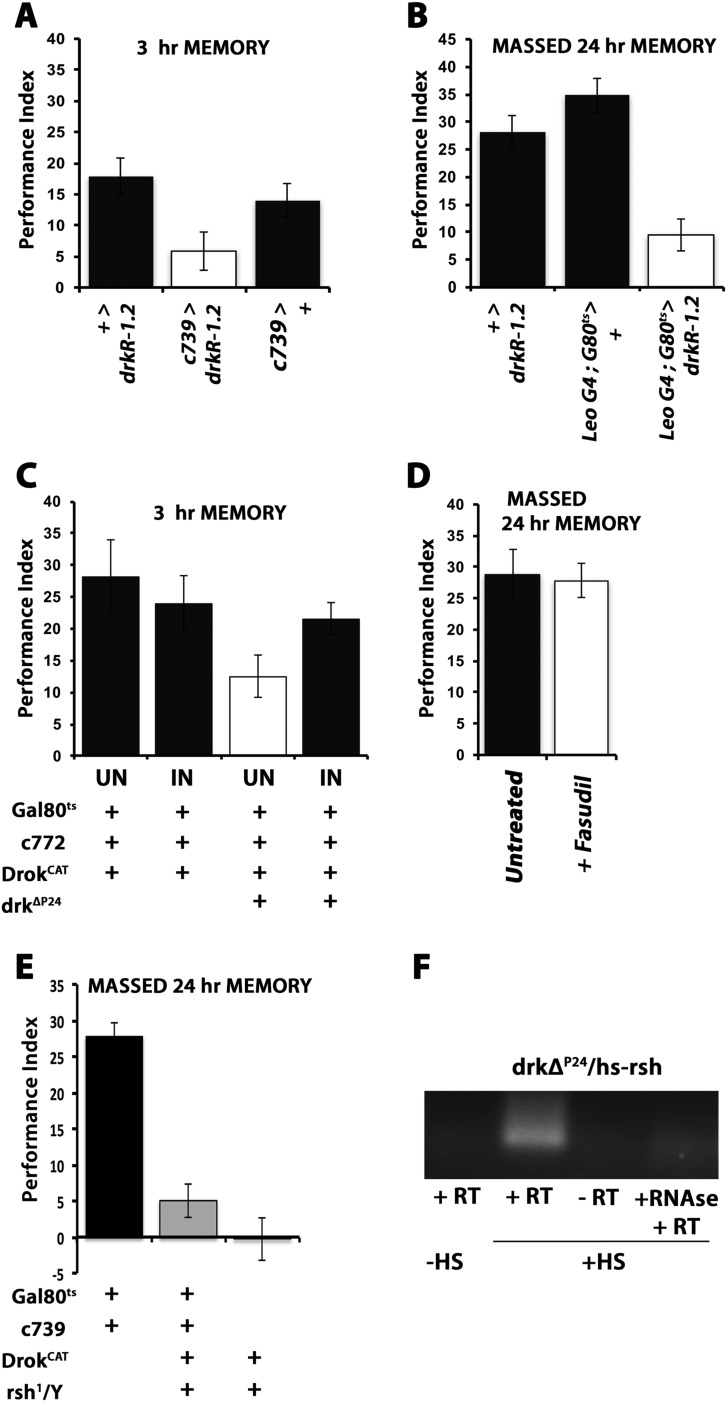
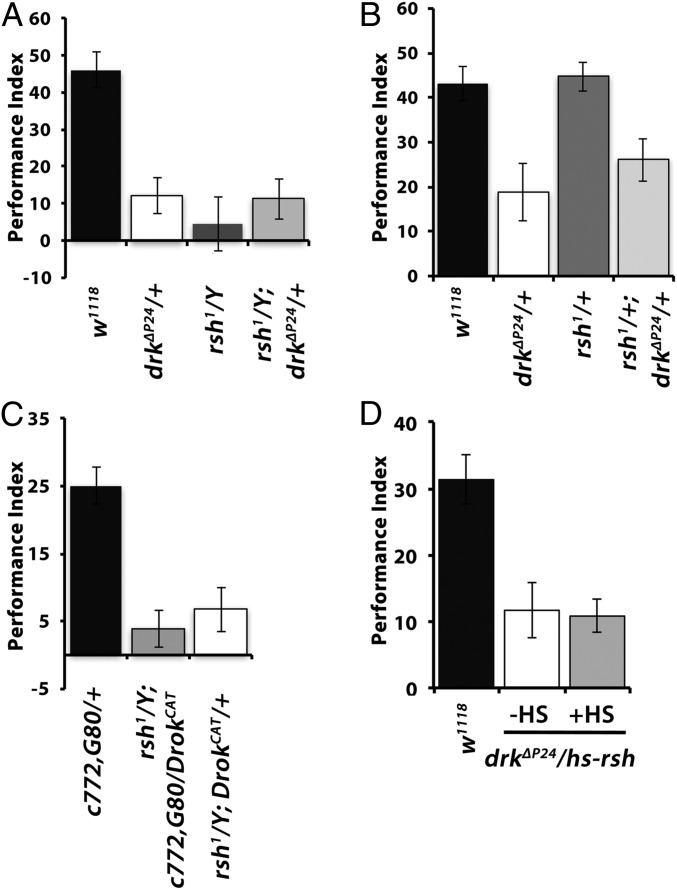
The requirement of Drk within αβ neurons for normal ARM is in agreement with the preferential expression and functional requirement of Rsh, which is specifically implicated in this form of consolidated olfactory memory (5) within these neurons (8). The exact function of Rsh is still unclear but appears to possess a GTPase-activating domain (Flybase Curators, 2008; flybase.org/reports/FBgn0265597.html). ARM is specifically impaired in rsh mutants (5, 18) and appears to be independent of octopaminergic (9) inputs to the MBs for ARM formation. Therefore, we asked whether Drk and Rsh act in the same molecular cascade by investigating whether they interact genetically. Hence, we tested 24-h ARM after massed training in males hemizygous for rsh1 and heterozygous for drkΔP24 (rsh1; drkΔP24/+) and in doubly heterozygous rsh1/+; drkΔP24/+ females. Reducing Drk levels by 50% did not alter the ARM deficit of rsh1 males (Fig. 5A), while ARM in the doubly heterozygous females was similar to those of drkΔP24/+ females (Fig. 5B). Although the low performance levels of male flies potentially hindered resolution, the results suggest either that Drk is upstream of Rsh or the two proteins act in different molecular ARM-mediating pathways.
Fig. 5.
Drk-mediated signaling to Drok for ARM does not engage Rsh. Mean performances ± SEM are shown for A–D. Following ANOVA, differences were assessed for significance least square means contrast analyses as necessary. (A) Twenty-four–hour ARM of control males (black bars) was significantly different (P < 0.0001) from that of drkΔP24/+ (open bars), rsh1 hemizygous males (dark gray bars), and rsh1; drkΔP24/+ (light gray bars). However, the performance of rsh1 hemizygous males was not significantly different from that of drkΔP24/+ (P = 03428) or rsh1; drkΔP24/+ (P = 0.4023). (B) Twenty-four–hour ARM of control females (black bars) was significantly different (P < 0.0006) from that of drkΔP24/+ and rsh1/+; drkΔP24/+ (P < 0.008) but not from rsh1/+ females (P = 0.8318, n > 10). (C) DrokCAT expression in their MBs does not rescue the 24-h ARM deficit of rsh1 mutants; 24-h ARM of control (black bars) males was significantly different from that of rsh1/Y; c772 Gal4,G80ts/+ (P < 0.0001) but also from rsh1/Y; c772-Gal4,G80ts/UASDrokCAT flies (P < 0.0001). In addition, the performance of rsh1/Y; c772 Gal4,G80ts/+ was not different from that of flies of the same genotype but was with those expressing DrokCAT (P = 0.4944, n > 9). (D) Twenty-four–hour ARM for drkΔP24heterozygotes carrying a conditional heat shock-inducible rsh transgene. Induction of the transgene (+HS) did not improve (P = 0.8527) their performance over that of their siblings without induction (−HS), which remained significantly different from that of (not heat shocked) controls (P < 0.001, n > 9).
If Drk, Drok, and Rsh were in the same cascade, Drok activation could reverse the deficient ARM of rsh1 males. This possibility is supported by the putative GTPase activator function of Rsh, which could be involved in Drok activation. Therefore, we introduced the DrokCAT transgene that rescued the drkΔP24/+ ARM deficit into rsh1 mutant flies. However, conditional expression of DrokCAT within the MBs of adult rsh1 males under c772Gal4 or c739Gal4 failed to rescue their deficient ARM (Fig. 5C and Fig. S1E). The results suggest that Rsh does not act upstream of Drk or between Drk and Drok in a single signaling pathway. To test the alternative possibility that Rsh is downstream of Drk and Drok, we attempted to rescue the ARM deficit of drkΔP24/+ flies with an inducible rsh transgene. Although the transgene was shown to rescue the deficient ARM of rsh1 mutants (5) and was highly induced by a brief heat shock (Fig. S1F), it was unable to reverse the deficit of drkΔP24 heterozygotes (Fig. 5D). These data strongly suggest that Drk and Rsh operate in distinct, potentially parallel molecular pathways serving ARM within αβ neurons.
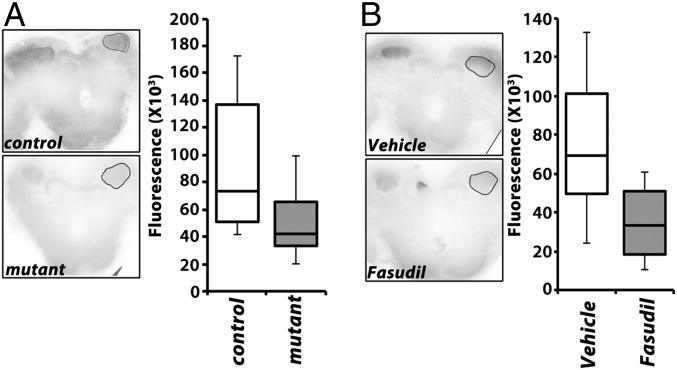
Because Drok-mediated signals likely engage actin and the actin cytoskeleton (26, 29, 31), we investigated whether changes in actin polymerization could be detected in animals with genetic or pharmacologically-induced attenuated ARM. We assessed filamentous actin (F-actin) levels in drk mutants and in control animals treated with the Drok inhibitor Fasudil. Brains were dissected from animals with strongly reduced Drk levels in the MBs [drkΔP24/LeoGal4; drkR-1.2/+ (17)], stained with phalloidin, and quantification of the signal within the calyces revealed a significant reduction in filamentous actin compared with controls (Fig. 6A). A similarly highly significant reduction in filamentous actin levels was observed upon treatment with Fasudil before dissection (Fig. 6B), a treatment that nearly abolishes ARM (Fig. 4E), which collectively with the results from the mutants strongly implicate actin cytoskeleton dynamics in the process.
Fig. 6.
Decreased filamentous actin in the MBs of drk mutants and upon acute pharmacological inhibition of Drok. (A) Representative confocal images of whole-mount brains at the level of the calyces used to quantify fluorescence from the marked regions of interest (ROI) after rhodamine-conjugated phalloidin staining. Control indicates LeoGal4/+, while mutant indicates the genotype drkΔP24/LeoGal4; drkR-1.2/+. Quantification (Right) of multiple experiments revealed significant differences in fluorescence in the calyces control and mutant animals (Wilkoxon test, χ2 = 12.4910, P < 0.0004, n > 20). (B) Representative confocal images of whole-mount brains at the level of the calyces used to quantify fluorescence from the marked ROIs after rhodamine-conjugated phalloidin staining of vehicle and Fasudil-treated w1118 animals. Quantification (Right) revealed significant differences in fluorescence in the calyces control and mutant animals (Wilkoxon test, χ2 = 22.6722, P < 0.0001, n > 28).
Discussion
Elucidation of the molecular pathways specific to ARM is essential to understanding this translation-independent consolidated memory form, how it differs from LTM, and what underlies their apparent inverse relationship (2, 4). Our evidence indicates that the small adaptor protein Drk/Grb2 is essential for ARM in a Rsh-independent manner. Genetically, Drk signals to Drok, but it is unlikely that they also interact physically. Drok lacks significant polyproline stretches targeted by the SH3 domain of Drk, and none of its regulatory phosphorylations are on Tyrosines residues, which could engage the SH2 domain (30).
Drk and Rsh are both present within MB neurons (5, 8, 17), but our evidence indicates that Drk/Drok-dependent ARM does not require Rsh. This is consistent with two signaling pathways serving ARM within αβ neurons, where Rsh expression overlaps that of Drk. These two cascades may be independent or converging downstream of Drok in a coincidence detection manner, but we favor the former based on the following: Serotonergic signals required for ARM formation in αβ neurons may engage Rsh (8), but not Drk/Drok required therein for structural plasticity as suggested below. Serotonergic signaling in Drk/Drok-mediated plasticity is currently under investigation. Another possibility consistent with independent cascades is that Rsh may only be involved in ARM retrieval within αβ neurons (16), while Drk/Drok in its formation or maintenance. In this model, rsh mutations will occlude rescue attempts with activated Drok, and Rsh overexpression will not rescue the drk/+ ARM deficits, as we describe (Fig. 5).
The ubiquitous Serine/Threonine kinase Drok is activated by the Rho1 GTPase, whose activation in turn requires Rho GTPase Activating Proteins (Rho-GAPs), and Drk physically interacts with multiple Rho-GAPs (33), likely bringing it in proximity with Drok. Finally, the Pleckstin homology domain of Drok indicates its membrane association and potential interaction with the actin cytoskeleton. Drk attenuation or inhibition of Drok activity precipitated reduction in F-actin and deficient ARM, suggesting that the deficit results from reduced ability to establish or maintain structural changes. In accord with its known functions (31), we propose that Drok mediates polymerization or stabilization, possibly of cortical actin filaments. We further propose that this activity-dependent cytoskeletal remodeling alters synaptic strength or properties, which underlies ARM formation, a hypothesis currently under investigation.
The structural plasticity model for ARM we propose being dependent on Drk/Drok-mediated actin dynamics is supported by known functions of the Rho1/Rho-GAP/Drok module. Rho1, Rho-GAPp190, and Drok activities have been shown to transduce Integrin-originating signals to the cytoskeleton essential for axonal growth of MB α lobes during development (27). Interestingly, Integrins are known to regulate RTK signaling by recruiting adaptors such as Drk to the membrane (34). Upon RTK activation, Integrins and their associated signaling molecules colocalize at focal adhesion sites and signal to the cytoskeleton, mediating its remodeling (34), likely via the Rho1/Rho-GAP/Drok module. Rok/Rho-GAPp190 and Integrins are involved in neuroplasticity because they are essential for fear memory in the rat (26) and for fly olfactory learning (35), respectively. Furthermore, multiple reports detail neurotransmitter-mediated structural synaptic plasticity in adult vertebrate neurons via Rho and Ras GTPases (summarized in ref. 36). In addition, the reported role of dopamine and serotonin in ARM (8, 10, 11) is consistent with the proposed structural plasticity model, as both neurotransmitters have been implicated in spine dynamics in both insects (37) and vertebrates (38, 39).
Collectively then, our results and the biochemical and interactome evidence detailed above lead us to propose that a molecular hallmark of ARM formation is activity-dependent localized structural and functional changes in the neuronal cytoskeleton that alter synaptic strength or properties, stable enough to last at least 24 h. Testing this hypothesis will provide essential insights into understanding not only the nature and function of the ARM form of consolidated memory and its relationship to LTM in flies but also its analogous process in vertebrates.
Materials and Methods
Drosophila culture, strains, genetics, and conditioning have been described before (17) and along with strains used are detailed in SI Materials and Methods. Fasudil (HA-1077; Sigma-Aldrich) dissolved in water was used to abrogate ARM. Confocal microscopy was performed using standard methods. Untransformed (raw) data were analyzed parametrically with the JMP 7.1 statistical software package (SAS Institute Inc.) as before (17). Detailed methods are presented in SI Materials and Methods.
SI Materials and Methods
Drosophila Culture, Strains, and Genetics.
Drosophila were raised and crosses set up in standard wheat–flour–sugar food supplemented with soy flour and CaCl2 (17) and cultured at 25 °C and 50% humidity with a 12-h light/dark cycle. The drkΔP24/CyO and drkE0A/CyO as well as the rsh1 mutant have been described previously (5, 17). Transgenic lines used in this study were as follows: UAS-drkRNAi1.2 (drkR-1.2) (17), UAS-Drok-CAT, and UAS-Drok-CAT-KG (28). The Gal4 driver lines c772-Gal4, c739-Gal4, NP1131-Gal4, and H24-Gal4 and one stock carrying a Drok RNAi encoding transgene (#28797) were obtained from the Bloomington Stock Center, whereas an independent Drok RNAi-encoding transgene (# 9774R-3) was obtained from NIG-FLY. Leo-Gal4 has been described previously (23). All transgenic lines were normalized to the w1118 genetic background with repeated backcrosses for at least six generations. The UAS-Drok-CAT and UAS-Drok-CAT-KG transgenes were introduced into the drkΔP24 mutant background by standard genetic crosses.
For behavioral experiments, non–balancer-bearing progeny from crosses of CyO-balanced drk males to w1118 females were used. To abrogate DRK levels, c772-Gal4, NP1131-Gal4, and H24-Gal4 males were crossed en masse to drkR-1.2 virgin females. Progeny of the cross between w1118 females and drkR-1.2 males were used as controls. Flies were collected, placed at 29 °C for 24 h for maximal transgene induction, and allowed 1 h recovery at 25 °C before training. For conditional abrogation of DRK in the adult MBs and rescue experiments with UAS-DrokCAT transgenes, the TARGET system was used (25). To that end, c772-Gal4;Tub-Gal80ts and c739-Gal4,Tub-Gal80ts and control w1118 females were crossed en masse to drkΔP24/CyO;UAS-DrokCAT or drkΔP24, UAS-DrokCAT/CyO or UAS-DrokCAT males. Progeny were reared at 18 °C, and the resultant nonbalanced 4–6-d-old flies were used for behavioral experiments. For conditional Drok abrogation, UAS-Drok RNAi encoding transgenes were crossed in the same manner to these drivers. To maximally induce the transgenes, flies were moved from 18 °C to 31 °C for 30–48 h, followed with a transfer to fresh vials and recovery at 25 °C for 1 h before conditioning. For the assessment of genetic interaction of drk and rsh, rsh1 homozygous females were crossed to drkΔP24/CyO males, and nonbalanced rsh1 hemizygous males or rsh1/+ heterozygous females for rsh were used. Male rsh1; hs-rsh flies (9) were obtained from S. Waddell, Oxford University, Oxford, and were crossed with drkΔP24/CyO females to obtain non–balancer-bearing males and rsh1/+ females for testing. The transgene was induced by incubation at 37 °C for 40 min, and such flies were tested behaviorally 40 min postinduction. Induction of the transgene was monitored by reverse transcription followed by PCR (RT-PCR) as described previously (17).
Drug Treatment.
Two- to 3-d-old adult w1118 flies were introduced in groups of 60–80 individuals to empty vials that contained ∼1 mL of Torula yeast in water (vehicle) or Fasudil (HA-1077; Sigma-Aldrich) dissolved in water at 200 μΜ. Flies were allowed to feed for 16 h and ∼30–40 min before conditioning were transferred to fresh normal media. Lower Fasudil concentrations were tested in pilot experiments but were ineffective.
Behavioral Analyses and Conditioning.
Behavioral experiments were performed under dim red light at 23–25 °C and 70–78% humidity as described before (17). All animals used were 3–6 d old and collected under light CO2 anesthesia 1 d before testing and kept in food vials in groups of 50–70 at 23–25 °C or 18 °C as appropriate for strains with Gal80ts temporal restriction of transgene expression. In all drug experiments, the experimenter was blind to the treatment. One cycle of olfactory conditioning training consisted of 12 CS/US pairings as described previously (10). For assessment of 3-h ARM, trained flies were transferred back to their vials at 25 °C, and 2 h posttraining, they were cold-shocked in prechilled glass vials on ice for 2 min. Complete anesthesia judged by immobility of the flies in the bottom of the glass tube was ascertained. After 2 min, flies were transferred back to vials at 25 °C and maintained until testing. They recovered from anesthesia within 20–30 s.
To assess 24-h memory after spaced training, flies were subjected to five cycles of conditioning using 12 CS/US pairing as detailed before (40). After training, the flies were transferred back to food vials and kept at 18 °C in a dark box. One hour before testing, they were transferred to 25 °C in the behavior room. For massed training, the same procedure was followed, except that flies were subjected to five consecutive cycles of 12 CS/US.
Phalloidin Staining and Confocal Microscopy.
Adult brains were dissected in cold PBS, fixed in 4% paraformaldehyde for 15 min, and permeabilized with 0.3% Triton X-100 in PBS. The brains were incubated with Rhodamine-Phalloidin (#R415; Invitrogen Molecular Probes) for 45 min at 25 °C to stain for F-actin. Confocal laser microscopy was performed using the Leica TCS SP8 system, and images at 40× magnification were obtained. Before acquisition, laser parameters were adjusted to obtain nonsaturating conditions, and samples were processed simultaneously using identical confocal acquisition parameters (laserpower, gain, and pinhole settings), as previously described (41). Serial optical sections of 0.5 μm thickness were obtained from the fixed whole-mount adult brain samples. Quantification of fluorescent staining in the MB calyces was obtained from one z-stack of each calyx per brain, and each stack was taken approximately at the same depth. Image processing was performed with ImageJ software. To obtain flies with maximally reduced Drk levels, LeoGal4 virgins were crossed en masse with drkΔP24/CyO; drkR-1.2 males, which in addition to the deletion allele bear a strong RNAi transgene for drk (5). Progeny raised at 25 °C were kept for 14–16 h at 30 °C before dissection. Fasudil was administered as described above. Multiple biological and technical replicates were performed (see sample numbers in the figure legends).
Data Analysis.
Untransformed (raw) data were analyzed parametrically with the JMP 7.1 statistical software package (SAS Institute Inc.) as described before (17). Following initial ANOVA, planned multiple comparisons were performed, using α = 0.05. The level of significance was adjusted for the experiment-wise error rate using Bonferroni correction. Detailed results of planned comparisons are mentioned in the figure legends. Data are shown as mean ± SEM. Imaging data were analyzed nonparametrically using Wilcoxon/Kruskal Wallis Rank Sums tests.
Acknowledgments
We thank Dr. L. Luo (Stanford University) for sharing Drok transgenic strains, Dr. S. Waddell (Oxford University) for the rsh mutant, the Bloomington Stock Center for Gal4 driver strains, and the Japanese National Institute of Genetics Fly Stock Center for stocks. We are indebted to Dr. E. M. Georganta for help with confocal microscopy.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1704835114/-/DCSupplemental.
References
- 1.Tully T, Preat T, Boynton SC, Del Vecchio M. Genetic dissection of consolidated memory in Drosophila. Cell. 1994;79:35–47. doi: 10.1016/0092-8674(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 2.Isabel G, Pascual A, Preat T. Exclusive consolidated memory phases in Drosophila. Science. 2004;304:1024–1027. doi: 10.1126/science.1094932. [DOI] [PubMed] [Google Scholar]
- 3.Busto GU, Cervantes-Sandoval I, Davis RL. Olfactory learning in Drosophila. Physiology (Bethesda) 2010;25:338–346. doi: 10.1152/physiol.00026.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lagasse F, Moreno C, Preat T, Mery F. Functional and evolutionary trade-offs co-occur between two consolidated memory phases in Drosophila melanogaster. Proc Biol Sci. 2012;279:4015–4023. doi: 10.1098/rspb.2012.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Folkers E, Waddell S, Quinn WG. The Drosophila radish gene encodes a protein required for anesthesia-resistant memory. Proc Natl Acad Sci USA. 2006;103:17496–17500. doi: 10.1073/pnas.0608377103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drier EA, et al. Memory enhancement and formation by atypical PKM activity in Drosophila melanogaster. Nat Neurosci. 2002;5:316–324. doi: 10.1038/nn820. [DOI] [PubMed] [Google Scholar]
- 7.Knapek S, Sigrist S, Tanimoto H. Bruchpilot, a synaptic active zone protein for anesthesia-resistant memory. J Neurosci. 2011;31:3453–3458. doi: 10.1523/JNEUROSCI.2585-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee PT, et al. Serotonin-mushroom body circuit modulating the formation of anesthesia-resistant memory in Drosophila. Proc Natl Acad Sci USA. 2011;108:13794–13799. doi: 10.1073/pnas.1019483108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu CL, Shih MF, Lee PT, Chiang AS. An octopamine-mushroom body circuit modulates the formation of anesthesia-resistant memory in Drosophila. Curr Biol. 2013;23:2346–2354. doi: 10.1016/j.cub.2013.09.056. [DOI] [PubMed] [Google Scholar]
- 10.Qin H, et al. Gamma neurons mediate dopaminergic input during aversive olfactory memory formation in Drosophila. Curr Biol. 2012;22:608–614. doi: 10.1016/j.cub.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scholz-Kornehl S, Schwärzel M. Circuit analysis of a Drosophila dopamine type 2 receptor that supports anesthesia-resistant memory. J Neurosci. 2016;36:7936–7945. doi: 10.1523/JNEUROSCI.4475-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horiuchi J, Yamazaki D, Naganos S, Aigaki T, Saitoe M. Protein kinase A inhibits a consolidated form of memory in Drosophila. Proc Natl Acad Sci USA. 2008;105:20976–20981. doi: 10.1073/pnas.0810119105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heisenberg M. Mushroom body memoir: From maps to models. Nat Rev Neurosci. 2003;4:266–275. doi: 10.1038/nrn1074. [DOI] [PubMed] [Google Scholar]
- 14.Davis RL. Traces of Drosophila memory. Neuron. 2011;70:8–19. doi: 10.1016/j.neuron.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crittenden JR, Skoulakis EMC, Han K-A, Kalderon D, Davis RL. Tripartite mushroom body architecture revealed by antigenic markers. Learn Mem. 1998;5:38–51. [PMC free article] [PubMed] [Google Scholar]
- 16.Yang CH, et al. Additive expression of consolidated memory through Drosophila mushroom body subsets. PLoS Genet. 2016;12:e1006061. doi: 10.1371/journal.pgen.1006061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moressis A, Friedrich AR, Pavlopoulos E, Davis RL, Skoulakis EM. A dual role for the adaptor protein DRK in Drosophila olfactory learning and memory. J Neurosci. 2009;29:2611–2625. doi: 10.1523/JNEUROSCI.3670-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Folkers E, Drain P, Quinn WG. Radish, a Drosophila mutant deficient in consolidated memory. Proc Natl Acad Sci USA. 1993;90:8123–8127. doi: 10.1073/pnas.90.17.8123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guven-Ozkan T, Davis RL. Functional neuroanatomy of Drosophila olfactory memory formation. Learn Mem. 2014;21:519–526. doi: 10.1101/lm.034363.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang T, Branch A, Shen P. Octopamine-mediated circuit mechanism underlying controlled appetite for palatable food in Drosophila. Proc Natl Acad Sci USA. 2013;110:15431–15436. doi: 10.1073/pnas.1308816110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aso Y, et al. The mushroom body of adult Drosophila characterized by GAL4 drivers. J Neurogenet. 2009;23:156–172. doi: 10.1080/01677060802471718. [DOI] [PubMed] [Google Scholar]
- 22.Scheunemann L, et al. Consolidated and labile odor memory are separately encoded within the Drosophila brain. J Neurosci. 2012;32:17163–17171. doi: 10.1523/JNEUROSCI.3286-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Messaritou G, Leptourgidou F, Franco M, Skoulakis EM. A third functional isoform enriched in mushroom body neurons is encoded by the Drosophila 14-3-3zeta gene. FEBS Lett. 2009;583:2934–2938. doi: 10.1016/j.febslet.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 24.McGuire SE, Le PT, Davis RL. The role of Drosophila mushroom body signaling in olfactory memory. Science. 2001;293:1330–1333. doi: 10.1126/science.1062622. [DOI] [PubMed] [Google Scholar]
- 25.McGuire SE, Mao Z, Davis RL. Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci STKE. 2004;2004:pl6. doi: 10.1126/stke.2202004pl6. [DOI] [PubMed] [Google Scholar]
- 26.Lamprecht R, Farb CR, LeDoux JE. Fear memory formation involves p190 RhoGAP and ROCK proteins through a GRB2-mediated complex. Neuron. 2002;36:727–738. doi: 10.1016/s0896-6273(02)01047-4. [DOI] [PubMed] [Google Scholar]
- 27.Billuart P, Winter CG, Maresh A, Zhao X, Luo L. Regulating axon branch stability: The role of p190 RhoGAP in repressing a retraction signaling pathway. Cell. 2001;107:195–207. doi: 10.1016/s0092-8674(01)00522-0. [DOI] [PubMed] [Google Scholar]
- 28.Winter CG, et al. Drosophila Rho-associated kinase (Drok) links frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell. 2001;105:81–91. doi: 10.1016/s0092-8674(01)00298-7. [DOI] [PubMed] [Google Scholar]
- 29.Ng J, Luo L. Rho GTPases regulate axon growth through convergent and divergent signaling pathways. Neuron. 2004;44:779–793. doi: 10.1016/j.neuron.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 30.Moon W, Matsuzaki F. Aurora A kinase negatively regulates Rho-kinase by phosphorylation in vivo. Biochem Biophys Res Commun. 2013;435:610–615. doi: 10.1016/j.bbrc.2013.05.028. [DOI] [PubMed] [Google Scholar]
- 31.Loirand G. Rho kinases in health and disease: From basic science to translational research. Pharmacol Rev. 2015;67:1074–1095. doi: 10.1124/pr.115.010595. [DOI] [PubMed] [Google Scholar]
- 32.Gentry EG, et al. Rho kinase inhibition as a therapeutic for progressive supranuclear palsy and corticobasal degeneration. J Neurosci. 2016;36:1316–1323. doi: 10.1523/JNEUROSCI.2336-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friedman AA, et al. Proteomic and functional genomic landscape of receptor tyrosine kinase and ras to extracellular signal-regulated kinase signaling. Sci Signal. 2011;4:rs10. doi: 10.1126/scisignal.2002029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim SH, Turnbull J, Guimond S. Extracellular matrix and cell signalling: The dynamic cooperation of integrin, proteoglycan and growth factor receptor. J Endocrinol. 2011;209:139–151. doi: 10.1530/JOE-10-0377. [DOI] [PubMed] [Google Scholar]
- 35.Grotewiel MS, Beck CD, Wu KH, Zhu XR, Davis RL. Integrin-mediated short-term memory in Drosophila. Nature. 1998;391:455–460. doi: 10.1038/35079. [DOI] [PubMed] [Google Scholar]
- 36.Ponimaskin E, Voyno-Yasenetskaya T, Richter DW, Schachner M, Dityatev A. Morphogenic signaling in neurons via neurotransmitter receptors and small GTPases. Mol Neurobiol. 2007;35:278–287. doi: 10.1007/s12035-007-0023-0. [DOI] [PubMed] [Google Scholar]
- 37.Kloppenburg P, Mercer AR. Serotonin modulation of moth central olfactory neurons. Annu Rev Entomol. 2008;53:179–190. doi: 10.1146/annurev.ento.53.103106.093408. [DOI] [PubMed] [Google Scholar]
- 38.Yagishita S, et al. A critical time window for dopamine actions on the structural plasticity of dendritic spines. Science. 2014;345:1616–1620. doi: 10.1126/science.1255514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Castillo-Gómez E, Varea E, Blasco-Ibáñez JM, Crespo C, Nacher J. Effects of chronic dopamine D2R agonist treatment and polysialic acid depletion on dendritic spine density and excitatory neurotransmission in the mPFC of adult rats. Neural Plast. 2016;2016:1615363. doi: 10.1155/2016/1615363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pavlopoulos E, Anezaki M, Skoulakis EMC. Neuralized is expressed in the α/β lobes of adult Drosophila mushroom bodies and facilitates olfactory long-term memory formation. Proc Natl Acad Sci USA. 2008;105:14674–14679. doi: 10.1073/pnas.0801605105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fulga TA, et al. Abnormal bundling and accumulation of F-actin mediates tau-induced neuronal degeneration in vivo. Nat Cell Biol. 2007;9:139–148. doi: 10.1038/ncb1528. [DOI] [PubMed] [Google Scholar]