Significance
Carotenoids are plant-derived pigment molecules that cannot be synthesized de novo by higher organisms. These physiologically relevant compounds function as potent antioxidants and light screening compounds, and their supplementation has been shown to ameliorate the progression of such diseases as age-related macular degeneration. Hundreds of carotenoids are present in the plant world, but the primate macula contains only three: lutein, zeaxanthin, and meso-zeaxanthin. The presence of meso-zeaxanthin in the foveal region of primates is an unexplained phenomenon, given its lack of dietary sources. We show that RPE65 is responsible for the conversion of lutein to meso-zeaxanthin in vertebrates, a unique role for RPE65 in carotenoid metabolism beyond its well-known retinoid isomerohydrolase function in the vertebrate visual cycle.
Keywords: carotenoid, isomerase, retina, lutein, zeaxanthin
Abstract
Carotenoids are plant-derived pigment molecules that vertebrates cannot synthesize de novo that protect the fovea of the primate retina from oxidative stress and light damage. meso-Zeaxanthin is an ocular-specific carotenoid for which there are no common dietary sources. It is one of the three major carotenoids present at the foveal center, but the mechanism by which it is produced in the eye is unknown. An isomerase enzyme is thought to be responsible for the transformation of lutein to meso-zeaxanthin by a double-bond shift mechanism, but its identity has been elusive. We previously found that meso-zeaxanthin is produced in a developmentally regulated manner in chicken embryonic retinal pigment epithelium (RPE)/choroid in the absence of light. In the present study, we show that RPE65, the isomerohydrolase enzyme of the vertebrate visual cycle that catalyzes the isomerization of all-trans-retinyl esters to 11-cis-retinol, is also the isomerase enzyme responsible for the production of meso-zeaxanthin in vertebrates. Its RNA is up-regulated 23-fold at the time of meso-zeaxanthin production during chicken eye development, and we present evidence that overexpression of either chicken or human RPE65 in cell culture leads to the production of meso-zeaxanthin from lutein. Pharmacologic inhibition of RPE65 function resulted in significant inhibition of meso-zeaxanthin biosynthesis during chicken eye development. Structural docking experiments revealed that the epsilon ring of lutein fits into the active site of RPE65 close to the nonheme iron center. This report describes a previously unrecognized additional activity of RPE65 in ocular carotenoid metabolism.
The rare carotenoid meso-zeaxanthin is present only in the eyes of higher vertebrates (1, 2). This is a unique phenomenon in nature, especially since vertebrates normally obtain carotenoids through their diet and are incapable of producing these molecules de novo (2, 3). meso-zeaxanthin is not commonly found in dietary sources; besides the eyes of vertebrates, this carotenoid is present in shrimp shells, turtle fat, and fish skin (2, 4, 5). Hundreds of carotenoids are present in nature, and even though primates consume more than 50 of them, meso-zeaxanthin is one of only three carotenoids present in the foveal center of the retina, the region responsible for sharp, central vision. Degeneration of the retina and retinal pigment epithelium (RPE) surrounding the fovea occurs in the disease state known as age-related macular degeneration (AMD). Carotenoid supplementation has been shown to be effective in curtailing the progression of this disease, because these molecules are capable of protecting the fovea from blue light damage and reactive oxygen species (2, 6). Despite the abundance of meso-zeaxanthin in the foveal center, its specific function relative to dietary lutein and zeaxanthin remains unknown.
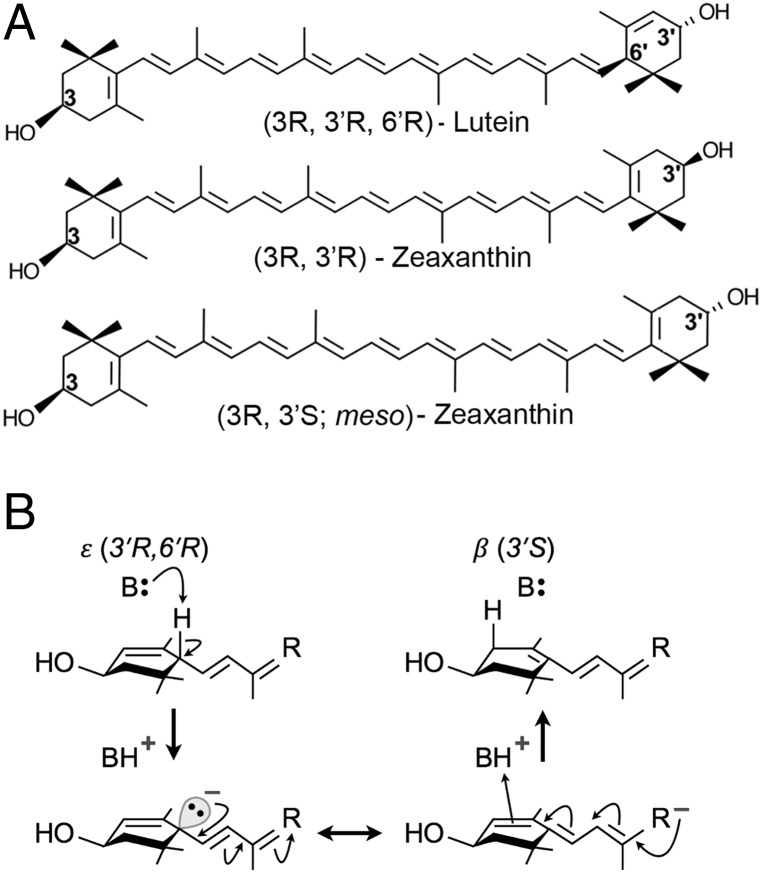
Lutein, zeaxanthin, and meso-zeaxanthin are the three carotenoids present at the foveal center (2). These molecules share the same molecular formula, C40H56O2 (Fig. 1A). Since lutein and zeaxanthin are abundant in a normal diet, it has long been hypothesized that an isomerase enzyme may be responsible for the metabolic transformations of either lutein or zeaxanthin to produce meso-zeaxanthin (2). In vivo studies from our laboratory using quail have shown that birds fed with deuterium-labeled lutein produce labeled meso-zeaxanthin, while labeled zeaxanthin feed did not have the same effect (7). Similar results have been observed in primates as well. When carotenoid-deficient monkeys were fed lutein, meso-zeaxanthin was present in their retinas, but no meso-zeaxanthin was detected when these animals were maintained on feed enriched with zeaxanthin (8). Both of these studies indicate that lutein undergoes metabolic transformations to form meso-zeaxanthin in vivo, but the biochemical mechanism by which this reaction occurs is unknown, although it is efficiently produced under harsh industrial conditions, such as high temperature and strong base (9). Conversion of dietary zeaxanthin to meso-zeaxanthin would require inversion of a chiral center at the 3′ position, a reaction rarely encountered in biological systems. In contrast, conversion of lutein to meso-zeaxanthin proceeds by the migration of just one double bond from the 4′-5′ position to the 5′-6′ position, a reaction that should be readily accomplished by coordinated acid-base catalysis as illustrated in Fig. 1B. Other mechanisms involving radical chemistry are also plausible.
Fig. 1.
Structures of macular xanthophylls (A) and simple mechanism of coordinated acid-base catalysis of lutein to meso-zeaxanthin (B). The first step involves base-catalyzed proton abstraction from the ε-ionone ring at position C6′. The resulting negative charge on the intermediate is expected to be resonance-stabilized (double-headed arrow). In the final step, BH+ acts as a source of proton for attachment to the ionone ring at the new position, C4′. The mechanism shown here illustrates how conversion might occur in vivo; alternate mechanisms involving radical chemistry (e.g., hydrogen atom transfer) are also possible.
In an effort to determine the biochemistry behind meso-zeaxanthin formation, our laboratory undertook studies in developing chicken embryos. In this isolated system, we determined that meso-zeaxanthin is produced in a developmentally regulated manner in the RPE/choroid of chicken embryos from the lutein and zeaxanthin naturally present in egg yolk (10). In a previous study, we detected the presence of meso-zeaxanthin in the RPE/choroid of E17 embryos, with increasing levels as the embryo neared hatching at E21 (10). Retinal detection of meso-zeaxanthin occurred only at E19, and all other tissues examined (brain, liver, serum, and yolk) were devoid of this carotenoid. Since the eggs were incubated in the dark, we could rule out the role of light in meso-zeaxanthin production. In the current study, we present evidence that RPE65, the isomerohydrolase enzyme of the vertebrate visual cycle responsible for the isomerization of all-trans-retinyl palmitate to 11-cis-retinol (11–14), is the lutein to meso-zeaxanthin isomerase.
Results
RPE65 Transcript and Protein Levels Are Significantly Up-Regulated in E21 Chicken RPE/Choroid.
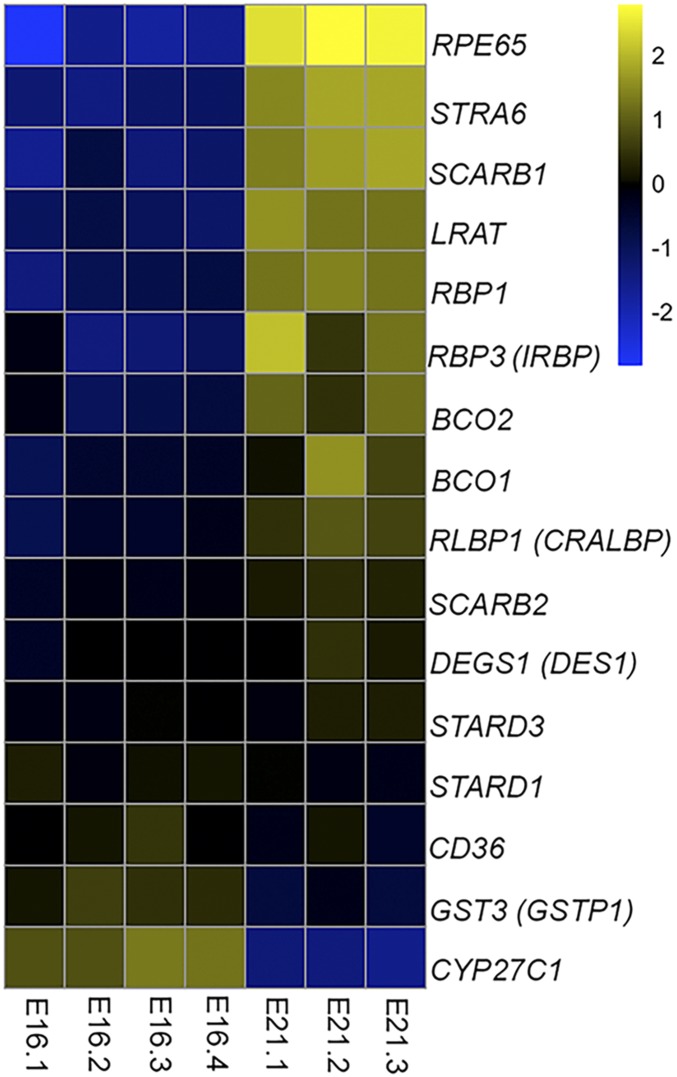
To identify the enzyme responsible for the production of meso-zeaxanthin in chicken RPE/choroid, we performed RNA sequencing to ascertain which transcripts of likely candidates were up-regulated. We used total RNA isolated from E16 RPE/choroid, a stage at which meso-zeaxanthin is not detectable in the RPE/choroid, and compared the expression profile of mRNA transcripts with those of E21 RPE/choroid, a stage at which substantial amounts of meso-zeaxanthin are present. Log2 FPKM values of at least three embryos from each stage were compared, and the relative abundance of gene transcripts normally involved in either carotenoid metabolism and transport (GSTP1, STARD3, STARD1, BCO1, BCO2, SCARB1, SCARB2, and CD36) or retinoid metabolism and transport (RBP1, IRBP, CRALBP, RPE65, LRAT, CYP27C1, STRA6, and DES1) are plotted in Fig. 2. Among the genes that we considered, RPE65 was the most highly up-regulated between E16 and E21. Its transcript levels were 23-fold higher at E21 compared with at E16. GSTP1 and STARD3 are zeaxanthin- and lutein-binding proteins, respectively (15, 16), and their transcript levels did not show any significant increase between E16 and E21. BCO1 and BCO2 are carotenoid oxygenases (17–20), and both of their genes were slightly up-regulated at E21. STRA6 is a retinol transport protein (21), and DES1 is a vitamin A isomerase expressed in the Müller cells of the retina (22). While STRA6 mRNA was up-regulated at E21, its magnitude of increase was lower than that observed for RPE65. DES1 mRNA levels were not changed during development. SCARB1, SCARB2, and CD36 are carotenoid transport proteins in the eye (23). While SCARB1 showed increased levels between E16 and E21, it is an unlikely candidate to catalyze the production of meso-zeaxanthin. LRAT, the acyl transferase enzyme required for visual pigment regeneration, was moderately up-regulated at E21 (12–14). CYP27C1, a protein known to convert vitamin A1 to vitamin A2 (24), showed a decrease in transcript abundance between E16 and E21. No significant differences in expression were observed for retinoid transporters, such as IRBP, CRALBP, and RBP1. From the RNA sequencing data, we concluded that among the relevant genes involved in the visual cycle and carotenoid metabolism, RPE65 is most likely to be responsible for meso-zeaxanthin production, especially since it is a relative of two carotenoid metabolic enzymes, BCO1 and BCO2.
Fig. 2.
Comparison of gene expression profiles in E16 and E21 chicken RPE/choroid. The FPKM value of each gene is compared with its expression across all samples to obtain the average expression. The ratio of gene expression in each sample is compared with the average, and the values are plotted on a log base2 scale. Positive values indicate above-average expression; negative values, below-average expression.
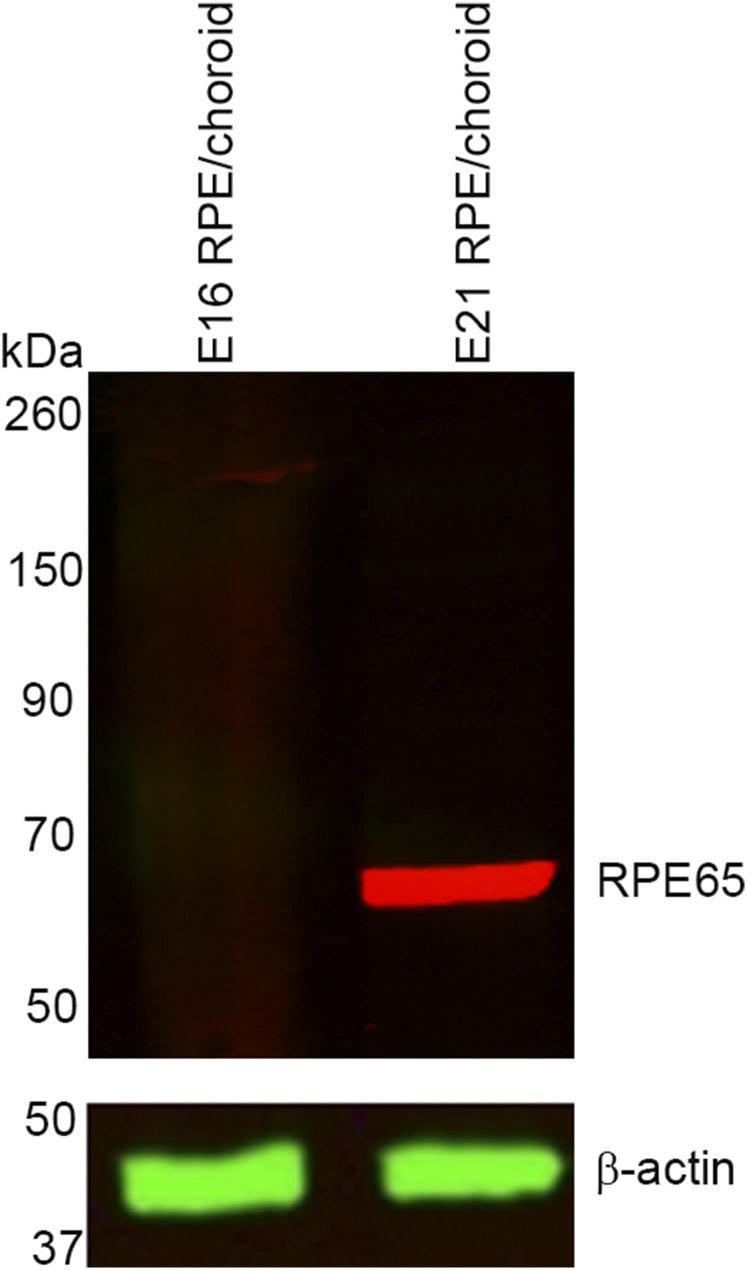
We next determined whether the protein expression profile of RPE65 showed a similar trend as the mRNA levels. No RPE65 protein was detected in E16 chicken RPE/choroid, whereas strong expression was observed in E21 tissue (Fig. S1).
Fig. S1.
No RPE65 protein is expressed in E16 RPE/choroid, whereas E21 RPE/choroid shows abundant presence of this protein.
Overexpression of RPE65 Leads to the Production of meso-Zeaxanthin in HEK293T Cells.
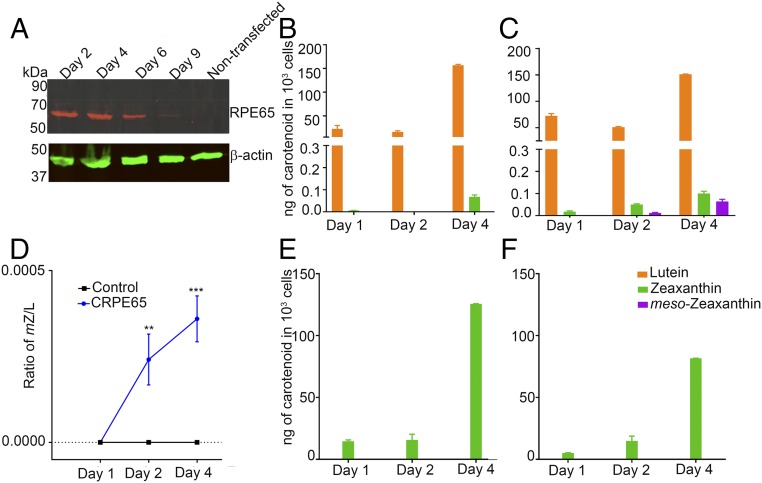
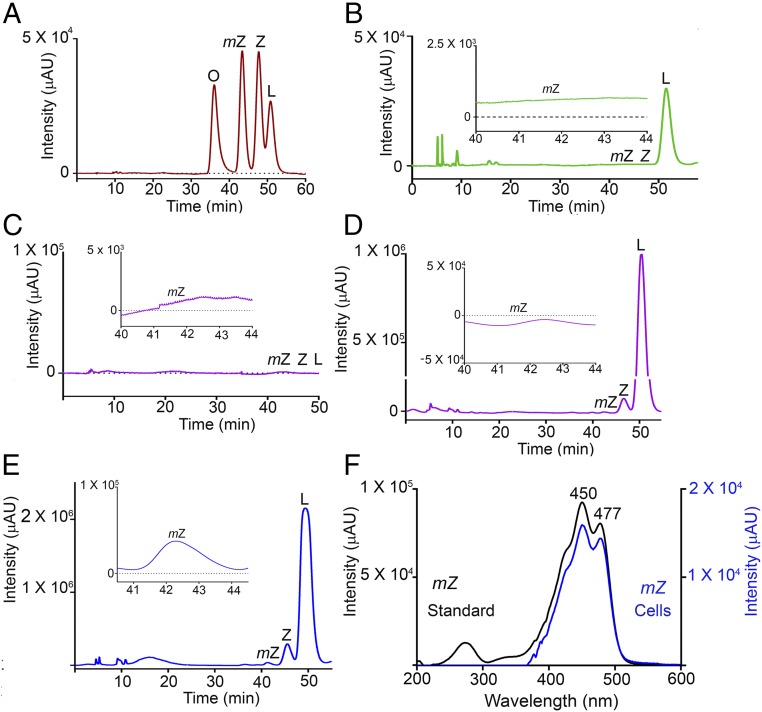
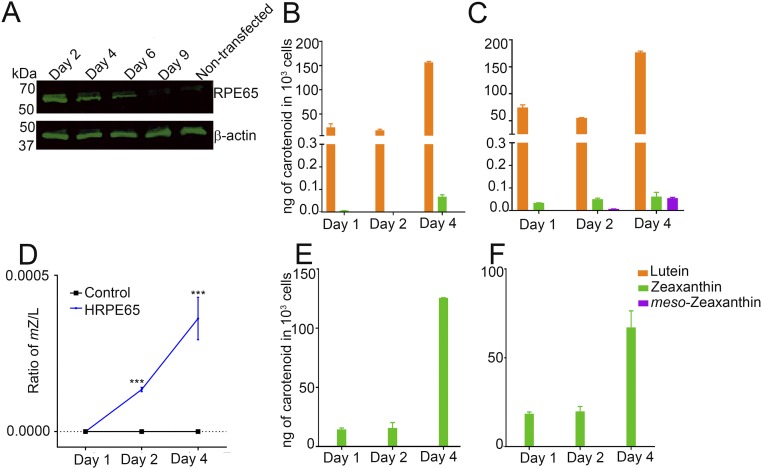
To determine whether RPE65 can catalyze the conversion of lutein to meso-zeaxanthin, we used the nonocular cell line HEK293T, which is derived from human embryonic kidney and does not express RPE65 or LRAT (12, 13). Overexpression of pCDNA3.1-CRPE65 (chicken RPE65) resulted in strong expression of RPE65 at 48 h posttransfection that was sustained for another 4 d, while nontransfected cells had no RPE65 (Fig. 3A). We supplied HPLC-purified lutein with no detectable meso-zeaxanthin and <0.5% zeaxanthin (Fig. 4B) to HEK293T cells with no endogenous carotenoids (Fig. 4C). Treatment with 4 μM lutein for up to 4 d resulted in the production of progressively higher levels of meso-zeaxanthin in RPE65-overexpressing cells, while control cells transfected with pCDNA3.1-GFP did not show the presence of meso-zeaxanthin (Figs. 3 B and C and 4 D and E). The identity of biosynthesized meso-zeaxanthin was confirmed by comparing its retention time with that of authentic carotenoid standards (Fig. 4A) and by the observation of its characteristic tripeak spectrum with the highest peak at 450 nm using in-line photodiode array detection (Fig. 4F and Fig. S2). We observed small amounts of zeaxanthin in our experimental and control cells that increased in a time-dependent manner (Fig. 3 B and C). This is likely because our lutein stock, even though devoid of meso-zeaxanthin, contains ∼0.5% zeaxanthin. To rule out zeaxanthin as the precursor to meso-zeaxanthin in CRPE65-overexpressing cells, we treated these cells with 4 μM isomerically pure zeaxanthin with no detectable lutein. Neither the control nor the experimental cells showed any detectable levels of meso-zeaxanthin or lutein (Fig. 3 E and F).
Fig. 3.
CRPE65 overexpression followed by lutein treatment gives rise to meso-zeaxanthin in HEK293T cells. Nontransfected HEK293T cells do not express RPE65, but transient transfection results in expression of this gene for several days (A). Treatment of CRPE65-transfected cells with 4 μM lutein resulted in meso-zeaxanthin production (C), whereas the cells overexpressing control plasmid were devoid of meso-zeaxanthin (B). The ratio of meso-zeaxanthin to lutein shows an increase in the latter in a time-dependent manner (D). Treatment with 4 μM zeaxanthin in control plasmid-overexpressing cells (E) or CRPE65-overexpressing cells did not result in meso-zeaxanthin production (F). n = 3. Error bars represent SEM. **P < 0.005; ***P < 0.0005.
Fig. 4.
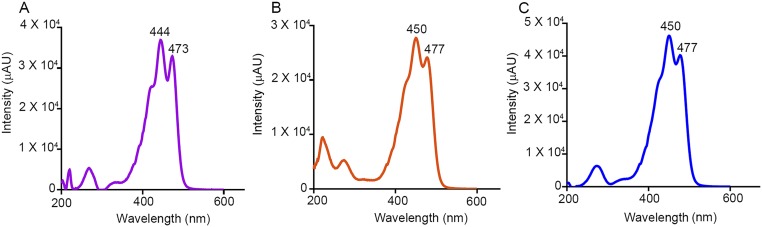
Chromatograms showing the presence of carotenoids in HEK293T cells. (A) Retention times of authentic carotenoid standard mixture. (B) The HPLC-purified lutein used in our incubations did not contain any meso-zeaxanthin. (C) HEK293T cells were free of carotenoids. (D and E) Overexpression of pCDNA3.1-GFP followed by treatment with 4 μM lutein for 4 d did not result in meso-zeaxanthin production (D), whereas cells overexpressing CRPE65 when treated with 4 μM lutein for 2 d gave rise to detectable levels of meso-zeaxanthin (E). (F) Characteristic tripeak spectrum obtained for meso-zeaxanthin from authentic standard (black) and CRPE65-overexpressing cells (blue) (Fig. S2). (Insets) Zoom-in views (20×). L, lutein; mZ, meso-zeaxanthin; O, oxo-lutein; Z, zeaxanthin.
Fig. S2.
Representative spectra of carotenoid standards in HPLC running solvent measured by an in-line photodiode array detector. Characteristic peaks of lutein are detected at 444 nm and 473 nm (A), whereas zeaxanthin (B) and meso-zeaxanthin (C) peaks are detected at 450 nm and 477 nm.
Previous studies have shown that CRPE65 is a better isomerohydrolase than its human counterpart (25). We conducted our next set of experiments to determine whether there were any differences between human RPE65 (HRPE65) and CRPE65 in meso-zeaxanthin isomerization (Fig. S3). We observed that HRPE65 transfection resulted in slightly lower meso-zeaxanthin production compared with CRPE65 at day 2; however, by day 4, both CRPE65- and HRPE65-overexpressing cells contained similar levels of meso-zeaxanthin (Fig. 3C and Fig. S3C). As with CRPE65, treatment of HRPE65-overexpressing cells with 4 μM zeaxanthin resulted in no detectable meso-zeaxanthin, and no meso-zeaxanthin was present in the control cells (Fig. S3 E and F). Comparison of the ratio of lutein to meso-zeaxanthin in CRPE65- and HRPE65-overexpressing cells revealed a conversion rate of <0.05% over a 4-d period (Fig. 3D and Fig. S3D).
Fig. S3.
HRPE65 overexpression followed by lutein treatment results in meso-zeaxanthin production in HEK293T cells. (A) No endogenous RPE65 expression was detected in HEK293T cells, whereas overexpression of pCDNA3.1-human RPE65 resulted in gene expression that was detectable until 6 d posttransfection. (B and C) Control plasmid-transfected cells treated with lutein did not produce meso-zeaxanthin (B), whereas HRPE65 cells gave rise to this carotenoid (C). (D) The ratio of meso-zeaxanthin to lutein increased in HRPE65-overexpressing cells In a time-dependent manner. (E and F) Treatment of control plasmid-overexpressing cells (E) or HRPE65-overexpressing cells (F) with zeaxanthin did not result in the production of meso-zeaxanthin. n = 3. Error bars represent SEM. ***P < 0.0005.
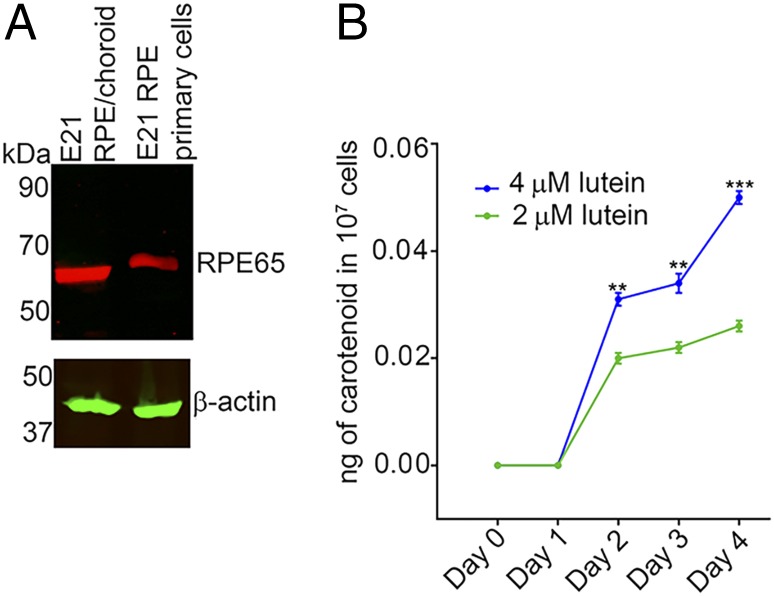
E21 Chicken RPE Primary Cells Retain RPE65 Expression and Can Produce Meso-Zeaxanthin.
In these experiments, we explored whether chicken RPE primary cultures from E21 embryos are capable of producing meso-zeaxanthin on lutein treatment. Unlike human primary RPE cells, these cells retain the expression of RPE65 even after five passages (26) (Fig. 5A). Treatment of 107 cells with 2 μM lutein for 2 d resulted in detectable levels of meso-zeaxanthin (Fig. 5B). Between days 2 and 4, we observed a progressive increase in meso-zeaxanthin levels. To determine whether a higher precursor concentration will result in increased production of meso-zeaxanthin, we treated these cells with 4 μM lutein. They produced significantly higher amounts of meso-zeaxanthin at days 2, 3, and 4 relative to the cells treated with 2 μM lutein (Fig. 5B).
Fig. 5.
Endogenous RPE65 in chicken RPE primary cells can catalyze the production of meso-zeaxanthin. (A) RPE primary cells from E21 chicken embryos retain RPE65 expression. (B) When treated with 2 μM lutein for 2 d, these cells produce meso-zeaxanthin, and higher levels of meso-zeaxanthin production are observed when cells are treated with 4 μM lutein. n = 3. Error bars represent SEM. **P < 0.005; ***P < 0.0005.
Pharmacologic Inhibition of RPE65 in the Developing Chicken Embryo Decreases meso-Zeaxanthin Levels.
We next examined whether pharmacologic inhibition of RPE65 activity could specifically inhibit meso-zeaxanthin production. Our previous studies have shown that meso-zeaxanthin is first present at detectable levels in the chicken RPE/choroid at E17. Therefore, we decided to introduce a competitive inhibitor of RPE65 activity into the yolk sac of chicken embryos at E17.
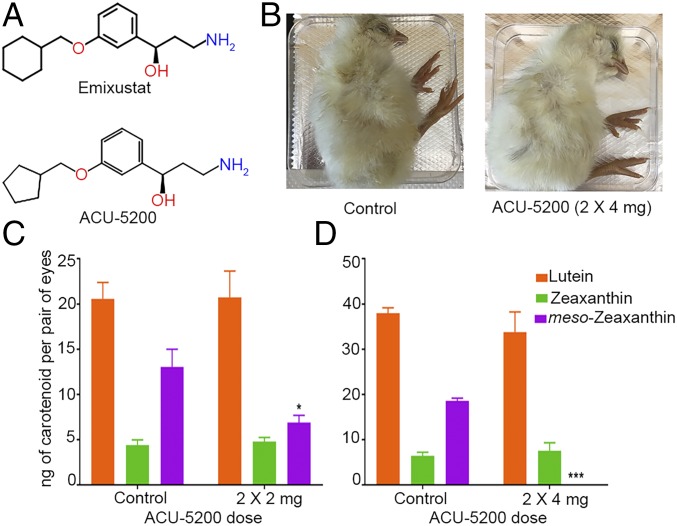
We used the pharmacologic inhibitor ACU-5200-HCl (ACU-5200) to knock down RPE65 function. ACU-5200 is an analog of emixustat, a highly specific RPE65 inhibitor that is currently undergoing clinical trials as a treatment for Stargardt disease (27–29) (Fig. 6A). ACU-5200 has been shown to inhibit production of 11-cis-retinoids in animals at very low oral doses (ED50 = 0.27 mg/kg in mice; proprietary data provided by Acucela Inc.). We injected various doses of ACU-5200 into the yolk sac of the developing embryo at E17 and then again at E19. The ACU-5200–injected embryos developed normally and had no obvious phenotypic abnormalities (Fig. 6B). Injection of two 2-mg doses (2 × 2 mg) of ACU-5200 resulted in significant down-regulation of RPE/choroid meso-zeaxanthin levels (Fig. 6C). Doubling this dose resulted in complete absence of meso-zeaxanthin in the injected embryos’ RPE/choroid (Fig. 6D). The RPE/choroid lutein and zeaxanthin contents of the ACU-5200–injected embryos remained comparable to those of control embryos (Fig. 6 C and D). These results indicate that in an in vivo system, the function of RPE65 is necessary for the production of meso-zeaxanthin.
Fig. 6.
ACU-5200 injection inhibits meso-zeaxanthin production in the RPE/choroid of developing chicken embryos. (A) ACU-5200 is an analog of emixustat. (B) The development of ACU-5200–injected embryos was comparable to that of control embryos. (C and D) No significant differences in lutein or zeaxanthin levels were observed in ACU-5200–injected embryos compared with corresponding control embryos. meso-zeaxanthin levels were either significantly down-regulated or completely absent following ACU-5200 injection. n ≥ 5. Error bars represent SEM. *P < 0.05; ***P < 0.0005.
Structural Docking Experiments Reveal That the Epsilon Ring of Lutein Can Fit into the Active Site of RPE65.
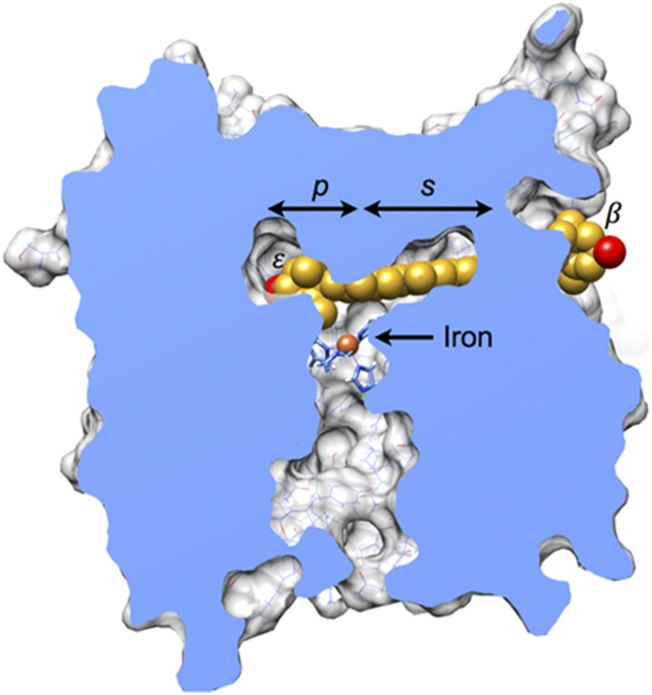
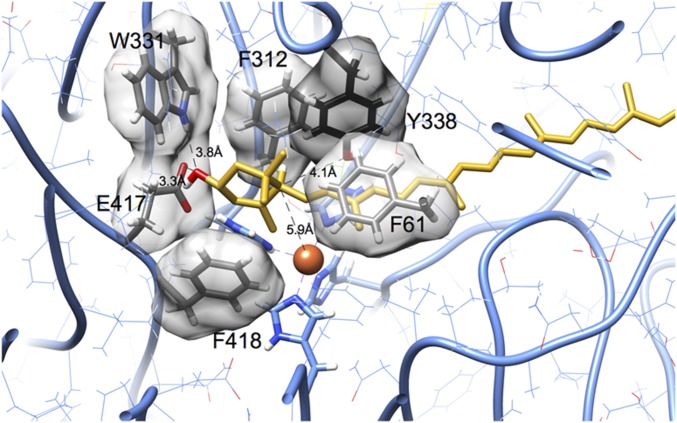
To determine whether lutein fits into the substrate tunnel of RPE65, we docked lutein molecules into a homology model for chicken RPE65. Fig. 7 shows a representative outcome that minimizes steric conflicts and maximizes hydrogen bonds. The ε-ionone ring of lutein rests on a ledge comprising the iron-coordinating histidine residues, and the β-ionone ring protrudes from an opening at the surface of the enzyme. The buried hydroxyl group is positioned in proximity with two hydrogen-bonding groups (indole amine of Trp-331 and carboxylate of Glu-417; Fig. S4). Steric complementarity is evident, with phenylalanine residues making a close approach on either side of the ε-ionone ring and also stacking with the polyene chain. The edge of the ionone ring containing atoms C4′, C5′, and C6′, which are involved in isomerization, was consistently found closer to the iron center and histidine residues. Docking outcomes with these atoms pointed away from the iron center were not observed among well-fitting outcomes, likely because in this orientation, the curvature observed for lutein molecules does not match the curvature of the substrate tunnel found in RPE65.
Fig. 7.
Model of RPE65 complexed with lutein. A molecule of lutein (carbon, gold; oxygen red) is shown docked into a homology model of chicken RPE65. In this view, much of the protein structure has been cut away to reveal the substrate tunnel found in the interior. The palmitate-binding pocket (p) is above and to the left of the iron center, overlapping with the ε-ionone ring of lutein. The polyene chain extends through the presumed substrate-binding pocket (s), defined by the structure of the enzyme in complex with its competitive inhibitor emixustat (29). The β-ionone ring emerges at an opening found on the surface of the enzyme. A more detailed view of molecular interactions is provided in Fig. S4. Energy minimization was not used for these coarse-grid rigid-body docking experiments. The representative result shown here was selected from >33,000 trials because it has few steric clashes (n = 9; clash defined as interatomic distance less than 3 Å) and makes two hydrogen bonds. Steric conflicts would likely resolve on subtle adjustments of torsion angles in the lutein molecule and repositioning of sidechains. For comparison, lutein docked into the tunnel-like cavity of StARD3 with the same rigid-body protocol experiences 14 clashes (42), ligands palmitate and emixustat bound to RPE65 have two clashes, and lutein molecules found in structures of chlorophyll-binding proteins frequently have no clashes (42). This figure was prepared with UCSF Chimera (43, 44).
Fig. S4.
Closeup view of lutein (gold) complexed with RPE65 (gray). The ε-ionone ring fits into a pocket lined with aromatic sidechains. The hydroxyl group is close to potential hydrogen bond donors and acceptors in residues Trp-331 and Glu-417. These residues participate in a hydrogen-bonded network that could be rearranged to include the hydroxyl group of lutein through modest adjustments of side chain rotamers. During the course of isomerization, a hydrogen atom attached to C6′ moves to C4′. The iron center (orange sphere) is closest to ring carbon C5′, and approximately equidistant to C6′ and C4′. The C6′ atom is 4.1 Å from the hydroxyl group of Tyr-338 and 5.9 Å from the iron center (orange sphere). This figure was prepared with UCSF Chimera (43, 44).
Discussion
RPE65 is an important enzyme in the visual cycle, responsible for the key all-trans to 11-cis-retinoid isomerization step of the visual cycle (11–13). Such diseases as Leber congenital amaurosis and retinitis pigmentosa arise from the loss of function of this gene (12, 13). Here we show that RPE65 is capable of carrying out an additional function in which it converts lutein to meso-zeaxanthin, an eye-specific carotenoid with no common dietary sources. Since meso-zeaxanthin is accumulated in high concentrations at the fovea of the retina, a region crucial for visual acuity, its hypothesized function is to protect the region from blue light damage and oxidative stress and to potentially enhance visual function. In support of this hypothesis, a previous study has shown that meso-zeaxanthin has stronger antioxidant properties than lutein and zeaxanthin (30), and another study has shown that oral supplementation with all three macular carotenoids can improve contrast sensitivity in normal individuals (31, 32). The process by which meso-zeaxanthin is produced in the eye has been a mystery. In the present study, we show in both in vitro and in vivo systems that RPE65 is the enzyme that catalyzes the conversion of lutein to meso-zeaxanthin.
After identifying RPE65 as a prime candidate for the meso-zeaxanthin isomerase in chicken RPE by RNA sequencing studies, we conducted overexpression experiments using both chicken and human RPE65 plasmids in a nonocular cell culture system. Our studies in HEK293T cells show that RPE65 of both species is capable of producing meso-zeaxanthin from lutein, but not from zeaxanthin. HEK293T cells do not endogenously express LRAT, the acyl transferase enzyme essential to provide all-trans fatty acid ester retinoid substrates for RPE65 to catalyze their conversion into 11-cis-retinol (12, 13, 25). By overexpressing RPE65 in a system free of LRAT and treating these cells with HPLC-purified lutein, we were able to produce meso-zeaxanthin independent of LRAT’s catalytic activity. The reaction is slow in cell culture, with no detectable product observed until several days after the addition of lutein. This is consistent with the relatively slow formation of meso-zeaxanthin during chicken eye development, which also takes several days (10).
Our structural modeling studies show that the epsilon ring of lutein can coordinate with the active site histidines and iron of RPE65 in a manner that could facilitate the double-bond shift reaction required to convert lutein to meso-zeaxanthin by a mechanism involving acid-base catalysis (Fig. 1B) or some other mechanism. We also found that an RPE65 inhibitor, ACU-5200, was able to specifically inhibit formation of meso-zeaxanthin during chicken eye development without affecting lutein or zeaxanthin uptake into the RPE/choroid. Its close analog, emixustat, is currently in clinical trials as a visual cycle inhibitor for various eye diseases (27–29). Our finding of RPE65’s additional role in macular carotenoid metabolism suggests that it may be of interest to examine whether this compound detectably alters macular pigment levels or distributions in the participants in these clinical trials.
Mutations in human RPE65 are quite rare and typically cause severe visual function deficits, and we suspect that individuals with deleterious mutations in RPE65 may also have abnormalities in their macular pigment levels and distributions. Interestingly, SNPs in human RPE65, along with other carotenoid-associated genes, such as GSTP1, BCO1, and SCARB1, were identified as determinants of macular optical density in women participating in the CAREDS study (33).
The notion that RPE65 is the meso-zeaxanthin isomerase is appealing, since its carotenoid oxygenase family members BCO1 and BCO2 are known carotenoid cleavage enzymes. In fact, RPE65’s alternate name is BCO3. These three proteins share significant sequence homology, and each plays a crucial role in vertebrate retinoid and carotenoid physiology. BCO1 cleaves β-carotene at the central 15, 15′ site to produce two molecules of retinal (18). This newly formed all-trans-retinal undergoes reduction and conversion into retinyl esters that are substrates for RPE65-mediated production of 11-cis-retinol; alternatively, retinal can be oxidized to retinoic acid, which is used for cell signaling and gene regulation. BCO2 cleaves a variety of xanthophyll carotenoid substrates at the 9′, 10′ double bond and is involved in the homeostasis of non-provitamin A carotenoids (17).
In other species, a single enzyme can perform the functions of BCO1, BCO2, and RPE65. Arthropods encode a single carotenoid cleavage enzyme, NinaB, that performs the functions of all three BCO family members (34). Carotenoid cleavage enzymes in lower organisms have a range of substrate specificities. ACO from cyanobacteria is capable of cleaving carotenoids of various lengths, ranging from C20 to C27. This enzyme binds to substrates with either aldehydes or alcohols at their terminal ends distal to the ionone ring, and it also can accept apocarotenoids with or without 3-hydroxyl groups on the ionone rings (35–37). Therefore, it is not unprecedented that RPE65, whose known interactions until now have been only with retinoids, may interact with structurally similar molecules, such as carotenoids.
Our findings show that meso-zeaxanthin production from lutein occurs in the RPE, and that it is catalyzed by RPE65. The specific accumulation of this carotenoid in the fovea may be mediated by specific transporters as well as binding proteins. IRBP and class B scavenger receptor proteins are capable of shuttling carotenoids to the retinal layers from the RPE via the interphotoreceptor space (38, 39). GSTP1 is a known zeaxanthin-binding protein present in the primate RPE and foveal regions that binds meso-zeaxanthin with equally high affinity (15). It is plausible to hypothesize that newly formed meso-zeaxanthin from the RPE is shuttled into the subretinal space and then into the retinal layers by means of transport proteins, and that once in the retina, it may be held in place in the foveal region by specific binding proteins.
In the present study, we have described a novel function of RPE65 as the lutein to meso-zeaxanthin isomerase. We have shown that both chicken and human RPE65 are capable of converting lutein to meso-zeaxanthin. The reaction rate is slow, and meso-zeaxanthin isomerization is likely a secondary function of RPE65. The foveal presence of meso-zeaxanthin, especially given its lack of common dietary sources, has been a conundrum in the field of carotenoid biology. With the identification of RPE65 as the enzyme responsible for the production of meso-zeaxanthin, future studies can further delineate the physiological role of this macula-specific carotenoid.
Materials and Methods
Total RNA Isolation and RNA Sequencing.
Total RNA was isolated from E16 and E21 chicken embryos with the Qiagen RNeasy Kit. Intact poly(A) RNA was purified from total RNA samples (100–500 ng) with oligo(dT) magnetic beads, and stranded mRNA sequencing libraries were prepared as described using the Illumina TruSeq Stranded mRNA Library Preparation Kit. Details are provided in SI Materials and Methods.
Cell Culture and Transient Transfection.
A primary cell culture of E21 chicken RPE was established, and HEK293T cells were transiently transfected with RPE65 or GFP for overexpression experiments, as described in SI Materials and Methods.
Carotenoid Treatment.
HPLC-purified carotenoid stocks were prepared. Tween 40 was added to the dried stocks before the addition of medium. Cells were treated with the carotenoid-containing medium for 0, 1, 2, or 4 d. More details are provided in SI Materials and Methods.
Carotenoid Extraction and HPLC Analysis.
Carotenoid extraction and chiral HPLC analyses were carried out as described previously (10) and in SI Materials and Methods.
Protein Isolation and Western Blot Analysis.
Cell lysis, protein isolation, and Western blot analysis were done following standard protocols, as described in SI Materials and Methods.
Inhibitor Treatment of Chicken Embryos.
E17 and the same chicken embryos at E19 were injected with appropriate concentrations of ACU-5200 (Acucela Inc.) diluted in Ringer’s solution containing 1% penicillin-streptomycin (SI Materials and Methods).
Structural Modeling of Lutein Docking into RPE65.
Lutein molecules were docked into a homology model of RPE65 obtained with Phyre2 (40), by threading the amino acid sequence of CRPE65 into the structure of bovine RPE65 [Protein Data Bank (PDB) ID codes 4RSC and 3FSN] (29, 41). Additional details are provided in SI Materials and Methods.
SI Materials and Methods
RNA Sequencing.
Purified libraries were qualified on an Agilent 2200 TapeStation using a D1000 ScreenTape assay (catalog nos. 5067–5582 and 5067–5583). The molarity of adapter-modified molecules was defined by quantitative PCR using the Kapa Library Quant Kit (Kapa Biosystems; catalog no. KK4824). Individual libraries were normalized to 10 nM, and equal volumes were pooled in preparation for Illumina sequence analysis. Reads were aligned to the galGal4 genome and all possible splices using Novoalign. Spliced alignments were converted back to genomic space using USeq’s SamTranscriptomeParser. This application also removes low-quality reads and converts the sam format files to bam format. Normalized coverage tracks were generated using USeq Sam2USeq and USeq2UCSC applications. Differential expression was determined using USeq’s DefinedRegionDifferentialSeq. This method uses the DESeq2 statistics package. Data were clustered using custom R scripts. Significant genes were analyzed using Qiagen Ingenuity Pathway Analysis software. Only genes with a log2 ratio >1 were analyzed using this method.
Cell Culture and Transient Transfections.
Eyes were removed from E21 chicken embryos, and RPE/choroid was dissected out of retina with the help of trypsin. Following this, the released RPE cells were carefully plated onto Corning Primaria tissue culture flasks (Thermo Fisher Scientific) and then cultured in DMEM containing 20% FBS with 1% antibiotic-antimycotic mixture. After 5 d, this medium was removed, and 10% FBS containing DMEM was added to the cells. On confluency, cells were passaged using TrypLE Express (Thermo Fisher Scientific) and plated on T-25 flasks. Cells at P5 were used for the experiments. The HEK-293T human embryonic kidney cell line (American Type Culture Collection) was cultured in DMEM containing 10% FBS and 1% antibiotic mixture (Thermo Fisher Scientific). Overexpression was carried out by transient transfection of plasmids pCDNA3.1 chicken RPE65, pCDNA3.1 human RPE65 (both generous gifts from Jian-Xing Ma), or pCDNA3.1 GFP (OriGene). Cells were transfected with jetPRIME reagent (Polyplus Transfection) following the manufacturer’s instructions.
Carotenoid Treatment.
HPLC-purified lutein was supplied by Kemin Health, zeaxanthin was obtained from Zeavision, and meso-zeaxanthin was provided by DSM. Carotenoid stocks were prepared in hexanes. 0.3% Tween 40 (Sigma-Aldrich) was added to appropriate volume of stocks, and the mixture was dried under nitrogen gas. Complete medium was added to this mixture, and this solution was sonicated on ice for 30 min and then vortexed at room temperature for 20 min. The medium containing carotenoids was added to the cells to initiate carotenoid uptake. The dishes were placed into a 37 C incubator for 0, 1, 2, or 4 d. The cells were subsequently washed with ice-cold 10 mM sodium taurocholate to remove any adsorbed carotenoids and then with 1× PBS. Cells were scraped in 1× PBS and centrifuged at 16,200 × g for 5 min, excess liquid was removed, and carotenoid extraction was conducted.
Carotenoid Extraction and HPLC Analysis.
Carotenoids were extracted following the addition of tetrahydrofuran (THF) containing 0.1% butylated hydroxytoluene. Here 1 mL of THF was added to the cell pellet from above, and this mixture was sonicated on ice for 10 min, followed by vortexing for 5 min. The cell homogenates were centrifuged at maximum speed for 10 min, and the supernatant containing carotenoids was removed and dried under nitrogen gas. The foregoing process was carried out three times to remove all carotenoid content in the cells.
Protein Isolation and Western Blot Analysis.
Cells and tissues were homogenized at 4 C in RIPA buffer (25 mM NaCl, 0.5 mM EDTA, 25 mM Tris⋅HCl pH 7.2, and 0.1% Tween 20) containing protease inhibitors. Cell debris was removed by centrifugation. A BCA assay (Thermo Fisher Scientific) was carried out, and 20 μg of protein was resolved by 9% SDS/PAGE. Transfer to a 0.45-μm nitrocellulose membrane was done using a Trans-Blot SD semidry transfer cell (Bio-Rad) at 25 V for 1 h. Membranes were subsequently washed in TBS with 0.01% Tween 20 and then blocked using Odyssey Blocking Buffer (LI-COR) containing 0.01% Tween 20 for 1 h. Primary antibodies were diluted in the foregoing blocking buffer, and the membranes were incubated overnight at 4 C. The antibodies used and their dilutions were as follows: 1:1,000 dilution of DALEED chicken RPE65, 1:1,000 dilution of PETLET human RPE65 antibody (both kind gifts from Jian-Xing Ma) and 1:5,000 dilution of mouse monoclonal β-actin (8H10D10; Cell Signaling Technology). Proteins were visualized using an Odyssey Image Analyzer (LI-COR) following incubation with IR dye-conjugated secondary antibodies (LI-COR) at 1:10,000 dilutions for 1 h at room temperature.
Inhibitor Treatment of Chicken Embryos.
The shells were wiped with 70% ethanol and placed on their side to accurately target the yolk sac. Each egg was taped on the side with electrical tape, and a small hole was made through the tape using a sterile 18-gauge needle. Then 0.1 mL of Ringer’s solution or Ringer’s solution with ACU-5200 was introduced into the yolk sac of the developing embryo using a 21-gauge needle. The injection site was taped over using electrical tape, and the egg was incubated with the injection site facing upward.
Rigid-Body Docking of Lutein.
A homology model of the enzyme from chicken was constructed using Phyre2 (40), threading the chicken sequence into the bovine structure (PDB ID code 3FSN). The chicken and bovine enzymes share 89% identity with no insertions or deletions. All positions of variation were found to be excluded from the substrate-binding tunnel-like cavity. Structures of lutein found in complex with chlorophyll-binding proteins were harvested from structures of photosystems from plants (PDB ID codes 1RWT, 3JCU, 3PL9, and 4XK8). The locations of emixustat and palmitate in the inhibitor-complexed structure of RPE65 (PDB ID code 4RSC) provided initial guidepost coordinates that were augmented by manually identifying nearby positions in steric compliance (>3.5 Å from nonhydrogen atoms). A total of 33,738 docking trials were obtained by least squares superposition of several different lutein structures, selecting several sterically accessible coordinates and pairing these with atoms in the ε-ionone ring and the polyene chain. The docked complexes were scored by counting the number of steric conflicts (<3 Å between nonhydrogen atoms) and potential hydrogen bonds (2.0–4.2 Å between electronegative atoms) using a C language computer program written by M.P.H. (42).
Acknowledgments
We thank Acucela Inc. for generously providing the RPE65 inhibitor ACU-5200, Kemin Health for supplying HPLC-purified lutein, Dr. Jian-Xing Ma and Dr. Yusuke Takahashi (University of Oklahoma) for providing RPE65 antibodies and plasmids, and Dr. Wolfgang Baehr (University of Utah) for providing feedback on the manuscript. This work was supported by National Institutes of Health Grants EY11600 and EY14800 (to P.S.B.) and Ruth L. Kirschstein Training Grant T32EY024234 (to R.S.), and an unrestricted departmental grant from Research to Prevent Blindness.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 10818.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1706332114/-/DCSupplemental.
References
- 1.Maoka T, Arai A, Shimizu M, Matsuno T. The first isolation of enantiomeric and meso-zeaxanthin in nature. Comp Biochem Physiol B. 1986;83:121–124. doi: 10.1016/0305-0491(86)90341-x. [DOI] [PubMed] [Google Scholar]
- 2.Bernstein PS, et al. Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog Retin Eye Res. 2016;50:34–66. doi: 10.1016/j.preteyeres.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li B, Vachali P, Bernstein PS. Human ocular carotenoid-binding proteins. Photochem Photobiol Sci. 2010;9:1418–1425. doi: 10.1039/c0pp00126k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katsuyama M, Komori T, Matsuno T. Metabolism of three stereoisomers of astaxanthin in the fish, rainbow trout and tilapia. Comp Biochem Physiol B. 1987;86:1–5. doi: 10.1016/0305-0491(87)90165-9. [DOI] [PubMed] [Google Scholar]
- 5.Nolan JM, et al. Verification of meso-zeaxanthin in fish. J food Process Technol. 2014;5:335. doi: 10.4172/2157-7110.1000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chew EY, et al. Age-Related Eye Disease Study 2 (AREDS2) Research Group Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report no. 3. JAMA Ophthalmol. 2014;132:142–149. doi: 10.1001/jamaophthalmol.2013.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhosale P, Serban B, Zhao DY, Bernstein PS. Identification and metabolic transformations of carotenoids in ocular tissues of the Japanese quail Coturnix japonica. Biochemistry. 2007;46:9050–9057. doi: 10.1021/bi700558f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson EJ, Neuringer M, Russell RM, Schalch W, Snodderly DM. Nutritional manipulation of primate retinas, III: Effects of lutein or zeaxanthin supplementation on adipose tissue and retina of xanthophyll-free monkeys. Invest Ophthalmol Vis Sci. 2005;46:692–702. doi: 10.1167/iovs.02-1192. [DOI] [PubMed] [Google Scholar]
- 9.Bone RA, Landrum JT, Hime GW, Cains A, Zamor J. Stereochemistry of the human macular carotenoids. Invest Ophthalmol Vis Sci. 1993;34:2033–2040. [PubMed] [Google Scholar]
- 10.Gorusupudi A, et al. Developmentally regulated production of meso-zeaxanthin in chicken retinal pigment epithelium/choroid and retina. Invest Ophthalmol Vis Sci. 2016;57:1853–1861. doi: 10.1167/iovs.16-19111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernstein PS, Law WC, Rando RR. Isomerization of all-trans-retinoids to 11-cis-retinoids in vitro. Proc Natl Acad Sci USA. 1987;84:1849–1853. doi: 10.1073/pnas.84.7.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moiseyev G, Chen Y, Takahashi Y, Wu BX, Ma J-X. RPE65 is the isomerohydrolase in the retinoid visual cycle. Proc Natl Acad Sci USA. 2005;102:12413–12418. doi: 10.1073/pnas.0503460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin M, Li S, Moghrabi WN, Sun H, Travis GH. Rpe65 is the retinoid isomerase in bovine retinal pigment epithelium. Cell. 2005;122:449–459. doi: 10.1016/j.cell.2005.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redmond TM, et al. Mutation of key residues of RPE65 abolishes its enzymatic role as isomerohydrolase in the visual cycle. Proc Natl Acad Sci USA. 2005;102:13658–13663. doi: 10.1073/pnas.0504167102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhosale P, et al. Identification and characterization of a Pi isoform of glutathione S-transferase (GSTP1) as a zeaxanthin-binding protein in the macula of the human eye. J Biol Chem. 2004;279:49447–49454. doi: 10.1074/jbc.M405334200. [DOI] [PubMed] [Google Scholar]
- 16.Li B, Vachali P, Frederick JM, Bernstein PS. Identification of StARD3 as a lutein-binding protein in the macula of the primate retina. Biochemistry. 2011;50:2541–2549. doi: 10.1021/bi101906y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu K-Q, et al. The biochemical characterization of ferret carotene-9′,10′-monooxygenase catalyzing cleavage of carotenoids in vitro and in vivo. J Biol Chem. 2006;281:19327–19338. doi: 10.1074/jbc.M512095200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiefer C, et al. Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A. J Biol Chem. 2001;276:14110–14116. doi: 10.1074/jbc.M011510200. [DOI] [PubMed] [Google Scholar]
- 19.Wyss A, et al. Expression pattern and localization of β,β-carotene 15,15′-dioxygenase in different tissues. Biochem J. 2001;354:521–529. doi: 10.1042/0264-6021:3540521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Redmond TM, et al. Identification, expression, and substrate specificity of a mammalian β-carotene 15,15′-dioxygenase. J Biol Chem. 2001;276:6560–6565. doi: 10.1074/jbc.M009030200. [DOI] [PubMed] [Google Scholar]
- 21.Kawaguchi R, et al. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science. 2007;315:820–825. doi: 10.1126/science.1136244. [DOI] [PubMed] [Google Scholar]
- 22.Kaylor JJ, et al. Identification of DES1 as a vitamin A isomerase in Müller glial cells of the retina. Nat Chem Biol. 2013;9:30–36. doi: 10.1038/nchembio.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borel P, et al. CD36 and SR-BI are involved in cellular uptake of provitamin A carotenoids by Caco-2 and HEK cells, and some of their genetic variants are associated with plasma concentrations of these micronutrients in humans. J Nutr. 2013;143:448–456. doi: 10.3945/jn.112.172734. [DOI] [PubMed] [Google Scholar]
- 24.Enright JM, et al. Cyp27c1 red-shifts the spectral sensitivity of photoreceptors by converting vitamin A1 into A2. Curr Biol. 2015;25:3048–3057. doi: 10.1016/j.cub.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moiseyev G, Takahashi Y, Chen Y, Kim S, Ma J-X. RPE65 from cone-dominant chicken is a more efficient isomerohydrolase compared with that from rod-dominant species. J Biol Chem. 2008;283:8110–8117. doi: 10.1074/jbc.M703654200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamel CP, et al. Molecular cloning and expression of RPE65, a novel retinal pigment epithelium-specific microsomal protein that is post-transcriptionally regulated in vitro. J Biol Chem. 1993;268:15751–15757. [PubMed] [Google Scholar]
- 27.Dugel PU, et al. Phase II randomized, placebo-controlled, 90-day study of emixustat hydrochloride in geographic atrophy associated with dry age-related macular degeneration. Retina. 2015;35:1173–1183. doi: 10.1097/IAE.0000000000000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jack LS, Sadiq MA, Do DV, Nguyen QD. Emixustat and lampalizumab: Potential therapeutic options for geographic atrophy. Dev Ophthalmol. 2016;55:302–309. doi: 10.1159/000438954. [DOI] [PubMed] [Google Scholar]
- 29.Kiser PD, et al. Catalytic mechanism of a retinoid isomerase essential for vertebrate vision. Nat Chem Biol. 2015;11:409–415. doi: 10.1038/nchembio.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li B, Ahmed F, Bernstein PS. Studies on the singlet oxygen scavenging mechanism of human macular pigment. Arch Biochem Biophys. 2010;504:56–60. doi: 10.1016/j.abb.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crosby-Nwaobi R, Hykin P, Peto T, Sivaprasad S. An exploratory study evaluating the effects of macular carotenoid supplementation in various retinal diseases. Clin Ophthalmol. 2016;10:835–844. doi: 10.2147/OPTH.S102798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nolan JM, et al. Enrichment of macular pigment enhances contrast sensitivity in subjects free of retinal disease: Central retinal enrichment supplementation trials. Report 1. Invest Ophthalmol Vis Sci. 2016;57:3429–3439. doi: 10.1167/iovs.16-19520. [DOI] [PubMed] [Google Scholar]
- 33.McKay GJ, et al. Investigation of genetic variation in scavenger receptor class B, member 1 (SCARB1) and association with serum carotenoids. Ophthalmology. 2013;120:1632–1640. doi: 10.1016/j.ophtha.2013.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Babino D, et al. The biochemical basis of vitamin A3 production in arthropod vision. ACS Chem Biol. 2016;11:1049–1057. doi: 10.1021/acschembio.5b00967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kloer DP, Schulz GE. Structural and biological aspects of carotenoid cleavage. Cell Mol Life Sci. 2006;63:2291–2303. doi: 10.1007/s00018-006-6176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kloer DP, Ruch S, Al-Babili S, Beyer P, Schulz GE. The structure of a retinal-forming carotenoid oxygenase. Science. 2005;308:267–269. doi: 10.1126/science.1108965. [DOI] [PubMed] [Google Scholar]
- 37.Sui X, Kiser PD, Lintig Jv, Palczewski K. Structural basis of carotenoid cleavage: From bacteria to mammals. Arch Biochem Biophys. 2013;539:203–213. doi: 10.1016/j.abb.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vachali PP, Besch BM, Gonzalez-Fernandez F, Bernstein PS. Carotenoids as possible interphotoreceptor retinoid-binding protein (IRBP) ligands: A surface plasmon resonance (SPR)-based study. Arch Biochem Biophys. 2013;539:181–186. doi: 10.1016/j.abb.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Voolstra O, et al. The Drosophila class B scavenger receptor NinaD-I is a cell surface receptor mediating carotenoid transport for visual chromophore synthesis. Biochemistry. 2006;45:13429–13437. doi: 10.1021/bi060701u. [DOI] [PubMed] [Google Scholar]
- 40.Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJE. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc. 2015;10:845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kiser PD, Golczak M, Lodowski DT, Chance MR, Palczewski K. Crystal structure of native RPE65, the retinoid isomerase of the visual cycle. Proc Natl Acad Sci USA. 2009;106:17325–17330. doi: 10.1073/pnas.0906600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horvath MP, et al. Structure of the lutein-binding domain of human StARD3 at 1.74 Å resolution and model of a complex with lutein. Acta Crystallogr F Struct Biol Commun. 2016;72:609–618. doi: 10.1107/S2053230X16010694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pettersen EF, et al. UCSF Chimera: A visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 44.Huang CC, Meng EC, Morris JH, Pettersen EF, Ferrin TE. Enhancing UCSF Chimera through web services. Nucleic Acids Res. 2014;42:W478–W484. doi: 10.1093/nar/gku377. [DOI] [PMC free article] [PubMed] [Google Scholar]