Significance
We identified truncation of tooth development during postnatal ontogeny in two theropod dinosaurs, a caenagnathid oviraptorosaur and the Early Cretaceous bird Sapeornis. Developmental and paleontological evidence each suggests dental reduction and beak evolution are coupled, and a sequence of common morphologies is identified that characterizes the multiple transitions to toothless beaks in theropod dinosaurs and birds. Shifts toward earlier cessation of postnatal tooth development can be identified in fish, amphibians, and mammals that are edentulous as adults; therefore the identification of similar transitions in multiple Mesozoic theropod dinosaur lineages strongly implies that heterochronic truncation of odontogenesis played an important role in the macroevolution of beaks in modern birds.
Keywords: ontogenetic edentulism, beak evolution, tooth reduction, Caenagnathidae, Sapeornis
Abstract
Beaks are innovative structures characterizing numerous tetrapod lineages, including birds, but little is known about how developmental processes influenced the macroevolution of these important structures. Here we provide evidence of ontogenetic vestigialization of alveoli in two lineages of theropod dinosaurs and show that these are transitional phenotypes in the evolution of beaks. One of the smallest known caenagnathid oviraptorosaurs and a small specimen of the Early Cretaceous bird Sapeornis both possess shallow, empty vestiges of dentary alveoli. In both individuals, the system of vestiges connects via foramina with a dorsally closed canal homologous to alveoli. Similar morphologies are present in Limusaurus, a beaked theropod that becomes edentulous during ontogeny; and an analysis of neontological and paleontological evidence shows that ontogenetic reduction of the dentition is a relatively common phenomenon in vertebrate evolution. Based on these lines of evidence, we propose that progressively earlier postnatal and embryonic truncation of odontogenesis corresponds with expansion of rostral keratin associated with the caruncle, and these progenesis and peramorphosis heterochronies combine to drive the evolution of edentulous beaks in nonavian theropods and birds. Following initial apomorphic expansion of rostral keratinized epithelia in perinatal toothed theropods, beaks appear to inhibit odontogenesis as they grow postnatally, resulting in a sequence of common morphologies. This sequence is shifted earlier in development through phylogeny until dentition is absent at hatching, and odontogenesis is inhibited by beak formation in ovo.
At least seven transitions to edentulism occurred independently in theropod dinosaurs (1–3), all presumably accompanied by the appearance of a horny beak (1). Although the structure and morphogenetic events of beak formation have been well studied in extant birds (4–6), evolutionary developmental mechanisms linking beak formation and tooth loss have proven difficult to test, given extant models. Previous authors hypothesized that avian tooth loss was due to inactivation of odontogenic signaling pathways (7), but acknowledged that regional tooth loss initially accompanied various acquisition of beaks in Cretaceous birds (8, 9). Therefore, degradation of the odontogenic program alone cannot provide a developmental explanation for the coupling of tooth loss and beak formation (9). Macroevolutionary hypotheses for these phenomena have included weight-saving in response to the evolution of flight (1, 10) and efficient processing of herbivorous diets (3). Weight-saving hypotheses have been rejected by recent studies (11, 12) and fail to explain the tradeoff between tooth loss and beak development in nonvolant theropod lineages, while hypotheses related to dietary specialization appear salient for at least initial rostral beak formation in theropods.
Here we provide fossil evidence consistent with postnatal truncation of odontogenesis in several lineages of theropod dinosaurs that eventually reach complete edentulism. We hypothesize that BMP4 (bone morphogenetic protein 4) mediated peramorphic expansions of keratinized epithelia underlying caruncles (“egg teeth”) are linked with progenetic truncation of odontogenesis in beaked theropod lineages, and these heterochronic processes combined to generate the repeated evolution of nonavian and avian beaks when induced by selection for specialized diets.
Results
Postnatal Dental Reduction.
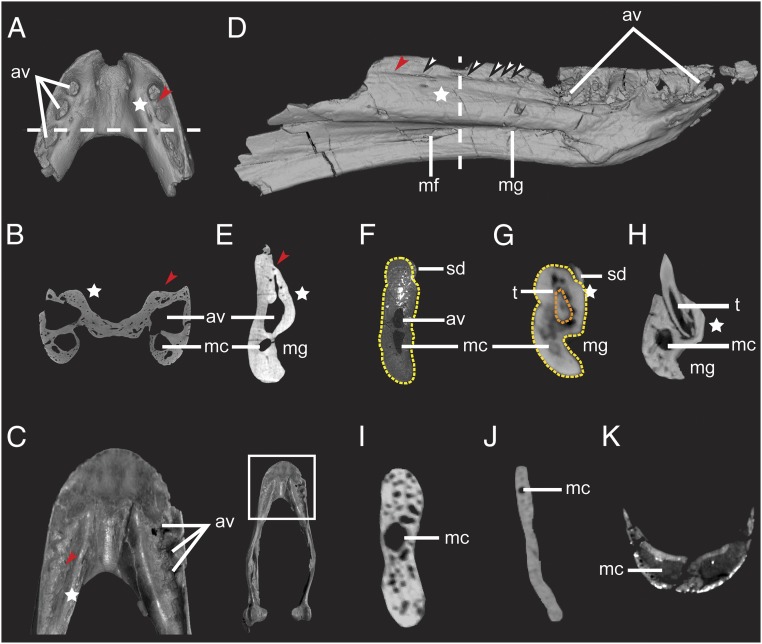
Among oviraptorosaurian theropods, teeth are only present in some Early Cretaceous taxa (13–17), whereas all other oviraptorosaurs are edentulous (18). Caenagnathidae is a group of edentulous oviraptorosaurs that is characterized by a series of lateral grooves separate from each other by lateral ridges (sensu ref. 19) present on the occlusal surface of the dentary (Fig. 1 A–C) (19), which together constitute the lingual groove. Our computed tomography (CT) results reveal that in addition to the dentary’s mandibular canal (inferior alveolar canal, V3), a neomorphic canal is present in caenagnathid oviraptorosaurs situated above the Meckelian groove and below the system of lateral occlusal grooves and ridges (Fig. 1 A and B) (2). In small caenagnathid specimens [e.g., IVPP V20377 (20) and CCMGE 401/12457 (21); see SI Appendix for the institutional abbreviations] the lateral grooves are relatively deep, the lateral ridges extend across the whole lingual groove to contact the lingual ridge, and the neomorphic canal communicates dorsally with lateral grooves via several small foramina (Fig. 1A in ref. 20). In larger caenagnathid dentaries (e.g., Caenagnathus collinsi CMN 8776) (22) the lateral grooves are bounded medially by a shallow lingual ridge, the lateral ridges fail to reach the lingual ridge, and the grooves resemble the fossa-like alveolar vestiges present in the anterior dentary of young juvenile Limusaurus (e.g., IVPP V15301) (2) and the Early Cretaceous bird Sapeornis (Fig. 1D and SI Appendix, Table S1) (23). Rather than exiting vertically from the floor of lateral grooves as in IVPP 20377, foramina communicating with the interior dentary in larger specimens (Fig. 1C) (22, 24) are minute and extend onto the medial face of the dentary’s buccal edge as anastomosing vascular grooves, an osteological correlate of a keratinous rhamphotheca (25).
Fig. 1.
Dinosaurian and crocodilian dentaries illustrated by CT data, showing the presence of the alveolar canal and other alveolar vestiges. (A) Dorsal view and (B) coronal section of Caenagnathasia sp. (IVPP V20377). (C) Dorsal view of rostral dentary of cf. Chirostenotes TMP 2012.12.12, and (Inset) entire mandible. Photographs in Fig. 1C are reproduced with permission from ref. 24. Copyright 2008 Canadian Science Publishing or its licensors. (D) Mirror symmetry of right dentary of Sapeornis chaoyangensis (LPM B00015) in lingual view showing vestigial alveoli and foramina present on the dorsolingual aspect of the dentary. (E–K) Coronal dentary sections of (E) S. chaoyangensis (LPM B00015), (F) subadult Limusaurus inextricabilis (IVPP V15923), (G) juvenile L. inextricabilis (IVPPV15301), (H) extant Alligator sinensis (IVPP 1361), (I) extant Pavo sp. (IVPP 1032), (J) Confuciusornis sp. (IVPP V23275), and (K) Khaan mckennai (IGM 100/973). av, alveolar vestige; mc, mandibular canal (V3); mf, Meckelian foramen; mg, Meckelian groove; sd, supradentary; t, tooth. White stars, swollen ridge present on the lingual aspect of the dentary; red arrows, lingual groove; white arrows, foramina piercing the lingual groove on the medial aspect of the dentary; dashed lines in A and D mark the positions of the slices shown in B and E, respectively; light yellow dashed lines in F and G mark the dentary; orange dashed line in G marks a replacement tooth present inside an enclosed dentary alveolus. (Not to scale.)
Although extant birds lack dentition, most known Mesozoic birds possess teeth and exhibit various tooth reduction patterns (1). Some specimens of Sapeornis (STM 16–18 and STM 15–7) (23) possess a combination of two dentary teeth and three fossa-like alveolar homologs anterior to those teeth, whereas larger specimens have a toothless dentary (e.g., BMNH C-PH1067; SI Appendix, Table S2). The specimen LPM B00015 preserves no dentary teeth (SI Appendix, Fig. S1), but there are four alveoli or alveolar homologs at the anterior end of the dentary which are shallowest anteriorly and deepest posteriorly (Fig. 1D). These features are shallower than the alveolar homologs in IVPP V20377 and instead resemble the condition in large caenagnathid dentaries (22). The posteriormost alveolar homolog is interpreted as the position of the rostral-most dentary tooth in subadult Sapeornis (e.g., STM 16–18) based on the relative anterior extension of the Meckelian groove (23). Posterior to this alveolus there is a lingual groove situated above a canal similar in morphology and position to the one present in the caenagnathid IVPP V20377 (Fig. 1 B and E). Several concentrated foramina pierce the lingual groove to connect with the neomorphic canal where the second dentary tooth is present in other Sapeornis specimens (Fig. 1 D and E), suggesting the alveolus for the second dentary tooth was remodeled in life. In LPM B00015 and other Sapeornis specimens with dentary teeth (e.g., STM 16–18, figure 6A in ref. 23), several slit-like foramina enter the lingual groove posteriorly to connect with the neomorphic canal, each of them slightly shorter than the anteroposterior length of the anterior alveolar homologs. The length and regular spacing of these foramina suggest they are also alveolar vestiges.
An unusual form of edentulism known as ontogenetic edentulism occurs in some extant vertebrates (26), and we reported it recently in the Jurassic ceratosaurian theropod Limusaurus inextricabilis, whereby toothed jaws in the juvenile individuals transitioned to a completely toothless beaked jaw in more mature individuals during ontogeny (2). In mature Limusaurus, vestigial dentary alveoli remain after tooth loss but are enclosed dorsally and modified into an alveolar canal dorsal to the mandibular canal (Fig. 1F) (2), as in the theropods described above. A swollen ridge medial to the lingual grooves in both caenagnathids and Sapeornis (Fig. 1 A–E) resembles a similar structure that accommodates the teeth in other archosaurs, including juvenile Limusaurus (Fig. 1G), but is reduced in subadult Limusaurus (Fig. 1F). Unlike these taxa, most edentulous vertebrates lack an alveolar canal bounded medially by this projecting ridge and instead possess only the mandibular canal (Fig. 1 I–K).
These lines of evidence confirm the vestigial alveoli seen in Sapeornis and suggest that the lateral grooves and ridges present in caenagnathid oviraptorosaurs (Fig. 1 A and C) are vestigial alveoli and interdental septa, respectively. The isolated occurrence of most known caenagnathid mandibles and absence of ontogenetic series from a limited stratigraphic horizon in both Caenagnathidae and Sapeornis complicates ontogenetic comparisons, though differences in dentary tooth count in Sapeornis (2, 1, or 0) correspond roughly with body size and osteohistological indicators (see also SI Appendix, Table S2). Given the recent description of postnatal ontogenetic edentulism in Limusaurus (2), the identification of alveolar homologs in Caenagnathidae and Sapeornis consistent with morphologies occurring in Limusaurus suggests that ontogenetic truncation of odontogenesis produced the observed morphologies in these theropod taxa.
Alveolar remodeling occurs primarily on the occlusal and dorsolingual margins of the alveoli in Limusaurus, Sapeornis, and Caenagnathidae. In the latter two there are no preserved remnants of replacement teeth (Fig. 1 B and E), so a replacement tooth present inside an enclosed dentary alveolus in young juvenile Limusaurus deserves special note (Fig. 1G). Since the alveolus is completely enclosed on the occlusal surface, inhibiting normal tooth replacement, a signal extrinsic to the odontogenic program likely initiated remodeling of the alveolus in this case. Midtooth row alveolar closure is unusual in reptiles but is known to result from traumatic pathology and has been observed in extant (27) and fossil Archosauromorpha (28), including a nonavian theropod (29). These examples confirm that epigenetic factors such as wound healing or other tissue interactions can inhibit odontogenesis and result in permanent closure of alveoli with normally continuous tooth replacement. Though the precise mechanism by which keratinized oral epithelia would affect the aborally located dental lamina is unclear, acceleration of postnatal growth of the keratinized beak could overgrow alveoli and would likely induce alveolar remodeling. Keratinous epithelial appendages are associated with delayed tooth eruption and truncation of odontogenesis in a range of tetrapods at various embryonic and postnatal ontogenetic stages (26, 30–32), and cytokeratin expression is up-regulated during degradation of the successional dental lamina in monophyodont (33, 34) and diphyodont (35) amniotes. Given that a functional dental lamina and appropriate expression of odontogenic pathways are essential prerequisites for vertebrate tooth formation (5, 7, 36), dental lamina damage, loss of epithelial–mesenchymal interaction, or even misexpression of odontogenic signals caused by jaw bone remodeling and/or rhamphotheca growth could have led to the tooth reduction in theropods.
Embryonic Dental Reduction.
During the evolution of birds, odontogenic truncation could be achieved progressively early through heterochronic development. Just as postnatal growth of beaks and other keratinous oral structures serves to inhibit tooth replacement, the embryonic development of these structures appears to play a role in the loss of early tooth generations (null and/or first-generation teeth) as well. Previous authors noticed the broad coincidence of rhamphothecae and perinatal caruncles in amniotes and hypothesized that the two structures are iterative homologs (37, 38). A survey of embryonic/larval dentitions fails to reject this hypothesis and shows that development of the superficially initiated null-generation dentition is inhibited in all extant tetrapods with rhamphothecae or occlusal keratinization of embryonic mouthparts (SI Appendix and SI Appendix, Fig. S2). Among Archosauromorpha, the cessation of null-generation tooth development occurs penecontemporaneously with first keratinization of the caruncle in birds (7) and turtles (39), whereas keratinization of the Alligator caruncle begins simultaneous with initiation of the final null-generation premaxillary teeth (40). All extant amniotes that possess nonvestigial true egg teeth [a hypothesized serial homolog of null-generation teeth (41, 42)] either lack caruncles and rhamphothecae altogether, or keratinize the rhamphotheca postnatally [e.g., monotremes (43)], suggesting that transitions between aquatic and terrestrial oviparity, and perhaps hard-shelled and soft-shelled oviparity or viviparity, involve trade-offs between egg-pipping mechanisms that are consequential for the phylogenetic distribution of rhamphothecae.
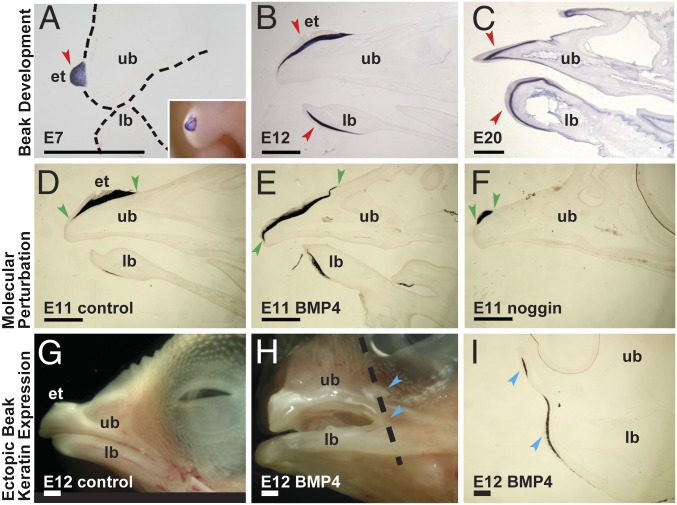
We conducted tests for character correlation (44) between terrestrial oviparity, mineralized columnar shell units, the presence of caruncles, and the presence of rhamphothecae on a species-level time-calibrated supertree of extant Tetrapoda (45). The independent evolution of caruncles in amniotes and multiple lineages of direct-developing frogs was significantly correlated with terrestrial oviparity; and the evolution of rhamphothecae was significantly correlated with the presence of a caruncle rather than a true egg-tooth (Table 1). These results should be interpreted in light of the susceptibility of character correlation tests to Type I error (46), especially given the infrequent origins of rhamphothecae among extant taxa; however, independent acquisitions of rhamphothecae in the fossil record are heavily concentrated within Archosauromorpha, nearly all extant members of which possess caruncles (SI Appendix and SI Appendix, Fig. S3). Beak development in birds is initiated by cornification of epithelium underlying the premaxillary caruncle on the premaxilla and a corresponding proliferative zone on the rostral end of the mandible (Fig. 2 A–C) and proceeds posteriorly to form the premaxillary and mandibular nails (38). Growth of rhamphothecae can be modulated in vivo by overexpression of BMP4 or its antagonist noggin (NOG), producing peramorphic or paedomorphic beak phenotypes at comparable ontogenetic stages (Fig. 2 D–F), respectively, and/or producing ectopic keratin growth (Fig. 2 G–I). Though BMP4 is a necessary odontogenic agonist, the absence of BMP4 antagonists is known to cause incisor agenesis or malformation in Nog−/− (47) and Grem2−/− (48) mice, suggesting that rhamphotheca hypertrophy induced by BMP4 overexpression could cause an imbalance in agonist/antagonist relationships necessary for normal tooth development. In addition, the spatiotemporal pattern of BMP4 expression in mouse oral epithelium matches apoptosis associated with regression of vestigial tooth germs in murine diastemata (49), and BMPs are known to regulate apoptosis in the chicken mandible (50). Therefore, both alteration of epithelial–mesenchymal interactions due to keratinization of oral epithelium and early disruption of odontogenic developmental pathways are potential mechanisms for inhibition of embryonic tooth development by rhamphothecae.
Table 1.
Tests of phylogenetic character correlations
| Characters | I (LnL) | D (LnL) | p |
| Terrestrial oviparity—caruncle | −1139.8 | −1047.7 | 9.34e−39 |
| Caruncle—beak | −147.9 | −137.2 | 0.0003 |
D, dependent model; I, independent model; LnL, mean log-likelihood.
Fig. 2.
Development of chicken beak keratin. (A–C) Sagittal sections of the snout showing expression patterns of beak keratin in wild type chicken: (A) E7, (B) E12, and (C) E20. Inset in A shows whole-mount in situ hybridization of beak keratin, and red arrows in A–C mark beak keratin expression in E7 egg tooth, and E12 and E20 upper and lower beaks. (D–F) Sagittal sections of the snout showing expression patterns of beak keratin in developing chicken with molecular perturbation: (D) control; (E) BMP4 overexpression; and (F) noggin overexpression. (G and H) Stereophotos showing ectopic expression of beak keratin caused by BMP4 overexpression: (G) control (left lateral view); (H) ectopic expression of beak keratin on face (left lateral view); (I) coronal section of the snout in H showing in situ hybridization of beak keratin. Dashed line in H marks the position of the slice shown in I. Red arrows mark positions where normal keratin is present, green arrows mark the anteroposterior length of the keratin expression domain in the upper beak, and blue arrows mark ectopic expression of beak keratin on the face. et, egg tooth; lb, lower beak; ub, upper beak. (Scale bars, 1 mm.)
Macroevolutionary Model for Edentulism.
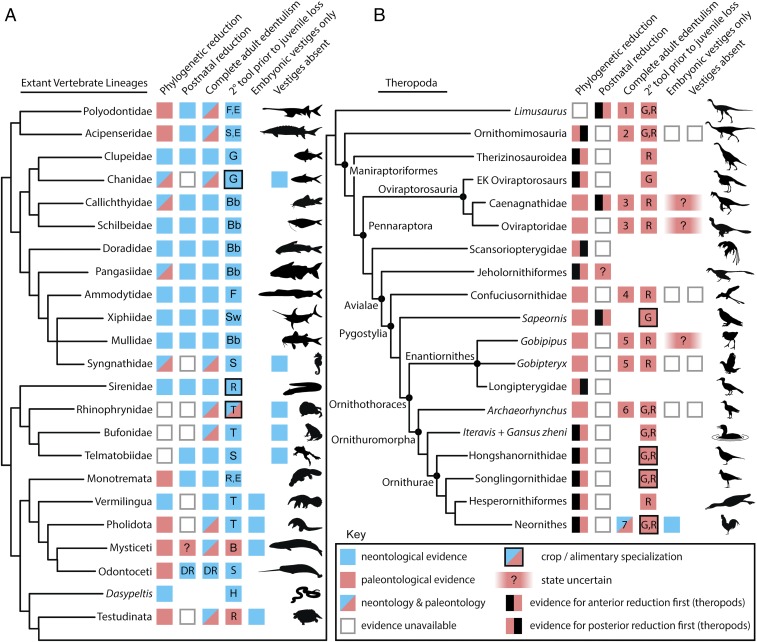
Evidence for postnatal and embryonic ontogenetic reduction of dental tissues can be observed in numerous extant vertebrate lineages (Fig. 3A), including the embryonic appearance of dental developmental homologs and their subsequent resorption in embryos of modern birds (7). In most observable cases, a lineage’s initial phylogenetic reduction in tooth number, size, tooth generations, and/or location is followed by a stage of postnatal reduction and eventual adult edentulism while juveniles retain teeth [e.g., Limusaurus (2), Ornithorhynchus (51), and Acipenser (52, 53)].
Fig. 3.
Summary of evidence for a macroevolutionary model of edentulism in vertebrates. (A) Evidence from extant nonavian vertebrate lineages with complete or near edentulism, showing phylogenetic and trophic diversity of ontogenetic tooth loss. (B) Evidence from select theropod dinosaur lineages focusing on Coelurosauria and showing independent evolution of adult edentulism at least seven times (1–7). ?, hypothesized state/partial evidence; B, baleen; Bb, barbels; DR, sexually dimorphic reduction pattern; E, electroreception; F, filtration; G, gastric mill/ gizzard; H, expanded hypapophyses; R, rhamphotheca; S, suction feeding; Sw, sword; T, projectile tongue. (Taxon silhouettes not to scale.)
This process is usually aided by a secondary morphological and/or ethological tool for food acquisition or processing, the appearance of which occurs phylogenetically before the loss of teeth in young juveniles (1, 26). The repeated evolution of rhamphothecae and gastric mills in nonavian theropods and the addition of the crop in Cretaceous birds (Fig. 3B) provided ample tools for food acquisition and mechanical digestion before complete tooth loss in each lineage. Loss of the early tooth generations present in young juveniles can be facilitated by parental behaviors that obviate juvenile foraging such as regurgitation feeding in altricial birds, nursing in mammals, or early competence of secondary foraging tools as in precocial birds and anuran larvae.
Once juvenile tooth loss has occurred, relaxation of selection leads to loss-of-function mutations in dental developmental genes and the successive vestigialization of the odontogenic program (9); and the final stages of embryonic reduction may persist for tens of millions of years, as in birds and turtles (7, 39). Cases of complete embryonic or larval loss are only readily apparent in anurans, where larval keratinous structures overlie what will become dentigerous mouth parts (26), or where early competence of secondary foraging or digestive features occurs, as in syngnathid and chanid fishes (54, 55).
Discussion
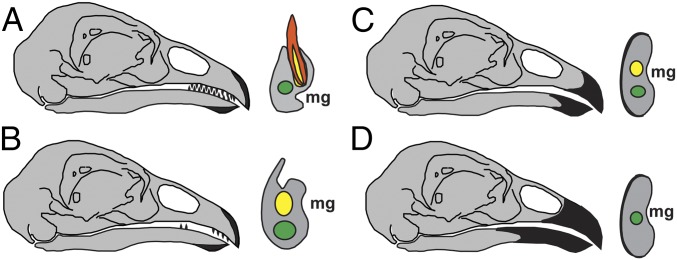
The ubiquity of our model’s phenotypic succession in extant lineages with complete or near edentulism (Fig. 3A) and the identification of these stages in various theropod dinosaur lineages (Fig. 3B) suggest that this heterochronic model of odontogenic truncation should be the null hypothesis for the macroevolution of edentulism in crown birds. Heterochronic tooth reduction processes in nonavian and avian theropod lineages likely included the following transformations: (i) normal tooth development and tooth replacement with an apomorphic keratinized rhamphotheca covering only the rostral-most portion of the jaws; (ii) external closure and/or constriction of alveoli impeding tooth replacement and adjacent growth of the rhamphotheca with regional tooth reduction; (iii) enlargement of the keratinized rhamphotheca with either functional reduction or redundancy of the remaining teeth; and (iv), complete or nearly complete remodeling of alveoli with complete coverage of the edentulous beak by the rhamphotheca (Fig. 4). Morphologies consistent with this phenotypic sequence can be observed in both ontogenetic and phylogenetic dimensions in theropods, including Limusaurus, Caenagnathidae, and Sapeornis, as well as other Cretaceous birds (1), and it is likely only the rarity of stratigraphically controlled ontogenetic series and preservation biases against young juveniles hindering more frequent documentation of these phenomena in the fossil record.
Fig. 4.
Transformations involved in theropod tooth reduction. Right lateral view of the head (Left) and transverse view of the dentary (Right). (A) Normal tooth development and tooth replacement with apomorphic keratinized rhamphotheca covering only the rostral-most portion of the jaws. (B) Tooth replacement is impeded by external closure and/or constriction of alveoli and regional tooth reduction occurs. (C) As the keratinized rhamphotheca enlarges, the remaining teeth are either functionally reduced or redundant. (D) Edentulous beak completely covered by rhamphotheca. Green, mandibular canal; yellow, alveolus and alveolar canal; orange, tooth. (Not to scale)
This integrated developmental framework for ontogenetic tooth loss and beak formation is consistent with prevailing life-history characteristics of Mesozoic dinosaurs, including terrestrial oviparity, multiyear ontogenies with juvenile sociality, and ontogenetic niche partitioning (2, 56). The results of the character correlation analysis show that the evolution of keratinous rhamphothecae is correlated with the presence of a caruncle, which is in turn correlated with terrestrial oviparity. These state-dependent correlations are supported by the absence of beaks in oviparous squamates (which have true egg teeth) and all viviparous tetrapods, including mammals, squamates, and marine reptiles. BMP4-mediated expansion of keratinous caruncles in ovo appears to have played an important role in initial rostral beak formation and accompanying tooth loss, as well as the final embryonic truncation of odontogenesis as lineages transition to complete edentulism. Whereas birds (57) and turtles (58) begin expanding the keratinized epithelium underlying the caruncle relatively early (shortly after the first elements ossify, Fig. 2 B, C, and G), crocodilians have formed multiple tooth generations in ovo, and most of the skeleton has ossified by the time rostral keratinization begins to expand beyond the caruncle (59, 60).
Multiyear maturation and the large neonate-to-adult body size disparity of many dinosaurs and other archosauromorphs is predictive of ontogenetic niche partitioning and/or dietary changes. Evidence for these phenomena have been observed directly in Limusaurus (2) and are probably widespread in dinosaurs given ontogenetic social segregation in many dinosaur clades (56) and examples of ontogenetic dietary change in many extant species within Archosauromorpha (61–63). These life-history characteristics may have predisposed terrestrial archosauromorphs transitioning from carnivory to omnivory or herbivory (3, 26) toward the evolution of rhamphothecae (Figs. 3B and SI Appendix, Fig. S3B). Tooth reduction is not as frequent or complete in nonarchosauromorph amniotes, only rarely includes a rhamphotheca (e.g., Ornithorhynchus and Dicynodontia), and is mostly limited to aquatic, fossorial, oophagous, and myrmecophagous habits (Figs. 3A and SI Appendix, Fig. S3A).
Materials and Methods
Details of the CT and synchrotron scanning and development experiment protocols are available in SI Appendix. For the character correlation analysis, a species-level time-calibrated supertree was taken from Hedges et al. (45) (www.biodiversitycenter.org/ttol, “TTOL_animals_unsmoothed”) and pruned to include only Tetrapoda. The node ages of crown group Archosauromorpha and Archosauria were modified to conform with minimum divergence dates based on the fossil record. Bayesian models of independent and dependent character evolution were estimated in BayesTraits V3.0 and compared with likelihood ratio tests. See SI Appendix for additional information on tree construction, sources of character information, and character modeling.
Supplementary Material
Acknowledgments
We thank X. Ding for preparing the specimens; Y. Feng and Y. Hou for Mi-CT imaging and 3D reconstruction; and X. Ni, J. Clark, and B. Zhu for discussion. S.W. was supported by the National Natural Science Foundation of China (Grant 41602013), the Youth Innovative Research Team Project of Capital Normal University, and the State Key Laboratory of Palaeobiology and Stratigraphy, Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences (Grant 173125); X.X. and S.W. were supported by the National Natural Science Foundation of China (Grants 41688103 and 41120124002); J.S. was supported by the U.S. National Science Foundation (Grants NSF EAR 0310217 and 0228559); D.H. was supported by the National Natural Science Foundation of China (Grant 41172026); P.W. and C.-M.C. were supported by the National Institutes of Health (Grants AR47364 and AR60306); and A.B. was supported by the National Science Foundation (Grant NSF DEB 1457181).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1708023114/-/DCSupplemental.
References
- 1.Louchart A, Viriot L. From snout to beak: The loss of teeth in birds. Trends Ecol Evol. 2011;26:663–673. doi: 10.1016/j.tree.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Wang S, et al. Extreme ontogenetic changes in a ceratosaurian theropod. Curr Biol. 2017;27:144–148. doi: 10.1016/j.cub.2016.10.043. [DOI] [PubMed] [Google Scholar]
- 3.Zanno LE, Makovicky PJ. Herbivorous ecomorphology and specialization patterns in theropod dinosaur evolution. Proc Natl Acad Sci USA. 2011;108:232–237. doi: 10.1073/pnas.1011924108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abzhanov A, Protas M, Grant BR, Grant PR, Tabin CJ. Bmp4 and morphological variation of beaks in Darwin’s finches. Science. 2004;305:1462–1465. doi: 10.1126/science.1098095. [DOI] [PubMed] [Google Scholar]
- 5.Wu P, Jiang TX, Shen JY, Widelitz RB, Chuong C-M. Morphoregulation of avian beaks: Comparative mapping of growth zone activities and morphological evolution. Dev Dyn. 2006;235:1400–1412. doi: 10.1002/dvdy.20825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu P, Jiang TX, Suksaweang S, Widelitz RB, Chuong C-M. Molecular shaping of the beak. Science. 2004;305:1465–1466. doi: 10.1126/science.1098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, et al. Conservation of early odontogenic signaling pathways in Aves. Proc Natl Acad Sci USA. 2000;97:10044–10049. doi: 10.1073/pnas.160245097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris MP, Hasso SM, Ferguson MWJ, Fallon JF. The development of archosaurian first-generation teeth in a chicken mutant. Curr Biol. 2006;16:371–377. doi: 10.1016/j.cub.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 9.Meredith RW, Zhang G, Gilbert MTP, Jarvis ED, Springer MS. Evidence for a single loss of mineralized teeth in the common avian ancestor. Science. 2014;346:1254390. doi: 10.1126/science.1254390. [DOI] [PubMed] [Google Scholar]
- 10.Zhou ZH, Li ZH, Zhang FC. A new Lower Cretaceous bird from China and tooth reduction in early avian evolution. Proc R Soc Lond B Biol Sci. 2010;277:219–227. doi: 10.1098/rspb.2009.0885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou YC, Corwin S, Zhang FC. Tooth reduction in Mesozoic birds had a negligible effect on body mass. Vertebrata PalAsiatica. 2017 in press. [Google Scholar]
- 12.Lautenschlager S, Witmer LM, Altangerel P, Rayfield EJ. Edentulism, beaks, and biomechanical innovations in the evolution of theropod dinosaurs. Proc Natl Acad Sci USA. 2013;110:20657–20662. doi: 10.1073/pnas.1310711110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balanoff AM, Xu X, Kobayashi Y, Matsufune Y, Norell MA. Cranial osteology of the theropod dinosaur Incisivosaurus gauthieri (Theropoda: Oviraptorosauria) Am Mus Novit. 2009;3651:1–35. [Google Scholar]
- 14.Zhou ZH, Wang XL, Zhang FC, Xu X. Important features of Caudipteryx—evidence from two nearly complete new specimens. Vertebrata PalAsiatica. 2000;38:241–254. [Google Scholar]
- 15.Ji Q, Lü JC, Wei XF, Wang XR. A new oviraptorosaur from the Yixian Formation of Jianchang, Western Liaoning Province, China. Geol Bull China. 2012;31:2102–2107. [Google Scholar]
- 16.Watabe M, Weishampel DB, Barsbold R, Tsogtbataar K, Suzuki S. New nearly complete skeleton of the bird-like theropod, Avimimus, from the Upper Cretaceous of the Gobi Desert, Mongolia. J Vertebrate Paleontol. 2000;20:77A. [Google Scholar]
- 17.Ji Q, Currie PJ, Norell MA, Ji SA. Two feathered dinosaurs from northeastern China. Nature. 1998;393:753–761. [Google Scholar]
- 18.Osmólska H, Currie PJ, Barsbold R. Oviraptorosauria. In: Weishampel DB, Dodson P, Osmólska H, editors. The Dinosauria. 2nd Ed Univ of Calif Press; Berkeley: 2004. [Google Scholar]
- 19.Lamanna MC, Sues H-D, Schachner ER, Lyson TR. A new large-bodied oviraptorosaurian theropod dinosaur from the latest Cretaceous of western North America. PLoS One. 2014;9:e92022. doi: 10.1371/journal.pone.0092022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao X, et al. Caenagnathasia sp. (Theropoda: Oviraptorosauria) from the Iren Dabasu Formation (Upper Cretaceous: Campanian) of Erenhot, Nei Mongol, China. Vertebrata PalAsiatica. 2015;53:291–298. [Google Scholar]
- 21.Sues H-D, Averianov A. New material of Caenagnathasia martinsoni (Dinosauria: Theropoda: Oviraptorosauria) from the Bissekty Formation (Upper Cretaceous: Turonian) of Uzbekistan. Cretac Res. 2015;54:50–59. [Google Scholar]
- 22.Currie PJ, Godfrey SJ, Nessov L. New caenagnathid (Dinosauria: Theropoda) specimens from the Upper Cretaceous of North America and Asia. Can J Earth Sci. 1993;30:2255–2272. [Google Scholar]
- 23.Wang Y, et al. A previously undescribed specimen reveals new information on the dentition of Sapeornis chaoyangensis. Cretac Res. 2017;74:1–10. [Google Scholar]
- 24.Funston GF, Currie PJ. A previously undescribed caenagnathid mandible from the late Campanian of Alberta, and insights into the diet of Chirostenotes pergracilis (Dinosauria: Oviraptorosauria) Can J Earth Sci. 2014;51:156–165. [Google Scholar]
- 25.Hieronymus TL, Witmer LM, Tanke DH, Currie PJ. The facial integument of centrosaurine ceratopsids: Morphological and histological correlates of novel skin structures. Anat Rec (Hoboken) 2009;292:1370–1396. doi: 10.1002/ar.20985. [DOI] [PubMed] [Google Scholar]
- 26.Davit-Béal T, Tucker AS, Sire J-Y. Loss of teeth and enamel in tetrapods: Fossil record, genetic data and morphological adaptations. J Anat. 2009;214:477–501. doi: 10.1111/j.1469-7580.2009.01060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erickson GM. Toothlessness in American alligators. Copeia. 1996;3:739–743. [Google Scholar]
- 28.Hungerbühler A. Heterodonty in the European phytosaur Nicrosaurus kapffi and its implications for the taxonomic utility and functional morphology of phytosaur dentitions. J Vertebr Paleontol. 2000;20:31–48. [Google Scholar]
- 29.Xing LD, et al. Tooth loss and alveolar remodeling in Sinosaurus triassicus (Dinosauria: Theropoda) from the Lower Jurassic strata of the Lufeng Basin, China. Chin Sci Bull. 2013;15:1931–1935. [Google Scholar]
- 30.Lanyon JM, Sanson GD. Degenerate dentition of the dugong (Dugong dugon), or why a grazer does not need teeth: Morphology, occlusion and wear of mouthparts. J Zool. 2006;268:133–152. [Google Scholar]
- 31.Davit-Béal T, Chisaka H, Delgado S, Sire J-Y. Amphibian teeth: Current knowledge, unanswered questions, and some directions for future research. Biol Rev Camb Philos Soc. 2007;82:49–81. doi: 10.1111/j.1469-185X.2006.00003.x. [DOI] [PubMed] [Google Scholar]
- 32.Thewissen JGM, et al. Evolutionary aspects of the development of teeth and baleen in the bowhead whale. J Anat. 2017;230:549–566. doi: 10.1111/joa.12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dosedělová H, et al. Fate of the molar dental lamina in the monophyodont mouse. PLoS One. 2015;10:e0127543. doi: 10.1371/journal.pone.0127543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buchtová M, Stembírek J, Glocová K, Matalová E, Tucker AS. Early regression of the dental lamina underlies the development of diphyodont dentitions. J Dent Res. 2012;91:491–498. doi: 10.1177/0022034512442896. [DOI] [PubMed] [Google Scholar]
- 35.Buchtová M, Zahradníček O, Balková S, Tucker AS. Odontogenesis in the Veiled Chameleon (Chamaeleo calyptratus) Arch Oral Biol. 2013;58:118–133. doi: 10.1016/j.archoralbio.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 36.Richman JM, Handrigan GR. Reptilian tooth development. Genesis. 2011;49:247–260. doi: 10.1002/dvg.20721. [DOI] [PubMed] [Google Scholar]
- 37.Lee MSY. The evolution of beaks in reptiles: A proposed evolutionary constraint. Evolutionary Theory Rev. 1997;11:249–256. [Google Scholar]
- 38.Hieronymus TL, Witmer LM. Homology and evolution of avian compound rhamphothecae. Auk. 2010;127:590–604. [Google Scholar]
- 39.Tokita M, Chaeychomsri W, Siruntawineti J. Developmental basis of toothlessness in turtles: Insight into convergent evolution of vertebrate morphology. Evolution. 2013;67:260–273. doi: 10.1111/j.1558-5646.2012.01752.x. [DOI] [PubMed] [Google Scholar]
- 40.Westergaard B, Ferguson MWJ. Development of the dentition in Alligator mississippiensis: Upper jaw dental and craniofacial development in embryos, hatchlings, and young juveniles, with a comparison to lower jaw development. Am J Anat. 1990;187:393–421. doi: 10.1002/aja.1001870407. [DOI] [PubMed] [Google Scholar]
- 41.Hill JP, de Beer GR. Development of the monotremata.—PART VII. The development and structure of the egg-tooth and the caruncle in the monotremes and on the occurrence of vestiges of the egg-tooth and caruncle in marsupials. Trans Zool Soc Lond. 1950;26:503–544. [Google Scholar]
- 42.Peterková R, Lesot H, Peterka M. Phylogenetic memory of developing mammalian dentition. J Exp Zoolog B Mol Dev Evol. 2006;306:234–250. doi: 10.1002/jez.b.21093. [DOI] [PubMed] [Google Scholar]
- 43.Manger PR, Hall LS, Pettigrew JD. The development of the external features of the platypus (Ornithorhynchus anatinus) Philos Trans R Soc Lond B Biol Sci. 1998;353:1115–1125. doi: 10.1098/rstb.1998.0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pagel M. Detecting correlated evolution on phylogenies: A general method for the comparative analysis of discrete characters. Proc R Soc Lond B Biol Sci. 1994;255:37–45. [Google Scholar]
- 45.Hedges SB, Marin J, Suleski M, Paymer M, Kumar S. Tree of life reveals clock-like speciation and diversification. Mol Biol Evol. 2015;32:835–845. doi: 10.1093/molbev/msv037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maddison WP, FitzJohn RG. The unsolved challenge to phylogenetic correlation tests for categorical characters. Syst Biol. 2015;64:127–136. doi: 10.1093/sysbio/syu070. [DOI] [PubMed] [Google Scholar]
- 47.Hu X, et al. Noggin is required for early development of murine upper incisors. J Dent Res. 2012;91:394–400. doi: 10.1177/0022034511435939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vogel P, et al. Malformation of incisor teeth in Grem2−/− mice. Vet Pathol. 2015;52:224–229. doi: 10.1177/0300985814528218. [DOI] [PubMed] [Google Scholar]
- 49.Peterková R, et al. Correlation between apoptosis distribution and BMP-2 and BMP-4 expression in vestigial tooth primordia in mice. Eur J Oral Sci. 1998;106:667–670. doi: 10.1046/j.0909-8836..t01-5-.x. [DOI] [PubMed] [Google Scholar]
- 50.Ekanayake S, Hall BK. The in vivo and in vitro effects of bone morphogenetic protein-2 on the development of the chick mandible. Int J Dev Biol. 1997;41:67–81. [PubMed] [Google Scholar]
- 51.Green HLHH. The development and morphology of the teeth of Ornithorhynchus. Philos Trans R Soc Lond B Biol Sci. 1937;228:367–420. [Google Scholar]
- 52.Hilton EJ, Grande L, Bemis WE. Skeletal anatomy of the shortnose sturgeon, Acipenser brevirostrum Lesueur, 1818, and the systematics of sturgeons (Acipenseriformes, Acipenseridae) Fieldiana Life Earth Sci. 2011;3:1–168. [Google Scholar]
- 53.Bemis WE, Findeis EK, Grande L. An overview of Acipenseriformes. Environ Biol Fishes. 1997;48:25–71. [Google Scholar]
- 54.Franz-Odendaal TA, Adriaens D. Comparative developmental osteology of the seahorse skeleton reveals heterochrony amongst Hippocampus sp. and progressive caudal fin loss. Evodevo. 2014;5:45. doi: 10.1186/2041-9139-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kohno H, Ordonio-Aguilar R, Ohno A, Taki Y. Morphological aspects of feeding and improvement in feeding ability in early stage larvae of the milkfish, Chanos chanos. Ichthyol Res. 1996;43:133–140. [Google Scholar]
- 56.Varricchio DJ. A distinct dinosaur life history? Hist Biol. 2011;23:91–107. [Google Scholar]
- 57.Kingsbury JW, Allen VG, Rotheram BA. The histological structure of the beak in the chick. Anat Rec. 1953;116:95–115. doi: 10.1002/ar.1091160109. [DOI] [PubMed] [Google Scholar]
- 58.Alibardi L. Microscopic and immunohistochemical study on the cornification of the developing beak in the turtle Emydura macquarii. J Morphol. 2016;277:1309–1319. doi: 10.1002/jmor.20576. [DOI] [PubMed] [Google Scholar]
- 59.Ferguson MW. 1985. Reproductive biology and embryology of the crocodilians. Biology of the Reptilia, eds Gans C, Billett F, Maderson PFA (Wiley, NY), pp 329–491, vol. 14.
- 60.Rieppel O. Studies on skeleton formation in reptiles. V. Patterns of ossification in the skeleton of Alligator mississippiensis DAUDIN (Reptilia, Crococlylia) Zool J Linn Soc. 1993;109:301–325. [Google Scholar]
- 61.Morais RA, et al. Direct evidence for gradual ontogenetic dietary shift in the green turtle, Chelonia mydas. Chelonian Conserv Biol. 2014;13:260–266. [Google Scholar]
- 62.Giroux JF, Bedard J. Age differences in the fall diet of greater snow geese in Quebec. Condor. 1988;90:731–734. [Google Scholar]
- 63.Tucker AD, Limpus CJ, McCallum HI, McDonald KR. Ontogenetic dietary partitioning by Crocodylus johnstoni during the dry season. Copeia. 1996;4:978–988. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.