Significance
Ketocarotenoids are high-value pigments used in the food and feed industry to confer color. Aquaculture is a good example, where the addition of carotenoids to the feed is essential for the coloration of trout or salmon flesh, and thus product viability. In this study, complex engineering has been carried out to produce a renewable source of ketocarotenoids for use as feed additives. Production in tomato fruit has enabled the testing of this “generally recognized as safe” material with low-energy minimal bioprocessing in aquaculture trials to demonstrate production, technical, and economic feasibility of the system. This achievement represents a potential paradigm in the bioproduction of specialty and bulk chemicals without our reliance on fossil fuel-derived chemical processes.
Keywords: carotenoids, genetic intervention, tomato, aquaculture, industrial biotechnology
Abstract
Ketocarotenoids are high-value pigments used commercially across multiple industrial sectors as colorants and supplements. Chemical synthesis using petrochemical-derived precursors remains the production method of choice. Aquaculture is an example where ketocarotenoid supplementation of feed is necessary to achieve product viability. The biosynthesis of ketocarotenoids, such as canthaxanthin, phoenicoxanthin, or astaxanthin in plants is rare. In the present study, complex engineering of the carotenoid pathway has been performed to produce high-value ketocarotenoids in tomato fruit (3.0 mg/g dry weight). The strategy adopted involved pathway extension beyond β-carotene through the expression of the β-carotene hydroxylase (CrtZ) and oxyxgenase (CrtW) from Brevundimonas sp. in tomato fruit, followed by β-carotene enhancement through the introgression of a lycopene β-cyclase (β-Cyc) allele from a Solanum galapagense background. Detailed biochemical analysis, carried out using chromatographic, UV/VIS, and MS approaches, identified the predominant carotenoid as fatty acid (C14:0 and C16:0) esters of phoenicoxanthin, present in the S stereoisomer configuration. Under a field-like environment with low resource input, scalability was shown with the potential to deliver 23 kg of ketocarotenoid/hectare. To illustrate the potential of this “generally recognized as safe” material with minimal, low-energy bioprocessing, two independent aquaculture trials were performed. The plant-based feeds developed were more efficient than the synthetic feed to color trout flesh (up to twofold increase in the retention of the main ketocarotenoids in the fish fillets). This achievement has the potential to create a new paradigm in the renewable production of economically competitive feed additives for the aquaculture industry and beyond.
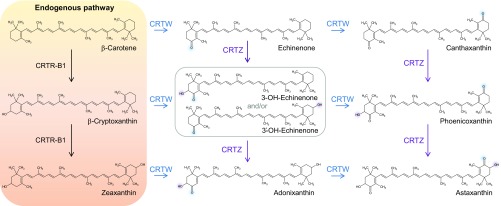
Carotenoids represent one of the largest classes of pigments found in nature (1); however, only a small number are used commercially. Ketocarotenoids, such as astaxanthin or canthaxanthin, are among the highest-value carotenoid pigments on the market (2). These carotenoids possess a characteristic chemical keto moiety on the 4 or 4′ position on the β-ionone ring and can also exhibit hydroxyl groups on the 3 and 3′ positions (Fig. 1). The decoration of the β-ionone ring present in cyclic carotenoids can only be performed by a limited number of enzymes. These enzymes are promiscuous, and thus a myriad of intermediates/products can arise. The best-characterized ketocarotenoid-forming enzymes are those from marine bacteria (3).
Fig. 1.
Representative scheme of the ketocarotenoid pathway introduced in plant. Enzyme names are as follow: CRTR-B1, plant carotene β-hydroxylase 1; CRTW, bacterial carotene ketolase; and CRTZ, bacterial carotene hydroxylase. The purple and blue shadings depict the position of the newly added functional group (hydroxyl or ketone, respectively).
The predominant commercial uses of ketocarotenoids are as feed supplements in the aquaculture and poultry industry to convey aesthetic color and nutritional benefit. Without these supplements, adequate coloration of fish flesh cannot be achieved and an economically viable product cannot be obtained (4). In addition, the pigments also confer beneficial animal husbandry aspects that enable intensification of the industry (5). It is estimated that 15–25% of the total feed costs associated with aquaculture production are due to the price of the carotenoid feed supplements required.
To date, chemical synthesis has been the production method of choice. Like many such processes, it is intrinsically linked to the chemical refining of fossil fuels, using by-products as precursors. The procedures are expensive, have detrimental environmental impact, and lead to a final product that contains reaction contaminants and a mixture of stereoisomers of which the nonnatural form typically predominates. The consumer’s demand for “nonartificial” colorants has driven the industry to identify and develop new sources of carotenoids to replace chemical synthesis (6). For example, algal platforms have been used but logistical problems linked to their slow growth have inhibited broad implementation (7). Other microbial sources, such as Xanthophyllomyces dendrorhous (formally Phaffia rodozyma) and Paracoccus carotinifaciens (Panaferd-AX), have been and are presently used. However, on a production cost-basis, a plant-based source remains the most economically viable (8, 9). The only plant capable of ketocarotenoid (astaxanthin/phoenicoxanthin) formation is Adonis aestivalis, which is not amenable to agricultural production and contains toxic alkaloids (10, 11). Thus, a genetic engineering approach of an agricultural crop offers a viable alternative.
To date, numerous proof-of-concept studies have been reported that have shown how complex pathway and cellular engineering can deliver dramatic changes in desirable compounds. However, very few reports exist that actually show the effectiveness of the approaches under “real-life” scenarios. In the present article, natural variation in combination with complex engineering has been performed to create a plant-based renewable source of ketocarotenoids. The production, technical, and economic feasibility of the material has been demonstrated in comparison with existing products presently used in the aquaculture industry. The data generated have generic implications for the production of high-value specialty and bulk chemical production for renewable sources.
Results
Generation of a High Ketocarotenoid Tomato Line (ZWRI).
A stable ZWRI tomato line was generated from the genetic crossing of ZW and RI tomato. ZW lines overexpress the bacterial genes carotene hydroxylase (CrtZ) and carotene ketolase (CrtW), which are essential for the production of ketocarotenoids from endogenous plant carotenoids, primarily β-carotene (Fig. 1). Interestingly, ZW-expressing lines do not produce high levels of ketocarotenoids [e.g., ZWRIØ ∼ 70 µg/g dry weight (DW)] (SI Appendix, Table S1) due to the lack of the biosynthetic precursor β-carotene. RI are orange-fruited recombinant inbred lines accumulating high levels of β-carotene. They derive from crossing the cultivated tomato Solanum lycopersicum with the wild Solanum galapagense accession (12, 13). Analysis of this collection identified concurrent high β-carotene fruit content with the presence of high comparative expression of the fruit ripening enhanced lycopene β cyclase (β-Cyc).
Two ZW events were crossed with two RI lines. The best combination in terms of ketocarotenoid levels were selected and kept as a hemizygous state for ZW genes, to prevent detrimental effects on plant vigor, and a homozygous state for the S. galapagense lycopene cyclase (β-Cyc) gene. The greater supply of immediate precursor (β-carotene) in ZWRI overcame biosynthetic limitations to ketocarotenoid formation and high ketocarotenoid lines containing about 3 mg/g DW in the fruit material (40-fold increase compared with ZWRIØ) were generated (SI Appendix, Table S1).
Biochemical Characterization of ZWRI.
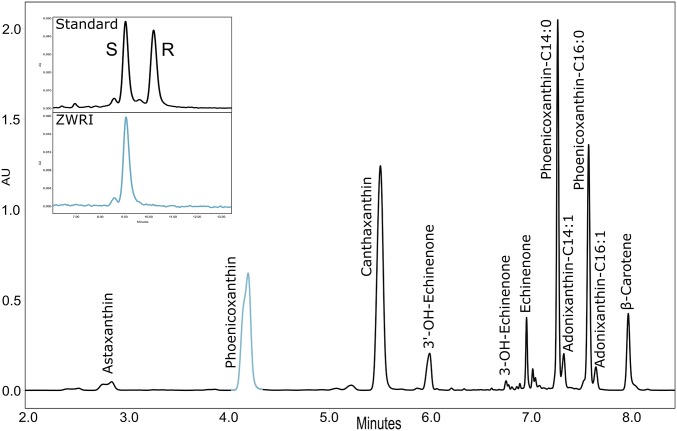
In addition to the ZWRI line, the double azygous control (ZWØRIØ), which lost both the CrtZ and CrtW genes (ZW) plus the S. galapagense β-Cyc promoter (RI), and the azygous controls (ZWØRI and ZWRIØ) were studied. ZWRIØ deep red fruit were defined by a high level of lycopene (77% of total carotenoids) and a small level of ketocarotenoids (2%) (SI Appendix, Table S1). ZWØRIØ were red tomatoes predominantly accumulating lycopene (68% of total carotenoids), ZWØRI tomatoes had an orange color representative of their β-carotene content (66%), and the ZWRI tomatoes had a deep red color reflecting the presence of the ketocarotenoids (87%) (SI Appendix, Fig. S1 and Table S1). Chromatographic analysis of the ZWRI line revealed a complex ketocarotenoid profile (Fig. 2). The main ketocarotenoids found were phoenicoxanthin (in its free and esterified forms, ∼45%) and canthaxanthin (∼35%) (SI Appendix, Table S1). The stereoisomer of phoenicoxanthin was determined as an S configuration (Fig. 2). High-resolution MS/MS was used to identify phoenicoxanthin esters (C14:0 and C16:0). No statistically significant differences of total fatty acid content of the tomatoes were observed (SI Appendix, Fig. S2). Astaxanthin, phoenicoxanthin, and canthaxanthin will be described in this study as the coloring ketocarotenoids, as they all harbor two ketone moieties, giving them the greatest spectra characteristic (λmax > 470 nm) of all of the ketocarotenoids and therefore the most intense red hue.
Fig. 2.
Chromatographic profiles of ZWRI tomato carotenoids and phoenicoxanthin chirality. The chromatographic carotenoids profile was obtained by UPLC and recorded at 470 nm. The Inset shows that the chiral carbon of the ZWRI phoenicoxanthin has an S configuration.
Scalability of the Production Platform.
Following robust glasshouse production of ketocarotenoids from the ZWRI lines, production scalability was assessed. Cultivation of over 200 plants under rudimentary polytunnel containment devoid of supplementary lighting and heating was performed over one growing cycle in the United Kingdom with an early and late season, as defined by commercial growers. The composition of the ketocarotenoid profiles of the ZWRI tomatoes grown in different conditions did not alter (SI Appendix, Table S1). There was a noticeable decrease of total ketocarotenoid content under polytunnel cultivation compared with greenhouse conditions. However, the greatest differences were observed between the early and late seasons of the crop. Despite this change arising from environmental effectors, the levels of ketocarotenoids in the ripe fruit still reached levels of 2.0 mg/g DW total ketocarotenoids in the late season crop (SI Appendix, Table S1). Over the ZWRI tomatoes growth cycle in the polytunnel, an average yield of 12 tons per hectare could be extrapolated, which represents 23 kg of coloring ketocarotenoids [astaxanthin, phoenicoxanthin (free and esterified) and canthaxanthin] per hectare.
Trout Feeding Trials.
The potential of the ZWRI-derived tomato material as feed supplements for the coloring of rainbow trout (Oncorhynchus mykiss) fillets was investigated in two geographical locations (Germany and Chile), where different conditions (fresh and brackish water, respectively) were used to assess the robustness of the platform. Four to five feed treatments were tested (basic, control tomato, ZWRI tomato, ZWRI extract, and commercial feeds). Their compositions are described in SI Appendix, Fig. S3A and Tables S2 and S3. A standard tomato variety devoid of ketocarotenoids but containing a similar total carotenoid content compared with ZWRI tomatoes (2–3 mg/g DW) was used as control tomatoes (SI Appendix, Table S1). The tomato feeds were made using freeze-dried tomato powder (SI Appendix, Fig. S3B). The ZWRI extract feed was based on an oily carotenoid extract of the ZWRI tomatoes (SI Appendix, Fig. S3C). The synthetic BioMar supplement and carophyll pink pigments were used for the commercial feed in the fresh and brackish experiment, respectively. Levels of total ketocarotenoids in the ZWRI and commercial feeds were targeted at 75–80 ppm and clarified after feed processing (SI Appendix, Table S4). Trout with a starting weight of 100 g and 40 g were fed with the different treatments for 50 d and up to 80 d under fresh and brackish conditions, respectively.
ZWRI Tomatoes Color Trout Fillets.
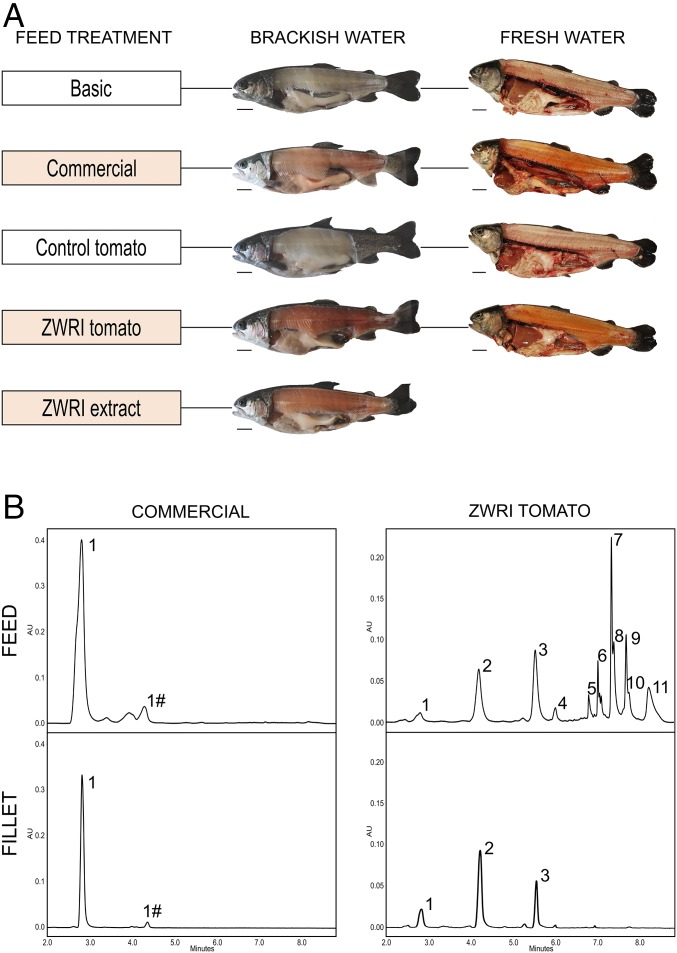
Following the feeding trials, a pink stripe along the lateral line of the fish (from gills to tail) was observed on the fish fed with the ZWRI tomato, ZWRI extract, and commercial treatments. These same fish harbored colored fillets with an orange to pink hue, whereas the fish fed with the basic and control tomato feeds had white fillets (Fig. 3A). Fillet color estimation using the DSM SalmoFan lineal showed that ZWRI feeds provided comparable fillet color compared with the commercial feeds (SI Appendix, Fig. S4), despite the commercial feed for the fresh water trial having a greater initial ketocarotenoid content (SI Appendix, Table S4). The ketocarotenoid composition in the feed and in the fillet remained the same for the commercial treatments and was predominantly astaxanthin. However, for the ZWRI treatments, the main change was the loss of the ketocarotenoid esters in the fillet compared with the feed (Fig. 3B). The ketocarotenoids found in the ZWRI fillets were phoenicoxanthin, canthaxanthin, and some astaxanthin. Most of the endogenous tomato carotenoids, such as lycopene and β-carotene, were also not found in the trout fillet (SI Appendix, Table S5). The retention of (keto) carotenoid indicates quantitatively how these compounds were retained in the fillet, compared with their initial amount in the feed, and is represented as a percentage. For the trout trial in fresh water, the retention of the coloring ketocarotenoids (phoenicoxanthin, canthaxanthin, and astaxanthin) in the fillet was more than twofold greater in the ZWRI treatment compared with the commercial supplement (Table 1). In particular, astaxanthin and phoenicoxanthin had exceptionally high retention, while in the case of the brackish water experiment the retention of the coloring ketocarotenoids was similar when comparing the ZWRI tomato, ZWRI extract, and the commercial treatments (Table 1). Carotenoids were also quantified in the feces of the fresh water trout. Levels of coloring ketocarotenoids were seven times greater in the feces of trout fed with the commercial treatment compared with the ZWRI tomato one, and the retention was 1.2-fold higher for the commercial treatment (SI Appendix, Table S6).
Fig. 3.
ZWRI tomatoes color trout fillets. (A) Photographs of the trout fed with the basic, commercial, control tomato, ZWRI tomato, and ZWRI extract feeds, taken at the end of the fresh and brackish water trials (50 and 80 d, respectively). (B) Chromatographic profiles of carotenoids in the feed and fillet corresponding to the commercial and ZWRI tomato treatments. 1, astaxanthin; 1#, unknown ketocarotenoid-1; 2, phoenicoxanthin; 3, canthaxanthin; 4, 3′-OH-echinenone; 5, 3-OH-echinenone; 6, echinenone; 7, phoenicoxanthin-C14:0; 8, adonixanthin-C14:1; 9, phoenicoxanthin-C16:0; 10, adonixanthin-C16:1; 11, β-carotene.
Table 1.
Ketocarotenoid retention in trout fillet
| Ketocarotenoid | ZWRI tomato | ZWRI extract | Commercial | ||
| Fresh | Brackish | Brackish | Fresh | Brackish | |
| Echinenone | 2 | nq | nq | ||
| 3′-OH-Echinenone | 11 | 10 | 7 | ||
| Canthaxanthin | 44 | 34 | 20 | ||
| Phoenicoxanthin | 127 | 29 | 20 | ||
| Astaxanthin | 158 | 51 | 43 | 27 | 16 |
| Unknown keto-1 | 8 | 16 | |||
| Total coloring keto. | 61 | 26 | 15 | 27 | 16 |
| Total ketocarotenoids | 40 | 20 | 11 | 25 | 16 |
| n | 15 | 5 | 5 | 15 | 5 |
Ketocarotenoid retention is presented for the fresh and brackish water trials for the fish that were fed with ketocarotenoid containing feeds (ZWRI tomato, ZWRI extract, and commercial feeds). Values for the 50- and 80-d-old fish for the fresh and brackish water trial are shown, respectively. Retention was calculated by dividing the quantity of a ketocarotenoid in the fillet by its content in the feed and multiplying by 100. The retention is expressed as percentage. n is the number of trout fillets analyzed. “nq” means that the compound was detected but under the limit of quantification. keto., ketocarotenoids.
Chemical and Physiological Analysis of the Trout Showed Substantial Equivalents.
No significant difference was observed when comparing the weight of the fish across the experiments (SI Appendix, Fig. S5). Levels of carotenoids deposited in the eyes and livers of the trout obtained in the fresh water trial were minimal (∼10 µg/g DW) and actually lower in ZWRI compared with the commercial treatment (SI Appendix, Table S6). No significant difference was detected in cholesterol contents in the fillets and livers of the fresh water trout from the various feed conditions (SI Appendix, Fig. S6). The retinoid, retinyl acetate and apocarotenal, β-apo-14′-carotenal were found at similar levels in the trout livers from the basic and control tomato conditions. However, their levels were both increased in livers of trout fed with the ZWRI tomato and commercial feeds (2.4- to 4-fold and 3.3-fold, respectively). No significant difference in retinyl acetate and β-apo-14′-carotenal contents was noticed between the two latter treatments (SI Appendix, Fig. S7). Fatty acids were also quantified in the different feeds and fillets from the fresh water trial. No significant difference in total fatty acid content was observed between the different feeds (SI Appendix, Fig. S8A). The main fatty acids in the feeds were C18:1, C18:2, and C16:0. In the fillets, these fatty acids were still predominant but C22:6 increased considerably in the fillets compared with the feeds (∼3.3-fold on average). The fatty acid composition of the fillets reflected that of the feeds used (SI Appendix, Figs. S3A and S8B and Table S3B). Moreover, a global analysis of the nonpolar metabolites present in the fillets, derived from the different feed treatments tested, displayed no discernable clustering/separation following principal component analysis on the basis of the feed supplement used (SI Appendix, Fig. S9).
Discussion
Over the last decades, biotechnology has successfully delivered agronomical input traits (14). The consumers demand for improved quality, global food security issues, and the dwindling reserves of fossil fuels, has provided the economic and social impetus to switch from chemical refining to a bioeconomy-based structure. To achieve this goal the development of output traits represent a major agricultural objective. “Golden Rice” and high oleic acid soya are two examples of output traits with the potential to make a difference. In addition to these two examples, numerous proof-of-concept studies have been reported that confer enhanced output traits associated with quality. However, only a few had the opportunity to show technical and production feasibility (15).
In the present study, a plant-based source of ketocarotenoids has been achieved and scalability demonstrated in a contained manner under a field-like environment with low-resource input (SI Appendix, Table S1). The effectiveness of the tomato-based material or its derived ketocarotenoid extract to act as an aquaculture feed supplement, responsible for coloring salmonid flesh, has been demonstrated and bench-marked against two existing chemically synthesized products on the market. Over two trials in different geographical locations, in both brackish and fresh water conditions, the addition of the tomato material as an admix outperformed existing industry products in terms of ketocarotenoid retention in the trout fillets (Table 1). No adverse effects on animal husbandry or yield parameters were observed and chemical substantial equivalence was determined (SI Appendix, Figs. S5, S6, and S9). The high ketocarotenoid tomato extracts also had the potential to color trout fillets (Fig. 3). However, the tomato matrix seemed to improve the retention of the ketocarotenoids in the fillets by nearly twofold (Table 1). Additional work is required to ascertain the mechanism underlying these important phenomena, although we speculate that the lipid microenvironment within the material may enhance ketocarotenoid absorption into the gastrointestinal tract of the trout, as fat and oil are known to improve carotenoid solubilization into micelles and therefore their bioavailability (16). Furthermore, analysis of the carotenoid distribution in the specific organs/tissues of the trout (fillet, eye, and liver) showed that distinct chemical classes of carotenoids were deposited in a differential manner (SI Appendix, Tables S5 and S6). These phenomena could be due to the different lipid transport mechanisms that exist but also other factors, such as carotenoid concentration and composition or the presence of other competing molecules. In the present study, carotenes were exclusively found in the liver while nonesterified xanthophylls were deposited in the fillet, with the exception of echinenone and canthaxanthin that could also be found in the liver and eye. In a generic manner, these data corroborate previous reports describing carotenoid distribution in chicken tissues fed on carotenoid enhanced maize (17). The ketocarotenoids present in the tomato material and derived extracts are predominantly esterified, but those ketocarotenoids present in the flesh of the trout are nonesterified. This observation supports previous findings where esterified ketocarotenoids have been shown to be cleaved in the intestine of the trout before deposition in the flesh (18). To date, the literature is inconclusive with regard to the potential of the esterified carotenoid forms being more bioavailable to fish (19, 20).
The rudimentary approach to formulation used in this study and the results achieved suggest that further optimization of the process will deliver an improved product beyond the prototype used to date. The use of an admix also greatly improves the environmental impact of the process, as no organic solvents are required in the downstream processing or formulation process. In addition to its improved environmental credentials, the reduction in costs is significant. Although a full life cycle analysis is necessary, our estimates suggest that the keto tomato admix could provide an approximate 10-fold cost saving, as presently the production cost of the synthetic feed is in the range of US$1,000–2,000 per kilogram. Using the data generated in this study, the production costs for tomato material containing a kilogram of coloring ketocarotenoids are in the region of US$150. It is important to note that the reason such an admix can be effective is because of the levels reached in the selected tissues used. In this particular case, tomato fruit is the ideal sink tissue because it is intrinsically adapted to isoprenoid production. Although ketocarotenoids have been produced in lettuce (21), potato (22), maize (23), canola (24), and soybean seeds (25), the levels are over two orders-of-magnitude lower than those achieved in tomato. These low levels make the vast amounts of seed material required for incorporation into feed formulation impractical. Presumably, a major reason why these sources are limited is because they have evolved and been selected for starch and oil accumulation. It is interesting that the nonendogenous ketocarotenoids produced existed, where chemically possible, in an esterified form. Precisely how these phenomena arise and their potential to facilitate sequestration awaits further elucidation. Tomato fruit is also an established food, which is readily digestible and regarded as “generally recognized as safe” (GRAS). Nonfood sources, such as tobacco, do not have these credentials and no sink organs are readily amenable for production; this means that pleotropic effects are likely to occur in vegetative tissues when high levels (above 3% DW) are reached, and substantial downstream processes are essential.
The present study also shows that a mixture of ketocarotenoids can have the same coloring potential as astaxanthin solely, the main ketocarotenoid used in aquaculture feeds. Other natural colorants approved by the European Food Safety Authority, such as Panaferd-AX (26) made from Paracoccus carotinifaciens, a red carotenoid-rich soil bacterium, is also constituted of a mixture of carotenoids (27) [astaxanthin (2.2%), phoenicoxanthin (1.3%), and canthaxanthin (0.4%) besides other carotenoids]. The main ketocarotenoid in the ZWRI tomato preparation is phoenicoxanthin. Although not abundant in nature, it can be found in mollusks, crustaceans (28), green alga [such as Haematococcus pluvalis (29) and Chlorococcum (30)], and Adonis flowers (31). The synthetic astaxanthin preparations used contain unidentified reaction contaminants and a mixture of stereoisomers, whereas the biosynthetically derived phoenicoxanthin used in the present study was exclusively present in its biologically active S configuration (Fig. 2), as found in A. aestivalis petals (32). One of the main concerns of novel foods is traceability. The salmonid fillets are the end-products for the food chain, which are effectively nongenetically modified (GM) products with no foreign DNA. In effect, the approach is synonymous with the marketing of livestock products fed on GM feedstuffs, such as soya and corn. One advantage of the present phoenicoxanthin product is that it offers an auditable biochemical marker. Previously, to achieve deregulation of GM peppermint varieties, unnatural stereoisomers had to be generated to create a traceable product within the market place (33).
The targeted chemical analysis of the experimental trout tissues has indicated no significant changes in steady-state metabolite levels or composition between the trout consuming the present commercial product and the experimental tomato-derived ketocarotenoid material. They both prove to enhance the level of one of the natural forms of vitamin A, retinyl acetate in the trout livers, compared with the trout fed with ketocarotenoid free feeds. This demonstrates that ketocarotenoids can also be used as vitamin A precursors in agreement with a previous in vitro study on rainbow trout intestine (34). Although further metabolomic analysis will help to confer the existence of substantial equivalents, based on the present chemical and physical data acquired, the end-product would appear equivalent to similar products in the market, in line with the US Food and Drug Administration terms. It could then be designated as GRAS under the Federal Food, Drug, and Cosmetic Act and therefore avoid premarket approval in the United States (35).
The approach described in this article demonstrates that using a combination of technologies presently available, new technology pipelines can be established to deliver renewable sources of high-value specialty chemicals. In this case, ketocarotenoids have been chosen and tomato fruit exploited as the production platform. Technical and production feasibility have been demonstrated. The pipeline developed is scalable, requires minimal downstream processing, has improved environmental credentials, and is economically competitive.
Materials and Methods
Plant Material and Cultivation.
The Moneymaker variety of tomato S. lycopersicum had been previously transformed with the ZW construct, harboring the bacterial Brevundimonas sp strain SD212 genes carotene hydroxylase (CrtZ) and carotene ketolase (CrtW), both under the cauliflower mosaic virus 35S constitutive promoter (36), using the Agrobacterium tumefaciens strain LBA 4404. The high β-carotene line used in this study (RI) derives from the crossing of the cultivated tomato S. lycopersicum [LA4024 in the Tomato Genetics Resource Center (TGRC) database] with the wild S. galapagense accession (LA0483 in the TGRC database) (12, 13). Two ZW events (10–12, 10–17) were crossed with two RI lines (RI33 and RI1). The lines were cross-pollinated. The best combination in terms of ketocarotenoid levels were selected (10–12 × RI33). The ZWRI plants were greenhouse grown (25 °C day/15 °C night), with supplementary lighting (16-h light/8-h dark) or under polytunnel containment devoid of supplementary lighting and heating, over one growing cycle in the United Kingdom with an early (June–July, ∼24 °C day) and late season (August–September, ∼21 °C day). Analyses were made on three pooled fruits from each studied plant in the greenhouse and on three samples from four individual batches of several kilos of tomatoes (∼5 kg) from each season for the large-scale study in the polytunnel.
Extraction and Analysis of Metabolites.
Carotenoids.
Carotenoids were extracted from freeze-dried tomatoes, freeze-dried trout parts (fillets, livers, and eyes), feces, and feeds. Extractions and analyses were carried out following a protocol previously published (37). A detailed description is shown in SI Appendix, SI Text.
Fatty acids.
Fatty acids were extracted from 20 mg of freeze-dried tomato powder or 50 mg of freeze-dried trout fillet powder and feed and analyzed following the protocol from Menard et al. (38). Details are described in SI Appendix, SI Text.
Retinoids.
A retinoid extraction method was adapted from Gesto et al. (39). A detailed description is given in SI Appendix, SI Text.
Nonpolar compounds (including cholesterol).
Nonpolar compound extraction from the trout fillets and livers was performed as described above for the carotenoids. The extracts were analyzed by gas chromatography-MS analysis. A detailed protocol is given in SI Appendix, SI Text.
Phoenicoxanthin esters fatty acid determination.
The ketocarotenoid esters found in the tomato UPLC chromatogram profile were individually isolated for further characterization. First, the ketocarotenoid esters were saponified using the cholesterol esterase from Pseudomonas (Sigma). The protocol was adapted from Jacobs et al. (40) and Stålberg et al. (41) and is described in SI Appendix, SI Text. The saponified ketocarotenoids were identified as pheonicoxanthin by comparison of spectral characteristic and retention time value of the authentic standard. To determine the fatty acids attached to the phoenicoxanthin esters, the compounds were analyzed using MS. Separations were performed by HPLC (Ultimate 3000; Dionex) before on-line MS using a RP C30 3-μm column (150 × 2.1-mm i.d., YMC) coupled to a 20 × 4.6-mm C30 guard column. The column temperature was maintained at 30 °C. The mobile phase was comprised of (A) methanol containing 0.1% formic acid (by volume) and (B) tert-butyl methyl ether containing 0.1% formic acid (by volume). These solvents were used in a gradient mode starting at 100% (A) for 5 min, then stepped to 95% (A) for 4 min, followed by a linear gradient over 30 min to 25% (A). After this, gradient (A) was a step down to 10% over 10 min. Initial conditions (100% A) were restored for 10 min after the gradient to reequilibrate the system. The flow rate used was 0.2 mL/min. The HPLC system was coupled to maXis quadrupole-time-of-flight (Bruker). The ionization mode used was Atmospheric Pressure Chemical Ionization (APCI) operating in positive mode. Capillary and APCI vaporization temperatures were set at 250 °C and 450 °C, respectively, and the gas flow (nitrogen) at 4 L/min. APCI source settings were as follows: nebulizer pressure 2.5 bar, corona current 4 μA, and a capillary voltage of 4.5 kV. A full MS scan was performed from 300 to 1,500 m/z and MS/MS spectra were recorded at the isolation width of 0.2 m/z. Identification of the fatty acids attached to the phoenicoxanthin was done by comparison with the expected mass in the MS and MS/MS profiles of the phoenicoxanthin esters. Instrument calibration was performed externally before each sequence with APPI/APCI calibrant solution (Agilent Technologies). Automated postrun internal calibration was performed by injecting the same APPI/APCI calibrant solution at the end of each sample run via a six-port divert valve equipped with a 20-µL loop.
Phoenicoxanthin optical isomerism analysis.
Fractions of phoenicoxanthin were collected and optical isomerism studied using a liquid chromatography method adapted from Wang et al. (42), which is detailed in SI Appendix, SI Text.
Trout Trials.
Feed preparation.
A detailed description of the feed preparation is given in SI Appendix, SI Text. The composition of the feeds are described in SI Appendix, Tables S2 and S3. SI Appendix, Fig. S3A gives an overview of the composition of the different feeds.
Feeding trout trial.
The experiments were conducted in compliance with the 3Rs (replacement, reduction, refinement) principles and the 2010/63/EU directives. Ethical approval on animal experiments from the internal Royal Holloway University of London and the DISCO (from DISCOvery to products: A next-generation pipeline for the sustainable generation of high-value plant products) steering committees were obtained.
Fresh water experiment.
Rainbow trout (O. mykiss) of about 100 g were grown in 400-L tanks filled with 260 L of fresh water (flow rate:1.2–2 L/min, temperature: 11–13 °C, oxygen content: 8–10 mg/L). They were fed for 7 wk with the different feed conditions [basic, control tomato, ZWRI tomato, and commercial (BioMar Efico alpha Color 717 42/22) feeds], first with 2% of their weight and afterward with 1.5%. Each feed was tested in three tanks containing 10 fish each. Feeds were delivered from the top of the tank at the surface of the water. Feeds were stored at 4 °C in a dark room during the length of the experiment to prevent carotenoid degradation. Fish were sampled at the end of the experiment and the fillets, eyes, livers, and feces of each trout were collected and kept at −80 °C under N2 atmosphere until analysis.
Brackish water experiment.
Rainbow trout (O. mykiss) of about 30–40 g were grown in 130-L tanks filled with brackish water (salt concentration: 30 PSU, flow rate: 8–9 L/min, temperature: 14–15 °C, oxygen content: 7.405 mg/L). Each tank contained 25 fish, 20 of whom were fed for 60 d and then sampled; the other 5 fish were fed for an extra 20 d, so in total 80 d. Each tank corresponded to one feed condition [basic, control tomato, ZWRI tomato, ZWRI extract, and commercial (Carophyll pink) feeds]. Fillets of each fish were collected for analysis at the end of the experiments and kept at −80 °C under N2 atmosphere until the analyses were performed.
Statistical power of the study.
A post hoc power analysis was performed to assess the statistical power of the study (SI Appendix, SI Text).
Fillet color assessment.
Color of the fish fillets were assessed by three individuals in natural light, right after the slaughtering of the fish, using the DSM SalmoFan as a reference. The color indices of the fan associated with the different hues were used to estimate the color. The average of the indices from the three individuals were calculated and used as a representation of the fillet color of each trout.
Statistical Analysis.
For the study of plant material, three to five biological replicates with three technical replicates per biological replicates were analyzed for every experiment. For the study of the trout material, 5 to 15 biological replicates with three technical replicates per biological replicate were investigated for each experiment unless stated otherwise. IBM SPSS Statistics 21 software was used to determine significant differences between groups. A detailed explanation of the statistical tests performed is given in SI Appendix, SI Text. P values were calculated and represented in figures as follow: *P < 0.05, **P < 0.01, and ***P < 0.001, when appropriate. SI Appendix, Table S7 describes all of the statistical tests performed in this paper and all of the P values obtained with the SPSS software.
A randomization technique was used whenever possible (SI Appendix, SI Text).
Supplementary Material
Acknowledgments
We thank Dr. Perez-Fons for MS assistance; Prof. Bramley for his continued support and advice; and Anne Beiermeister and Ulfert Focken from the Johann Heinrich von Thunen-Institut for their assistance with the trout trial in Germany. This work was supported through the European Union Framework Program DISCO (from DISCOvery to products: a next-generation pipeline for the sustainable generation of high-value plant products; Project 613513), the Biotechnology and Biological Sciences Research Council OPTICAR Project (optimisation of tomato fruit carotenoid content for nutritional improvement and industrial exploitation; Project BB/P001742/1), KBBE III (Plant Knowledge-Based Bioeconomy) high-carotenoid maize CAROMAIZE Project PIM2010PKB-00746, and European Cooperation in Science and Technology (COST) EUROCAROTEN (European network to advance carotenoid research and applications in agro-food and health; COST Action 15136).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1708349114/-/DCSupplemental.
References
- 1.Fraser PD, Bramley PM. The biosynthesis and nutritional uses of carotenoids. Prog Lipid Res. 2004;43:228–265. doi: 10.1016/j.plipres.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Marz U. 2015 The global market for carotenoids. Available at https://www.bccresearch.com/market-research/food-and-beverage/carotenoids-global-market-report-fod025e.html. Accessed September 12, 2017.
- 3.Fraser PD, Miura Y, Misawa N. In vitro characterization of astaxanthin biosynthetic enzymes. J Biol Chem. 1997;272:6128–6135. doi: 10.1074/jbc.272.10.6128. [DOI] [PubMed] [Google Scholar]
- 4.Alfnes F, Guttormsen AG, Steine G, Kolstad K. Consumers’ willingness to pay for the color of salmon: A choice experiment with real economic incentives. Am J Agric Econ. 2006;88:1050–1061. [Google Scholar]
- 5.Steven DM. Studies on animal carotenoids; carotenoids of the brown trout (Salmo trutta Linn.) J Exp Biol. 1948;25:369–387. doi: 10.1242/jeb.25.4.369. [DOI] [PubMed] [Google Scholar]
- 6.Olesen I, Alfnes F, Rora MB, Kolstad K. Eliciting consumers’ willingness to pay for organic and welfare-labelled salmon in a non-hypothetical choice experiment. Livest Sci. 2010;127:218–226. [Google Scholar]
- 7.Lorenz RT, Cysewski GR. Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends Biotechnol. 2000;18:160–167. doi: 10.1016/s0167-7799(00)01433-5. [DOI] [PubMed] [Google Scholar]
- 8.Ausich RL. Commercial opportunities for carotenoid production by biotechnology. Pure Appl Chem. 1997;69:2169–2174. [Google Scholar]
- 9.Gatlin DM, et al. Expanding the utilization of sustainable plant products in aquafeeds: A review. Aquacult Res. 2007;38:551–579. [Google Scholar]
- 10.Cunningham FX, Jr, Gantt E. Elucidation of the pathway to astaxanthin in the flowers of Adonis aestivalis. Plant Cell. 2011;23:3055–3069. doi: 10.1105/tpc.111.086827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maksiutova SS, Lazareva DN. [Pharmacological properties of Adonis sibir and A. vernalis growing in Bashkiria] Farmakol Toksikol. 1978;41:223–226. Russian. [PubMed] [Google Scholar]
- 12.Goldman IL, Paran I, Zamir D. Quantitative trait locus analysis of a recombinant inbred line population derived from a Lycopersicon esculentum x Lycopersicon cheesmanii cross. Theor Appl Genet. 1995;90:925–932. doi: 10.1007/BF00222905. [DOI] [PubMed] [Google Scholar]
- 13.Paran I, Goldman I, Tanksley SD, Zamir D. Recombinant inbred lines for genetic mapping in tomato. Theor Appl Genet. 1995;90:542–548. doi: 10.1007/BF00222001. [DOI] [PubMed] [Google Scholar]
- 14.James C. 2014. Global Status of Commercialized Biotech/GM Crops: 2014 (International Service for the Acquisition of Agri-Biotech Applications, Manila, Philippines), ISAAA Brief 49.
- 15.Newell-McGloughlin M. Nutritionally improved agricultural crops. Plant Physiol. 2008;147:939–953. doi: 10.1104/pp.108.121947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagao A. Bioavailability of dietary carotenoids: Intestinal absorption and metabolism. Jarq-Jpn Agr Res Q. 2014;48:385–391. [Google Scholar]
- 17.Moreno JA, et al. The distribution of carotenoids in hens fed on biofortified maize is influenced by feed composition, absorption, resource allocation and storage. Sci Rep. 2016;6:35346. doi: 10.1038/srep35346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White DA, Page GI, Swaile J, Moody AJ, Davies SJ. Effect of esterification on the absorption of astaxanthin in rainbow trout, Oncorhynchus mykiss (Walbaum) Aquacult Res. 2002;33:343–350. [Google Scholar]
- 19.White DA, et al. The degree of carotenoid esterification influences the absorption of astaxanthin in rainbow trout, Oncorhynchus mykiss (Walbaum) Aquacult Nutr. 2003;9:247–251. [Google Scholar]
- 20.Bowen J, et al. Utilization of (3S,3′S)-astaxanthin acyl esters in pigmentation of rainbow trout (Oncorhynchus mykiss) Aquacult Nutr. 2002;8:59–68. [Google Scholar]
- 21.Harada H, et al. Construction of transplastomic lettuce (Lactuca sativa) dominantly producing astaxanthin fatty acid esters and detailed chemical analysis of generated carotenoids. Transgenic Res. 2014;23:303–315. doi: 10.1007/s11248-013-9750-3. [DOI] [PubMed] [Google Scholar]
- 22.Mortimer CL, et al. Product stability and sequestration mechanisms in Solanum tuberosum engineered to biosynthesize high value ketocarotenoids. Plant Biotechnol J. 2016;14:140–152. doi: 10.1111/pbi.12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farré G, et al. Metabolic engineering of astaxanthin biosynthesis in maize endosperm and characterization of a prototype high oil hybrid. Transgenic Res. 2016;25:477–489. doi: 10.1007/s11248-016-9943-7. [DOI] [PubMed] [Google Scholar]
- 24.Fujisawa M, et al. Pathway engineering of Brassica napus seeds using multiple key enzyme genes involved in ketocarotenoid formation. J Exp Bot. 2009;60:1319–1332. doi: 10.1093/jxb/erp006. [DOI] [PubMed] [Google Scholar]
- 25.Pierce EC, et al. Ketocarotenoid production in soybean seeds through metabolic engineering. PLoS One. 2015;10:e0138196. doi: 10.1371/journal.pone.0138196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.EFSA Safety and efficacy of Panaferd-AX (red carotenoid-rich bacterium Paracoccus carotinifaciens) as feed additive for salmon and trout. EFSA J. 2007;546:1–30. [Google Scholar]
- 27.Osterlie M, Lerfall J. Pigments for aquaculture of salmonids. A comparative model study of carophyll pink and Panaferd AX in cod liver oil. J Am Oil Chem Soc. 2015;92:1321–1331. [Google Scholar]
- 28.Matsuno T. Aquatic animal carotenoids. Fish Sci. 2001;67:771–783. [Google Scholar]
- 29.Yuan JP, Chen F. Chromatographic separation and purification of trans-astaxanthin from the extracts of Haematococcus pluvialis. J Agric Food Chem. 1998;46:3371–3375. [Google Scholar]
- 30.Liu BH, Lee YK. Secondary carotenoids formation by the green alga Chlorococcum sp. J Appl Phycol. 2000;12:301–307. [Google Scholar]
- 31.Cunningham FX, Jr, Gantt E. A study in scarlet: Enzymes of ketocarotenoid biosynthesis in the flowers of Adonis aestivalis. Plant J. 2005;41:478–492. doi: 10.1111/j.1365-313X.2004.02309.x. [DOI] [PubMed] [Google Scholar]
- 32.Maoka T, Etoh T, Kishimoto S, Sakata S. Carotenoids and their fatty acid esters in the petals of Adonis aestivalis. J Oleo Sci. 2011;60:47–52. doi: 10.5650/jos.60.47. [DOI] [PubMed] [Google Scholar]
- 33.Lange BM, et al. Improving peppermint essential oil yield and composition by metabolic engineering. Proc Natl Acad Sci USA. 2011;108:16944–16949. doi: 10.1073/pnas.1111558108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White DA, Ørnsrud R, Davies SJ. Determination of carotenoid and vitamin A concentrations in everted salmonid intestine following exposure to solutions of carotenoid in vitro. Comp Biochem Physiol A Mol Integr Physiol. 2003;136:683–692. doi: 10.1016/s1095-6433(03)00222-8. [DOI] [PubMed] [Google Scholar]
- 35.Price WD, Underhill L. Application of laws, policies, and guidance from the United States and Canada to the regulation of food and feed derived from genetically modified crops: Interpretation of composition data. J Agric Food Chem. 2013;61:8349–8355. doi: 10.1021/jf401178d. [DOI] [PubMed] [Google Scholar]
- 36.Mortimer CL, et al. The formation and sequestration of nonendogenous ketocarotenoids in transgenic Nicotiana glauca. Plant Physiol. 2017;173:1617–1635. doi: 10.1104/pp.16.01297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nogueira M, Mora L, Enfissi EMA, Bramley PM, Fraser PD. Subchromoplast sequestration of carotenoids affects regulatory mechanisms in tomato lines expressing different carotenoid gene combinations. Plant Cell. 2013;25:4560–4579. doi: 10.1105/tpc.113.116210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Menard GN, et al. Genome wide analysis of fatty acid desaturation and its response to temperature. Plant Physiol. 2017;173:1594–1605. doi: 10.1104/pp.16.01907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gesto M, Castro LFC, Reis-Henriques MA, Santos MM. Tissue-specific distribution patterns of retinoids and didehydroretinoids in rainbow trout Oncorhynchus mykiss. Comp Biochem Physiol B Biochem Mol Biol. 2012;161:69–78. doi: 10.1016/j.cbpb.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 40.Jacobs PB, Leboeuf RD, Mccommas SA, Tauber JD. The cleavage of carotenoid esters by cholesterol esterase. Comp Biochem Physiol B. 1982;72:157–160. [Google Scholar]
- 41.Stålberg K, Lindgren O, Ek B, Höglund AS. Synthesis of ketocarotenoids in the seed of Arabidopsis thaliana. Plant J. 2003;36:771–779. doi: 10.1046/j.1365-313x.2003.01919.x. [DOI] [PubMed] [Google Scholar]
- 42.Wang C, Armstrong DW, Chang CD. Rapid baseline separation of enantiomers and a mesoform of all-trans-astaxanthin, 13-cis-astaxanthin, adonirubin, and adonixanthin in standards and commercial supplements. J Chromatogr A. 2008;1194:172–177. doi: 10.1016/j.chroma.2008.04.063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.