Significance
Type 1 diabetes (T1D) is known to be caused by immune destruction of insulin-producing β cells, but the disease pathogenesis remains poorly understood largely because of limitations in animal models to study the immunopathology. Here we established a humanized mouse T1D model, in which diabetes is driven by human T cells recognizing the HLA-DQ8–restricted insulin B chain peptide consisting of amino acids 9–23 (InsB:9–23). This study not only demonstrates the capacity of InsB:9–23-specific human CD4 T cells to initiate diabetes but also provides a preclinical humanized mouse model that has the potential to be used in studies of the immunopathogenesis and immunotherapy of T1D.
Keywords: type 1 diabetes, insulin, humanized mice
Abstract
There is an urgent and unmet need for humanized in vivo models of type 1 diabetes to study immunopathogenesis and immunotherapy, and in particular antigen-specific therapy. Transfer of patient blood lymphocytes to immunodeficient mice is associated with xenogeneic graft-versus-host reactivity that complicates assessment of autoimmunity. Improved models could identify which human T cells initiate and participate in beta-cell destruction and help define critical target islet autoantigens. We used humanized mice (hu-mice) containing robust human immune repertoires lacking xenogeneic graft-versus-host reactivity to address this question. Hu-mice constructed by transplantation of HLA-DQ8+ human fetal thymus and CD34+ cells into HLA-DQ8–transgenic immunodeficient mice developed hyperglycemia and diabetes after transfer of autologous HLA-DQ8/insulin-B:9–23 (InsB:9–23)-specific T-cell receptor (TCR)-expressing human CD4+ T cells and immunization with InsB:9–23. Survival of the infused human T cells depended on the preexisting autologous human immune system, and pancreatic infiltration by human CD3+ T cells and insulitis were observed in the diabetic hu-mice, provided their islets were stressed by streptozotocin. This study fits Koch’s postulate for pathogenicity, demonstrating a pathogenic role of islet autoreactive CD4+ T-cell responses in type 1 diabetes induction in humans, underscores the role of the target beta-cells in their immunological fate, and demonstrates the capacity to initiate disease with T cells, recognizing the InsB:9–23 epitope in the presence of islet inflammation. This preclinical model has the potential to be used in studies of the pathogenesis of type 1 diabetes and for testing of clinically relevant therapeutic interventions.
Type 1 diabetes mellitus (T1D) is an autoimmune disease resulting from immune destruction of insulin-producing β cells in the pancreatic islets. Autoreactive T cells recognizing β-cell antigens (e.g., insulin) are believed to play a critical role in disease onset and progression, but direct evidence for this is still lacking (1). The loss of β cells and the subsequent reduction or lack of insulin result in chronic elevation of blood glucose levels, leading to severe complications such as heart disease, stroke, and kidney failure. Patients with T1D rely on exogenous insulin to control the disease (2), but insulin therapy does not eliminate the risk for T1D complications and may be associated with life-threatening hypoglycemia (3). Thus, new therapies are urgently needed for T1D treatment. Recent insight into the human disease lesion in the pancreas indicated profound differences in immunopathology between patients with T1D and the nonobese diabetic mouse model that is regarded as the nearest preclinical model of autoimmune diabetes (4). These observations, combined with a range of other differences in the immune systems of humans versus mice, prompted us to design preclinical models in which the immune system is humanized to study both disease mechanisms and immunotherapeutic strategies (5).
In this study, we sought to overcome this issue, using humanized mice (hu-mice), by infusing autologous hu-mouse–derived human T cells engineered with a TCR that recognizes the HLA-DQ8–restricted insulin B chain peptide consisting of amino acids 9–23 (InsB:9–23). InsB:9–23 is a dominant MHC class II-restricted antigen recognized by islet-infiltrating insulin-specific T cells and serves as an essential target of the immune destruction of pancreatic β cells in nonobese diabetic (NOD) mice (6, 7). Previous observations suggested this epitope may also serve as a key autoantigenic target in humans, as it does in mice. HLA class II-restricted T-cell response to InsB:9–23 peptide is highly associated with T1D in humans (8). A recent study using HLA-DQ8/B:11–23R22E tetramers further confirmed the presence of CD4 T cells recognizing the HLA-DQ8–restricted B:11–23 peptide in patients with T1D (9). More recently, 2.35% of CD4 T-cell clones isolated from inflamed islets of patients with T1D were found to recognize InsB:9–23 (10). However, there has been no direct evidence for human InsB:9–23-reactive T-cell–mediated in vivo destruction of pancreatic β cells in humans. Our data showed that adoptive transfer of HLA-DQ8–restricted InsB:9–23-specific human CD4 T cells is capable of inducing diabetes in HLA-DQ8–Tg hu-mice, consistent with the potential of T-cell responses to the InsB:9–23 epitope to initiate T1D in humans.
Results
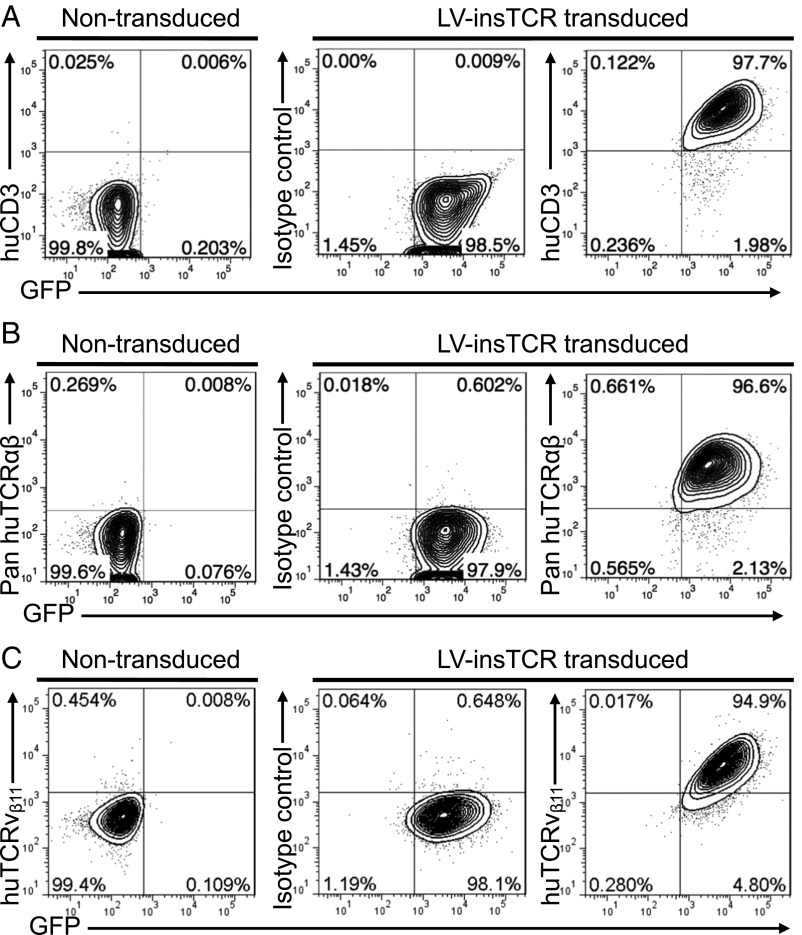
The TCR α (Vα21) and β (Vβ11) chain cDNA was extracted from an InsB:9–23-specific human T-cell line (clone #5), which was established from blood from an 18-y-old Caucasoid HLA-DQ8 homozygous man diagnosed with T1D at the age of 8 y (11, 12), and linked by a P2A self-cleaving peptide gene. The TCRα-P2A-β gene fragment was then linked to a F2A-AcGFP gene fragment, and then cloned into a lentiviral vector (LV-insTCR; Fig. S1). To assess the efficacy of cell surface TCR expression of the engineered human T cells, we measured TCR expression on the TCR-negative human cell line J.RT3-T3.5 cells after transduction with LV-insTCR. Flow cytometric (FCM) analysis revealed that all transduced, that is, GFP+, J.RT3-T3.5, cells gained cell surface expression of human CD3 (Fig. S2A) and αβTCR (Fig. S2B). All GFP+ cells were also stained positively for human TCRvβ11, further confirming the expression of the engineered B:9–23-specific TCR on the transduced cells (Fig. S2C). Together, these results confirmed that efficient cell surface expression of the diabetogenic TCR in human T cells can be achieved via lentiviral transduction.
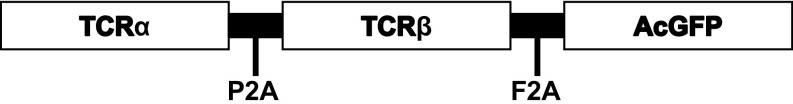
Fig. S1.
Schematic of the LV-insTCR vector. Shown is the B:9–23-specific TCR-AcGFP fragment cloned into the pRRLSIN lentiviral vector.
Fig. S2.
Transgenic TCR expression in lentivirally transduced human TCR-deficient T-cell line cells. J.RT3-T3.5 cells were transduced with LV-insTCR lentiviruses (at a multiplicity of infection = 25). Shown are representative FCM profiles of human CD3 (A), TCRαβ (B), and TCRvβ11 (C) expression on nontransduced control and virally transduced (GFP+) cells.
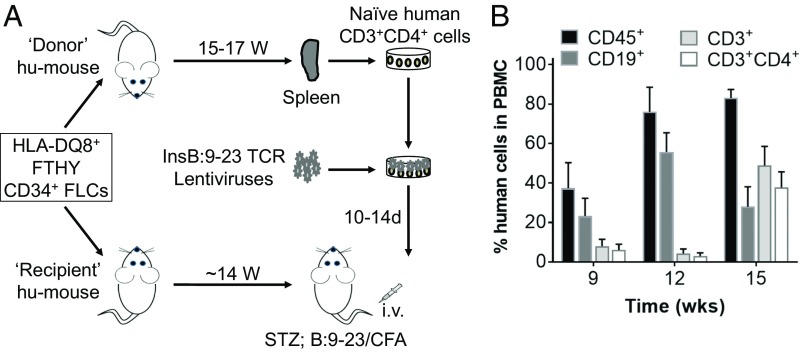
InsB:9–23 is a dominant epitope recognized by autoreactive T cells in both patients with T1D and NOD diabetic mice (8, 13). Because the TCR used in this study recognizes an HLA-DQ8–rectricted InsB:9–23 peptide, we used HLA-DQ8+ human fetal thymus (FTHY) and CD34+ cells isolated from fetal liver cells (FLCs) to construct hu-mice and used HLA-DQ8–Tg hu-mice as the recipients of the engineered human T cells (Fig. 1A). Adoptive transfer of peripheral blood mononuclear cells (PBMCs) from patients with T1D (14, 15) or human T cells that were virally transduced to express β-cell-specific TCRs (16) into immunodeficient mice has been explored previously to characterize the β-cell-specific autoimmune responses of human T cells, and the results demonstrated the potential of these models to facilitate understanding of the immunopathogenesis of human T1D. However, although survival and islet infiltration of the transferred human T cells were clearly detected in the recipient mice carrying the diabetogenic HLA transgene matched to the diabetic patient donors, none of the recipient mice developed diabetes. The presence of human T cells reactive to xenogeneic mouse antigens and varying degrees of graft-versus-host disease (GVHD) were confounding factors in the evaluation of antigen-specific immune responses in these studies (14–16). Indeed, previous studies have shown that xeno-GVH responses can result in global T-cell anergy of transfused human T cells even when they do not cause overt GVHD (17). To avoid both anti-human allogenic and anti-recipient mouse xenogeneic immune responses, self- and mouse-tolerant human T cells (18) from autologous hu-mice were used to generate InsB:9–23-specific T cells, which were then adoptively transferred into HLA-DQ8–Tg hu-mice with an autologous immune system (i.e., hu-mice used as the recipients and those used as the source of InsB:9–23-specific T cells were made by FTHY and CD34+ FLCs from the same fetus; Fig. 1A). Human lymphohematopoietic cell reconstitution in hu-mice was monitored by FCM analysis of peripheral blood human cells. Fig. 1B shows the kinetics of human CD45+, CD19+ B, CD3+, and CD3+CD4+ T cells in PBMCs from a representative experiment, in which the level of human CD3+ T cells in all hu-mice examined was greater than 30% in PBMCs by 15 wk after humanization.
Fig. 1.
Preparation of hu-mice for generating InsB:9–23-specific T cells and for induction of diabetes. (A) Schematic showing preparation of both donor hu-mice with HLA-DQ8+ human immune reconstitution, from which splenic human CD4+-naive T cells are prepared, transduced with LV-insTCR, expanded, and injected into recipient hu-mice for diabetes induction, and recipient HLA-DQ8–Tg hu-mice reconstituted with human immune cells autologous to the donor hu-mice, which are used as the recipients of LV-insTCR–transduced CD4+ T cells. (B). Levels (mean ± SEM) of human hematologic and immune cell chimerism in PBMCs of hu-mice from a representative experiment.
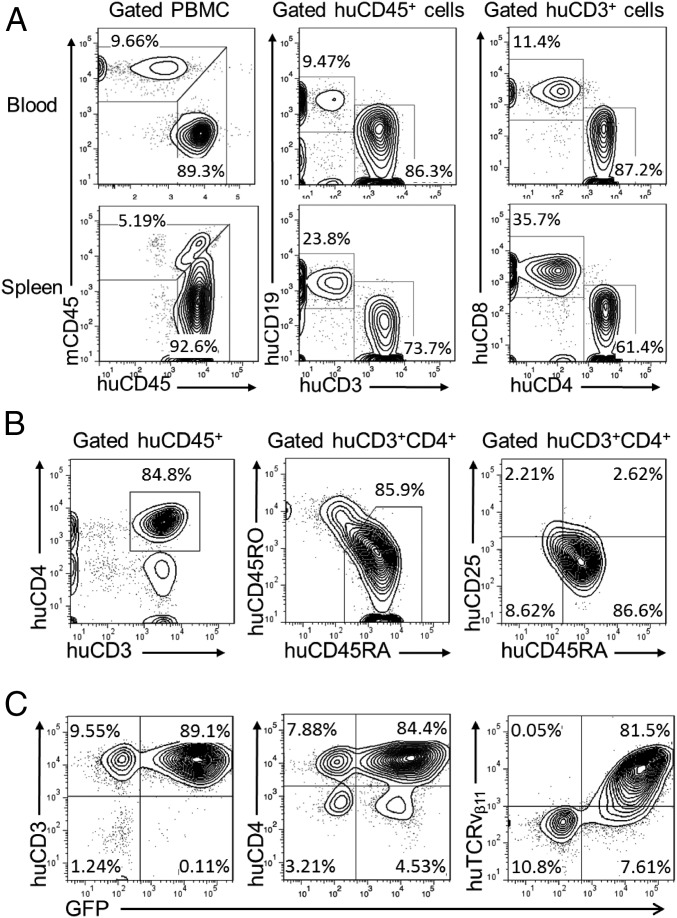
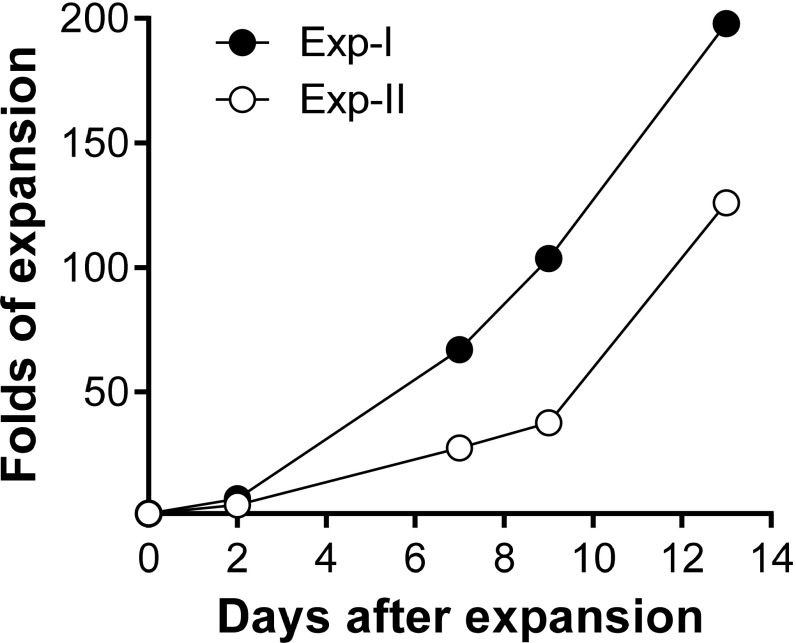
To collect human CD4+ T cells for genetic engineering, spleens were harvested from hu-mice between 15 and 17 wk after humanization. After confirming high levels of human T-cell reconstitution by FCM analysis of PBMCs and spleen cells (Fig. 2A), human CD4+-naive T cells were enriched by depletion of mouse cells and human CD14+, CD19+, CD8+, CD45RO+, and CD25+ cells. The purified cell population consisted of ≈85% human CD3+CD4+ T cells, with the majority of the cells expressing a CD45RA+CD45RO−CD25− naive phenotype (Fig. 2B). The human naive CD4+ T-cell–enriched cells were preactivated by anti-human CD3/CD28 Dynabeads, followed by incubation with LV-insTCR. The transduced human T cells were then expanded for 10–14 d with irradiated feeder cells plus human cytokines (IL-2, IL-7, and IL-15) and PHA (phytohemagglutinin) or OKT3 (ortho Kung T-cell 3). Using this protocol, we were able to achieve ≈130–200-fold expansion in numbers (Fig. S3). At the end of culture, the majority of the expanded cells were CD3+CD4+ T cells with more than 80% of the cells expressing the engineered TCR, as identified by coexpression of GFP and TCRvβ11 (Fig. 2C). The expanded human CD4+ T cells were used immediately in an effort to induce diabetes in HLA-DQ8–Tg hu-mice.
Fig. 2.
Purification and lentiviral transduction of hu-mouse–derived human CD3+CD4+CD45RO−CD25−-naive T cells. Spleen cells were prepared from donor hu-mice between 15 and 17 wk after humanization, as indicated in Fig. 1A, and human CD4+-naive T cells were prepared by MACS negative selection, using antibodies against mouse cells (anti-mCD45 and Ter119) and human cells expressing CD8, CD14, CD19, CD25, or CD45RO and transduced with LV-insTCR. (A) FCM profiles of human CD45+, CD3+, CD19+, CD3+CD4+, and CD4+CD8+ cells in the pooled spleen cells from donor hu-mice. (B) Purity of the MACS-selected hu-mouse spleen cells. Shown are the percentage of human CD3+CD4+ T cells and their expression of CD45RO, CD45RA, and CD25. (C) Expression of human CD3, CD4, and TCRvβ11 on the in vitro expanded LV-insTCR–transduced GFP+ cells.
Fig. S3.
Expansion of lentivirally transduced human CD4+ T cells in vitro. LV-insTCR–transduced hu-mouse–derived human CD4 T cells were expanded in vitro for 13 d. Data from two representative experiments are shown and presented as fold of expansion in cell numbers.
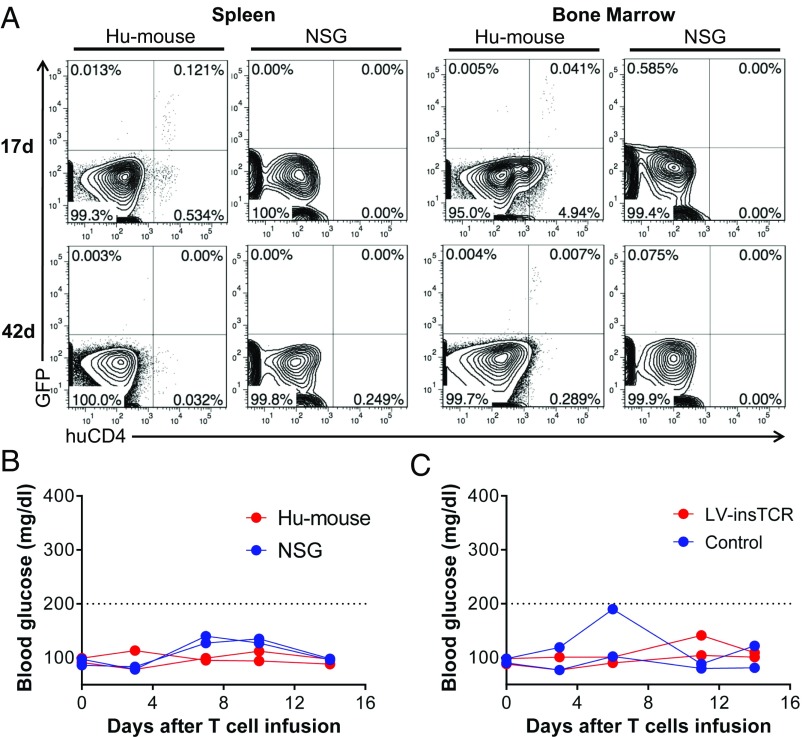
We initially injected the InsB:9–23-TCR-engineered human CD4+ T cells into HLA-DQ8–Tg NSG mice or hu-mice that were grafted 14 wk earlier with autologous human CD34+ FLCs (without FTHY; these hu-mice contained human antigen-presenting cells, but not T cells). FCM analysis revealed that the infused human GFP+CD4+ T cells were detectable for at least 17 d in the spleen and 42 d in bone marrow from hu-mice grafted with human CD34+ FLCs, but not in these tissues from control NSG mice (Fig. S4A). The lack of detectable GFP+CD4+ T cells in NSG mice is consistent with our previous studies showing that the engrafted human CD34+ FLC-derived cells, likely antigen-presenting cells, play an important role in the survival and/or expansion of infused human T cells when there is no xeno-GVH reactivity (19). However, neither HLA-DQ8–Tg hu-mice nor NSG mice developed diabetes or hyperglycemia after infusion of InsB:9–23-TCR-engineered human CD4+ T cells (Fig. S4B). Injection of InsB:9–23-TCR-engineered human CD4+ T cells also failed to induce diabetes or hyperglycemia in low-dose streptozotocin-treated HLA-DQ8–Tg hu-mice that were grafted 14 wk earlier with autologous human CD34+ FLCs and FTHY (Fig. S4C).
Fig. S4.
Survival of infused human T cells and levels of blood glucose in mice after infusion of human CD4 T cells. (A and B) LV-insTCR–transduced hu-mouse–derived human CD4 T cells were expanded for 13 d and injected into HLA-DQ8–Tg NSG mice (n = 2) or HLA-DQ8–Tg hu-mice grafted 14 wk earlier with human CD34+ FLCs (n = 2). (A) FCM analysis of surviving LV-insTCR–transduced GFP+CD4+ human T cells in spleen and bone marrow from hu-mouse (Left) and NSG mouse (Right) recipients at the indicated times after human T-cell transfer. (B) Blood glucose levels. (C) HLA-DQ8–Tg hu-mice grafted 14 wk earlier with human CD34+ FLCs and FTHY were treated by two injections of low-dose streptozotocin, followed 1 d later by infusion of expanded LV-insTCR–transduced (n = 2) or control (n = 2) hu-mouse–derived human CD4 T cells. Shown are blood glucose levels.
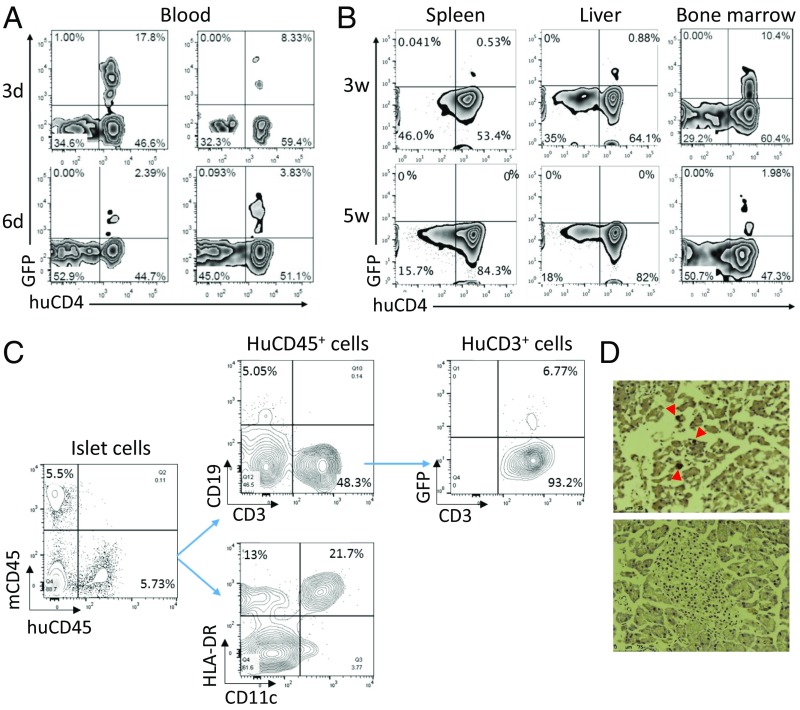
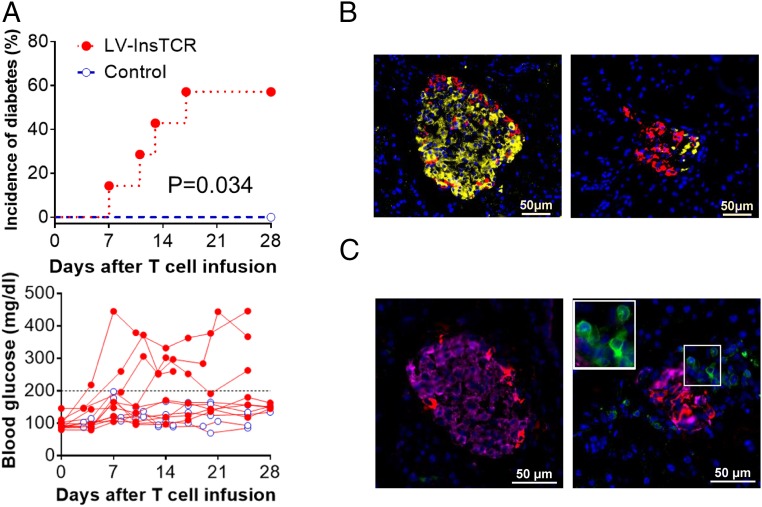
Considering the fact that the TCR used in this study was isolated from a blood-derived T-cell clone that may not respond to endogenously processed peptides (10), in the subsequent experiments, we immunized the HLA-DQ8–Tg hu-mice (grafted 14 wk earlier with human CD34+ FLCs and FTHY) with InsB:9–23 peptides in CFA adjuvant 1 d after injection of InsB:9–23-TCR-engineered or control human CD4+ T cells. FCM analysis confirmed the presence of the infused LV-insTCR–transduced (i.e., GFP+) human CD4 T cells in blood and tissues, including pancreas from the recipient hu-mice (Fig. 3). The infused LV-insTCR–transduced (i.e., GFP+) CD4+ T cells were detectable for days in peripheral blood (Fig. 3A), and for at least 3 wk in the spleen and liver and 5 wk in bone marrow (Fig. 3B) in the diabetic hu-mice. Moreover, human CD45+ cells, including CD11c+HLA-DR+ DCs, CD19+ B cells, and CD3+ T cells (nearly 50%, including both GFP+ and GFP− T cells) were detected in the islets 11 d after infusion of LV-insTCR–transduced (GFP+) human CD4 T cells (Fig. 3C). GFP+ T cells were also detected by histology in the pancreatic islets from the hu-mice 3–4 wk postinfusion of LV-insTCR–transduced T cells (Fig. 3D). Importantly, ≈60% of the hu-mice receiving InsB:9–23-TCR-engineered human T cells developed hyperglycemia and diabetes, whereas none of the similarly conditioned hu-mice receiving control human T cells and InsB:9–23 immunization had hyperglycemia (Fig. 4A). Histological analysis demonstrated severe destruction of mouse pancreatic islets and associated human CD3+ T-cell infiltration in the diabetic, but not the control, hu-mice (Fig. 4 B and C). Collectively, these data indicate that human T cells engineered with InsB:9–23-TCR are functional and capable of inducing insulitis and diabetes after adoptive transfer into HLA-DQ8–Tg hu-mice.
Fig. 3.
Survival of the infused LV-insTCR–transduced human CD4+ T cells in hu-mice. (A) Survival of GFP+CD4+ T cells in peripheral blood from hu-mice at 3 and 6 d after cell transfer. Data from two hu-mice at each point are shown. (B) Presence of GFP+CD4+ human T cells in spleen, liver, and bone marrow cells of two representative hu-mice analyzed at 3 and 5 wk, respectively, after cell transfer. (C) Pancreatic islets were prepared from hu-mice 11 d after infusion of LV-insTCR–transduced human CD4+ T cells, digested with trypsin, and stained with the indicated antibodies to detect human cell infiltration by flow cytometry. (D) Pancreatic tissue sections were prepared from hu-mice between 3 and 4 wk after injection of LV-InsTCR–transduced GFP+ human T cells (n = 3), and stained with anti-GFP antibodies. Shown are representative immunohistochemistry images of pancreas sections from hu-mice receiving LV-InsTCR–transduced GFP+ (Top) or control (Bottom) human T cells.
Fig. 4.
Induction of diabetes in HLA-DQ8–Tg hu-mice by adoptive transfer of autologous diabetogenic human CD4+ T cells. HLA-DQ8–Tg hu-mice were treated with low-dose streptozotocin and injected 1–2 d later with 5 × 106 expanded LV-insTCR–transduced or control (i.e., the same hu-mouse–derived human CD4+ T cells that were similarly expanded in vitro as the LV-insTCR–transduced CD4+ T cells) human CD4+ T cells, followed 1 d later by immunization with InsB:9–23 peptides. (A) Cumulative incidence of diabetes (Top) and levels of blood glucose (Bottom) in hu-mice receiving LV-insTCR–transduced (solid symbol; n = 7) or control (opened symbol; n = 6) human T cells. Mice were defined as hyperglycemia if two consecutive blood glucose measurements >200 mg/dL (B and C) Immunofluorescent staining of pancreas samples prepared between 3 and 4 wk after injection of CD4+ T cells from hu-mice receiving control (Left) or LV-InsTCR–transduced (Right) human T cells (n = 3 per group). (B) Staining of mouse insulin (yellow) and glucagon (red). (C) Staining of human CD3+ cells (green), mouse insulin (pink) and glucagon (red). Nuclear is stained blue by DAPI.
Discussion
Animal models have been crucial in understanding the pathogenesis and testing of therapeutic interventions for T1D. However, because of differences between animals and humans, information learned from animal models often does not apply to humans, and most therapeutic approaches effective in animals are not successful in the clinic (20). For these reasons, hu-mouse models have been increasingly used in the study of human diseases and therapies. Efforts have been made to model T1D by transferring peripheral blood of patients with T1D to immunodeficient mice with the diabetes-prone NOD background. Although some of these approaches have indeed led to insulitis (14, 21, 22), the models are limited by the restriction to blood, where the most relevant T cells may not reside (23), as the source of human T cells. An additional limitation is the propensity of human T cells to cause xenogeneic GVHD when transferred to immunodeficient mice (24, 25). Although GVHD may be prevented by using autoantigen-expanded T-cell lines or clones (21), this approach has not allowed assessment of the role of recruitment of other human T cells and immune cells in disease pathogenesis. Our study describes a hu-mouse model of T1D in mice with established robust immune systems and which lack GVHD. Direct study of human T1D pathogenesis is thereby afforded through insulin-reactive human T-cell–mediated disease induction in the presence of an autologous human lymphohematopoietic system. Despite its complexity and the other limitations of the rodent host, this model may prove useful for testing clinically relevant therapeutic interventions.
Autoreactive T cells recognizing β-cell antigens (e.g., insulin) are believed to play a critical role in disease onset and progression, but the primary antigens eliciting disease in humans remain unknown, and this question cannot be investigated. Using hu-mice, this study provides definitive proof that human islet autoreactive T cells carrying a TCR isolated from a patient with T1D can be diabetogenic in vivo, and demonstrates a possible pathogenic role of CD4+ T-cell responses to the InsB:9–23 epitope in T1D induction in humans. The TCR used in this study was isolated from a blood-derived T-cell clone, which, unlike islet-derived T-cell clones, does not recognize islets directly (10). According to Unanue’s classification, this is a type B clone that escapes from negative selection in the thymus and gets activated in periphery (26). These observations suggest that in our model, the peptide immunization may play an important role in activating the transferred InsB:9–23-reactive human CD4 T cells.
Our observation that streptozotocin treatment precipitated T-cell–mediated beta-cell destruction is in line with the popular notion that beta cells are involved in their own demise through their dialogue with the immune system (27). Indeed, recent data point out that beta-cell stress increases their vulnerability, immunogenicity, and attraction by the immune system (28). The beta-cell stress induced by perturbation with streptozotocin in our hu-mice model would act as an important checkpoint in the induction of beta-cell destruction mediated by islet autoreactive T-cells after loss of immune tolerance.
An important difference from previous studies (14–16) is that in the present study, diabetogenic human T cells were injected into hu-mice with an established human immune system. These hu-mice have high levels of human lymphohematopoietic cell reconstitution and formation of secondary lymphoid organs (e.g., white pulp in the spleen and follicular structure in lymph nodes), and are capable of mounting in vivo antigen-specific immune responses after antigen immunization or transplantation (29–31). The human immune cells from recipient hu-mice might also be involved in the immune destruction of pancreatic β cells. They were clearly essential for the prolonged survival of infused autoreactive T cells in our study (Figs. S4A and S5), consistent with the role that human antigen-presenting cells were shown to play in facilitating the survival, expansion, and phenotypic conversion of human T cells in hu-mice when xeno-GVH reactivity is absent (19). In addition, the presence of both GFP+ and GFP− human CD3+ T cells in the pancreatic islets from hu-mice receiving LV-insTCR–transduced (i.e., GFP+) human CD4 T cells (Fig. 3) suggests a possible contribution of recipient endogenous human T cells to the disease development. T cells recognizing numerous antigenic epitopes, including others of insulin, glutamate decarboxylase, islet specific glucose 6 phosphatase catalytic subunit related protein, and the islet tyrosine phosphatase IA-2, are associated with T1D in humans and NOD mice (32). Although T cells specific for InsB:9–23 may be required for ignition of T1D, the development and progression of the disease might also involve functional epitope spreading (7, 33). Further studies are needed to precisely understand the role of the recipient human immune cells in the development of diabetes in hu-mice infused with human diabetogenic T cells.
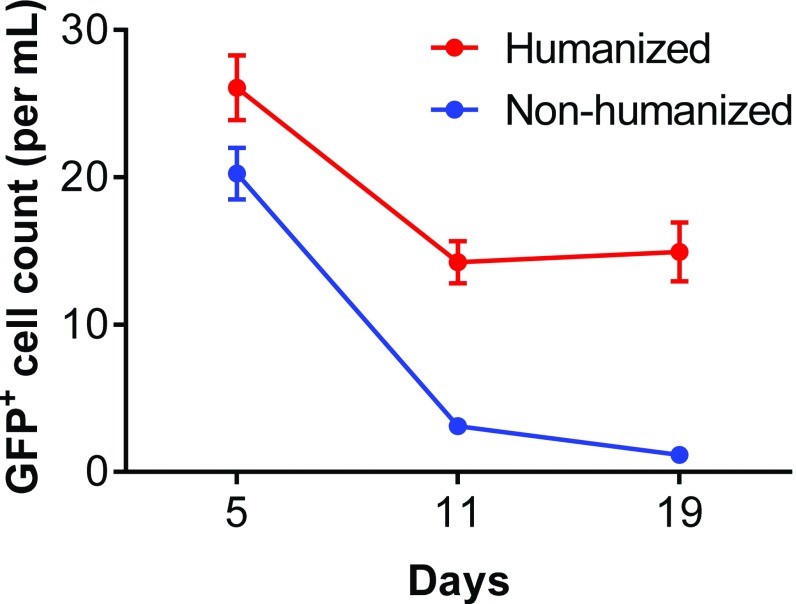
Fig. S5.
Comparison of the survival of infused human T cells in humanized versus nonhumanized NSG mice. Ex vivo expanded hu-mouse–derived human T cells, which were transduced with insB:9–23-specific TCR/GFP, were injected i.v. to hu-mice with autologous human lymphohematopoietic cells or nonhumanized NSG mice. Blood was collected at days 5, 11, and 19 after adoptive transfer, and numbers of the transferred (GFP+) T cells were determined. Shown are GFP+ T-cell counts (mean ± SEM; n = 5 per group).
Our hu-mice model will allow investigation of the pathogenicity and recruitment of human islet autoreactive T-cells, as well as identify their potentially initiating or pathogenic target beta-cell autoantigens, rendering this model uniquely suited to investigate antigen-specific immunotherapy in T1D in preclinical models in vivo that hitherto was impossible with any other animal model.
Materials and Methods
Animals and Human Tissues and Cells.
The NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice and NOD.Cg-Prkdcscid H2-Ab1tm1Doi Tg(HLA-DQA1,HLA-DQB1)1Dv/SzJ (HLA-DQ8–transgenic NOD/SCID) mice were purchased from the Jackson Laboratory. HLA-DQ8–transgenic NSG mice were generated by crossing HLA-DQ8–Tg NOD/SCID mice with NSG mice. All mice were housed in a specific pathogen-free microisolator environment and used between 6 and 12 wk of age. Human FTHY and liver tissues of gestational age of 17–21 wk were obtained from Advanced Bioscience Resource. J.RT3-T3.5 cell line, a TCRβ chain-deficient human T-cell line derived from the E6-1 clone of Jurkat cells that does not express CD3 or TCRαβ heterodimers on the surface (34), was purchased from American Type Culture Collection (ATCC TIB-153). Protocols involving the use of discarded human tissues and animals were approved by the Columbia University Medical Center Institutional Review Board and the Institutional Animal Care and Use Committee, respectively, and all experiments were performed in accordance with these protocols.
Humanized Mouse Preparation.
Hu-mice were constructed as described in our previous studies (29, 35). Briefly, NSG and HLA-DQ8–Tg NOD/SCID or NSG mice were conditioned with sublethal (1.5 Gy) total body irradiation and received human CD34+ FLCs (1 × 105, i.v.) and a FTHY tissue fragment (≈1 mm3; under the kidney capsule) from the same fetus.
HLA-DQ8/InsB:9–23-Specific TCR Isolation and Lentiviral Vector Construction.
After informed consent, peripheral blood was drawn from an 18-y-old Caucasoid HLA-DQ8/8 homozygous man diagnosed with T1D at the age of 8 y, and a T-cell line (clone #5) was generated by stimulation with InsB6-22 (the epitope being identical in B:9–23, and the T cells cross-react with B:9–23) (11, 12). The TCR α and β chain cDNA were extracted and linked by a P2A self-cleaving peptide gene (36). The TCR α-P2A-β gene fragment was then linked with a F2A-AcGFP (aequorea coerulescens green fluorescent protein) gene fragment and then cloned into a lentiviral vector (LV-insTCR; Fig. S1). Pseudotyped lentiviruses were produced by transfection, using Lipofectamine 2000 (Invitrogen) of 293T cells with a four-plasmid system consisting of the transfer vector (LV-insTCR) and three packaging plasmids (pMD2.G, pMDLg/pRRE, and pRSV-Rev). The supernatant with viral particles was collected 48 and 72 h posttransfection and concentrated by ultracentrifugation at 50,000 × g for 2 h. Lentiviruses were stored at −80 °C until use.
Flow Cytometric Analysis.
Levels of human hematopoietic cells in humanized mice were determined by FCM analysis of PBMCs and splenic cells, using various combinations of the following monoclonal antibodies: anti-human CD45, CD19, CD3, CD4, CD8, CD45RA, CD45RO; anti-mouse CD45 and Ter119; and corresponding isotype controls (all purchased from Biolegend). Human T cells transduced with B:9–23-specific TCR (using Vα21 and Vβ11) were identified by staining with anti-human TCRvβ11-FITC (mouse IgG2a; Beckman Coulter) followed by anti-mouse IgG2a-PE secondary mAb (Biolegend). The secondary anti-mouse IgG2a-PE was required to distinguish between GFP-positive cells with and without TCRVβ11 expression, as all virally transduced cells are expected to express AcGFP (Fig. S1). To detect human immune cell infiltration in pancreatic islets, islets were prepared and dissociated with trypsin and stained with anti-human CD45, CD3, CD19, CD11c, HLA-DR, and anti-mouse CD45. Samples were collected on a FACS LSR II (Becton Dickinson) and analyzed with FlowJo software (TreeStar). Dead cells were excluded from the analysis by gating out DAPI-positive cells.
Lentiviral Transduction of Human CD4+ T Cells from Humanized Mice.
Hu-mouse spleen cells enriched for human CD4+-naive T cells were prepared by negative separation using magnetic-activated cell sorting (MACS; Miltenyi Biotec). Briefly, spleen cells were stained with PE-conjugated mAbs against mouse Ter119, mouse CD45, huCD14, huCD19, huCD8, huCD45RO, and huCD25, followed by incubation with anti-PE magnetic beads (Miltenyi) and MACS column purification. The purified human CD4+-naive T cells were stimulated for 3.5 d in medium containing human T-Activator CD3/CD28 Dynabeads (Gibco Life Technologies), followed by incubation with lentiviruses in 96-well plates precoated with retronectin (Takara Bio Inc) for 24 h at a multiplicity of infection of 40. Cells were washed twice and expanded for 10–14 d in vitro in T-cell expansion medium (RPMI 1640 supplemented with 10% human AB serum), irradiated feeder cells, recombinant human cytokines (20 U/mL IL-2, 10 ng/mL IL-7, and 10 ng/mL IL-15; R&D), plus PHA (1.5 µg/mL, Cat# L1668-5 mg; Sigma) or anti-CD3 mAb (OKT3; 30 ng/mL, Cat# NC9195482; Thermo Fisher). Feeder cells were 35-Gy-irradiated pooled allogeneic PBMCs (3 × 106/mL) and 60-Gy-irradiated Epstein-Barr virus-transformed lymphoblastoid cell lines (1.5 × 105/mL). The expanded cells were injected into hu-mice immediately in efforts to induce diabetes.
Induction of Diabetes in HLA-DQ8–Tg Hu-Mice.
The HLA-DQ8–Tg hu-mice on the NOD/SCID or NSG background were conditioned with two successive low doses of streptozotocin (50 mg/kg/d × 2 d, i.p.; Sigma) starting 1 or 2 d before adoptive transfer of lentivirally transduced human CD4+ T cells derived from preestablished hu-mice with an autologous (same fetal tissue donor) immune system (i.e., hu-mice made with the same fetal tissues as the adoptive recipient hu-mice). In some experiments, the HLA-DQ8–Tg hu-mice were also immunized with 100 µL (100 µg) InsB:9–23 peptide (SHLVEALYLVCGERG; AnaSpec, Inc.) in 100 µL Freud’s Complete Adjuvant (InvivoGen) by s.c. injection 1 d after human CD4+ T-cell transfer. Blood glucose levels were monitored twice a week, using FreeStyle blood glucose test strips and a blood glucose meter (Abbott Diabetes Care Inc.). Mice were considered diabetic after two consecutive blood glucose measurements >200 mg/dL (37).
Immunofluorescent Staining of Pancreatic Tissues.
Pancreata were harvested from the hu-mouse recipients between 3 and 4 wk after injection of CD4+ T cells, and fixed in 4% paraformaldehyde for histological analysis. Briefly, frozen sections were prepared and stained with anti-insulin (guinea pig IgG; Dako), anti-glucagon (rabbit IgG; Sigma), anti-human CD3-Alexa Fluor 488 (Biolegend), followed by staining with donkey anti-guinea pig IgG-Cy5 (H+L; Jackson ImmunoResearch) and donkey anti-rabbit IgG-Dylight (H+L; Jackson ImmunoResearch). All sections were mounted in Vectorshield with DAPI (Vector Laboratories). Images were obtained using Leica DMI 6000B wide-field microscope.
Statistical Analyses.
The log-rank (Mantel-Cox) test was used analyze the difference in the incidence of diabetes between groups. A P value of ≤0.05 was considered significant.
Acknowledgments
This work was supported by grants from Ministry of Science and Technology of China (2015CB964400), National Natural Science Foundation of China (81273334, 91642208), and NIH (P01AI045897, R01AI064569, UC4DK104207, and R01DK103585). S.T. and C.-H.J. are supported in part by the China State Scholarship Fund (201306170106 and 201406170139). B.O.R. is supported by grants from the Juvenile Diabetes Research Foundation, the Dutch Diabetes Research Foundation, the DON Foundation, and the European Commission. Flow cytometric analysis was performed in part in the Columbia Center for Translational Immunology Flow Cytometry Core, funded in part through an NIH Shared Instrumentation Grant (1S10RR027050).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 10821.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1710415114/-/DCSupplemental.
References
- 1.Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464:1293–1300. doi: 10.1038/nature08933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383:69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cryer PE. The barrier of hypoglycemia in diabetes. Diabetes. 2008;57:3169–3176. doi: 10.2337/db08-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coppieters KT, et al. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J Exp Med. 2012;209:51–60. doi: 10.1084/jem.20111187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roep BO, Atkinson M, von Herrath M. Satisfaction (not) guaranteed: Re-evaluating the use of animal models of type 1 diabetes. Nat Rev Immunol. 2004;4:989–997. doi: 10.1038/nri1502. [DOI] [PubMed] [Google Scholar]
- 6.Simone E, et al. T cell receptor restriction of diabetogenic autoimmune NOD T cells. Proc Natl Acad Sci USA. 1997;94:2518–2521. doi: 10.1073/pnas.94.6.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakayama M, et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature. 2005;435:220–223. doi: 10.1038/nature03523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alleva DG, et al. A disease-associated cellular immune response in type 1 diabetics to an immunodominant epitope of insulin. J Clin Invest. 2001;107:173–180. doi: 10.1172/JCI8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang J, et al. Autoreactive T cells specific for insulin B:11-23 recognize a low-affinity peptide register in human subjects with autoimmune diabetes. Proc Natl Acad Sci USA. 2014;111:14840–14845. doi: 10.1073/pnas.1416864111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michels AW, et al. Islet-derived CD4 T-cells targeting proinsulin in human autoimmune diabetes. Diabetes. 2017;66:722–734. doi: 10.2337/db16-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eerligh P, et al. Functional consequences of HLA-DQ8 homozygosity versus heterozygosity for islet autoimmunity in type 1 diabetes. Genes Immun. 2011;12:415–427. doi: 10.1038/gene.2011.24. [DOI] [PubMed] [Google Scholar]
- 12.Michels AW, et al. Structure-based selection of small molecules to alter allele-specific MHC class II antigen presentation. J Immunol. 2011;187:5921–5930. doi: 10.4049/jimmunol.1100746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daniel D, Wegmann DR. Protection of nonobese diabetic mice from diabetes by intranasal or subcutaneous administration of insulin peptide B-(9-23) Proc Natl Acad Sci USA. 1996;93:956–960. doi: 10.1073/pnas.93.2.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitfield-Larry F, et al. HLA-A2-matched peripheral blood mononuclear cells from type 1 diabetic patients, but not nondiabetic donors, transfer insulitis to NOD-scid/γc(null)/HLA-A2 transgenic mice concurrent with the expansion of islet-specific CD8+ T cells. Diabetes. 2011;60:1726–1733. doi: 10.2337/db10-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viehmann Milam AA, et al. A humanized mouse model of autoimmune insulitis. Diabetes. 2014;63:1712–1724. doi: 10.2337/db13-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Babad J, et al. Generation of β cell-specific human cytotoxic T cells by lentiviral transduction and their survival in immunodeficient human leucocyte antigen-transgenic mice. Clin Exp Immunol. 2015;179:398–413. doi: 10.1111/cei.12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tary-Lehmann M, Saxon A. Human mature T cells that are anergic in vivo prevail in SCID mice reconstituted with human peripheral blood. J Exp Med. 1992;175:503–516. doi: 10.1084/jem.175.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalscheuer H, et al. Xenograft tolerance and immune function of human T cells developing in pig thymus xenografts. J Immunol. 2014;192:3442–3450. doi: 10.4049/jimmunol.1302886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onoe T, et al. Homeostatic expansion and phenotypic conversion of human T cells depend on peripheral interactions with APCs. J Immunol. 2010;184:6756–6765. doi: 10.4049/jimmunol.0901711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roep BO, Buckner J, Sawcer S, Toes R, Zipp F. The problems and promises of research into human immunology and autoimmune disease. Nat Med. 2012;18:48–53. doi: 10.1038/nm.2626. [DOI] [PubMed] [Google Scholar]
- 21.Unger WWJ, et al. Islet-specific CTL cloned from a type 1 diabetes patient cause beta-cell destruction after engraftment into HLA-A2 transgenic NOD/scid/IL2RG null mice. PLoS One. 2012;7:e49213. doi: 10.1371/journal.pone.0049213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao Y, et al. Selective destruction of mouse islet beta cells by human T lymphocytes in a newly-established humanized type 1 diabetic model. Biochem Biophys Res Commun. 2010;399:629–636. doi: 10.1016/j.bbrc.2010.07.128. [DOI] [PubMed] [Google Scholar]
- 23.Nepom GT, et al. HLA class II tetramers: Tools for direct analysis of antigen-specific CD4+ T cells. Arthritis Rheum. 2002;46:5–12. doi: 10.1002/1529-0131(200201)46:1<5::AID-ART10063>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 24.King MA, et al. Human peripheral blood leucocyte non-obese diabetic-severe combined immunodeficiency interleukin-2 receptor gamma chain gene mouse model of xenogeneic graft-versus-host-like disease and the role of host major histocompatibility complex. Clin Exp Immunol. 2009;157:104–118. doi: 10.1111/j.1365-2249.2009.03933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ali N, et al. Xenogeneic graft-versus-host-disease in NOD-scid IL-2Rγnull mice display a T-effector memory phenotype. PLoS One. 2012;7:e44219. doi: 10.1371/journal.pone.0044219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohan JF, Calderon B, Anderson MS, Unanue ER. Pathogenic CD4+ T cells recognizing an unstable peptide of insulin are directly recruited into islets bypassing local lymph nodes. J Exp Med. 2013;210:2403–2414. doi: 10.1084/jem.20130582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roep BO, Tree TI. Immune modulation in humans: Implications for type 1 diabetes mellitus. Nat Rev Endocrinol. 2014;10:229–242. doi: 10.1038/nrendo.2014.2. [DOI] [PubMed] [Google Scholar]
- 28.Kracht MJ, et al. Autoimmunity against a defective ribosomal insulin gene product in type 1 diabetes. Nat Med. 2017;23:501–507. doi: 10.1038/nm.4289. [DOI] [PubMed] [Google Scholar]
- 29.Lan P, Tonomura N, Shimizu A, Wang S, Yang YG. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood. 2006;108:487–492. doi: 10.1182/blood-2005-11-4388. [DOI] [PubMed] [Google Scholar]
- 30.Tonomura N, Habiro K, Shimizu A, Sykes M, Yang YG. Antigen-specific human T-cell responses and T cell-dependent production of human antibodies in a humanized mouse model. Blood. 2008;111:4293–4296. doi: 10.1182/blood-2007-11-121319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tonomura N, et al. Pig islet xenograft rejection in a mouse model with an established human immune system. Xenotransplantation. 2008;15:129–135. doi: 10.1111/j.1399-3089.2008.00450.x. [DOI] [PubMed] [Google Scholar]
- 32.Di Lorenzo TP, Peakman M, Roep BO. Translational mini-review series on type 1 diabetes: Systematic analysis of T cell epitopes in autoimmune diabetes. Clin Exp Immunol. 2007;148:1–16. doi: 10.1111/j.1365-2249.2006.03244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prasad S, Kohm AP, McMahon JS, Luo X, Miller SD. Pathogenesis of NOD diabetes is initiated by reactivity to the insulin B chain 9-23 epitope and involves functional epitope spreading. J Autoimmun. 2012;39:347–353. doi: 10.1016/j.jaut.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohashi PS, et al. Reconstitution of an active surface T3/T-cell antigen receptor by DNA transfer. Nature. 1985;316:606–609. doi: 10.1038/316606a0. [DOI] [PubMed] [Google Scholar]
- 35.Hu Z, Yang YG. Human lymphohematopoietic reconstitution and immune function in immunodeficient mice receiving cotransplantation of human thymic tissue and CD34(+) cells. Cell Mol Immunol. 2012;9:232–236. doi: 10.1038/cmi.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holst J, et al. Generation of T-cell receptor retrogenic mice. Nat Protoc. 2006;1:406–417. doi: 10.1038/nprot.2006.61. [DOI] [PubMed] [Google Scholar]
- 37.Li X, Chen H, Epstein PN. Metallothionein and catalase sensitize to diabetes in nonobese diabetic mice: Reactive oxygen species may have a protective role in pancreatic beta-cells. Diabetes. 2006;55:1592–1604. doi: 10.2337/db05-1357. [DOI] [PubMed] [Google Scholar]