Introduction
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of death worldwide and in the United States (US). Health, life expectancy, and medical care have improved dramatically in the US over the last century. However, the distribution of benefits arising from the decreases in heart disease and stroke has not occurred equitably. The current mortality gap between blacks and whites in the US, although decreasing, has been persistent since 19601. Black men and women are more likely to die of heart disease and stroke than white men and women. This population remains at higher risk for hypertension, type 2 diabetes, obesity (especially in females), myocardial infarction, stroke morbidity and mortality, chronic kidney disease (CKD), end-stage renal disease (ESRD), and cardiovascular mortality2.
Race as a social concept and disparities in US life expectancy
Although this review specifically describes the extent and severity of ASCVD and associated risk factors in blacks, race is not a true biological or scientific category. While there may be individual aspects of biology and genotype that contribute to increased burden of heart disease and stroke in African Americans, chronic diseases are usually greatly impacted by environmental factors such as adverse diet, lifestyle, socioeconomic status, and exposures that lead to stress in disadvantaged areas. Patients, regardless of race or ethnicity, manifest disease within a cultural context. Hence, specific aspects of the patients’ disease burden are greatly impacted by family, health care providers, the community, and the health care system at large3.
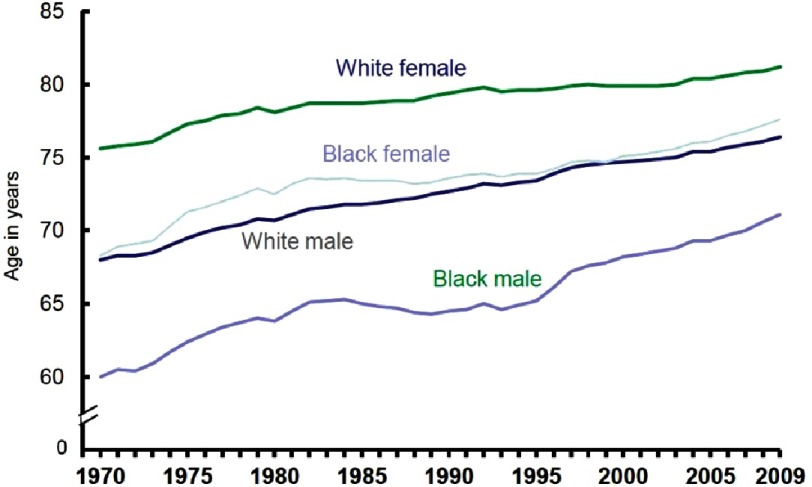
Most importantly, this excessive burden of heart disease and stroke in US blacks accounts for the largest portion of inequality in life expectancy between whites and blacks, despite existence of low-cost, highly effective preventive treatment (Figure 1).
Figure 1. US life expectancy by race and gender5.
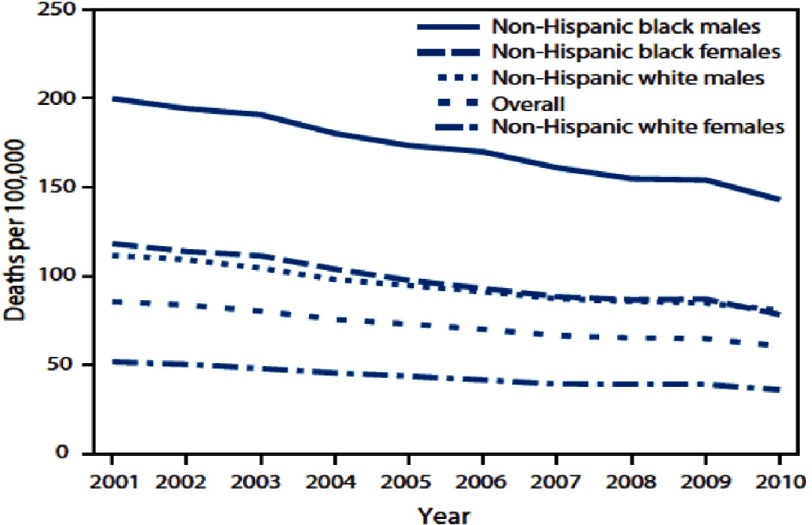
In March of 1966, while speaking at the Medical Committee for Human Rights in Chicago, Illinois, Martin Luther King Jr. said, “Of all the forms of inequality, injustice in health care is the most inhumane.”4 In consideration of the above, cardiovascular disease (CVD) disparities by race and ethnicity are sizeable, likely multifactorial, and preventable (Figure 2).
Figure 2. Avoidable death from heart disease, stroke, and CVD 2001–20106.
The burden of ASCVD is primarily a result of excessive levels of cardiac risk factors, which are often preventable. Nevertheless, the social determinants of health are the circumstances in which people are born, grow up, live, work, and age, as well as the systems put in place to deal with illness. These circumstances are in turn shaped by a wider set of forces: economics, social policies, and politics. A recent American Heart Association (AHA) Scientific Statement confirmed the impact of these social determinants on disparate CVD burden7.
Cardiometabolic risks in African Americans
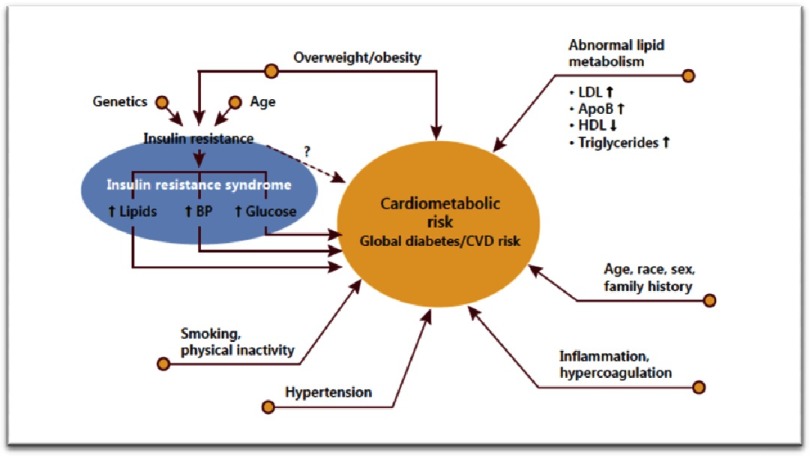
The multiple conditions or behaviors that significantly contribute to CVD morbidity and mortality not only include traditional CVD risk factors, but also encompass a constellation of conditions termed cardiometabolic risk (CMR). The concept of CMR incorporates a wide range of characteristics such as obesity, abnormal lipid metabolism, insulin resistance, smoking, physical inactivity, hypertension, inflammation, hypercoagulation, and patient history (Figure 3)8.
Figure 3. Contributions to CMR, global diabetes and CVD risk8.
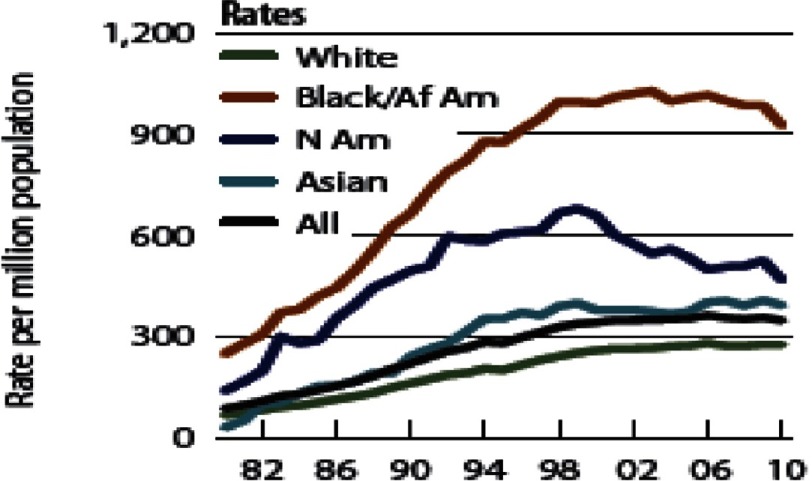
Hence, based on a variety of conditions as depicted below, African Americans show increased incidence of target organ damage associated with stroke, myocardial infarction mortality, left ventricular hypertrophy, heart failure, retinopathy, CKD, ESRD, and premature onset of morbidity and mortality (Figure 4)9.
Figure 4. Rates of kidney disease by race/ethnicity.
End-stage renal disease incidence has risen significantly among African-Americans9.
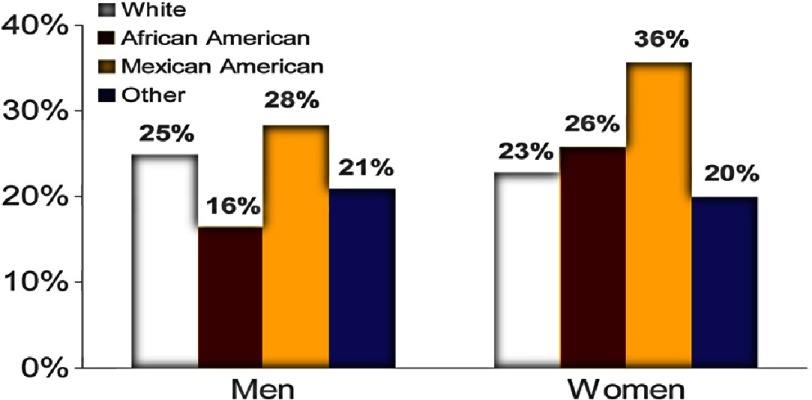
A study by Heidenreich et al. demonstrates the projected percentage of heart failure according to race. The data show that by 2030, the projected risk of heart failure will increase to above 3.5% in US blacks, while the projected percentage is significantly less for US whites at 2.7%10. Perhaps one of the primary factors for the increase in ASCVD and CMR in US blacks is the increased rate of overweight and obesity status. African Americans have the highest rates of obesity in the US: 39% of black men and 57% of black women are considered obese. Additionally, 7% of black men and 17% of black women are considered morbidly obese (BMI >40). Among Mexican-Americans, another often disadvantaged ethnic minority, 38% of men and 43% of women are obese. Whites have the lowest obesity rates with 35% of white men and 34% of white women considered obese (Table 1)11.
Table 1. Prevalence of overweight and obesity in the US 2007–201211.
| Sociodemographic Characteristics of Person 25 Years or Older From the NHANES, 2007-2012a | ||||||||
|---|---|---|---|---|---|---|---|---|
| Percentage | ||||||||
| Study Population, No. | Obesity Class | |||||||
| Characteristic | Sample | Weighted | Underweight | Normal weight | Overweight | 1 | 2 | 3 |
| Men | ||||||||
| Race/ethnicity | ||||||||
| Mexican American | 1845 | 12316214 | 0.35 | 18.75 | 43.17 | 24.83 | 8.21 | 4.70 |
| Non-Hispanic black | 1577 | 9245105 | 1.73 | 25.67 | 33.44 | 21.80 | 9.90 | 7.46 |
| Non-Hispanic white | 3427 | 63145888 | 0.62 | 23.35 | 40.74 | 23.36 | 7.80 | 4.13 |
| Other | 629 | 6187710 | 1.36 | 42.33 | 35.47 | 15.58 | 2.09 | 3.17 |
| Age,y | ||||||||
| 25-54 | 4143 | 59105817 | 0.69 | 25.05 | 39.38 | 22.51 | 7.78 | 4.59 |
| ≥55 | 3335 | 31789101 | 0.84 | 22.78 | 41.05 | 23.54 | 7.50 | 4.29 |
| Women | ||||||||
| Race/ethnicity | ||||||||
| Mexican American | 2024 | 11983246 | 0.68 | 22.43 | 33.58 | 24.16 | 12.34 | 6.81 |
| Non-Hispanic black | 1653 | 11484735 | 1.66 | 15.79 | 25.77 | 26.03 | 13.45 | 17.30 |
| Non-Hispanic white | 3417 | 67131553 | 2.28 | 33.77 | 30.02 | 17.58 | 9.37 | 6.98 |
| Other | 636 | 6556840 | 3.23 | 50.02 | 26.84 | 10.80 | 4.76 | 4.35 |
| Age,y | ||||||||
| 25-54 | 4291 | 59578408 | 2.29 | 33.45 | 28.58 | 17.64 | 9.82 | 8.22 |
| ≥55 | 3439 | 37577965 | 1.73 | 28.01 | 31.58 | 20.98 | 10.05 | 7.65 |
Notes.
TITLE
- NHANES
- National Health and Nutrition Examination Survey.
Patients are divided by National Heart, Lung, and Blood Institute recommaended weight group and sex. Weight groups were defined by body mass index (calculated as weight in kilograms divided by height in meters squared): underweight (<18.5), normal weight (18.5-24.9), overweight (25.0-29.9), obesity class 1 (30.0-34.9), obesity class 2 (35.0-39.9), and obesity class 3 (≥40).
Surprisingly, according to the National Health and Nutritional Examination Survey (NHANES), blacks have lower triglycerides and higher high density lipoprotein-cholesterol (HDL-C). Thus, prevalence of metabolic syndrome defined by arbitrary cut-points is actually determined as lower in blacks than whites (Figure 5)12.
Figure 5. Prevalence of the National Cholesterol Education Program’s metabolic syndrome NHANES III by sex and race/ethnicity13.
These racial differences in metabolic syndrome prevalence, in view of lower triglyceride levels and relatively higher HDL-C, are more prominent in men than in women. Considering higher rates of stroke and myocardial infarction in blacks versus whites, the favorable lipid profile of low triglycerides and high HDL-C in blacks is both surprising and paradoxical. Therefore, the widespread use of triglyceride levels to predict insulin resistance, CVD, and type 2 diabetes needs reevaluation. Yu and colleagues have coined the term triglyceride paradox in people of African descent12.
Safford et. al. conducted a study investigating the race-sex differences in the management of hyperlipidemia. Since coronary heart disease (CHD) risk influences physician prescribing, Adult Treatment Panel III CHD risk categories were constructed using baseline data from Reasons for Geographic and Racial Differences in Stroke study participants (recruited 2003–2007). Prevalence, awareness, treatment, and control of hyperlipidemia were examined for race-sex groups across CHD risk categories. Multivariable models conducted in 2013 estimated prevalence ratios adjusted for predisposing enabling and need factors influencing health services utilization. The study described several potentially actionable findings that likely contribute to racial disparities in acute CHD. Black males were less likely to be aware of hyperlipidemia compared with other race-sex groups, supporting the need for interventions to improve their awareness. This study also found that among those at highest CHD risk, white males were significantly more likely to be treated and controlled, and blacks in general, but especially black women, were less likely to be treated or controlled14.
To date, the Antihypertensive and Lipid-Lowering treatment to prevent Heart Attack Trial (ALLHAT) lipid arm with pravastatin has the largest subpopulation of black patients, with non-Hispanic black patients accounting for one-third of the trial population; however, the clinical implications of the apparently favorable subgroup results in African American patients remain unclear15. Clinical-event trials comparing different statins have been predominantly white or European (approximately 90%) and the subgroup data are often inadequate or unavailable. Therefore, while the risk of CHD is clearly greater in African American men and women, not enough data exist to aid physicians in treating their African American patients to lower this risk (Table 2)15–22.
Table 2. Percentage of black patients in statin clinical event trials15–22.
| Trial | Statin | Total Patients, n | African Americans, n or % |
|---|---|---|---|
| 4S | Simvastatin | 4444 | N/A |
| WOSCOPS | Pravastatin | 6595 | N/A |
| CARE | Pravastatin | 4159 | Others, 7-8% |
| LIPIED | Pravastatin | 9014 | N/A |
| AFCAPS/TEXCAPS | Lovastatin | 6605 | 206 |
| HPS | Simvastatin | 20536 | N/A |
| ALLHAT | pravastatin | 10355 | 3491 |
| ASCOT | Atorvastatin | 10305 | Others, 5-5.5% |
It is known that baseline creatine kinase (CK) levels are higher in African Americans than in whites and that they are higher in men than in women23. The Justification for the Use of Statins in prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) found 12,683 whites and 5,117 non-whites had low density lipoprotein-cholesterol (LDL-C) less than 130 mg/dL and high-sensitivity C-reactive protein (hs-CRP) greater than or equal to 2.0 mg/L. Rosuvastatin significantly decreased the rate of first MI, stroke, arterial revascularization, hospitalization for unstable angina, and cardiovascular death among whites and nonwhites24. Therefore, JUPITER is a welcomed addition to clinical evidence on statin therapy in blacks.
Based on limited statistical power, lipoprotein(a) (Lp(a)) was previously not considered a risk factor for CVD in blacks. However, Lp(a) is significantly higher among blacks versus whites, and in both, increased Lp(a) correlates positively with LDL-C and negatively with triglycerides. Increasing quintiles of Lp(a) was equally as predictive of future CVD in blacks as in whites25.
The Coronary Artery Risk Development In Young Adults (CARDIA) study found that for blacks, LDL-C levels in are adults significantly lower (P < 0.001) with three genetic variations—L253F, C679X, and Y142X—(81.5 mg/dL) and A443T (95.5 mg/dL) compared to non-carriers (109.6 mg/dL). The three genetic variants and the A443T variant in black men associated with lower carotid intima media thickness and lower prevalence of coronary calcification measured at ages 38 to 5026.
The Jackson Heart Study: A paradigm of cardiovascular risk assessment in African Americans
The Jackson Heart Study (JHS), a National Heart, Lung, and Blood Institute (NHLBI) sponsored epidemiologic study, followed 5,301 individuals since 2000. This landmark study seeks to explore the reasons for racial disparities in ASCVD and cardiac risk and to uncover new approaches to reduce them27. By identifying factors that influence the development and worsening of CVD, JHS would potentially be best able to prevent this high burden of ASCVD in US blacks in the future.
Distressingly, although 88% of JHS participants were ideal in risk factor status for smoking, fewer than half met the ideal criteria for fasting plasma glucose (45.3%) or total cholesterol (45.2%). Moreover, fewer than a quarter of the JHS cohort met the ideal criteria for physical activity (19.3%), blood pressure (17.8%), and BMI (13.7%), and only 0.9% met the ideal dietary intake27.
The very low prevalence of ideal cardiovascular health among participants of the JHS, and particularly the extremely low prevalence of ideal BMI, physical activity, and diet, underscores the need for reassessment of tools and strategies available to achieve the AHA 2020 goal among African Americans. In this manner, data from JHS also provide specific targets for clinical and public health interventions (i.e., diet, physical activity, and adiposity) as well as help to explain the burden of CVD in stroke-belt regions across the US. Identification of those harboring subclinical atherosclerosis will supplement current risk stratification strategies.
Perhaps due to the profound effects of hypertension in African Americans, despite having an observed lower frequency of elevated calcium artery calcification (CAC) scores, the survival rates remain disproportionally decreased when compared to other groups. The prevalence of CAC is 66% for non-Hispanic whites, 58% for African Americans, 55% for Hispanics and 55% for Asians (p<0.0001). As compared with non-Hispanic whites, all ethnic groups had lower odds of having an increasing burden of CAC, but blacks, nevertheless, had higher mortality rates28.
Unique aspects of antihypertensive pharmacotherapy in blacks: What is the evidence?
Hypertension prevalence in African Americans is among the highest in the world29–31. Compared to US whites, blacks develop hypertension at an earlier age, and average blood pressure is much higher. Potential physiologic and hemodynamic determinants of this hypertension could be obesity, higher salt sensitivity, low levels of plasma renin, vascular function (sympathetic over-activity), attenuated nocturnal fall in blood pressure, greater comorbidity (especially with diabetes), inactivity, and family history38–40.
Materson et. al. conducted a study in 1993 to uncover the effects of age and race on the effectiveness of single-drug antihypertensive medications in US veterans. The study included six antihypertensive medications and a placebo, one of which was randomly given to each of the 1292 men. Of these men, 48% were black and their ages ranged from 59–69. Results of this study found that age and race are indeed influential factors in the effectiveness of certain antihypertensive medications. Black men, both young and old, responded best to treatment with diltiazem. However, younger whites responded best to treatment with captopril, and older whites with atenolol. Blacks did not respond as well to captopril. Therefore, the study demonstrated that age and race should be considered when making treatment decisions32.
These findings were supported by another study in 2004, the Losartan Intervention For Endpoint reduction in hypertension (LIFE) study, which compared treatment with losartan and atenolol in black and non-black patients with hypertension and left ventricular hypertrophy (LVH). The study found that blacks had better outcomes after treatment with atenolol regimens, while the opposite was true for non-blacks in whom losartan was superior33.
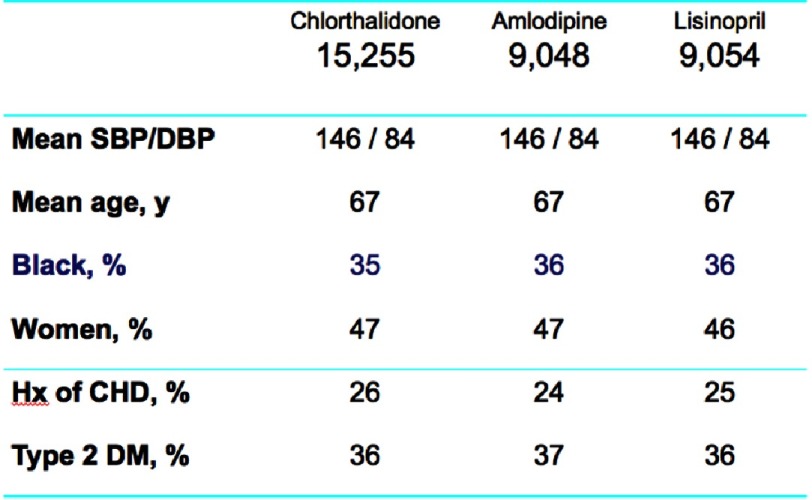
The NHLBI sought out to further investigate racial differences in the pharmacologic treatment of hypertension. The ALLHAT study was initiated in over 42,000 patients at high risk for elevated blood pressure. The proportion of blacks was approximately 35%, which enabled the investigators to compare black versus non-black participants in response to medications and CVD endpoints (Figure 6)34.
Figure 6. Baseline characteristics for ALLHAT participants34.
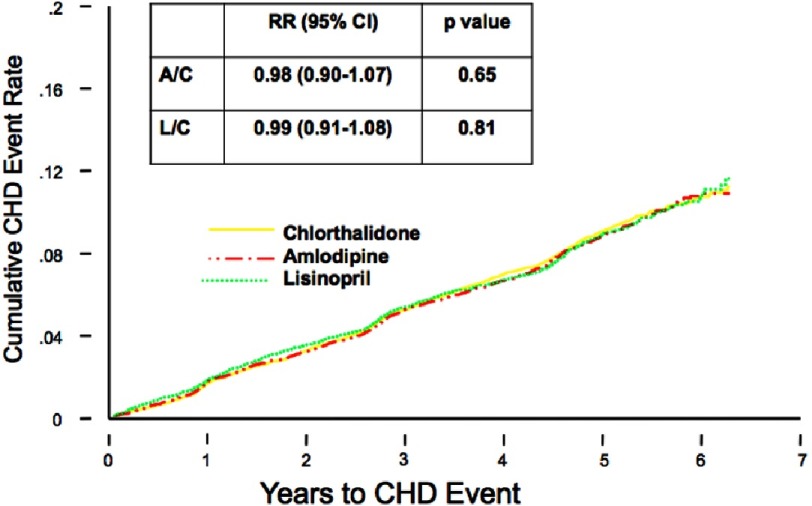
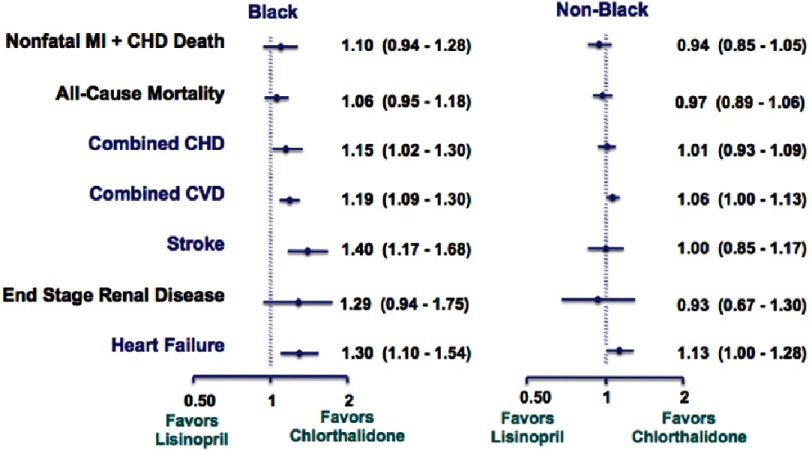
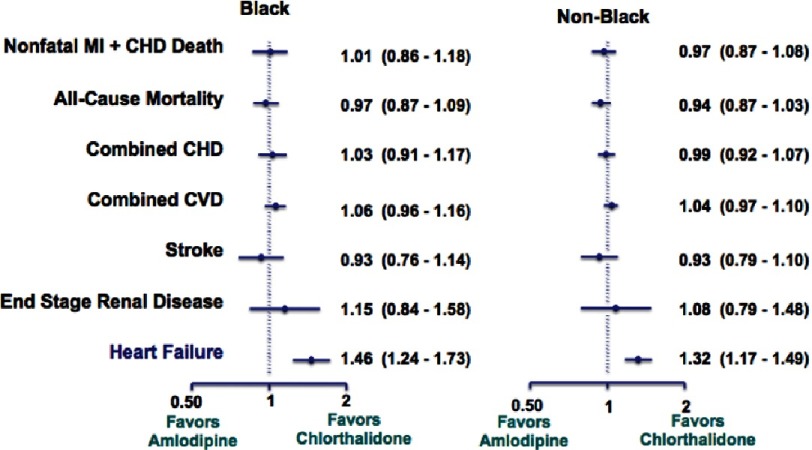
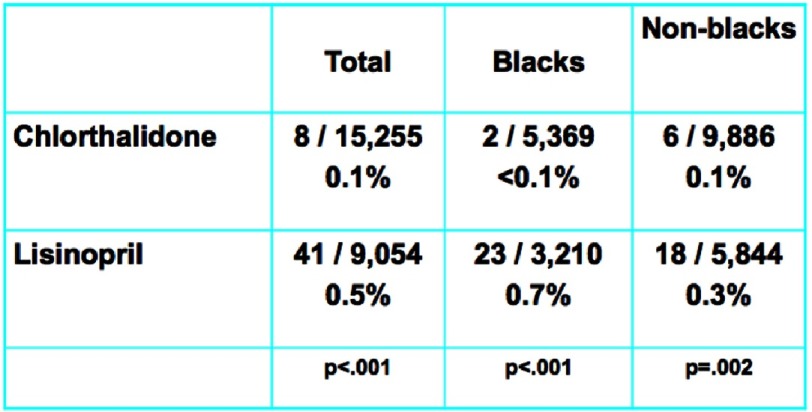
Chlorthalidone, amlodipine, and lisinopril were shown to have no significant difference in the primary endpoint effect on CHD event rate, but demonstrated differences were noted among certain diseases (Figure 7). In the lisinopril–chlorthalidone comparison, there were no differences in either sub-group for major CHD or total mortality. However, there were some differences by race, primarily for stroke, for which lisinopril-based treatment was significantly less effective in blacks by 40%. Heart failure was also prevented more effectively by chlorthalidone-based treatment than lisinopril, and the magnitude of this effect was not significantly different by race. Primarily as a consequence of these outcomes, combined CVD was lower in the chlorthalidone group for both races, although the effect was more pronounced in the black sub-group. Interestingly, the risk of ESRD was not reduced by lisinopril compared with chlorthalidone in either sub-group, although the confidence limits were somewhat wide (Figures 8 and 9). As well, while all patients experienced less angioedema with treatment with chlorthalidone compared to lisinopril, blacks had significantly higher rates of angioedema due to lisinopril compared to whites (Figure 10)35.
Figure 7. Primary outcome (fatal CHD or nonfatal MI) by treatment group45.
Figure 8. Black versus non-black lisinopril vs. chlorthalidone35.
Figure 9. Black versus non-black amlodipine vs. chlorthalidone35.
Figure 10. Angioedema differences in chlorthalidone vs. lisinopril35.
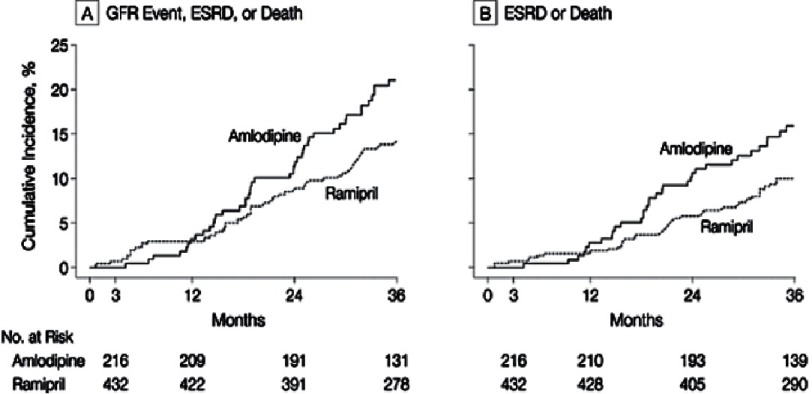
On the other hand, in blacks, the benefits of angiotensin converting enzyme inhibitors (ACEIs) have been shown in CKD. A study on the effects of ramipril and amlodipine on renal outcomes in hypertensive nephrosclerosis was conducted by the African American Study of Kidney Disease and Hypertension (AASK) study group. The study found that those treated with ramipril were at lower risk both initially and in the long-term for a glomerular filtration rate (GFR) event, ESRD, or death when compared to those treated with amlodipine36 (Figure 11).
Figure 11. AASK outcomes36.
Initial medications for the management of hypertension in blacks should include a thiazide-type diuretic and/or a calcium channel blocker (CCB). Most patients will require a two or three-drug combination to control their hypertension. If a diuretic is contraindicated or not tolerated, a CCB plus an ACEI or angiotensin receptor blocker (ARB) is required. However, ACEIs (and likely other RAS blockers) are less effective than other classes in lowering BP and preventing cardiovascular events in African Americans. For this reason, ACEIs are not the preferred initial agents unless combined with a thiazide-type diuretic or CCB. Chlorthalidone 12.5–25 mg/d is the thiazide-type diuretic of choice, especially is high risk blacks, since it is more potent and longer-acting than hydrochlorothiazide. Based on ALLHAT results and its long action and high tolerability, amlodipine is the CCB of choice. A loop diuretic (e.g., furosemide) may be needed with advanced CKD (eGFR <30). Regardless of needed pharmacotherapy, lifestyle modifications such as a low sodium diet, DASH eating plan, and adequate physical activity are the bedrock of appropriate hypertension therapy. Beta-blockers should be included in the regimen if there is compelling indication for a beta-blocker, such as recent myocardial infarction or heart failure with reduced ejection fraction37.
Treatment of resistant hypertension
Resistant hypertension is blood pressure above goal despite adhering to full doses of an appropriate three-drug antihypertensive regimen including a diuretic. Withdrawal or down titration interfering substances use adequate long-acting thiazide, preferably chlorthalidone, and combine different mechanisms of action. The recommended treatment of resistant hypertension includes a triple regimen of ACEIs (or ARB), CCB, and thiazide diuretic. Additional effects of BP reduction have also been shown with spironolactone in resistant hypertension38. In view of approximately half of adults with hypertension in the US are uncontrolled, perhaps, 12 % to 15 % are considered resistant. Moreover, compared with men, women have more prevalent resistant hypertension with greater older age status and obesity, two of the strongest predictors. Resistant hypertension is more common in African Americans and more frequent with conditions such as heart disease, heart failure, diabetes, stroke, and CKD. All patients require lifestyle modifications and effective treatment mandates combination therapy, including appropriate diuretics, renin-angiotensin system blockers, CCB, and underutilized aldosterone antagonists. In the future, emerging, innovative interventions may include renal artery denervation and carotid stimulation treatment, which are potentially effective approaches to BP control39.
Eliminating disparities
Efforts for the elimination of disparities can come in many forms, such as raising public and provider awareness of racial and ethnic disparities in health care, expanding health insurance coverage, improving capacity and number of providers in underserved communities, and increasing the knowledge based on causes and interventions to reduce disparities40. Moreover, the Centers for Disease Control (CDC) and Centers for Medicare and Medicaid Services (CMS) came together to create and lead the Million Hearts® initiative. This initiative, which was launched in 2012, aims to prevent one million heart attacks and strokes by 2017. The Million Hearts® approaches the ABCs of eliminating disparities that include appropriate aspirin therapy for those who need it, BP control, cholesterol management, and smoking cessation41. The National Forum for Heart Disease and Stroke Prevention, a coalition of over 80 organizations, aims to eliminate disparities and addresses the most significant population-level interventions to prevent heart disease, including reduction of sodium intake and tobacco use. In the US population, consumption of sodium far surpasses safe levels and contributes to hypertension, a great deal of which is uncontrolled. By lowering the amount of sodium in processed foods and products used for food preparation in the service industry, the consumption of sodium by the population will decrease. Overall, the US population consumes a diet consisting of excessive sodium and sugar intake and lacking in fish, fresh fruits, and vegetables. To better prevent the incidence of heart disease and stroke, the prevailing typical American diet, high in saturated fat, sugar and sodium, needs to be reconsidered42.
There is a need to develop, implement, and evaluate interventions to prevent CVD and risk factors. The department of Health and Human Services (HHS) will implement interventions ranging from quality of care improvements to potential reimbursement incentives for policy and health system changes, working both with minority providers and providers serving minority populations. These efforts will expand upon outreach toward minorities and aid in the adoption of certified electronic health records (EHR)43.
At the individual level, influences include biological effectiveness of medications, adherence to medications/lifestyle, mental health and substance abuse, reactions to discriminations, health literacy, English proficiency, employment status, and health insurance coverage. At the provider/clinical team level, influences include knowledge, communication skills, awareness of disparities, cultural competency, and trustworthiness44. Almost half (48%) of patients with hypertension or diabetes have inadequate health literacy, and therefore lack knowledge about their disease, important lifestyle modifications, and essential self-management skills45. Furthermore, evidence suggests that the issue of disparities in health outcomes of CVD, including acute coronary syndromes and chronic conditions, is not simply a result of the bias of clinicians, but rather additionally a “systems” problem that can be abolished with adequate performance improvement initiatives.
References
- 1.Unequal treatment: Confronting racial and ethnic disparities in health care (with CD) Washington, D.C.: National Academies Press; 2003. http://www.nap.edu/catalog/12875 . Accessed May 5, 2016. [PubMed] [Google Scholar]
- 2.Wong MD, Shapiro MF, Boscardin WJ, Ettner SL. Contribution of major diseases to disparities in mortality. N Engl J Med. 2002;347(20):1585–1592. doi: 10.1056/NEJMsa012979. doi:10.1056/NEJMsa012979 . [DOI] [PubMed] [Google Scholar]
- 3.Frieden TR, Centers for Disease Control and Prevention (CDC) Forward: CDC Health Disparities and Inequalities Report - United States, 2011. MMWR supplements. 2011;60(1):1–2. [PubMed] [Google Scholar]
- 4. Justice, health, and status: Moral theory and the new epidemiology of health disparities. ProQuest; 2008.
- 5.Kochanek KD, Xu J, Murphy SL, Miniño AM, Kung H-C. Deaths: Final data for 2009. Natl Vital Stat Rep. 2011;60(3):1–116. [PubMed] [Google Scholar]
- 6.Frieden T. MMWR. 2013;60(35):721–27. [Google Scholar]
- 7.Social determinants of health. (2015, October 19). Retrieved November 15, 2015, from http://www.cdc.gov/socialdeterminants/
- 8.Ferdinand KC, Rodriguez F, Nasser SA, et al. Cardiorenal metabolic syndrome and cardiometabolic risks in minority populations. Cardiorenal Medicine. 2014;4(1):1–11. doi: 10.1159/000357236. doi:10.1159/000357236 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.United States Renal Data System. 2012 USRDS annual data report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2015.
- 10.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Piña IL, Trogdon JG. Forecasting the impact of heart failure in the United States. A policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606–619. doi: 10.1161/HHF.0b013e318291329a. doi:10.1161/HHF.0b013e318291329a . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang L, Colditz GA. Prevalence of overweight and obesity in the United States, 2007–2012. JAMA Intern Med. 2015;175(8):1412–1413. doi: 10.1001/jamainternmed.2015.2405. doi:10.1001/jamainternmed.2015.2405 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu SSK, Castillo DC, Courville AB, Sumner AE. The triglyceride paradox in people of African descent. Metab Syndr Relat Disord. 2012;10(2):77–82. doi: 10.1089/met.2011.0108. doi:10.1089/met.2011.0108 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: Findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287(3):356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 14.Safford MM, Andreae S, Cherrington AL, Martin MY, Halanych J, Lewis M, Patel A, Johnson E, Clark D, Gamboa C, Richman JS. Peer coaches to improve diabetes outcomes in rural Alabama: A cluster randomized trial. Ann Fam Med. 2015;13(Suppl 1):S18–S26. doi: 10.1016/j.amepre.2014.10.025. doi:10.1370/afm.1798 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The antihypertensive and lipid-lowering treatment to prevent heart attack trial (allhat-llt) JAMA. 2002;288(23):2998. doi: 10.1001/jama.288.23.2998. doi:10.1001/jama.288.23.2998 . [DOI] [PubMed] [Google Scholar]
- 16.Sever PS, Dahlöf B, Poulter NR, Wedel H, Beevers G, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O’Brien E, Ostergren J, ASCOT investigators Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm (ASCOT-LLA): A multicentre randomised controlled trial. Lancet. 2003;361361(9364):1149–1158. doi: 10.1016/S0140-6736(03)12948-0. doi:10.1016/S0140-6736(03)12948-0 . [DOI] [PubMed] [Google Scholar]
- 17.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344(8934):1383–1389. [PubMed] [Google Scholar]
- 18.Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, McKillop JH, Packard CJ. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333(20):1301–1307. doi: 10.1056/NEJM199511163332001. doi:10.1056/NEJM199511163332001 . [DOI] [PubMed] [Google Scholar]
- 19.Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JM, Wun CC, Davis BR, Braunwald E. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335(14):1001–1009. doi: 10.1056/NEJM199610033351401. doi:10.1056/NEJM199610033351401 . [DOI] [PubMed] [Google Scholar]
- 20.Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med. 1998;339(19):1349–1357. doi: 10.1056/NEJM199811053391902. doi:10.1056/NEJM199811053391902 . [DOI] [PubMed] [Google Scholar]
- 21.Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, Langendorfer A, Stein EA, Kruyer W, Gotto AM. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: Results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279(20):1615–1622. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 22.Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet. 2002;360(9326):7–22. doi: 10.1016/S0140-6736(02)09327-3. doi:10.1016/S0140-6736(02)09327-3 . [DOI] [PubMed] [Google Scholar]
- 23.Neal RC, Ferdinand KC, Yčas J, Miller E. Relationship of ethnic origin, gender, and age to blood creatine kinase levels. The American Journal of Medicine. 2009;122(1):73–78. doi: 10.1016/j.amjmed.2008.08.033. doi:10.1016/j.amjmed.2008.08.033 . [DOI] [PubMed] [Google Scholar]
- 24.Albert MA, Glynn RJ, Fonseca FAH, Lorenzatti AJ, Ferdinand KC, MacFadyen JG, Ridker PM. Race, ethnicity, and the efficacy of rosuvastatin in primary prevention: The Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) trial. American Heart Journal. 2011;162(1):106-114.e2. doi: 10.1016/j.ahj.2011.03.032. doi:10.1016/j.ahj.2011.03.032 . [DOI] [PubMed] [Google Scholar]
- 25.Virani SS, Brautbar A, Davis BC, Nambi V, Hoogeveen RC, Sharrett AR, Coresh J, Mosley TH, Morrisett JD, Catellier DJ, Folsom AR, Boerwinkle E, Ballantyne CM. Associations between lipoprotein(a) levels and cardiovascular outcomes in black and white subjects: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2012;125(2):241–249. doi: 10.1161/CIRCULATIONAHA.111.045120. doi:10.1161/CIRCULATIONAHA.111.045120 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang C-C, Fornage M, Lloyd-Jones DM, Wei GS, Boerwinkle E, Liu K. Longitudinal association of PCSK9 sequence variations with low-density lipoprotein cholesterol levels: The Coronary Artery Risk Development in Young Adults Study. Circ Cardiovasc Genet. 2009;2(4):354–361. doi: 10.1161/CIRCGENETICS.108.828467. doi:10.1161/CIRCGENETICS.108.828467 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Djoussé L, Petrone AB, Blackshear C, Griswold M, Harman JL, Clark CR, Talegawkar S, Hickson DA, Gaziano JM, Dubbert PM, Correa A, Tucker KL, Taylor HA. Prevalence and changes over time of ideal cardiovascular health metrics among African–Americans: The Jackson Heart Study. Preventive Medicine. 2015;74:111–116. doi: 10.1016/j.ypmed.2015.02.006. doi:10.1016/j.ypmed.2015.02.006 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whittle J, Kressin NR, Peterson ED, Orner MB, Glickman M, Mazzella M, Petersen LA. Racial differences in prevalence of coronary obstructions among men with positive nuclear imaging studies. J Am Coll Cardiol. 2006;47(10):2034–2041. doi: 10.1016/j.jacc.2005.12.059. doi:10.1016/j.jacc.2005.12.059 . [DOI] [PubMed] [Google Scholar]
- 29.Nesbitt SD. Hypertension in black patients: Special issues and considerations. Curr Hypertens Rep. 2005;7(4):244–248. doi: 10.1007/s11906-005-0020-5. [DOI] [PubMed] [Google Scholar]
- 30.Gadegbeku CA, Lea JP, Jamerson KA. Update on disparities in the pathophysiology and management of hypertension: Focus on African Americans. Med Clin North Am. 2005;89(5):921–933. doi: 10.1016/j.mcna.2005.05.003. 930. doi:10.1016/j.mcna.2005.05.003 . [DOI] [PubMed] [Google Scholar]
- 31.Profant J, Dimsdale JE. Race and diurnal blood pressure patterns. A review and meta-analysis. Hypertension. 1999;33(5):1099–1104. doi: 10.1161/01.HYP.33.5.1099. [DOI] [PubMed] [Google Scholar]
- 32.Materson BJ, Reda DJ, Cushman WC, Massie BM, Freis ED, Kochar MS, Hamburger RJ, Fye C, Lakshman R, Gottdiener J. Single-drug therapy for hypertension in men. A comparison of six antihypertensive agents with placebo. The Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. N Engl J Med. 1993;328(13):914–921. doi: 10.1056/NEJM199304013281303. doi:10.1056/NEJM199304013281303 . [DOI] [PubMed] [Google Scholar]
- 33.Julius S, Alderman MH, Beevers G, Dahlöf B, Devereux RB, Douglas JG, Edelman JM, Harris KE, Kjeldsen SE, Nesbitt S, Randall OS, Wright J, Jackson T. Cardiovascular risk reduction in hypertensive black patients with left ventricular hypertrophy. The life study. J Am Coll Cardiol. 2004;43(6):1047–1055. doi: 10.1016/j.jacc.2003.11.029. doi:10.1016/j.jacc.2003.11.029 . [DOI] [PubMed] [Google Scholar]
- 34.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288(23):2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 35.Wright JT, Dunn J, Cutler JA, et al. Outcomes in hypertensive black and nonblack patients treated with chlorthalidone, amlodipine, and lisinopril. JAMA. 2005;293(13):1595–1608. doi: 10.1001/jama.293.13.1595. doi:10.1001/jama.293.13.1595 . [DOI] [PubMed] [Google Scholar]
- 36.Agodoa LY, Appel L, Bakris GL, et al. Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: A randomized controlled trial. JAMA. 2001;285(21):2719–2728. doi: 10.1001/jama.285.21.2719. doi:10.1001/jama.285.21.2719 . [DOI] [PubMed] [Google Scholar]
- 37.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC, Svetkey LP, Taler SJ, Townsend RR, Wright JT, Narva AS, Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311(5):507–520. doi: 10.1001/jama.2013.284427. doi:10.1001/jama.2013.284427 . [DOI] [PubMed] [Google Scholar]
- 38.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, Ferdinand K, Giles TD, Falkner B, Carey RM, American Heart Association Professional Education Committee Resistant hypertension: Diagnosis, evaluation, and treatment: A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation. 2008;117(25):e510–e526. doi: 10.1161/CIRCULATIONAHA.108.189141. doi:10.1161/CIRCULATIONAHA.108.189141 . [DOI] [PubMed] [Google Scholar]
- 39.Ferdinand KC, Nasser SA. Improved understanding and innovative approaches for an aging dilemma: Resistant hypertension in women with existing vascular disease. Curr Cardiovasc Risk Rep. 2012;6(5):450–458. doi: 10.1007/s12170-011-0206-0. doi:10.1007/s12170-012-0252-2 . [DOI] [Google Scholar]
- 40. 2014 National Healthcare Quality & Disparities Report. http://www.ahrq.gov/research/findings/ nhqrdr/nhqdr14/index.html . Published May 19, 2015. Accessed June 2, 2016.
- 41.Prevention. Million Hearts. Centers for Disease Control and Prevention. Retrieved November 15, 2015, from http://millionhearts.hhs.gov/learn-prevent/prevention.html .
- 42.Labarthe D, Grover B, Galloway J, Gordon L, Moffatt S, Pearson T, Schoeberl M, Sidney S. The public health action plan to prevent heart disease and stroke: Ten-year update. Washington, DC: National Forum for Heart Disease and Stroke Prevention; 2014. [Google Scholar]
- 43.HHS action plan to reduce racial and ethnic disparities: A nation free of disparities in health and health care. Washington, D.C.: U.S. Department of Health and Human Services; 2011. U.S. Department of Health and Human Services. [Google Scholar]
- 44.Mueller M, Purnell TS, Mensah GA, Cooper LA. Reducing racial and ethnic disparities in hypertension prevention and control: What will it take to translate research into practice and policy? Am J Hypertens. 2015;28(6):699–716. doi: 10.1093/ajh/hpu233. doi:10.1093/ajh/hpu233 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams MV, Baker DW, Honig EG, Lee TM, Nowlan A. Inadequate literacy is a barrier to asthma knowledge and self-care. Chest. 1998;114(4):1008–1015. doi: 10.1378/chest.114.4.1008. [DOI] [PubMed] [Google Scholar]