Abstract
Introduction
The use of adequate self-management strategies for people with chronic obstructive pulmonary disease (COPD) reduces healthcare use, improves health-related quality of life (HRQoL) and recovery after acute exacerbations. However, not many people with COPD receive support that promotes the use of such strategies and therefore new methods to facilitate and promote the use of self-management strategies are highly warranted. This pilot trial aims to evaluate the feasibility of the study design and study procedures considering effectiveness of the novel intervention, the COPD-web.
Methods and analysis
The overall design is a pragmatic controlled pilot trial with preassessments and postassessments and a parallel process evaluation. Patients with the diagnosis of COPD will be eligible for the study. The intervention group will be recruited when visiting one of the six participating primary care units in Sweden. The control group will be identified from the unit's computerised registers. The intervention, the COPD-web, is an interactive web page with two sections; one directed at people with COPD and one at healthcare professionals. The sections aim to support patients’ self-management skills—and to facilitate the provision of support for self-management strategies, respectively. Effectiveness with regard to patients’ symptoms, HRQoL, knowledge of and readiness for COPD-related self-management, health literacy, self-efficacy for physical activity and time spent in physical activity and time being sedentary, and further, healthcare professionals’ knowledge of and readiness to support COPD-related self-management strategies will be assessed using questionnaires at 3 and 12 months. The process evaluation will include observations and interviews.
Ethics and dissemination
Ethical approval has been obtained. Findings will be presented at conferences, submitted for publication in peer-reviewed publications and presented to the involved healthcare professionals, patients and to patient organisations.
Trial registration number
ClinicalTrials.gov: NCT02696187
Keywords: primary care, self management strategies, chronic obstructive pulmonary disease, eHealth
Strengths and limitations of this study.
The process evaluation will increase the understanding of the functioning of the chronic obstructive pulmonary disease (COPD)-web and thereby contribute to a more robust conclusion.
The pragmatic design will increase the applicability of the findings.
The long-term follow-up will add valuable information about sustainability of potential effectiveness and use of the COPD-web.
The combination of qualitative and quantitative data collection will give a deeper comprehension of the results.
The non-randomised design limits the strength of the study's conclusions.
Introduction
Background and rationale
Chronic obstructive pulmonary disease (COPD) is one of the most common chronic diseases and a major cause of morbidity and mortality throughout the world.1 In 2012, COPD accounted for 6% of all deaths globally.2 The disease places a significant burden on the individual patient as well as the society. In the European Union, the yearly cost of COPD is estimated to be €38.6 billion, while direct and indirect cost of COPD in the USA is estimated to be over €50 billion.2
The symptom burden of the disease, the impaired functional performance and the decreased quality of life in patients with COPD is a consequence of the underlying condition, and depends on the patient's adaptation to the illness and their ability to manage their disease.3 4 Self-management strategies, including strategies to promote self-efficacy through increasing the patients’ knowledge and skills and their confidence in successfully managing their disease, is therefore now an important part of COPD management.3 The use of such strategies have shown to reduce healthcare use, increase quality of life, improve recovery time after acute exacerbations and reduce overall health-related costs.3 5–7 Self-management strategies have also shown to increase adherence to medication, increase physical activity and physical performance, increase use of breathing regulation techniques and energy-saving strategies during activities of daily living as well as to reduce the impact of COPD in daily life and breathlessness for individuals with COPD.8 9 Even though the education and promotion of self-management strategies for patients with COPD could be performed independently using a case manager, it is often promoted through pulmonary rehabilitation.3 Despite the proven effectiveness of pulmonary rehabilitation including self-management interventions for patients with COPD, only a limited proportion of patients with COPD gain access to such services.2 10 Lack of knowledge and insight in their diagnosis, transportation challenges and changing in health status have been found to be individual barriers for participating in pulmonary rehabilitation programmes, thus reducing access to self-management strategies.11–13 In Sweden, inadequate staffing as well as insufficient COPD-specific knowledge among healthcare professionals have also been reported.14 Overcoming these barriers and finding new and alternative strategies to facilitate the provision of self-management strategies to patients with COPD is therefore highly warranted. eHealth solutions are a promising way of delivering health services and have previously been shown to have the ability to increase the level of physical activity15 as well as reduce the use of healthcare services in COPD.16 Whether or not an eHealth solution could be used to promote self-management strategies for patients with COPD remains unclear. To address this question, our research group has developed the COPD-web, which is an internet-based tool aimed at increasing access to self-management strategies for patients with COPD and to support and facilitate for healthcare professionals in promoting such self-management strategies. In Sweden, the vast majority of the population has access to internet at home.17 eHealth solutions have recently been implied as a valuable option for increasing the availability of pulmonary rehabilitation including self-management.18 Development of internet-based tools with potential to meet the needs of people with COPD and the healthcare professionals, might therefore be a beneficial strategy.
Objectives
Conducting a pilot trial is highly recommended as a step in the development and evaluation of complex interventions and should preferably precede a randomised controlled trial (RCT).19 The aim of this pilot trial is to evaluate the feasibility of the study design and study procedures thus refining the study protocol for a prospective RCT of an intervention consisting of an internet-based tool, the COPD-web. The aim is also to increase the understanding of effectiveness of the tool with regard to aspects of health, knowledge and physical activity and furthermore the functioning of the intervention considering aspects of the implementation, mechanisms of impact and the context. In order to meet the aim, the following specific research questions will be addressed:
Are the intervention and the study procedures acceptable and feasible from the perspective of patients with COPD and healthcare professionals?
What is the effectiveness of the COPD-web with regard to self-rated COPD-related symptoms, dyspnoea, health-related quality of life (HRQoL), knowledge of and readiness for COPD-related self-management strategies, health literacy, self-efficacy for physical activity, time spent in physical activity and physical training, time being sedentary and level of physical activity among people with COPD at 3 and 12 months?
What is the effectiveness of the COPD-web with regard to healthcare professional's self-rated knowledge of and readiness to support COPD-related self-management strategies at 3 and 12 months and the provision of interventions to promote such strategies to patients with COPD at 6 months?
How is the COPD-web implemented and maintained in clinical practice at 12 months? How is healthcare professionals’ fidelity to the delivery of the COPD-web and what proportion of the target population was introduced to the COPD-web?
How do healthcare professionals and people with COPD respond to and interact with the COPD-web? Are there any unexpected events or consequences of delivering or receiving the COPD-web?
How does the context influence the implementation and the healthcare professionals’ and patients’ responses to and interaction with the COPD-web?
Methods and analysis
Trial design
The overall trial design is a pragmatic controlled pilot trial with preassessments and postassessments and a parallel process evaluation. As the COPD-web is a complex intervention that cannot easily be evaluated in a controlled trial, the process evaluation is an important complement that will be performed in order to understand how the intervention and the study procedures, such as recruitment of participants, delivery and acceptability of the intervention works in clinical practice. Thus, directed by the guidance for process evaluation suggested by the Medical Research Council (MRC),20 we will assess aspects of the components implementation, mechanism of impacts and context in order to identify and understand core components of the COPD-web, to understand the process of delivering and receiving the interventions and to draw more of a robust conclusion with regard to acceptability, feasibility and potential effectiveness of the intervention. The study is designed as a pragmatic trial21 meaning that the intervention will be delivered by healthcare professionals in ordinary healthcare settings in order to maximise the applicability of the findings to other healthcare settings. This paper complies with the SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) recommendations for protocol reporting22 23 and the study will report against CONSORT (Consolidated Standards of Reporting Trials) guidelines for pragmatic21 and pilot24 trials.
Participants, interventions and outcome
Study settings
Two primary healthcare units in a region in the north of Sweden and four units in a region in the middle of Sweden will constitute the study sites. Four of the units are situated in urban areas with 38 000–120 000 inhabitants and the other units are located in rural areas with 1800–2500 inhabitants. Like almost all healthcare services in Sweden, the included units are publicly funded.
Eligibility criteria
Persons with COPD: the intervention group
The trial will be conducted between 15 January 2016 and 15 May 2017. All persons with a diagnosis of COPD (ICD-10:J44:9) who visit the primary care units due to their COPD during a 4-month period are eligible for inclusion in the study if they 1) can read and understand Swedish or 2) can be assisted in their use of the COPD-web by a person who reads and understand Swedish. Recruitment of potentially eligible patients will be performed by healthcare professionals at each primary care unit, respectively.
Persons with COPD: the control group
All persons with a diagnosis of COPD who have visited the primary care centres during a 4-month period prior to the inclusion of the intervention group are eligible for inclusion in the study if they read and understand Swedish. Potentially eligible patients will, by two of the responsible researchers (AN and MT), be identified from the primary care units’ computerised registers and recruited continuously until an equal amount of participants as in the intervention group is enrolled.
Healthcare professionals
One or two healthcare professionals at each primary care unit will be identified and included in the study. Eligible for inclusion are healthcare professionals who meet patients with COPD in their clinical practice.
The intervention
The COPD-web
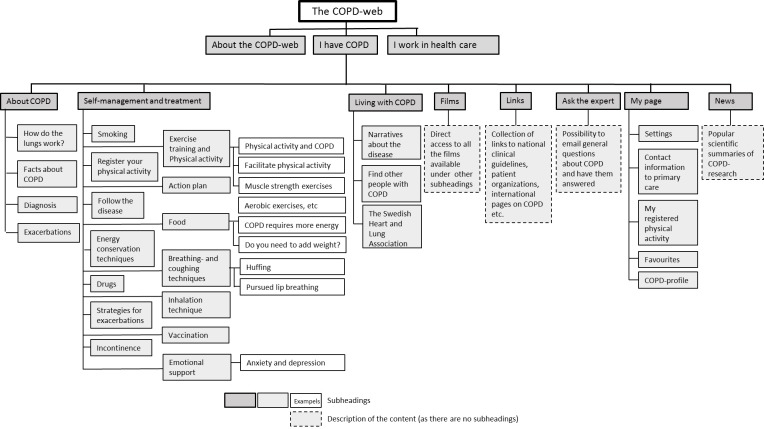
The intervention, the COPD-web, is an interactive website with two sections; one directed at people with COPD and one at healthcare professionals. A website map of the section for people with COPD is shown in figure 1. The section aims at supporting self-management skills including activities such as physical activity, physical training, breathing techniques and knowledge of the exacerbation symptoms to be aware of and advice on when to contact healthcare and how to make everyday activities less strenuous. The section includes factual texts, pictures, films and interactive components, such as pages for registering physical activity (steps) and receiving automatic personalised feedback. The website content directed at healthcare professionals complies to a large extent with the contents directed at people with COPD. However, the healthcare professional section of the website aims at facilitating the provision of support for self-management strategies to people with COPD, thereby facilitating the implementation of such services. The section includes factual texts, pictures, films as well as recommended and validated evaluation and screening tools. The content on the COPD-web refers to and is aligned with the National Guidelines for COPD-care recently developed and published by the National Board of Health and Welfare in Sweden.25 The material on the website will be permanent, although the contents will be updated when new national guidelines for COPD are released. In addition, we will continuously add new material such as links to reportages in media where COPD is covered and research news related to COPD. When new material is added, registered users will receive an email notification about the updates. The COPD-web was developed in collaboration with people with COPD and their relatives, healthcare professionals in the primary care and external researchers within the area of COPD.
Figure 1.
A website map of the chronic obstructive pulmonary disease (COPD)-web showing the section aimed at people with COPD.
The intervention group
The COPD-web will, after informed consent, be introduced by healthcare professionals at the primary care unit according to a prespecified routine (box 1).
Box 1. Routine for introduction of the chronic obstructive pulmonary disease (COPD)-web by the healthcare professionals.
Registration and creation of an account in order for the patient to use the COPD-web
Introducing the website structure, the content in the main menus and functions of the website, for example, how to enlarge or reduce the text, listen to the text or bookmark information of special interest
Introducing the section on ‘physical activity and exercise training’ to the patient. The healthcare professional will discuss the importance of physical activity/exercise training, point out the films with muscle strength exercises and the page for registering physical activity (steps) with automated feedback
Introduction of two to four additional topics on the website of special interest for the specific patient in question
Topics of special interest for the patient will be noted on a leaflet with information about the COPD-web. The patient will receive the leaflet and a card with the COPD-web's URL address, user name and password on it
In order to test the patients’ interest for and acceptability of the function of registering physical activity (steps) on the COPD-web, the included patients will (in addition to the introduction of the COPD-web) receive a pedometer with instructions on how it is used as well as an information leaflet on the importance of physical activity. In addition to the prespecified routine (box 1) and in line with the pragmatic approach, the healthcare professionals are free to use the COPD-web as they deem suitable, for example, by reinforcing the use of the COPD-web during follow-up visits or by recommending additional topics when suitable. No extra resources will be provided to the primary care units as healthcare professionals will deliver the intervention as a part of their regular work practice.
The control group
The control group will receive usual care. However, in order to evaluate the effectiveness of the COPD-web and minimise the influence of the pedometer on physical activity, the control group will also receive a pedometer with instructions on how it is used as well as an information sheet on the importance of physical activity, identical to the one given to the intervention group.
Training of healthcare professionals
The healthcare professionals will take part in two intervention training sessions prior to the trial. The first session (2 hours) will introduce the COPD-web and its content, how the content is related to the national evidence-based clinical guidelines for COPD,25 and how the COPD-web can promote the delivery of self-management strategies to people with COPD. Additionally, the study procedures such as routines for informed consent, inclusion protocols and questionnaires will be introduced. The healthcare professionals will be given the task to reflect on how they can use the COPD-web in their daily practice. During the second session, (1 hour) parts of the first session will be repeated and the participants will reflect on how the COPD-web fits into their daily work. The healthcare professionals will receive detailed written information on the COPD-web and the study procedures.
The healthcare professionals will be informed about that they will be able to continue using the COPD-web even after the inclusion of participants to the study has finished. Furthermore, they will receive information with preliminary data on patient outcomes between the short-term follow-up (3 months) and the long-term follow-up (12 months).
Outcomes and process evaluation
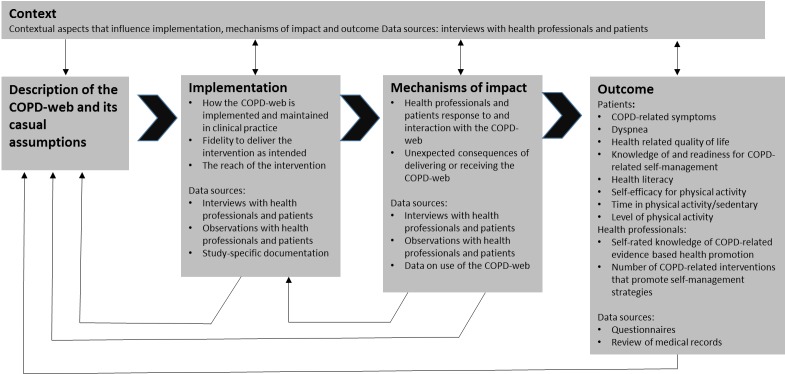
Data will be collected on outcomes and as a part of the process evaluation also on the components implementation, mechanisms of impact and context as shown in figure 2.
Figure 2.
Outcomes and components of the process evaluation (adapted from Moore et al20). COPD, chronic obstructive pulmonary disease.
Primary outcome measures
The primary outcome of effectiveness of the COPD-web is the difference in COPD-related symptoms between the intervention and control groups at the follow-ups at 3 and 12 months after the baseline assessment. COPD-related symptoms will be measured using COPD Assessment Test (CAT).26 The CAT was chosen as the assessment test covers all the symptomatic areas of COPD and has shown to be responsive to healthcare interventions.27
Secondary outcome measures
The secondary outcomes are the differences between the intervention and control groups at the follow-ups at 3 and 12 months after the baseline assessment regarding patients’ dyspnoea, HRQoL, knowledge of and readiness for COPD-related self-management, health literacy, self-efficacy for physical activity and time spent in physical activity and time being sedentary. In a subgroup of patients, level of physical activity will also be measured using an accelerometer (DynaPort, McRoberts BV, The Netherlands). Additional secondary outcomes are the difference in the healthcare professional's knowledge of and readiness to support COPD-related self-management strategies between baseline and follow-up at 3 months and the difference in the number of documented COPD-related interventions that promote management strategies provided to patients with COPD.
The secondary outcomes were chosen as they cover specific aspects of the content of the COPD-web and most of them have previously been used in COPD and/or in a Swedish context. The range of outcomes will ensure assessment of relevant aspects of patient's symptoms and HRQoL, readiness for use of self-management strategies as well as potential use of such strategies. Additionally, the outcomes will cover healthcare professionals’ readiness to support self-management strategies and actually change in delivery of such interventions.
Process evaluation
Aspects of the implementation component that will be assessed are implementation and maintenance of the COPD-web, fidelity to the prespecified routine during the introduction of the COPD-web and the reach, that is, the proportion of the target population that will receive the intervention. With regard to mechanisms of impact, the patients’ and healthcare professionals’ response to and interaction with the COPD-web will be examined as well as unexpected events or consequences of delivering or receiving the COPD-web. Finally, contextual influences on the implementation and mechanism of impact will be examined.
Participant timeline
As displayed in table 1, patients will be screened for eligibility and asked for informed consent when visiting their primary care centre (-t1). Patients will, during the visit, receive the questionnaire that constitutes the baseline assessment (0). Follow-ups will be conducted at 3 months (t1) and at 12 months (t2) after baseline. The time points for the assessment of healthcare professionals’ knowledge of COPD-related self-management strategies and the number of interventions that promote COPD-related self-management strategies provided to people with COPD differ from the patients’ baseline and follow-up and will be conducted at –t3,that is, before the enrolment of patients starts. Follow-up will be conducted at t2, when the patient enrolment is completed.
Table 1.
Participant timeline for enrolment, the intervention and assessments
| Timepoint | -t3 baseline (health professionals) | -t2 baseline | -t1 screening/consent | 0 baseline | t1 follow-up 3 months | t2 follow-up(health professionals) | t3 followup 12 months |
| Enrolment | |||||||
| Eligibility screen | X | ||||||
| Informed consent | X | ||||||
| Interventions | |||||||
| The COPD-web |

|
||||||
| Assessments | |||||||
| Sociodemographics (age, sex, civil status, occupation, smoking habits, diagnosis)1 | X | ||||||
| Pulmonary function1 | X3 | ||||||
| COPD-related symptoms1 | X | X | X | ||||
| Dyspnoea1 | X | X | X | ||||
| Health-related quality of life (HRQoL)1 | X | X | X | ||||
| Knowledge of and readiness for COPD-related self-management strategies1 | X | X | X | ||||
| Health literacy* | X | X | X | ||||
| Self-efficacy for physical activity1 | X | X | X | ||||
| Time spent in physical activity and training1 | X | X | X | ||||
| Time being sedentary1 | X | X | X | ||||
| Physical activity level (accelerometer)1 | X | X | X | ||||
| Healthcare professionals’ knowledge of and readiness to support COPD-related self management strategies2 | X | X | |||||
| The number of interventions that promote COPD-related self-management strategies provided by healthcare professionals3 | X | X | |||||
| Implementation and maintenance1 2 4 | X | X | X | X | |||
| Fidelity to the intervention2 | X | ||||||
| Reach5 | X | ||||||
| Response to and interaction with the COPD-web1 2 | X | X | X | ||||
| Unexpected events or consequences of delivering or receiving the COPD-web1 2 | X | X | X | ||||
| Contextual influences1 2 | X | X | |||||
Data collection, management and analysis
Sample size and blinding
Since this is a planned pragmatic controlled pilot trial, a sample size calculation is not necessary. The final sample size in this trial will be influenced by the total number of patients with COPD visiting any of the included primary care settings during the 3-month intervention period. We estimate an enrolment of 96 participants (16 per unit) based on information provided by the primary care units which would be a sufficient sample size for the primary outcome and for subgroup analyses.28 29 All outcome measures, except assessment of level of physical activity collected with an accelerometer in a subgroup of patients, will be collected through questionnaires. Due to the character of the intervention, blinding of trial participants will not be applicable. Furthermore, as all data are self-reported, neither is blinding of outcome assessors applicable. Randomisation is not applicable as both patients and healthcare professionals at included healthcare units will be introduced and instructed to use the COPD-web. The control group will therefore be recruited among patients with a visit to any of the included primary care units, prior to the introduction of the COPD-web.
Data collection methods
In order to guide the choice of outcome measures for evaluation of effectiveness in a future RCT and to evaluate the effectiveness and increase the understanding of the functioning of the COPD-web in the present trial, various methods for data collections including questionnaires, review of medical records, observations, qualitative interviews, study log books and user data from the COPD-web will be used. Box 2 provides an overview of the questionnaires and methods for data collection in this study.
Box 2. Questionnaires and methods for data collection.
COPD-related symptoms
-
The questionnaire COPD Assessment Test (CAT)30
The severity of eight COPD-related symptoms (coughing, presence of phlegm, feeling of tightness in the chest, breathlessness when walking, limitation in activities, confidence in leaving home, sleep, energy) is rated on a six-graded scale
Evaluated for internal consistency, stability over time in stable patients and ability to discriminate between stable and exacerbation patients with excellent or very good results30
Dyspnoea
-
The questionnaire Medical Research Council Scale31
Perceived dyspnoea is rated on a 5-graded Likert scale ranging from 0 ("I just get out of breath when I exert myself greatly", not when "I take a quick walk or walk uphill") to 4 ("I get out of breath when I wash myself or get dressed")
Evaluated for categorising patients with COPD in terms of disability with good results32
Health-related quality of life
-
The questionnaire EQ-5D33
Health status is rated on five items; three items relate to problems in mobility, self-care and usual activities and two items cover presence and severity of pain and anxiety/depression. Each item is rated on a three-grade scale corresponding to no problem/some or moderate problems/extreme problems.
Health state is rated on a scale ranging from 0 (worst imaginable health state) to 100 (best imaginable health state)
EQ-5D has ability to discriminate between groups of patients with different severity of COPD34
Knowledge of and readiness for COPD-related self-management
-
Questionnaire in three sections developed specifically for the study
The activities/methods considered as self-management in the questionnaire correspond to the interventions recommended by the Swedish evidence-based national Guidelines for Asthma and COPD25
Section A: the perceived importance of performing different self-management activities/methods in order to feel good (eg, “in order for you to feel as good as possible with your disease how important is it for you with daily physical activities?”) is rated on a 5-graded Likert scale ranging from 1 (not important at all) to 5 (very important).
Section B: perceived agreement with statements about knowledge related to the performance of the self-management activities/methods (eg, "I have enough knowledge to know how to be physically active in my daily life?”) is rated on a scale ranging from 1 (do not agree alt all) to 5 (agree completely).
Section C: the self-management activities/methods that currently are performed in order to manage their COPD is noted on a prespecified list
The questionnaire is tested for face validity.
Health literacy
-
The questionnaire communicative and critical health literacy scale35
The agreement regarding five items related to perceived capacity to collect, extract, understand, judge the reliability of the information and to apply health information is rated on a 5-graded scale from 1 (do not agree alt all) to 5 (agree completely).
Evaluated for content validity and stability over time with satisfactory results.35
Self-efficacy for physical activity
-
The questionnaire Exercise Self-Efficacy Scale36
Confidence for carrying out regular physical activities and exercise is rated on 10 items on a 4-point scale (1=not at all true, 2=rarely true, 3=moderately true, 4=always true).
Internal consistency and scale integrity have proven to be high and content validity satisfactory.36
Time spent in physical activity and training
-
Questionnaire from the National Board of Health and Welfare37
The time spent in physical activities such as taking a walk or working in the garden during last week, is rated by choosing between prespecified options (no time at all/30–60 min/60–90 min/9–120 min/>120 min)
The time spent in physical exercises such as running or doing exercise to keep fit during last week, is rated by choosing between prespecified options (no time at all/30–60 min/60–90 min/9–120 min/>120 min)
The categorical mode of the scale has shown low-to-moderate associations with objectively measured physical activity, maximal oxygen uptake, physical performance, balance, cardiovascular biomarkers and self-rated health37
-
The questionnaire Grimsby's Activity Scale38
The level of activity the last 6 months is rated on a 6-graded scale ranging from 1 (hardly any activity at all) to 6 (hard exercise regularly and several times a week, where the physical effort is large, such as running, skiing)
Weak-to-moderate relation with objectively measured physical activity39
Time being sedentary
-
Questionnaire from the National Board of Health and Welfare
The question "How much do you sit during a normal day not counting sleep?" is answered by choosing between prespecified options (rate dyspnoea on a 5-graded Likert scale ranging from 0 (almost all day/13–15 hours/10–12 hours/7–9 hours/4–6 hours/1–3 hours, never)
No psychometric properties have been published yet
Physical activity level
-
Accelerometer (DynaPort, McRoberts BV (DynaPort, McRoberts BV, The Netherlands) placed on the lower back 24 hours a day over seven consecutive days.40 41
Has shown to be accurate for measuring the number of steps of people with COPD40
The quantity of physical activity will be assessed using the mean number of steps per day and the number of days per week that the patient could be considered physically active. Physically active is operationally defined as ≥5000 steps per day.
Health professionals’ knowledge of and readiness to support COPD-related self-management strategies
-
Questionnaire developed specifically for the study and tested for face validity
Agreement with the importance of providing a number of prespecified interventions as support for self-management strategies to people with COPD in the primary care (tobacco cessation, education about the disease, education about self-management, physical activity/physical training, physical activity on prescription, breathing techniques, nutrition and energy conservation strategies is rated on a 4-graded scale ranging from (do not agree at all) to 5 (agree completely).
Questions on if healthcare professionals 1) experience that they have enough knowledge to provide support for the self-management strategies, 2) consider the provision of such interventions as part of their work assignment and 3) have experienced any difficulties in providing such interventions. The questions are answered with yes/no.
The number of interventions provided by healthcare professionals to support COPD-related self-management strategies
-
Review of medical records
A protocol for reviewing medical records covering the interventions included in the Swedish national guidelines for COPD has been developed for the study
Implementation
-
Implementation and maintenance of the COPD-web
Qualitative interviews and user statistics from the website
-
Fidelity to the intervention
Qualitative interviews and observations
-
Reach
Study-specific documentation including the number of patients who decline to take part in the intervention. When appropriate, the reasons to decline will also be noted.
Mechanisms of impact
-
Patients’ and healthcare professionals’ response to, and interaction with the COPD-web
Observations and qualitative interviews
-
Unexpected events or consequences of delivering or receiving the COPD-web
Qualitative interviews
Context
-
Contextual influence on the implementation and the healthcare professionals and patients response to and interaction with the COPD-web
Qualitative interviews
COPD, chronic obstructive pulmonary disease; EQ-5D, EuroQol five dimensions questionnaire.
When recruited at the primary care unit, the patients will receive the questionnaires for the baseline assessment together with a stamped return envelope. In case of non-response, the patient will be reminded by mail after 2 weeks. People with COPD will be considered included when he/she has given a written informed consent and returned/filled in baseline questionnaires. The follow-up questionnaires will be distributed by mail at 3 and 12 months after inclusion. The same procedure for reminders will be used.
The questionnaire to the healthcare professionals will be distributed prior to giving them access to the COPD-web. The follow-up questionnaire will be distributed once the inclusion of patients is completed.
For a subgroup of eight patients at each unit, the level of physical activity will be assessed using accelerometers. A baseline assessment of 7 days will be conducted before the patients receive the intervention and a follow-up assessment of 7 days will be conducted at 3 and at 12 months.
A review of medical records will be performed and the number of evidence-based health promotion interventions will be counted. The review of the medical record will be conducted for visits to the primary care centres for all patients with a diagnosis of COPD during a 2-month period before the start of the study. A follow-up review for a corresponding time period will be conducted a year later.
For the process evaluation,20 data will be collected using qualitative interviews, review of medical records, observations, study log books and user data from the website (figure 2 and box 2). Observations will be performed during three consecutive consultations between healthcare professionals and patients at each unit when patients are introduced to the COPD-web. Three patients from each unit, where at least one male/female, will be included in qualitative semi-structured interviews. The interviews will be conducted at 3 months after inclusion and a follow-up interview will be conducted at 12 months after inclusion. The healthcare professionals will participate in a qualitative semi-structured interview when the inclusion of patients is concluded and a follow-up interview will be conducted after 12 months.
Study-specific documentation and automatised data on the healthcare professionals and the patients’ use of the COPD-web will be collected.
Analysis
Statistical methods
For data management and statistical analysis, the IBM Statistical Package for Social Sciences (SPSS) V.23.1 will be used. Number of patients screened and asked for inclusion and number of patients analysed in each group for the pilot objective will be presented. Data analyses will include all patients using intention-to-treat analysis. In the case of missing data, any missing data due to dropouts will be considered Missing Not At Random (MNAR). On the other hand, missing answers in the different questionnaires will be considered Missing At Random (MAR).42 MNAR and MAR data will be imputed the overall mean43 except for activities/methods/measures currently performed to manage their COPD in the knowledge of and readiness for COPD-related self-management questionnaire in which the last observation carried forward method will be used to impute missing data. A sensitivity analysis will be performed comparing the results with imputed data to a complete case analysis, if necessary.44 Independent and paired sample T-tests as well as Mann-Whitney U test and Wilcoxon signed rank test will be used when appropriate. A p value of <0.05 will be considered statistically significant.
Analysis of qualitative data
The qualitative interviews will be recorded and transcribed verbatim. The observations will be captured in concurrent field notes. Data from the interviews and the observations will be inductively analysed using qualitative content analysis considering the process evaluation components implementation and mechanisms of impact.45 The data will be read through in order to capture ‘a sense of the whole’ and will then be inductively coded. The codes will be organised in subcategories and categories and finally collated on a conceptual level.45 Considering healthcare professionals’ fidelity to the prespecified routine for delivery of the intervention, data from the interviews and the observations will be analysed using deductive qualitative content analysis based on a matrix developed from the routine.
Acceptability and feasibility
In order to assess acceptability and feasibility of the intervention and the study procedures, findings from the statistical analyses and the qualitative content analyses will be synthesised and will contribute to refinement of the intervention and inform the study design and study procedures in the future study.
Ethics and dissemination
Research ethics approval
Ethical approval have been received from the Regional Ethical Review Board in Umeå. Dnr 2014-319-31M, 2015-392-32M and 2015-457-32M.
Consent
All patients and healthcare professionals will be informed orally and in writing about the present study and written consents will be obtained. Patients will be given informationand asked for consent by healthcare professionals. All participants are able to decline taking part in the study and they will be informed that they can withdraw from the study at any time.
Confidentiality
Patients will be get a unique identification (ID) number when included in the study. The code list linking the patient and the ID number will be kept separate from the data. Data will be analysed by ID number only.
Only the researchers will have access to the final trial dataset. Confidentiality to units and healthcare professionals through the entire process from data collection to analysis and presentation of results will be ensured.
Dissemination
The results of this study will be presented at national and international conferences and submitted for publication in peer-reviewed publications. The findings will also be presented to the involved healthcare professionals and patients as well as to patient organisations.
Discussion
This study protocol represents a pragmatic controlled pilot trial with preassessments and postassessments and a parallel process evaluation aimed at determining the feasibility and effects of an internet-based tool intended at increasing access to self-management strategies for patients with COPD and to support and facilitate for healthcare professionals in promoting self-management strategies to patients with COPD. Currently, despite its proven effectiveness, access to self-management interventions is limited2 10 and alternative ways of promoting self-management for patients with COPD are warranted. Even though eHealth interventions have been used to increase access to pulmonary rehabilitation, it remains unclear whether an eHealth tool could be used to increase access and provision of self-management strategies in this population. The proposed trial will provide new knowledge to this research area by investigating the use of an internet-based tool, the COPD-web, as a tool for increasing access to self-management strategies for patients with COPD and to support and facilitate for healthcare professionals in promoting self-management strategies to patients with COPD. The results from the study will determine its effect on clinically relevant outcomes such as symptoms, dyspnoea, quality of life as well as physical activity among patients with COPD. The latter is of utmost importance, as the level of physical activity is one of the strongest predictor of mortality in this group of patients.46 The results from the study will also provide novel information on the effects on self-efficacy to perform physical activity, health literacy as well as knowledge of and readiness for use of self-management strategies.
As the COPD-web is a complex intervention that cannot easily be evaluated in a controlled trial, our intention to use a process evaluation will provide novel information and understanding on how the COPD-web works in clinical practice. The process evaluation will assess components of implementation, mechanism of impacts and context in order to identify and understand core components of the COPD-web. This will increase knowledge on how the process of delivering and receiving the intervention can be understood. It will also help us draw a more robust conclusion with regard to if the COPD-web is accepted by patients and healthcare professionals and about the intervention's effectiveness. As the study is designed as a pragmatic trial,21 the intervention will be delivered by healthcare professionals in ordinary healthcare settings in order to maximise the clinical applicability of the findings to other healthcare settings. The fact that the intervention is delivered by healthcare professionals also include challenges with regard to the fidelity to the delivery of the intervention. However, the observations, which form a part of the process evaluation, will provide valuable information on the implementation of the intervention and on the need of enhanced training of the healthcare professionals in the future study. The pragmatic approach, with a focus on how the intervention works when used in clinical practice also means that the inclusion criterion are wide. In line with this approach,21 no selection beyond diagnosed COPD will be made thus increasing the clinical applicability of the findings in healthcare. A limitation in the design of the pilot trial is the non-randomised control group.
In conclusion, the pragmatic pilot trial will provide clinically relevant information on the acceptability, feasibility and potential effectiveness of the intervention and study procedures from the perspective of patients with COPD and healthcare professionals. The findings will inform a future large-scale study.
Supplementary Material
Footnotes
Contributors: AN has made direct and substantial contribution to this work by contributing to the conception and design of the study, designing and writing of the protocol and approving the final version of the protocol. KW is the principal investigator and has made direct and substantial contribution to this work by providing the project idea, contributing to the conception and design of the study and by providing critical revisions that are important for the intellectual content of the protocol and approving the final version of the protocol. HL has made direct and substantial contribution to this work in providing critical revisions that are important for the intellectual content of the protocol and approving the final version of the manuscript. MT has made direct and substantial contribution to this work by having a leading role in the design of the study and process evaluation, designing and writing the protocol and approving the final version of the protocol.
Competing interests: None declared.
Ethics approval: Regional Ethical Review Board in Umeå.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: n.a.
References
- 1. World Health Organization. Fact sheet on 10 top causes of death. http://www.who.int/mediacentre/factsheets/fs310/en/ (Accessed March 13, 2017).
- 2. Vogelmeier CF, Criner GJ, Martinez FJ, et al. . Global strategy for the diagnosis, Management, and Prevention of chronic obstructive lung disease 2017 Report: gold Executive Summary. Am J Respir Crit Care Med 2017. 10.1016/j.arbres.2017.06.001 [DOI] [PubMed] [Google Scholar]
- 3. Spruit MA, Singh SJ, Garvey C, et al. . An Official American Thoracic Society/European Respiratory Society statement: key concepts and advances in Pulmonary rehabilitation. Am J Respir Crit Care Med 2013;188:e13–e64. 10.1164/rccm.201309-1634ST [DOI] [PubMed] [Google Scholar]
- 4. Effing TW, Bourbeau J, Vercoulen J, et al. . Self-management programmes for COPD: moving forward. Chron Respir Dis 2012;9:27–35. 10.1177/1479972311433574 [DOI] [PubMed] [Google Scholar]
- 5. Bischoff EW, Hamd DH, Sedeno M, et al. . Effects of written action plan adherence on COPD exacerbation recovery. Thorax 2011;66:26–31. 10.1136/thx.2009.127621 [DOI] [PubMed] [Google Scholar]
- 6. Effing T, Kerstjens H, van der Valk P, et al. . (Cost)-effectiveness of self-treatment of exacerbations on the severity of exacerbations in patients with COPD: the COPE II study. Thorax 2009;64:956–62. 10.1136/thx.2008.112243 [DOI] [PubMed] [Google Scholar]
- 7. Cannon D, Buys N, Sriram KB, et al. . The effects of chronic obstructive pulmonary disease self-management interventions on improvement of quality of life in COPD patients: a meta-analysis. Respir Med 2016;121:81–90. 10.1016/j.rmed.2016.11.005 [DOI] [PubMed] [Google Scholar]
- 8. Apps LD, Mitchell KE, Harrison SL, et al. . The development and pilot testing of the self-management programme of activity, coping and education for chronic obstructive pulmonary disease (SPACE for COPD). Int J Chron Obstruct Pulmon Dis 2013;8:317–27. 10.2147/COPD.S40414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Velloso M, Jardim JR. Study of energy expenditure during activities of daily living using and not using body position recommended by energy conservation techniques in patients with COPD. Chest 2006;130:126–32. 10.1378/chest.130.1.126 [DOI] [PubMed] [Google Scholar]
- 10. Wadell K, Janaudis Ferreira T, Arne M, et al. . Hospital-based pulmonary rehabilitation in patients with COPD in Sweden--a national survey. Respir Med 2013;107:1195–200. 10.1016/j.rmed.2013.04.019 [DOI] [PubMed] [Google Scholar]
- 11. Thorpe O, Johnston K, Kumar S. Barriers and enablers to physical activity participation in patients with COPD: a systematic review. J Cardiopulm Rehabil Prev 2012;32:359–69. 10.1097/HCR.0b013e318262d7df [DOI] [PubMed] [Google Scholar]
- 12. Cicutto LC, Brooks D. Self-care approaches to managing chronic obstructive pulmonary disease: a provincial survey. Respir Med 2006;100:1540–6. 10.1016/j.rmed.2006.01.005 [DOI] [PubMed] [Google Scholar]
- 13. Guo SE, Bruce A. Improving understanding of and adherence to pulmonary rehabilitation in patients with COPD: a qualitative inquiry of patient and health professional perspectives. PLoS One 2014;9:e110835 10.1371/journal.pone.0110835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lundell S, Tistad M, Rehn B, et al. . Building COPD care on shaky ground - healthcare professional perspectives. A mixed methods study in swedish primary care. Accepted for publication in BMC Health Services Research 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lundell S, Holmner Å, Rehn B, et al. . Telehealthcare in COPD: a systematic review and meta-analysis on physical outcomes and dyspnea. Respir Med 2015;109:11–26. 10.1016/j.rmed.2014.10.008 [DOI] [PubMed] [Google Scholar]
- 16. Adams SG, Smith PK, Allan PF, et al. . Systematic review of the chronic care model in chronic obstructive pulmonary disease prevention and management. Arch Intern Med 2007;167:551–61. 10.1001/archinte.167.6.551 [DOI] [PubMed] [Google Scholar]
- 17. Statistiska centralbyrån [Statistics Sweden] Privatpersoners användning av datorer och internet Use of computers and the internet by private persons]. 2013. http://www.scb.se/Statistik/_Publikationer/LE0108_2013A01_BR_IT01BR1401.pdf (Accessed March 13, 2017).
- 18. Arne M, Emtner M, Lisspers K, et al. . Availability of pulmonary rehabilitation in primary care for patients with COPD: a cross-sectional study in Sweden. Eur Clin Respir J 2016;3:31601 10.3402/ecrj.v3.31601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Craig P, Dieppe P, Macintyre S, et al. . Developing and evaluating complex interventions: the New Medical Research Council guidance. BMJ 2008;337:a1655 10.1136/bmj.a1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moore GF, Audrey S, Barker M, et al. . Process evaluation of complex interventions: medical Research Council guidance. BMJ 2015;350:h1258 10.1136/bmj.h1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zwarenstein M, Treweek S, Gagnier JJ, et al. . Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ 2008;337:a2390 10.1136/bmj.a2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chan AW, Tetzlaff JM, Altman DG, et al. . SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chan AW, Tetzlaff JM, Gøtzsche PC, et al. . SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586 10.1136/bmj.e7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eldridge SM, Chan CL, Campbell MJ, et al. . CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ 2016;355:i5239 10.1136/bmj.i5239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Socialstyrelsen [The National Board of Health and Welfare]. Nationella riktlinjer för vård vid astma och KOL 2015. [National guidelines for asthma and COPD care]. http://www.socialstyrelsen.se/publikationer2015/2015-11-3 (Accessed March 13, 2017). [Google Scholar]
- 26. Jones PW. COPD assessment test --rationale, development, validation and performance. COPD 2013;10:269–71. 10.3109/15412555.2013.776920 [DOI] [PubMed] [Google Scholar]
- 27. Jones PW, Harding G, Wiklund I, et al. . Tests of the responsiveness of the COPD assessment test following acute exacerbation and pulmonary rehabilitation. Chest 2012;142:134–40. 10.1378/chest.11-0309 [DOI] [PubMed] [Google Scholar]
- 28. Billington J, Coster S, Murrells T, et al. . Evaluation of a Nurse-Led Educational Telephone intervention to support Self-Management of patients with chronic obstructive pulmonary disease: a Randomized Feasibility Study. COPD 2015;12:395–403. 10.3109/15412555.2014.974735 [DOI] [PubMed] [Google Scholar]
- 29. Gloeckl R, Damisch T, Prinzen J, et al. . Validation of an activity monitor during sleep in patients with chronic respiratory disorders. Respir Med 2015;109:286–8. 10.1016/j.rmed.2014.12.017 [DOI] [PubMed] [Google Scholar]
- 30. Jones PW, Harding G, Berry P, et al. . Development and first validation of the COPD Assessment Test. Eur Respir J 2009;34:648–54. 10.1183/09031936.00102509 [DOI] [PubMed] [Google Scholar]
- 31. Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest 1988;93:580–6. 10.1378/chest.93.3.580 [DOI] [PubMed] [Google Scholar]
- 32. Bestall JC, Paul EA, Garrod R, et al. . Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999;54:581–6. 10.1136/thx.54.7.581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095–108. 10.1097/00005650-199711000-00002 [DOI] [PubMed] [Google Scholar]
- 34. Rutten-van Mölken MP, Oostenbrink JB, Tashkin DP, et al. . Does quality of life of COPD patients as measured by the generic EuroQol five-dimension questionnaire differentiate between COPD severity stages? Chest 2006;130:1117–28. 10.1378/chest.130.4.1117 [DOI] [PubMed] [Google Scholar]
- 35. Wångdahl JM, Mårtensson LI. The communicative and critical health literacy scale--swedish version. Scand J Public Health 2014;42:25–31. 06:00] 10.1177/1403494813500592 [DOI] [PubMed] [Google Scholar]
- 36. Kroll T, Kehn M, Ho PS, Ps H, et al. . The SCI Exercise Self-Efficacy Scale (ESES): development and psychometric properties. Int J Behav Nutr Phys Act 2007;4:34 34 09:00] 10.1186/1479-5868-4-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Olsson SJ, Ekblom Ö, Andersson E, et al. . Categorical answer modes provide superior validity to open answers when asking for level of physical activity: a cross-sectional study. Scand J Public Health 2016;44:70–6. 10.1177/1403494815602830 [DOI] [PubMed] [Google Scholar]
- 38. Grimby G. Physical activity and muscle training in the elderly. Acta Med Scand 1986;220:233–7. 10.1111/j.0954-6820.1986.tb08956.x [DOI] [PubMed] [Google Scholar]
- 39. Ekblom Ö, Ekblom-Bak E, Bolam KA, et al. . Concurrent and predictive validity of physical activity measurement items commonly used in clinical settings--data from SCAPIS pilot study. BMC Public Health 2015;15:978 10.1186/s12889-015-2316-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Andersson M, Janson C, Emtner M. Accuracy of three activity monitors in patients with chronic obstructive pulmonary disease: a comparison with video recordings. COPD 2014;11:560–7. 06:00] 10.3109/15412555.2014.898033 [DOI] [PubMed] [Google Scholar]
- 41. Demeyer H, Burtin C, Van Remoortel H, et al. . Standardizing the analysis of physical activity in patients with COPD following a pulmonary rehabilitation program. Chest 2014;146:318–27. 10.1378/chest.13-1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Altman DG, Bland JM. Missing data. BMJ 2007;334:424 10.1136/bmj.38977.682025.2C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shrive FM, Stuart H, Quan H, et al. . Dealing with missing data in a multi-question depression scale: a comparison of imputation methods. BMC Med Res Methodol 2006;6:57 10.1186/1471-2288-6-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vandenbroucke JP, von Elm E, Altman DG, et al. . Strengthening the Reporting of Observational studies in Epidemiology (STROBE): explanation and elaboration. Epidemiology 2007;18:805–35. 10.1097/EDE.0b013e3181577511 [DOI] [PubMed] [Google Scholar]
- 45. Elo S, Kyngäs H. The qualitative content analysis process. J Adv Nurs 2008;62:107–15. 10.1111/j.1365-2648.2007.04569.x [DOI] [PubMed] [Google Scholar]
- 46. Waschki B, Kirsten A, Holz O, et al. . Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest 2011;140:331–42. 10.1378/chest.10-2521 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.