Abstract
Fruits of Sonneratia apetala (Buch.-Ham.), (English: mangrove apple, Bengali: keora) both seeds and pericarps, are largely consumed as food besides their enormous medicinal application. The fruit seeds have high content of nutrients and bioactive components. The seeds powder of S. apetala was successively fractionated using n-hexane, diethyl ether, chloroform, ethyl acetate, and methanol. The fractions were used to evaluate antibacterial, anti-diarrhoeal, analgesic, and cytotoxic activities. Methanol fraction of seeds (MeS) stronly inhibited Escherichia coli strains, Salmonella Paratyphi A, Salmonella Typhi, Shigella dysenteriae, and Staphylococcus aureus except Vibrio cholerae at 500 μg/disc. All the fractions strongly inhibited castor oil induced diarrhoeal episodes and onset time in mice at 500 mg extract/kg body weight (P<0.001). At the same concentration, MeS had the strongest inhibitory activity on diarrhoeal episodes, whereas the n-hexane fraction (HS) significantly (P<0.05) prolonged diarrhoeal onset time as compared to positive control. Similarly, HS (P<0.005) inhibited acetic acid induced writhing in mice at 500 mg extract/kg, more than any other fraction. HS and diethyl ether fractions of seed strongly increased reaction time of mice in hot plate test at 500 mg extract/kg. All the fractions showed strong cytotoxic effects in brine shrimp lethality tests. Gas chromatography-mass spectrometry analysis of HS led to the identification of 23 compounds. Linoleic acid (29.9%), palmitic acid (23.2%), ascorbyl palmitate (21.2%), and stearic acid (10.5%) were the major compounds in HS. These results suggest that seeds of S. apetala could be of great use as nutraceuticals.
Keywords: analgesic, anti-diarrhoea, keora, mangrove apple, Sonneratia apetala
INTRODUCTION
Sonneratia apetala (Buch.-Ham.) is a true mangrove, belonging to the family Lythraceae. It is called keora in Bengali and mangrove apple in English. It is a native plant abundantly grown in the world’s largest tidal halophytic forest, the Sundarbans’ along with coastal areas in Bangladesh, India, Myanmar, Thailand, Malaysia, Indonesia, Sri Lanka, and China, etc. The plants grow well on newly accreted soil in moderate to strong saline areas, and are considered as a pioneer species in ecological succession. Its fruits are berry-like, generally 1.5 to 2 cm in diameter, globose with persistent leathery calyx. Each fruit contains numerous seeds, even up to a hundred. Seeds are compactly arranged in 6 to 8 locules, yellowish, mostly U or V shaped, 0.88±0.07×0.65±0.09 cm in size, and 0.09±0.02 g in weight (1–3).
The whole fruit, both pericarp and seed, is extensively consumed following cooking and through other preparations. People adjacent to the Sundarbans’ generally consume the fruits by cooking with pulses and small shrimps. The fruits are also processed to produce pickles and sour sauce to be sold commercially. In addition, coastal people in Bangladesh use the fruit juice as tonic and to treat diarrhoea-like diseases (4). Reportedly, ripe fruits are also used to expel intestinal parasites (Malay) and half- ripe fruits are believed to be good for coughs. Fermented juice of this fruit is useful in arresting haemorrhaging (5). It was reported that fruits and barks of the plants have remedial activities against asthma, febrifuge, ulcers, swellings, sprains, bleeding, hemorrhages, and piles (5). Leaves of S. apetala showed anti-hepatitis activity (5). Ethanol extract of S. apetala plant (leaves, barks, and pneumatophores) showed antimicrobial activity against Gram-positive, Gram-negative bacteria, and Candida albicans with the inhibitory zones ranged from 15~20 mm at the concentration of 100 mg/mL (6). Different solvent (acetone, ethanol, methanol, and water) extracts showed antibacterial activity against nine Gram-positive and Gram-negative bacteria [minimum inhibitory concentrations (MICs) 1.25 to 5.0 mg/mL for active extracts], had antioxidant activity [2,2-diphenyl-1-picrylhydrazyl, nitric oxide, and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) scavenging assay, ferrous ion chelating, and reducing power], α-glucosidase inhibitory activity (IC50 values 0.29~0.64 mg/mL), and in vivo anticancer activity of methanol extract against Ehrlich ascites carcinoma (EAC) cells in Swiss albino mice at the dose of 0.2 mmol/kg body weight (b.w.) (7). Nevertheless, little scientific attention has been paid to the fruits. Earlier, antioxidant, antidiabetic, and antibacterial activities of methanol extract of the fruit were reported (4). It was also reported that the whole fruit ethanol extract showed some biological activities (8). Recently, nutrient compositions, antioxidant activity, and common phenolics of the fruit have been reported (9). In addition, cooking time (optimum 20 min) of fruit was found to influence the yield of phenolics and antioxidants (10). These investigations revealed that seeds of S. apetala consist of high amounts of polyphenols, flavonoids, anthocyanins, vitamin C, and antioxidants as compared to fruit pericarps. The present study was designed to prepare different fractions of S. apetala seeds to evaluate their antibacterial, anti-diarrhoeal, analgesic, and cytotoxic activities as well as to detect and quantify compounds in the fraction showing promising activity. The study was also a continuation of previous work (4,9) to clarify the active metabolites according to their polarity through the screening of different fractions and then chemically analysing the active fractions by gas chromatography-mass spectrometry (GC-MS).
MATERIALS AND METHODS
Chemicals and reagents
n-Hexane, diethyl ether, chloroform, ethyl acetate, methanol, dimethyl sulfoxide (DMSO), acetic acid, and Tween-80 were obtained from Merck (Darmstadt, Germany). Nutrient broth and nutrient agar media were purchased from Hi Media Laboratories Pvt. Ltd. (Mumbai, India). Morphine was obtained from Gonosastha Pharmaceuticals Limited (Dhaka, Bangladesh).
Sample collection
The mature unripe fruits of S. apetala (Buch.-Ham.) were collected during August 2015 from Munshiganj Forest Office area of the Sundarbans. The seeds, packed inside the fruits, were separated by removing the pericarps and shed dried. Dried seeds were ground into fine powder and stored in an air tight container at room temperature (24°C).
Extraction
Ten grams of powdered seeds was fractionated with solvents, starting with 200 mL of 100% n-hexane with vigorous shaking and kept in air tight bottle. After 30 min, it was filtered using Whatman no. 1 filter paper. The filtrate was air dried in the fume cupboard until the solvent evaporated, the solid was taken in a screw cap vial and kept in the freezer (−15°C) as hexane fraction (HS). Diethyl ether, chloroform, ethyl acetate, and methanol fractions were successively prepared using the residues on the filter paper and designated as DS, CS, ES and MeS, respectively. In addition, 10 g of untreated seed powder was also extracted with methanol (99%) to prepare the crude methanol extract (CMeS). For different experiments, extracts were dissolved in water at defined concentrations with the aid of DMSO where the concentration of DMSO did not exceed 1%. Control was prepared in the same fashion without the addition of extract.
Antibacterial activity assay
Bacteria Staphylococcus aureus, Salmonella Paratyphi A, Salmonella Typhi, Shigella dysenteriae, and Vibrio cholera were obtained from ICDDR’B (International Centre for Diarrheal Disease Research’ Bangladesh), Dhaka, Bangladesh. Strains of Escherichia coli were avirulent (containing only PHO gene); virulent and enterotoxigenic (containing PHO gene along with ST1 gene; and LT2 gene); virulent and enterophathogenic (having PHO gene and EAE gene). They were isolated from contaminated fish farms, and identified using polymerase chain reaction (PCR) based techniques. The bacterial strains were consecutively sub-cultured in nutrient agar media at least three times with 24 h intervals before use. Ten μL of culture was transferred to a 10 mL nutrient broth medium and incubated at 37°C for 24 h.
For susceptibility tests, nutrient agar plates were seeded by pouring the culture broth to make a bacterial lawn. The bacterial population was adjusted through comparison with 0.5% McFarland standard to make 5×106 CFU/mL. The extracts were dissolved in respective solvent to prepare the stock and added to the paper discs (6 mm) using a micropipette so that the disc contained 500 μg extract. Discs impregnated with the fractions/extract were placed at proportionate distance from each other using a sterile needle. The plates were incubated for 12 h at 37°C and checked for clear zone of inhibition around the disc. Tube dilution method was used to determine IC50 and MIC of the fractions. The lowest concentration of the fraction required to inhibit the growth of tested bacteria was reported as the MIC.
Animals
Young Swiss albino mice (16~22 g) of either sex were purchased from the ICDDR’B. They were provided with ICDDR’B formulated food and tap water ad libitum and maintained in polypropylene cages (5 mice in each cage of 30 cm×15 cm×15 cm dimension) with natural day-night cycle. All experiments were conducted in an isolated and noiseless conditions located in the animal house section in accordance with the guidelines of the Animal Ethics Committee, Khulna University, Khulna, Bangladesh (Research Ref. No.: KUAEC-2017/07/01), which agrees with the EU Directive 2010 for animal experiments.
Antidiarrhoeal activitiy assay
Castor oil induced diarrhoea was studied as described by Shoba and Thomas (11). The test animals fasted with water for 24 h were randomly divided into control, positive control and test groups (n=6). Each mouse was placed in an individual cage, the floor of which was lined with absorbent paper. Suspensions of the fractions were prepared in water with the assistance of 0.1% Tween-80. The control group received distilled water containing 0.1% Tween-80; the positive control group received loperamide hydrochloride (3 mg/kg b.w.); the test groups received the S. apetala seed fractions at 250 and 500 mg/kg b.w. After 1 h each mouse was given 0.5 mL of castor oil with the help of a feeding needle for mice. The time elapsed between the administration of castor oil and the excretion of the first diarrhoeal stool, and the total numbers of the characteristics diarrhoeal droppings were noted every half an hour over a time period of 5 h.
Analgesic activity assay
Acetic acid induced writhing was studied as described by Koster et al. (12). The test animals fasted for 18 h with water were randomly divided into control, positive control, and test groups (n=6). Each mouse was placed in an individual cage, the floor of which was lined with absorbent paper. Suspensions of the fractions were prepared in water with the assistance of 0.1% Tween-80 which were orally administered to mice 30 min before intraperitoneal injection of 0.7% (v/v) acetic acid, at a dose of 10 mg/kg b.w., while the control group received distilled water containing 0.1% Tween-80 and the positive control group received diclofenac sodium (25 mg/kg b.w.). Writhings, i.e., stretching or bending of the body, that occurred between 5 and 15 min after acetic acid administration were counted. Dose-dependent inhibition of writhings was also measured.
The method of Eddy and Leimbach (13) was used with slight modification to study analgesic activity by hot plate test. The test animals fasted with water for 12 h were randomly divided into control, positive control, and test groups (n=6). Prior to the experiments, the hot plate was set to 55°C. Mice of different groups were treated with control vehicle (0.1% Tween-80 in distilled water), morphine (10 mg/kg b.w.), and different fractions of seeds (500 mg/kg b.w.) with the help of a feeding needle. The animal was placed on the hot plate (Ugo Basile, Varese, Lombardy, Italy) and the time for licking paws or jumping on the hot plate, which was appeared first had been recorded by using a stop watch. To observe the effect of the fractions with the passage of time, mice were placed on the hot plate at 0, 30, 60, 90, 120, 180, and 240 min after the administration of the respective drugs and the reaction time was recorded. The cut off time for the reaction time recording was set to 20 s to minimize undue tissue damage as a result of over exposure to heat.
Cytotoxicity test
Brine shrimp lethality bioassay was studied as described by McLaughlin (14). The eggs of Artemia salina was collected from Fisheries and Marine Resource Technology Discipline, Khulna University, Khulna, Bangladesh. They were hatched in a rectangular tank of two unequal compartments containing artificial sea water (20 g of NaCl and 18 g of table salt in 1 L of distilled water). Eggs hatched into larvae in the large compartment attracted by the light and moved to the small compartment where they were collected to investigate the cytotoxicity of different fractions of seeds. Different concentrations (μg/mL) of the fractions were tested in triplicate. Fractions were dissolved in distilled water with the assistance of DMSO in such a way that the final concentration of DMSO did not exceed 1%. As positive control, vincristine sulphate (Cipla Pharmaceuticals, Hyderabad, Telangana, India) was used; and 1% DMSO in distilled water served as the negative control. Survivors were counted after 24 h, and % mortality was calculated. The values of the lethal concentration in which 50% (LC50) or 90% (LC90) of the larvae died were determined from the best-fit linear line plotted of concentration verses percentage mortality.
GC-MS analysis
The HS was analyzed on a Shimadzu GC system QP2010 Ultra equipped with a FID detector using a fused capillary column Rtx-5MS, length 30 m, internal diameter 0.25 mm, and film thickness 0.25 μm (Shimadzu, Kyoto, Japan). Ultra-high purity helium (99.99%) (National Industrial Gas Plants, Salwa, Sudan) was used as the carrier gas at a constant flow rate of 1 mL/min. The column oven temperature was programmed at 35°C having a holding time of 3 min whereas the injection temperature was set at 250°C. The injection mode was split with a ratio of 100 and the flow control mode had a linear velocity of 39.4 cm. The total flow rate was 124 mL/min, whereas the column flow rate and purge flow rate were 1.2 and 3.0 mL/min, respectively, at 61.8 kPa pressure. The other parameters include ion source temperature of 200°C, interface temperature of 250°C and solvent cut time of 3.5 min. The MS start time was 4 min and the end time was 61.3 min. The acquisition mode was operated at 1,666 scan speed with 0.5 s event time. Sample was run in the 35~800 m/z range and the total ion chromatogram obtained was auto-integrated using ChemStation. The components were identified by comparison with the National Institute of Standards and Technology (NIST) and Wiley 7 libraries. The relative percentage amount of each component was calculated by comparing its average peak area to the total areas.
Statistical analysis
Statistical analysis was performed using SPSS (version 16, Chicago, IL, USA). Results were expressed as mean±standard deviation. One way analysis of variance (ANOVA) was used to analyze the statistical difference between several groups. Differences with P values <0.05 were considered statistically significant.
RESULTS AND DISCUSSION
Antibacterial activity
Antibacterial activities of the different fractions of seeds were determined using disc diffusion method. Diameter of inhibition zones was measured in mm against the test bacteria. Among the fractions, MeS strongly inhibited all the E. coli strains used, S. Paratyphi A, S. Typhi, S. Dysenteriae, and S. aureus at 500 μg/disc (Table 1). Results showed that CMeS had the stronger inhibitory activity than MeS. At the same concentration, CS inhibited V. cholerae (9.4±0.2 mm) strongly, whereas HS, DS, and ES showed smaller inhibitory effects than MeS. Control disc diameter was 6.5 mm, and streptomycin (30 μg/disc) was used as a positive control. In the antibacterial assay, IC50 and MIC of CMeS, MeS, ES, and HS were determined. The IC50 values of MeS for inhibition of E. coli, S. Paratyphi A, S. Typhi, S. dysenteriae, and S. aureus were 0.59, 0.28, 1.63, 0.83, and 1.1 mg/mL, respectively (Table 1), whereas IC50 of CS for S. aureus and V. cholerae were 1.32 and 1.22 mg/mL, respectively (data not shown). The study showed that among the fractions, MeS had the lowest IC50 and MIC for S. Paratyphi A followed by E. coli and S. dysenteriae. MeS potentially inhibited all the bacterial species except V. cholerae. CMeS showed smaller IC50 values and MICs than MeS. This may be due to the effects of extraction methods; extraction that used only methanol as a solvent, extracted mainly polar and some parts of semi-polar components. Successive extraction sequentially extracted the components depending on their polarity. Therefore, components of seeds of S. apetala probably have, at least in part, synergistic antibacterial effects. Hence, CMeS should be the choice of extract to control common pathogenic bacteria followed by MeS. Potential antimicrobial effects of aerial parts other than fruits of S. apetala have also been reported (15,6). This may be due to the presence of high content of polyphenols, especially caffeic acid, (+)-catechin, (−)-epicatechin, ellagic acid, gallic acid, and quercetin (9). Arif et al. (16) reported that antidiabetic medicinal plants with high contents of polyphenols have strong antibacterial activities. Phenolics and polyphenols have antimicrobial effects through various mechanisms (17).
Table 1.
Antibacterial activities of different solvent [n-hexane (HS), diethyl ether (DS), chloroform (CS), ethyl acetate (ES), methanol (MeS), and crude methanol (CMeS)] fractions of Sonneratia apetala seeds, their IC50, and minimum inhibitory concentration (MIC)
| Name of bacteria | Zone of inhibition (mm±SD) | Fractions of seed (mg/mL) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||||
| Seed fractions (500 μg/disc) | IC50 | MIC | ||||||||||||
|
|
|
|
||||||||||||
| HS | DS | CS | ES | MeS | CMeS | HS | ES | MeS | CMeS | HS | ES | MeS | CMeS | |
| Escherichia coli (avirulent: PHO gene) | 9.5±0.0 | 8.3±0.4 | 7.5±1.4 | 8.8±0.4 | 12.0±0.0 | 13.3±1.1 | 1.25 | 0.65 | 0.61 | 0.35 | 3.5 | 3.0 | 2.5 | 2.0 |
| E. coli (virulent: PHO gene and ST1 gene) | 9.5±0.0 | 8.5±0.0 | 7.5±1.4 | 9.5±0.0 | 12.3±0.4 | 13.0±0.0 | 1.30 | 0.71 | 0.60 | 0.46 | 4.0 | 3.0 | 2.5 | 2.0 |
| E. coli (virulent: PHO gene and LT2 gene) | 9.3±0.4 | 7.5±0.0 | 8.3±0.3 | 9.3±0.4 | 12.0±0.0 | 12.3±1.1 | 1.14 | 0.66 | 0.57 | 0.49 | 4.0 | 3.0 | 2.5 | 2.0 |
| E. coli (virulent: PHO gene and EAE gene) | 9.0±1.0 | 8.5±0.0 | 7.0±0.7* | 9.0±1.0 | 12.3±0.4 | 13.0±1.4 | 1.12 | 0.67 | 0.59 | 0.47 | 3.5 | 3.0 | 2.5 | 2.0 |
| Salmonella Paratyphi A | 6.7±0.5* | 7.2±0.5 | 6.9±0.0 | 7.8±0.3 | 14.5±0.3 | 16.3±2.0 | − | − | 0.28 | 0.19 | − | − | 2.0 | 1.5 |
| Salmonella Typhi | 6.6±0.3* | 6.6±0.2* | 6.8±0.0 | 7.2±0.1 | 11.3±0.5 | 12.4±1.0 | − | − | 1.63 | 0.82 | − | − | 3.5 | 3.0 |
| Shigella dysenteriae | 6.8±0.2 | 6.5±0.0* | 6.5±0.0* | 6.9±0.0 | 11.8±0.5 | 12.6±1.3 | − | − | 0.83 | 0.54 | − | − | 2.5 | 2.0 |
| Staphylococcus aureus | 9.2±0.2 | 6.7±0.2* | 9.8±0.3 | 8.8±0.3 | 12.6±0.4 | 13.4±1.0 | 1.53 | 0.92 | 1.10 | 0.88 | 4.0 | 3.5 | 3.0 | 2.5 |
| Vibrio cholerae | 6.5±0.4* | 6.6±0.1* | 9.4±0.2 | 7.3±0.3 | 7.8±0.4 | 8.6±1.0 | − | − | − | − | − | − | − | − |
Non-significant when compared to control, 6.5 mm.
IC50, inhibition concentration 50; MIC, minimum inhibitory concentration; −, not detected.
Anti-diarrhoeal activity
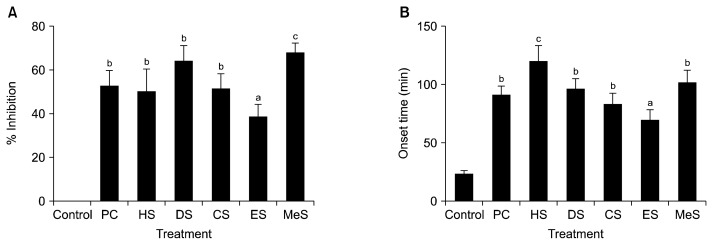
At the dose of 500 mg/kg, all fractions significantly (P<0.05 vs. control as Student’s t-test) inhibited castor oil induced diarrhoea in mice. MeS showed the highest inhibition (68%) followed by DS (65%), CS (51%), HS (50%), and ES (39%) whereas loperamide (3 mg/kg), an anti-diarrhoeal drug showed 52.6% inhibition (Fig. 1A). The average number of characteristics diarrhoeal stool for control group was 16±2. Significant differences (P<0.05) were observed between positive control group (PC, loperamide treated) and test groups treated with ES, and MeS. At that concentration, all the fractions significantly (P<0.05 vs. control as Student’s t-test) delayed the onset of diarrhoeal episodes. It was the highest for HS (114 min), followed by MeS (101 min), DS (96 min), CS (92 min), and ES (67 min), whereas those for loperamide and control were 91 and 22 min, respectively (Fig. 1B). HS showed significantly higher and ES showed smaller prolongation of diarrhoea onset time than loperamide treated group and other fractions. The fractions showed dose dependent inhibition of diarrhoea (data not shown). People in coastal Bangladesh and some other countries use this fruit juice as tonic and also to treat abdominal pain and diarrhea-like diseases (4). In this study, castor oil was used to induce diarrhoea in mice model since its ricinoleic acid had produced irritation and inflammation of the intestinal mucosa resulting in the release of prostaglandins that induces motility and secretion (18). This condition increases the permeability of the mucosal cells and decreases Na+ and K+ absorption, stimulating peristaltic activity and diarrhoea. The observed anti-diarrhoeal activity of the fractions of seed may be due to the inhibition of prostaglandin biosynthesis and/or reduction of gastrointestinal motility. Reportedly, tannins have anti-diarrhoeal activity since protein-tannate makes mucosa more resistant and reduces intestinal secretion. Flavonoids also inhibit intestinal motility and hydro-electrolytic secretion. Therefore, the anti-diarrhoeal activity of the fractions may be attributed to its content of tannins, flavonoids, polyphenols, and/or antioxidants. It was shown that polyphenol extract from apples dose-dependently inhibited cholera toxin induced diarrhoea and the fraction containing polymerized catechins most effectively inhibited the toxin-mediated fluid secretion (19).
Fig. 1.
Effects of different solvent [n-hexane (HS), diethyl ether (DS), chloroform (CS), ethyl acetate (ES), and methanol (MeS)] fractions of Sonneratia apetala seeds on castor oil induced diarrhoea in mice. (A) Inhibition (%) and (B) onset time (min) of diarrhoea in different treatment groups at 500 mg extract/kg body weight (b.w). All data were significant when compared to control, and different letters (a–c) indicate significant differences when compared to positive control (PC, 3 mg loperamide/kg b.w.) using one way analysis of variance (ANOVA) at P<0.05.
Analgesic activity
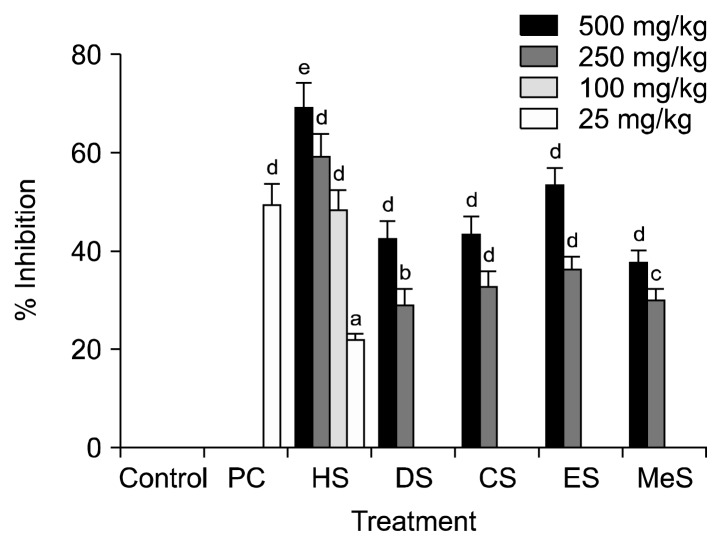
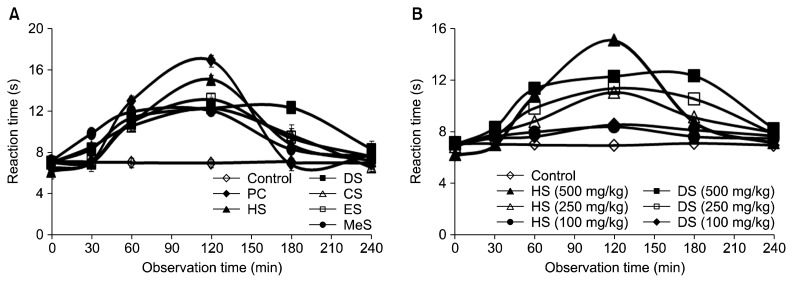
In acetic acid induced writhing test, each of the five fractions of seed when given orally (500 mg/kg b.w.), significantly (P<0.05) inhibited the frequency of acetic acid induced abdominal constrictions in mice (Fig. 2). Among them, only HS strongly inhibited (P<0.05) the abdominal constrictions in mice more than the positive control, diclofenac sodium (25 mg/kg b.w). At that concentration, DS, CS, ES, and MeS showed similar inhibitory effects as that of positive control. Control group showed the average writhing numbers of 60±6. The dose-dependent inhibition of acetic acid induced writhing numbers in mice was also shown in Fig. 2. Increasing the reaction time (paw licking/jumping) by different fractions of seed on mice was tested using hot plate test. Fig. 3A showed reaction time increasing effects of different fractions of seed at 500 mg/kg b.w. on mice. All the fractions increased reaction time with the highest effect at 120 min. At that time, HS showed the highest reaction time (15.1 s), whereas morphine (10 mg/kg b.w.) used as a positive control, showed 16.9 s. The reaction time for all groups started to decline after 120 min except DS treated group, which showed similar effect until 180 min and then declined. All treatment groups significantly (P<0.05) increased the reaction time at 60, 120, and 180 min. Since HS showed the highest reaction time followed by DS, their dose-dependent effects were shown in Fig. 3B. Intraperitoneal administration of acetic acid stimulates the tissue to produce prostaglandins (PGs) and sympathomimetic mediators like PGE2 and PGF2α (20), bradykinin, histamine, and 5-hydroxytryptamine (21) in the peritoneal fluid, which stimulates peripheral nociceptive neurons. Hot plate test measures nociceptive response latencies of test animals to thermal stimulus, which is sensitive to opioid receptors located in the central nervous system (22). All the fractions at 500 mg/kg, inhibited acetic acid induced writhes, and delayed the reaction time in mice significantly. Among the fractions, HS showed the highest analgesic activity through both the peripheral and the central nervous system. DS prolonged (30~180 min) analgesic effect through opioid receptors. These observations suggest that seeds of S. apetala possess peripherally and centrally mediated analgesic properties. The peripheral analgesic action of these fractions may be mediated via inhibition of cyclo-oxygenases (COXs) and/or lipoxygenases (LOXs), whereas the central analgesic action may be mediated through the inhibition of central pain receptors. Since the seed of S. apetala is a rich source of both hydrophilic and lipophilic antioxidants (9), they could effectively ameliorate inflammatory responses through the reduction of oxidative stress and the inhibition of pro-inflammatory enzymes activities. Phenolic-enriched extracts of copao fruits effectively inhibited pro-inflammatory enzymes-LOX and COX-2, and thus showed anti-inflammatory activity (23). The antihistaminic and/or anti-inflammatory activities of citrus fruit peels, and various edible fruits were also reported in different studies (24,25).
Fig. 2.
Dose-dependent inhibitory effects of different solvent [n-hexane (HS), diethyl ether (DS), chloroform (CS), ethyl acetate (ES), and methanol (MeS)] fractions of Sonneratia apetala seeds on acetic acid induced writhing in mice. All data were significant when compared to control, and different letters (a–e) indicate significant differences when compared to positive control (PC, 2.5 mg diclofenac sodium/kg body weight) at P<0.05.
Fig. 3.
Reaction time increasing of mice with different solvent [n-hexane (HS), diethyl ether (DS), chloroform (CS), ethyl acetate (ES), and methanol (MeS)] fractions of Sonneratia apetala seeds. Treatment at 500 mg extract/kg body weight (b.w.) and PC [positive control (morphine, 10 mg/kg b.w.)], (A) and dose-dependent effects of HS and DS on reaction time of mice (B). All data were significant (P<0.05) at 60, 120, and 180 min when compared to control group.
Cytotoxicity
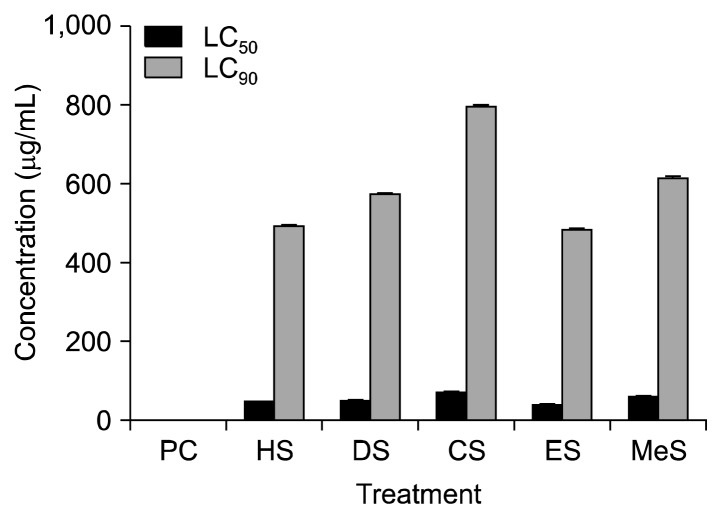
In brine shrimp lethality bioassay, LC50 and LC90 were determined. ES had the lowest LC50 (36 μg/mL) followed by HS (46 μg/mL) (Fig. 4). DS, CS, and MeS showed LC50 values of 48, 68, and 56 μg/mL, respectively whereas vincristine sulphate, a positive control, had 0.45 μg/mL. The LC90 values of HS, DS, CS, ES, and MeS were 493, 563, 794, 482, and 616 μg/mL, respectively, and that for vincristine sulphate was 3.6 μg/mL. The brine shrimp lethality test is a simple bioassay used for the preliminary screening of large number of crude extracts in the drug discovery process. It is an indicator of cytotoxicity, antifungal, pesticidal and various pharmacologic actions (14). This in vitro test is highly correlated with in vivo tests and it is a useful alternative model for predicting toxicity of plant extracts (26). In the present investigation, all the fractions of S. apetala seeds showed dose-dependent lethality of brine shrimp nauplii with LC50 values much lower than 250 μg/mL. It was postulated that extracts having LC50 values less than 250 μg/mL were significantly active and potential for further investigation (27). Seeds of S. apetala could be a potential source of cytotoxic compounds due to the presence of various functional phytochemicals such as polyphenols, flavonoids, saponins, steroids, and alkaloids, etc. It has been reported that some polyphenols destruct the membrane structure (28,29).
Fig. 4.
LC50 and LC90 values of cytotoxic activity of different solvent [n-hexane (HS), diethyl ether (DS), chloroform (CS), ethyl acetate (ES), and methanol (MeS)] fractions of Sonneratia apetala seeds on brine shrimp nauplii. All data were significant at P<0.001 when compared to PC (positive control, vincristine) by Student’s t-test.
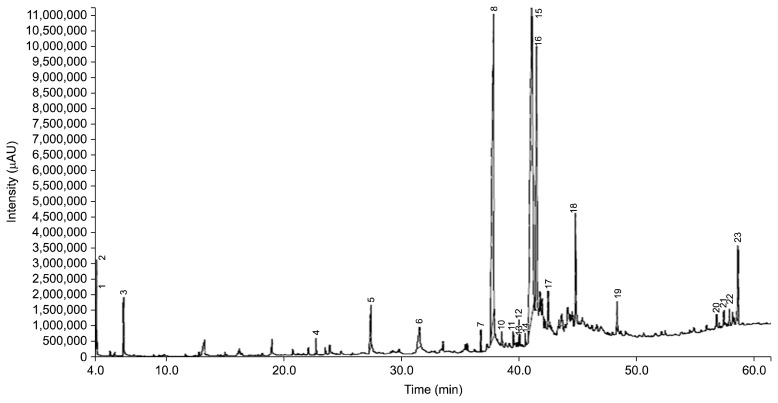
GC-MS analysis of HS
The GC-MS chromatograms of HS (Fig. 5) enabled the identification of 23 compounds (Table 2). A mixture of different classes of compounds such as ester, aldehyde, and fatty acids composed the n-hexane fraction of S. apetala seed. The mass spectrum of each compound was compared with the NIST library and the Wiley 7 library. Among the 23 compounds, four of them constitute 85% of n-hexane fraction. They are linoleic acid (30%), palmitic acid (23%), ascorbyl palmitate (21%), and stearic acid (11%). Linoleic acid is a polyunsaturated omega-6 fatty acid known as cis-9, cis-12-octadecadienoic acid, whereas oleic acid (2%) is a monounsaturated omega-9 fatty acid recognized as cis-9-octadecenoic acid. They are essential for maintaining health of humans. Reportedly, omega fatty acids show various health benefits such as antimicrobial, anti-inflammatory, anticancer, hypocholesterolemic, hypotensive, hepatoprotective, antihistaminic, and antiarthritic activities. Palmitic acid is known as n-hexadecanoic acid, widely used in processed foods for improving texture and as natural additive. Anti-inflammatory activity of palmitate was also reported (30). Ascorbyl palmitate called as L-(+)-ascorbic acid 2,6-dihexadecanoate is a fat soluble form of vitamin C, which is used as a source of vitamin C and as an antioxidant food additive in many countries. Stearic acid known as octadecanoic acid, a common saturated fatty acid in nature is used to produce dietary supplements, cosmetics and detergents. It is also associated with lowering low-density lipoprotein cholesterol (31). Other compounds identified in n-hexane fraction, though they are in small amounts, also reportedly have antimicrobial, anti-diarrhoeal, analgesic, and cytotoxic activities. In addition, esters and aldehydes such as ethyl propanoate, propyl acetate, hexanal, and decadienal have characteristics aroma and thus are commonly used in fragrances and as a flavor additive in a wide varieties of products including foods and beverages. Thus, functional and pharmacological properties of S. apetala seeds are possibly due to the presence of polyphenols, flavonoids, anthocyanins, vitamin C, antioxidants (4), and the identified chemical compounds in HS. In previous work, health promoting polyphenols such as caffeic acid, (+)- catechin, (−)-epicatechin, ellagic acid, gallic acid, and quercetin were detected and quantified at 88.1, 1459.3, 310.1, 616.9, 416.7, and 71.8 mg/100 g of methanol fraction of seed (MeS), respectively (9). Reports showed that dietary fruit intake increased the concentration of serum salicylic acid and had beneficial effects on postprandial metabolic and inflammatory responses (32).
Fig. 5.
GC-MS chromatogram of the n-hexane fraction (HS) of Sonneratia apetala seed.
Table 2.
Chemical composition of n-hexane fraction (HS) of Sonneratia apetala seeds analyzed by GC-MS
| Peak no. | Retention time (min) | Final time (min) | Name of compound | Area (%) |
|---|---|---|---|---|
| 1 | 4.124 | 4.150 | Ethyl propanoate | 0.6 |
| 2 | 4.184 | 4.225 | n-Propyl acetate | 1.0 |
| 3 | 6.457 | 6.517 | n-Hexanal | 0.8 |
| 4 | 22.740 | 22.808 | 2,4-Decadienal | 0.3 |
| 5 | 27.390 | 27.450 | Oleic acid | 1.6 |
| 6 | 31.496 | 31.642 | 2-Methyldecahydronaphthalene | 1.3 |
| 7 | 36.696 | 36.742 | Methyl palmitate | 0.4 |
| 8 | 37.787 | 37.825 | Ascorbylpalmitate | 21.2 |
| 9 | 37.787 | 37.908 | Palmitic acid | 23.2 |
| 10 | 38.471 | 38.533 | Cyclodecacyclotetradecene, 14,15-didehydro-1,4,5,8,9,10,11,12,13,16,17,18,19,20-tetradecahydro– | 0.3 |
| 11 | 39.439 | 39.525 | Margaric acid | 0.4 |
| 12 | 39.926 | 39.967 | 8,11-Octadecadienoic acid, methyl ester | 0.4 |
| 13 | 40.024 | 40.067 | Oleic acid, methyl ester | 0.2 |
| 14 | 40.479 | 40.517 | Stearic acid, methyl ester | 0.2 |
| 15 | 41.005 | 41.217 | Linoleic acid | 29.9 |
| 16 | 41.433 | 41.542 | Stearic acid | 10.5 |
| 17 | 42.403 | 42.500 | Linoleic acid, methyl ester | 1.1 |
| 18 | 44.718 | 44.775 | Arachidic acid | 2.5 |
| 19 | 48.221 | 48.292 | Tetracosanoic acid | 0.7 |
| 20 | 56.656 | 56.733 | Cholesteryl bromide | 0.5 |
| 21 | 57.281 | 57.333 | Stigmasta-5,22-dien-3-ol, acetate | 0.3 |
| 22 | 57.734 | 57.800 | Stigmast-5-en-3-ol, oleate | 0.4 |
| 23 | 58.454 | 58.533 | β-Sitosterol acetate | 2.3 |
Nutraceutical and pharmacological properties of edible mangrove fruit of S. apetala are attributed to presence of various active compounds. Seeds fractions found to have potential as antibacterial, anti-diarrhoeal, analgesic, and cytotoxic constituents. Linoleic acid, palmitic acid, ascorbyl palmitate, and stearic acid constitute 85% of n-hexane fraction of S. apetala seed. Hence, seeds can be used as a good source of linoleic acid, a polyunsaturated omega-6 fatty acid, and oleic acid, a monounsaturated omega-9 fatty acid. Moreover, the seeds may also be used as a natural preservative owing to their significant activity against food spoilage organisms. Considering food security, primary health care, income generation, and coastal environment protection, such fruit crops cultivation could be a boom for farmers in the tidal sea-water intruded vast tropical coastal regions of the world. Also, availability of such raw ingredient will help in meeting the rising consumer demand for functional foods and dietary supplements.
ACKNOWLEDGEMENTS
The present research was supported by a grant received in 2012~2013 from the Ministry of Science and Technology, Government of the Peoples Republic of Bangladesh, which is gratefully acknowledged.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Das S, Siddiqi NA. Mangrove silviculture division, bulletin No. 2. Bangladesh Forest Research Institute; Chittagonge, Bangladesh: 1985. The mangroves and mangrove forests of Bangladesh. [Google Scholar]
- 2.Tomlinson PB. The botany of mangroves. Cambridge University Press; Cambridge, UK: 1994. [Google Scholar]
- 3.Das S, Ghose M. Seed structure and germination pattern of some Indian mangroves with taxonomic relevance. Taiwania. 2003;48:287–298. [Google Scholar]
- 4.Hossain SJ, Basar MH, Rokeya B, Arif KMT, Sultana MS, Rahman MH. Evaluation of antioxidant, antidiabetic and antibacterial activities of the fruit of Sonneratia apetala (Buch.-Ham.) Orient Pharm Exp Med. 2013;13:95–102. doi: 10.1007/s13596-012-0064-4. [DOI] [Google Scholar]
- 5.Bandaranayake WM. Traditional and medicinal uses of mangroves. Mangroves and Salt Marshes. 1998;2:133–148. doi: 10.1023/A:1009988607044. [DOI] [Google Scholar]
- 6.Teja VP, Ravishankar K. Preliminary phytochemical investigation and in vitro antimicrobial activity of ethanol extract of Sonneratia apetala. Int Res J Pharm. 2013;4:84–87. doi: 10.7897/2230-8407.04619. [DOI] [Google Scholar]
- 7.Patra JK, Das SK, Thatoi H. Phytochemical profiling and bioactivity of a mangrove plant, Sonneratia apetala, from Odisha Coast of India. Chin J Integr Med. 2015;21:274–285. doi: 10.1007/s11655-014-1854-y. [DOI] [PubMed] [Google Scholar]
- 8.Shefa AA, Baishakhi FS, Islam S, Sadhu SK. Phytochemical and pharmacological evaluation of fruits of Sonneratia apetala. GJMR-B–Pharma, Drug Discovery, Toxicology and Medicine. 2014;14:1–6. [Google Scholar]
- 9.Hossain SJ, Iftekharuzzaman M, Haque MA, Saha B, Moniruzzaman M, Rahman MM, Hossain H. Nutrient compositions, antioxidant activity, and common phenolics of Sonneratia apetala (Buch.-Ham.) fruit. Int J Food Prop. 2016;19:1080–1092. doi: 10.1080/10942912.2015.1055361. [DOI] [Google Scholar]
- 10.Hossain SJ, Pervin T, Suma SA. Effects of cooking methods at different time durations on total phenolics and antioxidant activities of fresh and dried-stored fruits of Sonneratia apetala (Buch.-Ham.) Int Food Res J. 2016;23:556–563. [Google Scholar]
- 11.Shoba FG, Thomas M. Study of antidiarrhoeal activity of four medicinal plants in castor-oil induced diarrhoea. J Ethnopharmacol. 2001;76:73–76. doi: 10.1016/S0378-8741(00)00379-2. [DOI] [PubMed] [Google Scholar]
- 12.Koster R, Anderson M, De Beer EJ. Acetic acid-induced analgesic screening. Fed Proc. 1959;18:412. [Google Scholar]
- 13.Eddy NB, Leimbach D. Synthetic analgesics. II. Dithienylbutenyl-and dithienylbutylamines. J Pharmacol Exp Ther. 1953;107:385–393. [PubMed] [Google Scholar]
- 14.McLaughlin JL. Crown gall tumors on potato disc and brine shrimp lethality: two simple assays for higher plant screening and fractionation. In: Dey PM, Harborne JB, Hostettman K, editors. Methods in Plant Biochemistry Assays for Bioactivity. Vol. 6. Academic Press; San Diego, CA, USA: 1991. pp. 1–32. [Google Scholar]
- 15.Bobbarala V, Vadlapudi V, Naidu KC. Mangrove plant Sonneratia apetala antimicrobial activity on selected pathogenic microorganisms. Orient J Chem. 2009;25:445–447. [Google Scholar]
- 16.Arif KMT, Datta RK, Sarower MG, Hossain SJ. Comparison of phenolics and antibacterial activity of commonly used antidiabetic medicinal plants in Bangladesh. PharmacologyOnline. 2014;1:7–15. [Google Scholar]
- 17.Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pierce NF, Carpenter CC, Jr, Elliott HL, Greenough WB., 3rd Effects of prostaglandins, theophylline, and cholera exotoxin upon transmucosal water and electrolyte movement in the canine jejunum. Gastroenterology. 1971;60:22–32. [PubMed] [Google Scholar]
- 19.Saito T, Miyake M, Toba M, Okamatsu H, Shimizu S, Noda M. Inhibition by apple polyphenols of ADP-ribosyltransferase activity of cholera toxin and toxin-induced fluid accumulation in mice. Microbiol Immunol. 2002;46:249–255. doi: 10.1111/j.1348-0421.2002.tb02693.x. [DOI] [PubMed] [Google Scholar]
- 20.Collier HO, Dinneen LC, Johnson CA, Schneider C. The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br J Pharmacol Chemother. 1968;32:295–310. doi: 10.1111/j.1476-5381.1968.tb00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whittle BA. The use of changes in capillary permeability in mice to distinguish between narcotic and nonnarcotic analgesics. Br J Pharmacol Chemother. 1964;22:246–253. doi: 10.1111/j.1476-5381.1964.tb02030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yaksh TL, Rudy TA. Studies on the direct spinal action of narcotics in the production of analgesia in the rat. J Pharmacol Exp Ther. 1977;202:411–428. [PubMed] [Google Scholar]
- 23.Jiménez-Aspee F, Alberto MR, Quispe C, Soriano Mdel P, Theoduloz C, Zampini IC, Isla MI, Schmeda-Hirschmann G. Anti-inflammatory activity of copao (Eulychnia acida Phil., Cactaceae) fruits. Plant Foods Hum Nutr. 2015;70:135–140. doi: 10.1007/s11130-015-0468-7. [DOI] [PubMed] [Google Scholar]
- 24.Hossain SJ, Tsujiyama I, Takasugi M, Islam MA, Biswas RS, Aoshima H. Total phenolic content, antioxidative, anti-amylase, anti-glucosidase, and antihistamine release activities of Bangladeshi fruits. Food Sci Technol Res. 2008;14:261–268. doi: 10.3136/fstr.14.261. [DOI] [Google Scholar]
- 25.Tsujiyama I, Mubassara S, Aoshima H, Hossain SJ. Anti-histamine release and anti-inflammatory activities of aqueous extracts of citrus fruits peels. Orient Pharm Exp Med. 2013;13:175–180. doi: 10.1007/s13596-012-0093-z. [DOI] [Google Scholar]
- 26.Logarto Parra A, Silva Yhebra R, Guerra Sardiñas I, Iglesias Buela L. Comparative study of the assay of Artemia salina L. and the estimate of the medium lethal dose (LD50 value) in mice, to determine oral acute toxicity of plant extracts. Phytomedicine. 2001;8:395–400. doi: 10.1078/0944-7113-00044. [DOI] [PubMed] [Google Scholar]
- 27.Rieser MJ, Gu ZM, Fang XP, Zeng L, Wood KV, McLaughlin JL. Five novel mono-tetrahydrofuran ring acetogenins from the seeds of Annona muricata. J Nat Prod. 1996;59:100–108. doi: 10.1021/np960037q. [DOI] [PubMed] [Google Scholar]
- 28.Hossain SJ, Kato H, Aoshima H, Yokoyama T, Yamada M, Hara Y. Polyphenol-induced inhibition of the response of Na+/glucose cotransporter expressed in Xenopus oocytes. J Agric Food Chem. 2002;50:5215–5219. doi: 10.1021/jf020252e. [DOI] [PubMed] [Google Scholar]
- 29.Hossain SJ, Aoshima H, Koda H, Kiso Y. Review of functional studies of beverage components acting on the recombinant GABAA neuroreceptor, and Na+/glucose cotransporter-response using the Xenopus oocyte expression system and electrophysiological measurements. Food Biotechnol. 2007;21:237–270. doi: 10.1080/08905430701534081. [DOI] [Google Scholar]
- 30.Saeed NM, El-Demerdash E, Abdel-Rahman HM, Algandaby MM, Al-Abbasi FA, Abdel-Naim AB. Anti-inflammatory activity of methyl palmitate and ethyl palmitate in different experimental rat models. Toxicol Appl Pharmacol. 2012;264:84–93. doi: 10.1016/j.taap.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 31.Hunter JE, Zhang J, Kris-Etherton PM. Cardiovascular disease risk of dietary stearic acid compared with trans, other saturated, and unsaturated fatty acids: a systematic review. Am J Clin Nutr. 2010;91:46–63. doi: 10.3945/ajcn.2009.27661. [DOI] [PubMed] [Google Scholar]
- 32.Rinelli S, Spadafranca A, Fiorillo G, Cocucci M, Bertoli S, Battezzati A. Circulating salicylic acid and metabolic and inflammatory responses after fruit ingestion. Plant Foods Hum Nutr. 2012;67:100–104. doi: 10.1007/s11130-012-0282-4. [DOI] [PubMed] [Google Scholar]