Abstract
The individual Maillard reactions of glucose, glucosamine, cyclohexylamine, and benzylamine were studied at a fixed temperature of 120°C under different durations by monitoring the absorbance of the final products at 425 nm. Glucosamine was the most individually reactive compound, whereas the reactions of glucose, cyclohexylamine, and benzylamine were not significantly different from each other. Maillard reactions of reaction mixtures consisting of glucosamine-cyclohexylamine, glucosamine-benzylamine, glucose-cyclohexylamine, and glucose-benzylamine were also studied using different concentration ratios under different durations at a fixed temperature of 120°C and pH 9. Maillard reactions in the pairs involving glucosamine were observed to be more intense than those of the pairs involving glucose. Finally, with respect to the concentration ratios, it was observed that in most instances, optimal activity was realized, when the reaction mixtures were in the ratio of 1:1.
Keywords: Maillard reaction, glucose, glucosamine, cyclohexylamine, benzylamine
INTRODUCTION
Maillard reaction is a non-enzymatic browning reaction typically involving a reducing sugar and an amino acid, in the presence of heat, that is of paramount importance to the food flavouring industry. It is one of the most important and complex processes in food chemistry because many components are able to participate through different pathways that form a complex mixture of products (1).
The open chain form of the reducing sugar molecules present in aqueous solutions is very minimal (in the case of glucose, the open chain form present is less than 1% of the total sugar). It is therefore thought that the ring opening reaction is initiated by the presence of amino acids (2). The initial step involves the nucleophilic attack by the nitrogen atom of an amino compound on the electrophilic carbonyl group of an aldehyde or ketone leading to the formation of an N-substituted glycosylamine or a ketosamine. The N-substituted glycosylamine or ketosamine then undergoes rearrangement to form an Amadori rearrangement product if the reducing sugar is an aldose or a Heyns rearrangement product in case the reducing sugar is a ketose. Further reactions give rise to the formation of different intermediate products including reductones, furfurals, pyrazines, and a variety of other cyclic substances. In the last stages of Maillard reaction, brown high molecular polymers, known as melanoidins, are formed (3).
In the process, hundreds of different flavour compounds are created. These compounds, in turn, break down to form new flavour compounds. Each type of food has a very distinctive set of flavour compounds that are formed during Maillard reaction. These compounds are used by flavour scientists to create reaction flavours.
These series of reactions leading to non-enzymatic browning are the basis of the flavouring industry since the type of the amino acid determines the resulting flavour. Thus, the modern food industry relies on the application of Maillard reactions to produce many different types of food products (4). However, some changes caused by Maillard reaction may be undesirable and reduce the quality of a food product, making them both unpleasant to eat and in appearance.
The course of Maillard reaction is strongly affected by factors which influence the different chemical reactions involved. These include temperature, time, water activity, reactant source, and concentration (5), the type and ratio of reducing sugar (6,7), amino acids (7,8), pH (9), and food composition (10,11).
Ashoor and Zent (12) classified amino acids into three groups depending on the intensity of their browning when they were heated in an autoclave at 121°C for 10 min, under identical conditions, with each of the sugars such as D-ribose, D-glucose, D-fructose, α-lactose, and sucrose. The amino compounds were grouped into high, intermediate, and low browning producing groups. The most reactive amino acids were lysine, glycine, tryptophan, and tyrosine. O’Brien and Morrissey (13) reported that lysine appears to be the most reactive amino acid due to the fact that it has two available amino groups.
This study involved four compounds, namely glucose, glucosamine, cyclohexylamine, and benzylamine (14,15). Glucose has an aldehyde moiety that provides an electrophilic carbonyl group whereas cyclohexylamine and benzylamine have a nucleophilic amino group. Glucosamine is special in this case since it has both an aldehyde as well as an amino group. It was envisaged that under proper conditions, Maillard reaction can occur under different combinations of the four compounds.
The objective of this study was therefore to study the development of Maillard reactions between glucose, glucosamine, cyclohexylamine, and benzylamine at a constant temperature of 120°C and initial pH of 9 under varied experimental conditions of time and initial reactant concentration ratios.
MATERIALS AND METHODS
Materials
D-Glucose, sodium hydroxide, and hydrochloric acid were purchased from Junsei Chemical Co., Ltd. (Tokyo, Japan). D-Glucosamine was purchased from Jiangsu Jiushoutang Organisms Manufacture Co., Ltd. (Xinghua, Jiangsu, China). Cyclohexylamine and benzylamine were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA).
Individual Maillard reactions
Aliquots of 5 mL of each of the reactants were prepared in concentrations of 0.1 M by dissolving their respective quantities in distilled water. They were then kept in covered test tubes in order to protect the aqueous solutions from evaporation. The reactants were then reacted individually at reaction times ranging from 1, 2, and 3 h. The temperature in this series of experiments was kept constant at 120°C by the use of a dry oven (Dongwon Scientific System, Busan, Korea). The reactions were conducted in triplicate.
Maillard reactions of reactions at different values
The reactants were grouped into four reaction mixtures: glucosamine-cyclohexylamine, glucosamine-benzylamine, glucose-cyclohexylamine, and glucose-benzylamine. In these experiments, the main factors for investigation were two folds of reaction times and initial reaction concentration ratios. The reaction times ranged from 0.5, 1.0, 1.5, 2.0, and 2.5 h. The ratio of the initial reactant concentrations ranged from 10:1, 5:1, 1:1, 1:5, and 1:10. The initial reaction pH was fixed at pH 9.0 using 0.1 M NaOH and 0.1 M HCl accordingly and verified by the use of a pH meter (Metrohm 827 pH lab, Metrohm AG, Herisau, Switzerland). The reactants were prepared at concentrations of 0.1 M by dissolving their respective quantities in distilled water. For each concentration ratio, the final concentration of the reactant with the smaller ratio was achieved by appropriate dilution using distilled water. Equivalent volumes of the reactants were then combined together in order to come up with the final reactants mixture to undergo the Maillard reaction. Aliquots of 5 mL of the reactants mixture at the different concentration ratios were prepared and kept in covered test tubes. The temperature was kept constant at 120°C in a dry oven (Dongwon Scientific System). The reactions were conducted in triplicate.
Measurement of browning degree of Maillard reaction
The degree of Maillard reaction was determined by reading the absorbance of the reaction media at the wavelength of 425 nm using a spectrophotometer (Ultrospec 2000, Amersham Pharmacia Biotech Ltd., Buckinghamshire, UK) after cooling down. In instances where the readings were too high, tenfold dilutions were made in order to achieve an optical density of less than 1.0. Distilled water was used as the standard for reference.
Statistical analysis
Data were subjected to two-way analysis of variance (ANOVA) to evaluate significant difference within and between factors using the Statistical Analysis System (SAS) software (version 9.1.3, SAS Institute Inc., Cary, NC, USA). The Fisher Least Significant Difference (LSD) test was used for multiple comparisons of the factors with the level of significance fixed at 5%.
RESULTS AND DISCUSSION
Individual Maillard reactions
Maillard reactions of the individual reactants consisting of glucosamine, glucose, cyclohexylamine, and benzylamine in a way proceeded following the 120°C and different times of 1, 2 and 3 h. The main effects (Fig. 1) showed significant differences between the reactions of the individual reactants, whereas the LSD multiple comparison tests confirmed the reaction of glucosamine to be significantly different from the rest (P<0.05). The reactions of cyclohexylamine, glucose, and benzylamine were not significantly different from each other (P>0.05).
Fig. 1.
Absorbance of individual Maillard reaction products of glucose, glucosamine, cyclohexylamine, and benzylamine, at concentrations of 0.1 M, different times, and a fixed temperature of 120°C.
The plots of the change in absorbance against time (Fig. 1) show a general increase in the rate of the reaction for all the reactants. After 3 h of the Maillard reaction, there was a marked difference in the plots. Glucosamine is clearly pictured as the most individually reactive of the four reactants, and this attribute can readily be explained by its possession of both an amino acid as well as an aldehyde moiety. Hence, the nucleophilic attack by the nitrogen atom of the amino group on the electrophilic carbonyl group of the aldehyde was most intense in glucosamine. It is worthwhile to note that the Maillard reactions of cyclohexylamine and benzylamine seemed to be more intense than that of glucose, albeit not significantly different. This may be accounted for by the presence of the amino groups in the amines which seem to be more reactive upon application of heat than the aldehyde group in glucose.
Glucosamine and cyclohexylamine
The Maillard reactions of glucosamine and cyclohexylamine at different concentration ratios showed some very interesting trends. The main effects (Fig. 2A) showed significant differences between the concentration ratios (P<0.05). The LSD multiple comparison tests showed the 10:1 concentration ratio to be the best followed by the 1:1, 5:1, 1:5, and 1:10 concentration ratios respectively. The 1:5 and 1:10 concentration ratios were not significantly different from each other (P>0.05). Again, it is easily noted that the Maillard reaction of the reaction pair was directly influenced by concentration.
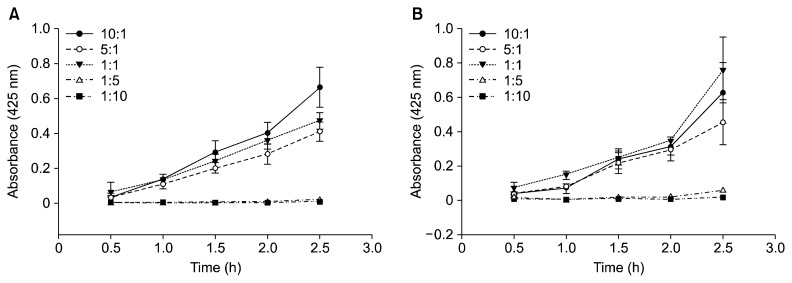
Fig. 2.
The absorbance of Maillard reaction products of glucosamine and cyclohexylamine (A) and glucosamine and benzylamine (B) at different concentration ratios at different times, the pH and temperature being fixed at pH 9.0 and 120°C respectively. The aqueous solutions of the products of the latter were diluted tenfold prior to taking the readings.
It is worthwhile to note that the absorbance readings of the final products in this reaction were higher than 1.0 and hence they had to be diluted tenfold in order to obtain accurate readings. The extent of the Maillard reaction in this particular reaction pair may be attributed to the amino groups present in each of the reactants and the aldehyde group inherent in glucosamine. The trend of the effect of the concentration ratios in this model may be compared to that of the glucose-lysine model system studied by Warmbier et al. (16) that showed that the rate of the Maillard reaction was optimum when the concentration ratio of glucose to lysine was 1:3.
Glucosamine and benzylamine
Like the case of Maillard reactions of glucosamine and cyclohexylamine, the main effects in this case (Fig. 2B) also showed significant differences between the concentration ratios (P<0.05). In this case, however, the LSD multiple comparison tests showed the 1:1 concentration ratio to be the best, followed by the 10:1, 5:1, 1:5, and 1:10 concentration ratios, respectively. The 10:1 and 5:1 concentration ratios were not significantly different from each other (P>0.05).
Maillard reactions of this reaction mixture also exhibited absorbance readings of the final products higher than 1.0 and hence they also had to be diluted tenfold in order to obtain accurate readings. Cyclohexylamine and benzylamine have similar structures, and it was anticipated that their reactions with glucosamine would follow a similar pattern. Minor deviations would be expected in their reactions with glucosamine due to the major structural difference between the two compounds. This is brought about by the presence of a benzene ring in benzylamine, a feature that is missing in the structure of cyclohexylamine. This could account for the minor deviations pertaining to the order of the reactivity of their different concentration ratios with respect to glucosamine.
Glucose and cyclohexylamine
In the Maillard reactions of glucose and cyclohexylamine, the main effects (Fig. 3A) showed significant differences between the concentration ratios (P<0.05). The LSD multiple comparison tests showed the 1:1 concentration ratio to be the best followed by the 5:1, 10:1, 1:10, and 1:5 concentration ratios, respectively. The 5:1 and 10:1 concentration ratios were not significantly different from each other (P>0.05).
Fig. 3.
The absorbance of Maillard reaction products of glucose and cyclohexylamine (A) and glucose and benzylamine (B), at different concentration ratios at different times, the pH, and temperature being fixed at pH 9.0 and 120°C respectively. The aqueous solutions of the products of the latter were diluted tenfold prior to taking the readings.
Unlike in the reactions of glucosamine and cyclohexylamine, these reactions generally proceeded at a slower rate as there was no need to subject the final products to dilutions as their optical densities were less than 1.0. This was in accordance with the expectations, and it is probably explained by the fact that glucose possesses only the aldehyde group unlike glucosamine, which has the amino group in addition to the aldehyde group. The Maillard reactions of glucose have also been generally observed to be slow compared to those of other sugars (17).
Glucose and benzylamine
In the Maillard reactions of glucose and benzylamine, the main effects (Fig. 3B) showed significant differences between the concentration ratios (P<0.05). The LSD multiple comparison tests showed the 1:1 concentration ratio to be the best followed by the 5:1, 1:5, 10:1, and 1:10 concentration ratios, respectively. All the concentration ratios were significantly different from each other (P<0.05).
These results are very similar to those of the Maillard reaction between glucose and cyclohexylamine. One thing in common between the two reactions is the concurrence with the 1:1 concentration ratio being the most significant. Again, there is a slight variation in the order of significance of the other concentration ratios which could be due to the structural differences between cyclohexylamine and benzylamine. Another similarity is that of the slowness of the Maillard reactions of both reaction pairs. As was the case with the reaction between glucose and cyclohexylamine, the optical densities of the reaction products in the reaction of glucose and benzylamine were also less than 1.0.
In terms of individual Maillard reactions, the reaction of glucosamine is significantly higher than those of cyclohexylamine, benzylamine and glucose at 120°C. As for the Maillard reactions of pairs, the reactions of glucosamine paired with either of cyclohexylamine and benzylamine were significantly more intense than those of glucose paired with the same amines at a temperature of 120°C and pH 9. With respect to the concentration ratios, it was observed that, in most instances, optimal activity is realized when the reaction pairs are in the ratio of 1:1.
ACKNOWLEDGEMENTS
This work was supported by a research grant from the Pukyong National University (2016).
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Moreno FJ, Molina E, Olano A, López-Fandiño R. High-pressure effects on Maillard reaction between glucose and lysine. J Agric Food Chem. 2003;51:394–400. doi: 10.1021/jf025731s. [DOI] [PubMed] [Google Scholar]
- 2.Yaylayan VA, Huyghues-Despointes A. Chemistry of Amadori rearrangement products: analysis, synthesis, kinetics, reactions, and spectroscopic properties. Crit Rev Food Sci Nutr. 1994;34:321–369. doi: 10.1080/10408399409527667. [DOI] [PubMed] [Google Scholar]
- 3.Rufián-Henares JA, Morales FJ. Effect of in vitro enzymatic digestion on antioxidant activity of coffee melanoidins and fractions. J Agric Food Chem. 2007;55:10016–10021. doi: 10.1021/jf0718291. [DOI] [PubMed] [Google Scholar]
- 4.Ames JM. Applications of the Maillard reaction in the food industry. Food Chem. 1998;62:431–439. doi: 10.1016/S0308-8146(98)00078-8. [DOI] [Google Scholar]
- 5.Jing H, Kitts DD. Chemical and biochemical properties of casein-sugar Maillard reaction products. Food Chem Toxicol. 2002;40:1007–1015. doi: 10.1016/S0278-6915(02)00070-4. [DOI] [PubMed] [Google Scholar]
- 6.Naranjo GB, Malec LS, Vigo MS. Reducing sugars effect on available lysine loss of casein by moderate heat treatment. Food Chem. 1998;62:309–313. doi: 10.1016/S0308-8146(97)00176-3. [DOI] [Google Scholar]
- 7.Yeboah FK, Alli I, Yaylayan VA. Reactivities of D-glucose and D-fructose during glycation of bovine serum albumin. J Agric Food Chem. 1999;47:3164–3172. doi: 10.1021/jf981289v. [DOI] [PubMed] [Google Scholar]
- 8.Morales FJ, Jiménez-Pérez S. Free radical scavenging capacity of Maillard reaction products as related to colour and fluorescence. Food Chem. 2001;72:119–125. doi: 10.1016/S0308-8146(00)00239-9. [DOI] [Google Scholar]
- 9.Ajandouz EH, Tchiakpe LS, Dalle Ore F, Benajiba A, Puigserver A. Effects of pH on caramelization and Maillard reaction kinetics in fructose-lysine model systems. J Food Sci. 2001;66:926–931. doi: 10.1111/j.1365-2621.2001.tb08213.x. [DOI] [Google Scholar]
- 10.Lerici CR, Barbanti D, Manzano M, Cherubin S. Early indicators of chemical changes in foods due to enzymic or non-enzymic browning reactions 1. Study on heat treated model systems. Lebensm Wiss Technol. 1990;23:289–294. [Google Scholar]
- 11.Tanaka M, Chiba N, Ishizaki S, Takai R, Taguchi T. Influence of water activity and Maillard reaction on the polymerization of myosin heavy chain in freeze-dried squid meat. Fish Sci. 1994;60:607–611. doi: 10.2331/fishsci.60.607. [DOI] [Google Scholar]
- 12.Ashoor AH, Zent JB. Maillard browning of common amino acids and sugars. J Food Sci. 1984;49:1206–1207. doi: 10.1111/j.1365-2621.1984.tb10432.x. [DOI] [Google Scholar]
- 13.O’Brien J, Morrissey PA. Nutritional and toxicological aspects of the Maillard browning reaction in foods. Crit Rev Food Sci Nutr. 1989;28:211–248. doi: 10.1080/10408398909527499. [DOI] [PubMed] [Google Scholar]
- 14.Nursten HE. Key mechanistic problems posed by the Maillard reaction. In: Finot PA, Aeschbacher HU, Hurrell RF, Liardon R, editors. The Maillard Reaction in Food Processing, Human Nutrition and Physiology. Birkhäuser Verlag GmbH; Basel, Switzerland: 1990. pp. 145–196. [DOI] [Google Scholar]
- 15.Josef K, Chris W, Tomas D, Imre B. Basic chemistry and process conditions for reaction flavors with particular focus on maillard-type reactions. In: Taylor AJ, Linforth RST, editors. Food Flavour Technology. Wiley-Blackwell; Oxford, UK: 2010. pp. 2–14. [Google Scholar]
- 16.Warmbier HC, Schnickles RA, Labuza TP. Effect of glycerol on nonenzymatic browning in a solid intermediate moisture model food system. J Food Sci. 1976;41:528–531. doi: 10.1111/j.1365-2621.1976.tb00663.x. [DOI] [Google Scholar]
- 17.Komthong P, Katoh T, Igura N, Shimoda M, Hayakawa I. Effect of high hydrostatic pressure combined with pH and temperature on glucose/fructose-leucine/lysine/glutamate browning reactions. J Fac Agric Kyushu Univ. 2003;48:135–142. [Google Scholar]