Abstract
Antioxidant rich green leafy vegetables including garden spinach leaf, water spinach leaf, Indian spinach leaf, and green leaved amaranth were selected to evaluate the effects of water boiling and oil frying on their total phenolic content (TPC), total flavonoid content (TFC), reducing power (RP), and antioxidant capacity. The results revealed that there was a significant increase in TPC, TFC, and RP in all the selected vegetables indicating the effectiveness of the cooking process on the antioxidant potential of leafy vegetables. Both cooking processes enhanced significantly (P<0.05) the radical scavenging ability, especially the oil fried samples showed the highest values. There is a significant reduction in the vitamin C content in all the vegetables due to boiling and frying except in the Indian spinach leaf. However, the present findings suggest that boiling and frying can be used to enhance the antioxidant ability, by increasing the bioaccessibility of health-promoting constituents from the four vegetables investigated in this study.
Keywords: leafy vegetables, boiling, frying, antioxidant
INTRODUCTION
Reactive oxygen species (ROS), including free radicals such as singlet oxygen, superoxide anion, hydroxyl radical, and hydrogen peroxide (H2O2) are continuously formed in the human body during cellular metabolism, such as energy production in the mitochondria, electron transport chain, phagocytosis, arachidonic acid metabolism, ovulation, fertilization, and in xenobiotic metabolism (1). These reactive species cause oxidative damage by reacting with nearly every molecule found in living cells (2) and play an important role in the development of several pathological conditions such as lipid peroxidation, protein oxidation, DNA damage, and cellular degeneration. Diseases such as atherosclerosis, carcinogenesis, diabetes, rheumatoid arthritis, osteoporosis, ulcers, sunburn, cataracts, and aging have been linked to oxygen radicals and ROS (3). Antioxidants constitute a diverse group of compounds with different properties. Antioxidants enzymes (naturally produced in the body) and antioxidant nutrients (found in foods) can scavenge or deactivate the reactive free radicals and repair oxidant-induced injury (4). Although there effective endogenous (catalase, superoxide dismutase, and glutathione peroxidase/reductase) and exogenous (vitamin C, E, and β-carotene) antioxidant defense mechanisms to protect the body against oxidant attacks, sometimes it cannot cope with the oxidant load in the body, and additional dietary antioxidants are needed. As a result, much attention focused on the use of antioxidants especially natural antioxidants to inhibit and prevent damage from free radicals and ROS. Plant derived foods are recognized as sources of natural antioxidants such as flavonoids and related phenolic compounds that combat oxidative stress in the body by maintaining a balance between oxidants and antioxidants (5,6). Among plant foods, green leafy vegetables and grains are rich sources of antioxidants apart from energy, protein, and selected micronutrients. Epidemiological studies have shown that intake of vegetables and fruits can protect humans against oxidative damage by inhibiting or quenching free radicals and reactive oxygen species (7).
In general, most of the green leafy vegetables undergo a cooking process prior to consumption on the basis of convenience and taste preference rather than retention of nutrient and health promoting compounds. Cooking can make food microbiologically safer to eat as well as to improve the edibility of the food. It also can induce significant changes in physical characteristics, chemical composition, and influence the concentration and bioavailability of bioactive compounds in vegetables (8–10). The nutritional value is increased or decreased depending on the cooking method. The cooking method not only affects the nutritional composition of the food, but also the level of available bioactive compounds.
However, information abounds on the antioxidant capacity of tropical fruits and vegetables (8,9,11,12), but data on the effects of cooking on the nutritional properties and antioxidant capacities of green leafy vegetables are still limited. It is important to know what happens to their antioxidant activity during common domestic cooking processes and how much of it is really retained after cooking. Therefore, this study was carried out to determine the effects of domestic cooking practices (boiling and frying) on the antioxidant qualities of four types of green leafy vegetables (garden spinach, water spinach, Indian spinach, and green leaved amaranth) with the hope that the results would guide to an optimum cooking method that results in the highest retention of the antioxidant capacity and radical scavenging activity of leafy vegetables to improve their functional activity.
MATERIALS AND METHODS
Plant materials
Four freshly harvested leafy vegetables: Spinacia oleracea L. (garden spinach leaf), Ipomoea aquatic Forssk (water spinach leaf), Basella rubra L. (Indian spinach leaf), and Amaranthus gangeticus L. (green leaved amaranth) were purchased from the local market of Dhaka, Bangladesh (Table 1) and transported to the laboratory. The taxonomy of the vegetables (13) shown in Table 1.
Table 1.
Experimental leafy vegetables
| Scientific name | English name | Local name |
|---|---|---|
| Spinacia oleracea L. | Garden spinach leaf | Palong Shak |
| Ipomoea aquatic Forssk | Water spinach leaf | Kalmi Shak |
| Basella rubra L. | Indian spinach leaf | Pui Shak |
| Amaranthus gangeticus L. | Green leaved amaranth | Data Shak |
Preparation of vegetable samples
Edible parts of leafy vegetables were washed with tap water, dried on a socking paper for 20 min, and chopped into small pieces. The chopped leaves were divided into three parts: 1) fresh (uncooked served as control), 2) boiled (100 g vegetables were boiled in 150 mL of water in a stainless steel pan and cooked until tender), and 3) fried (100 g vegetables were fried in a frying pan with 10 mL hot refined soybean oil until the sample became crisp-tender).
Preparation of vegetable extract
Fresh and cooked vegetables were blended for 5 min and 5 g blended sample was put in a homogenizer tube and 50 mL of 80% methanol was added. The mixture was homogenized and centrifuged at 10,000 rpm for 10 min. The supernatant was filtered through Whatman No. 1 filter paper. The filtrate was collected in amber bottles and stored at refrigerated temperature until analysis.
Reagents and standards
Methanol (80%), Folin-Ciocalteau’s reagent (10%), sodium carbonate (7.5%), standard gallic acid, ammonium chloride (10%), potassium acetate (1 M), tandard quercetin, 1,1-diphenyl-2-picryl hydrozyl radical (DPPH), standard Trolox, phosphate buffer (0.2 M, pH 6.6), potassium ferricyanide (1.0%), trichloroacitic acid (10%), and ferric chloride (0.1%) were prepared. All reagents and standards were supplied by Sigma-Aldrich Co., Munich, Germany.
Determination of total phenol content (TPC)
The TPC of the extracts was determined using the method reported by Singleton et al. (14). The extract was oxidized with 2.5 mL of 10% Folin-Ciocalteau’s reagent (v/v) and neutralized 2.0 mL of 7.5% sodium carbonate. The reaction mixture was incubated for 1.5 h at room temperature, and the absorbance was measured at 765 nm using a Spectro UV-Vis Dual Beam Model UVS-2700 (Labomed, Inc., Los Angeles, CA, USA). The total phenol content was subsequently calculated using gallic acid as standard. Results were expressed as milligram gallic acid equivalents (GAE)/100 g of sample.
Determination of total flavonoid content (TFC)
The TFC of the extracts was determined by the method of Ebrahimzadeh et al. (15). A 0.5 mL of appropriately prepared extract was mixed with 1.5 mL methanol, 0.1 mL of 10% AlCl3, 0.1 mL of 1 M potassium acetate, and 2.8 mL distilled water and incubated at room temperature for 30 min. The absorbance was measured at 415 nm using a Spectro UV-Vis Dual Beam Model UVS-2700 (Labomed, Inc.). The TFC was calculated using quercetin as standard, and values were expressed in terms of quercetin equivalents (mg QE/100 g) of the samples.
DPPH radical scavenging activity
The free radical scavenging ability of the extracts against DPPH radical was evaluated as described by Ebrahimzadeh et al. (16). A 450 μL of the extract was mixed with 4.5 mL of 300 μM methanolic solution containing the DPPH radical. The mixture was left in the dark for 30 min, and the absorbance was measured at 517 nm using a Spectro UV-Vis Dual Beam Model UVS-2700 (Labomed, Inc.). The antioxidant activity was expressed as the percentage of reduction of the initial DPPH absorption by test samples as follows:
where A0 is absorbance of the control and At is absorbance of the sample.
Determination of reducing power (RP)
The RP of the extract was determined according to the method of Oyaizu (17). A 2.0 mL aliquot was mixed with 2.0 mL of 0.2 M sodium phosphate buffer (pH 6.6) and 2.0 mL of 1% potassium ferricyanide. The mixture was incubated at 50°C for 30 min and after cooling, 2.0 mL of trichloroacetic acid (10%) was added to the mixture, which was then centrifuged at 3,000 rpm for 10 min. The upper layer of solution (5.0 mL) was mixed with 1.0 mL ferric chloride (0.1%), and the absorbance was measured at 700 nm using a Spectro UV-Vis Dual Beam Model UVS-2700 (Labomed, Inc.). The RP content was calculated using butylated hydroxytoluene (BHT) as standard. Results were expressed as milligram BHT equivalents/100 g of the samples.
Statistical analysis
Results are expressed as the means±standard deviations of three replicates and statistically analyzed using SPSS for Windows version 20.0 (SPSS Inc., Chicago, IL, USA). Differences between variables were tested for significance at the P<0.05 level using analysis of variance.
RESULTS AND DISCUSSION
Effect of cooking on TPC and TFC
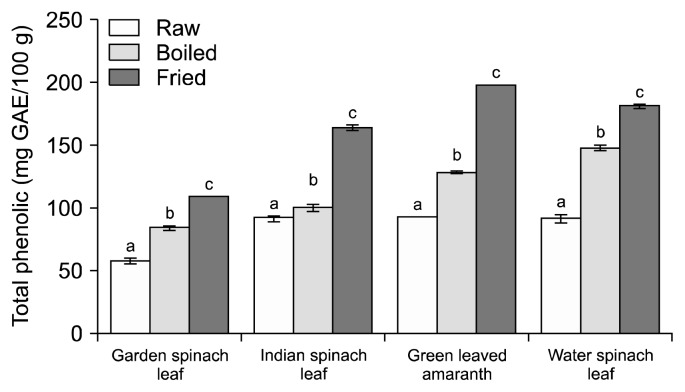
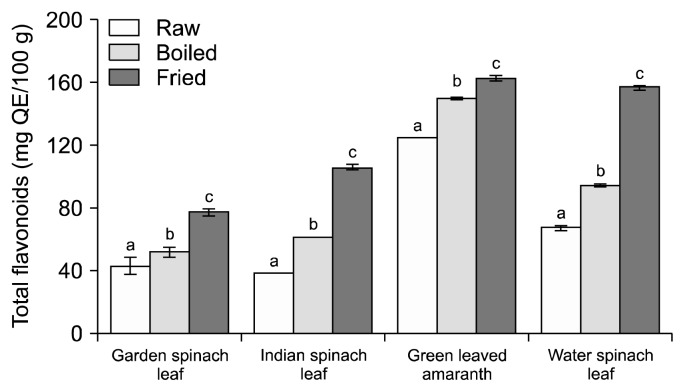
It is well known that phenolic compounds as well as flavonoids are the most abundant antioxidants in fruits and vegetables that reduce the incidence of chronic diseases, such as cardiovascular disease, diabetes, and cancer (18). Fig. 1 and 2 illustrate the effects of boiling and frying on the TPC and TFC in methanolic extracts from different green leafy vegetables.
Fig. 1.
Effect of cooking methods on total phenol content of green leafy vegetables. Data are means±standard deviations of three replicates. Different letters (a–c) for each vegetable are significantly different (P<0.05). GAE, gallic acid equivalents.
Fig. 2.
Cooking effects on flavonoids content of green leafy vegetables. Data are means±standard deviations of three replicates. Different letters (a–c) for each vegetable are significantly different (P<0.05). QE, quercetin equivalents.
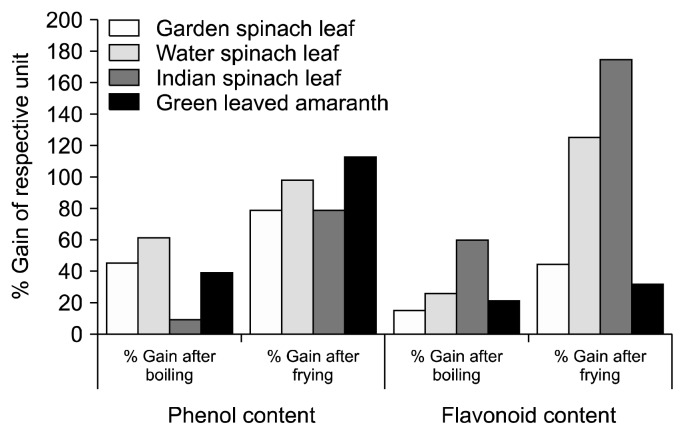
In fresh conditions, 3 leafy vegetables (green leaved amaranth, water spinach leaf, and Indian spinach leaf) had similar TPC (93.33, 92.14, and 91.95 mg GAE/100 g) whereas garden spinach leaf had the lowest (58.14 mg GAE/100 g). Accordingly, raw green leaved amaranth had the highest TFC (128.54 mg QE/100 g) followed by water spinach leaf, garden spinach leaf, and Indian spinach leaf. Cooking processes increased the TPC and TFC in all the studied leafy vegetables. Frying showed significantly higher TPC and TFC compared to raw and water boiling (Fig. 1 and 2) because the heating process makes available most of the phenolic compounds trapped in fiber of green leafy vegetables as explained by Adefegha and Oboh (19). The researchers also found that steam cooking resulted in a significant increase in TPC and TFC of 8 types of tropical green leafy vegetables. Accordingly, the percentage gain of TPC and TFC was significantly higher in cooked vegetables, especially in fried vegetables compared to boiled vegetables (Fig. 3). This might be due to the breakdown of cell walls and the release of bioactive compounds for easier absorption in the small intestine (6). Fried green leaved amaranth and Indian spinach leaf showed the highest percent gain of phenol content (111.99%) and flavonoid content (174.11%), respectively. Our results are in agreement with an earlier report by Dewanto et al. (20) where ferrulic acid, a phenolic found in the cell wall of grains such as corn, wheat, and oats, doubled after 10 min of cooking and increased by as much as 90% after 50 min of cooking. Several researchers have found similar trends of the changes in phenolics upon different types of cooking or heating systems. Ferracane et al. (21) observed that common cooking practices (i.e., boiling, steaming, and frying) increase the overall caffeoylquinic acid concentration of artichoke due to the formation of different dicaffeoylquinic acid isomers as evaluated by liquid chromatography-mass spectrometry (MS)/MS analysis. Moreover, heat treatment could increase free phenolic acids as detected by high-performance liquid chromatography in citrus peel extracts (22).
Fig. 3.
Effect of cooking methods on percentage of gain (% gain) of total phenol and flavonoids content in green leafy vegetables.
Effect of cooking on antioxidant activity of leafy vegetables
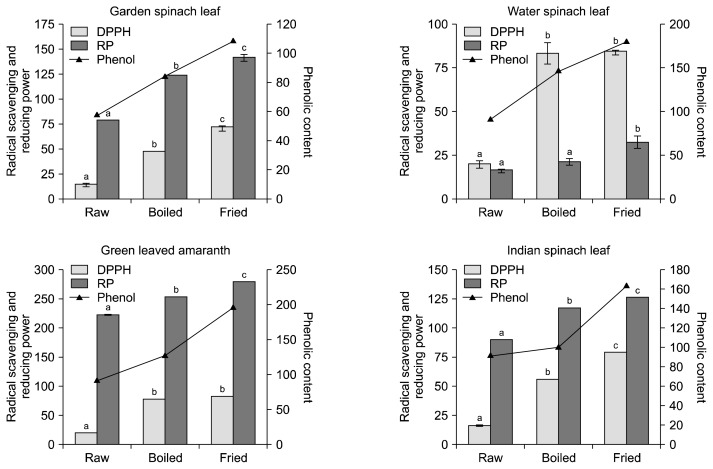
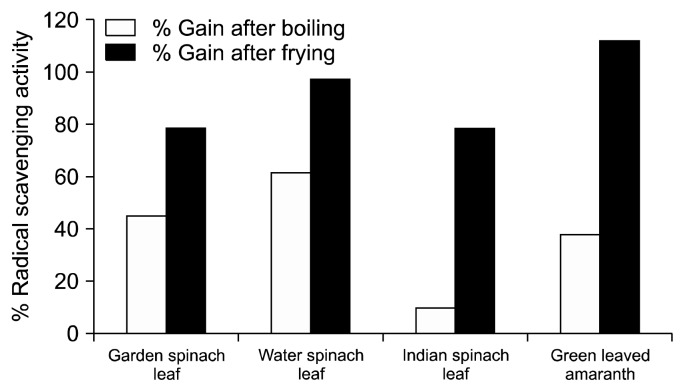
Phytochemicals present in foods protect our body from the deleterious effects of free radicals produced in the body by preventing their production or neutralizing free radicals or chelating the transition metal composition of foods (23). The DPPH free radical scavenging ability of raw and cooked extracts of the leafy vegetables are presented in Fig. 4. The results revealed that among the raw leafy vegetables, the methanolic extracts of water spinach leaf and green leaved amaranth had significantly higher DPPH radical scavenging activity than the other vegetables (Indian spinach leaf and garden spinach leaf). Both, water boiling and oil frying processes significantly increased (P<0.05) the radical scavenging ability compared to raw leafy vegetables, especially the fried samples showed the highest value. Fried green leaved amaranth showed highest percent gain in radical scavenging capacity (111.98%) among the 4 leafy vegetables (Fig. 5). Morales and Babel (24) suggested four possible reasons for the increase in antioxidant activity of vegetables after cooking: 1) the liberation of high amounts of antioxidant components due to the thermal destruction of cell walls and sub cellular compartments, 2) the production of stronger radical-scavenging antioxidants by thermal chemical reaction, and/or 3) suppression of the oxidation capacity of antioxidants by thermal inactivation of oxidative enzymes, 4) production of new non-nutrient antioxidants or the formation of novel compounds such as Maillard reaction products with antioxidant activity. In the present study, an increment in radical scavenging activity was found to be correlated with the increase of the TPC of the green leafy vegetables as illustrated in Fig. 4. This result also indicates that phenolics could be the main antioxidant phytochemicals in leafy vegetables. Several researchers found this correlation between the total phenol content of some plant foods and their antioxidant capacity (9,25–29). Hence, the results indicate that the cooked, especially fried vegetables, would be better for enhancing the antioxidant potential of the diet.
Fig. 4.
Effects of cooking on radical scavenging activity (%) and reducing power (mg butylated hydroxytoluene equivalents/100 g) of leafy vegetables in relation to total phenolic content (mg gallic acid equivalents/100 g). Data are means±standard deviations of three replicates. Different letters (a–c) for each vegetable are significantly different (P<0.05).
Fig. 5.
Percentage of gain of antioxidant activity in leafy vegetables subjected to cooking.
Effect of cooking on RP
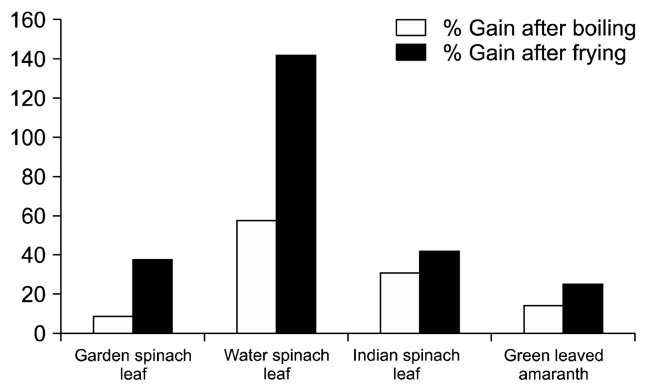
The antioxidants present in the vegetable extracts possess functional properties such as free radical scavenging and RP, along with other activities mitigating the devastating action of free radicals produced in the body. The reducing property is a significant mechanism for anti-oxidation potential of a food item (30). In the present study, the RP of raw and cooked extracts was assessed based on the ability to reduce Fe (III) to Fe (II), and the results are presented in Fig. 4. The results revealed that all the boiled and fried vegetables possesed significantly higher capacity to reduce free radicals than the raw samples. The increased RP might be due to the increase in TPC and TFC during cooking as shown in Fig. 1 and 2. Adefegha and Oboh (19) also found increased RP in 8 types of cooked tropical vegetables. The value of percent gain in RP also indicates the effectiveness of cooking process on the antioxidant potential of leafy vegetables. Among the 4 leafy vegetables, green leaved amaranth showed the highest DPPH in raw (19.73), boiled (77.03), and fried (81.99) extract indicating that the green leaved amaranth had the highest free radical-reducing potential (Fig. 4). Water spinach leaf showed maximum gain in RP after boiling and frying (57.66 and 141.99%), respectively (Fig. 6).
Fig. 6.
Percentage of gain of reducing power of different leafy vegetables after cooking.
Effect of cooking on vitamin C content
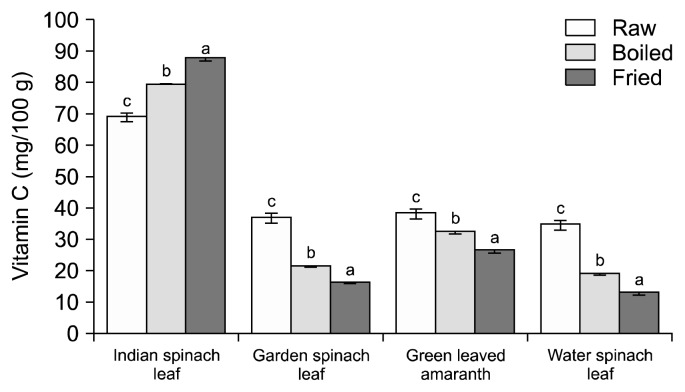
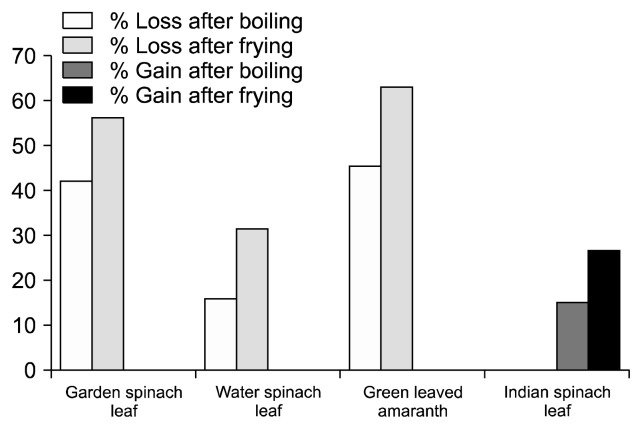
Vitamin C, a well-known potent dietary water soluble antioxidant, can react with peroxyl radicals, and help control lipid peroxidation of cellular membranes (23). Green leafy vegetables are excellent sources of vitamin C, although processing methods affect its stability because of its high water solubility and low resistance to heat treatment (31,32). Thus, the fate of the vitamin C levels in the studied 4 types of leafy vegetables is presented in Fig. 7. Among the 4 leafy vegetables, fresh (raw) Indian spinach leaf had the highest amount of vitamin C (69.33 mg/100 g) compared to the other vegetables. Whereas raw leaves of garden spinach leaf, green leaved amaranth, and water spinach leaf had vitamin C contents of 36.93, 38.33, and 34.66 mg/100 g, respectively. However, the three samples (garden spinach leaf, green leaved amaranth, and water spinach leaf) showed a similar trend as reported earlier that both boiling and frying significantly reduced the vitamin C content for some tropical vegetables and reported 47.5~82.4% loss in vitamin C content after blanching of vegetables (12,33). In our study, the maximum loss of vitamin C due to cooking was found in water spinach leaf followed by garden spinach leaf and green leaved amaranth. In all cases, frying caused greater loss than boiling. The only one green leafy vegetable, Indian spinach leaf, showed a reverse effect. There was a significant increase of vitamin C content in both, boiled and fried Indian spinach leaf samples (Fig. 7), and the percent gain due to boiling and frying were 14.81 and 26.43, respectively (Fig. 8). The apparent increase may be attributed to loss of soluble solids: the rate of diffusion of ascorbic acid out of the cell may be slower than that of other solids such as sugars (34). Another possible reason for the increment of vitamin C is that Indian spinach leaf leaves are an excellent source of mucilage (non-starch polysaccharide) that might slow down vitamin C degradation during cooking practices. However, there is no literature where any clear distinctions are drawn in favor of this finding. This poses an avenue for future research.
Fig. 7.
Effect of cooking methods on vitamin C content of leafy vegetables. Data are means±standard deviations of three replicates. Different letters (a–c) for each vegetable are significantly different (P<0.05).
Fig. 8.
Percentage of gain of vitamin C of different vegetables after cooking.
Finally, it can be concluded that the present study provides further evidence that leafy vegetables are rich in phenolics and flavonoid as antioxidants. Cooking has a determining effect on the levels of bioactive components and antioxidant capacities of vegetables. Although cooking results in vitamin C loss, this study provides the first report on the increase of vitamin C in cooked leafy vegetables (Indian spinach leaf). This study demonstrated that the oil frying process would be better for enhancing antioxidants and the free-radical scavenging potential of the 4 green leafy vegetables investigated in this study.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. Oxford University Press; Oxford, UK: 2007. pp. 440–487. [Google Scholar]
- 2.Cooke MS, Evans MD, Dizdaroglu M, Lunec J. Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J. 2003;17:1195–1214. doi: 10.1096/fj.02-0752rev. [DOI] [PubMed] [Google Scholar]
- 3.Gülçın İ, Oktay M, Kıreçcı E, Küfrevıoǧlu Öİ. Screening of antioxidant and antimicrobial activities of anise (Pimpinella anisum L.) seed extracts. Food Chem. 2003;83:371–382. doi: 10.1016/S0308-8146(03)00098-0. [DOI] [Google Scholar]
- 4.Chu YF, Sun J, Wu X, Liu RH. Antioxidant and antiproliferative activities of common vegetables. J Agric Food Chem. 2002;50:6910–6916. doi: 10.1021/jf020665f. [DOI] [PubMed] [Google Scholar]
- 5.Scalbert A, Manach C, Morand C, Rémésy C, Jiménez L. Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr. 2005;45:287–306. doi: 10.1080/1040869059096. [DOI] [PubMed] [Google Scholar]
- 6.Oboh G, Rocha JBT. Antioxidant in foods: a new challenge for food processors. In: Panglossi HV, editor. Leading Edge Antioxidants Research. Nova Science Publishers; New York, NY, USA: 2007. pp. 35–64. [Google Scholar]
- 7.Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci USA. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zia-ur-Rehman Z, Islam M, Shah WH. Effect of microwave and conventional cooking on insoluble dietary fibre components of vegetables. Food Chem. 2003;80:237–240. doi: 10.1016/S0308-8146(02)00259-5. [DOI] [Google Scholar]
- 9.Zhang D, Hamauzu Y. Phenolics, ascorbic acid, carotenoids and antioxidant activity of broccoli and their changes during conventional and microwave cooking. Food Chem. 2004;88:503–509. doi: 10.1016/j.foodchem.2004.01.065. [DOI] [Google Scholar]
- 10.Miglio C, Chiavaro E, Visconti A, Fogliano V, Pellegrini N. Effects of different cooking methods on nutritional and physicochemical characteristics of selected vegetables. J Agric Food Chem. 2008;56:139–147. doi: 10.1021/jf072304b. [DOI] [PubMed] [Google Scholar]
- 11.Oboh G, Akindahunsi AA. Change in the ascorbic acid, total phenol and antioxidant activity of sun-dried commonly consumed green leafy vegetables in Nigeria. Nutr Health. 2004;18:29–36. doi: 10.1177/026010600401800103. [DOI] [PubMed] [Google Scholar]
- 12.Oboh G. Effect of blanching on the antioxidant properties of some tropical green leafy vegetables. LWT-Food Sci Technol. 2005;38:513–517. doi: 10.1016/j.lwt.2004.07.007. [DOI] [Google Scholar]
- 13.Karmakar K, Muslim T, Rahman MA. Chemical composition of some leafy vegetables of Bangladesh. Dhaka Univ J Sci. 2013;61:199–201. doi: 10.3329/dujs.v61i2.17070. [DOI] [Google Scholar]
- 14.Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- 15.Ebrahimzadeh MA, Pourmorad F, Bekhradnia AR. Iron chelating activity, phenol and flavonoid content of some medicinal plants from Iran. Afr J Biotechnol. 2008;7:3188–3192. [Google Scholar]
- 16.Ebrahimzadeh MA, Hosseinimehr SJ, Hamidinia A, Jafari M. Antioxidant and free radical scavenging activity of Feijoa sellowiana fruits peel and leaves. Pharmacologyonline. 2008;1:7–14. [Google Scholar]
- 17.Oyaizu M. Studies on products of browning reaction: antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr Diet. 1986;44:307–315. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- 18.Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009;2:270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adefegha SA, Oboh G. Enhancement of total phenolics and antioxidant properties of some tropical green leafy vegetables by steam cooking. J Food Process Preserv. 2011;35:615–622. doi: 10.1111/j.1745-4549.2010.00509.x. [DOI] [Google Scholar]
- 20.Dewanto V, Wu X, Liu RH. Processed sweet corn has higher antioxidant activity. J Agric Food Chem. 2002;50:4959–4964. doi: 10.1021/jf0255937. [DOI] [PubMed] [Google Scholar]
- 21.Ferracane R, Pellegrini N, Visconti A, Graziani G, Chiavaro E, Miglio C, Fogliano V. Effects of different cooking methods on antioxidant profile, antioxidant capacity, and physical characteristics of artichoke. J Agric Food Chem. 2008;56:8601–8608. doi: 10.1021/jf800408w. [DOI] [PubMed] [Google Scholar]
- 22.Xu G, Ye X, Chen J, Liu D. Effect of heat treatment on the phenolic compounds and antioxidant capacity of citrus peel extract. J Agric Food Chem. 2007;55:330–335. doi: 10.1021/jf062517l. [DOI] [PubMed] [Google Scholar]
- 23.Rice-Evans C, Miller NJ. Antioxidants–the case for fruit and vegetables in the diet. Br Food J. 1995;97:35–40. doi: 10.1108/00070709510100163. [DOI] [Google Scholar]
- 24.Morales FJ, Babbel MB. Antiradical efficiency of Maillard reaction mixtures in a hydrophilic media. J Agric Food Chem. 2002;50:2788–2792. doi: 10.1021/jf011449u. [DOI] [PubMed] [Google Scholar]
- 25.Crozier A, Lean MEJ, McDonald MS, Black C. Quantitative analysis of the flavonoid content of commercial tomatoes, onions, lettuce, and celery. J Agric Food Chem. 1997;45:590–595. doi: 10.1021/jf960339y. [DOI] [Google Scholar]
- 26.Stewart AJ, Bozonnet S, Mullen W, Jenkins GI, Lean ME, Crozier A. Occurrence of flavonols in tomatoes and tomato-based products. J Agric Food Chem. 2000;48:2663–2669. doi: 10.1021/jf000070p. [DOI] [PubMed] [Google Scholar]
- 27.Ismail A, Marjan ZM, Foong CW. Total antioxidant activity and phenolic content in selected vegetables. Food Chem. 2004;87:581–586. doi: 10.1016/j.foodchem.2004.01.010. [DOI] [Google Scholar]
- 28.Sahlin E, Savage GP, Lister CE. Investigation of the antioxidant properties of tomatoes after processing. J Food Compos Anal. 2004;17:635–647. doi: 10.1016/j.jfca.2003.10.003. [DOI] [Google Scholar]
- 29.Turkmen N, Sari F, Velioglu YS. The effect of cooking methods on total phenolics and antioxidant activity of selected green vegetables. Food Chem. 2005;93:713–718. doi: 10.1016/j.foodchem.2004.12.038. [DOI] [Google Scholar]
- 30.Meir S, Kanner J, Akiri B, Philosoph-Hadas S. Determination and involvement of aqueous reducing compounds in oxidative defense systems of various senescing leaves. J Agric Food Chem. 1995;43:1813–1819. doi: 10.1021/jf00055a012. [DOI] [Google Scholar]
- 31.El-Ishaq A, Obirinakem S. Effect of temperature and storage on vitamin C content in fruits juice. Int J Chem Biomol Sci. 2015;1:17–21. [Google Scholar]
- 32.Chuah AM, Lee YC, Yamaguchi T, Takamura H, Yin LJ, Matoba T. Effect of cooking on the antioxidant properties of coloured peppers. Food Chem. 2008;111:20–28. doi: 10.1016/j.foodchem.2008.03.022. [DOI] [Google Scholar]
- 33.Kao FJ, Chiu YS, Chiang WD. Effect of water cooking on antioxidant capacity of carotenoid-rich vegetables in Taiwan. J Food Drug Anal. 2014;22:202–209. doi: 10.1016/j.jfda.2013.09.010. [DOI] [Google Scholar]
- 34.Rickman JC, Barrett DM, Bruhn CM. Nutritional comparison of fresh, frozen and canned fruits and vegetables. Part 1. Vitamins C and B and phenolic compounds. J Sci Food Agric. 2007;87:930–944. doi: 10.1002/jsfa.2825. [DOI] [Google Scholar]