Abstract
Amygdalin contents of the seeds, endocarps, and mesocarps from three peach cultivars (i.e., Stone Peach, Hikawa Hakuho, and Bakhyang) were measured at three stages of fruit development (stone-hardening, fruit enlargement, and ripening). The peach samples were dried and defatted with a Soxhlet apparatus, reflux extracted with methanol, and analyzed using reverse phase high-performance liquid chromatography. During all fruit development stages, the amygdalin contents in the seeds were higher than those in the endocarps and mesocarps. The amygdalin contents of the Stone Peach were comparatively higher than the Hikawa Hakuho and Bakhyang (P<0.05). Further, the amygdalin contents during ripening were very low or not detected. Overall, the amygdalin contents of the three peach cultivar samples (seed, endocarp, and mesocarp) increased until the fruit enlargement stage and either remained constant or decreased during ripening.
Keywords: amygdalin, fruit development stages, peaches, HPLC, seeds
INTRODUCTION
Due to the abundance of sugar and organic acids, peaches are primarily consumed as fresh produce in Korea. In 2015, the peach production in Korea was 238,000 M/T, and 0.4% was processed (1). Peaches are processed as jam and jelly and fermented to alcoholic drinks (2–4). Unripe peaches contain more nutrients than ripe peaches, such as organic acids, minerals, and polyphenols (5). In Korea, unripe peaches are thinned out and discarded in the orchard mostly from early April to late May. Some studies were carried out on the chemical composition (5–7) and utilization of unripe peaches as functional materials (8,9). Peach seeds were also studied in regard to their use in the prevention of atherosclerosis (10), and were also processed to functional materials (11). Amygdalin (D-mandelonitrile-β-D-glucoside-6-β-D-glucoside) is a cyanogenic glycoside plant toxin contained in relatively high concentrations in the kernels and seeds of apples, apricots, almonds, cherries, and peaches (12,13), and it is abundant in plum seeds (14). Notably, amygdalin is hydrolyzed to HCN, benzaldehyde, and D-glucose and can cause acute intoxication and chronic human central nervous system maladies (15,16). The quantitative profile of amygdalin content during peach development should be studied in order to utilize unripe peaches as functional materials, and to enhance the quality of processed peach products. In this study, the amygdalin contents of three cultivars of peach seeds, endocarps, and mesocarps were analyzed during fruit development (stone-hardening stage, fruit enlargement stage, and ripening period).
MATERIALS AND METHODS
Sample materials and reagents
Three cultivars of peaches [Stone Peach (P1), Hikawa Hakuho (P2), and Baekhyang (P3)] were obtained from the Cheongdo Peach Experimental Station of Gyeongsangbuk-do Agricultural Research Extension Services (Cheongdo, Korea). Table 1 shows the harvest time of each cultivar. Fruits with uniform colors and sizes were selected and divided into mesocarps, endocarps, and seeds. The samples were then air-dried in the shade. Amygdalin standards were purchased from Sigma Co. (St. Louis, MO, USA), and solvents for high-performance liquid chromatography (HPLC) analysis were purchased from J.T. Baker (Phillipsburg, NJ, USA). All other chemicals used in this study were obtained from Duksan Co. (Ansan, Korea).
Table 1.
Harvest date of peaches at different fruit development stages
| Cultivar1) | Fruit development stages | |||
|---|---|---|---|---|
|
| ||||
| Stone-hardening | Fruit enlargement | Ripening | ||
| P1 | Harvest date | Jun 17th | Aug 8th | Sep 16th |
| Average fruit weight | 18.2 | 49.2 | 84.2 | |
| P2 | Harvest date | Jun 17th | Jul 2nd | Jul 8th |
| Average fruit weight | 61.4 | 139.1 | 193.1 | |
| P3 | Harvest date | Jun 17th | Jul 29th | Aug 26th |
| Average fruit weight | 56.9 | 178.3 | 337.9 | |
P1, Stone Peach; P2, Hikawa Hakuho; P3, Baekhyang.
Amygdalin extraction and HPLC analysis
The air-dried peach seeds, endocarps, and mesocarps were pulverized, screened with a 60-mesh sieve, and defatted with ethyl ether for 3 h using a Soxhlet apparatus. After evaporating the solvent and drying in an oven, the defatted powders were reflux extracted with methanol at 60°C for 6 h, filtered, concentrated with a rotary evaporator, filtered, and then injected into an HPLC instrument (LC-10A, Shimadzu Co., Kyoto, Japan). The HPLC column was a SupelcosilTM LC-18-S (φ 4.6×250 mm, Supelco, Bellefonte, PA, USA), and the samples were analyzed at a UV wavelength of 210 nm with 20% methanol (0.7 mL/min) as the mobile phase (17). The amygdalin contents were determined with linear regression methods using an amygdalin standard curve.
Statistical analysis
All measured values are reported as mean±standard deviation, and evaluated by the analysis of variance (ANOVA), followed by Duncan’s multiple range test (P<0.05) using the Statistical Analysis System software package (version 9.3, SAS Institute Inc., Cary, NC, USA).
RESULTS AND DISCUSSION
Amygdalin analysis by HPLC
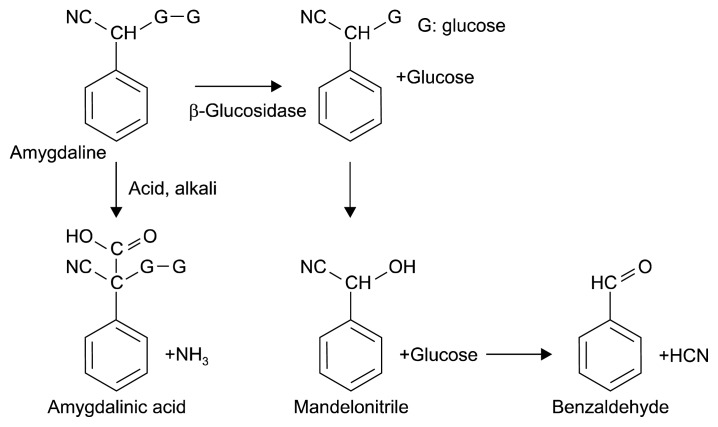
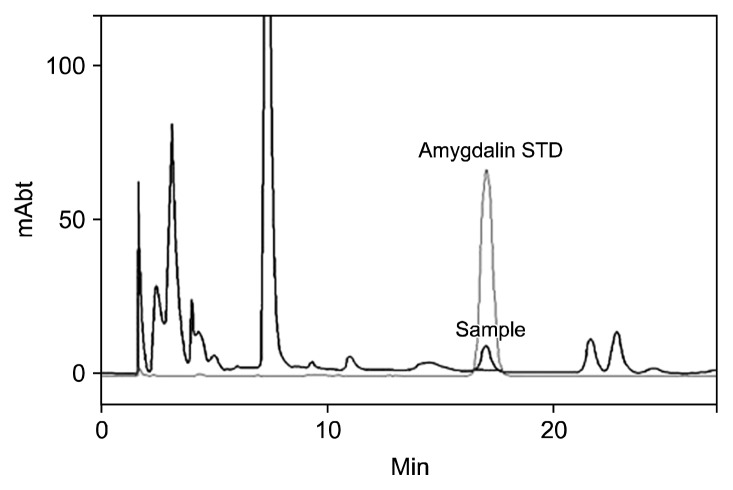
As a cyanogenic glycoside, amygdalin is enzymatically hydrolyzed and ultimately transformed into benzaldehyde and HCN (Fig. 1). The total cyanide content used to be determined using the picrate and acid hydrolysis method for the quantification of cyanogenic glycosides (18). However, since the development of reverse phase HPLC (19), this newer approach has been used to quickly determine the amygdalin content in a variety of matrices (20). Fig. 2 shows the reverse phase HPLC chromatograms of the amygdalin standard and a P1 seed. Amygdalin was isolated fairly well in the reverse phase C18 column with a 20% methanol mobile phase. Amygdalin contents were obtained from the linear regression curve of the standard amygdalin contents.
Fig. 1.
Amygdalin decomposition.
Fig. 2.
HPLC chromatograms of amygdalin standard (amygdalin STD) and Stone Peach seed extract (sample; June 17th).
Amygdalin contents of peaches during fruit development
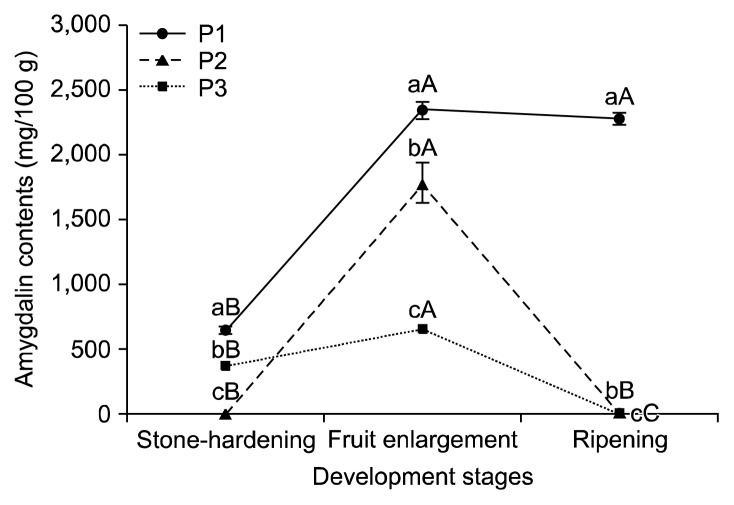
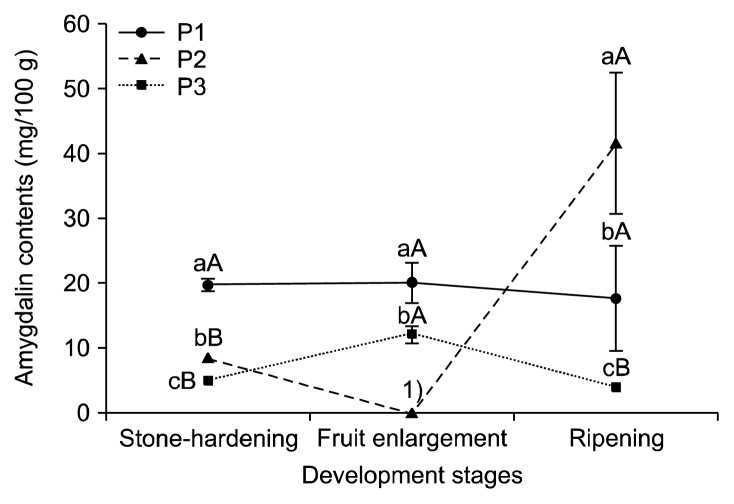
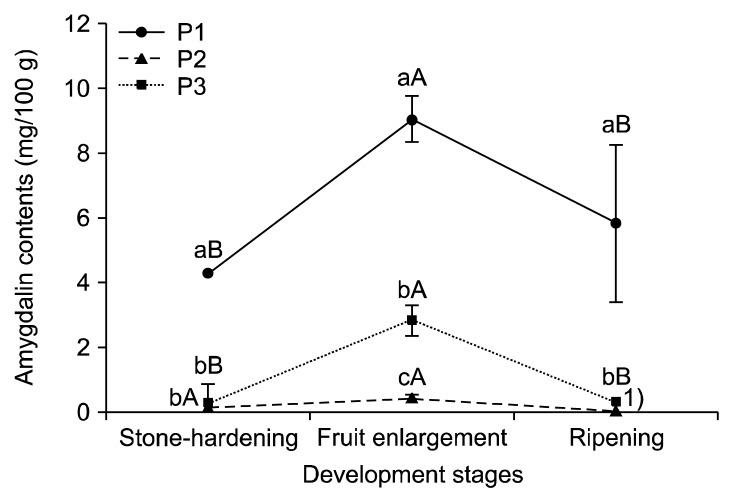
Amygdalin is mainly contained in the seeds of Rosaceae and is catabolized upon germination as a source of nitrogen and carbon (21) for later use during flowering and fruit development (22). The amygdalin contents in the seeds, endocarps, and mesocarps from three peach cultivars during stone-hardening, fruit enlargement, and ripening stages are shown in Fig. 3, 4, and 5, respectively. The amygdalin contents in the seeds of the three cultivars increased until the fruit enlargement stage. During the ripening period, the amygdalin contents of the P1 remained constant, but decreased largely for the P2 and P3. In the endocarp of P1, the amygdalin contents showed no significant differences among the fruit development stages. In P3, the amygdalin contents increased slightly to the fruit enlargement stage. But the amygdalin contents were not detected in P2. During the ripening period, the amygdalin contents increased in P2, but were not detected in P3. The amygdalin contents of the P1 and P3 mesocarps (flesh) increased until the fruit enlargement stage, and then decreased slightly during ripening. In contrast, no significant differences between the stone-hardening and fruit enlargement stages were observed for the amygdalin contents of the P2 mesocarps, and no amygdalin was detected during the ripening period. The amygdalin contents of the three peach cultivars depending on their parts at different development stages are shown in Table 2. Overall, the amygdalin contents in the seeds were reasonably higher than those in the endocarp and mesocarp. Relative to P2 and P3, P1 had the greatest amygdalin content (P<0.05). Additionally, the amygdalin contents increased until the fruit enlargement stage and kept constant or decreased slightly during ripening. Likewise, the amygdalin contents of four stone fruit species (apricot, peach, plum, and bitter apricot trees) also increased during fruit development and remained stable or decreased slightly when they ripened (17,23).
Fig. 3.
Amygdalin contents in the seeds of three peach cultivars during different fruit development stages. Means followed by the same letters within the same peaches (a–c) and the same stages (A–C) are not significantly different (P<0.05). P1, Stone Peach; P2, Hikawa Hakuho; P3, Baekhyang.
Fig. 4.
Amygdalin contents in the endocarps of three peach cultivars during different fruit development stages. Means followed by the same letters within the same peaches (a–c) and the same stages (A–C) are not significantly different (P<0.05). P1, Stone Peach; P2, Hikawa Hakuho; P3, Baekhyang. 1)Not detected.
Fig. 5.
Amygdalin contents in the mesocarps of three peach cultivars during different fruit development stages. Means followed by the same letters within the same peaches (a–c) and the same stages (A–C) are not significantly different (P<0.05). P1, Stone Peach; P2, Hikawa Hakuho; P3, Baekhyang. 1)Not detected.
Table 2.
Amygdalin contents of three peach cultivars at different fruit development stages (unit: mg/100 g, DW)
| Fraction | Cultivar1) | Fruit development stage | ||
|---|---|---|---|---|
|
| ||||
| Stone-hardening | Fruit enlargement | Ripening | ||
| Seed | P1 | 654.19±51.46aB | 2,349.16±79.45aA | 2,311.88±62.57aA |
| P2 | 0.20±0.02fB | 1,792.65±158.20bA | 21.16±3.00cB | |
| P3 | 372.36±24.92bB | 669.67±66.58cA | 12.14±4.80cC | |
| Endocarp | P1 | 19.77±1.47c | 19.99±3.14d | 17.90±8.11c |
| P2 | 8.56±0.12dB | ND2) | 41.64±10.77bA | |
| P3 | 5.12±0.10eB | 12.04±1.75eA | 3.96±1.05dB | |
| Mesocarp | P1 | 4.29±0.10eB | 9.09±0.71fA | 5.85±2.45dB |
| P2 | 0.19±0.72f | 0.46±0.23h | ND | |
| P3 | 0.19±0.18fB | 2.88±0.48gA | 0.46±0.02eB | |
Means followed by the same letters within the column (a–h) and the row (A–C) are not significantly different (P<0.05).
P1, Stone Peach; P2, Hikawa Hakuho; P3, Baekhyang.
Not detected.
In summary, the amygdalin contents in the seeds, endocarps, and mesocarps of three peach cultivars (P1, P2, and P3) were determined during fruit development by reverse phase HPLC. For every stage and cultivar, the amygdalin contents in the peach seeds were greater than those in the endocarps and mesocarps (P<0.05). The amygdalin contents of the P1 were moderately greater than those of the other cultivars (P2 and P3). Notably, amygdalin contents were very low or not detected during the ripening periods of the peaches. Further, the amygdalin contents of the peaches increased until the fruit enlargement stage and remained constant or decreased slightly during the ripening periods.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Ministry of Agriculture, Food and Rural Affairs. The status of fruits processing in 2015. Sejong, Korea: 2016. [Google Scholar]
- 2.Yi SH, Ann YG, Choi JS, Lee JS. Development of peach fermented wine. Korean J Food Nutr. 1996;9:409–412. [Google Scholar]
- 3.Jo JW, Kim JK, Kim ID, Kim SD. Characteristics of peach wine prepared by using different cultivars. Korean J Postharvest Sci Technol. 2000;7:84–88. [Google Scholar]
- 4.Chung JH, Mok C, Lim S, Park YS. Changes of physicochemical properties during fermentation of peach wine and quality improvement by ulfilteration. J Korean Soc Food Sci Nutr. 2003;32:506–512. doi: 10.3746/jkfn.2003.32.4.506. [DOI] [Google Scholar]
- 5.Lee JB, Chung HS. Studies on the components of unripe peaches. Korean J Food Preserv. 2008;15:79–83. [Google Scholar]
- 6.McCready RM, McComb EA. Pectic constituents in ripe and unripe fruit. J Food Res. 1954;19:530–535. doi: 10.1111/j.1365-2621.1954.tb17485.x. [DOI] [Google Scholar]
- 7.Kim DM, Kim KH, Choi IJ, Yook HS. Composition and physicochemical properties of unripe Korean peaches according to cultivars. J Korean Soc Food Sci Nutr. 2012;41:221–226. doi: 10.3746/jkfn.2012.41.2.221. [DOI] [Google Scholar]
- 8.Kim HJ. MS Thesis. Korea University; Seoul, Korea: 2007. Isolation and characterization of active whitening compound from Prunus persica. [Google Scholar]
- 9.Kim DM, Kim KH, Kim YS, Koh JH, Lee KH, Yook HS. A study on the development of cosmetic materials using unripe peaches seed extracts. J Korean Soc Food Sci Nutr. 2012;41:110–115. doi: 10.3746/jkfn.2012.41.1.110. [DOI] [Google Scholar]
- 10.Yoon IH, Seo BI, Kim SH. The effect of Persicae Semen on the atherosclerosis in rabbit. J Herbol. 1996;11:79–98. [Google Scholar]
- 11.Ryu HM, Jeon DK, Kim SA, Chung HJ. Antioxidant and quality characteristics of mungbean starch gel added with peach seed powder. Korean J Food Preserv. 2013;20:372–378. doi: 10.11002/kjfp.2013.20.3.372. [DOI] [Google Scholar]
- 12.Nahrstedt A. Zur cyanogenese von Prunus avium. Phytochemistry. 1972;11:3121–3126. doi: 10.1016/S0031-9422(00)86360-8. [DOI] [Google Scholar]
- 13.Jones MB, Fleming ZW, Bailey LF. Cyanide as a growth inhibiting substance in extracts of peach leaves, bark, and flower buds. Proc Amer Soc Hort Sci. 1957;69:152–157. [Google Scholar]
- 14.Bolarinwa IF, Orfila C, Morgan MRA. Amygdalin content of seeds, kernels and food products commercially-available in the UK. Food Chem. 2014;152:133–139. doi: 10.1016/j.foodchem.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Concon JM. Food toxicology: principles and concepts. Marcel Dekker, Inc; New York, NY, USA: 1988. p. 74. [Google Scholar]
- 16.Kwon H, Jo Y. A study on the decomposition of amygdalin using an in vitro assay. J Toxicol Pub Health. 2007;23:47–53. [Google Scholar]
- 17.Viorica-Mirela G, Socaciu C, Jianu I, Florica R, Florinela F. Identification and quantitative evaluation of amygdalin from apricot, plum and peach oils and kernels. Buletin USAMV-CN. 2006;62:246–253. [Google Scholar]
- 18.Haque MR, Bradbury JH. Total cyanide determination of plants and foods using the picrate and acid hydrolysis methods. Food Chem. 2002;77:107–114. doi: 10.1016/S0308-8146(01)00313-2. [DOI] [Google Scholar]
- 19.Hwang EY, Lee JH, Lee YM, Hong SP. Reverse-phase HPLC separation of D-amygdalin and neoamygdalin and optimum conditions for inhibition of racemization of amygdalin. Chem Pharm Bull. 2002;50:1373–1375. doi: 10.1248/cpb.50.1373. [DOI] [PubMed] [Google Scholar]
- 20.Kim EJ, Lee HJ, Jang JW, Kim IY, Kim DH, Kim HA, Lee SM, Jang HW, Kim SY, Jang YM, Im DK, Lee SH. Analytical determination of cyanide in maesil (Prunus mume) extracts. Korean J Food Sci Technol. 2010;42:130–135. [Google Scholar]
- 21.Selmar D, Lieberei R, Biehl B. Mobilization and utilization of cyanogenic glycosides: the linustatin pathway. Plant Physiol. 1988;86:711–716. doi: 10.1104/pp.86.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swain E, Poulton JE. Utilization of amygdalin during seedling development of Prunus serotina. Plant Physiol. 1994;106:437–445. doi: 10.1104/pp.106.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao Y. Amygdalin content in four stone fruit species at different developmental stages. ScienceAsia. 2012;38:218–222. doi: 10.2306/scienceasia1513-1874.2012.38.218. [DOI] [Google Scholar]