Abstract
Objective
This systematic review was carried out to determine the effectiveness of continuous isoniazid (given for at least 36 months) for the treatment of latent tuberculosis infection (LTBI) in people living with HIV (PLHIV).
Methods
Six databases and HIV and tuberculosis (TB) conference abstract books were searched for randomized controlled trials that compared the effectiveness of continuous isoniazid treatment with 6 months of isoniazid application. Outcomes of interest were TB incidence, mortality, adverse events and risk of drug resistance. Data were pooled using fixed-effects meta-analysis.
Results
Three studies were included, from Botswana, South Africa and India. The risk of active TB was 38% lower among patients receiving continuous isoniazid compared with isoniazid regimen for 6 months [relative risk (RR) 0.62, 95% confidence interval (CI): 0.42–0.89; I2 = 0%], and 49% lower for those with a positive tuberculin skin test (TST) (RR 0.51, 95% CI: 0.30–0.86; I2 = 7%). Similarly, individuals with positive TST had a 50% lower chance of death (RR 0.50, 95% CI: 0.27–0.91; I2 = 3%). Two studies found no evidence of an increase in adverse events in the continuous isoniazid group, whereas a third study, that used a different definition for adverse events, found strong evidence of increase. There was no evidence of increased drug resistance when continuous isoniazid was given.
Conclusion
For PLHIV in settings with high TB and HIV prevalence and transmission, continuous isoniazid for at least 36 months is beneficial and probably outweighs the risk of increased adverse events compared with isoniazid regimen for 6 months.
Keywords: antiretroviral therapy, chemoprophylaxis, HIV/AIDS, isoniazid, prevention, re-infection, tuberculosis
Introduction
HIV infection is the strongest risk factor for tuberculosis (TB) disease in those with latent Mycobacterium tuberculosis infection [1]. Isoniazid preventive therapy (IPT) is a key intervention for the prevention of active TB infection among people living with HIV (PLHIV) [2], and is usually given for a period of 6 to 12 months [3]. However, the effectiveness of IPT in PLHIV in high burden TB settings may be limited to the period during which IPT is given, may have suboptimal efficacy in immunocompromised individuals [4] and does not protect against reinfection after therapy cessation [3]. Moreover, high rates of transmission and re-infection among PLHIV in settings with high prevalence of TB increase the risk of development of active TB [5]. In such settings, lifelong IPT may be beneficial for PLHIV.
We assessed whether providing IPT for at least 36 months to PLHIV reduced the risk of active TB compared with the standard 6-month course, and determined the impact of tuberculin skin test (TST) and antiretroviral therapy (ART) status. This study was undertaken to inform WHO guidelines for the delivery of IPT in settings with high prevalence of TB and HIV [6].
Methods
Using a predefined study protocol, the following databases were searched: Medline via Pubmed, Embase, Global Health Library (Regional databases), Central (Cochrane Library) and TRIP. Ongoing trials were searched via the WHO International Clinical Trials Registry Platform. The reference lists of relevant articles were checked for additional studies. We further searched the abstract books from the following conferences: Conference on Retroviruses and Opportunistic Infections, International AIDS Society Conferences on HIV, Interscience Conference on Antimicrobial Agents and Chemotherapy and the International Union against TB and Lung Diseases. We limited the search period from 1 January 2005 to 1 May 2015, and restricted to English, French and Spanish.
We included randomized controlled trials (RCTs) that evaluated the effectiveness of continuous isoniazid compared with 6 months of isoniazid application in adults living with HIV in preventing active TB infection. Article eligibility and data extraction were done in duplicate (S.D.B., A.M.), with risk of bias assessed using standard Cochrane methods.
The number of events of each prespecified outcome was compared between those receiving continuous isoniazid and those that received isoniazid for 6 months. For dichotomous data, a risk ratio with 95% confidence intervals (CIs) was calculated. We did fixed-effects meta-analysis for studies that were clinically and methodologically homogeneous using RevMan version 5. We explored heterogeneity between studies using the I2 statistic and visual inspection of forest plots. We carried out stratified analysis based on TST result. We used GRADE to assess the quality of the evidence.
Results
From an initial screen of 1120 records, 49 articles were reviewed in full and three studies, from Botswana [7], South Africa [8] and India [9], were included for review (Fig. 1). One study had a control group that received 300 mg isoniazid and 800 mg ethambutol for 6 months [9], whereas in the other two studies the control group received 300 mg isoniazid for 6 months [7,8]. In all the three studies, the intervention consisted of 300 mg isoniazid for 36 months. One study included only participants with TST not less than 5 mm and excluded those on ART [8]; participants in this study had a higher median CD4+ cell count at baseline (484 vs. 297 [7] and 325 cells/μl [9]). The other two studies were balanced with respect to TST status at baseline [7,9].
Fig. 1. Study selection process.
Two studies required a positive TB culture to confirm TB disease [8,9], whereas the third defined confirmed TB as either a positive culture or two or more sputum smears positive for acid fast bacilli (AFB) [7]. Probable TB was defined as the presence of signs and symptoms with AFB in a sputum smear in one study [8]; one sputum smear or biopsy specimen positive for AFB in another study [7]; and clinical, radiographic, histopathologic or biochemical features in the third study [9]. Two studies had a category of possible TB based on clinical presentation and response to anti-TB treatment [7,8].
Adherence was not consistently defined or reported. Overall, study quality was rated as high. The main concern with respect to risk of bias was that two out of three studies were not blinded, which may lead to ascertainment bias [10]. In one study, there was a 2 : 1 randomization of 6 month vs. continuous INH [8].
Meta-analysis
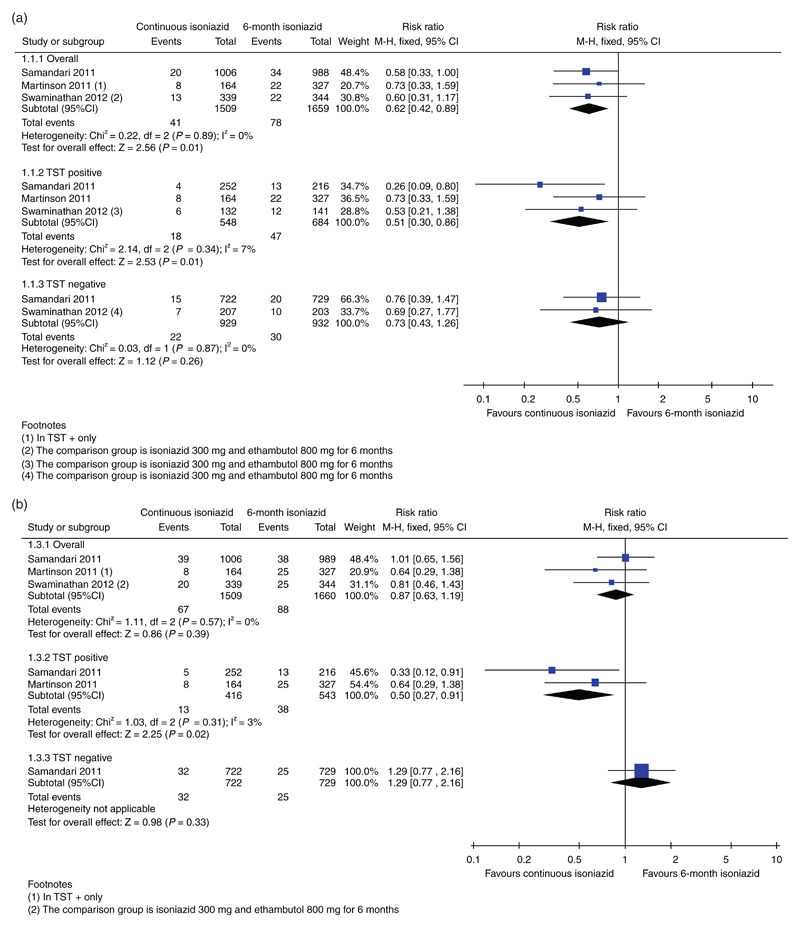
Active TB was diagnosed in 41 (2.7%) of 1509 individuals in the continuous isoniazid group and 78 (4.7%) of 1659 participants in the control group. The pooled relative risk (RR) of incident TB infection was 0.62 (95% CI: 0.42–0.89). There was no sign of heterogeneity (I2 = 0%). There was a stronger protective effect of continuous isoniazid on those with a positive TST (RR 0.51, 95% CI: 0.30–0.86); conversely, in those with negative TST, there was no evidence of a difference (RR 0.73, 95% CI: 0.43–1.26) (Fig. 2a). In sensitivity analysis assessing confirmed TB results only, the protective benefit of continuous isoniazid was similar, but there was no statistical evidence of a difference. The number needed to treat to prevent one case of active TB was 50 overall, 28 for TST positives and 125 for TST negatives.
Fig. 2.
(a) Forest plot showing the results of the fixed-effects meta-analysis for active TB (confirmed, probable and possible TB), overall results and stratified according to TST status. (b) Forest plot showing the results of the fixed-effects meta-analysis for death, overall results and stratified according to TST status.
There was no difference in mortality risk overall (RR 0.87, 95% CI: 0.63–1.19) but continuous isoniazid reduced mortality by 50% in TST-positive individuals (RR 0.50, 95% CI: 0.27–0.91) (Fig. 2b). Heterogeneity was low (I2 = 0%). Numerically, there were fewer deaths due to TB in the continuous isoniazid group (4/1345) compared with the 6-month group (8/1333) but this was not statistically different (RR 0.52, 95% CI: 0.17–1.64). The quality of the evidence for the outcomes resulting in active TB and death was considered to be low.
Isoniazid preventive therapy and antiretroviral therapy
Two studies excluded those who were eligible for ART [9,10]. In the third study few (2–3%) were on ART at enrolment; however, this increased to nearly 50% by month 36 [7]. In the short course isoniazid group, 360 days of ART led to a 50% reduction in TB incidence compared with participants who did not receive ART [adjusted hazard ratio (aHR) 0.50, 95% CI: 0.26–0.97]. In those with negative TST, continuous isoniazid plus ART did not reduce the incidence of TB much further (54% reduction, aHR 0.45, 95% CI: 0.16–1.30), whereas, in participants with a positive TST, continuous isoniazid plus ART led to a 96% reduction in TB incidence (aHR 0.04, 95% CI: 0.005–0.35) [8]. Owing to the fact that data on the interaction between IPT and ART came from only one study, and the ART data was observational, evidence for the efficacy of IPT co-administered with ART is considered to be of very low quality. Cotrimoxazole use was not described in any of the three studies.
Adverse events
Two studies reported a slight increase in the risk of adverse events in the continuous isoniazid group but this was not statistically different (India [9]: 2.4 vs. 1.2%, RR: 2.03, 95% CI: 0.83–2.30; Botswana [7]: 2.7 vs. 2.1%, RR: 1.26, 95% CI: 0.72–2.22). The third study reported grade 3 or grade 4 elevation in the aspartate or alanine aminotransferase level and provided strong evidence of more adverse events in the continuous isoniazid group (32 vs. 9.5%, RR: 3.41, 95% CI: 2.28–5.09) [8]. Temporarily or permanent discontinuation due to adverse events was much higher in one study [8] compared with the other two that showed no difference between the two groups [7,9]. The pooled RR of discontinuation was 5.96 (95% CI: 4.12–8.62). Meta-analysis was not performed due to clinical diversity.
Risk of drug resistance development
One study reported resistance rates in both the continuous isoniazid and 6-month isoniazid group. This study found one case of isoniazid resistance among 164 (0.6%) receiving isoniazid for 36 months and 0 (0%) cases of isoniazid resistance among 327 receiving isoniazid for 6 months [8]. The other two studies reported that the observed proportion of resistant cases among confirmed TB cases was similar as the expected rate [7,9].
Discussion
Our systematic review and meta-analysis shows that in PLHIV in high TB transmission settings, continuous isoniazid reduced the risk of developing active TB by 38% compared with 6 months of isoniazid treatment. The effect was stronger in those with a positive TST (49% for active TB and 50% for death) but there was no evidence of a difference in those with negative TST. Based on the findings of one study, ART and IPT had additional effect in reducing TB incidence in TST positive but not in TST negative individuals.
There are several possible explanations for the fact that TST negative individuals did not have added protection over prolonged time on INH treatment: first, we only had baseline TST data and some TST negatives may have converted to being TST positive; second, TST negatives may never have been infected by MTB, and they may have been had a lower baseline risk of infection; and last, there are cases of TB in TST negatives, most likely in people that were anergic, that is exposed to TB but unable to mount an adequate immune response to it [11].
In settings with high TB incidence and transmission, 36 months of IPT or longer should be recommended in all adult HIV-infected patients receiving ART and with an unknown or positive TST result. TST should be encouraged whenever feasible, but it should not be a prerequisite for IPT. If TST is performed, those with a negative TST should not receive 36 months of IPT.
In moderate TB incidence settings 6 months of IPT is likely to be durable and sufficient to prevent active TB infection in HIV-infected individuals [12]. In high-burden TB settings, isoniazid for 36 months or longer should be seen as a proxy for lifelong isoniazid, particularly because the protective effect of IPT diminishes after completing 36 months of isoniazid treatment [8,13].
Publication bias is a concern for all reviews. We used a comprehensive search that included multiple databases and conferences sites across multiple languages; however, our search was limited to studies published in the last 10 years and in three languages, and it is possible that not all studies were identified. At the study level, there were some limitations that increased the risk of bias, most importantly that two out of the three included studies were not blinded. Detailed information on adherence was often not reported in the individual studies.
In conclusion, for PLHIV in settings with high TB prevalence and transmission, continuous isoniazid is beneficial and probably outweights the risk of adverse events, and the results of this review have supported a recent WHO recommendation to provide continuous isoniazid in these settings [5]. Further research is needed to define public health parameters to identify settings with high transmission of TB that would benefit from the implementation of continuous IPT, particularly in settings where ART is provided to all HIV-positive individuals irrespective of immune status.
Acknowledgements
S.D.B wrote the study protocol, carried out the database search, study selection and data extraction, performed data analysis and wrote the manuscript. A.M. contributed to the writing of the study protocol, carried out study selection and data extraction and helped writing the manuscript. N.F. contributed to the writing of the study protocol, and helped writing the manuscript. H.G. supervised the writing of the study protocol, data collection and analysis and helped writing the manuscript. All authors read and approved the final manuscript.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.World Health Organization (WHO) Guidelines for intensified case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings. Geneva, Switzerland: WHO; 2011. [Google Scholar]
- 2.Tuberculosis preventive therapy in HIV-infected individuals. A Joint Statement of the WHO and IUATLD. Wkly Epidemiol Rec. 1993;68:361–364. [PubMed] [Google Scholar]
- 3.Getahun H, Matteelli A, Chaisson RE, Raviglioni M. Latent Mycobacterium tuberculosis Infection. N Engl J Med. 2015;372:2127–2135. doi: 10.1056/NEJMra1405427. [DOI] [PubMed] [Google Scholar]
- 4.Houben RM, Sumner T, Grant AD, White RG. Ability of preventive therapy to cure latent Mycobacterium tuberculosis infection in HIV-infected individuals in high-burden settings. Proc Natl Acad Sci USA. 2014;111:5325–5330. doi: 10.1073/pnas.1317660111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sonnenberg P, Murray J, Glynn JR, Shearer S, Kambashi B, Godfrey-Faussett P. HIV-1 and recurrence, relapse and reinfection of tuberculosis after cure: a cohort in South African mineworkers. Lancet. 2001;358:1687–1693. doi: 10.1016/S0140-6736(01)06712-5. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Recommendation on 36 months isoniazid preventive therapy to adults and adolescents living with HIV in resource-constrained and high TB- and HIV-prevalence settings – 2015 update. Geneva, Switzerland: WHO; 2015. [PubMed] [Google Scholar]
- 7.Samandari T, Agizew TB, Nyirenda S, Tedla Z, Sibanda T, Shang N, et al. 6-month versus 36-month isoniazid preventive treatment for tuberculosis in adults with HIV infection in Botswana: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377:1588–1598. doi: 10.1016/S0140-6736(11)60204-3. [DOI] [PubMed] [Google Scholar]
- 8.Martinson NA, Barnes GL, Moulton LH, Msandiwa R, Hausler H, Ram M, et al. New regimens to prevent tuberculosis in adults with HIV infection. N Engl J Med. 2011;365:11–20. doi: 10.1056/NEJMoa1005136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swaminathan S, Menon PA, Gopalan N, Perumal V, Santhanakrishnan RK, Ramachandran R, et al. Efficacy of a six-month versus a 36-month regimen for prevention of tuberculosis in HIV-infected persons in India: a randomized clinical trial. PLoS One. 2012;7:e47400. doi: 10.1371/journal.pone.0047400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273:408–412. doi: 10.1001/jama.273.5.408. [DOI] [PubMed] [Google Scholar]
- 11.Rangaka MX, Wilkinson RJ, Boulle A, Glynn JR, Fielding K, van Cutsem G, et al. Isoniazid plus antiretroviral therapy to prevent tuberculosis: a randomised double-blind placebo-controlled trial. Lancet. 2014;384:682–690. doi: 10.1016/S0140-6736(14)60162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golub JE, Cohn S, Saraceni V, Cavalcante SC, Pacheco AG, Moulton LH, et al. Long-term Protection From Isoniazid Preventive Therapy for Tuberculosis in HIV-Infected Patients in a Medium-Burden Tuberculosis Setting: The TB/HIV in Rio (THRio) Study. Clin Infect Dis. 2015;60:639–645. doi: 10.1093/cid/ciu849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samandari T, Agizew T, Nyirenda S, Tedla Z, Sibanda T, Mosimaneotsile OI, et al. Tuberculosis incidence increase after 36 months’ isoniazid prophylaxis in HIV-infected adults in Botswana: a posttrial observational analysis. AIDS. 2015;29:351–359. doi: 10.1097/QAD.0000000000000535. [DOI] [PMC free article] [PubMed] [Google Scholar]