Abstract
Background and aims:
Regional oxygen saturation (rSO2) monitoring of the brain by near-infrared spectroscopy (NIRS) has been mainly used during carotid endarterectomy. The present study was conducted in volunteers and investigates the rSO2 values of the brain, heart and liver tissue as assessed by NIRS in the supine and the sitting position.
Methods:
After obtaining written informed consent from forty-nine healthy volunteers, rSO2 values were recorded in the heart and liver areas in the supine and the sitting position, while simultaneously the rSO2 values of the brain.
Results:
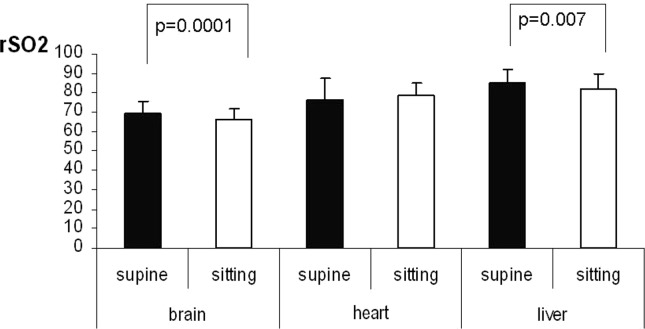
The rSO2 brain values in the supine and the sitting position were 69 ± 6.0 and 66 ± 6.1 respectively (p = 0.0001). The rSO2 values in the supine and the sitting position were 76 ± 10.5 and 79 ± 6.7 for the heart (p > 0.05) and 85 ± 6.8 and 82 ± 7.2 for the liver, (p = 0.007). Heart rSO2 values were higher than the brain rSO2 values in both the supine (76 ± 10.4 versus 69 ± 6.6; p = 0.0001) and the sitting position (79 ± 6.7 versus 66 ± 6.1; p = 0.0001). The liver rSO2 values were also higher than the brain rSO2 values in the supine (85 ± 6.8 versus 69 ± 6.0; p = 0.0001) and in the sitting position (82 ± 7.2 versus 66 ± 5.7; p = 0.0001). Arterial blood pressure and arterial oxygen saturation (SpO2) did not differ between the two positions but the heart rate was higher in the sitting position (p = 0.030).
Conclusions:
We conclude that brain and liver (but not heart) rSO2 values are higher in the supine than sitting position. Additionally, NIRS may be used to assess oxygenation of the heart and liver.
Keywords: tissue oxygenation, near infrared spectroscopy (NIRS), brain oxygenation, heart oxygenation, liver oxygenation
Introduction
Cerebral oximetry by near-infrared spectroscopy (NIRS) measures intracerebral oxygen saturation (rSO2) continuously and non-invasively [1]. The method has been validated and used extensively during carotid endarterectomy to monitor the rSO2 values during carotid clamping [2, 3]. It has also been used in stroke and cardiac arrest patients to detect desaturation of the metabolically active brain but may exhibit normal values in the absence of cerebral perfusion [4]. Cerebral oximetry is useful during coronary artery bypass surgery as its use is associated with a shorter length of stay in the ICU, lower incidence of stroke, renal failure, deep sternal infection, prolonged ventilation, reoperation and death [5].
In elderly patients undergoing major abdominal surgery, monitoring of cerebral oxygen saturation revealed that cerebral desaturation is common, and it is associated with early postoperative cognitive dysfunction that can be corrected by blood transfusion [6, 7]. The rSO2 values depend on blood flow and on the perfusion pressures and oxygen extraction by the different tissues. Our hypothesis was that regional hemoglobin saturation is posture dependent, thus rSO2 values will change from supine to sitting position and vice versa. We also hypothesized that cerebral oximetry may be used to assess the oxygenation of other vital organs like heart and liver.
The aim of this prospective observational study was to evaluate changes in the rSO2 in volunteers due to changes in position (from sitting to supine or the other way around) using the INVOS 4100 cerebral oximeter (Somanetics, Troy, MI, USA). A secondary aim was to investigate the potential of monitoring the regional oxygenations of other organs such as the liver and heart by means of NIRS in both the supine and sitting positions.
Methods
After obtaining approval from the Institutional Review Board (IRB), 36 women and 13 men gave written informed consent to participate in the study. All volunteers were ASA I physical status with a mean age of 30 (min–max: 20–61) years. Exclusion criteria included intake of drugs with effect on the nervous system (such as benzodiazepines and opioids), obesity, smoking and alcoholism. The study was performed between June 2006 and November 2009.
Measurements included simultaneous rSO2 recordings of the brain and heart in the supine and sitting positions. Similarly, rSO2 recordings of the liver in both supine and sitting position were performed simultaneously with rSO2 recordings of the brain, as NIRS is primarily the method used to measure the regional hemoglobin saturation in the brain. All measurements for heart and liver along with the brain measurements as a standard comparator were performed on the same day. The rSO2 of each organ was measured with the INVOS 4100 cerebral oximeter (Somanetics, Troy, MI, USA) and the Somanetics disposable sensor.
Systolic and diastolic arterial pressure as well as arterial oxygen saturation by means of pulse oximeter (SpO2) was also measured along with the rSO2 measurements. The order of the position for measurements, supine versus sitting, was determined by a coin toss. Regarding liver versus heart rSO2 measurements, the order was determined with opaque sealed envelopes containing the code for the relevant tissue. Systolic and diastolic arterial blood pressure, heart rate and SpO2 were recorded before and after rSO2 measurements in each position.
The left disposable INVOS sensor was applied on the ipsilateral temporal area of the brain, specifically on the left forehead with the caudal border about 1 cm above the eyebrow and the medial edge on the midline, between the two eyebrows. The right sensor was attached over the sternum area, or over the liver area according to the order of randomization (Fig. 1). The volunteer was allowed to relax in the predetermined position at least 5 minutes and subsequently remained in this position to accomplish the series of measurements, thus rSO2 values of the brain and the predetermined organ. Subsequently the right sensor was applied over the next tissue while the left sensor remained on the temporal area. The rSO2 values of the brain and the next tissue, liver or heart were recorded. When measurements were completed at the supine or the sitting position the volunteer was asked to change position and to remain relaxed for more than 5 minutes. Then the whole series of measurements was repeated in the new position.
Fig. 1.
Sensor placement over the brain and heart areas
The primary endpoint of the study was determination of the rSO2 values in each organ in the supine versus the sitting position. The secondary end point was to compare the rSO2 values obtained from the sensor applied on the sternum area representing the heart and the right sub costal area representing the liver with the values recorded simultaneously from the brain.
A priori power analysis (GPower 3.0.8, Universität Düsseldorf, Germany) showed that in order to find a medium size (0.25) of effect according to d Cohen’s coefficient, between the brain rSO2 in the supine and sitting positions, which corresponds to 6% variance, with a power of 0.90 and an error a = 0.5, a sample of 45 subjects was needed.
Statistical analysis was performed with SPSS, IBM statistical package (version 17.0). According to Kolmogorov Smirnov test all rSO2 values followed normal distribution.
The heart rate and the rSO2 values in the supine versus the sitting position obtained from each individual organ were analyzed with paired t-test. Similarly, paired t-test was used to compare the rSO2 values between the brain and the heart and between the brain and the liver in both supine and sitting position. Systolic and diastolic arterial pressure and arterial oxygen saturation (SpO2) values in the supine versus the sitting position did not follow normal distribution and were analyzed with Wilcoxon Signed Ranks test. A value of p < 0.05 was considered statistically significant.
Results
Measurements were performed in all volunteers and their characteristics are shown in Table 1. Incomplete measurements were one rSO2 value in the heart recordings with the volunteer in the supine position, and six and four rSO2 values during measurements with the sensor over the liver at the supine and sitting position, respectively. These values were not obtained due to technical reasons.
Table 1.
Age, body weight and height in the 49 volunteers recruited for the study
| Age (years) | 30 (20–61) |
| Body Weight (kg) | 65 (47–100) |
| Height (cm) | 169 (150–180) |
Values are mean (range)
The rSO2 comparisons for the same organ between the supine and the sitting position are shown in table 2 and figure 2. The rSO2 values obtained for the brain and the liver were lower in the sitting when compared to the supine position (p = 0.0001 and p = 0.007, respectively). However, we found no significant differences in the rSO2 values between the supine and sitting position in the heart (p = 0.212).
Table 2.
The rSO2 values in the supine and sitting position for the brain, heart and liver tissue
| Brain | Heart | Liver | |||
|---|---|---|---|---|---|
| Supine | Sitting | Supine | Sitting | Supine | Sitting |
| (N = 49) | (N = 49) | (N = 48) | (N = 49) | (N = 43) | (N = 45) |
| 69 ± 6.0 | 66 ± 5.7 | 76 ± 10.5 | 79 ± 6.7 | 85 ± 6.8 | 82 ± 7.2 |
| 67.7–71.1 | 64.6–67.9 | 73.5–79.6 | 76.7–80.6 | 82.9–87.1 | 80.1–84.4 |
| t = −5.837, p = 0.0001 | t = 1.26, p = 0.212 | t = −2825, p = 0.007 | |||
Values are mean ± SD, 95% confidence interval and t score
Fig. 2.
The rSO2 values of the brain, heart and liver in the supine versus the sitting position
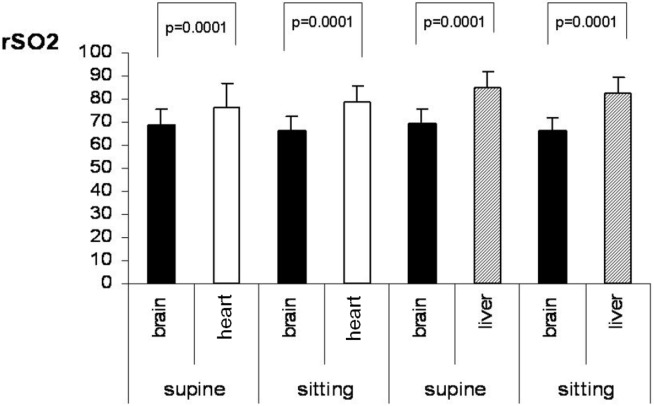
The rSO2 comparisons between brain and heart and brain and liver in the supine and the sitting position are shown in table 3 and figure 3. The heart area exhibited higher rSO2 values when compared to the brain in both the supine and the sitting position (p = 0.0001 and p = 0.0001, respectively). This might be due to the venous blood return to the heart, which is increased in the supine position, while in the same position the brain receives more arterial blood. With regard to the brain and liver comparisons, the liver rSO2 values are higher in both the supine (p = 0.0001) and sitting position (p = 0.0001). The rSO2 difference between brain and liver in both positions is the same (16%) considering that both organs receive more arterial blood in the supine position.
Table 3.
The rSO2 values of the brain compared to the rSO2 values obtained from heart and to the rSO2 values obtained from the liver in the supine and sitting position
| Supine | Sitting | Supine | Sitting | ||||
|---|---|---|---|---|---|---|---|
| Brain | Heart | Brain | Heart | Brain | Liver | Brain | Liver |
| (N = 49) | (N = 48) | (N = 49) | (N = 49) | (N = 49) | (N = 43) | (N = 49) | (N = 45) |
| 69 ± 6.6 | 76 ± 10.4 | 66 ± 6.1 | 79 ± 6.7 | 69 ± 6.0 | 85 ±6.8 | 66 ± 5.7 | 82 ± 7.2 |
| 67.1 – 70.9 | 73.5 – 79.6 | 64.3 – 67.8 | 76.7 – 80.6 | 67.7 – 71.1 | 82.9 – 87.1 | 64 – 67.9 | 80 – 84.4 |
| t = −5.079, p = 0.0001 | t = −10.114, p = 0.0001 | t = −10.542, p = 0.0001 | t = −11.576, p = 0.0001 | ||||
Values are mean ± SD and 95% confidence interval
Fig. 3.
The rSO2 values of the brain versus heart and of the brain versus liver in the sitting and in the supine position
We found no difference in the SpO2 values recorded during the rSO2 measurements of the heart and the liver between the supine and the sitting position (p = 0.738 and p = 0.434 respectively). Similarly systolic and diastolic blood pressures did not differ significantly (p = 0.311 and p = 0.73 respectively). Heart rate was higher in the sitting versus the supine position (p = 0.030).
Discussion
The results of the present study demonstrate that rSO2 values for the brain and for other areas of the body measured by NIRS vary with the patient’s posture. The data also showed that rSO2 measured in the brain and heart area in the sitting position differ. The rSO2 values between brain and liver differ and this difference is the same in both positions (16%).
Several factors may interfere and influence the rSO2 measurements by NIRS. Kishi et al. investigated the rSO2 values in the brain obtained after a sensor position at different sites on the forehead and also the impact of factors such as patient characteristics and hemoglobin concentration [8]. The sensor location, age and hemoglobin all are determinants of the rSO2 values. An inverse relationship was found between age and rSO2 values and a direct relationship between hemoglobin and rSO2 values [8]. In fact in elderly patients undergoing prolonged major abdominal surgery the significant decreases observed in rSO2 during major hemorrhage, despite maintenance of blood pressure responded only to blood transfusion [7]. We did not include elderly volunteers so we do not expect age to have an impact to the rSO2 values we measured. We did not measure the hemoglobin of our volunteers with free history for disease as our interest was focused on possible changes in the rSO2 with changes in posture and not on absolute rSO2 values.
Yoshitani et al. evaluated clinically the skull thickness, the area of the cerebrospinal fluid layer, the mean arterial pressure and the hemoglobin concentration, and found that all are determinants of the NIRS values [9]. These parameters had no effect on the tissue oxygen index, which is the ratio of oxyhemoglobin to total hemoglobin.
Nonetheless, these factors are dependent upon the technique of measurement and not upon the patient’s factors as in the rSO2 measurements the calculations include the optical path length, while in the tissue oxygen index algorithm the optical path length is not included in the formula [10]. For this reason the same parameters had no effect on the tissue oxygen index.
In our study the impact of the different factors that may influence the rSO2 measurements, such as hemoglobin and patients’ characteristics are constant and do not change during the measurements obtained in the different positions or in the different body areas. Therefore, these variables are not expected to affect the measurements in different ways.
The rSO2 was found to increase significantly when NIRS assessments were made with the subjects placed from the supine to the 20° Trendelenburg position, but not in the 20° reverse Trendelenburg position [11]. Similarly, in our study the supine position is associated with increased rSO2 values in both the brain and liver compared to the sitting position. This change, which may not be clinically significant, is likely related to increased cardiac output due to increased venous return in the supine position. In contrast, in the heart we found higher rSO2 values in the sitting position. This could be explained by the fact that in the supine position the increased return of venous blood goes directly to the heart decreasing so the rSO2 value. The finding that rSO2 values in the heart are higher in the sitting than in the supine position suggests that the sternum area may be appropriate location for the sensor to monitor the heart rSO2. Additionally other locations for placement of the sensor to monitor the rSO2 of the heart (such as the apical area of the chest) are not always feasible particularly in women due to the breasts.
Arterial blood pressure and SpO2 may also influence the NIRS measurements. Yoshitani et al. found that mean arterial pressure was a significant factor that determines the SpO2 [9]. In a retrospective study Paquet et al. reported that rSO2 brain values correlated significantly to the central venous pressure, the pulmonary capillary wedge pressure, the mean pulmonary artery pressure, the mean arterial pressure/mean pulmonary artery pressure ratio, the left fractional area change and the regional and motion score index and the diastolic function [12]. The authors conclude that NIRS may have a place in cardiac function assessment.
Although we found changes in the rSO2 between the supine and the sitting position systolic and diastolic arterial pressure did not change. This finding may be explained by the fact that the volunteers were young subjects and compensated by the increase in heart rate. Sitting position is rarely used today during surgery, aside from neurological (sitting craniotomy) and orthopedic (shoulder surgery) procedures. However the changes in the rSO2 values we observed imply possible changes of this variable when positions other than the supine are adopted intraoperatively.
Regarding the rSO2 in areas other than the brain, to our knowledge no previous studies used INVOS to investigate the feasibility of monitoring the rSO2 in other organs such as heart or liver. The rSO2 values we recorded in the sternum area are consistent with those close to the SvO2 values in healthy subjects. Considering the rSO2 of the brain as the gold standard we found higher rSO2 values primarily in the liver and secondarily in the heart areas. Nonetheless, each tissue exhibits its own rSO2 values and the importance may be the changes in rSO2, particularly in critical situations like multiple organ failure.
The consistent difference between the rSO2 values in the brain and liver in both positions suggests that the method and the medical device may be useful to monitor oxygenation of the liver but the rSO2 baseline values should be higher. Also the fact that the rSO2 values obtained in the heart do not differ between the two positions, evidently due to the increased return of venous blood to the heart in the supine position suggests that the method may be useful to monitor the global oxygenation of the heart.
Conclusion
The position of the patient influences the rSO2 values measured by NIRS in the brain and liver but not in the heart. However, our results are limited to healthy young subjects not undergoing surgery. More studies are required to validate and expand these data to patients undergoing major surgery as well as to older, sicker surgical patients. The method and the apparatus may be useful to monitor the global oxygenation of the heart and the liver as well.
Footnotes
Conflict of interest
Nothing to declare
Funding statement
This work has been done without any financial support or sponsorship.
References
- 1.Jöbsis FF. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science. 1977;198:1264–1267. doi: 10.1126/science.929199. [DOI] [PubMed] [Google Scholar]
- 2.Moritz S, Kasprzak P, Arlt M, Taeger K, Metz C. Accuracy of cerebral monitoring in detecting cerebral ischemia during carotid endarterectomy: a comparison of transcranial Doppler sonography, near-infrared spectroscopy, stump pressure, and somatosensory evoked potentials. Anesthesiology. 2007;107:563–569. doi: 10.1097/01.anes.0000281894.69422.ff. [DOI] [PubMed] [Google Scholar]
- 3.Samra SK, Dy EA, Welch K, Dorje P, Zelenock GB, Stanley JC. Evaluation of a cerebral oximeter as a monitor of cerebral ischemia during carotid endarterectomy. Anesthesiology. 2000;93:964–970. doi: 10.1097/00000542-200010000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Nemoto EM, Yonas H, Kassam A. Clinical experience with cerebral oximetry in stroke and cardiac arrest. Crit Care Med. 2000;28:1052–1054. doi: 10.1097/00003246-200004000-00023. [DOI] [PubMed] [Google Scholar]
- 5.Murkin JM, Adams SJ, Novick RJ, Quantz M, Bainbridge D, Iglesias I, et al. Monitoring brain oxygen saturation during coronary bypass surgery: a randomized, prospective study. Anesth Analg. 2007;104:51–58. doi: 10.1213/01.ane.0000246814.29362.f4. [DOI] [PubMed] [Google Scholar]
- 6.Casati A, Fanelli G, Pietropaoli P, Proietti R, Tufano R, Montanini S, et al. Monitoring cerebral oxygen saturation in elderly patients undergoing general abdominal surgery: a prospective cohort study. Eur J Anaesthesiol. 2007;24:59–65. doi: 10.1017/S0265021506001025. [DOI] [PubMed] [Google Scholar]
- 7.Green DW. A retrospective study of changes in cerebral oxygenation using a cerebral oximeter in older patients undergoing prolonged major abdominal surgery. Eur J Anaesthesiol. 2007;24:230–234. doi: 10.1017/S0265021506001645. [DOI] [PubMed] [Google Scholar]
- 8.Kishi K, Kawaguchi M, Yoshitani K, Nagahata T, Furuya H. Influence of patient variables and sensor location on regional cerebral oxygen saturation measured by INVOS 4100 near-infrared spectrophotometers. J Neurosurg Anesthesiol. 2003;15:302–306. doi: 10.1097/00008506-200310000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Yoshitani K, Kawaguchi M, Miura N, Okuno T, Kanoda T, Ohnishi Y, et al. Effects of hemoglobin concentration, skull thickness, and the area of the cerebrospinal fluid layer on near-infrared spectroscopy measurements. Anesthesiology. 2007;106:458–462. doi: 10.1097/00000542-200703000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Okada E, Delpy DT. Near-infrared light propagation in an adult head model. II. Effect of superficial tissue thickness on the sensitivity of the near-infrared spectroscopy signal. Appl Opt. 2003;42:2915–2922. doi: 10.1364/AO.42.002915. [DOI] [PubMed] [Google Scholar]
- 11.Pollard V, Prough DS, DeMelo E, Deyo DJ, Uchida T, Widman R. The influence of carbon dioxide and body position on near-infrared spectroscopic assessment of cerebral hemoglobin oxygen saturation. Anesth Analg. 1996;82:278–287. doi: 10.1097/00000539-199602000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Paquet C, Deschamps A, Denault AY, Couture P, Carrier M, Babin D, et al. Baseline regional cerebral oxygen saturation correlates with left ventricular systolic and diastolic function. J Cardiothorac Vasc Anesth. 2008;22:840–846. doi: 10.1053/j.jvca.2008.02.013. [DOI] [PubMed] [Google Scholar]