Abstract
Many clinical features of lung cancer are different in women and men. Sex steroid hormones exert effects in non-reproductive organs, such as the lungs. The association between menstrual and childbearing factors and the risk of lung cancer among women is still debated. We performed a pooled analysis of eight studies contributing to the International Lung Cancer Consortium (ILCCO; 4,386 cases and 4,177 controls). Pooled associations between menstrual or reproductive factors and lung cancer were estimated using multivariable unconditional logistic regression. Subgroup analyses were done for menopause status, smoking habits and histology. We found no strong support for an association of age at menarche and at menopause with lung cancer, but peri/postmenopausal women were at higher risk compared to premenopausal (OR 1.47, 95% CI 1.11–1.93). Premenopausal women showed increased risks associated with parity (OR 1.74, 95% CI 1.03–2.93) and number of children (OR 2.88 95% CI 1.21–6.93 for more than 3 children; P for trend 0.01) and decreased with breastfeeding (OR 0.54, 95% CI 0.30–0.98). In contrast, peri/postmenopausal subjects had ORs around unity for the same exposures. No major effect modification was exerted by smoking status or cancer histology. Menstrual and reproductive factors may play a role in the genesis of lung cancer, yet the mechanisms are unclear, and smoking remains the most important modifiable risk factor. More investigations in large well-designed studies are needed to confirm these findings and to clarify the underlying mechanisms of gender differences in lung cancer risk.
Keywords: Case-control studies, Lung neoplasms, Menopause, Reproductive History, Women
Introduction
Lung cancer in women was a rare disease until the 1970s: clinical and etiological knowledge has been therefore acquired from studies that included mainly men. Clinical features are different between genders and have led some researchers to consider lung cancer in women as a distinct biological entity 1, 2. Tumours are more often localised in women than in men. Squamous cell carcinoma has been the predominant subtype among men for decades, but it has been overtaken by adenocarcinoma in many countries in the new millennium, while among women the latter has always been to most common histotype, irrespective of smoking status3. Women have better survival rates than men, a fact that is still poorly understood 4, 5. A Scandinavian study conducted on over 40,000 lung cancer cases showed that this difference is independent of lung cancer stage at diagnosis, age at diagnosis, period of diagnosis, and histological type 6, while a French study demonstrated that 1-year mortality has significantly decreased in both genders over ten years, but less so in men than in women, leading to an increased difference 7.
There is increasing evidence for the effects of sex steroid hormones in non-reproductive organs such as the lungs. Acknowledgment of this kind of interplay is fundamental for innovative integrated health care approaches like systems medicine, that consider the individual’s complexity for the best therapeutic and preventive strategies 8. Recent reports suggest that sex hormones may play a role in many chronic respiratory diseases, including asthma, lung fibrosis, COPD and lung cancer. Sex steroid receptors have been reported in human bronchial epithelium, airway smooth muscle and alveolar epithelium. Most studies have focused on estrogen receptors (ER α and ER β), but some evidence exists also for progesterone and androgen receptors. Local metabolism of sex steroids may also be important, as suggested by the presence of sex steroids synthesising enzymes in lung parenchyma 9, 10.
A possible role of hormonal factors in the aetiology of lung cancer in women was first suggested after the finding of an increased risk of lung adenocarcinoma in association with shorter menstrual cycle lengths 11. Since then, several studies have evaluated the association of lung cancer risk with menstrual and reproductive factors, with findings generally inconsistent. Increased, null or decreased risks have been reported to be associated with the factors investigated, possibly reflecting differences in study populations and design, or random associations due to small sample size and multiple comparisons. Two meta-analyses have summarized some of these heterogeneous results, showing a protective effect of longer menstrual cycles and no association for parity, number of pregnancies, or age at menarche, at first live birth, and at menopause 12, 13.
Previous studies investigating the association between lung cancer risk and hormone therapy after menopause and/or for contraceptive purposes have shown conflicting results 12, while a pooled analysis conducted in the International Lung Cancer Consortium (ILCCO) dataset based on six studies has found that hormone use was inversely associated with lung cancer 14. To investigate the role of menstrual and reproductive factors in the onset of lung cancer, we conducted a pooled analysis based on 8 studies from the ILCCO, with a total of 4,386 lung cancer patients and 4,177 controls.
Methods
Study design and population
A pooled analysis was conducted from 8 independent case-control studies participating in ILCCO, a consortium established in 2004 with the aim to pool comparable data and maximize statistical power of lung cancer epidemiological studies. Further details regarding the aims, guidelines and policies are described in Hung et al. 15 and available on the consortium portal (http://ilcco.iarc.fr). A de-identified dataset was provided by each study and was checked for inconsistencies, before harmonizing variables and coding data uniformly across studies.
Eight case-control studies that included hormonal and reproductive variables agreed to participate and were pooled, including one case-control study nested in a cohort (ESTHER16) (table 1). Six out of the 8 individual studies were conducted in North America (i.e., LCSS17, MLCS18, TORONTO19, WELD20, NELCS21, MLCCCS22) and two came from Europe (EAGLE-Italy23, ESTHER-Germany). Two case-control studies included both hospital and population-based control groups (MLCS and TORONTO), while the others had only population-based controls. Enrollment periods dated back to the early 1990s and were quite comparable. All studies except the TORONTO study frequency matched controls to cases on age. Moreover, the TORONTO study performed matching on ethnicity and residential area, and the MLCS study additionally matched hospital controls on smoking status. Finally, the pooled analysis included eight studies with 4,386 cases and 4,177 controls. Institutional approval and written informed consent from all subjects was obtained by the investigators at each study site.
TABLE 1.
Characteristics of ILCOO studies
| Study name | Country | Controls | Period of enrolment |
Cases
|
Controls
|
Age range (years) in protocol |
||
|---|---|---|---|---|---|---|---|---|
| Principal investigator | (location) | n | Participation Rate* (%) | n | Participation Rate * (%) |
|||
| EAGLE | Italy | Population | 2002–2005 | 407 | 86.6 | 498 | 72.4 | 35–79 |
| MT Landi | (Lombardy region) | |||||||
|
| ||||||||
| ESTHER | Germany | Population | 2000–2003 | 52 | Nested case-control study | 52 | Nested case-control study | 50–75 |
| H Brenner | (Saarland) | |||||||
|
| ||||||||
| LCSS | USA | Population | 1992–2009 | 2005 | 85 | 993 | 80 | More than 18A |
| D Christiani | (Boston, Massachusetts) | |||||||
|
| ||||||||
| MLCCCS | Canada | Population | 1996–1997 | 422 | 81.7 | 576 | 69.4 | More than 35B |
| A Koushik | (Montreal area) | |||||||
|
| ||||||||
| MLCS | USA | Population/Hospital | 1998–2010 | 540 | 80 | 777 | 80 | No age restrictionC |
| CC Harris | (Baltimore City and Eastern Shore of Maryland) | |||||||
|
| ||||||||
| NELCS | USA | Population | 2005–2008 | 149 | 61 | 148 | 46 | 30–74 |
| E Duell | (New Hampshire and Vermont) | |||||||
|
| ||||||||
| TORONTO | Canada | Population/Hospital | 1997–2002 | 235 | 62 | 559 | 60 | 20–84 |
| RJ Hung; J McLaughlin | (Metropolitan Toronto) | |||||||
|
| ||||||||
| WELD | USA | Population | 1984–2005 | 576 | 83 | 574 | 70.6 | 18–74 |
| AG Schwartz | (Wayne, Macomb and Oakland counties) | |||||||
|
| ||||||||
| Total | 4386 | 4177 | ||||||
Participation Rate as mentioned in the published study protocols.
Age range (years) in ILCCO: (23–93).
Age range (years) in ILCCO: (33–75).
Age range (years) in ILCCO: (27–97).
Study variables
From each study participating in ILCCO, we obtained data on case/control status, age at interview, ethnicity, education level, smoking status and cancer histology. In each study, ever smokers were defined as subjects who reported having smoked at least 100 cigarettes in their lifetime, and former smokers as subjects who declared smoking cessation at least 2 years prior to interview. Lifetime smoking history was estimated in each study by the Comprehensive Smoking Index (CSI) that has been previously used in other case-control studies on lung cancer 24, 25. CSI incorporates measures of smoking duration, time since cessation, and smoking intensity into one aggregate measure. The lung cancer histological subtypes were classified according to the International Classification of Diseases in Oncology, Third Edition 26.
Women were asked about their reproductive histories. Information available included menstrual factors (age of menarche, menopause status and reason, age at natural menopause, oophorectomy status), and childbearing factors (age at first child, parity, number of children, breastfeeding).
The variable “Age at menopause” was built on the replies to questions like “At what age was your last menstrual period?” among subjects that declared not to be menstruating anymore. In addition, women experiencing episodic amenorrhea could have self-attributed a definite menopausal status even if they were rather in perimenopause. Consequently, mean age at menopause was relatively early (47 years, rather similar in the various studies collecting this information) and menopause status was defined as “premenopausal” or “peri/postmenopausal”.
Menopause was considered as non-natural if both ovaries were surgically removed or ovarian function was abolished by radiation or drugs. Oophorectomy was defined as the surgical removal of the two ovaries. Missing values for oophorectomy were considered as no oophorectomy.
Single datasets were eligible for a specific analysis only if information on reproductive factors was available for at least 70% of the subjects. For a given variable, data were pooled according to its availability in each study.
Statistical analysis
All data were quality checked for inadmissible values, inconsistencies and missing variables. Questions regarding data were resolved by the original study principal investigators.
To estimate differences between cases and controls in socio-demographic characteristics, a single pooled database was built from all studies. Pooled odds ratios (OR) and the corresponding 95% confidence intervals (CI) were estimated using unconditional logistic regression models that included study center, age at interview or diagnosis (categorized into 4 groups, ≤53, 54–62, 63–70 and >70 years based on the distribution among the control population), ethnicity (Caucasians, non Caucasians), education level (categorized into 3 groups: primary, secondary, university) and CSI (as a continuous variable). We conducted analyses stratified by menopause (pre- or peri/post-) and smoking status (never, ex- and current smokers), and tests of interaction were assessed using log-likelihood test statistics, comparing models with and without the interaction term. We also performed multinomial logistic regression to test homogeneity of the association between the menstrual and reproductive factors and lung cancer risk across histological types (adenocarcinoma, squamous cell carcinoma and small cell carcinoma), using Wald test.
In addition we calculated I2, the percentage of the variability across studies in effect that is due to heterogeneity 27, using the Cochrane Handbook for systematic Review interventions 28. The influence of each study on the overall analysis estimate was evaluated by an influence analysis, where the analysis estimates are computed before and after omitting each study that was found to be a source of heterogeneity.
Statistical analyses were performed with SAS (© SAS Institute Inc.; North Carolina, USA; version 9.3). All p values were two-sided, and a p value ≤ 0.05 was the threshold for statistical significance.
Results
ILCCO pooled analysis
Table 2 describes demographic and lifestyle characteristics of cases and controls included in the analysis. Mean ages of cases and controls were 63.3 years (range: 26–93) and 59.6 years (range: 20–97) respectively. The majority of the population was Caucasian (more than 80%). Compared with controls, cases had lower education levels and were more likely to be smokers. Regarding histological subtypes of lung cancer cases, 47% of the tumors were classified as adenocarcinomas, followed by squamous cell carcinomas (14%), while small cell lung cancers represented 7% of all cases.
TABLE 2.
Demographic characteristics of the study population among cases and controls and distribution of histological subtypes of lung cancer
| Characteristics | Cases (n=4386) | Controls (n=4177) | OR | 95%Cl | Pχ2* | ||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| n | % | n | % | ||||
| Age at interview (years)A | |||||||
|
| |||||||
| ≤ 53 | 834 | 19.0 | 1202 | 28.8 | 0.71 | (0.61–0.82) | |
| 54–62 | 1100 | 25.1 | 1098 | 26.3 | 0.81 | (0.70–0.93) | |
| 63–70 | 1226 | 28.0 | 1096 | 26.2 | 1.0 | Reference | |
| >70 | 1218 | 27.8 | 778 | 18.6 | 1.62 | (1.40–1.87) | <0.0001 |
| Missing | 8 | 0.2 | 3 | 0.1 | - | - | |
| Mean (SD) | 63.3 (10.7) | 59.6 (11.8) | P<0.0001 | ||||
|
| |||||||
| EthnicityB | |||||||
|
| |||||||
| Caucasian | 3837 | 87.5 | 3400 | 81.4 | 1.0 | Reference | |
| No-Caucasian | 536 | 12.2 | 761 | 18.2 | 1.14 | (0.98–1.32) | <0.0001 |
| Missing | 13 | 0.3 | 16 | 0.4 | - | - | |
|
| |||||||
| EducationC E | |||||||
|
| |||||||
| Primary | 584 | 24.5 | 564 | 17.7 | 1.23 | (1.03–1.47) | |
| Secondary | 1143 | 48.0 | 1238 | 38.9 | 1.0 | Reference | |
| University | 632 | 26.5 | 1330 | 41.8 | 0.68 | (0.58–0.79) | <0.0001 |
| Missing | 22 | 0.9 | 52 | 1.6 | - | - | |
|
| |||||||
| Smoking statusD | |||||||
|
| |||||||
| Never smoker | 636 | 14.5 | 1923 | 46.0 | 1.0 | Reference | |
| Former smoker | 1684 | 38.4 | 1210 | 29.0 | 3.58 | (3.17–4.05) | |
| Current smoker | 2030 | 46.3 | 993 | 23.8 | 6.64 | (5.86–7.52) | <0.0001 |
| Missing | 36 | 0.8 | 51 | 1.2 | - | - | |
|
| |||||||
| CSID | |||||||
|
| |||||||
| 0 | 636 | 14.5 | 1923 | 46.0 | 1.0 | Reference | |
| ≤0.92 | 474 | 10.8 | 949 | 22.7 | 1.44 | (1.24–1.67) | |
| 0.93–1.56 | 839 | 19.1 | 594 | 14.2 | 4.17 | (3.61–4.82) | |
| 1.57–2.03 | 1025 | 23.4 | 398 | 9.5 | 8.30 | (7.11–9.69) | |
| >2.03 | 1137 | 25.9 | 226 | 5.4 | 14.6 | (12.2–17.44) | <0.0001 |
| Missing | 275 | 6.3 | 87 | 2.1 | - | - | |
|
| |||||||
| Histological type | |||||||
|
| |||||||
| SCLC | 322 | 7.3 | - | - | |||
| NSCLC | 2901 | 66.1 | - | - | |||
| Squamous cell | 605 | 13.8 | - | - | |||
| Large cell | 223 | 5.1 | - | - | |||
| Adenocarcinoma | 2073 | 47.3 | - | - | |||
| OthersF | 1011 | 23.1 | - | - | |||
| Missing | 152 | 3.5 | - | - | |||
Abbreviations: CI = Confidence interval; CSI = Comprehensive Smoking Index
P values are derived from the χ2 test for categorical variables.
Adjustment for center, ethnicity, education and CSI
Adjustment for center, age at interview, education and CSI
Adjustment for center, age at interview, ethnicity and CSI
Adjustment for center, age at interview, education and ethnicity
Not available in LCSS
carcinoid/Barrett’s carcinoma/not otherwise specified/information not available
Pooled ORs of lung cancer associated with menstrual and reproductive factors are shown in table 3. No association was observed with age of menarche. Late age at natural menopause (>51 years) was associated with significantly decreased odds ratio, but this association was lost when the EAGLE study was removed because of high heterogeneity (I2=61%).
TABLE 3.
Pooled analysis of the association of menstrual and reproductive factors with lung cancer
| Factors (cases/controls)A | Cases | Controls | Pooled ORB | 95%Cl | P trendC | ||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Studies | n | % | n | % | |||
| MENSTRUAL FACTORS | |||||||
|
| |||||||
| Age of menarche (years) (2381/3184) | |||||||
| EAGLE - ESTHER - MLCCCS - MLCS - NELCS - TORONTO - WELD | |||||||
|
| |||||||
| ≤ 12 | 983 | 41.3 | 1384 | 43.5 | 1.0 | Reference | 0.9 |
| ]12–13] | 556 | 23.4 | 823 | 25.9 | 0.99 | (0.84–1.16) | |
| ]13–14] | 357 | 15.0 | 476 | 15.0 | 1.11 | (0.92–1.35) | |
| > 14 | 346 | 14.5 | 447 | 14.0 | 0.98 | (0.81–1.2) | |
| Missing | 139 | 5.8 | 54 | 1.7 | |||
|
| |||||||
| Age at natural menopause (years)D (1575/1901) | |||||||
| EAGLE - ESTHER - MLCS - WELD | |||||||
|
| |||||||
| ≤ 43 | 507 | 32.2 | 492 | 25.9 | 1.0 | Reference | 0.1 |
| ]44–48] | 358 | 22.7 | 372 | 19.6 | 0.98 | (0.78–1.22) | |
| ]48–51] | 257 | 16.3 | 323 | 17.0 | 0.97 | (0.76–1.24) | |
| > 51 | 243 | 15.4 | 422 | 22.2 | 0.78 | (0.62–0.99) | |
| Missing | 210 | 13.3 | 292 | 15.4 | |||
|
| |||||||
| Menopause status (2381/3184)E | |||||||
| EAGLE - ESTHER - MLCCCS - MLCS - NELCS - TORONTO - WELD | |||||||
|
| |||||||
| Premenopausal | 230 | 9.7 | 604 | 19.0 | 1.0 | Reference | |
| Peri/Postmenopausal | 2078 | 87.3 | 2523 | 79.2 | 1.92 | (1.5–2.46) | |
| Missing | 73 | 3.1 | 57 | 1.8 | |||
|
| |||||||
| Menopause reason (1945/2425) | |||||||
| EAGLE - MLCCCS - MLCS - WELD | |||||||
|
| |||||||
| Premenopausal | 152 | 7.8 | 308 | 12.7 | 1.0 | Reference | |
| Peri/Postmenopausal | |||||||
| natural | 1078 | 55.4 | 1358 | 56.0 | 1.64 | (1.2–2.23) | |
| non natural | 666 | 34.2 | 734 | 30.3 | 1.89 | (1.38–2.58) | |
| Missing | 49 | 2.5 | 25 | 1.0 | |||
|
| |||||||
| Oophorectomy (1116/1685)F | |||||||
| EAGLE - ESTHER - MLCCCS - TORONTO | |||||||
|
| |||||||
| No | 934 | 83.7 | 1483 | 88.0 | 1.0 | Reference | |
| Yes | 182 | 16.3 | 202 | 12.0 | 1.45 | (1.12–1.87) | |
| REPRODUCTIVE FACTORS | |||||||
|
| |||||||
| Age at first child (years) (2232/3036) | |||||||
| EAGLE - ESTHER - MLCCCS - MLCS - TORONTO – WELD | |||||||
|
| |||||||
| ≤ 20 | 647 | 29.0 | 645 | 21.3 | 1.0 | Reference | 0.6 |
| ]20–23] | 493 | 22.1 | 575 | 18.9 | 1.16 | (0.95–1.41) | |
| ]23–27] | 428 | 19.2 | 668 | 22.0 | 1.05 | (0.56–1.28) | |
| > 27 | 318 | 14.3 | 624 | 20.6 | 0.94 | (0.75–1.17) | |
| Missing | 346 | 15.5 | 524 | 17.3 | |||
|
| |||||||
| Parity (4237/4029) | |||||||
| EAGLE - ESTHER - LCSS - MLCCCS - MLCS - TORONTO - WELD | |||||||
|
| |||||||
| No | 563 | 13.3 | 650 | 16.1 | 1.0 | Reference | 0.42 |
| Yes | 3489 | 82.3 | 3319 | 82.4 | 1.07 | (0.89–1.29) | |
| Missing | 185 | 4.4 | 60 | 1.5 | |||
|
| |||||||
| Number of childrenG (4237/4029) | |||||||
| EAGLE - ESTHER - LCSS - MLCCCS - MLCS - TORONTO - WELD | |||||||
|
| |||||||
| 0 | 563 | 13.3 | 650 | 16.1 | 1.0 | Reference | |
| 1 | 528 | 12.5 | 569 | 14.1 | 1.1 | (0.88–1.4) | |
| 2 | 1053 | 24.9 | 1142 | 28.3 | 1.19 | (0.97–1.45) | |
| 3 | 739 | 17.4 | 795 | 19.7 | 0.95 | (0.76–1.2) | |
| >3 | 803 | 19.0 | 739 | 18.3 | 0.98 | (0.78–1.23) | |
| Missing | 551 | 13.0 | 134 | 3.3 | |||
|
| |||||||
| Breastfeeding (1997/2477)H | |||||||
| EAGLE - ESTHER - MLCCCS - MLCS - WELD | |||||||
|
| |||||||
| No | 1049 | 52.5 | 1111 | 44.9 | 1 | Reference | |
| yes | 670 | 33.6 | 1021 | 41.2 | 0.88 | (0.74–1.04) | |
| Missing | 278 | 13.9 | 345 | 13.9 | |||
Abbreviations: Cl = Confidence interval; OR = odds ratio
(Number of cases/number of controls) included in the logistic regression
Adjustment for center, age at interview, ethnicity, education and CSI
Test for ordinal variables was performed only when the assumption of linearity was satisfied.
Heterogeneity: I2=61%
Heterogeneity: I2=65%
Missing was considered as No Oophorectomy
Heterogeneity: I2=67%
Adjustment for center, age at interview, ethnicity, education, CSI and number of children
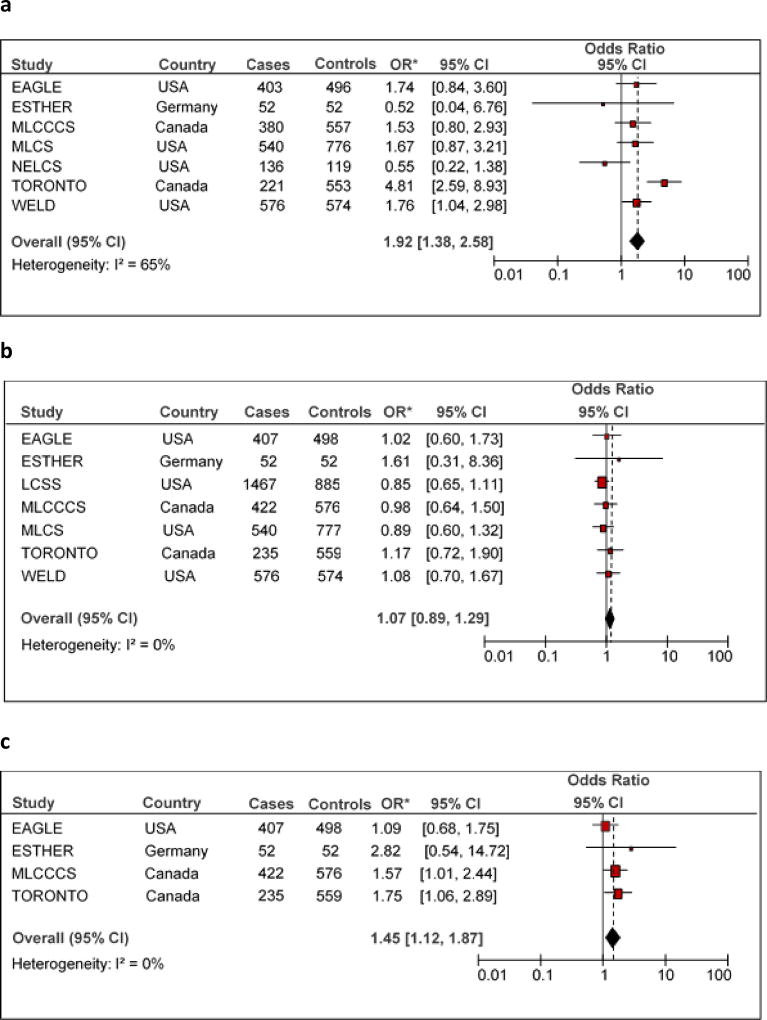
Peri/postmenopausal women had a 90% higher risk of lung cancer as compared to premenopausal women (statistically significant). After removing the TORONTO study in order to eliminate significant heterogeneity (I2=65%), the risk estimate was reduced, but still significant (Pooled OR = 1.47, 95% CI 1.11 – 1.93). Figure 1a shows the study-specific ORs for menopause status, along with the overall estimate. When accounting for menopause reason, the association was slightly stronger for non-natural than natural menopause. The positive association was confirmed in the subgroup of studies that reported on oophorectomy, as is shown in Figure 1b.
Figure 1.
*Odds ratios for lung cancer were adjusted for age at interview, ethnicity, education and CSI using multivariable unconditional logistic regression models.
I2 were calculated using the Cochrane Handbook for systematic Review interventions
No association with lung cancer was found for age at first child. Pooled ORs for parity and for number of children were around unity, even after removing the MLCS and LCSS studies, that gave a high heterogeneity (I2=67%). Figure 1c is dedicated to parity and shows the ORs with confidence intervals of the single studies, together with the pooled results.
Breastfeeding was associated with a slightly decreased lung cancer risk, although without reaching statistical significance.
Subgroup analyses
The associations with menstrual and reproductive factors were stratified by menopausal status, as is shown in table 4.
TABLE 4.
Pooled analysis of the association of menstrual and reproductive factors with lung cancer stratified by menopause status
| Premenopausal | Peri/Postmenopausal | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factors (cases/controls)A | Cases | Controls | Pooled ORB | 95%Cl | Cases | Controls | Pooled ORB | 95%Cl | P for interactionC | ||||
|
|
|
||||||||||||
| Studies | n | % | n | % | n | % | n | % | |||||
| MENSTRUAL FACTORS | |||||||||||||
|
| |||||||||||||
| Age of menarche (years)(230/604) | (2078/2523) | ||||||||||||
| EAGLE - ESTHER - MLCCCS - MLCS - NELCS - TORONTO - WELD | |||||||||||||
|
| |||||||||||||
| ≤ 12 | 110 | 47.8 | 266 | 44.0 | 1.0 | 858 | 41.3 | 1091 | 43.2 | 1.0 | |||
| ]12–13] | 49 | 21.3 | 181 | 30.0 | 0.74 | (0.46–1.2) | 497 | 23.9 | 636 | 25.2 | 1.02 | (0.86–1.21) | |
| ]13–14] | 37 | 16.1 | 87 | 14.4 | 0.97 | (0.55–1.7) | 313 | 15.1 | 382 | 15.1 | 1.13 | (0.92–1.38) | |
| > 14 | 27 | 11.7 | 64 | 10.6 | 1.23 | (0.65–2.36) | 313 | 15.1 | 379 | 15.0 | 0.98 | (0.8–1.20) | 0.3 |
| Missing | 7 | 3.0 | 6 | 1.0 | 97 | 4.7 | 35 | 1.4 | |||||
| p trend E=0.9 | p trend E=0.9 | ||||||||||||
| REPRODUCTIVE FACTORS | |||||||||||||
|
| |||||||||||||
| Age at first child (years) (180/568) | (1992/2440) | ||||||||||||
| EAGLE - ESTHER - MLCCCS - MLCS - TORONTO - WELD | |||||||||||||
|
| |||||||||||||
| ≤ 20 | 56 | 31.1 | 69 | 12.2 | 1.0 | Reference | 576 | 28.9 | 570 | 23.4 | 1.0 | Reference | |
| ]20–23] | 34 | 18.9 | 45 | 7.9 | 1.2 | (0.6–2.38) | 455 | 22.8 | 528 | 21.6 | 1.17 | (0.96–1.44) | |
| ]23–27] | 26 | 14.5 | 125 | 22.0 | 0.49 | (0.26–0.94) | 391 | 19.6 | 542 | 22.2 | 1.16 | (0.93–1.45) | |
| > 27 | 38 | 21.1 | 161 | 28.4 | 0.73 | (0.38–1.41) | 272 | 13.7 | 461 | 18.9 | 1.01 | (0.79–1.28) | 0.006 |
| Missing | 26 | 14.4 | 168 | 29.6 | 298 | 15.0 | 339 | 13.9 | |||||
| p trend E=0.8 | |||||||||||||
|
| |||||||||||||
| Parity (272/606) | (2634/2491) | ||||||||||||
| EAGLE - ESTHER - LCSS - MLCCCS - MLCS - TORONTO - WELD | |||||||||||||
|
| |||||||||||||
| No | 48 | 17.7 | 192 | 31.7 | 1.0 | Reference | 349 | 13 | 331 | 13.3 | 1.0 | Reference | |
| Yes | 223 | 82.0 | 405 | 66.8 | 1.74 | (1.03–2.93) | 2266 | 86 | 2147 | 86.2 | 0.92 | (0.75–1.12) | 0.001 |
| Missing | 1 | 0.4 | 9 | 1.5 | 19 | 0.7 | 13 | 0.5 | |||||
|
| |||||||||||||
| Number of children (272/606) | (2634/2491) | ||||||||||||
| EAGLE - ESTHER - LCSS - MLCCCS - MLCS - TORONTO - WELD | |||||||||||||
|
| |||||||||||||
| 0 | 48 | 17.7 | 192 | 31.7 | 1.0 | Reference | 349 | 13.3 | 331 | 13.3 | 1.0 | Reference | |
| 1 | 50 | 18.4 | 122 | 20.1 | 1.41 | (0.75–2.67) | 390 | 14.8 | 358 | 14.4 | 1.01 | (0.78–1.3) | |
| 2 | 93 | 34.2 | 188 | 31.0 | 1.55 | (0.87–2.76) | 775 | 29.4 | 735 | 29.5 | 1.03 | (0.82–1.3) | |
| 3 | 48 | 17.7 | 61 | 10.1 | 2.63 | (1.3–5.34) | 530 | 20.1 | 529 | 21.2 | 0.78 | (0.61–0.99) | 0.0002 |
| >3 | 32 | 11.8 | 34 | 5.6 | 2.87 | (1.28–6.44) | 571 | 21.7 | 525 | 21.1 | 0.82 | (0.64–1.05) | |
| Missing | 1 | 0.4 | 9 | 1.5 | 19 | 0.7 | 13 | 0.5 | |||||
| p trend E=0.002 | |||||||||||||
|
| |||||||||||||
| BreastfeedingD (154/311) | (1797/2144) | ||||||||||||
| EAGLE - ESTHER - MLCCCS - MLCS - WELD | |||||||||||||
|
| |||||||||||||
| No | 86 | 55.8 | 97 | 31.2 | 1.0 | Reference | 936 | 52.1 | 1007 | 47.0 | 1.0 | Reference | |
| yes | 50 | 32.5 | 147 | 47.3 | 0.54 | (0.3–0.98) | 614 | 34.2 | 870 | 40.6 | 0.94 | (0.78–1.12) | 0.04 |
| Missing | 18 | 11.7 | 67 | 21.5 | 247 | 13.8 | 267 | 12.5 | |||||
Abbreviations: Cl = Confidence interval; OR = odds ratio
(Number of cases/number of controls) included in the logistic regression.
Adjustment for center, age at interview, ethnicity, education and CSI.
P-value for interaction between the corresponding variable and menopause status using the likelihood ratio test.
Adjustment for center, age at interview, ethnicity, education, CSI and number of children.
Test for ordinal variables was performed only when the assumption of linearity was satisfied.
Age at menarche was not related to lung cancer, neither in pre- nor in peri/postmenopausal women.
The reproductive factors appeared to exert a different, stronger influence on lung cancer risk among the premenopausal women than the peri/postmenopausal, as confirmed by the statistically significant interactions that have been found for all the variables considered.
In particular, the risk of lung cancer seemed to decrease with age at first child among premenopausal women, although no clear trend could be demonstrated, but it was null for women that were not menstruating anymore. In comparison to nulliparity, the risk associated with parity was 74% higher among premenopausal women (statistically significant), while it was slightly decreased in peri/postmenopausal subjects, showing a highly significant interaction. In addition, among premenopausal women the risk increased with the number of children (p for trend <0.002 and <0.01 respectively in analysis with and without heterogeneity), up to an almost triple risk for women with 3 children or more, whereas borderline decreased risks were observed among peri/postmenopausal women in the same categories (p for interaction 0.0002). After removing heterogeneous studies (LCSS and MLCS), a significant increased risk was confirmed only among women with more than 3 children (Pooled OR = 2.88, 95% CI 1.21 – 6.93), while no clear trend was found among peri/postmenopausal women. Finally, breastfeeding was associated with half the risk of lung cancer among the women still menstruating at interview, but showed no major effect among those after menopause.
The results according to histology of lung cancer are presented in table 5. We did not observe any major difference with respect to the overall results presented in table 3. Oophorectomy was negatively associated with small cell lung cancer and positively associated with the other histologies. However, even in this case, histological types were statistically homogeneous. Test of homogeneity was significant (P=0.04) only for menopause status, which was more strongly associated with small cell lung cancer than with adenocarcinoma and squamous cell carcinoma histotypes.
TABLE 5.
Association between menstrual and reproductive factors and lung cancer risk by histologic types
| Small cell carcinoma | Adenocarcinoma | Squamous cell carcinoma | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Factors (cases/controls)A | Controls | Cases | Pooled | 95%Cl | Cases | Pooled | 95%Cl | Cases | Pooled | 95%Cl | PC |
|
|
|
|
|||||||||
| Studies | n | n | n | n | |||||||
| MENSTRUAL FACTORS | |||||||||||
|
| |||||||||||
| Age of menarche (years) (3184/157) | (1188) | (349) | |||||||||
| EAGLE - ESTHER - MLCCCS - MLCS - NELCS - TORONTO - WELD | |||||||||||
|
| |||||||||||
| ≤ 12 | 1384 | 60 | 1 | Reference | 501 | 1 | Reference | 131 | 1 | Reference | |
| ]12–13] | 823 | 33 | 1.03 | (0.63–1.69) | 273 | 0.97 | (0.81–1.17) | 84 | 1.08 | (0.79–1.49) | |
| ]13–14] | 476 | 17 | 0.8 | (0.44–1.47) | 193 | 1.19 | (0.96–1.49) | 53 | 1.12 | (0.77–1.63) | 0.9 |
| > 14 | 447 | 21 | 0.83 | (0.45–1.51) | 172 | 0.97 | (0.80–1.17) | 49 | 0.92 | (0.62–1.34) | |
| Missing | 54 | 26 | 49 | 32 | |||||||
|
| |||||||||||
| Age at natural menopause (years) (1901/51) | (843) | (206) | |||||||||
| EAGLE - ESTHER - MLCS - WELD | |||||||||||
|
| |||||||||||
| ≤ 43 | 492 | 6 | 1 | Reference | 273 | 1 | Reference | 59 | 1 | Reference | |
| ]44–48] | 372 | 9 | 1.85 | (0.56–6.06) | 170 | 0.84 | (0.65–1.09) | 63 | 1.65 | (1.09–2.51) | |
| ]48–51] | 323 | 14 | 4.14 | (1.33–12.84) | 144 | 0.98 | (0.74–1.28) | 33 | 1.24 | (0.76–2.03) | 0.25 |
| > 51 | 422 | 13 | 3.67 | (1.15–11.67) | 126 | 0.7 | (0.53–0.93) | 28 | 0.91 | (0.55–1.51) | |
| Missing | 292 | 9 | 130 | 23 | |||||||
|
| |||||||||||
| Menopause status (3184/157) | (1188) | (349) | |||||||||
| EAGLE - ESTHER - MLCCCS - MLCS - NELCS - TORONTO - WELD | |||||||||||
|
| |||||||||||
| Premenopausal | 604 | 12 | 1 | Reference | 129 | 1 | Reference | 22 | 1 | Reference | |
| Peri/Postmenopausal | 2523 | 133 | 2.9 | (1.31–6.4) | 1027 | 1.82 | (1.36–2.43) | 313 | 1.83 | (0.98–3.44) | 0.04 |
| Missing | 57 | 12 | 32 | 14 | |||||||
|
| |||||||||||
| Menopause reason (2425/112) | (1019) | (278) | |||||||||
| EAGLE - MLCCCS - MLCS - WELD | |||||||||||
|
| |||||||||||
| Premenopausal | 308 | 6 | 1 | Reference | 100 | 1 | Reference | 9 | 1 | Reference | |
| Peri/Postmenopausal | |||||||||||
| natural | 1358 | 78 | 4.41 | (1.57–12.38) | 544 | 1.47 | (1.05–2.08) | 174 | 2.06 | (0.9–4.71) | 0.2 |
| non natural | 734 | 16 | 3.06 | (1.01–9.36) | 352 | 1.75 | (1.23–2.48) | 85 | 1.92 | (0.83–4.46) | |
| Missing | 25 | 12 | 23 | 10 | |||||||
|
| |||||||||||
| OophorectomyD (1483/140) | (540) | (172) | |||||||||
| EAGLE - ESTHER - MLCCCS - TORONTO | |||||||||||
|
| |||||||||||
| No | 1483 | 129 | 1 | Reference | 451 | 1 | Reference | 141 | 1 | Reference | |
| yes | 202 | 11 | 0.56 | (0.28–1.11) | 89 | 1.5 | (1.1–2.01) | 31 | 1.35 | (0.85–2.14) | 0.2 |
| REPRODUCTIVE FACTORS | |||||||||||
|
| |||||||||||
| Age at first child (years) (3036/141) | (1137) | (322) | |||||||||
| EAGLE - ESTHER - MLCCCS - MLCS - TORONTO - WELD | |||||||||||
|
| |||||||||||
| ≤ 20 | 645 | 31 | 1 | Reference | 297 | 1 | Reference | 107 | 1 | Reference | |
| ]20–23] | 575 | 28 | 1.02 | (0.57–1.82) | 257 | 1.34 | (1.06–1.68) | 71 | 0.95 | (0.66–1.37) | |
| ]23–27] | 668 | 26 | 0.92 | (0.5–1.68) | 231 | 1.22 | (0.96–1.55) | 62 | 0.95 | (0.64–1.4) | 0.3 |
| > 27 | 624 | 25 | 1.05 | (0.57–1.94) | 184 | 1.15 | (0.88–1.5) | 36 | 0.67 | (0.43–1.06) | |
| Missing | 524 | 31 | 168 | 46 | |||||||
|
| |||||||||||
| Parity (4029/306) | (2022) | (578) | |||||||||
| EAGLE - ESTHER- LCSS - MLCCCS - MLCS - TORONTO - WELD | |||||||||||
|
| |||||||||||
| No | 650 | 43 | 1 | Reference | 265 | 1 | Reference | 68 | 1 | Reference | |
| yes | 3319 | 250 | 1.02 | (0.62–1.69) | 1671 | 1.15 | (0.92–1.43) | 483 | 1.07 | (0.74–1.56) | 0.83 |
| Missing | 60 | 13 | 86 | 27 | |||||||
|
| |||||||||||
| Number of children (4029/306) | (2022) | (578) | |||||||||
| EAGLE - ESTHER- LCSS - MLCCCS - MLCS - TORONTO - WELD | |||||||||||
|
| |||||||||||
| 0 | 650 | 43 | 1 | Reference | 265 | 1 | Reference | 68 | 1 | Reference | |
| 1 | 569 | 35 | 0.99 | (0.53–1.85) | 267 | 1.18 | (0.9–1.55) | 73 | 1.02 | (0.64–1.62) | |
| 2 | 1142 | 68 | 0.86 | (0.47–1.55) | 534 | 1.3 | (1.01–1.64) | 124 | 1.21 | (0.8–1.84) | |
| 3 | 795 | 50 | 1.04 | (0.55–1.97) | 362 | 1.05 | (0.8–1.37) | 106 | 0.86 | (0.54–1.37) | 0.93 |
| >3 | 739 | 70 | 1.31 | (0.71–2.43) | 368 | 0.97 | (0.74–1.28) | 133 | 1.11 | (0.71–1.72) | |
| Missing | 134 | 40 | 226 | 74 | |||||||
|
| |||||||||||
| BreastfeedingE (2477/124) | (1044) | (289) | |||||||||
| EAGLE - ESTHER - MLCCCS - MLCS - WELD | |||||||||||
|
| |||||||||||
| No | 1111 | 63 | 1 | Reference | 541 | 1 | Reference | 160 | 1 | Reference | |
| yes | 1021 | 36 | 0.73 | (0.41–1.31) | 367 | 0.88 | (0.73–1.07) | 95 | 0.94 | (0.68–1.31) | 0.3 |
| Missing | 345 | 25 | 136 | 34 | |||||||
Abbreviations: Cl = Confidence interval; OR = odds ratio
(Number of controls/number of cases) included in the logistic regression.
Adjustment for center, age at interview, ethnicity, education and CSI using multinomial logistic regression models.
P-value for homogeneity using the Wald test
Missing was considered as no oophorectomy
Adjustment for center, age at interview, ethnicity, education, CSI and number of children.
We also examined the possibility of effect modification by smoking status, and the results are reported in supplementary materials. No significant interaction could be demonstrated.
Moreover, there were no major influences of smoking status on the associations between lung cancer and the menstrual variables, except for oophorectomy, whose positive association with lung cancer was statistically significant only among current smokers. For most reproductive factors, never smokers showed somehow increased risks with respect to smokers, namely for age at first children, parity and number of children.
Four studies never caused any statistically significant heterogeneity in our analysis, namely MLCCCS, ESTHER,WELD and NELCS. After excluding the NELCS study, due to its relatively low participation rate, we run a sensitivity analysis with the three remaining studies, and we found that the results were in line with those presented above.
Discussion
We explored whether menstrual and reproductive factors might be associated with the risk of lung cancer among women, using data pooled from eight studies in the framework of the ILCCO collaboration. Menopausal status represented a risk factor, while we found no strong support for an association of age at menarche and age at menopause with lung cancer. A statistically significant association with reproductive factors was found among premenopausal women, who showed increased risks for parity and number of children. Premenopausal women who breastfed had lower risks, compared to those who did not. In contrast, peri/postmenopausal subjects had ORs around unity for the same exposures. No major effect modification was exerted by smoking status or cancer histology.
Biology of sex hormones in women is undoubtedly complex, and includes the enzymes involved in their metabolism, their receptors, their regulation and the cross-talk with other signalling pathways. The interplays of these factors in normal and neoplastic lung have not been fully clarified yet, but in vitro data, animal models, and functional or physiologic evidence provide support for a role of steroid hormones in lung carcinogenesis. The presence of receptors for both estrogen (ER α, ER β and GPER) and progesterone in lung cells29, 30, the pulmonary physiologic abnormalities associated with the targeted inactivation of ER β receptors in female mice 31, the ER β receptor most often expressed in human non-small cell lung cancer cell lines and in normal pulmonary tissue of sick patients 30, illustrate the plausibility of the role of steroid hormones in lung carcinogenesis. Furthermore, some studies have indicated differences in the expression of ERs depending on the sex of the subject and the cancer histology 10.
Tens of studies have evaluated the association of lung cancer risk with several menstrual and reproductive factors, but their results have been generally inconsistent.
Late age of menarche resulted in a slightly, non significantly decreased risk of lung cancer in the meta-analysis of 19 studies (including LCSS, MLCCCS, MLCS and WELD), conducted by Zhang and colleagues 12. No association was shown in four studies published thereafter 14, 20, 31, 32 and in the present pooled analysis. Tan and colleagues recently found a doubling of risk limited to lung adenocarcinoma in Chinese women 33, a result that we did not confirm in our study population, which predominantly included Caucasian women.
Summary results for late age at menopause pointed to a weak protective or null effect in a meta-analysis of 15 studies12, and so did both our pooled analysis and the Singapore cohort 33. Among the other recent studies, two gave an indication of an increased risk in Chinese women 31, 32, and two of a protective effect in Caucasians 14, 20.
Menopausal status was associated with a statistically significant 50% increased risk of lung cancer in our analysis, with minor differences according to menopause reason (natural, induced, oophorectomy), smoking behaviour or cancer histology. The risk was similar or even higher in the subset of women from the EAGLE study 14 and from MLCCCS 22 and in two other study groups that were included in this analysis, namely MLCS and WELD (partially published in 18 and 34 respectively), but an inverse association was found in two smaller datasets (ESTHER and NELCS).
Our pooled result is particularly important because menopausal status has been rarely examined in previous studies with adequate statistical power. Lung cancer is diagnosed typically at late ages and many published studies included only (e.g. Schwartz 2015) 20 or almost exclusively peri/postmenopausal women (e.g. Tan 2015) 31, 33. In the case-control studies that examined this issue 14, 18, 22, 34–38, the number of premenopausal cases was less than 50, with one exception 37. As a consequence, even if they consistently reported higher risks for peri/postmenopausal with respect to premenopausal women, particularly in the case of induced menopause, their results rarely reached statistical significance 22, 37. In the cohort studies, menopausal status is often recorded only at entry, and no information at time of cancer diagnosis is generally available. An exception is represented by a study on a cohort that was updated every two years and whose results pointed once more to a positive association of peri/postmenopausal status with lung cancer 39.
Our result should be interpreted cautiously, because residual confounding by smoking was still possible after adjustment by CSI, however the OR was increased even among the never smokers. Some peculiarities in smoking patterns of pre- and peri/postmenopausal women in our pooled dataset may have influenced the results. The premenopausal women reported smoking less than the peri/postmenopausal, and the proportion of never smokers was higher (43% vs. 31%). Moreover in the premenopausal there was a shift towards low CSI levels, an index that includes a duration term and is therefore linked to the subjects’ age. In addition, the age of menopause has been shown to be 1 to 2 years earlier among current and former smokers with respect to never smokers40. The anti-estrogenic effect of smoking was confirmed in our study, with a mean age at natural menopause of 50.3, 48.9 and 48.6 years respectively for never, former and current smokers.
Bilateral oophorectomy is known to be associated with lower risks of ovarian and breast cancers, but few studies have examined its association with lung cancer. Our results are consistent with some previous studies that have observed an increased lung cancer risk among women with oophorectomies 37, 41, 42, while one study showed no statistically significant association 43. The effect of a sudden and rapid decrease in circulating estrogen levels that occurs after a bilateral oophorectomy has been proposed as a possible hypothesis to justify the increased risk 22. Some studies hypothesized that the association could be explained by long-term use of hormone replacement therapy prescribed to oophorectomized women 22, 44. However, this explanation seems to be unlikely, as a previously published ILCCO pooled study demonstrated a protective effect of exogenous hormones, either oral contraceptives or hormone replacement therapy 45.
Parity was not associated with lung cancer in our analysis, when considering the whole pooled dataset. However, after stratifying by menopausal status, a statistically significant 74% increased risk was found among premenopausal women, and was corroborated by a significant trend with the number of children, with an almost tripling risk for those who had had 3 children or more. On the contrary, a weak inverse association between lung cancer and parity was shown among peri/postmenopausal subjects, with no dose-response trend. Moreover, the tests for interaction were highly significant. The effect exerted by parity was more evident in premenopausal women, who were younger and smoked less, while it seemed to be masked in subjects not menstruating anymore, who were already at higher risk because of their menopausal status. However, our results in women still menstruating must be interpreted cautiously, because they appeared to be mainly driven by two of the pooled studies (WELD and MLCS). Should this association be confirmed in other studies, mechanisms are likely complex and may depend on many different factors. At each childbirth, parous women experience a multitude of changes that may have a transient influence on their risk of cancer: first of all a huge hormonal derangement, but also radical changes in smoking habits, exposure to infectious agents and occupational hazards, diet, hours of sleep and other lifestyle or biological conditions.
The lack of association of parity with lung cancer in the whole study population, and the weak inverse association among peri/postmenopausal women that we observed in the present pooled analysis confirm most of the existing literature, considering that the studies published to date included mainly peri/postmenopausal women, as explained above. It should be noted, however, that the endpoints may differ among studies (e.g., pregnancies instead of parity, or different categorizations of the number of children), therefore any comparison should be cautious. Non-significant inverse associations were reported in a recent cohort study 32 and in the meta-analysis of 19 studies performed by Zhang and co-workers 12. The relative risk per livebirth was close to unity in a meta-analysis of 16 studies 13 (including LCSS, MLCCCS, MLCS and WELD), and in the EAGLE study 14. Statistically significant inverse associations were shown instead in two recent Singaporean studies 31, 33.
Regarding the studies conducted exclusively on peri/postmenopausal women, three studies have found no association with parity 44, 46, with a modest significantly increased risk in those with five children or more 20.
Two studies examined the association between lung cancer and parity after stratifying by age, which might be a proxy of menopausal status: a Canadian cohort study did not observe any difference by age (40–49 vs. 50–59 years) 47, while a preliminary analysis of the LCSS case-control study (performed on half the subjects that have been included in the present pooled analysis) found no risk in the subgroup of women aged less than 50 years, and a statistically significant negative association in those 50 years old or more 17.
Only one published study to date carefully examined the effect of parity by menopausal status. It reported on a part of the WELD subjects included in the present pooled analysis, and found a statistically significant positive association with lung cancer in premenopausal women, with no effect in peri/postmenopausal women 34.
In our analysis, breastfeeding was associated with a slightly reduced risk, when considering the whole study population, but the risk was halved, and statistically significant, in the subgroup of premenopausal women. Few studies have examined this issue to date, and they found no significant association, in substantial agreement with the result we obtained in the whole group 32, 44 22, 36.
Some associations seemed to be slightly strengthened when attention was restricted to never smokers, in particular those with menopause reason, parity and number of children, although no significant interaction could be demonstrated according to smoking status. It is unclear whether this result could be related to the fact that about 20% of never smokers were premenopausal. On the other hand, the positive association with oophorectomy was statistically significant only in current smokers. No major differences were found in our analysis according to cancer histology as well. Sparse and inconsistent results have been shown in the published literature, when stratifying by smoking habits or cancer histology.
In conclusion, the effects of gynecologic and obstetric factors on lung cancer development are difficult to disentangle, possibly due to their complex interactions with other host or environmental factors. For example, cigarette smoking decreases hormone levels and causes early menopause, while estrogens produced in adipose tissue after menopause may partly explain the relationship between BMI and lung cancer. Multiple pathways of estrogen action exist and estrogen levels have never been measured in lung cancer patients, so that their role remains an open question20. The pattern that we found in lung cancer is in contrast with what has been demonstrated for breast cancer, for which late menopause and low parity are well-established risk factors. On the other hand, it appears to be similar to what has been observed among thyroid cancer patients: the risk decreased with increasing age at menopause and ovariectomy, and decreased with breastfeeding48.
Biological mechanisms are far from being elucidated, but it is possible that exposure to fluctuating levels of hormones for decades plaid different roles in different tissues, according to their specific hormone receptors.
Strengths and limitations of the study
The main strength of our study lies in its size: 4386 cases and 4177 controls were pooled from eight different studies. This allowed us to have considerable statistical power to conduct separate analyses by menopausal status and by lung cancer histological type and to examine potential effect modification by smoking status.
Bias due to differential reporting of hormonal and reproductive factors by cases and controls was unlikely, because a potential role for these factors in lung cancer risk has rarely been mentioned in popular media, and questionnaires investigated a variety of factors.
Another major strength of this study was the detailed assessment of smoking history across studies. This is important in an analysis of hormonal factors and lung cancer, as women smokers generally have lower estrogen levels 49. We used one parsimonious measure (i.e., the CSI), which incorporates various measures of smoking: duration, intensity and time since cessation. Nonetheless, residual confounding by smoking is still possible.
The principal limitation of data from epidemiological studies by using pooled analysis is heterogeneity 50. One of the main potential sources of heterogeneity is the design of studies to be combined, however almost all those in our sample had a case-control design, except one case-control study nested in a cohort, and no cohort study has been included. The exposure under investigation and the covariables may also vary across studies, most commonly because of differing approaches to definition or measurement. To overcome this potential heterogeneity, we performed a careful standardization of the original data and we excluded studies which had not precisely collected the information we needed.
Another limitation of our study was the inclusion of studies conducted exclusively in North American and European countries, where the population is largely of Caucasian descent and tobacco consumption levels among women were typically higher than in other parts of the world.
Lastly, we lacked information regarding occupational exposures. However, this did not seem a great concern, because occupational exposures possibly posing an increased lung cancer risk have generally a very low prevalence among women.
In summary, there is evidence that menstrual and reproductive factors may play a role in the genesis of lung cancer, yet the mechanisms are unclear. While smoking remains the most important modifiable factor associated with lung cancer, understanding the role of hormones and the potential effect modification by smoking in lung carcinogenesis may provide further insights into the etiology of the disease, particularly for women. Our results suggest that menopause may increase the risk, while a positive association of parity with lung cancer is suggested among premenopausal women. Nonetheless, more investigations in large well-designed studies are needed to confirm these findings and to clarify the underlying mechanisms.
Supplementary Material
Novelty and Impact.
There is increasing evidence for the effects of sex steroid hormones in non-reproductive organs. Our study suggests that menstrual and reproductive factors may play a role in lung carcinogenesis. In particular, menopausal status represented a risk factor, while premenopausal women showed increased risks with parity and number of children.
Acknowledgments
EAGLE study was funded by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics, with partial funding from the Lombardy Region (Environmental Epidemiology Program) and CARIPLO Foundation, Milan, Italy.
LCSS Study (Harvard Lung Cancer Study) was supported by the National Institutes of Health, and National Cancer Institute (Grants CA092824, CA074386, CA090578, ES00002)
Curtis C Harris was supported by The Intramural Research Program of the National Cancer Institute, NIH, Bethesda, Md
Monica Neri’s work was partially supported by the University of Genoa, Genoa, Italy
NELCS: The New England Lung Cancer Study was funded by Grant Number P20RR018787 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH).
TORONTO Study was supported by Canadian Cancer Society Research Institute (no. 020214)
WELD Study was supported by NIH R01CA87895, P30CA022453 and HHSN261201300011I.
The ILCCO data management was supported by Cancer Care Ontario and Lunenfeld-Tanenbaum Research Institute, Sinai Health System
Abbreviations
- OR
odds ratio
- CI
confidence interval
- CSI
comprehensive smoking index
Footnotes
Conflict of interest:
The authors declare that they have no conflict of interest.
References
- 1.Remon J, Molina-Montes E, Majem M, Lianes P, Isla D, Garrido P, Felip E, Vinolas N, de Castro J, Artal A, Sanchez MJ. Lung cancer in women: an overview with special focus on Spanish women. Clin Transl Oncol. 2014;16:517–28. doi: 10.1007/s12094-013-1137-7. [DOI] [PubMed] [Google Scholar]
- 2.Patel JD. Lung Cancer in Women. Journal of Clinical Oncology. 2005;23:3212–8. doi: 10.1200/JCO.2005.11.486. [DOI] [PubMed] [Google Scholar]
- 3.Lortet-Tieulent J, Soerjomataram I, Ferlay J, Rutherford M, Weiderpass E, Bray F. International trends in lung cancer incidence by histological subtype: Adenocarcinoma stabilizing in men but still increasing in women. Lung Cancer. 2014;84:13–22. doi: 10.1016/j.lungcan.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Belani CP, Marts S, Schiller J, Socinski MA. Women and lung cancer: epidemiology, tumor biology, and emerging trends in clinical research. Lung cancer (Amsterdam, Netherlands) 2007;55:15–23. doi: 10.1016/j.lungcan.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Francisci S, Minicozzi P, Pierannunzio D, Ardanaz E, Eberle A, Grimsrud TK, Knijn A, Pastorino U, Salmeron D, Trama A, Sant M. European journal of cancer. Oxford, England: 2015. Survival patterns in lung and pleural cancer in Europe 1999–2007: Results from the EUROCARE-5 study. 1990. [DOI] [PubMed] [Google Scholar]
- 6.Sagerup CM, Smastuen M, Johannesen TB, Helland A, Brustugun OT. Sex-specific trends in lung cancer incidence and survival: a population study of 40,118 cases. Thorax. 2011;66:301–7. doi: 10.1136/thx.2010.151621. [DOI] [PubMed] [Google Scholar]
- 7.Debieuvre D, Oster JP, Riou R, Berruchon J, Levy A, Mathieu JP, Dumont P, Leroy-Terquem E, Tizon-Couetil V, Martin F, Grivaux M. The new face of non-small-cell lung cancer in men: Results of two French prospective epidemiological studies conducted 10 years apart. Lung Cancer. 2016;91:1–6. doi: 10.1016/j.lungcan.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Schmeck B, Bertrams W, Lai X, Vera J. Systems Medicine for Lung Diseases: Phenotypes and Precision Medicine in Cancer, Infection, and Allergy. Methods in molecular biology (Clifton, NJ) 2016;1386:119–33. doi: 10.1007/978-1-4939-3283-2_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sathish V, Martin YN, Prakash YS. Sex steroid signaling: Implications for lung diseases. Pharmacology & Therapeutics. 2015;150:94–108. doi: 10.1016/j.pharmthera.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siegfried JM, Stabile LP. Estrongenic steroid hormones in lung cancer. Semin Oncol. 2014;41:5–16. doi: 10.1053/j.seminoncol.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao YT, Blot WJ, Zheng W, Ershow AG, Hsu CW, Levin LI, Zhang R, Fraumeni JF., Jr Lung cancer among Chinese women. International journal of cancer Journal international du cancer. 1987;40:604–9. doi: 10.1002/ijc.2910400505. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Yin Z, Shen L, Wan Y, Zhou B. Menstrual factors, reproductive factors and lung cancer risk: a meta-analysis. Zhongguo Fei Ai Za Zhi. 2012;15:701–19. doi: 10.3779/j.issn.1009-3419.2012.12.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dahabreh IJ, Trikalinos TA, Paulus JK. Parity and risk of lung cancer in women: systematic review and meta-analysis of epidemiological studies. Lung Cancer. 2012;76:150–8. doi: 10.1016/j.lungcan.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pesatori AC, Carugno M, Consonni D, Caporaso NE, Wacholder S, Tucker M, Landi MT. Reproductive and hormonal factors and the risk of lung cancer: the EAGLE study. International journal of cancer Journal international du cancer. 2013;132:2630–9. doi: 10.1002/ijc.27926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hung RJ, Christiani DC, Risch A, Popanda O, Haugen A, Zienolddiny S, Benhamou S, Bouchardy C, Lan Q, Spitz MR, Wichmann HE, LeMarchand L, et al. International Lung Cancer Consortium: pooled analysis of sequence variants in DNA repair and cell cycle pathways. Cancer Epidemiol Biomarkers Prev. 2008;17:3081–9. doi: 10.1158/1055-9965.EPI-08-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raum E, Rothenbacher D, Low M, Stegmaier C, Ziegler H, Brenner H. Changes of cardiovascular risk factors and their implications in subsequent birth cohorts of older adults in Germany: a life course approach. European journal of cardiovascular prevention and rehabilitation : official journal of the European Society of Cardiology, Working Groups on Epidemiology & Prevention and Cardiac Rehabilitation and Exercise Physiology. 2007;14:809–14. doi: 10.1097/HJR.0b013e3282eeb308. [DOI] [PubMed] [Google Scholar]
- 17.Paulus JK, Asomaning K, Kraft P, Johnson BE, Lin X, Christiani DC. Parity and risk of lung cancer in women. Am J Epidemiol. 2010;171:557–63. doi: 10.1093/aje/kwp441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meinhold CL, Berrington de Gonzalez A, Bowman ED, Brenner AV, Jones RT, Lacey JV, Jr, Loffredo CA, Perlmutter D, Schonfeld SJ, Trivers GE, Harris CC. Reproductive and hormonal factors and the risk of nonsmall cell lung cancer. International journal of cancer Journal international du cancer. 2011;128:1404–13. doi: 10.1002/ijc.25434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brenner DR, Hung RJ, Tsao MS, Shepherd FA, Johnston MR, Narod S, Rubenstein W, McLaughlin JR. Lung cancer risk in never-smokers: a population-based case-control study of epidemiologic risk factors. BMC Cancer. 2010;10:285. doi: 10.1186/1471-2407-10-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartz AG, Ray RM, Cote ML, Abrams J, Sokol RJ, Hendrix SL, Chen C, Chlebowski RT, Hubbell FA, Kooperberg C, Manson JE, O’Sullivan MJ, et al. Hormone Use, Reproductive History, and Risk of Lung Cancer: The Women’s Health Initiative Studies. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2015;10:1004–13. doi: 10.1097/JTO.0000000000000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heck JE, Andrew AS, Onega T, Rigas JR, Jackson BP, Karagas MR, Duell EJ. Lung cancer in a U.S. population with low to moderate arsenic exposure. Environmental health perspectives. 2009;117:1718–23. doi: 10.1289/ehp.0900566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koushik A, Parent ME, Siemiatycki J. Characteristics of menstruation and pregnancy and the risk of lung cancer in women. Int J Cancer. 2009;125:2428–33. doi: 10.1002/ijc.24560. [DOI] [PubMed] [Google Scholar]
- 23.Landi MT, Consonni D, Rotunno M, Bergen AW, Goldstein AM, Lubin JH, Goldin L, Alavanja M, Morgan G, Subar AF, Linnoila I, Previdi F, et al. Environment And Genetics in Lung cancer Etiology (EAGLE) study: an integrative population-based case-control study of lung cancer. BMC public health. 2008;8:203. doi: 10.1186/1471-2458-8-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leffondre K, Abrahamowicz M, Xiao Y, Siemiatycki J. Modelling smoking history using a comprehensive smoking index: application to lung cancer. Stat Med. 2006;25:4132–46. doi: 10.1002/sim.2680. [DOI] [PubMed] [Google Scholar]
- 25.Papadopoulos A, Guida F, Leffondré K, Cénée S, Cyr D, Schmaus A, Radoï L, Paget-Bailly S, Carton M, Menvielle G, Woronoff AS, Tretarre B, et al. Heavy smoking and lung cancer: Are women at higher risk? Result of the ICARE study. British journal of cancer. 2014;110:1385–91. doi: 10.1038/bjc.2013.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fritz AGPC, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, Whelan S. International Classification of Diseases for Oncology. 3. World Health Organization; Geneva, Switzerland: 2000. [Google Scholar]
- 27.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.RevMan RM. In: Copenhagen: The Nordic Cochrane Centre. Collaboration TC, editor. 2012. Version 5.2. [Google Scholar]
- 29.Slowikowski BK, Lianeri M, Jagodzinski PP. Exploring estrogenic activity in lung cancer. Molecular biology reports. 2016 doi: 10.1007/s11033-016-4086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siegfried JM, Hershberger PA, Stabile LP. Estrogen receptor signaling in lung cancer. Semin Oncol. 2009;36:524–31. doi: 10.1053/j.seminoncol.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim WY, Chen Y, Chuah KL, Eng P, Leong SS, Lim E, Lim TK, Ng A, Poh WT, Tee A, Teh M, Salim A, et al. Female reproductive factors, gene polymorphisms in the estrogen metabolism pathway, and risk of lung cancer in Chinese women. Am J Epidemiol. 2012;175:492–503. doi: 10.1093/aje/kwr332. [DOI] [PubMed] [Google Scholar]
- 32.Gallagher LG, Rosenblatt KA, Ray RM, Li W, Gao DL, Applebaum KM, Checkoway H, Thomas DB. Reproductive factors and risk of lung cancer in female textile workers in Shanghai, China. Cancer Causes Control. 2013;24:1305–14. doi: 10.1007/s10552-013-0208-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan HS, Tan MH, Chow KY, Chay WY, Lim WY. Reproductive factors and lung cancer risk among women in the Singapore Breast Cancer Screening Project. Lung cancer (Amsterdam, Netherlands) 2015;90:499–508. doi: 10.1016/j.lungcan.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz AG, Wenzlaff AS, Prysak GM, Murphy V, Cote ML, Brooks SC, Skafar DF, Lonardo F. Reproductive factors, hormone use, estrogen receptor expression and risk of non small-cell lung cancer in women. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25:5785–92. doi: 10.1200/JCO.2007.13.3975. [DOI] [PubMed] [Google Scholar]
- 35.Wu AH, Yu MC, Thomas DC, Pike MC, Henderson BE. Personal and family history of lung disease as risk factors for adenocarcinoma of the lung. Cancer research. 1988;48:7279–84. [PubMed] [Google Scholar]
- 36.Taioli E, Wynder EL. Re: Endocrine factors and adenocarcinoma of the lung in women. Journal of the National Cancer Institute. 1994;86:869–70. doi: 10.1093/jnci/86.11.869. [DOI] [PubMed] [Google Scholar]
- 37.Kreuzer M, Gerken M, Heinrich J, Kreienbrock L, Wichmann HE. Hormonal factors and risk of lung cancer among women? International Journal of Epidemiology. 2003;32:263–71. doi: 10.1093/ije/dyg064. [DOI] [PubMed] [Google Scholar]
- 38.Matsuo K, Ito H, Yatabe Y, Hiraki A, Hirose K, Wakai K, Kosaka T, Suzuki T, Tajima K, Mitsudomi T. Risk factors differ for non-small-cell lung cancers with and without EGFR mutation: assessment of smoking and sex by a case-control study in Japanese. Cancer science. 2007;98:96–101. doi: 10.1111/j.1349-7006.2006.00347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiss JM, Lacey JV, Jr, Shu XO, Ji BT, Hou L, Yang G, Li H, Rothman N, Blair A, Gao YT, Chow WH, Zheng W. Menstrual and reproductive factors in association with lung cancer in female lifetime nonsmokers. American journal of epidemiology. 2008;168:1319–25. doi: 10.1093/aje/kwn257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Midgette AS, Baron JA. Cigarette smoking and the risk of natural menopause. Epidemiology. 1990;1:474–80. doi: 10.1097/00001648-199011000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Brinton LA, Gierach GL, Andaya A, Park Y, Schatzkin A, Hollenbeck AR, Spitz MR. Reproductive and hormonal factors and lung cancer risk in the NIH-AARP Diet and Health Study cohort. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20:900–11. doi: 10.1158/1055-9965.EPI-10-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parker WH, Broder MS, Chang E, Feskanich D, Farquhar C, Liu Z, Shoupe D, Berek JS, Hankinson S, Manson JE. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the nurses’ health study. Obstet Gynecol. 2009;113:1027–37. doi: 10.1097/AOG.0b013e3181a11c64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jacoby VL, Grady D, Wactawski-Wende J, Manson JE, Allison MA, Kuppermann M, Sarto GE, Robbins J, Phillips L, Martin LW, O’Sullivan MJ, Jackson R, et al. Oophorectomy vs ovarian conservation with hysterectomy: cardiovascular disease, hip fracture, and cancer in the Women’s Health Initiative Observational Study. Arch Intern Med. 2011;171:760–8. doi: 10.1001/archinternmed.2011.121. [DOI] [PubMed] [Google Scholar]
- 44.Liu Y, Inoue M, Sobue T, Tsugane S. Reproductive factors, hormone use and the risk of lung cancer among middle-aged never-smoking Japanese women: a large-scale population-based cohort study. International journal of cancer Journal international du cancer. 2005;117:662–6. doi: 10.1002/ijc.21229. [DOI] [PubMed] [Google Scholar]
- 45.Pesatori AC, Carugno M, Consonni D, Hung RJ, Papadoupolos A, Landi MT, Brenner H, Muller H, Harris CC, Duell EJ, Andrew AS, McLaughlin JR, et al. Hormone use and risk for lung cancer: a pooled analysis from the International Lung Cancer Consortium (ILCCO) British journal of cancer. 2013;109:1954–64. doi: 10.1038/bjc.2013.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baik CS, Strauss GM, Speizer FE, Feskanich D. Reproductive factors, hormone use, and risk for lung cancer in postmenopausal women, the Nurses’ Health Study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2010;19:2525–33. doi: 10.1158/1055-9965.EPI-10-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kabat GC, Miller AB, Rohan TE. Reproductive and hormonal factors and risk of lung cancer in women: a prospective cohort study. International journal of cancer Journal international du cancer. 2007;120:2214–20. doi: 10.1002/ijc.22543. [DOI] [PubMed] [Google Scholar]
- 48.Cordina-Duverger E, Leux C, Neri M, Tcheandjieu C, Guizard A-V, Schvartz C, Truong T, Guénel P. Hormonal and reproductive risk factors of papillary thyroid cancer: A population-based case-control study in France. Cancer Epidemiology. 48:78–84. doi: 10.1016/j.canep.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 49.Westhoff C, Gentile G, Lee J, Zacur H, Helbig D. Predictors of ovarian steroid secretion in reproductive-age women. American journal of epidemiology. 1996;144:381–8. doi: 10.1093/oxfordjournals.aje.a008939. [DOI] [PubMed] [Google Scholar]
- 50.Colditz GA, Burdick E, Mosteller F. Heterogeneity in meta-analysis of data from epidemiologic studies: a commentary. American journal of epidemiology. 1995;142:371–82. doi: 10.1093/oxfordjournals.aje.a117644. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.