Abstract
Background
Given that continued smoking after a cancer diagnosis increases the risk for adverse health outcomes, cancer patients are strongly advised to quit. Despite a current lack of evidence regarding their safety and effectiveness as a cessation tool, electronic cigarettes (e-cigarettes) are becoming increasingly popular. In order to guide oncologists’ communication with their patients about e-cigarette use, this paper provides the first published clinical data about e-cigarette use and cessation outcomes among cancer patients.
Methods
Participants (n=1074) included smokers (cancer patients) who recently enrolled in a tobacco treatment program at a comprehensive cancer center. Standard demographic, tobacco use history and follow-up cessation outcomes were assessed.
Results
A threefold increase in e-cigarette use was observed from 2012 to 2013 (10.6% vs. 38.5%). E-cigarette users were more nicotine dependent than non-users, had more prior quit attempts, and were more likely to be diagnosed with thoracic and head or neck cancers. Using a complete case analysis, e-cigarette users were as likely to be smoking at follow-up as non-users, (OR: 1.0; 95%CI 0.5–1.7). Using an intention to treat analysis, e-cigarette users were twice as likely to be smoking at follow-up as non-users, (OR: 2.0; 95%CI 1.2–3.3).
Conclusions
The high rate of e-cigarette use observed is consistent with recent papers highlighting increased e-cigarette use in the general population. Our longitudinal findings raise doubt about the utility of e-cigarettes for facilitating smoking cessation among cancer patients. Further research is needed to evaluate the safety and efficacy of e-cigarettes as a cessation treatment for cancer patients.
Keywords: Smoking Cessation, Tobacco, Cancer, E-cigarettes
Introduction
Continued smoking after a cancer diagnosis increases the risk for adverse health outcomes including treatment-related complications, disease recurrence, poor quality of life, second primary cancers and mortality.1–4 As a result of these known risks, leading oncology professional organizations recommend that all cancer patients should be asked about their smoking status, advised to quit, and assisted with smoking cessation.5–8 Nonetheless, approximately two-thirds of adult cancer survivors who were smoking at the time of their cancer diagnosis continue to smoke after diagnosis.9 The prevalence of continued smoking and the growing awareness of the importance of integrating smoking cessation into routine cancer care have prompted the American Society of Clinical Oncology (ASCO) to launch a campaign for oncologists to deliver evidence-based tobacco treatment consistent with the USDHHS Clinical Practice Guideline for Treating Tobacco Use and Dependence (2008).10 Several patient, provider and systems-level barriers must be addressed to insure that best practices for treating tobacco dependence are implemented in routine cancer care.11–13
Identified as a “disruptive technology” in the tobacco control field,14 there is much current debate on whether e-cigarettes will facilitate or impede smoking cessation and reduction of known hazards of traditional cigarettes and other combustible tobacco products.15, 16 Clinical anecdotes suggest that the increased popularity of e-cigarettes has prompted uncertainty within oncology and other medical specialties about how to best advise patients about the safety and efficacy of e-cigarettes as a cessation tool. E-cigarettes are battery-powered devices that mimic the hand-to-mouth sensory experience of smoking and typically deliver nicotine to the user. E-cigarettes have gained rapid popularity since 2007 when they were first introduced into the U.S. marketplace.17 As of April 2014, the U.S. Federal Drug Administration (FDA) does not have the authority to regulate e-cigarettes; however, they have issued a statement that they intend to issue a proposed rule extending their tobacco product authority to include e-cigarettes. With limited data to guide e-cigarette regulation, establishing public health policy has been vexing for tobacco control. Despite limited data about e-cigarette safety,15 adult ever use nearly quadrupled from 2010 to 2012, with 10.6% of all adults in the United States and 44.6% of current smokers report having ever used e-cigarettes.18
Cigarette smokers report using e-cigarettes to manage nicotine cravings and withdrawal symptoms, to reduce daily smoking consumption, and to quit smoking or avoid relapsing.19 E-cigarette users report perceptions that e-cigarettes are less harmful than traditional cigarettes.19 There are also a growing number of dual tobacco users who supplement conventional cigarette smoking with e-cigarettes in environments where traditional combustible smoking is prohibited.20 Many smokers report using e-cigarettes to help them quit using traditional cigarettes and smokers with strong intentions to quit are significantly more likely to have ever used e-cigarettes than those with no intentions of quitting21, 22. Vickerman and colleagues found that 31% of Quitline callers (n=2,758) had tried e-cigarettes, and of those 52% reported using e-cigarettes to help them quit smoking traditional cigarettes. 23
Although smokers report expectations that e-cigarettes facilitate cessation, there is currently a lack of adequate evidence that actual use of e-cigarettes actually helps smokers cut down or quit smoking. Two recent clinical trials, one conducted in New Zealand24 and the other conducted in Italy,25 have examined the potential utility of e-cigarettes for helping smokers quit. Based on a relatively small sample, Bullen and colleagues demonstrated that e-cigarettes were about as useful as the nicotine patch in helping people quit smoking.24 Caponnetto and colleagues demonstrated that e-cigarettes may be useful in helping smokers to reduce the number of cigarettes per day, but the study results are not definitive.25
As of April 2014, e-cigarettes are not approved by the FDA for use as a cessation treatment in the U.S. In the absence of adequate clinical data on the safety and efficacy of e-cigarettes, the Tobacco Control and Smoking Cessation Subcommittee of the International Association for the Study of Lung Cancer (IASLC) has recently published a policy statement counseling physicians against recommending the use of e-cigarettes among cancer patients advised to quit.26
In order to guide oncologists’ communication with smokers about e-cigarettes, this paper is intended to provide much-needed data about e-cigarette use among cancer patients. The specific objectives were: 1) to identify the prevalence of e-cigarette use among smokers referred to an on-site, tobacco treatment program at a comprehensive cancer center; 2) to examine recent temporal trends in e-cigarette use; 3) to identify the characteristics of e-cigarette users and compare these characteristics with non e-cigarette users, and 4) to examine whether e-cigarette use is associated with smoking cessation outcomes.
Methods
Participants
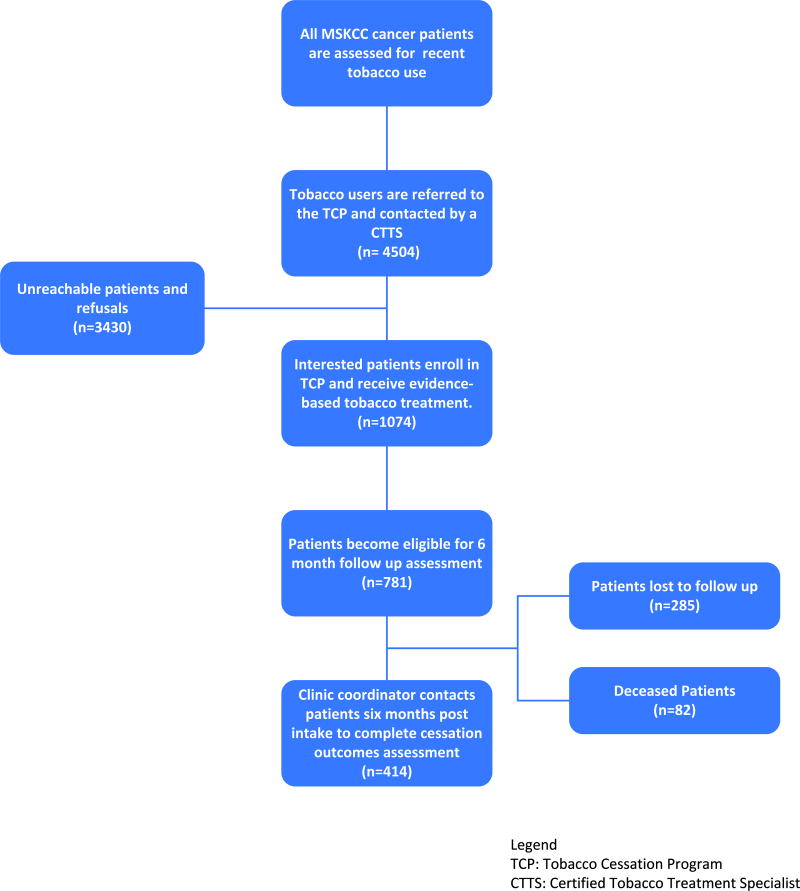
All patients who presented for cancer treatment at Memorial Sloan Kettering Cancer Center (MSKCC) from January, 2012 to December, 2013, were screened and identified as current smokers. Referrals are made to the Tobacco Cessation Program (TCP) from all oncology clinics and those patients who completed a TCP intake assessment are the focus of this investigation. Figure 1 presents patient flow from patient referral to the TCP through program intake and outcomes assessment.
Figure 1.
Patient Flow
Procedures
This study was approved by the Institutional Review Board at MSKCC. As a standard of care, all new MSKCC patients are screened for current smoking status and asked whether they have smoked cigarettes or used other tobacco products within the past 30 days. Current smoking status is documented in the electronic medical record. Patients who report tobacco use within the past 30 days are systematically referred to the MSKCC Tobacco Cessation Program. TCP staff make up to three attempts to contact referrals either by telephone or bedside for hospitalized patients. Multi-component, evidence-based behavioral and pharmacologic treatment for tobacco dependence is provided. Patients who accept tobacco treatment are offered up to five sessions of individual telephone or in-person behavioral counseling with one of MSKCC’s Certified Tobacco Treatment Specialists (CTTS), designated oncology nurses and nurse practitioners who have undergone specialized training and met requirements for certification as a tobacco treatment specialist (http://attudaccred.org/). An intake assessment is completed and an evidence-based, tobacco dependence treatment plan including behavior modification and cessation medication recommended by the Clinical Practice Guidelines for Treating Tobacco Use and Dependence10 are developed in coordination with the patients’ cancer treatment team. Approximately six to twelve months after each patient enrolls in the TCP, tobacco cessation outcomes data is collected by a Clinic Coordinator.
Measures
The intake assessment includes standard demographic characteristics, tobacco use history (including e-cigarette use), and clinical characteristics as well as the following measures:
Assessment of e-cigarette use
Current e-cigarette use was assessed at the intake for all newly referred patients. Patients were asked, “Have you used e-cigarettes in the past 30 days?” with the response options being yes/no. For the sake of brevity, henceforth we call this variable “e-cigarette use” with the understanding that it was assessed for the past 30 days.
Fagerström Test for Nicotine Dependence (FTND)
Nicotine dependence was assessed by using the Fagerstrom Test for Nicotine Dependence27 a 6-item scale (range 0–10) with scores 0–4 coded as lower and scores of 5 or greater27 indicating higher nicotine dependence.
Cessation Outcome
At about six to twelve months after the initial intake, a trained Clinical Coordinator made up to three attempts to contact patients by telephone to collect smoking cessation outcomes. Patients were asked, “Have you smoked even a puff of a (traditional) cigarette in the last seven days?” Patients answering yes were then asked “How many cigarettes per day do you currently smoke?” Patients were also asked “Since [date of intake assessment] have you gone at least 24 hours without a cigarette?” No biochemical verification of smoking cessation was obtained.
Data analysis
Data were first described graphically and by descriptive statistics. Abstinence rates were calculated with two methods for handling missing data: (1) modified Intent-to-Treat (ITT), assuming all participants lost to follow-up are smoking and, (2) Complete-Case Analysis (CCA), eliminating participants lost to follow-up from the analysis.28, 29 Consistent with recommendations for handling missing data when reporting cessation outcomes30, 31, we report both ITT and CCA because they represent, respectively, the full range of conservative and liberal interpretations of smoking abstinence outcomes. We used the χ2 statistic to examine associations between e-cigarette use reported at intake and putative baseline contributors (e.g., gender, education level, and cancer diagnosis). Continuous variables assessed at intake and outcome (e.g., age, Fagerstrom score, and average number of cigarettes per day) were evaluated using independent-sample t-tests between e-cigarette users and non-users. We also analyzed the influence of e-cigarette use at intake on tobacco abstinence assessed at 6–12 months follow-up in a logistic regression model. The logistic regression model also included participant characteristics that had a statistically discernible contribution to e-cigarette use, as shown in the χ2 statistics and t-tests. For example, nicotine dependence, quitting history and cancer diagnosis were included in the model. To minimize the impact of multi-collinearity in model predictors, variables that were highly correlated with each other were excluded from the model (e.g., nicotine dependence and average number of cigarettes per day). The logistic regression was intended to examine whether or not e-cigarette use, as well as additional contributing factors of e-cigarette use, were associated with follow-up smoking abstinence outcomes. Analyses were carried out using the PASW version 18 (SPSS Inc, Chicago, IL, USA).
Results
Patient Characteristics
From January 1, 2012 to December 31, 2013, the MSKCC Tobacco Cessation Program received 4,504 referrals, 1074 of these patients accepted the referral, enrolled and completed a clinical intake assessment. Table 1 presents patient characteristics.
Table 1.
Prevalence of e-cigarette use at intake by patient characteristics (n=1074).
| E-cigarette use | No e-cigarette use |
||||
|---|---|---|---|---|---|
|
|
|||||
| Total (n = 1074) | (n = 285)* | (n = 789) | p-values a | ||
|
|
|||||
| Gender | Female | 607 (56) | 172 (60) | 435 (55) | - |
| Education level | |||||
| 7-11th Grade | 21 (3) | 8 (4) | 13 (3) | - | |
| High School Graduate | 257 (42) | 86 (43) | 171 (41) | ||
| Partial College | 101 (16) | 32 (16) | 69 (16) | ||
| College Graduate | 200 (32) | 65 (32) | 135 (32) | ||
| Graduate Degree/Professional Training | 38 (6) | 9 (4) | 29 (7) | ||
| Number of past quit attempts | p = 0.012 | ||||
| Never or one time | 311 (31) | 60 (24) | 251 (33) | ||
| Two or more times | 698 (69) | 195 (76) | 503 (67) | ||
| Cancer diagnosis | - | ||||
| Breast | 158 (15) | 37 (13) | 121 (16) | ||
| Colorectal | 64 (6) | 20 (7) | 44 (6) | ||
| Genitourinary | 85 (8) | 25 (9) | 60 (8) | ||
| Gynecological | 59 (6) | 16 (6) | 43 (6) | ||
| Head and neck | 103 (10) | 35 (12) | 68 (9) | ||
| Hepatobiliary | 65 (6) | 16 (6) | 49 (6) | ||
| Thoracic | 210 (20) | 67 (24) | 143 (18) | ||
| Urology | 55 (5) | 10 (4) | 45 (6) | ||
| Other | 264 (25) | 56 (20) | 208 (27) | ||
|
|
|||||
| Average ± stdev | Average ± stdev | ||||
|
|
|||||
| Age (yrs) | 56.3 ± 11.8 | 55.6 ± 10.9 | - | ||
| Longest time without cigarette (days) | 5.8 ± 6.1 | 7.4 ± 9.8 | p = 0.012 | ||
| Number of cigarettes per day | 13.7 ± 9.8 | 12.4 ± 9.2 | p = 0.047 | ||
| Nicotine dependence (Fagerstrom score) | 4.6 ± 2.7 | 3.3 ± 2.7 | p < 0.001 | ||
Data are given as sample size and percentages except where noted. Counts do not always add up to the total sample size due to missing responses.
p-values were calculated using chi-square statistics for categorical variables and independent-sample t-test for continuous variables; p-values greater than 0.05 were omitted.
About half of the patients that completed an intake in 2012 and 2013 (n=1074), were female (56.5%, n=607), and 43.5% (n=467) were male. The mean age was 56 years old (SD ± 11.6; range 18–87). The majority of patients were high school graduates (41.7%, n=257) or college graduates (32.4%, n=200). Most patients had tried quitting at least twice before entering the program (69.2%, n=698). The mean Fagerstrom score was 3.7 (SD ± 2.7, range 0–10). About one third reported high nicotine dependence, as evidenced by Fagerstrom scores ≥ 5 (37.2%, n=371). At intake, the average number of cigarettes per day (CPD) was 13.0 (SD ± 9.4; range 0–50). The highest proportion of patients were diagnosed with thoracic cancer (19.8%, n=210), breast cancer (14.9%, n=158), head and neck cancers (9.7%, n=103) or genitourinary cancers (8%, n=85). Very few patients reported recent use of cigars (1.6%), and less than 1% reported recent pipe, snuff or chewing tobacco use.
Excluding deceased patients (n=82), smoking cessation outcomes were collected from 414 (59.5%) of the subsample of 699 participants who were eligible for the follow-up assessment at the close of the study period (12/31/2013). We examined potential correlates of lost to follow-up and found no differences in terms of age, gender, prior quit attempts, longest lifetime period of abstinence, nicotine dependence, or cancer diagnosis. A significantly higher proportion of e-cigarette users dropped out of tobacco treatment and were lost to follow-up than non e-cigarette users (66.3% vs 32.4%; P<0.01). Smoking cessation outcomes were collected on average about 10 months from the date of intake (M = 291.9 days, SD ± 86.5 days). There was no significant difference in smoking cessation outcome based on time to follow-up data collection.
Prevalence and temporal pattern of e-cigarette use
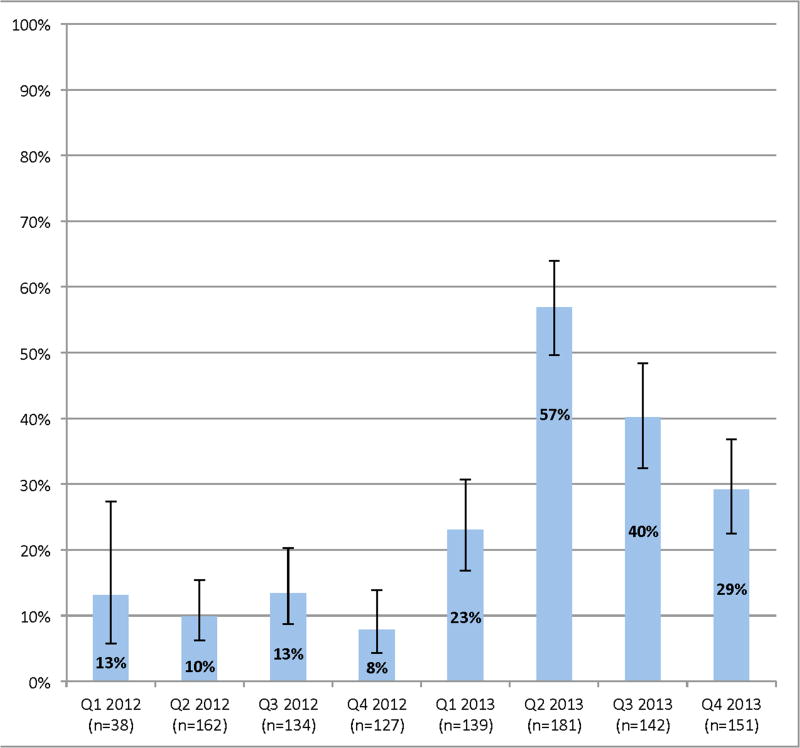
At enrollment, about one fourth (26.5%, n=285) of the total sample reported that they had used e-cigarettes in the past 30 days. Most e-cigarette users (92%) reported dual use of combustible (traditional) cigarettes. To examine how the percentage of e-cigarette use changed over time, Figure 2 depicts changes in percentages per quarter, using all available intake data (n=1074). Figure 2 shows a substantive increase in e-cigarette use between Q1 2012 and Q4 2013. In 2012, the quarterly prevalence of e-cigarette use was relatively stable and on average 10.6% of participants reported e-cigarette use. However, beginning in Q1 of 2013, the percentage of e-cigarette use more than doubled from 2012 and peaked in the Q2 of 2013 at 56.9%. During 2013, 38.5% of patients reported e-cigarette use, representing a threefold increase in self-reported e-cigarette use from 2012 to 2013. The non-overlapping confidence intervals indicate that the growth in e-cigarette use is statistically significant.
Figure 2.
Percentage of patients who report E-cigarette use (n=1074)
Differences between e-cigarette users and non-e-cigarette users
There were no significant demographic differences between e-cigarette users and non-users in terms of age, gender or education (Table 1). A significantly higher proportion of e-cigarette users were thoracic or head and neck cancer patients than were non e-cigarette users (36.2% vs 27.0%; P<0.01). E-cigarette users appeared to be smoking more cigarettes per day than non-users (M=13.7 (SD ± 9.8) versus 12.4 (SD ± 9.2; p <.05)) and reporting higher FTND scores than non-users (M=4.6 (SD ± 2.7) vs. M=3.3 (SD ± 2.7); p<.01). A significantly higher percentage of e-cigarette users were highly nicotine dependent (FTND score ≥ 5), when compared with non-users (51.8% vs 32.2%, respectively; p<0.01). In terms of time to first cigarette (TTFC), 55.1% of e-cigarette users reported smoking within 30 minutes of waking, compared with 39.3% of non-users (p<0.01). Of note, twice as many e-cigarette users reported that it was difficult to keep from smoking in places where it is forbidden (17.7% and 8.6%; p<0.01). In terms of lifetime history of prior quit attempts, e-cigarette users reported more frequent and longer duration of prior quit attempts than non-users. We found that 76.5% of e-cigarette users reported at least two prior quit attempts before entering the TCP, compared with 66.7% of non-users (p<0.01). E-cigarette users also reported longer duration of prior (pre-TCP enrollment) cigarette smoking abstinence than non-users (mean = 7.4 days (SD ± 9.8) for e-cigarette users compared with 5.8 days (SD ± 6.1) for non-users; p = 0.01). E-cigarette users were no different than non-users in terms of quitting motivation and quitting confidence.
E-cigarette use and smoking cessation outcomes
Using a complete case analysis (CCA) model (Table 2), self-reported, 7-day point prevalence smoking abstinence was equivalent for e-cigarette users and non-users (44.4% vs. 43.1%, respectively). Of note, only half of e-cigarette users reported that they had gone without smoking for 24 hours or more (made a 24 hour quit attempt) since enrolling in the TCP, compared with 76% of non e-cigarette users, (P=0.02). After adjusting for nicotine dependence, number of past quit attempts and cancer diagnosis, e-cigarette users were as likely to be smoking at follow-up as non-users (P=0.87), (OR: 1.0; 95% CI 0.5–1.7). At follow-up, e-cigarette users reported smoking a slightly higher average number of traditional cigarettes per day than non-users, but the difference was not statistically significant (P=0.23), (12.3 CPD (SD ± 8.0) vs. 10.1 CPD (SD ± 6.6)). Using an intention to treat (ITT) model of analysis, the self-reported, 7-day point prevalence abstinence rate was twice as high for non e-cigarette users compared with e-cigarette users (30% vs. 14.5%, P<0.01). After adjusting for nicotine dependence, number of past quit attempts and cancer diagnosis, e-cigarette users were twice as likely to be smoking at follow-up compared with non users (P<0.01), (OR: 2.0; 95% CI 1.2–3.3).
Table 2.
Results of two logistic regression models predicting smoking cessation outcomes
| Complete Case Analysis (n=414*)1 | Intent to Treat Analysis (n=699*)2 | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Odds Ratio | 95% Confidence Interval |
p-valuesa | Odds Ratio |
95% Confidence Interval |
p-valuesa | |
|
|
||||||
| E-cigarette | ||||||
| E-cigarette use | .95 | 0.53, 1.72 | - | 2.00 | 1.23, 3.26 | p = 0.005 |
| No e-cigarette use^ | (1.00) | - | (1.00) | - | ||
| Nicotine dependence | ||||||
| High nicotine dependence | 1.22 | 0.78, 1.91 | - | 1.14 | 0.77, 1.69 | - |
| Low nicotine dependence^ | (1.00) | - | (1.00) | - | ||
| Number of past quit attempts | ||||||
| Tried quitting at least twice | 1.77 | 1.14, 2.74 | p = 0.010 | 1.83 | 1.25, 2.67 | p = 0.002 |
| Tried quitting one time or less^ | (1.00) | - | (1.00) | - | ||
| Cancer diagnosis | ||||||
| Thoracic or head and neck | .56 | 0.36, 0.89 | p = 0.014 | 0.71 | 0.48, 1.04 | - |
| Other^ | (1.00) | - | (1.00) | - | ||
Data are given as sample size except where noted. Counts do not always add up to the total sample size due to missing responses and deceased patients.
Complete Case Analysis: Self-reported seven day point prevalence abstinence at six months, excluding lost to follow-up patients.
Intent to Treat analysis: Self-reported seven day point prevalence abstinence at six months, lost to follow-up patients assumed to be smoking.
p-values were calculated using chi-square statistics for categorical variables and independent-sample t-test for continuous variables; p-values greater than 0.05 were omitted.
Reference categories
Discussion
We analyzed data on e-cigarette use from a large, clinical sample of cancer patients who enrolled in a tobacco cessation program at a Comprehensive Cancer Center. To our knowledge, these longitudinal findings represent the first published data on e-cigarette use among cancer patients. We observed a threefold increase in the prevalence of recent (30 day) e-cigarette use from 2012 to 2013. Further, we found that e-cigarette users compared to non-users were more highly nicotine-dependent, diagnosed with cancers of the lung and head and neck and smoked more cigarettes per day than non e-cigarette users. Finally, using both CCA and ITT analyses, we found no evidence that e-cigarette use was associated at follow-up with superior cessation outcomes.
We now discuss the implications and limitations of these findings. The markedly increased rate of e-cigarette use observed is consistent with recently published papers highlighting dramatic rise in the use of e-cigarettes in the general population.32–34 Consistent with recently published observational studies examining use of e-cigarettes and smoking cessation outcomes in the general population,23, 35 our findings raise doubt about the utility of e-cigarettes for facilitating smoking cessation among cancer patients. Accounting for factors that are commonly associated with smoking cessation, using both CCA and ITT, our analyses showed that e-cigarette use does not appear to be associated with a greater likelihood of achieving abstinence from smoking traditional cigarettes. Indeed, in the more conservative ITT analyses, e-cigarette users were twice as likely as non-users to be persistent smokers of traditional cigarettes. There is also no evidence that use of e-cigarettes helped patients reduce their smoking rate. The data also shows that fewer e-cigarette users than non-users reported being abstinent for 24 hours or more (made a quit attempt) during the observation period, further suggesting that use of e-cigarettes may have averted or delayed quit attempts. All in all, although we speculate that patients may be drawn to e-cigarette use for harm reduction, our findings provide no evidence to support oncologists recommending e-cigarette use for cancer patients advised to quit smoking.
While e-cigarette users and non-users were similar in many demographic and clinical characteristics, e-cigarette users were more likely to be diagnosed with either thoracic or head and neck cancer, cancers widely associated with cigarette smoking. Indeed, the prevalence of e-cigarette use increased more rapidly for thoracic and head or neck cancer patients than for patients diagnosed with other cancers. Previous literature has shown that thoracic and head or neck cancer patients are highly motivated to quit smoking36, 37 and our findings suggest that thoracic and head and neck cancer patients who smoke cigarettes may be more influenced by any claims implying that e-cigarettes may help them quit or reduce harm associated with smoking traditional cigarettes. E-cigarette users were more nicotine dependent than non-users. Twice as many e-cigarette users than non-users indicated that it was difficult to keep from smoking in places where it was forbidden. Considering that the overwhelming majority reported dual use, it is plausible that these patients were using e-cigarettes in addition to traditional-cigarettes in order to manage nicotine withdrawal symptoms and the challenges of smoke-free environments. It is hard to know what to make of the observation that e-cigarette users reported more frequent and longer duration of prior quit attempts than non-users. On the one hand, e-cigarette users may be more motivated to quit. On the other hand, e-cigarette use may represent a well-intended effort to “try something new” after multiple failed quitting efforts.
This study shows that among cancer patients in this sample, dual tobacco use is common and e-cigarettes are much more commonly used in conjunction with traditional cigarettes than any other tobacco product. While the prevalence of e-cigarette use increased dramatically during the study period, the prevalence of other types of tobacco products remained quite low in comparison. Although we did not specifically assess risk perceptions regarding the use of e-cigarettes, these findings suggest that cancer patients may perceive e-cigarettes to be less harmful than traditional cigarettes.
The study is not without limitations. First, the findings are drawn from a clinical cohort of patients being treated by a single comprehensive cancer center with a well-established program for treating tobacco dependence in cancer patients. Second, cessation (abstinence) outcome was not biochemically verified reflecting routine clinical practice rather than outcomes procedures that would commonly be used in a controlled clinical trial. Third, although our follow-up rate exceeds or meets that observed in many clinical cohort cessation reports38, 39 (e.g., Quitline outcomes), a higher proportion of e-cigarette users were lost to follow-up such that the ITT analyses should be interpreted with caution. These findings should not be interpreted that e-cigarettes will never help patients quit smoking. There is a clear need for well-controlled research examining the efficacy of e-cigarette use to promote long-term cessation of traditional tobacco among cancer patients. There also remain unanswered questions about the toxicity of e-cigarettes and any specific safety concerns for e-cigarette use among cancer patients. Given the high rate of use observed, e-cigarette use should be included in comprehensive tobacco use assessment for both clinical trials and routine clinical care. Future studies should assess ever and current use (past 30 days) as well as frequency, patterns, reasons and duration of e-cigarette use.
In summary, e-cigarette use appears increasingly common among cancer patients who smoke. E-cigarette users are more nicotine-dependent and appear to have been more engaged in prior quitting efforts than non-users, but are equally or less likely to have quit smoking traditional cigarettes at follow-up. Given the increasing popularity and availability of e-cigarettes in the general population and the strong advice to quit smoking traditional cigarettes at the time of diagnosis, cancer patients are likely to consider use of e-cigarettes. Further controlled research is needed to evaluate the safety and efficacy of e-cigarettes as a cessation treatment for cancer patients. In the meantime, oncologists should advise smokers to quit smoking traditional cigarettes, encourage use of FDA approved cessation medications, refer patients for tobacco cessation counseling and provide education about the potential risks and lack of known benefits of e-cigarette use with regard to long-term cessation. Hospitals should include restrictions on e-cigarette use in their tobacco-free campus and clean indoor air policies. Environmental studies have identified nicotine and other constituents in the vapor emitted by e-cigarette users and some of these chemical constituents may result in respiratory irritation and other as yet unknown health effects.40 Until more is known about the risks and benefits of e-cigarettes for cancer patients, oncologists are likely to struggle with these complexities and have more questions than answers about e-cigarette use. The Tobacco Control and Smoking Cessation Committee of the International Association for the Study of Lung Cancer has recently published a commentary providing guidance for oncologists as to what to recommend to their patients who might be struggling to stop smoking or wondering about e-cigarettes.26 At our institution, in response to staff requests for guidance on how to respond to patient inquiries about the e-cigarette, we have prepared and disseminated responses to a series of Frequently Asked Questionsi (What is an electronic cigarette? Are e-cigarettes safe for use? Are e-cigarettes effective in helping patients quit smoking? Are patients permitted to use e-cigarettes in the hospital? What should I tell my patients about the e-cigarette? What are some of the other concerns about the e-cigarette? Given the importance of promoting tobacco cessation among cancer patients,1 it is imperative to examine whether e-cigarettes help or hinder these efforts.
Acknowledgments
The authors wish to thank Maureen O’Brien, CNS, CTTS, Will Wikle, NP, CTTS and Suhana De-Leon, NP, CTTS, the Tobacco Treatment Specialists and Dionne Birkbeck, Clinic Coordinator of the Memorial Sloan Kettering Tobacco Cessation Program and Lou-Anne David for her assistance with manuscript preparation.
NIH grant Grant support: P30 CA008748.
Footnotes
This work had no specific funding source. There are no financial disclosures from any authors.
Available from Authors
References
- 1.U.S. Department of Health and Human Services. The Health Consequences of Smoking – 50 years of progress: A report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 2.Gajdos C, Hawn MT, Campagna EJ, Henderson WG, Singh JA, Houston T. Adverse effects of smoking on postoperative outcomes in cancer patients. Ann Surg Oncol. 2012;19:1430–1438. doi: 10.1245/s10434-011-2128-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daniel M, Keefe FJ, Lyna P, et al. Persistent smoking after a diagnosis of lung cancer is associated with higher reported pain levels. J Pain. 2009;10:323–328. doi: 10.1016/j.jpain.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warren GW, Kasza KA, Reid ME, Cummings KM, Marshall JR. Smoking at diagnosis and survival in cancer patients. Int J Cancer. 2013;132:401–410. doi: 10.1002/ijc.27617. [DOI] [PubMed] [Google Scholar]
- 5.Toll BA, Brandon TH, Gritz ER, Warren GW, Herbst RS. Assessing tobacco use by cancer patients and facilitating cessation: an American Association for Cancer Research policy statement. Clin Cancer Res. 2013;19:1941–1948. doi: 10.1158/1078-0432.CCR-13-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morgan G, Schnoll RA, Alfano CM, et al. National cancer institute conference on treating tobacco dependence at cancer centers. J Oncol Pract. 2011;7:178–182. doi: 10.1200/JOP.2010.000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarna L, Brown JK, Lillington L, Wewers ME, Brecht ML. Tobacco-control attitudes, advocacy, and smoking behaviors of oncology nurses. Oncol Nurs Forum. 2000;27:1519–1528. [PubMed] [Google Scholar]
- 8.Hanna N. Helping patients quit tobacco: ASCO's efforts to help oncology care specialists. J Oncol Pract. 2013;9:263–264. doi: 10.1200/JOP.2013.001026. [DOI] [PubMed] [Google Scholar]
- 9.Tseng TS, Lin HY, Moody-Thomas S, Martin M, Chen T. Who tended to continue smoking after cancer diagnosis: the national health and nutrition examination survey 1999–2008. BMC Public Health. 2012;12:784. doi: 10.1186/1471-2458-12-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiore M. United States Tobacco Use and Dependence Guideline Panel: Treating tobacco use and dependence: 2008 update. 2008 update ed. Rockville, Md: U.S. Dept. of Health and Human Services, Public Health Service; 2008. [Google Scholar]
- 11.Goldstein AO, Ripley-Moffitt CE, Pathman DE, Patsakham KM. Tobacco use treatment at the U.S. National Cancer Institute's designated Cancer Centers. Nicotine Tob Res. 2013;15:52–58. doi: 10.1093/ntr/nts083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warren GW, Marshall JR, Cummings KM, et al. Addressing tobacco use in patients with cancer: a survey of American Society of Clinical Oncology members. J Oncol Pract. 2013;9:258–262. doi: 10.1200/JOP.2013.001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabatino SA, Coates RJ, Uhler RJ, Pollack LA, Alley LG, Zauderer LJ. Provider counseling about health behaviors among cancer survivors in the United States. J Clin Oncol. 2007;25:2100–2106. doi: 10.1200/JCO.2006.06.6340. [DOI] [PubMed] [Google Scholar]
- 14.Abrams DB. Promise and peril of e-cigarettes: can disruptive technology make cigarettes obsolete? JAMA. 2014;311:135–136. doi: 10.1001/jama.2013.285347. [DOI] [PubMed] [Google Scholar]
- 15.Cobb NK, Abrams DB. E-cigarette or drug-delivery device? Regulating novel nicotine products. N Engl J Med. 2011;365:193–195. doi: 10.1056/NEJMp1105249. [DOI] [PubMed] [Google Scholar]
- 16.Cobb NK, Byron MJ, Abrams DB, Shields PG. Novel nicotine delivery systems and public health: the rise of the "e-cigarette". Am J Public Health. 2010;100:2340–2342. doi: 10.2105/AJPH.2010.199281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang J. Rapid rise of electronic cigarettes: Public health and policy implications; 141st APHA Annual Meeting; November 2-November 6, 2013; APHA. 2013. [Google Scholar]
- 18.McMillen RC, Gottlieb MA, Winickoff JP, Klein J. Three Year Trends In The Use Of Emerging Tobacco Products. Age. 2013;18:24. [Google Scholar]
- 19.Etter JF, Bullen C. Electronic cigarette: users profile, utilization, satisfaction and perceived efficacy. Addiction. 2011;106:2017–2028. doi: 10.1111/j.1360-0443.2011.03505.x. [DOI] [PubMed] [Google Scholar]
- 20.Pearson JL, Richardson A, Niaura RS, Vallone DM, Abrams DB. e-Cigarette awareness, use, and harm perceptions in US adults. Am J Public Health. 2012;102:1758–1766. doi: 10.2105/AJPH.2011.300526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Popova L, Ling PM. Alternative tobacco product use and smoking cessation: a national study. Am J Public Health. 2013;103:923–930. doi: 10.2105/AJPH.2012.301070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adkison SE, O’Connor RJ, Bansal-Travers M, Hyland A, Borland R, Yong HH, Cummings KM, McNeill A, Thrasher JF, Hammond D, Fong GT. Electronic nicotine delivery systems: International tobacco control four-country survey. Am J Prev Med. 2013;44:207–215. doi: 10.1016/j.amepre.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vickerman KA, Carpenter KM, Altman T, Nash CM, Zbikowski SM. Use of electronic cigarettes among state tobacco cessation quitline callers. Nicotine Tob Res. 2013;15:1787–1791. doi: 10.1093/ntr/ntt061. [DOI] [PubMed] [Google Scholar]
- 24.Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382:1629–1637. doi: 10.1016/S0140-6736(13)61842-5. [DOI] [PubMed] [Google Scholar]
- 25.Caponnetto P, Campagna D, Cibella F, et al. Efficiency and Safety of an electronic cigarette (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One. 2013;8:e66317. doi: 10.1371/journal.pone.0066317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cummings KM, Dresler CM, Field JK, et al. E-Cigarettes and Cancer Patients. Journal of Thoracic Oncology. 2014 doi: 10.1097/JTO.0000000000000129. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 28.Hall SM, Delucchi KL, Velicer WF, et al. Statistical analysis of randomized trials in tobacco treatment: longitudinal designs with dichotomous outcome. Nicotine Tob Res. 2001;3:193–202. doi: 10.1080/14622200110050411. [DOI] [PubMed] [Google Scholar]
- 29.Nelson DB, Partin MR, Fu SS, Joseph AM, An LC. Why assigning ongoing tobacco use is not necessarily a conservative approach to handling missing tobacco cessation outcomes. Nicotine Tob Res. 2009;11:77–83. doi: 10.1093/ntr/ntn013. [DOI] [PubMed] [Google Scholar]
- 30.Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res. 2003;5:13–25. [PubMed] [Google Scholar]
- 31.Sheffer CE, Stitzer M, Payne TJ, Applegate BW, Bourne D, Wheeler JG. Treatment for tobacco dependence for rural, lower-income smokers: outcomes, predictors, and measurement considerations. Am J Health Promot. 2009;23:328–338. doi: 10.4278/ajhp.06031933. [DOI] [PubMed] [Google Scholar]
- 32.Dockrell M, Morrison R, Bauld L, McNeill A. E-cigarettes: prevalence and attitudes in Great Britain. Nicotine Tob Res. 2013;15:1737–1744. doi: 10.1093/ntr/ntt057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.King B, Alam S, Promoff G, Arrazola R, Dube S. Awareness and ever use of electronic cigarettes among U.S. Adults, 2010–2011. Nicotine & Tobacco Research. 2013 doi: 10.1093/ntr/ntt013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kasza KA, Bansal-Travers M, O'Connor RJ, et al. Cigarette Smokers' Use of Unconventional Tobacco Products and Associations with Quitting Activity: Findings From the ITC-4, U.S. Cohort. Nicotine Tob Res. 2013 doi: 10.1093/ntr/ntt212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grana RA, Popova L, Ling PM. A longitudinal analysis of electronic cigarette use and smoking cessation. JAMA Intern Med. 2014 doi: 10.1001/jamainternmed.2014.187. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park ER, Japuntich SJ, Rigotti NA, et al. A snapshot of smokers after lung and colorectal cancer diagnosis. Cancer. 2012;118:3153–3164. doi: 10.1002/cncr.26545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simmons VN, Litvin EB, Jacobsen PB, et al. Predictors of smoking relapse in patients with thoracic cancer or head and neck cancer. Cancer. 2013;119:1420–1427. doi: 10.1002/cncr.27880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maher JE, Rohde K, Dent CW, Stark MJ, Pizacani B, Boysun MJ, Dilley JA, Yepassis-Zembrou PL. Is a statewide tobacco quiteline an appropriate service for specific populations? Tob Control. 2007;(16 Suppl 1):i65–i70. doi: 10.1136/tc.2006.019786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheffer CS, Stitzer M, Payne TJ, Applegate BW, Bourne D, Wheeler JG. Treatment for tobacco dependence for rural, lower-income smokers: outcomes, predictors, and measurement considerations. Am J Health Promot. 2009;23:328–338. doi: 10.4278/ajhp.06031933. [DOI] [PubMed] [Google Scholar]
- 40.Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23:133–139. doi: 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]