Key Points
Addition of dasatinib to chemotherapy followed by allogeneic stem cell transplant is well tolerated in younger patients with Ph+ ALL.
Landmark analysis showed statistically superior advantages for relapse-free and overall survival for the transplanted patients.
Abstract
This multicenter trial was conducted to determine whether the addition of dasatinib to chemotherapy followed by an allogeneic hematopoietic cell transplant (HCT) in patients with Philadelphia chromosome positive (Ph+) acute lymphoblastic leukemia (ALL) was feasible. Patients ≥18 and ≤60 years of age with newly diagnosed Ph+ ALL received up to 8 cycles of alternating hyperfractionated cyclophosphamide, vincristine, Adriamycin, and dexamethasone and high-dose cytarabine and methotrexate with dasatinib. Patients with an available matched sibling or unrelated donor underwent an allogeneic HCT in first complete remission (CR1), followed by daily dasatinib starting from day 100. Others received maintenance therapy with vincristine and prednisone for 2 years and dasatinib indefinitely. Ninety-seven patients (94 evaluable) with a median age of 44 years (range, 20-60 years) and median white blood cells at presentation of 10 × 109/L (range, 1-410 × 109/L) were accrued. Eighty-three patients (88%) achieved CR or CR with incomplete count recovery (CRi), and 41 underwent allogeneic stem cell transplant in CR1. Median follow-up is 36 months (range, 9-63). For the overall population, overall survival (OS), event-free survival, and relapse-free survival (RFS) at 3 years were 69%, 55%, and 62%, respectively. The 12-month RFS and OS after transplant were 71% and 87%, respectively. Landmark analysis at 175 days from the time of CR/CRi (longest time to HCT) showed statistically superior advantages for RFS and OS (P = .038 and P = .037, respectively) for the transplanted patients. Addition of dasatinib to chemotherapy and HCT for younger patients with Ph+ ALL is feasible and warrants further testing.
Visual Abstract
Introduction
Treatment of patients with Philadelphia chromosome positive (Ph+) acute lymphoblastic leukemia (ALL) has changed because of the introduction of tyrosine kinase inhibitors (TKIs).1,2 Addition of TKIs to conventional chemotherapy regimens has not only permitted more patients to undergo allogeneic hematopoietic cell transplant (HCT) in first remission but has led to their improved outcome.3,4 Furthermore, this strategy has produced long-term relapse-free survival (RFS) in many individuals unable or unwilling to receive HCT.5-7
The trials conducted to date, utilizing mainly imatinib and dasatinib, have not led to consensus on the best strategy to incorporate them into the traditional combination chemotherapy regimens used in ALL.2 Early studies incorporated imatinib as a block given separately from the chemotherapy.4 However, there is now significant evidence suggesting the importance of early and continuous treatment with TKIs.8 Other investigators have examined the efficacy of initial therapy with TKI with minimal or no cytotoxic agents.9,10 Although this strategy has been extremely well tolerated, particularly in elderly patients, and the response rate has been very high, there are questions about the durability of the responses if patients are not consolidated with an allogeneic HCT.10 Furthermore, there is significant concern regarding the development of resistance-mediating mutations such as T315I after such strategies.10 A recent randomized trial suggested the superiority of reduced-intensity chemotherapy combined with imatinib over intensive induction, with a higher complete remission (CR) rate due to fewer induction deaths, but the 5-year event-free and overall survival (OS) were similar.11
An important question is whether an allogeneic HCT in first CR remains the preferred strategy. Children’s Oncology Group have reported long-term follow-up of 91 children ages 1 to 21 years old showing a similar 5-year disease-free survival for patients treated with chemotherapy and imatinib, sibling-donor, and unrelated-donor HCT.7 A French randomized study in patients 21 to 60 years of age suggested a benefit for those who underwent HCT, although the donor versus no-donor analysis failed to demonstrate a benefit for RFS or OS because of higher nonrelapse mortality in the transplanted patients.11
Second- and third-generation TKIs have been investigated in Ph+ ALL for potential superiority, given their increased in vitro potency as well as their ability to overcome most imatinib resistant mutations.12 Furthermore, preclinical studies have demonstrated synergy between traditional drugs used for treatment of ALL and dasatinib.13 We have previously conducted a single-institution phase II trial of hyperfractionated cyclophosphamide, vincristine, Adriamycin, and dexamethasone (hyperCVAD) plus dasatinib in patients 21 to 80 years old with newly diagnosed Ph+ ALL.14 Herein, we report the results for a multicenter trial using the same regimen but in an overall younger population. The objectives of the study were to determine the feasibility of such a strategy in a multi-institutional setting and to determine its ability to improve outcomes in patients who are and are not able to undergo allogeneic HCT in first CR. A secondary objective was to determine the feasibility of initiation of dasatinib post-HCT.
Patients and methods
Eligibility criteria
Patients were eligible to participate (ClinicalTrials.gov identifier: NCT00792948) if they had the diagnosis of Ph+ ALL based on morphological and flow cytometry assessment of a bone marrow or peripheral blood specimen as well as evidence of the Philadelphia translocation by cytogenetic (CG) analysis, fluorescent in situ hybridization (FISH), or polymerase chain reaction (PCR). They had to be previously untreated or had received one cycle of prior ALL-type induction therapy before the establishment of the Ph+ status. They had to be 18 years or older and 50 years or younger. The protocol was amended in January 2012 to allow participation of patients 60 years or younger. All patients had to have a performance status of 0 to 3 and adequate organ function with bilirubin, alanine aminotransferase, aspartate aminotransferase, and creatinine ≤ 3 × upper limit of normal. Patients who were pregnant or nursing and those with uncontrolled illness were also excluded. All patients provided a signed informed consent approved by the institutional review board of the participating institutions to be treated in the study, which was conducted in accordance with the principles of the Declaration of Helsinki.
Treatment plan
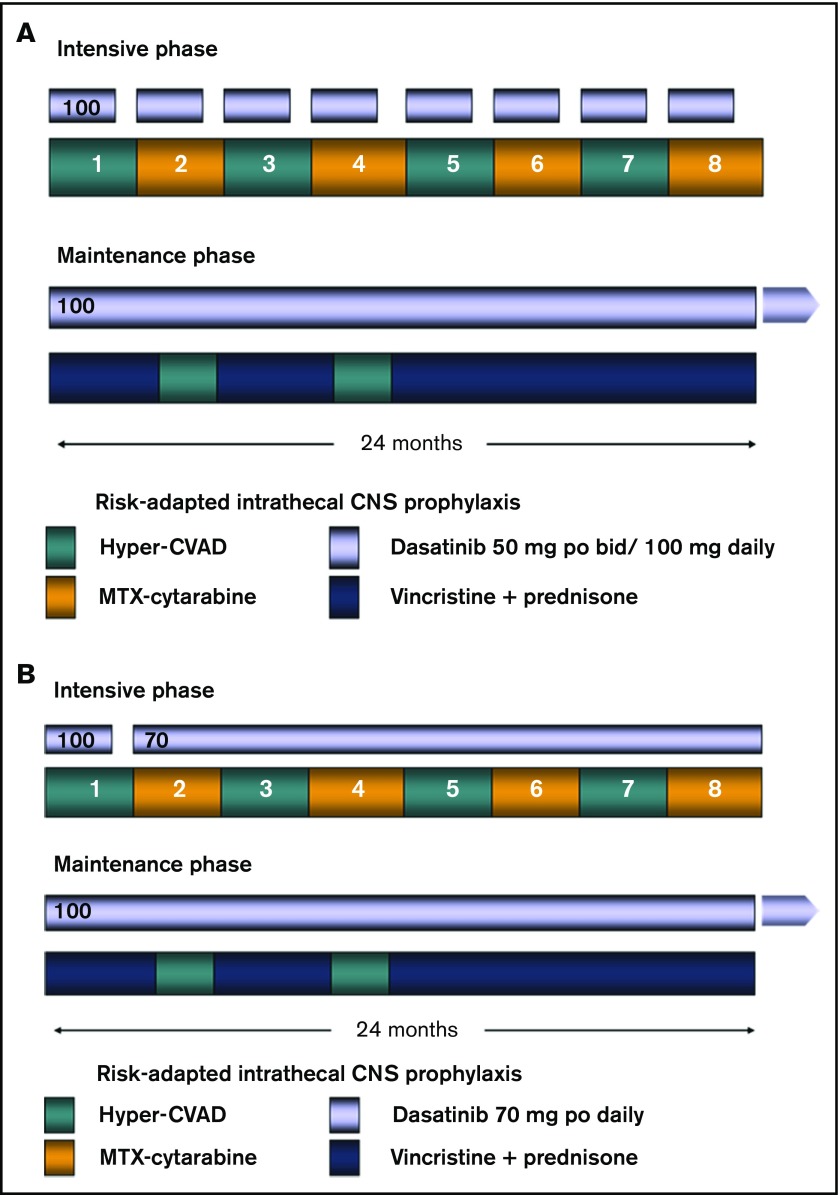
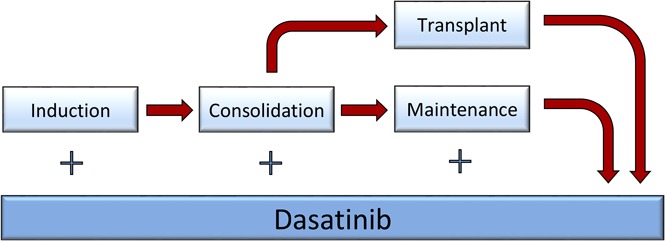
The details of the backbone hyperCVAD regimen have been published previously.14-16 Briefly, patients would receive up to 8 cycles of alternating hyperCVAD and high-dose cytarabine plus methotrexate as induction/consolidation courses. Dasatinib (initially 50 mg twice daily and, after an amendment, 100 mg once daily) was administered in the first 14 days of each of the 8 cycles. After a further amendment in February 2011, patients were treated with dasatinib 100 mg daily for the first 14 days of the first cycle, followed by dasatinib 70 mg daily, continuously started with the second cycle. All patients received central nervous system (CNS) prophylaxis with intrathecal cytarabine, alternating with methotrexate given twice on each of the first 4 cycles. Patients presenting with active CNS disease were treated with twice-weekly intrathecal chemotherapy until achievement of a negative cerebrospinal fluid (CSF) assessment; they would then revert to the above regimen. All patients who achieved CR and had an available matched sibling or 10/10 unrelated donor would be encouraged to proceed to HCT with the specified preparative regimen of total-body irradiation (TBI) (1200 cGy in 6 fractions over 3 days) and etoposide. They had to have recovered from all prior toxicity and have CR or CR with incomplete peripheral blood recovery (CRi) established within 14 days of the procedure. Transplants were all conducted in one of the Blood and Marrow Transplant Clinical Trials Network affiliated centers. All other patients remaining in CR after the completion of the 8 cycles of chemotherapy (or earlier, owing to intolerance or significant toxicity) would be enrolled in the maintenance portion of the study, which entailed continuous dasatinib 100 mg daily, monthly vincristine, and prednisone for 5 days per month, given for a total of 2 years. Dasatinib 100 mg daily was continued alone after the maintenance. Two cycles of intensification with hyperCVAD were permitted during months 6 and 13 of maintenance at the discretion of the treating physician, depending on the patients’ molecular data and tolerability. Patients who underwent HCT also received dasatinib 100 mg daily starting on day 100 posttransplant and continued for up to 5 years. Dose adjustments to all the agents in the study, including dasatinib, were permitted according to standard clinical practice. Figure 1 summarizes the treatment plan.
Figure 1.
Treatment regimen. (A) Original regimen. Dasatinib was administered at 100 mg daily for the first 14 days of each of the consolidation cycles. (B) Modified regimen after amendment. The dasatinib dose was modified to 70 mg orally daily continuously from course 2 onward during the consolidation cycles. MTX, methotrexate.
Study-related assessments
Requirements for baseline evaluations included a full history and physical examination, complete blood count (CBC), full chemistry panel, bone marrow aspirate examination for morphology, and flow cytometry, as well as CG, FISH, or PCR for the detection of BCR-ABL1 fusion transcripts. CBC and chemistry panel were repeated at least weekly during the intensive chemotherapy cycles and at least monthly during the maintenance phase. Bone marrow exam was repeated on day 21 of the first cycle to determine response, and then every 2 to 3 cycles during the intensive phase, and approximately every 3 months during the maintenance phase. Baseline CSF evaluation was conducted with the induction course and at any time there was clinical suspicion for CNS relapse. Initial and follow-up bone marrow samples for minimal residual disease (MRD) assessment were collected centrally.
Response criteria
Response was assessed on the basis of the established criteria for the definition of CR, CRi, and relapse. Cytogenetic and molecular responses were defined as previously reported.17
Endpoint definitions and statistical methods
The primary endpoint was 12-month RFS after transplant, which was measured from the date of transplant to the first relapse or death from any cause, with patients last known to be alive in remission censored at the date of last contact. Among all patients who achieved remission, RFS was measured from date of remission to relapse or death from any cause, with patients last known to be alive in remission censored at the date of last contact. Event-free survival (EFS) was measured from the date of study registration to the first of date of completion of protocol therapy without CR or CRi, relapse from CR or CRi, or death from any cause. Patients last known to be alive and in remission were censored at the date of last contact. Overall survival was measured from date of study registration to death from any cause, with patients last known to be alive censored at the date of last contact. The study was designed to test a null 12-month RFS after transplant rate of 40% and powered for an alternative rate of 65%. Thirty-four patients undergoing transplant would provide 90% power with a one-sided type 1 error 4.4% for this test; with this design, if 19 or more transplanted patients were alive and relapse-free at 12 months, the regimen would be considered effective. It was anticipated that 85 eligible patients would need to be accrued to have 34 patients transplanted.
Survival endpoints were estimated using the Kaplan-Meier method and analyzed with the log-rank test and Cox proportional hazard regression models. A two-sided α value of 5% denoted significance. The following results are based on data available as of 25 March 2016.
Results
Patient characteristics
Between September 2009 and October 2013, 97 patients were enrolled in the study from 30 participating centers across the United States (estimated yearly incidence, ∼1500 patients). Ninety-four patients were eligible and evaluable for this analysis. Their median age was 44 years (range, 20-60 years) and median white blood cell (WBC) count at presentation was 10 × 109/L (range, 1-410 × 109/L). Three patients had documented CNS involvement at the time of presentation. Thirty-four patients had received one course of therapy before enrollment in the study, including 16 who had achieved a CR or CRi. Table 1 summarizes enrollment characteristics of the patients.
Table 1.
Patient characteristics at enrollment
| Demographics | Median [range] or no (%) |
|---|---|
| Patients | 94 |
| Median age at diagnosis, y | 44 [20-60] |
| Age >50 y | 23 (24) |
| Sex: female | 52 (55) |
| Laboratory | |
| WBC (× 109/L) | 10 [1-410] |
| Marrow blast, % | 83 [0-100] |
| CNS disease at diagnosis | |
| Absent | 62 (66) |
| Not assessed | 29 (31) |
| Present | 3 (3) |
| Prior therapy before enrollment | |
| Untreated | 60 (64) |
| Previously treated; achieved CR/CRi | 16 (17) |
| Previously treated; remission status unknown | 7 (7) |
| Previously treated; refractory | 11 (12) |
CNS, central nervous system; CR, complete remission; CRi, CR with incomplete peripheral blood recovery; WBC, white blood cell.
Response and outcome
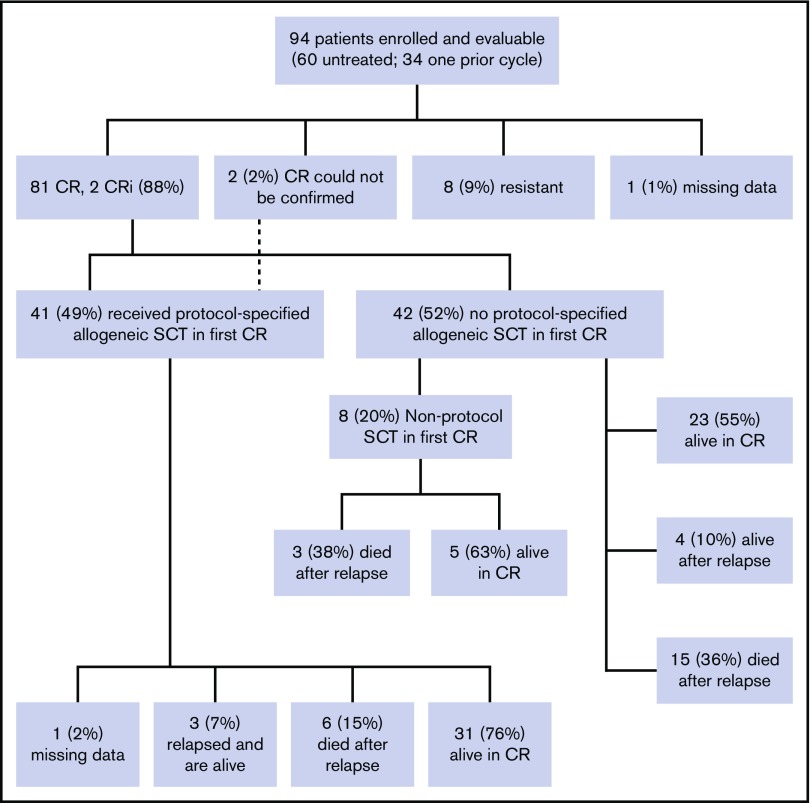
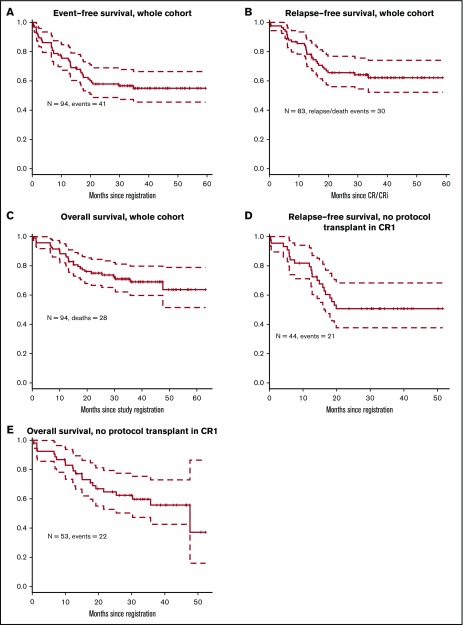
Overall, 83 patients (88%) achieved CR or CRi (81 with CR and 2 CRi); among patients without prior therapy, 90% (54 of 60) achieved CR or CRi, and among patients who were refractory to their first cycle of therapy, 81% achieved CR or CRi (9 of 11). Eight patients (9%) were unresponsive to the treatment, and the response is not available for 1 patient (Figure 2). Two patients had extramedullary disease at presentation, which was not reassessed (hence cannot be counted as CR), but achieved hematologic CR after induction. The median follow-up on the study for the censored patients is 36 months. Three-year EFS was 55% (95% confidence interval [CI], 46% to 66%) for the whole cohort (Figure 3A). For the 83 patients who achieved CR/CRi, 3-year relapse-free survival was 62% (CI, 52% to 74%; Figure 3B), and 3-year overall survival for the full cohort was 69% (CI, 52% to 79%; Figure 3C).
Figure 2.
Patient disposition and response. SCT, stem cell transplant.
Figure 3.
Survival outcomes. (A) Event-free survival for the whole cohort. (B) Relapse-free survival for the whole cohort. (C) Overall survival for the whole cohort. (D) Relapse-free survival in patients with no protocol transplant in first remission. (E) Overall survival in patients with no protocol transplant in first remission.
Fifty-three patients did not undergo a protocol prescribed HCT in first CR. Nine patients did not achieve CR/CRi; 2 of them died within 6 weeks of registering in the study (day 14 and day 42); the other 7 lived at least 1 year after study registration. Forty-four patients in this subset achieved CR or CRi. Landmark analysis was performed at 175 days after achieving CR or CRi (which was the longest time from achieving a response to undergoing HCT). The 3-year RFS after this landmark date is 51% (Figure 3D) and 3-year OS is 56% (Figure 3E).
Outcomes after allogeneic HCT
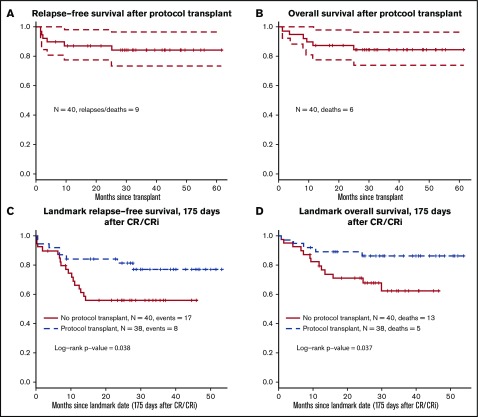
The primary end-point of the trial was 12-month RFS after undergoing HCT to estimate the outcome of combined therapy with HCT plus dasatinib. Forty-one patients underwent the protocol-related HCT in first remission. Among them, only 12 received the TBI + etoposide regimen; 22 received TBI + cyclophosphamide, and others received miscellaneous regimens. Figure 4A-B shows RFS and OS plots from the date of HCT. Twelve-month RFS was 83% (CI, 72% to 95%), significantly higher than the historical rate of 40% (P < .001). Three-year RFS after transplant was 76% (CI, 63% to 91%).
Figure 4.
Outcomes with protocol-specified HCT. (A) Relapse-free survival after protocol-specified HCT. (B) Overall survival after protocol-specified HCT. (C) Relapse free survival; landmark analysis HCT versus no HCT, in favor of HCT; hazard ratio (HR) 0.42, 95% CI (0.18-0.97). (D) Overall survival; landmark analysis HCT versus no HCT, in favor of HCT; HR 0.35, 95% CI (0.12-0.97).
There were no statistically significant differences between the characteristics at the time of diagnosis (including age, WBC, performance status, and CNS disease) of patients who did or did not receive an allogeneic HCT (supplemental Table 1). Acute graft versus host disease (GVHD) occurred in 18% (grade 1), 28% (grade 2), 10% (grade 3), and 3% (grade 4) of the transplanted patients. One patient (3%) had extensive chronic GVHD. Data on graft failure were not collected. Landmark analysis was performed at 175 days after achieving CR or CRi. When we used this analysis, there was a significant advantage for the patients who did undergo protocol HCT (P = .038 for RFS [Figure 4C] and P = .037 for OS [Figure 4D]). Cox regression models with transplant as a time-dependent covariate and controlling for prestudy age, WBC count, and prior therapy had similar results (P = .11 for RFS and P = .037 for OS).
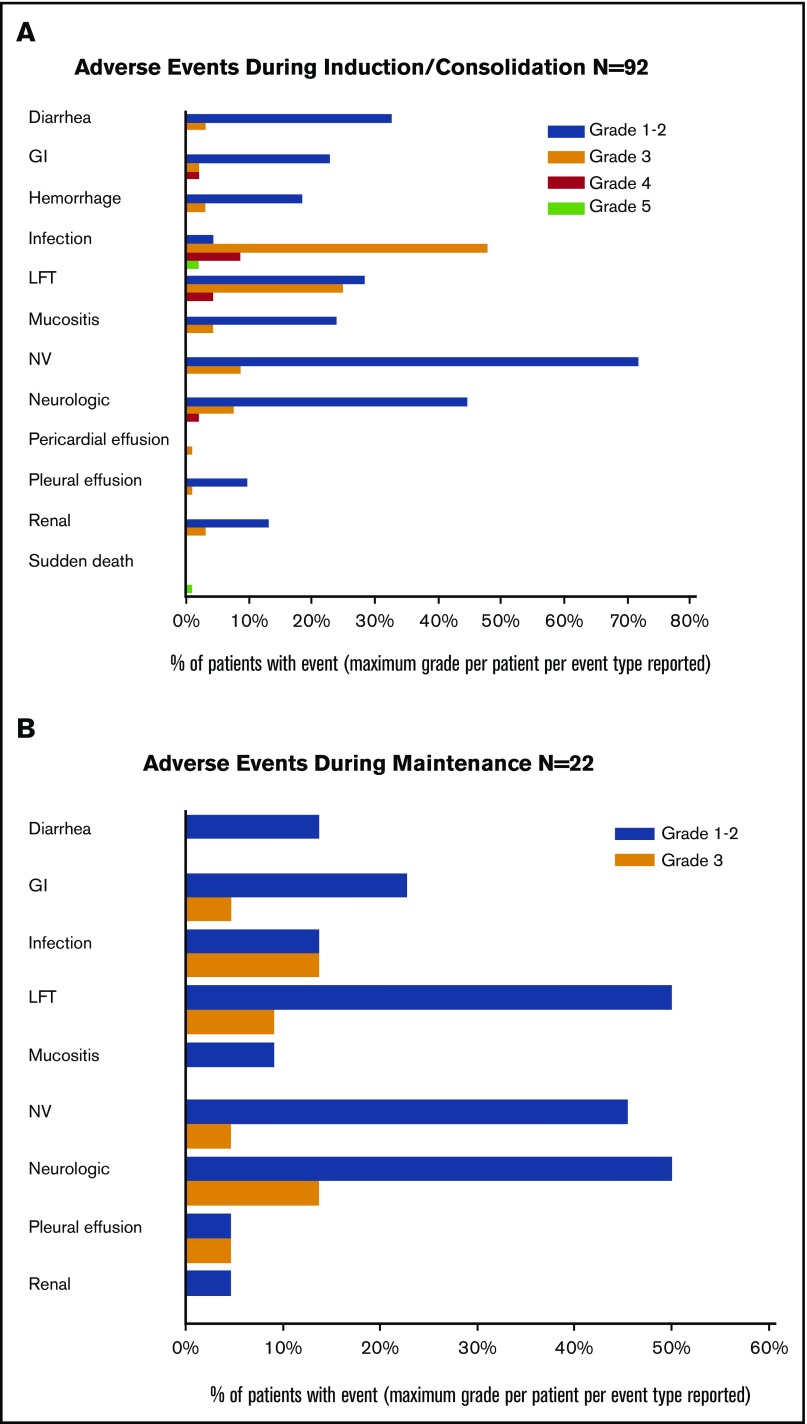
Toxicity
During the induction and consolidation courses, grade 3 and higher toxicities were mostly related to myelosuppression (Figure 5A). Similarly, during the maintenance phase, the adverse events were mostly grades 1 and 2 (Figure 5B). A total of 40 patients received maintenance dasatinib; 11 patients have had a grade 3 toxicity, and 1 patient had a grade 4 toxicity, deemed possibly, probably, or definitely related to treatment. Of the 33 patients receiving dasatinib post-HCT, 79% were on therapy for more than 6 months and 73% were on therapy more than 1 year. Twenty-four (73%) have reduced the dose of dasatinib for at least 1 cycle.
Figure 5.
Toxicity. (A) Grades 3 and 4 adverse events during induction/consolidation cycles. (B) Adverse events during maintenance cycles. GI, gastrointestinal; LFT, liver function tests; NV, nausea and vomiting.
Discussion
The availability of second- and third-generation TKIs with significantly more potent activity against the ABL kinase, including the more resistant mutant variants, has raised significant interest in their utilization in place of imatinib in regimens for Ph+ ALL, because the development of imatinib-resistant mutations is a significant cause of failure of such regimens.18-20 Recently reported studies demonstrate the feasibility of combining these agents with cytotoxic chemotherapy regimens.6,21,22
Deintensification of chemotherapy is associated with a significant reduction of mortality risk during induction, particularly in older patients.9,10 In a study by the Gruppo Italiano Malattie Ematologiche dell’Adulto Acute Leukemia Working Party, 53 patients with Ph+ ALL, who were older than 18 years with no upper age limit, received dasatinib combined with steroids and intrathecal methotrexate for their induction.10 All patients achieved a CR, with the vast majority achieving it after only 22 days of therapy and none dying during induction.10 Postremission treatment was not specified. At median follow-up of only 20 months, 23 (43%) had relapsed with a median time to relapse from CR of 5.9 months (range, 2.8-23.6 months). Relapses occurred in 14 of 19 patients treated with TKI alone, in 2 of 2 patients receiving no further therapy, in 5 of 14 patients receiving TKI plus chemotherapy, and in 2 of 18 patients receiving HCT. A T315I mutation was detected in 12 of 17 patients analyzed, with 8 occurring in patients who received TKI alone, demonstrating the inadequacy of this strategy for long-term disease control.10
The available literature suggests that even the second-generation ABL kinase inhibitors are not potent enough to eliminate the need for HCT and cytotoxic chemotherapy.6,10 This notion is particularly true for the younger patients in whom the benefits of HCT appear to outweigh the risks. We have previously reported that in our single-institution trials, HCT appeared to be beneficial in patients younger than 40 without reaching statistical significance, likely because of the limited patient numbers.5,6 Chalandon et al also reported that HCT was associated with a significant benefit in RFS and OS in their population of patients who were younger than 60 and received imatinib-based therapy.11 However, they were unable to show a benefit for the transplanted group among the patients who achieved molecular CR after the second course of therapy.11 Similarly, a donor versus no-donor analysis failed to show an advantage for the transplanted patients, despite a significant reduction in risk of relapse; this was likely because of a significantly increased risk of nonrelapse mortality in the transplanted group.
These data hint at the potential relevance of MRD monitoring in the selection of patients most likely to benefit from HCT. In a prior report, we showed that among patients with Ph+ ALL treated with the hyperCVAD plus imatinib or dasatinib without HCT, those who achieved a major molecular response at 3 months had an improved outcome.17 MRD monitoring has also been shown to be beneficial for the identification of patients most likely to benefit from HCT in Ph-negative ALL.23-25 This is particularly relevant in older patients with a greater risk of morbidity and mortality associated with HCT.
Another important consideration is the potential benefit of posttransplant TKI usage (reviewed recently by Giebel et al26). Pfeifer et al randomized 56 patients with Ph+ ALL to receive posttransplant imatinib either prophylactically or after detection of MRD.27 The first MRD analysis was conducted within 3 months after HCT, at which time the proportion of patients with detectable BCR-ABL1 transcripts was similar in the 2 arms. With an intention-to-treat analysis, prophylactic imatinib significantly reduced the incidence of molecular recurrence.27 However, the 5-year survival in both arms was similar. Another small study reported the feasibility of nilotinib given prophylactically after HCT for patients with Ph+ leukemias.28 In our study, all patients were assigned to receive dasatinib post-HCT with regular PCR monitoring during follow-up. Thirty-three patients actually received dasatinib post-HCT. Among them, 30 (91%) patients required at least 1 dose reduction.
In conclusion, this is the first US intergroup study examining the role of dasatinib plus chemotherapy in younger patients with Ph+ ALL; it confirms the overall tolerability and high efficacy of this regimen in a multicenter setting. It also demonstrated a beneficial effect of HCT in this younger population with better RFS and OS for the transplanted group on landmark analysis, though we note that this was not a randomized comparison, and the potential presence of unmeasured confounders makes this analysis not definitive. Future trials should examine the potential role of more potent TKIs such as ponatinib as well as monoclonal antibody-based strategies incorporating blinatumomab and inotuzumab ozogamycin.22,29-31 Similarly, the potential role of MRD monitoring both for selection of best candidates for HCT and for posttransplant TKI administration should be examined formally.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
Meir Wetzler is posthumously recognized for his important contributions to this study.
Supported by the National Institutes of Health, National Cancer Institute (NCI) grant numbers CA180888, CA180819, CA180821, CA180820, CA180858, CA189953, CA180816, CA180846, CA180818, CA189848, CA180835, CA46282, CA11083, CA46368, CA04919, CA12644, CA46136, and CA13612, and National Heart, Lung, and Blood Institute grant number HL069294, and by the Bristol-Myers Squibb Company. Dasatinib was distributed by the NCI Division of Cancer Treatment and Diagnosis, Cancer Therapy Evaluation Program.
Footnotes
Presented, in part, as an oral presentation at the 57th annual meeting of the American Society of Hematology, Orlando, FL, 5-8 December 2015.
Authorship
Contribution: F.R.A., H.P.E., C.S.H., M.H., R.L., S.M.O., J.P.R., F.R., M.S.T., S.J.F., and J.Y.C.W. conceived and designed the study; U.B., H.P.E., M.H., H.M.K., M.O., E.P., J.R., F.R., N.T., and G.L.U. collected and assembled the data; F.R.A., C.S.H., H.M.K., P.K., L.K., M.O., J.P.R., and F.R. analyzed and interpreted the data; F.R.A., H.P.E., C.S.H., H.M.K., P.K., L.K., R.L., S.M.O., M.O., E.P., J.P.R., F.R., M.S.T., G.L.U., and J.Y.C.W. wrote the manuscript; F.R.A., U.B., H.P.E., C.S.H., M.H., H.M.K., P.K., L.K., R.L., S.M.O., M.O., E.P., J.P.R., F.R., M.S.T., N.T., G.L.U., S.J.F., and J.Y.C.W. approved the final version of the manuscript.
Conflict-of-interest disclosure: U.B. received honoraria from Alexion, Novartis, and Amgen; has a consulting or advisory role with Alexion, Novartis, and Suneisis; has received funding for travel, accommodations, or expenses from and is a member of the speakers’ bureau at Novartis, Alexion, Amgen, and Jazz. E.P. received honoraria from, has a consulting or advisory role with, and has received funding for travel, accommodations, or expenses from Amgen. S.M.O. received honoraria from Gilead, Pharmacyclics, and Janssen; has a consulting or advisory role with Amgen, Celgene, CLL Global Research Foundation, and GSK; and has received research funding from Acerta, TG Therapeutics, Regeneron, Gilead, Pharmacyclics, and ProNAi. J.P.R. received honoraria from Novartis, BMS, and Ariad; has a consulting or advisory role with Novartis, BMS, and Ariad; has received funding for travel, accommodations, or expenses from Novartis and BMS; and has received research funding from Novartis. F.R. received honoraria from BMS and Ariad; has a consulting or advisory role with Ariad; and has received research funding from BMS. M.S.T. received honoraria from St. Vincent’s Hospital, Hartford Hospital, and Lundbeck Canada; has a consulting or advisory role with Igenica, Amgen, Pfizer, Neumedicine, Celator, Bioline, Biosight, and Agios; and has received research funding from Bioline, Epizyme, and Arog. J.Y.C.W. received honoraria, has received funding for travel, accommodations, or expenses, and has received research funding from Accuray, Inc. H.P.E. has a consulting or advisory role with Novartis, Incyte, Celgene, Amgen, Ariad, Seattle Genetics, Sunesis, Pfizer, Janssen, and Daiichi Sankyo; and has received research funding from Celator, Millennium/Takeda, Seattle Genetics, Astellas, Amgen, Agio, Juno, and Janssen. M.O. has a consulting or advisory role on the Data and Safety Monitoring Board (DSMB) with both Glycomemetics and Celgene. M.H. has received funding for travel, accommodations, or expenses from Novartis; and has received research funding from Sobi Pharmaceuticals. H.K. has received research funding from Ariad, BMS, Pfizer, Amgen, and Novartis. H.E. is a member of the speakers’ bureau at Novartis, Incyte, and Celgene; and has a relationship with Gyclomemetics, Inc. (DSMB Chair). F.R.A. owns stock or has other ownership in Adaptive Biotechnology. C.S.H. has patents in association with the University of Texas Health Science Center at San Antonio, but no royalty arrangements. The remaining authors declare no competing financial interests.
Correspondence: Farhad Ravandi, Department of Leukemia, Unit 428, University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: fravandi@mdanderson.org.
References
- 1.Fielding AK. Treatment of Philadelphia chromosome-positive acute lymphoblastic leukemia in adults: a broader range of options, improved outcomes, and more therapeutic dilemmas. Am Soc Clin Oncol Educ Book. 2015;35:e352-e359. [DOI] [PubMed] [Google Scholar]
- 2.Ravandi F, Kebriaei P. Philadelphia chromosome-positive acute lymphoblastic leukemia. Hematol Oncol Clin North Am. 2009;23(5):1043-1063, vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mizuta S, Matsuo K, Nishiwaki S, et al. . Pretransplant administration of imatinib for allo-HSCT in patients with BCR-ABL-positive acute lymphoblastic leukemia. Blood. 2014;123(15):2325-2332. [DOI] [PubMed] [Google Scholar]
- 4.Fielding AK, Rowe JM, Buck G, et al. . UKALLXII/ECOG2993: addition of imatinib to a standard treatment regimen enhances long-term outcomes in Philadelphia positive acute lymphoblastic leukemia. Blood. 2014;123(6):843-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daver N, Thomas D, Ravandi F, et al. . Final report of a phase II study of imatinib mesylate with hyper-CVAD for the front-line treatment of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Haematologica. 2015;100(5):653-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ravandi F, O’Brien SM, Cortes JE, et al. . Long-term follow-up of a phase 2 study of chemotherapy plus dasatinib for the initial treatment of patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Cancer. 2015;121(23):4158-4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schultz KR, Carroll A, Heerema NA, et al. ; Children’s Oncology Group. Long-term follow-up of imatinib in pediatric Philadelphia chromosome-positive acute lymphoblastic leukemia: Children’s Oncology Group study AALL0031. Leukemia. 2014;28(7):1467-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schultz KR, Bowman WP, Aledo A, et al. . Improved early event-free survival with imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia: a children’s oncology group study. J Clin Oncol. 2009;27(31):5175-5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vignetti M, Fazi P, Cimino G, et al. . Imatinib plus steroids induces complete remissions and prolonged survival in elderly Philadelphia chromosome-positive patients with acute lymphoblastic leukemia without additional chemotherapy: results of the Gruppo Italiano Malattie Ematologiche dell’Adulto (GIMEMA) LAL0201-B protocol. Blood. 2007;109(9):3676-3678. [DOI] [PubMed] [Google Scholar]
- 10.Foà R, Vitale A, Vignetti M, et al. ; GIMEMA Acute Leukemia Working Party. Dasatinib as first-line treatment for adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2011;118(25):6521-6528. [DOI] [PubMed] [Google Scholar]
- 11.Chalandon Y, Thomas X, Hayette S, et al. ; Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL). Randomized study of reduced-intensity chemotherapy combined with imatinib in adults with Ph-positive acute lymphoblastic leukemia. Blood. 2015;125(24):3711-3719. [DOI] [PubMed] [Google Scholar]
- 12.Shah NP, Tran C, Lee FY, Chen P, Norris D, Sawyers CL. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 2004;305(5682):399-401. [DOI] [PubMed] [Google Scholar]
- 13.Boulos N, Mulder HL, Calabrese CR, et al. . Chemotherapeutic agents circumvent emergence of dasatinib-resistant BCR-ABL kinase mutations in a precise mouse model of Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2011;117(13):3585-3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ravandi F, O’Brien S, Thomas D, et al. . First report of phase 2 study of dasatinib with hyper-CVAD for the frontline treatment of patients with Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia. Blood. 2010;116(12):2070-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kantarjian H, Thomas D, O’Brien S, et al. . Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (hyper-CVAD), a dose-intensive regimen, in adult acute lymphocytic leukemia. Cancer. 2004;101(12):2788-2801. [DOI] [PubMed] [Google Scholar]
- 16.Thomas DA, Faderl S, Cortes J, et al. . Treatment of Philadelphia chromosome-positive acute lymphocytic leukemia with hyper-CVAD and imatinib mesylate. Blood. 2004;103(12):4396-4407. [DOI] [PubMed] [Google Scholar]
- 17.Ravandi F, Jorgensen JL, Thomas DA, et al. . Detection of MRD may predict the outcome of patients with Philadelphia chromosome-positive ALL treated with tyrosine kinase inhibitors plus chemotherapy. Blood. 2013;122(7):1214-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ottmann O, Dombret H, Martinelli G, et al. . Dasatinib induces rapid hematologic and cytogenetic responses in adult patients with Philadelphia chromosome positive acute lymphoblastic leukemia with resistance or intolerance to imatinib: interim results of a phase 2 study. Blood. 2007;110(7):2309-2315. [DOI] [PubMed] [Google Scholar]
- 19.Kantarjian H, Giles F, Wunderle L, et al. . Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med. 2006;354(24):2542-2551. [DOI] [PubMed] [Google Scholar]
- 20.Cortes JE, Kim DW, Pinilla-Ibarz J, et al. ; PACE Investigators. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013;369(19):1783-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim DY, Joo YD, Lim SN, et al. ; Adult Acute Lymphoblastic Leukemia Working Party of the Korean Society of Hematology. Nilotinib combined with multiagent chemotherapy for newly diagnosed Philadelphia-positive acute lymphoblastic leukemia. Blood. 2015;126(6):746-756. [DOI] [PubMed] [Google Scholar]
- 22.Jabbour E, Kantarjian H, Ravandi F, et al. . Combination of hyper-CVAD with ponatinib as first-line therapy for patients with Philadelphia chromosome-positive acute lymphoblastic leukaemia: a single-centre, phase 2 study. Lancet Oncol. 2015;16(15):1547-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bassan R, Spinelli O, Oldani E, et al. . Improved risk classification for risk-specific therapy based on the molecular study of minimal residual disease (MRD) in adult acute lymphoblastic leukemia (ALL). Blood. 2009;113(18):4153-4162. [DOI] [PubMed] [Google Scholar]
- 24.Gökbuget N, Kneba M, Raff T, et al. ; German Multicenter Study Group for Adult Acute Lymphoblastic Leukemia. Adult patients with acute lymphoblastic leukemia and molecular failure display a poor prognosis and are candidates for stem cell transplantation and targeted therapies. Blood. 2012;120(9):1868-1876. [DOI] [PubMed] [Google Scholar]
- 25.Ravandi F, Jorgensen JL, O’Brien SM, et al. . Minimal residual disease assessed by multi-parameter flow cytometry is highly prognostic in adult patients with acute lymphoblastic leukaemia. Br J Haematol. 2016;172(3):392-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giebel S, Czyz A, Ottmann O, et al. . Use of tyrosine kinase inhibitors to prevent relapse after allogeneic hematopoietic stem cell transplantation for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: a position statement of the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Cancer. 2016;122(19):2941-2951. [DOI] [PubMed] [Google Scholar]
- 27.Pfeifer H, Wassmann B, Bethge W, et al. ; GMALL Study Group. Randomized comparison of prophylactic and minimal residual disease-triggered imatinib after allogeneic stem cell transplantation for BCR-ABL1-positive acute lymphoblastic leukemia. Leukemia. 2013;27(6):1254-1262. [DOI] [PubMed] [Google Scholar]
- 28.Shimoni A, Volchek Y, Koren-Michowitz M, et al. . Phase 1/2 study of nilotinib prophylaxis after allogeneic stem cell transplantation in patients with advanced chronic myeloid leukemia or Philadelphia chromosome-positive acute lymphoblastic leukemia. Cancer. 2015;121(6):863-871. [DOI] [PubMed] [Google Scholar]
- 29.Sanford DS, Kantarjian H, O’Brien S, Jabbour E, Cortes J, Ravandi F. The role of ponatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia. Expert Rev Anticancer Ther. 2015;15(4):365-373. [DOI] [PubMed] [Google Scholar]
- 30.Topp MS, Gökbuget N, Stein AS, et al. . Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16(1):57-66. [DOI] [PubMed] [Google Scholar]
- 31.Kantarjian H, Thomas D, Jorgensen J, et al. . Inotuzumab ozogamicin, an anti-CD22-calecheamicin conjugate, for refractory and relapsed acute lymphocytic leukaemia: a phase 2 study. Lancet Oncol. 2012;13(4):403-411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.