Abstract
Species abundances and distributions are inherently tied to individuals’ decisions about movement within their habitat. Therefore, integrating individual phenotypic variation within a larger ecological framework may provide better insight into how populations structure themselves. Recent evidence for consistent individual differences in behaviour prompts the hypothesis that variation in behavioural types might be related to variation in movement in natural environments. In a multiyear mark–recapture study, we found that individual sticklebacks exhibited consistent individual differences in behaviour both within a standardized testing arena designed to measure exploratory behaviour and within a river. Therefore, we asked whether individual differences in movement in a natural river were related to an individual’s exploratory behavioural type. We also considered whether body condition and/or the individual’s habitat or social environment use was related to movement. There was no evidence that an individual’s exploratory behavioural type was related to movement within the river. Instead, an individual’s habitat use and body condition interacted to influence natural movement patterns. Individuals in good condition were more likely to move further in the river, but only if they inhabited a vegetated complex part of the river; body condition had no influence on movement in those individuals inhabiting open areas of the river. Our results suggest that individual traits could help improve predictions about how populations may distribute themselves within patchy and complex environments.
1. INTRODUCTION
Understanding how populations structure themselves is a fundamental goal of ecology. Population processes are largely mediated by the individual animals within populations. For example, individual activity and movement patterns influence population abundance and distribution (Clobert et al., 2001). Intraspecific variation in traits related to these processes is therefore inherent to the study of population dynamics, yet classic ecological theory frequently simplifies this variation by describing populations by average traits. Recently, there has been a call for explicit consideration of individual variation in ecologically relevant traits in population and community ecology (Bolnick et al., 2011; Dall et al., 2012; Violle et al., 2012). Individual traits are broadly defined as any ‘measurable feature of an individual organism, including size, morphology, behaviour and physiology’ (Bolnick et al., 2011). Here, we consider traits to be any feature of an individual that is measurable and repeatable over time. There is growing appreciation that variation in individual traits can influence diverse population-level processes, from generating variation in predation pressures (Miller et al., 2014; Sherratt and Macdougall, 1995) to the non-random dispersal of individuals to novel environments (Cote et al., 2010; Duckworth and Badyaev, 2007). Therefore, there is a growing need to understand whether and how individual trait variation may scale up to influence larger scale dynamics in populations and communities.
One growing area of research on intraspecific variation is the study of animal personality. Consistent individual differences in behaviour, or behavioural types, are widespread across taxa (Bell et al., 2009), influenced by important ecological factors, such as predation and competition (Bell and Sih, 2007; Laskowski and Bell, 2013) and may have fitness consequences (Smith and Blumstein, 2008). In particular, there is evidence that individuals consistently differ in general activity levels and willingness to explore novel and potentially risky environments (Chapman et al., 2011; Cote et al., 2010; Dingemanse et al., 2003; Fraser et al., 2001; Lindström et al., 2013), behaviours which have been hypothesized to influence movement decisions and therefore population structure. These behaviours are frequently measured using standardized open-field assays to rapidly assess individual behavioural types. Behavioural types have been implicated in the spread of invasive species (Phillips and Suarez, 2012) and as a mechanism for species range expansion (Duckworth and Badyaev, 2007). Behaviourally mediated movement patterns are interesting because if certain behavioural types are more likely to move, the individuals that arrive at new resources or habitats will be a particular subset of the population, the most active or exploratory individuals, for example (Cote et al., 2010). Therefore, consistent individual differences in behaviour, such as activity and exploration, hold the potential to help explain species ranges and abundances.
It is likely, though, that movement is influenced not just by behavioural types, but also by other traits (Bowler and Benton, 2005; Clobert et al., 2001; Ronce, 2007). For example, differences in body condition have been related to differences in movement across a wide array of taxa with the general trend being that individuals in high condition move further than low condition individuals (Bonte et al., 2012). Condition is an index that is frequently used to approximate physiological wellness (Pope and Kruse, 2007) and usually does not change abruptly (Robb et al., 1992; Tosh et al., 2010). Biotic and abiotic environmental factors can also influence movement and often differ consistently between individuals. For example, consistent differences in habitat use are well known in threespine stickleback (Bentzen and McPhail, 1984). Those individuals using complex, vegetated habitats might be more likely to move greater distances if the abundance of refuges offer safety from predation (Gilliam and Fraser, 2001; Patrick et al., 2001). Similarly, movement decisions might also be related to the type of social environment that an individual uses (Magnhagen and Bunnefeld, 2009; Ward et al., 2013) and individuals often consistently occur in certain types of social environments (Brown and Brown, 2000; Saltz, 2011). For example, some lizards consistently avoid conspecifics, resulting in their further dispersal from groups (Cote and Clobert, 2007).
Each of these traits (body condition, habitat and social environment use) may by themselves influence on individual movement decisions and potentially interact with each other to influence movement. Body condition and habitat use may both influence movement decisions if individuals in poorer body condition are willing to move between refuges in a complex habitat because it is safe, compared to an open, unstructured environment, for example (Krause et al., 1998). Another possibility is that individuals in poor condition might instead rely on public information provided by the social group, which would limit their need to travel to sample patches (Templeton and Giraldeau, 1996; Valone, 1989; Valone and Templeton, 2002). A better understanding of the relative influence and interaction of these traits on an individual’s movement patterns can generate insight into larger scale ecological processes.
Therefore in this chapter, we present the results of two studies designed to answer a series of questions about the causes of intraspecific variation in movement within a natural population of threespine sticklebacks, a species well known for its consistent individual variation in behaviour (Bell, 2005; Dingemanse et al., 2007; Huntingford, 1976; Laskowski and Bell, 2014). This species is also a model for investigating several population-level processes, such as colonization of new habitats (Bell and Foster, 1994) and structured habitat use by particular individuals (Bolnick et al., 2009; Schluter and McPhail, 1992). In the first study, we ask (1) whether individual exploratory behaviour in an open-field arena is repeatable and (2) whether exploratory behaviour in an open-field predicts movement within a stream. In the second study, we expand our study to (3) compare the influence of exploratory behavioural type and other factors (condition, habitat and social environment) on individual movement patterns. Specifically, we compared seven a priori models: models with only the effect of either behavioural type, body condition, habitat use or social environment use; a model with the interaction between body condition and habitat use; a model with the interaction between body condition and social environment use; and finally, a null model with no fixed effects. We chose the open-field arena as our standardized behavioural assay because it is designed to measure exploratory behaviour and/or general activity levels, which are plausibly related to movement (Chapman et al., 2011; Cote et al., 2010; Dingemanse et al., 2003; Fraser et al., 2001; Lindström et al., 2013).
2. METHODS
2.1 Overview
The two studies were conducted over 3 years in the Navarro River in Mendocino County, California, USA. We used mark–recapture techniques to test whether exploratory behaviour in a standardized open-field arena predicted individual movement within the river. The study sites were divided into transects. We sampled each transect and measured the exploratory behaviour of each individual in an open-field assay. Then, the individuals were marked, released and recaptured. We estimated movement as the number of transects moved between captures. In the first study (2010 and 2011), individuals were remeasured for exploratory behaviour in the open-field assay after recapture. In the second study (2013), we expanded the scope by considering other traits, such as body condition, habitat use and social environment use as possible predictors of movement.
2.2 Population location and characteristics
The sites for both studies were fairly narrow (4–10 m wide) and shallow (maximum 2 m depth) stretches of the river. The site for Study 1 was characterized by clear water, a stone and pebble bottom with considerable vegetation (azalea and eucalyptus) overhanging the edges. The second study was conducted at a different but nearby (<3 km away) stretch of the river where there was a clearer demarcation between different habitat types. The Navarro River is not dammed and experiences regular seasonal variation in flow and depth. Both studies took place during the summer low point. Small riffles bounded each study site and extensive sampling up and downstream of the riffles recaptured no marked individuals so we assume that dispersal outside of the study sites was negligible. At each site, the river was divided into 15 m transects (total 240 m section of river for each site). All transects were the same size and there were no barriers to movement between any of transects.
2.3 Animal marking and capture techniques
2.3.1 Study 1
At the beginning of the capture period, we arbitrarily chose a transect near the centre of the study section of river where we used six silver wire minnow traps to capture adult sticklebacks (age 1 year). Any sticklebacks that were found in these traps were measured in a standardized open-field assay designed to measure exploratory behaviour (described below). After measuring each individual’s exploratory behaviour, we recorded standard length (to the nearest mm) and transect at capture, and then marked each individual with a unique identification using subcutaneous UV elastomer (Northwest Marine Technology, Inc.). Each individual was marked at up to six sites on their dorsal side using a combination of three different colours. The fish were then allowed to recover in a dark, aerated bucket until the end of the day and released at their point of capture.
We repeated this process until all transects had been sampled (one transect per day). We alternated sampling an upstream and a downstream transect each day from our starting transect in order to minimize disturbance in the same area. After all transects had been sampled once, we waited 2 days and then began our recapture efforts following the same schedule (average time between capture and recapture: 8.6 days; range 1–27).
During the recapture period, in addition to using minnow traps, we also seined each transect in an attempt to recapture as many individuals as possible and to avoid bias associated with particular capture methods, e.g., ‘trappability’ (Biro and Dingemanse, 2009; Wilson et al., 1993). A logistic regression also demonstrated that trapping technique had no effect on probability of recapture (Appendix), so we assume that our recaptured individuals were not biassed towards certain behavioural types. We remeasured the exploratory behaviour of recaptured, marked individuals. After the behavioural assay, we determined each individual’s identity and individuals were placed in dark aerated bucket to rest until the end of the day. The fish were then released where captured. Some individuals (n = 64 out of 246 recaptured individuals) were recaptured more than once. We counted the number of transects between consecutive captures as our measure of movement, hereafter referred to as ‘movement in the river’. For example, an individual that was initially caught in transect 3 and then recaptured in transect 6 was given a score of 3. If that individual was recaptured again in transect 4, it received a score of 2 (transects 6–4) for its second recapture.
2.3.2 Study 2
Each transect in Study 2 included a shallow bank that lacked vegetation (open habitat) and a relatively deep bank that was covered by patches of low hanging tree branches and grasses (cover habitat). These two habitat types were usually separated by a deep central area that stickleback did not occupy. The open habitat was a bed of sand and fine gravel that stretched uninterrupted from the furthest upstream transect to the furthest downstream transect. Stickleback were evenly distributed throughout this habitat type. The cover habitat was dominated by the submerged branches of the trees that lined the deep bank. Stickleback tended to aggregate at tree branches that were separated by as much as 10 m, i.e., the cover habitat was patchier. Thus, stickleback distribution was less evenly distributed across space in the cover habitat compared to the open habitat. Mark–recapture techniques were similar to those employed in Study 1 with a few modifications. We used snorkelling surveys and hand nets to capture individuals in order to assess each individual’s habitat and social environment use at the time of capture (Pearish et al., 2013). Starting at the edge of a randomly selected transect, we collected one, haphazardly selected individual at a time with a hand net, alternating between habitats (open vs. cover). Before approaching, we noted the focal individual’s social environment use by recording whether the focal fish was alone or in a shoal (<10 cm or four body lengths from another fish; Pitcher, 1993). No fish escaped capture so we assume our sample was not biassed towards more ‘catchable’ individuals (Biro and Dingemanse, 2009; Wilson et al., 1993). Each individual was placed into a separate 500-mL opaque container of river water and held overnight. The following day, individuals were observed in the standardized open-field assay to measure exploratory behaviour.
Following the behavioural assay, fish were weighed, measured for standard length and given unique markings using fluorescent visible implant elastomer. Each fish was released back into the river at the transect from which it was collected. After all transects were sampled once, we began the recapture period (average time between capture and recapture; 16.6 days; range 7–26 days). We started at the transect furthest downstream and moved methodically up the river using block nets to isolate each transect. This prevented fish from moving up or downstream in response to our activity. We used three methods for recapturing marked individuals (seining, snorkelling and electrofishing) in an effort to avoid biassing recapture towards particular behavioural types. We spent 3 h in each transect.
2.4 Standardized open-field assay to measure exploratory behaviour
Our open-field arena consisted of a 1.5-m diameter circular pool. The pool was divided into nine equally sized sections: eight sections around the outside with one central section. Rocks were placed within each perimeter section to provide cover. The arena was placed in the shade and we monitored the water temperature and changed it as needed to maintain the same temperature throughout the day.
An individual stickleback was placed into an acclimation chamber that was placed in the centre of the open-field arena. The chamber was made of PVC pipe end caps (height 8 cm, diameter 10 cm) with a hole that was plugged with a stopper on one side. After 3 min, we remotely pulled the stopper from the chamber and removed the stopper from the arena. We recorded latency to emerge from the chamber, the total number of sections crossed, and the number of unique sections (out of 9) that an individual swam through for 3 min after emergence. Results were similar for all three behaviours that were recorded during the open-field assay. Here, we focus on total number of sections as it exhibited the most interindividual variation (results for the other behaviours are in Appendix). We interpret the total number of sections as activity level and/or exploratory behaviour which has been hypothesized to influence natural movement behaviour (e.g., Cote et al., 2010; Fraser et al., 2001). Hereafter, we refer to this as ‘exploratory behaviour in the pool’. Trials where the individual did not exit the chamber after 5 min were terminated (occurred in 19% of trials). In Study 2, we increased the cutoff for emergence time from 5 to 10 min in order to maximize the number of individuals that emerged. If an individual did not emerge, we gently poured it from the chamber into the arena (occurred in 3% of trials). In Study 2, we did not remeasure behaviour at recapture as Study 1 indicated behaviour in the open-field arena was significantly repeatable (see Section 3, see also Pearish et al., 2013).
2.5 Data analysis
Exploratory behavioural data were normally distributed. Movement in the field was non-normal so we specified a Poisson error distribution and confirmed that this distribution was a good fit with Q–Q plots. We corrected for the anticonservative nature of the Poisson distribution by using quasi-likelihood estimation of parameters (see below).
2.5.1 Study 1
Our first question was whether there was evidence for consistent individual differences in behaviour, both in the open-field arena and in the river (for those individuals that were recaptured multiple times). To test this, we estimated the repeatability of exploratory behaviour in the pool and movement in the river separately. Because movement in the river was Poisson distributed, we corrected for the estimation of the residual error according to Nakagawa and Schielzeth (2010). We used (generalized) linear mixed models with exploratory behaviour in the pool or river as the response variable and individual (nested within year) as a random effect. Because we were interested in a general characterization of among individual variance in these behaviours, we did not include any other fixed effects. For exploratory behaviour in the pool we used data on all individuals in these models, even those that were not recaptured, as they still provide information about the behavioural variation present in this population (Martin et al., 2011). For movement in the river only, individuals that were recaptured at least once (N = 246) were included as we could not estimate movement in the river for nonrecaptured individuals. To determine whether any fixed effects could explain interindividual variation in exploratory behaviour in the pool, we ran a second linear mixed model for exploratory behaviour in the pool including the following fixed effects: time of day, individual standard length, number of capture (first, second, etc., capture) and year. We then reestimated the repeatability of exploratory behaviour in the pool using the variance components from the full model. We used (generalized) linear mixed models with the ‘lme’ command in the ‘nlme’ package (for total sections) and the ‘glmmPQL’ command in the ‘MASS’ package (distance) in Rv2.15 (http://www.r-project.org/). For exploratory behaviour in the pool, we tested for the significance of the individual effects using a log-likelihood ratio test comparing a model fit with the random individual effect to one without the individual effect. However, because of the nature of movement in the river (Poisson distributed) we could not accurately assess the significance of the individual effect (Nakagawa and Schielzeth, 2010).
Our second question was whether exploratory behaviour in the pool was related to natural movement in the river. To test this, we used a generalized linear mixed model with movement in the river as the response variable and exploratory behaviour in the pool at the previous capture as a predictor. We also included standard length and days since previous capture as predictors. We then reestimated the repeatability of distance moved from this full model to determine if the fixed effects could explain interindividual variation in behaviour in the river. Individual was included as a random effect as some individuals were recaptured more than once. Models were constructed using the ‘glmmPQL’ command in the lme4 package in R. Additionally, we tested whether exploratory behaviour in the pool predicted the probability of recapture. To test this, we used a binary regression with recapture (yes/no) as the response variable. We included exploratory behaviour in the pool, sex, standard length and year as fixed effects. For this model, we only used behavioural data from the initial capture.
2.5.2 Study 2
In Study 2, we measured exploratory behaviour in the pool as well as several other traits that might influence movement (condition, habitat and social environment at time of capture). These data allowed us to test the relative importance of different factors on movement within the river. This analysis was conducted in two steps. First, we developed a set of a priori models corresponding to the predictions that were developed from our knowledge of the stickleback system and relevant literature (Table 5). Average movement was upstream (Fig. 1) and fish in upstream transects were limited in how far upstream they could travel. To account for this, we used generalized linear mixed models with transect included as a random factor in all models. Models were constructed using the ‘lme4’ package in R.
Table 5.
Results of information theoretic analysis comparing seven different models from Study 2 ordered from most to least informative
| Model | K | QAICc | Δ | w | R2 |
|---|---|---|---|---|---|
| 1. Random effect only (transect) | 2 | 93.8 | 0 | 0.28 | 0.10 |
| 2. Body condition | 3 | 94.3 | 0.45 | 0.23 | 0.13 |
| 3. Social environment use | 3 | 95.3 | 1.51 | 0.13 | 0.11 |
| 4. Exploratory behaviour in the pool | 3 | 95.7 | 1.84 | 0.11 | 0.11 |
| 5. Habitat use | 3 | 95.7 | 1.85 | 0.11 | 0.11 |
| 6. Condition × habitat use | 5 | 95.9 | 2.07 | 0.10 | 0.18 |
| 7. Condition × social environment use | 5 | 97.8 | 4.00 | 0.04 | 0.13 |
Models 6 and 7 include both the main effects and the interaction term between the two factors. K is the number of parameters estimated. QAICc is a smaller-is-better measure of goodness of fit. Delta (Δ) is the difference between the ‘best’ model with the lowest QAICc and all other models. Akaike’s weight (w) is the relative probabilities of each model given the data. R2 is the conditional R2 that shows the variance explained by fixed and random effects.
Figure 1.
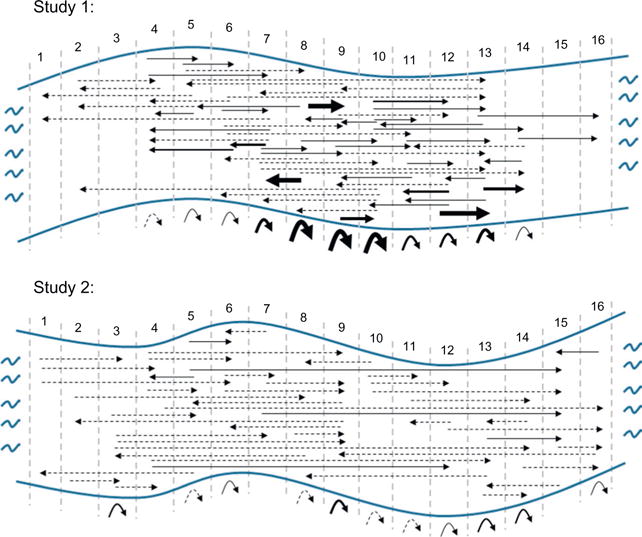
Stickleback movement in the stream in both studies. Shown is the study section of river divided into 15 m transects (vertical grey dashed lines) with a riffle at either end. Transect number is above the river. The direction of the arrowed line indicates the direction in which the fish moved between initial and subsequent captures. The thickness of the arrowed lines indicates the number of individuals that moved between those two transects (dashed line = 1 individual). The curved arrows below the river indicate the number of individuals that were recaptured within that same transect.
We tested seven models corresponding to our six a priori models and one model that contained only the random effect of transect (Table 5). Fixed factors included in models were body condition, habitat use, social environment use and exploratory behaviour in the pool. We used Fulton’s K calculated from body measurements taken during the initial capture as the body condition index. Fulton’s K is calculated by
where W is weight (g), L is length (mm) and values are multiplied by 100,000 to achieve an index with values close to 1 (Pope and Kruse, 2007). Fulton’s K is usually avoided in cases where comparisons across age classes, populations or species are desired but is appropriate for this application as we limited our study to threespine stickleback of a single population. The models that included two fixed factors also contained the interaction term between them (body condition × habitat use, body condition × social environment use).
The second step of our analysis was to use multimodel inferencing to compare our a priori models and calculated model-averaged estimates. This method is preferred over step-wise model selection because model selection uncertainty is accounted for in the model-averaged parameter estimates (Burnham et al., 2011). The ability of each model to predict movement was assessed using the second order, quasi-Akaike’s information criteria (QAICc) to account for overdispersion in the data and small sample size (Burnham and Anderson, 2002). For each model, we calculated the number of parameters estimated (K), QAICc, delta (Δ, difference in QAICc between the focal model and the model with the lowest QAICc), Akaike’s weight (w) and a conditional R2. Models with deltas less than 2 are considered to have ‘substantial support’ while models with deltas of 4 or more receive ‘considerably less support’ (Burnham and Anderson 2002). Akaike’s weight is the relative probabilities of each model given the data and sums to one over the set of models (Burnham et al., 2011; Johnson and Omland, 2004). Conditional R2 represents the variance explained by fixed and random factors (Nakagawa and Schielzeth, 2013). When no single model can be specified as the best model, a 95% confidence set can be constructed by summing Akaike’s weights from the largest to smallest until the sum is ≥ 0.95. This set can be used to calculate model-averaged estimates and 95% confidence intervals for fixed effects (Burnham and Anderson, 2002). We interpreted effects with estimates with confidence intervals that did not overlap zero as statistically significant. We used the ‘MuMIn’ package in R for analysis.
3. RESULTS
3.1 Study 1
3.1.1 General characteristics of captured fish
We marked 422 individual sticklebacks in the first study. Of these, we recaptured 246 individuals at least once (Table 1). Most individuals that were recaptured in the same or adjacent transect (distance = 0 or 1, Table 1), though one individual travelled a maximum of nine transects. Table 2 lists the general characteristics of all fish captured in each year.
Table 1.
Summary of sample sizes for each year in each study
| Study 1 | Study 2 | ||
|---|---|---|---|
| 2010 | 2011 | 2013 | |
| Number of individuals | |||
| Marked | 182 | 240 | 431 |
| Recaptured | 64 | 118 | 118 |
| x2 | 10 | 39 | 0 |
| x3 | 1 | 12 | 0 |
| x4 | 0 | 2 | 0 |
| Movement | Number of recaptures | ||
| 0 | 24 | 48 | 45 |
| 1 | 27 | 68 | 24 |
| 2 | 9 | 29 | 11 |
| 3 | 10 | 16 | 6 |
| 4 | 3 | 5 | 6 |
| 5 | 0 | 0 | 8 |
| 6 | 0 | 2 | 6 |
| 7 | 1 | 1 | 3 |
| ≥8 | 1 | 2 | 9 |
‘Marked’ lists the total number of individuals initially marked in each year and ‘Recaptured’ lists the number of individuals recaptured at least once; of those individuals that were recaptured, a number were recaptured two, three or four (x2, x3, x4) times. Movement indicates the number of transects an individual moved in the river between the previous and current captures.
Table 2.
Average values (±SE) for traits measured in both studies
| Study 1 | Study 2 | ||
|---|---|---|---|
| Measure | 2010 | 2011 | 2013 |
| SL (mm) | 42.31 (±0.33) | 40.13 (±0.29) | 25.12 (±0.16) |
| Number of transects | 1.28 (±0.15) | 1.28 (±0.09) | 2.43 (±0.28) |
| Days between captures | 8.54 (±0.55) | 8.71 (±0.45) | 16.60 (±0.51) |
| Total sections in pool | 15.91 (±0.93) | 20.63 (±0.87) | 13.19 (±0.43) |
| Latency to emerge(s) | 98.29 (±8.31) | 61.04 (±5.62) | 131.88 (±7.96) |
| Unique sections | 5.54 (±0.25) | 6.71 (±0.19) | 6.09 (±0.13) |
3.1.2 Consistent individual differences in behavior
In general, we found evidence for consistent individual variation in exploratory behaviour in the pool and in the river. Repeatability of exploratory behaviour in the pool was r = 0.29 and inclusion of the individual effect in the model was strongly supported by the log-likelihood ratio test (log-likelihood ratio = 23.95, p<0.001). The repeatability of movement in the river was r = 0.26.
Exploratory behaviour in the pool depended on time of day, size, number of recaptures and year (Table 3). For example, individuals that were measured later in the day and were relatively large were more active in the arena. An individual’s exploratory behaviour in the pool tended to decrease with increasing number of captures (Table 3). Including these fixed effects did not change the estimate of the total variation among individuals in exploratory behaviour in the pool (conditional repeatability estimate: r = 0.24 log-likelihood ratio = 16.65, p<0.001) or movement in the river (conditional repeatability: r = 0.23).
Table 3.
Summary of the effects on exploratory behaviour in the pool in Study 1
| Factor | Estimate | F value | p value |
|---|---|---|---|
| Time of day | 0.11 | 2.95 | 0.043* |
| Standard length | 0.58 | 16.48 | <0.001* |
| Capture # | −1.61 | 1.13 | 0.069 |
| Year: 2011 | 5.77 | 26.75 | <0.001* |
Effects significant at the p<0.05 level are marked with ‘*’.
3.1.3 Relationship between exploratory behaviour in the pool and movement in the river
Among those individuals that were recaptured, we found no evidence that exploratory behaviour in the pool predicted movement between capture and recapture (Fig. 1 and Table 4). Interestingly, we also found that there was no effect of days since previous capture on movement in the river (Table 4), suggesting that even if given more time, individuals would not have moved further in the river.
Table 4.
Summary of the effects on movement in the river between captures in Study 1
| Factor | Estimate | 95% CI | p value |
|---|---|---|---|
| Total sections in the pool | 0.006 | (−0.004, 0.017) | 0.218 |
| Standard length | 0.01 | (−0.018, 0.010) | 0.469 |
| Days since capture | 0.003 | (−0.020, 0.027) | 0.765 |
There was no influence of exploratory behaviour in the pool on probability of recapture (Appendix). Using the other two behaviours, latency to emerge from the acclimation chamber or unique sections as predictors did not change our results: there was no relationship between these two behaviours and movement in the river or probability of recapture (Appendix).
3.2 Study 2
3.2.1 General characteristics of captured fish
We marked 431 individual sticklebacks in the second study. Of these, we recaptured 118 individuals (Table 1). As in the first study, there was considerable variation in movement in the river (range 0–12 transects). Although movement in the river tended to be in the upstream direction, visual inspection of the data did not suggest that fish were converging on particular transects (Fig. 1).
3.2.2 Relative influence of different traits on movement in the river
Several of the a priori models received substantial support (delta<2), including models that contained the main effects of body condition, social environment use and habitat use, and the model that contained only the random effect of transect (Table 5). Only the ‘body condition × social environment use’ model received substantially less support (delta ≥ 4). Since there was not a single ‘best’ model, we created a 95% confidence set of models (which excluded the ‘body condition × social environment use’ model) from which we calculated model-averaged estimates and 95% confidence intervals (Fig. 2).
Figure 2.
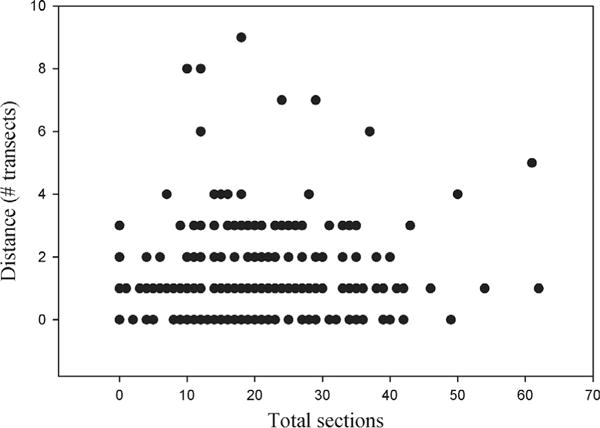
Relationship between exploratory behaviour in the pool and behaviour in the river in Study 1.
Based on the model-averaged estimates, as in the first study, we found no evidence that exploratory behaviour in the pool influenced behaviour in the stream (Table 6). Instead, we found a significant interaction between habitat use and body condition: fish in better condition moved further but only among individuals that occurred in the cover habitat (effect of condition × habitat type interaction = 1.42 [1.16, 1.69], Fig. 3). There was also a main effect of social environment use, where individuals that occurred in shoals tended to be more likely to be recaptured further away than fish that occurred alone (Table 6). We detected a non-significant trend for fish in the open to move further than fish that occurred in the cover habitat (Table 6). As in the first study, the amount of time between capture and recapture was not correlated with movement in the river (Kendall’s tau = −0.02, p = 0.75, n = 118).
Table 6.
Model-averaged estimates and 95% confidence intervals of factors predicting movement in Study 2 calculated from the 95% confidence set of models
| Factor | Estimate | 95% CI |
|---|---|---|
| Body condition | 0.41* | 0.12, 0.71 |
| Social environment | 0.21* | 0.13, 0.29 |
| Open-field behaviour | 0.01 | 0.00, 0.03 |
| Habitat use | −1.23 | −3.46, 1.00 |
| Condition × habitat use | 1.42* | 1.16, 1.69 |
Asterisks highlight significant factors where confidence intervals did not overlap zero. N = 82.
Figure 3.
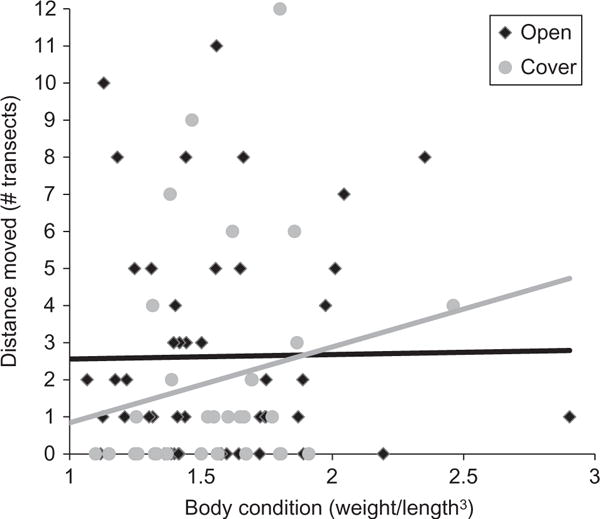
Interaction between body condition and movement in Study 2. Black diamonds and line represent individuals from open habitats. Grey circles and line represent fish from cover habitats. Among fish in cover habitats, individuals that were in better condition moved further. Condition was not related to distance moved among fish in the open. N, open = 48, cover = 34.
Similar to the first study, these results did not depend upon the behavioural measure from the open-field arena: neither latency to emerge nor unique sections influenced an individual’s movement within the river (Appendix).
4. DISCUSSION
Our studies tested how intraspecific phenotypic variation influences natural movement within a river population of sticklebacks. Consistent with the growing literature on animal personalities, we found strong evidence for consistent individual differences in behaviour in a standardized assay designed to measure exploratory behaviour and in behaviour in their natural environment. However, despite previous demonstrations that an individual’s behavioural type can influence movement patterns in the wild in other species (Chapman et al., 2011; Cote et al., 2010; Dingemanse et al., 2003; Duckworth and Badyaev, 2007; Fraser et al., 2001; Herborn et al., 2010), we found no evidence that a stickleback’s exploratory behaviour in the pool predicted how they moved within a river. Instead, we found that movement in the river was jointly influenced by an individual’s body condition and its habitat use.
Individuals that were in better condition moved further in the river, but only if they occurred in the cover habitat. The cover habitat in this study consisted of distinct patches of submerged grass and tree branches separated by areas that were not inhabited by stickleback. The open habitat, consisting of relatively homogeneous gravel beds, was much more continuous and stickleback inhabiting this habitat were more evenly distributed. This difference in space between patches (i.e., patch isolation sensu; Bowler and Benton, 2005) in the open versus cover habitats might explain why we only detected condition-dependent movement in the cover habitat. High condition individuals might have been better able than low condition individuals to afford the costs associated with moving between patches in the cover habitat (e.g., lost foraging time, energetic requirements of locomotion, risk of predation; Hanski, 1998). This is further supported by the fact that individual sticklebacks were found to consistently occupy the same habitat (cover vs. open) between capture and recapture (S. Pearish, Unpublished manuscript). An individual’s habitat use could be driven by a number of factors, such as food resource availability, predation risk or competitive exclusion and future work determining the relative influence of these could shed light on the mechanisms underlying this condition-dependent movement.
Our data support the hypothesis that group living encourages individuals to move further: fish that had been captured in shoals moved further in the river than fish that had been captured by themselves. Previous work has shown that fish in groups are less inhibited in the presence of a predator than fish that are alone (Magnhagen and Bunnefeld, 2009), perhaps due to the added safety from predation provided by group living (Krause and Ruxton, 2002). A plausible explanation for why shoaling individuals in our study moved further is that their perception of the predation risk of movement was lower compared to fish that occurred alone. Including the influence of the social environment and an individual’s propensity to shoal might then help improve predictions about dispersal and migration on a larger scale.
We found no evidence that exploratory behaviour in the pool predicted movement in the river at two different field sites, in three different years and for neither juvenile nor adult sticklebacks. The resounding failure to detect a relationship between exploratory behaviour in the pool and movement in the river urges us to be cautious about assuming that an individual’s behaviour in a standardized assay reflects natural behaviour in the field (Niemelä and Dingemanse, 2014). While some studies have shown that individual behaviour in a standardized open-field arena assay predicts behaviour and movement in the field (Chapman et al., 2011; Cote and Clobert, 2007; Dingemanse et al., 2003; Fraser et al., 2001; Herborn et al., 2010; Kobler et al., 2011; Wilson and McLaughlin, 2007), others have found no, or mixed, relationships between behaviour in standardized assays and natural behaviours in the field (Coleman and Wilson, 1998; Minderman et al., 2010; Wilson et al., 1993), a finding that is probably even more common but underreported due to publication bias.
One potential reason for the failure to detect a relationship between exploratory behaviour in the pool and movement in the river is because exploratory behaviour in the pool reflected a response to a novel and risky environment, while movement in the river reflected behaviour in a familiar environment. By switching to juveniles in the second study, we attempted to increase the likelihood that the movement we observed represented exploration of novel areas, but it is possible that even the youngest individuals had ample time to explore the entire study site prior to capture. This possibility is supported by the fact that fish that experienced more time between capture and recapture did not move further. A different behavioural assay, one designed to measure behaviour in a familiar environment, might have better success predicting movement in the river. It is also conceivable that exploratory behaviour in the pool might have better success predicting longer-range movement during the winter, when dispersal from the river is not limited by shallow riffles (Chapman et al., 2011). Instead, our studies were carried out during the summer, when male sticklebacks defend breeding territories. Finally, perhaps our measure of movement in the field (number of transects) is too coarse, i.e., it does not detect nonlinear movement, or movement over a smaller spatial scale. Studies utilizing internal acoustic or radio tags should be fruitful by providing more detailed and nuanced measures of individuals’ natural movement behaviours (e.g., Kobler et al., 2009; Taylor and Cooke, 2014).
Altogether our studies demonstrate that individual phenotypic variation has the potential to impact processes that occur on larger, population-level scales. However, we also show that not all measures of individual variation are informative about natural processes and we urge caution when making the assumption that behavioural types as assessed using standardized behavioural assays are predictive of behaviour in the wild. Further investigation of the potential influence of individual variation on larger scale processes, such as population structure and distribution, can only provide a more integrative understanding of ecological dynamics.
APPENDIX. SUPPLEMENTARY RESULTS WITH OTHER BEHAVIOURAL VARIABLES
1.1 Total sections
1.1.1 Study 1: Effect of total sections on probability of recapture
Table A.1.
Summary of factors influencing the probability of recapture in both years as estimated by a binary logistic regression
| Factor | Odds ratio | p value |
|---|---|---|
| Total sections | 1.01 | 0.487 |
| Standard length | 0.99 | 0.506 |
| Technique: seine | 1.02 | 0.551 |
1.2 Latency to emerge from the chamber
1.2.1 Study 1
Latency to emerge was log transformed to approximate a Gaussian distribution. We performed all the same analyses using the same methods as with ‘total sections’ (see Section 2).
Repeatability of latency to emerge
Conditional repeatability of latency to emerge
Similar to the results with total sections in the open-field arena, we found evidence for consistent individual differences in latency to emerge from the acclimation chamber (repeatability = 0.25; log-likelihood ratio: 21.18, p<0.001).
Latency to emerge was influenced by an individual’s standard length, capture number and year of capture (Table A.2). Individuals that were larger emerged more quickly from the acclimation chamber (larger scores indicate longer latencies). Additionally, individuals were quicker to emerge with subsequent captures and generally emerged more quickly in 2011. Inclusion of these fixed effects did not alter the amount of consistent individual variation (conditional repeatability = 0.24; log-likelihood ratio: 19.92, p<0.001).
Table A.2.
Summary of the effects on latency to emerge (log transformed) from the acclimation chamber into the open-field arena in Study 1
| Factor | Estimate | F value | p value |
|---|---|---|---|
| Study 1: 2010 and 2011 | |||
| Time of day | −0.003 | 1.46 | 0.228 |
| Standard length | −0.019 | 8.54 | 0.001* |
| Capture # | −0.063 | 5.40 | 0.021* |
| Year: 2011 | −0.264 | 29.43 | <0.001* |
Effects significant at the p<0.05 level are marked with ‘*’.
iii. Effect of latency on distance moved within stream
iv. Effect of latency on probability of recapture
We found no indication that variation in latency to emerge predicted an individual’s movement in the stream (Table A.3) or probability of recapture (Table A.4).
Table A.3.
Summary of the effects on distance moved in the stream between captures in Study 1 as estimated by a GLMM with quasi-Poisson distribution
| Factor | Estimate | 95% CI | p value |
|---|---|---|---|
| Latency to emerge | −0.148 | (−0.35, 0.05) | 0.163 |
| Standard length | 0.017 | (−0.01, 0.05) | 0.233 |
| Days since capture | 0.008 | (−0.01, 0.03) | 0.446 |
Table A.4.
Summary of factors influencing the probability of recapture in both years as estimated by a binary logistic regression
| Factor | Odds ratio | p value |
|---|---|---|
| Latency to emerge | 1.04 | 0.833 |
| Standard length | 1.00 | 0.878 |
| Technique: seine | 1.01 | 0.562 |
Males were less likely to be recaptured.
1.2.2 Study 2
Comparison of six models predicting movement
Model-averaged estimates of predictors
Table A.5.
Results of information theoretic analysis from Study 2 using an alternative behavioural trait, latency to emerge, in order from most to least informative
| Model | K | QAICc | Δ | w | R2 |
|---|---|---|---|---|---|
| Latency to emerge | 3 | 91.5 | 0 | 0.39 | 0.11 |
| Random effect only (transect) | 2 | 92.9 | 1.38 | 0.20 | 0.10 |
| Body condition | 3 | 93.3 | 1.85 | 0.15 | 0.13 |
| Social environment | 3 | 94.4 | 2.90 | 0.09 | 0.11 |
| Habitat use | 3 | 94.7 | 3.24 | 0.08 | 0.11 |
| Condition and habitat use | 5 | 95.0 | 3.51 | 0.07 | 0.18 |
| Condition and social environment | 5 | 96.9 | 5.41 | 0.03 | 0.13 |
K is the number of parameters estimated. QAICc is a smaller-is-better measure of goodness of fit. Delta (Δ) is the difference between the ‘best’ model with the lowest QAICc and all other models. Akaike’s weight (w) is the relative probabilities of each model given the data. R2 is the conditional R2 that shows the variance explained by fixed and random effects.
Table A.6.
Model-averaged estimates and 95% confidence intervals of factors predicting movement in Study 2 calculated from the 95% confidence set of models that included an alternative behavioral trait, latency to emerge
| Factor | Estimate | 95% CI |
|---|---|---|
| Body condition | 0.41* | 0.12, 0.71 |
| Social environment | 0.21* | 0.13, 0.29 |
| Latency to emerge | 0.00 | 0.00, 0.00 |
| Habitat use | −1.21 | −3.44, 1.02 |
| Condition × habitat use | 1.42* | 1.16, 1.69 |
Asterisks highlight significant factors where confidence intervals did not overlap zero. N = 82.
1.3 Unique sections
1.3.1 Study 1
Repeatability of latency to emerge
As a bounded count variable (0–9), unique sections were difficult to analyse. A majority (58%) of individuals explored at least eight out of the nine sections generating a high amount of skew in the data that could not be correctly by transformation, or adequately modelled with alternative error distributions. However, we attempted to estimate the repeatability of unique sections using a GLMM with quasi-Poisson error. If we did this, the estimated repeatability was r = 0.009. Therefore, given the lack of consistent individual variation in this behaviour we did not find it justified to use this variable as an explanatory variable for Study 1.
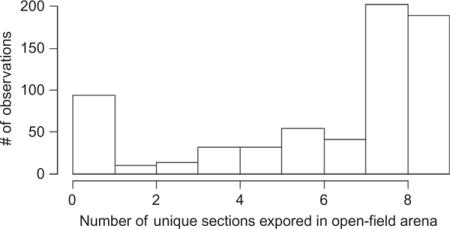
1.3.2 Study 2
Comparison of six models predicting movement
Model-averaged estimates of predictors
Table A.7.
Results of information theoretic analysis from Study 2 using an alternative behavioural trait, number of unique sections, in order from most to least informative
| Model | K | QAICc | Δ | w | R2 |
|---|---|---|---|---|---|
| Random effect only (transect) | 2 | 93.8 | 0 | 0.29 | 0.10 |
| Body condition | 3 | 94.3 | 0.45 | 0.23 | 0.13 |
| Social environment | 3 | 95.3 | 1.51 | 0.13 | 0.11 |
| Habitat use | 3 | 95.7 | 1.85 | 0.11 | 0.11 |
| Condition and habitat use | 5 | 95.9 | 2.07 | 0.10 | 0.18 |
| Number of unique sections | 3 | 96.0 | 2.15 | 0.10 | 0.11 |
| Condition and social environment | 5 | 97.8 | 4.00 | 0.04 | 0.13 |
K is the number of parameters estimated. QAICc is a smaller-is-better measure of goodness of fit. Delta (Δ) is the difference between the ‘best’ model with the lowest QAICc and all other models. Akaike’s weight (w) is the relative probabilities of each model given the data. R2 is the conditional R2 that shows the variance explained by fixed and random effects.
Table A.8.
Model-averaged estimates and 95% confidence intervals of factors predicting movement in Study 2 calculated from the 95% confidence set of models that included an alternative behavioural trait, number of unique sections
| Factor | Estimate | 95% CI |
|---|---|---|
| Body condition | 0.41* | 0.12, 0.71 |
| Social environment | 0.21* | 0.13, 0.29 |
| Number of unique sections | 0.02 | 0.00, 0.03 |
| Habitat use | −1.23 | −3.46, 1.00 |
| Condition and habitat use | 1.42* | 1.16, 1.69 |
Asterisks highlight significant factors where confidence intervals did not overlap zero. N = 82.
References
- Bell A. Behavioural differences between individuals and two populations of stickleback (Gasterosteus aculeatus) J Evol Biol. 2005;18:464–473. doi: 10.1111/j.1420-9101.2004.00817.x. [DOI] [PubMed] [Google Scholar]
- Bell MA, Foster SA. The Evolutionary Biology of the Threespine Stickleback. Oxford University Press; Oxford, UK: 1994. [Google Scholar]
- Bell AM, Sih A. Exposure to predation generates personality in threespined sticklebacks (Gasterosteus aculeatus) Ecol Lett. 2007;10:828–834. doi: 10.1111/j.1461-0248.2007.01081.x. [DOI] [PubMed] [Google Scholar]
- Bell AM, Hankison SJ, Laskowski KL. The repeatability of behaviour: a meta-analysis. Anim Behav. 2009;77:771–783. doi: 10.1016/j.anbehav.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzen P, McPhail J. Ecology and evolution of sympatric sticklebacks (Gasterosteus): specialization for alternative trophic niches in the Enos Lake species pair. Canadian J Zool. 1984;62:2280–2286. [Google Scholar]
- Biro PA, Dingemanse NJ. Sampling bias resulting from animal personality. Trends Ecol Evol. 2009;24:66–67. doi: 10.1016/j.tree.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Bolnick DI, Snowberg LK, Patenia C, Stutz WE, Ingram T, Lau OL. Phenotype-dependent native habitat preference facilitates divergence between parapatric lake and stream stickleback. Evolution. 2009;63:2004–2016. doi: 10.1111/j.1558-5646.2009.00699.x. [DOI] [PubMed] [Google Scholar]
- Bolnick DI, Amarasekare P, Araújo MS, Bürger R, Levine JM, Novak M, Rudolf VH, Schreiber SJ, Urban MC, Vasseur DA. Why intraspecific trait variation matters in community ecology. Trends Ecol Evol. 2011;26:183–192. doi: 10.1016/j.tree.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonte D, Van Dyck H, Bullock JM, Coulon A, Delgado M, Gibbs M, Lehouck V, Matthysen E, Mustin K, Saastamoinen M. Costs of dispersal. Biol Rev. 2012;87:290–312. doi: 10.1111/j.1469-185X.2011.00201.x. [DOI] [PubMed] [Google Scholar]
- Bowler DE, Benton TG. Causes and consequences of animal dispersal strategies: relating individual behaviour to spatial dynamics. Biol Rev. 2005;80:205–225. doi: 10.1017/s1464793104006645. [DOI] [PubMed] [Google Scholar]
- Brown CR, Brown MB. Heritable basis for choice of group size in a colonial bird. Proc Natl Acad Sci. 2000;97:14825–14830. doi: 10.1073/pnas.97.26.14825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. Springer; New York, NY: 2002. [Google Scholar]
- Burnham KP, Anderson DR, Huyvaert KP. AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behav Ecol Sociobiol. 2011;65:23–35. [Google Scholar]
- Chapman BB, Hulthén K, Blomqvist DR, Hansson LA, Nilsson JÅ, Brodersen J, Anders Nilsson P, Skov C, Brönmark C. To boldly go: individual differences in boldness influence migratory tendency. Ecol Lett. 2011;14:871–876. doi: 10.1111/j.1461-0248.2011.01648.x. [DOI] [PubMed] [Google Scholar]
- Clobert J, Danchin E, Dhondt AA, Nichols JD. Dispersal. Oxford University Press; Oxford: 2001. [Google Scholar]
- Coleman K, Wilson DS. Shyness and boldness in pumpkinseed sunfish: individual differences are context-specific. Anim Behav. 1998;56:927–936. doi: 10.1006/anbe.1998.0852. [DOI] [PubMed] [Google Scholar]
- Cote J, Clobert J. Social personalities influence natal dispersal in a lizard. Proc R Soc B: Biol Sci. 2007;274:383–390. doi: 10.1098/rspb.2006.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote J, Fogarty S, Weinersmith K, Brodin T, Sih A. Personality traits and dispersal tendency in the invasive mosquitofish (Gambusia affinis) Proc R Soc B: Biol Sci. 2010;277:1571–1579. doi: 10.1098/rspb.2009.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall SR, Bell AM, Bolnick DI, Ratnieks FL. An evolutionary ecology of individual differences. Ecol Lett. 2012;15:1189–1198. doi: 10.1111/j.1461-0248.2012.01846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemanse NJ, Both C, Van Noordwijk AJ, Rutten AL, Drent PJ. Natal dispersal and personalities in great tits (Parus major) Proc R Soc London Ser B: Biol Sci. 2003;270:741–747. doi: 10.1098/rspb.2002.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemanse NJ, Wright J, Kazem AJ, Thomas DK, Hickling R, Dawnay N. Behavioural syndromes differ predictably between 12 populations of three-spined stickleback. J Anim Ecol. 2007;76:1128–1138. doi: 10.1111/j.1365-2656.2007.01284.x. [DOI] [PubMed] [Google Scholar]
- Duckworth RA, Badyaev AV. Coupling of dispersal and aggression facilitates the rapid range expansion of a passerine bird. Proc Natl Acad Sci. 2007;104:15017–15022. doi: 10.1073/pnas.0706174104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser DF, Gilliam JF, Daley MJ, Le AN, Skalski GT. Explaining leptokurtic movement distributions: intrapopulation variation in boldness and exploration. Am Nat. 2001;158:124–135. doi: 10.1086/321307. [DOI] [PubMed] [Google Scholar]
- Gilliam JF, Fraser DF. Movement in corridors: enhancement by predation threat, disturbance, and habitat structure. Ecology. 2001;82:258–273. [Google Scholar]
- Hanski I. Metapopulation dynamics. Nature. 1998;396:41–49. [Google Scholar]
- Herborn KA, Macleod R, Miles WT, Schofield AN, Alexander L, Arnold KE. Personality in captivity reflects personality in the wild. Anim Behav. 2010;79:835–843. [Google Scholar]
- Huntingford FA. The relationship between anti-predator behaviour and aggression among conspecifics in the three-spined stickleback, Gasterosteus aculeatus. Anim Behav. 1976;24:245–260. [Google Scholar]
- Johnson JB, Omland KS. Model selection in ecology and evolution. Trends Ecol Evol. 2004;19:101–108. doi: 10.1016/j.tree.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Kobler A, Klefoth T, Mehner T, Arlinghaus R. Coexistence of behavioural types in an aquatic top predator: a response to resource limitation? Oecologia. 2009;161:837–847. doi: 10.1007/s00442-009-1415-9. [DOI] [PubMed] [Google Scholar]
- Kobler A, Maes GE, Humblet Y, Volckaert FA, Eens M. Temperament traits and microhabitat use in bullhead, Cottus perifretum: fish associated with complex habitats are less aggressive. Behaviour. 2011;148:5–6. [Google Scholar]
- Krause J, Ruxton GD. Living in Groups. Oxford University Press; Oxford: 2002. [Google Scholar]
- Krause J, Loader SP, McDermott J, Ruxton GD. Refuge use by fish as a function of body length-related metabolic expenditure and predation risks. Proc R Soc London Ser B: Biol Sci. 1998;265:2373–2379. [Google Scholar]
- Laskowski KL, Bell AM. Competition avoidance drives individual differences in response to a changing food resource in sticklebacks. Ecol Lett. 2013;16:746–753. doi: 10.1111/ele.12105. http://dx.doi.org/10.1111/ele.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski KL, Bell A. Strong personalities, not social niches, drive individual differences in social behaviour in sticklebacks. Anim Behav. 2014;90:287–295. doi: 10.1016/j.anbehav.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindström T, Brown G, Sisson S, Phillips B, Shine R. Rapid shifts in dispersal behavior on an expanding range edge. Proc Natl Acad Sci. 2013;110:13452–13456. doi: 10.1073/pnas.1303157110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnhagen C, Bunnefeld N. Express your personality or go along with the group: what determines the behaviour of shoaling perch? Proc R Soc B: Biol Sci. 2009;276:3369–3375. doi: 10.1098/rspb.2009.0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JG, Nussey DH, Wilson AJ, Reale D. Measuring individual differences in reaction norms in field and experimental studies: a power analysis of random regression models. Methods Ecol Evol. 2011;2:362–374. [Google Scholar]
- Miller JRB, Ament JM, Schmitz OJ. Fear on the move: predator hunting mode predicts variation in prey mortality and plasticity in prey spatial response. J Anim Ecol. 2014;83:214–222. doi: 10.1111/1365-2656.12111. http://dx.doi.org/10.1111/1365-2656.12111. [DOI] [PubMed] [Google Scholar]
- Minderman J, Reid JM, Hughes M, Denny MJ, Hogg S, Evans PG, Whittingham MJ. Novel environment exploration and home range size in starlings Sturnus vulgaris. Behav Ecol. 2010;21:1321–1329. [Google Scholar]
- Nakagawa S, Schielzeth H. Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol Rev. 2010;85:935–956. doi: 10.1111/j.1469-185X.2010.00141.x. http://dx.doi.org/10.1111/j.1469-185X.2010.00141.x. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Schielzeth H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol. 2013;4:133–142. [Google Scholar]
- Niemelä PT, Dingemanse NJ. Artificial environments and the study of ‘adaptive’ personalities. Trends Ecol Evol. 2014;29:245–247. doi: 10.1016/j.tree.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Patrick DC, Carlo R, Paul C. Field test for environmental correlates of dispersal in hedgehogs Erinaceus europaeus. J Anim Ecol. 2001;70:33–46. [Google Scholar]
- Pearish S, Hostert L, Bell AM. Behavioral type–environment correlations in the field: a study of three-spined stickleback. Behav Ecol Sociobiol. 2013;67:765–774. doi: 10.1007/s00265-013-1500-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips BL, Suarez AV. The role of behavioural variation in the invasion of new areas. In: Candolin U, Wong B, editors. Behavioural Responses to a Changing World: Mechanisms and Consequences. Oxford Universty Press; Oxford: 2012. pp. 190–200. [Google Scholar]
- Pitcher TJ. Behaviour of Teleost Fishes. Springer; New York, NY: 1993. [Google Scholar]
- Pope K, Kruse C. Analysis and Interpretation of Freshwater Fisheries Data. American Fisheries Society; Bethesda, MD: 2007. Condition; pp. 423–471. [Google Scholar]
- Robb LA, Martin K, Hannon SJ. Spring body condition, fecundity and survival in female willow ptarmigan. J Anim Ecol. 1992;61:215–223. [Google Scholar]
- Ronce O. How does it feel to be like a rolling stone? Ten questions about dispersal evolution. Annu Rev Ecol Evol Systemat. 2007;38:231–253. [Google Scholar]
- Saltz JB. Natural genetic variation in social environment choice: context-dependent gene–environment correlation in Drosophila melanogaster. Evolution. 2011;65:2325–2334. doi: 10.1111/j.1558-5646.2011.01295.x. [DOI] [PubMed] [Google Scholar]
- Schluter D, McPhail JD. Ecological character displacement and speciation in sticklebacks. Am Nat. 1992;140:85–108. doi: 10.1086/285404. [DOI] [PubMed] [Google Scholar]
- Sherratt TN, Macdougall AD. Some population consequences of variation in preference among individual predators. Biol J Linn Soc. 1995;55:93–107. [Google Scholar]
- Smith BR, Blumstein DT. Fitness consequences of personality: a meta-analysis. Behav Ecol. 2008;19:448–455. [Google Scholar]
- Taylor MK, Cooke SJ. Repeatability of movement behaviour in a wild salmonid revealed by telemetry. J Fish Biol. 2014;84:1240–1246. doi: 10.1111/jfb.12334. http://dx.doi.org/10.1111/jfb.12334. [DOI] [PubMed] [Google Scholar]
- Templeton JJ, Giraldeau LA. Vicarious sampling: the use of personal and public information by starlings foraging in a simple patchy environment. Behav Ecol Sociobiol. 1996;38:105–114. [Google Scholar]
- Tosh J, Garber A, Trippel E, Robinson J. Genetic, maternal, and environmental variance components for body weight and length of Atlantic cod at 2 points in life. J Anim Sci. 2010;88:3513–3521. doi: 10.2527/jas.2009-2676. [DOI] [PubMed] [Google Scholar]
- Valone TJ. Group foraging, public information, and patch estimation. Oikos. 1989;56:357–363. [Google Scholar]
- Valone TJ, Templeton JJ. Public information for the assessment of quality: a widespread social phenomenon. Philos Trans R Soc London Ser B: Biol Sci. 2002;357:1549–1557. doi: 10.1098/rstb.2002.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Violle C, Enquist BJ, McGill BJ, Jiang L, Albert CH, Hulshof C, Jung V, Messier J. The return of the variance: intraspecific variability in community ecology. Trends Ecol Evol. 2012;27:244–252. doi: 10.1016/j.tree.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Ward A, James R, Wilson A, Webster M. Site fidelity and localised homing behaviour in three-spined sticklebacks (Gasterosteus aculeatus) Behaviour. 2013;150:1689–1708. [Google Scholar]
- Wilson AD, McLaughlin RL. Behavioural syndromes in brook charr, Salvelinus fontinalis: prey-search in the field corresponds with space use in novel laboratory situations. Anim Behav. 2007;74:689–698. [Google Scholar]
- Wilson DS, Coleman K, Clark AB, Biederman L. Shy-bold continuum in pumpkinseed sunfish (Lepomis gibbosus): an ecological study of a psychological trait. J Comp Psychol. 1993;107:250. [Google Scholar]


