Abstract
The extended-synaptotagmins (tricalbins in yeast) derive their name from their partial domain structure similarity to the synaptotagmins, which are characterized by an N-terminal membrane anchor and cytosolically exposed C2 domains. However, they differ from the synaptotagmins in localization and function. The synaptotagmins tether secretory vesicles, including synaptic vesicles, to the plasma membrane (PM) via their C2 domains and regulate their Ca2+ triggered exocytosis. In contrast, the extended-synaptotagmins are resident proteins of the endoplasmic reticulum (ER), which tether this organelle to the plasma membrane via their C2 domains, but not as a premise to fusion of the two membranes. They transport glycerolipids between the two bilayers via their lipid-harboring SMP domains and Ca2+ regulate their membrane tethering and lipid transport function. Additionally, the extended-synaptotagmins are more widely expressed in different organisms, as they are present not only in animal cells, but also in fungi and plants, which do not express the synaptotagmins. Thus, they have a more general function than the synaptotagmins, whose appearance in animals species correlated with the occurrence of Ca2+ triggered exocytosis.
Keywords: Synaptotagmin, Tricalbin, TULIP, SMP, C2
1. Introduction
The discovery of synaptotagmin as an intrinsic synaptic vesicle membrane protein containing cytoplasmic Ca2+-binding C2 domains first suggested that this protein might be responsible for the Ca2+-dependent exocytosis of synaptic vesicles[1, 2]. Subsequent studies provided evidence for this hypothesis, identified numerous synaptotagmin paralogues and implicated this protein family in Ca2+-dependent exocytosis in a wide variety of animal cells and organisms [3, 4]. Synaptotagmins, however, are not present in yeast, nor in any other fungi or plants. All organisms of these kingdoms, however, express a class of closely related proteins, the extended-synaptotagmins (E-Syts), also known as tricalbins in yeast [5–8]. The E-Syts share a similar domain organization with bona fide synaptotagmin, but contain an additional domain, the Synaptotagmin-like, Mitochondrial and lipid-binding Protein (SMP) domain, and have a different subcellular localization and function: they are resident proteins of the endoplasmic reticulum (ER), and they mediate contacts, but not fusion, of ER membrane with the plasma membrane (PM) [5, 8, 9]. Thus, the E-Syts seem to have a more general function than the synaptotagmins in cell physiology. Here we briefly summarize their properties and what is known about their function in cell physiology.
2. E-Syts function as ER-PM tethers
Synaptotagmins are a family of intrinsic membrane proteins concentrated on secretory vesicles, including synaptic vesicles. They comprise a N-terminal transmembrane domain (with the N-terminus in the vesicle lumen) and two cytosolic C2 domains [1, 10]. They are present in all animals and human and mice express 16 synaptotagmin isoforms (Syt1-16) [11], which are encoded by different genes and are differentially expressed in different tissues with highest enrichment in the nervous system. C2 domains of some, but not all, synaptotagmins bind Ca2+ [12, 13] and interact with membrane phospholipids upon elevation of cytosolic Ca2+ [1]. These synaptotagmins are thought to function as the primary Ca2+ sensors for Ca2+-dependent exocytosis mediated by SNARE proteins [4, 14]. Lack of synaptotagmins in fungi and plants correlates with the absence of classical Ca2+-triggered exocytosis in cells of these organisms. Therefore, it would appear that synaptotagmins have emerged during evolution in parallel with Ca2+-triggered exocytosis, specifically in animal lineages [15].
The tricalbin/E-Syts, like bona fide synaptotagmins, are anchored to the membranes that host them via their N-terminal region and have the bulk of the protein localized in the cytosolic space [6, 8]. However, their insertion into the membrane occurs via an hydrophobic hairpin, rather than via a transmembrane segment, so that their N-terminus is also cytosolically exposed [8]. Additionally, while bona fide synaptotagmins exit the ER, the E-Syts are retained in the ER and are resident proteins of this organelle [8]. Downstream of the hairpin of E-Syts are in sequence, the SMP domain, which is followed by a variable number of C2 domains [7] (Figure 1a,b). Yeast expresses three tricalbins (TCB1, TCB2 and TCB3), which have 4 or 5 C2 domains [6, 16]. Mammals also express three E-Syts (E-Syt1, E-Syt2 and E-Syt3) (Figure 1a) [7]. E-Syt2 and E-Syt3 have 3 C2 domains with two closely juxtaposed C2 domains organized in tandem (C2A and C2B), followed by a C-terminal C2C domain. E-Syt1 has 5 C2 domains due to the additional presence, between the C2A-C2B domain pair and the C-terminal C2 domain (C2E), of a C2 domain doublet (C2C-C2D) that most likely originated by duplication of the C2A-C2B domain pair (Figure 1a) [7, 16, 17]. Drosophila and C. elegans only express one E-Syt [17]. Tricalbins/E-Syts occur as homo- and heterodimers due to dimerization of their SMP domains [8, 9]. They tether the ER to the PM via C2 domain-dependent interaction with PM phospholipids, in particular via an interaction of their positively charged C-terminal C2 domain with PI(4,5)P2, a phosphoinositide that is enriched in this membrane [8] (Figure 1a,b). Yeast tricalbins are selectively concentrated in the peripheral portion of the yeast ER that is apposed to the PM, also known as cortical ER, and contribute to staple it constitutively to the PM [5, 6]. In the mammalian E-Syt1 the interaction with the PM is enhanced by binding of Ca2+ to its central C2C domain. Thus, the fraction of E-Syt1 present throughout the ER or concentrated at ER-PM contacts in the cortical ER is controlled by cytosolic Ca2+ [8, 18, 19]. E-Syt2 and E-Syt3 mediate constitutive ER-PM tethering and are selectively concentrated in the cortical ER if expressed alone. As E-Syts form a heteromeric complex, E-Syts-dependent ER-PM contacts are regulated by both cytosolic Ca2+ and PM PI(4,5)P2 [8, 18, 20] (Figure 1b).
Figure 1. E-Syts regulate ER-PM tethering and transport/exchange of lipids.
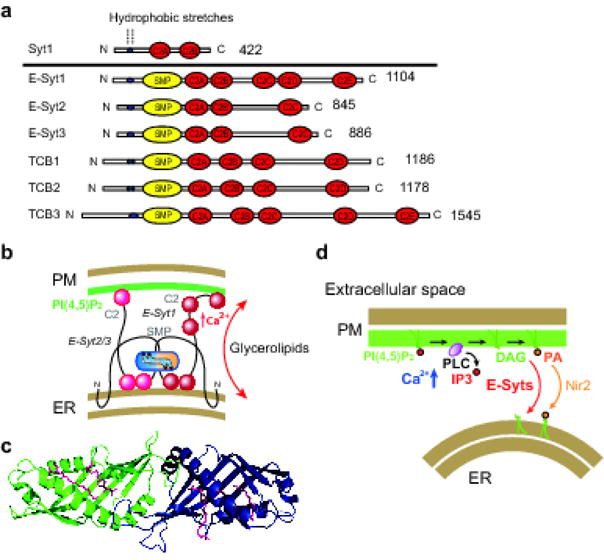
(a) Schematics showing the domain structure of synaptotagmin (Syt1), E-Syts (E-Syt1/2/3) and yeast tricalbins (TCB1/2/3). The amino acid lengths are indicated. (b) (Top) E-Syts, anchored to ER-membrane by a hydrophobic hairpin, form a dimeric complex and mediate ER-PM tethering via their C2 domain-dependent interaction with PM PI(4,5)P2. This tethering function is additionally regulated by cytosolic Ca2+, primarily via the Ca2+ sensing properties of the C2C domain of E-Syt1. The SMP dimer mediates lipid transfer likely by shuttling between the ER and the PM. The figure depicts binding of the C2A-C2B pair “in cis” to the ER, where its Ca2+ binding properties may help the SMP dimer extract lipids from the ER membrane. However, it cannot be excluded that it may bind “in trans” to the PM. From Saheki and De Camilli. Annual Review of Biochemistry, 2017. (c) Crystal structure of the SMP domain of E-Syt2 with bound hydrophobic molecules (diacyglycerol and Triton-X100 used for the purification of the module) in its hydrophobic groove. The SMP domain forms a dimer (green and blue indicate the two monomer within the dimer). Lipid molecules are shown in red. From Schauder et al. Nature, 2014 [9] (d) Schematics of the potential role of the E-Syts in the control of PM lipid homeostasis in response to PLC activation. E-Syts may cooperate with Nir2 in the transport PI(4,5)P2 metabolites from the PM to the ER. IP3, inositol 1,4,5-trisphosphate. DAG, diacylglycerol. PA, phosphatidic acid. PLC, phospholipase C. The cytosplasmic leaflet of the PM is depicted in green (rather than in brown as the other membrane leaflets) to indicate the presence of high concentration PI(4,5)P2 In this membrane.
Based on an assay involving semi-intact cells, the Ca2+ concentration required for E-Syt1-recruitment to the PM is in low micromolar Ca2+ range, similar to the concentration required for the interaction of bona fide synaptotagmins with the PM [18]. Accordingly, E-Syt1 accumulation at ER-PM contacts in intact cells occurred upon experimental conditions that lead to these high levels of Ca2+, but not upon manipulation resulting in release of Ca2+ from the ER [18]. These high levels of Ca2+ can be achieved by the activation of Ca2+ influx from extracellular media, such as Store-Operated Ca2+ Entry (SOCE) in all cells or membrane depolarization in neurons and neuroendocrine cells [18]. However, E-Syts-dependent contacts are not required for SOCE as reducing the levels of their expression did not affect this process [8]. Thus, ER-PM contacts mediated by E-Syts are functionally distinct from those mediated by the ER protein STIM and the PM protein Orai, which are key players in SOCE [8, 21–23]. Notably, STIM and Orai are not present in yeast and plant, while these organisms express E-Syts, further emphasizing the unrelated functions of ER-PM tethering mechanisms that control SOCE and those mediated by the E-Syts. However, E-Syts and Stim1-Orai share at least partially the same contact sites [8], suggesting the possible occurrence of constitutive ER-PM tethers where regulated ER-PM tethers differentially accumulate depending upon the functional state of the cell. As ER proteins, including the E-Syts, diffuse in the ER membrane, they are expected to randomly visit areas of close appositions between the ER and the PM. Thus, they can be trapped there under conditions that favor their binding “in trans” to the PM.
3. Lipid transport properties of the E-Syts
The presence of an SMP domain within the tricalbin/E-Syts is a defining element of this protein family. The predicted similarity of the SMP domain to lipid harboring protein modules of the TULIP (tubular lipid-binding) superfamily was the first clue suggesting a role in lipid binding and transport of the E-Syts [9, 24, 25]. A lipid transport function of the E-Syts was further supported by the localization at contacts between intracellular membranes not only of the tricalbin/E-Syts, but also of other known proteins with SMP domains [5], as membrane contacts are sites where lipid transfer between bilayers independent of membrane fusion can occur [26, 27]. A crystallographic study of the SMP domain of E-Syt2 confirmed its assignment to the TULIP domain superfamily, demonstrated its property to harbor lipids and revealed the structural basis of such binding [9]. The SMP domain has a β-barrel structure and dimerizes in an anti-parallel fashion to form a cylinder (~9 nm long) traversed by a deep hydrophobic groove [9] (Figure 1c). Mass spectrometry analysis of the lipids harbored by the SMP domain of E-Syt2 expressed and purified from mammalian cells demonstrated the presence of glycerolipids, without selectivity for specific head groups [9]. Similar lipid-harboring properties were reported for the SMP domains of a protein complex that localizes at ER-mitochondria contact sites, the endoplasmic reticulum (ER)-mitochondria encounter structure (ERMES) complex [28, 29]. The lack of selectivity against specific glycerolipid headgroups is in good agreement with the solved structure of the SMP domain where acyl chains of glycerolipids lie in its hydrophobic channel while their headgroups are exposed to the solvent [9] (Figure 1c).
In vitro analysis of the lipid transport properties of E-Syt1 showed that E-Syts could indeed transport glycerolipids between membrane bilayers (without bilayer fusion or hemifusion) regardless of their head groups, including diacylglycerol (DAG) [30, 31]. Transport was bidirectional, driven by the concentration gradient of the lipid in the membranes and stimulated by micromolar concentration of Ca2+ [30]. Importantly, deletion of the SMP domain completely abolished lipid transport properties. Further, amino acid mutations in the SMP domain expected to impair lipid-insertion in the hydrophobic groove strongly impaired the lipid transport ability of E-Syt1 without impairing its membrane tethering properties [30, 31]. Although bona fide synaptotagmin1 (Syt1) alone only tethered artificial membranes without mediating lipid transport, a soluble chimeric Syt1 protein where the transmembrane region of wild type Syt1 was replaced by the SMP domain of E-Syt1 could transport lipids [31]. Therefore, the SMP domain is necessary and sufficient for lipid transport, while the C2 domains mediate lipid tethering.
E-Syt1-dependent lipid transport was strongly stimulated by the presence of micromolar Ca2+ concentrations [30], i.e. the same Ca2+ concentrations required for its recruitment to the PM [18]. Further supporting the role of Ca2+ in this process, mutations in the Ca2+-binding site of the C2C domain of E-Syt1, i.e. the C2 domain responsible for Ca2+-dependent interaction with the PM, strongly reduced lipid transfer activity [31]. Thus, a major role of Ca2+ may be to mediate membrane tethering which is a prerequisite for lipid transport. However, additional roles of Ca2+, for example in controlling intra-molecular interactions that affect lipid transport may be considered. In this context it is of interest to note that while E-Syt2 and E-Syt3 do not contain the tandem C2C–C2D domains of E-Syt1, where the main Ca2+ binding site of E-Syt1 resides (in the C2C domain), all three mammalian E-Syts possess Ca2+ binding sites in their C2A domain [7, 8, 31–33]. Collectively, these studies suggest that a function of the E-Syts is to mediate regulated lipid transfer between the ER and the PM independent of vesicular traffic [30, 31].
4. A role of the E-Syts in the control of PM lipid homeostasis
Changes in PM lipid composition need to be reset to normal levels after acute perturbations. The transient recruitment to the PM of the E-Syts in response to experimental manipulations that elevate cytosolic Ca2+ raises the possibility that the E-Syts might participate in this homeostatic response. For example, stimulation of phospholipase C-dependent PI(4,5)P2 hydrolysis by activation of PM receptors or by Ca2+ ionophore (e.g. ionomycin) results in the generation of DAG which transiently accumulates in the PM bilayer. At least a pool of this DAG is rapidly metabolized to phosphatidic acid (PA), which is transported to the ER for metabolic recycling by another lipid transport protein, Nir2 [34, 35]. However, genome-edited cells lacking E-Syts exhibit enhanced/sustained accumulation of DAG in the PM following PI(4,5)P2 hydrolysis and re-expression of E-Syt1, but not of mutant E-Syt1 lacking the SMP domain, rescues this phenotype [30]. An attractive possibility is that the E-Syts may be part of the homeostatic response by transferring excess DAG from the PM to the ER for its metabolic recycling (Figure 1d).
5. Open questions
The strong evolutionary conservation of E-Syts from yeast to plants and animals, including humans, indicates important functions of these proteins in eukaryotes. In mammals, E-Syts have broad tissue distribution [36]. Surprisingly, their absence does not have a major disrupting effect on cellular life, as tricalbin triple knockout yeast cells and E-Syt triple knockout mammalian cells are viable [5, 6, 30]. Additionally, mutant mice lacking all the three E-Syts develop normally, are viable and fertile [36, 37]. Thus, while it was reported that E-Syt2 is required for rapid endocytosis and signaling of growth factor receptors, including Fibroblast Growth Factor (FGF) receptor, such role is clearly dispensable in live animals [38–40]. Interestingly, however, tricalbin deficient yeast cells exhibit enhanced fragility upon heat-induced PM damage [41], and plant cells lacking E-Syt homologs show reduced tolerance to freezing [42, 43] and mechanical stress [44]. These findings suggest that absence of these proteins lead to altered biophysical properties of the PM, possibly as a result of an abnormal lipid composition of this membrane. Lack of major phenotypes in cells and multicellular organisms where E-Syt function has been disrupted may be due, at least partially, to redundant roles of this protein family with other proteins that transfer lipids between the ER and the PM and/or to bypass of protein-mediated lipid transport by vesicular transport. As the E-Syts are expressed at particularly high concentration in some mammalian tissues, for example in the immune system and lungs, studies of these systems may help provide insights into the impact of their function(s) in physiology.
Finally, mammalian organisms express several membrane proteins with multiple C2 domains, including Ferlins and MCTPs (multiple C2-domain transmembrane proteins) [10]. At least some of such proteins were proposed to participate in Ca2+ triggered exocytosis of secretory vesicles [45–47]. The realization that at least one class of such proteins, the E-Syts, are not, directly, implicated in this process raise the possibility that functions of multiple C2 domain containing membrane proteins may be more heterogeneous than anticipated.
Highlights.
Extended-synaptotagmins (E-Syts) are proteins of the ER membrane.
They tether the ER to the plasma membrane (PM) via C2 domains
Their ER-PM tethering function is regulated by cytosolic Ca2+
They mediate lipid exchange between the ER and the PM via SMP domains.
They are expressed by all eukaryotic cells
Acknowledgments
We apologize to all the investigators whose work could not be cited due to space limitations. Work from the authors related to this review has been supported in part by a Nanyang Assistant Professorship grant and a Lee Kong Chian School of Medicine Start Up Grant, Nanyang Technological University, to Y.S. and by NIH grants (NS036251, DK45735 and DA018343), the Kavli Foundation and HHMI to P.D.C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Perin MS, Fried VA, Mignery GA, Jahn R, Sudhof TC. Phospholipid binding by a synaptic vesicle protein homologous to the regulatory region of protein kinase C. Nature. 1990;345:260–263. doi: 10.1038/345260a0. [DOI] [PubMed] [Google Scholar]
- 2.Brose N, Petrenko AG, Sudhof TC, Jahn R. Synaptotagmin: a calcium sensor on the synaptic vesicle surface. Science. 1992;256:1021–1025. doi: 10.1126/science.1589771. [DOI] [PubMed] [Google Scholar]
- 3.Chapman ER. How does synaptotagmin trigger neurotransmitter release? Annual review of biochemistry. 2008;77:615–641. doi: 10.1146/annurev.biochem.77.062005.101135. [DOI] [PubMed] [Google Scholar]
- 4.Sudhof TC. Calcium control of neurotransmitter release. Cold Spring Harbor perspectives in biology. 2012;4:a011353. doi: 10.1101/cshperspect.a011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toulmay A, Prinz WA. A conserved membrane-binding domain targets proteins to organelle contact sites. Journal of cell science. 2012;125:49–58. doi: 10.1242/jcs.085118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manford AG, Stefan CJ, Yuan HL, Macgurn JA, Emr SD. ER-to-plasma membrane tethering proteins regulate cell signaling and ER morphology. Developmental cell. 2012;23:1129–1140. doi: 10.1016/j.devcel.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Min SW, Chang WP, Sudhof TC. E-Syts, a family of membranous Ca2+-sensor proteins with multiple C2 domains. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3823–3828. doi: 10.1073/pnas.0611725104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giordano F, Saheki Y, Idevall-Hagren O, Colombo SF, Pirruccello M, Milosevic I, Gracheva EO, Bagriantsev SN, Borgese N, De Camilli P. PI(4,5)P2-Dependent and Ca(2+)-Regulated ER-PM Interactions Mediated by the Extended Synaptotagmins. Cell. 2013;153:1494–1509. doi: 10.1016/j.cell.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schauder CM, Wu X, Saheki Y, Narayanaswamy P, Torta F, Wenk MR, De Camilli P, Reinisch KM. Structure of a lipid-bound extended synaptotagmin indicates a role in lipid transfer. Nature. 2014;510:552–555. doi: 10.1038/nature13269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pang ZP, Sudhof TC. Cell biology of Ca2+-triggered exocytosis. Current opinion in cell biology. 2010;22:496–505. doi: 10.1016/j.ceb.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Craxton M. Synaptotagmin gene content of the sequenced genomes. BMC genomics. 2004;5:43. doi: 10.1186/1471-2164-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shao X, Davletov BA, Sutton RB, Sudhof TC, Rizo J. Bipartite Ca2+-binding motif in C2 domains of synaptotagmin and protein kinase C. Science. 1996;273:248–251. doi: 10.1126/science.273.5272.248. [DOI] [PubMed] [Google Scholar]
- 13.von Poser C, Ichtchenko K, Shao X, Rizo J, Sudhof TC. The evolutionary pressure to inactivate. A subclass of synaptotagmins with an amino acid substitution that abolishes Ca2+ binding. The Journal of biological chemistry. 1997;272:14314–14319. doi: 10.1074/jbc.272.22.14314. [DOI] [PubMed] [Google Scholar]
- 14.Sugita S, Shin OH, Han W, Lao Y, Sudhof TC. Synaptotagmins form a hierarchy of exocytotic Ca(2+) sensors with distinct Ca(2+) affinities. The EMBO journal. 2002;21:270–280. doi: 10.1093/emboj/21.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Craxton M. A manual collection of Syt, Esyt, Rph3a, Rph3al, Doc2, and Dblc2 genes from 46 metazoan genomes–an open access resource for neuroscience and evolutionary biology. BMC genomics. 2010;11:37. doi: 10.1186/1471-2164-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jimenez JL, Davletov B. Beta-strand recombination in tricalbin evolution and the origin of synaptotagmin-like C2 domains. Proteins. 2007;68:770–778. doi: 10.1002/prot.21449. [DOI] [PubMed] [Google Scholar]
- 17.Craxton M. Evolutionary genomics of plant genes encoding N-terminal-TM-C2 domain proteins and the similar FAM62 genes and synaptotagmin genes of metazoans. BMC genomics. 2007;8:259. doi: 10.1186/1471-2164-8-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Idevall-Hagren O, Lu A, Xie B, De Camilli P. Triggered Ca2+ influx is required for extended synaptotagmin 1-induced ER-plasma membrane tethering. The EMBO journal. 2015;34:2291–2305. doi: 10.15252/embj.201591565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang CL, Hsieh TS, Yang TT, Rothberg KG, Azizoglu DB, Volk E, Liao JC, Liou J. Feedback regulation of receptor-induced Ca2+ signaling mediated by E-Syt1 and Nir2 at endoplasmic reticulum-plasma membrane junctions. Cell Rep. 5:813–825. doi: 10.1016/j.celrep.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez-Busnadiego R, Saheki Y, De Camilli P. Three-dimensional architecture of extended synaptotagmin-mediated endoplasmic reticulum-plasma membrane contact sites. Proceedings of the National Academy of Sciences of the United States of America. 2015 doi: 10.1073/pnas.1503191112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carrasco S, Meyer T. STIM proteins and the endoplasmic reticulum-plasma membrane junctions. Annual review of biochemistry. 2011;80:973–1000. doi: 10.1146/annurev-biochem-061609-165311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis RS. Store-operated calcium channels: new perspectives on mechanism and function. Cold Spring Harbor perspectives in biology. 2011;3 doi: 10.1101/cshperspect.a003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hogan PG, Rao A. Store-operated calcium entry: Mechanisms and modulation. Biochemical and biophysical research communications. 2015;460:40–49. doi: 10.1016/j.bbrc.2015.02.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kopec KO, Alva V, Lupas AN. Homology of SMP domains to the TULIP superfamily of lipid-binding proteins provides a structural basis for lipid exchange between ER and mitochondria. Bioinformatics. 2010;26:1927–1931. doi: 10.1093/bioinformatics/btq326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reinisch KM, De Camilli P. SMP-domain proteins at membrane contact sites: Structure and function. Biochimica et biophysica acta. 2016;1861:924–927. doi: 10.1016/j.bbalip.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holthuis JC, Levine TP. Lipid traffic: floppy drives and a superhighway, Nature reviews. Molecular cell biology. 2005;6:209–220. doi: 10.1038/nrm1591. [DOI] [PubMed] [Google Scholar]
- 27.Lev S. Nonvesicular lipid transfer from the endoplasmic reticulum. Cold Spring Harbor perspectives in biology. 2012;4 doi: 10.1101/cshperspect.a013300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.AhYoung AP, Jiang J, Zhang J, Khoi Dang X, Loo JA, Zhou ZH, Egea PF. Conserved SMP domains of the ERMES complex bind phospholipids and mediate tether assembly. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:E3179–3188. doi: 10.1073/pnas.1422363112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kornmann B, Currie E, Collins SR, Schuldiner M, Nunnari J, Weissman JS, Walter P. An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science. 2009;325:477–481. doi: 10.1126/science.1175088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saheki Y, Bian X, Schauder CM, Sawaki Y, Surma MA, Klose C, Pincet F, Reinisch KM, De Camilli P. Control of plasma membrane lipid homeostasis by the extended synaptotagmins. Nature cell biology. 2016;18:504–515. doi: 10.1038/ncb3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu H, Liu Y, Gulbranson DR, Paine A, Rathore SS, Shen J. Extended synaptotagmins are Ca2+-dependent lipid transfer proteins at membrane contact sites. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:4362–4367. doi: 10.1073/pnas.1517259113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang CL, Hsieh TS, Yang TT, Rothberg KG, Azizoglu DB, Volk E, Liao JC, Liou J. Feedback regulation of receptor-induced Ca2+ signaling mediated by E-Syt1 and Nir2 at endoplasmic reticulum-plasma membrane junctions. Cell Rep. 2013;5:813–825. doi: 10.1016/j.celrep.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 33.Xu J, Bacaj T, Zhou A, Tomchick DR, Sudhof TC, Rizo J. Structure and Ca(2)(+)-binding properties of the tandem C(2) domains of E-Syt2. Structure. 2014;22:269–280. doi: 10.1016/j.str.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim YJ, Guzman-Hernandez ML, Wisniewski E, Balla T. Phosphatidylinositol-Phosphatidic Acid Exchange by Nir2 at ER-PM Contact Sites Maintains Phosphoinositide Signaling Competence. Developmental cell. 2015;33:549–561. doi: 10.1016/j.devcel.2015.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yadav S, Garner K, Georgiev P, Li M, Gomez-Espinosa E, Panda A, Mathre S, Okkenhaug H, Cockcroft S, Raghu P. RDGBalpha, a PtdIns-PtdOH transfer protein, regulates G-protein-coupled PtdIns(4,5)P2 signalling during Drosophila phototransduction. Journal of cell science. 2015;128:3330–3344. doi: 10.1242/jcs.173476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tremblay MG, Moss T. Loss of all 3 Extended Synaptotagmins does not affect normal mouse development, viability or fertility. Cell Cycle. 2016;15:2360–2366. doi: 10.1080/15384101.2016.1203494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sclip A, Bacaj T, Giam LR, Sudhof TC. Extended Synaptotagmin (ESyt) Triple Knock-Out Mice Are Viable and Fertile without Obvious Endoplasmic Reticulum Dysfunction. PloS one. 2016;11:e0158295. doi: 10.1371/journal.pone.0158295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jean S, Mikryukov A, Tremblay MG, Baril J, Guillou F, Bellenfant S, Moss T. Extended-synaptotagmin-2 mediates FGF receptor endocytosis and ERK activation in vivo. Developmental cell. 2010;19:426–439. doi: 10.1016/j.devcel.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 39.Jean S, Tremblay MG, Herdman C, Guillou F, Moss T. The endocytic adapter E-Syt2 recruits the p21 GTPase activated kinase PAK1 to mediate actin dynamics and FGF signalling. Biol Open. 2012;1:731–738. doi: 10.1242/bio.2012968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tremblay MG, Herdman C, Guillou F, Mishra PK, Baril J, Bellenfant S, Moss T. Extended synaptotagmin interaction with the fibroblast growth factor receptor depends on receptor conformation, not catalytic activity. The Journal of biological chemistry. 2016;291:3173. doi: 10.1074/jbc.A115.656918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Omnus DJ, Manford AG, Bader JM, Emr SD, Stefan CJ. Phosphoinositide kinase signaling controls ER-PM cross-talk. Molecular biology of the cell. 2016;27:1170–1180. doi: 10.1091/mbc.E16-01-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schapire AL, Voigt B, Jasik J, Rosado A, Lopez-Cobollo R, Menzel D, Salinas J, Mancuso S, Valpuesta V, Baluska F, Botella MA. Arabidopsis synaptotagmin 1 is required for the maintenance of plasma membrane integrity and cell viability. The Plant cell. 2008;20:3374–3388. doi: 10.1105/tpc.108.063859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamazaki T, Kawamura Y, Minami A, Uemura M. Calcium-dependent freezing tolerance in Arabidopsis involves membrane resealing via synaptotagmin SYT1. The Plant cell. 2008;20:3389–3404. doi: 10.1105/tpc.108.062679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perez-Sancho J, Vanneste S, Lee E, McFarlane HE, Esteban Del Valle A, Valpuesta V, Friml J, Botella MA, Rosado A. The Arabidopsis synaptotagmin1 is enriched in endoplasmic reticulum-plasma membrane contact sites and confers cellular resistance to mechanical stresses. Plant physiology. 2015;168:132–143. doi: 10.1104/pp.15.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Washington NL, Ward S. FER-1 regulates Ca2+ -mediated membrane fusion during C. elegans spermatogenesis. Journal of cell science. 2006;119:2552–2562. doi: 10.1242/jcs.02980. [DOI] [PubMed] [Google Scholar]
- 46.Bansal D, Miyake K, Vogel SS, Groh S, Chen CC, Williamson R, McNeil PL, Campbell KP. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature. 2003;423:168–172. doi: 10.1038/nature01573. [DOI] [PubMed] [Google Scholar]
- 47.Roux I, Safieddine S, Nouvian R, Grati M, Simmler MC, Bahloul A, Perfettini I, Le Gall M, Rostaing P, Hamard G, Triller A, Avan P, Moser T, Petit C. Otoferlin, defective in a human deafness form, is essential for exocytosis at the auditory ribbon synapse. Cell. 2006;127:277–289. doi: 10.1016/j.cell.2006.08.040. [DOI] [PubMed] [Google Scholar]


