Abstract
Lifelong generation of blood and immune cells depends on hematopoietic stem cells (HSCs). Their function is precisely regulated by complex molecular networks that integrate and respond to ever changing physiological demands of the body. Over the past several years, significant advances have been made in understanding the extrinsic regulation of HSCs during development and in homeostasis. Propelled by technical advances in the field, the cellular and molecular components of the microenvironment that support HSCs in vivo are emerging. In addition, the interaction of HSCs with their niches is appreciated as a critical contributor to the pathogenesis of a number of hematological disorders. Here, we review these advances in detail and highlight the extrinsic regulation of HSCs in the context of development, homeostasis and diseases.
Visual abstract
The interaction between hematopoietic stem cells (HSCs) and their environment is critical for the hematopoietic system in development, homeostasis and diseases.

Introduction
Multicellular organisms evolved tissue-specific stem cells to generate, sustain and repair diverse tissue and organ types. Stem cells are maintained in tissues through life-long self-renewal divisions, where one or two stem cells are generated in each round of cell division1. Stem cells also have multilineage differentiation potential. Thus stem cells are constantly balancing two seemingly opposed functions: maintaining the undifferentiated stem cell state and differentiating into cells of multiple lineages. Work from Drosophila has demonstrated that by providing adhesive interactions and biased signaling to stem cells, but not their immediate downstream progenies, stem cell microenvironmental niches provide a perfect solution to this issue2. Understanding how stem cells are regulated by their local niche and by other extrinsic mechanisms is fundamental to the field of stem cell biology.
Hematopoiesis has been a fruitful model for the study of stem cell biology. Multiple cell types constitute the hematopoietic system, including myeloid cells, lymphoid cells, erythroid cells and megakaryocytes. All of these lineages are ultimately generated from multipotent HSCs through a differentiation hierarchy that includes multiple levels of progenitors throughout life3. HSCs are also capable of regenerating the hematopoietic system after transplantation. In fact, HSC transplantation is the only cure available for a number of hematologic diseases. Their enormous medical potential aside, HSCs have served as the model tissue stem cell, having defined the rigorous standards of self-renewal and multilineage potential that characterize all tissue stem cells. This definition has provided the framework for understanding stem cell biology in general. Not surprisingly, the proposal of a stem cell niche was first suggested in the hematopoietic system for HSC maintenance4.
The high medical value and scarcity of HSCs prompted searches for conditions to culture or expand HSCs in vitro. Despite decades of effort, no culture system is able to robustly maintain or expand HSCs. However, these stem cells can clearly thrive within their native environment in vivo. Thus, defining the in vivo extrinsic regulatory mechanisms is a key step that will allow us to expand and augment the therapeutic utility of HSCs. Hematopoiesis and HSCs change organ sites several times throughout life to meet distinct physiological demands. The dynamic nature of the interaction between HSCs and their environments presents a fascinating yet challenging opportunity to understand HSC regulation. The fluid nature of the hematopoietic tissue and a lack of morphological or positional differences between HSCs and other hematopoietic cells have made the identification of these cells and their in vivo environment difficult. Despite these roadblocks, significant advancements have been made regarding the extrinsic regulation of HSCs in recent years. Here, we will summarize our understanding of the extrinsic regulation of HSCs in the context of development, homeostasis and disease. We will also highlight some of the outstanding questions in the field.
Overview of technical history
Our knowledge of HSCs is built on experimental evidence made possible by a number of technical advances, including two key innovations: transplantation and flow cytometry. During World War II, it was discovered that people exposed to lethal irradiation could be rescued by transplantation of cells from healthy donor bone marrow. This sparked the quest for cells that can replenish the hematopoietic system5. Work from Till and McCulloch showed that there are cells in the bone marrow that when transplanted can regenerate the blood system and form colonies on the spleens (colony forming unit-spleen or CFU-S) of mice exposed to lethal doses of irradiation6. It was later discovered that CFU-Ss are not HSCs but hematopoietic progenitors7,8. Nonetheless, using cytological methods, Till and McCulloch provided convincing evidence that these colonies contained multiple hematopoietic lineages and were derived from a single hematopoietic progenitor9. These observations have conceptually shaped the field of stem cell biology. The capability to stably reconstitute lethally irradiated recipient mice upon transplantation has become the gold standard in defining HSCs. Throughout the review, HSCs are defined by this criterion. Based on limiting dilution transplantation assays, it was estimated that about 5 cells in every 105 C57BL/6 bone marrow cells are HSCs10. But these rare stem cells are so potent that a single transplanted HSC can reconstitute the entire blood system of a lethally irradiated recipient mouse11,12.
Although HSCs were in the mixture of bone marrow cells used in early in vivo experiments, their exact identity remained elusive. No morphological features can distinguish rare HSCs from other hematopoietic cells, which was a major hurdle in the field. The invention of monoclonal antibodies and fluorescence activation cell sorting (FACS) made possible the isolation of HSCs based on the expression of specific cell surface antigens. Cell sorting combined with functional transplantation assays allowed for the development of a series of surface marker profiles that can be used to sort HSCs to high purity, particularly in the bone marrow3. The purification of HSCs has facilitated extensive research on intrinsic molecular mechanisms that regulate their self-renewal and differentiation. But how these mechanisms are integrated with the in vivo environment had not been clear.
Localizing HSCs in vivo is a prerequisite to elucidating their environmental regulation. Much of the focus in the field has been on the adult bone marrow. The complex markers used in FACS to purify HSCs were not suitable to identifying them on bone marrow tissue sections using microscopy, requiring the use of alternative markers in many early HSC niche studies. Simpler markers with low purity for HSCs13 and tracing assays for transplanted FACS-sorted fluorescent marker-labeled HSCs were initially used to localize HSCs in situ14,15. But it was not clear whether the localization of these cells reflected the niche of endogenous HSCs under steady state conditions. The development of Signaling Lymphocyte Activation Molecular (SLAM) markers allowed for the identification of HSCs on bone marrow sections in situ by simple two-color staining for cells with markers that also strictly defined HSCs by FACS and transplantation11. More recently developed genetically encoded fluorescent markers have also allowed detailed studies of HSC localization16,17. The advancement of other imaging techniques such as intra-vital live imaging and tissue clearing technology has provided even more comprehensive views of the natural environment of HSCs in the bone marrow18,19. The imaging of HSC emergence during development has also provided critical information on the niches that maintain HSCs. For example, live imaging of the aorta-gonad-mesonephros (AGM) has provided definitive evidence of hemogenic endothelium20–22. Similarly, imaging data regarding the niches in hematopoietic malignancies and other stress conditions are emerging.
Imaging has provided a solid framework for understanding the niche that regulates HSCs. However, functional studies in vivo are key to uncovering the mechanisms of HSC extrinsic regulation. Ideally, a defined perturbation of the candidate niche cell type should result in a perturbation of HSCs. Following this logic, several functional approaches have been taken to uncover the nature of bone marrow niche that maintains HSCs. We use the bone marrow as an example to highlight some functional methods to study HSC extrinsic regulation. Early genetic analysis of Steel (with mutated stem cell factor, SCF) and White (with mutated cKit receptor) mutants led to the identification of the key role of the SCF-cKit pathway in maintaining HSCs in vivo23. Additional genetic gain-of-function and loss-of-function studies have been used to elucidate the roles of other cell types and pathways in HSC maintenance24,25. Many of these studies used whole-body mutant mice where all cells are genetically perturbed. Thus, it is not clear what niche cells are relevant to observed HSC phenotypes. Cell ablation experiments have also been employed to functionally assess the requirement of distinct cell populations in supporting HSCs26. However, eliminating specific cells by inducing cell death may provoke non-specific HSC phenotypes related to other functions of the candidate niche cells. Ectopic bone marrow formation is another method employed to show the sufficiency of a given cell population in initiating an HSC-supporting environment27–29. Purified candidate niche organizing cells are transplanted subcutaneously or under the kidney capsule, sometimes with scaffolds. Over time, bone marrow will be developed and hematopoiesis will be sustained in these ectopic loci. But it is not clear whether these niche organizing cells or other cells recruited by them are directly supporting HSCs within the ectopic bone marrow. Ultimately, HSC maintenance factors need to be conditionally deleted from candidate niche cells to definitively test the hypothesis that these cells create the niche by elaborating key HSC-supporting factors30,31. Some of these functional studies have also been used to define cells and pathways that regulate HSCs extrinsically during development and in hematological disorders. In the following sections, we will discuss extrinsic HSC regulation with the emphasis on evidence from functional studies.
Developmental extrinsic regulation and migration
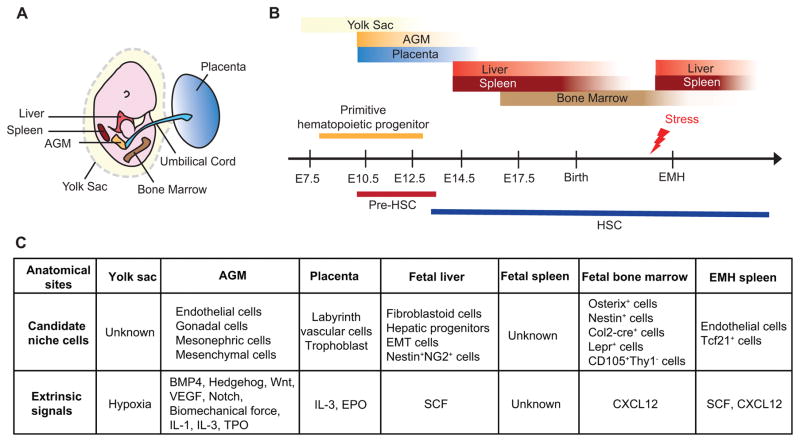
HSCs are formed during a narrow time window early during development in multiple anatomical sites (Figure 1). Thereafter, HSCs are maintained by self-renewal throughout adult life. As a consequence, the extrinsic regulation of HSCs during development is highly dynamic. Primitive hematopoiesis occurs in the yolk sac blood islands where lineage-restricted hematopoietic progenitors, but not transplantable HSCs, are generated. Definitive hematopoiesis follows with the emergence of HSCs that have self-renewal and multi-lineage potential in the AGM region. HSCs then colonize the fetal liver, where they mature and expand. The fetal spleen also serves as a transient reservoir of HSCs until birth, when HSCs migrate to the bone marrow, the predominant site of hematopoiesis in adults. The migration of HSCs through several organs suggests the sequential establishment of niches for HSCs. These niches may facilitate the maturation and expansion of definitive HSCs while eliminating primitive HSCs and other unwanted hematopoietic progenitors. Regardless of the exact mechanisms, the main purpose of extrinsic regulation of HSCs during development is to generate sufficient definitive HSCs for lifelong hematopoiesis. Conversely, HSCs can egress from the bone marrow to seed the liver and spleen and initiate extramedullary hematopoiesis (EMH) in adults, indicating that the niches can be reactivated in these extramedullary organs. The transient nature of these niches poses challenges to study the underpinning mechanisms, but it also provides an opportunity to study how a niche is switched on and off. This information will be important to devise means to amplify HSCs by activating niches in vivo.
Figure 1. Extrinsic regulation of HSCs during ontogeny.
A. An illustration depicting hematopoietic organs in mouse embryos. B. Timing and location of hematopoiesis during development. Hematopoiesis takes place in several anatomical sites. Primitive hematopoietic progenitors are found in the blood island of the yolk sac. AGM is the predominant site of HSC emergence although the placenta may also play a key role. HSCs migrate to, mature and expand in the fetal liver. Subsequently, HSCs transiently seed the fetal spleen and around birth, migrate to the bone marrow where HSCs reside throughout adult life. Under stress conditions, HSCs can egress from the bone marrow and take residence in the liver and spleen to initiate EMH. C. Candidate cellular components and extrinsic signals that regulate hematopoiesis during development. AGM, aorta-gonad-mesonephros; EMH, extramedullary hematopoiesis; EMT, epithelial-mesenchymal transition.
Yolk sac
The first blood cells in vertebrate embryos arise in the yolk sac. At embryonic day (E) 8.0 in mice, primitive erythrocytes and precursors of erythroid and myeloid cells with in vitro colony-forming potential are found in the blood islands of the yolk sac32–34. However, whether HSCs that contribute to adult hematopoiesis are generated in the yolk sac is controversial35–41. Different time points of analysis and different experimental techniques might explain conflicting results from varying studies. Nonetheless, the yolk sac does not seem to hold a large number of HSCs. Little is known regarding the extrinsic regulation in the yolk sac, although hypoxia is proposed to be critical for hematopoietic progenitors42.
Aorta-gonad-mesonephros (AGM)
Numerous studies have reported that HSCs arise de novo from the hemogenic endothelium of the dorsal aorta in the aorta-gonad-mesonephros (AGM) region through morphological cell transition43. Moreover, AGM-derived stromal cells can support HSCs and hematopoietic progenitors in vitro44,45. Although HSCs originate from the AGM, their poor bone marrow engraftment potential suggests that they require further maturation before seeding the bone marrow niche46.
Several pathways are important extrinsic regulators of HSC emergence in the AGM. Studies in zebrafish and mouse embryos have shown that BMP447,48, Hedgehog47,49, Wnt50, VEGF51 and Notch52,53 signaling is critical for the establishment of HSCs in the AGM. These factors are generally from the endothelial or mesenchymal cells that surround the hemogenic endothelium. Recently, sterile tonic proinflammatory signals have also been shown to promote the generation of HSCs in the AGM54–56. Consistent with this, proinfammatory cytokines IL-1 and IL-3 have been shown to constrain HSC differentiation57 and to promote HSC emergence58 in the AGM, respectively. The absence of the thrombopoietin (TPO)/MPL signaling results in a delayed production of HSCs in the AGM59, implying that this pathway regulates HSC emergence. Besides these cytokine pathways, several unconventional cues are also important for HSC emergence in the AGM. Biomechanical force generated by blood flow is also important to the production of HSCs in the AGM, likely through the nitric oxide pathway60,61 and cAMP-PKA-CREB pathway56,57. Several cell types make up the AGM, including endothelial cells, gonadal cells, mesonephric cells and mesenchymal cells. Identification of the cellular sources of the key extrinsic signals is required to gain a comprehensive understanding of the AGM niche.
Placenta
The placenta may also be an essential site for the generation and expansion of HSCs62–64. IL-3 seems to play a role in placental HSC emergence or maintenance as placental cells from IL-3 knockout mice failed to reconstitute adult recipients58. PDGFβ signaling in trophoblasts has been suggested to modulate erythropoiesis in the placenta by negatively regulating the expression of EPO65. Examination of the localization of Runx1+ (a master regulator of hematopoiesis) cells suggested that the large vessels in the chorioallantoic mesenchyme may be the origin of HSCs while small labyrinth vessels may provide a niche for HSC expansion66. Further investigation is needed to uncover how other pathways and cell types control the hematopoietic activity of the placenta.
Fetal liver
The fetal liver is the major site for HSC maturation and expansion. Circulating HSCs from different anatomical sites seed the fetal liver around E11.567. Fetal liver HSCs are actively cycling and have better repopulating capacity compared to adult bone marrow HSCs68–70. The SCF-cKit pathway is important for fetal liver HSCs. Administrating functional cKit blocking antibody to mouse embryos at fetal liver hematopoiesis stages disrupts fetal liver hematopoietic progenitors and hematopoiesis71. Consistent with this, fetal livers from Scf knockout mice harbor significantly less HSCs30,72. However, it is not clear what cells are the cellular source of SCF in the fetal liver in vivo.
Many cell types have been suggested to be critical components of the fetal liver niche. Murine fetal liver stromal cell lines73 and human fetal liver fibroblastoid cells74 have hematopoietic-supportive activity. Hepatic progenitors have also been implicated as niche cells based on expression of key HSC niche factors and their ability to support HSCs in vitro75. Cells with characteristics of undergoing epithelial-to-mesenchymal transition (EMT) have also been demonstrated to help maintain hematopoietic cells including HSCs in vitro76,77. Furthermore, endothelial protein C receptor (EPCR)-expressing cells enriched for HSC activity have been shown to localize near the sinusoidal network in fetal livers78, suggesting a perivascular niche. However, these studies were largely based on in vitro co-culture systems. Direct in vivo functional evidence supporting important roles of these putative fetal liver niche components still needs to be obtained.
Recently, Khan et al. have proposed Nestin+NG2+ periportal stromal cells as an important component of the fetal liver HSC niche through in vivo experiments. Fetal liver HSCs were found adjacent to portal vessels, and Nestin-GFP+ stromal cells had HSC maintaining activity in vitro79. Genetic ablation of NG2+ stromal cells led to a two-fold reduction in the total number of phenotypic fetal liver HSCs with more quiescence. However, fetal liver cells from these mice had better lymphoid reconstitution activity when transplanted (with normal myeloid cell potential – an accurate readout for HSC activity)79. It is not clear why ablation of a niche cell type reduces HSC numbers but increases lymphoid reconstitution activity of fetal liver cells. One possibility is that NG2+ cell ablation may selectively deplete some myeloid-biased or balanced HSCs, but does not affect lymphoid-biased HSCs. Nevertheless, as ablation of NG2+ pericytes did not lead to a profound change of HSCs and reconstitution activity in the fetal liver, other cellular components may also be critical contributors to the fetal liver HSC niche.
Fetal spleen
The fetal spleen is a transient niche for HSCs during embryogenesis. HSCs are detected in the spleen at E14.5 prior to their colonization of the bone marrow80. Fetal spleens lack HSC expansion capacity and fetal spleen stromal cells have been reported to fail to maintain multi-lineage potential of HSCs in vitro81. However, it seems unlikely that HSCs detected in the fetal spleen are merely passing through circulation as HSCs are found in the blood stream at a significant lower level than in the fetal spleen during embryogenesis80. It is not clear what cells or extrinsic factors regulate HSCs in the fetal spleen.
Fetal and neonatal bone marrow
HSCs start to seed the bone marrow at E17.580. This process depends on the expression of bone marrow C-X-C Motif Chemokine Ligand 12 (CXCL12), a known chemokine for HSCs82. Other than through attractive signals elaborated from the fetal bone marrow, the exit of HSCs from the fetal liver may be driven by the loss of HSC-supporting activity in the liver, although evidence for this has not yet been shown. Osterix+ cells83, neural crest-derived Nestin+ cells84, Col2-cre+ cells85, and Lepr+ cells86 have been suggested to be the cellular source of stromal populations that comprise the fetal bone marrow HSC niche. In addition, fetal mouse bone marrow CD105+Thy1− stromal cells have been shown to form HSC-supporting bone marrow28. However, the lineage and spatial relationship of these cells needs further investigation.
Bone marrow HSCs from 3-week old mice are more similar to fetal liver HSCs than adult bone marrow HSCs in their self-renewal capacity and gene expression87. At 4 weeks old, HSCs acquire characteristics of adult bone marrow HSCs, including transition to quiescence87. Therefore, it is speculated that the neonatal bone marrow niche undergoes a major transformation during this period. However, little is known about how this transition occurs, including the role of niche independent, HSC-intrinsic pathways.
Extramedullary hematopoiesis (EMH)
The developmental migration of HSCs from fetal liver to fetal spleen and bone marrow is reversible. In adults, HSCs can egress from the bone marrow to seed the spleen and liver to initiate EMH. This is proposed to be a response to hematopoietic stress, particularly when the bone marrow is not inhabitable for HSCs88.
A perisinusoidal niche comprised of endothelial cells and Tcf21+ perivascular stromal cells is activated in the spleen during EMH89. Key signals involved in HSC migration to the bone marrow during development, such as CXCL12, also play important roles in establishing spleen EMH89,90. It would be interesting to investigate whether the adult EMH niche is comprised of the same cellular components as the fetal HSC niche. Furthermore, whether EMH niches regulate HSCs differently than the bone marrow warrants future studies.
Homeostatic extrinsic regulation
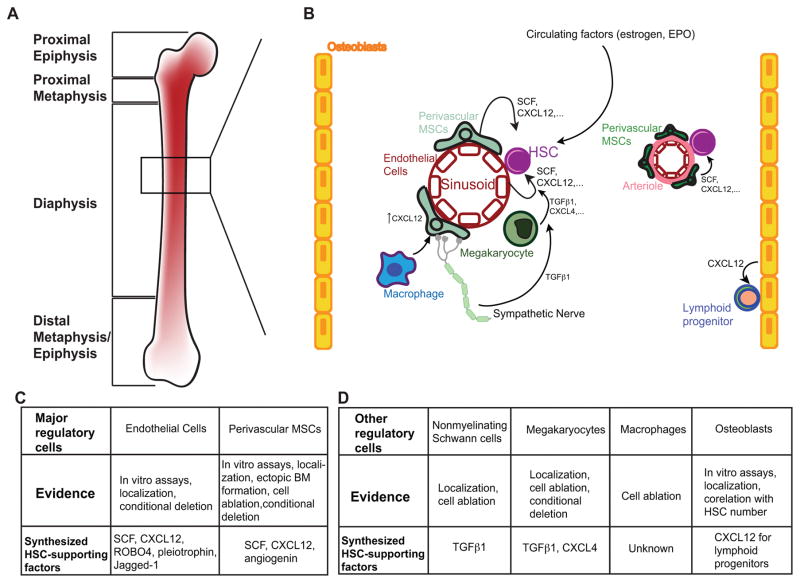
After development, mammalian HSCs take up residence in the bone marrow niche to sustain homeostatic hematopoiesis. The bone marrow niche is a specialized microenvironment that exquisitely regulates the quiescence, self-renewal, and differentiation of HSCs91. Studies have shown that bone marrow HSCs preferentially localize near the vasculature, and subsequent loss-of-function experiments have confirmed that both the vascular endothelium and perivascular stroma are crucial sources of HSC regulatory factors. In addition, mature hematopoietic cells and adipo-lineage cells within the bone marrow are able to regulate HSC behavior. Local oxygen levels, the sympathetic nervous system, and both local and circulating cytokines have also been implicated as regulators of HSCs. In total, these extrinsic signals are crucial to maintaining a functional HSC pool and lifelong hematopoiesis (Figure 2).
Figure 2. Homeostatic HSC extrinsic regulation.
A. Anatomy of a mammalian long bone, the major hematopoietic organ in adults. B. In the bone marrow, HSCs are regulated by a variety of cell types including endothelial cells, heterogeneous populations of mesenchymal stromal cells, non-myelinating Schwann cells, megakaryocytes, macrophages and osteoblasts. Signals from sympathetic nerves, circulating factors and possibly hypoxia (not pictured) are also important. Key locally synthesized factors for HSC maintenance include SCF, CXCL12, TGF-β1 and CXCL4. C and D. Major and other HSC regulatory cells in the bone marrow, where major regulatory cells are required to maintain the HSC pool, and other regulatory cells affect the cell cycle, localization and downstream progeny of HSCs through direct and indirect mechanisms.
The bone marrow HSC niche: location, location, location
Although the concept of an HSC niche was first proposed almost four decades ago4, robust characterization of the bone marrow niche was hindered by the lack of markers that could precisely label HSCs in situ. One of first studies performed by Lord et al. demonstrated that CFU-Ss (later shown to be hematopoietic progenitors, not HSCs) are enriched in endosteum, hinting this region may contain HSC niches92. Initial in situ visualization studies indeed showed that transplanted, flow cytometrically purified HSCs homed to the endosteum14,15, where osteoblasts were proposed to be a critical component of the niche. Recent work using SLAM or other genetic markers demonstrates that a vast majority of HSCs are directly in contact with the vascular endothelium16,17,19,26,93,94, with only a minority of them close to endosteum, pointing to the perivascular microenvironment as the bone marrow HSC niche.
While there is a growing agreement on the central role of the perivascular microenvironment, there is conflicting evidence as to whether HSCs prefer the endosteum. One should be careful to distinguish observations of endogeneous HSCs from those of transplanted HSCs, since the niches may differ in these conditions in several ways. First, it is possible that the niche is perturbed by the myeloablation used in many transplantation experiments, which is known to damage the sinusoidal vasculature86,95. Second, it has been shown that endosteal cells proliferate rapidly and up-regulate HSC maintenance pathways in response to myeloablation96,97. Finally, it is not clear whether the transplanted HSCs are engaged with their niche or in the process of finding the niche. Thus, it is possible that the endosteal localization frequently seen of transplanted HSCs reflects experimental conditioning rather than homeostatic physiology, although understanding how the niche differs in these states has important clinical implications for transplantation therapies. Discrepancies in the literature thus may in part reflect subtle differences between the ‘homeostatic niche’ and ‘post-transplant niche’. However, the cell types and signaling pathways implicated in HSC maintenance seem to play significant roles in both the homeostatic and post-transplant bone marrow – for example, angiogenin from mesenchymal stromal cells (MSCs) regulates HSC proliferation in homeostasis and promotes hematopoietic regeneration following myeloablative conditioning98,99.
Key cellular components of the bone marrow niche
Functional evidence suggests that a number of cell types contribute critically to the bone marrow niche. As the cellular components of the bone marrow niche have been extensively reviewed elsewhere91,100,101, we will only summarize some of the key in vivo functional evidence here. Early studies showed that osteoblast number was positively correlated with the number of HSCs in two mouse genetic models24,25, which in part led to the initial proposal of an endosteal bone marrow niche. Osteoblasts have been proposed to regulate HSCs by a variety of mechanisms including granulocyte colony stimulating factor (G-CSF)102, Notch24, Wnt103, Angiopoietin-1 (ANGPT1)13, and thrombopoietin (TPO)104 signaling and N-cadherin-mediated cell adhesion25. However, careful functional studies reveal conflicting evidence over the roles of Notch105, ANGPT1106, and N-cadherin107–109, and additional data complicates the picture of how osteoblasts regulate HSCs. In several other mouse models where osteoblast number was increased or decreased, HSC number was unchanged107,110–113. Furthermore, conditional deletion of critical HSC maintenance factors SCF and CXCL12 from osteoblasts has no effect on HSC function30,31. However, it has been shown that osteoblasts serve as an important niche component for early lymphoid progenitors by synthesizing CXCL1231,114. It is possible that osteoblasts act through other mechanisms to modify HSC behavior, but their role as a niche cell is not as prominent as initially proposed.
While the evidence about the role of osteoblasts in HSC maintenance is conflicting, there is strong evidence that the vascular endothelium is a crucial cellular component of the niche. Endothelial cells (ECs) drive HSC self-renewal in vivo through Notch signaling115–118, FGFR signaling119, gp130120 and production of SCF30 and CXCL1231, and HSC homing through release of CXCL1231, ROBO4121,122 and pleiotrophin123,124. The vasculature also plays a critical role in mediating a physiologic response to hematopoietic stress; following myeloablation, regeneration of VEGFR2+ sinusoidal ECs is required for effective hematopoietic reconstitution95,125. Moreover, endothelial cell-derived EGF is critical for HSC and hematopoietic regeneration after irradiation126.
Perivascular MSCs that lie in close contact with the bone marrow vasculature have also been identified as major regulators of HSCs. Several markers with overlapping expression patterns have been used to define these perivascular stromal cells, including platelet-derived growth factor receptor ζ (PDGFRA)127,128, leptin receptor (LEPR)30, Prx1-cre114, Osx-cre114, Osx-creER, FAP129, CD146 in humans27, SCF30, CXCL1231,114,130, and the Nestin-GFP transgene26. Using some of these markers as tools to manipulate these cells, a number of functional studies have provided strong evidence that the perivascular stromal cells are a critical component of the bone marrow niche. Ablation of Cxcl12-expressing or FAP-expressing bone marrow cells led to depletion of HSCs131 and compromised hematopoiesis131,132. Conditional deletion of Scf from perivascular stromal using Lepr-cre resulted in HSC depletion30, as did conditional deletion of Cxcl12 from perivascular stromal cells using Lepr-cre or Prx1-cre31,114. Interestingly, these stromal cells have mesenchymal progenitor activity with the capacity to differentiate into bone and adipocyte lineages in vitro and in vivo26,86. Conditional deletion of Foxc1, a transcriptional factor involved in mesenchymal cell fate determination, from perivascular stromal cells led to excessive adipogenesis and depletion of HSCs133. Recently, angiogenin has been identified as a novel factor from Osx+ mesenchymal cells for HSC maintenance99. Besides these genetic studies, subcutaneously transplanted human CD146+ bone marrow stromal cells have been shown to form ectopic HSC-supporting bone marrow27. Thus, the bone marrow perivascular mesenchymal stromal cells are a critical component of the bone marrow niche.
Other non-hematopoietic cells have also been implicated in HSC maintenance. HSCs from adipocyte-rich bone have decreased function and treatment with adipogenic inhibitors increases the reconstitution potential of HSCs, suggesting that adipocytes may negatively regulate HSCs134. Additionally, non-myelinating Schwann cells have been shown to activate latent TGF-β signaling required for HSC maintenance135.
Mature hematopoietic cells have also been connected to HSC regulation. Megakaryocytes physically associate with HSCs, maintain HSC quiescence through secretion of factors such as CXCL4 and TGF-β1, and promote hematopoietic recovery after myeloablation by driving HSC expansion136,137. Like megakaryocytes, bone marrow macrophages also modify HSC behavior. Depletion of macrophages leads to increased egress of HSCs from the bone marrow, suggesting that macrophages help retain HSCs in the bone marrow niche through cell homing and adhesion pathways, such as CXCL12-CXCR4 signaling138,139. Macrophages are believed to drive this retention of HSCs indirectly by acting on other niche cells including MSCs138,139. However, a recent study suggested that a subpopulation of macrophages which express Duffy antigen/receptor for chemokines (DARC) directly maintain HSC quiescence via binding of DARC to the HSC cell surface antigen KANGAI1 (KAI1)140. Thus, macrophages can directly and indirectly impact HSCs. In addition, Treg cells have been shown to provide immune privilege in the bone marrow niche141. In summary, these findings highlight the complex and interconnected regulatory interactions of the bone marrow niche.
Hypoxia and bone marrow niche
The cells of the perivascular bone marrow niche are critical extrinsic regulators of HSCs, but they are not the only factors that influence HSC biology. Numerous studies suggest that oxygen levels in the bone marrow have a major impact on HSC homing and function. In poorly perfused regions of the bone marrow that presumably have lower oxygen tension, the HSC population is enriched, more quiescent, and better able to serially reconstitute irradiated recipient mice142. Consistent with these findings, HSCs with high levels of reactive oxygen species (ROS), a feature associated with aerobic metabolism, have diminished self-renewal potential143. Indeed, HSC maintenance seems to require continuous activation of hypoxia-associated transcriptional and metabolic programs144–147. Interestingly, harvesting HSCs in a hypoxic environment better preserves transplantable HSCs148. The importance of these pathways to HSC function has driven many labs to investigate the location of the putative ‘hypoxic niche’ through indirect94 and direct149 measurements of oxygen levels throughout the bone marrow. The most recent and direct evidence shows that the peri-sinusoidal region in the bone marrow is the most hypoxic region of the bone at steady state149. However, it is possible that the association between HSCs and hypoxia is indirect. The ‘hypoxic’ profile of HSCs measured by pimonidazole may reflect their metabolic status independent of localization94. In addition, the hypoxia master regulator HIF-1α may be dispensable in HSCs150, although HIF-1α activity drives growth and expansion of vascular endothelium117. Thus, hypoxia may promote the development of the HSC niche, rather than provide direct extrinsic regulation of HSCs.
Heterogeneity of the bone marrow niche
The above data strongly suggest that HSCs reside in a perivascular niche with endothelial cells and mesenchymal stromal cells as critical components. Recent evidence points to potential heterogeneity in the perivascular niche. Perivascular NG2+LEPR−Nes-GFPbright mesenchymal cells that predominantly associate with bone marrow arterioles, not sinusoids, have been shown to play a role in HSC maintenance: partial ablation of NG2+ cells leads to decreased HSC quiescence and diminished capacity for functional reconstitution151. However, deletion of Scf and Cxcl12 using NG2-creER did not significantly affect HSCs19. Further investigation is needed to clarify this discrepancy. Mechanisms other than SCF and CXCL12 production may account for the HSC phenotype observed in the mice with NG2+ cells ablated. Recently, heterogeneity among endothelial cells in the bone marrow has been suggested to play important roles in regulating distinct HSC behaviors - arterial blood vessels maintain HSCs while sinusoids promote HSC activation and trafficking119. The functional evidence supporting this conclusion is partly based on evidence that conditional deletion of Fgfr from endothelial cells using Vecadherin-CreER leads to vasculature leakage and HSC reduction. However, Vecadherin-creER recombines in all bone marrow endothelial cells including arteriolar and sinusoidal endothelial cells152. Cre drivers specific to distinct vascular domains are needed to further elucidate the roles of different subsets of the vasculature.
Beyond the bone marrow: systemic regulation of HSCs
The bone marrow microenvironment is the most well-studied component of HSC extrinsic regulation, but extramedullary signals from outside the bone marrow also have a significant impact on HSCs. One prominent example of an extramedullary regulator is the sympathetic nervous system, which has a major influence on the release of HSCs into the blood stream153,154. Catecholaminergic signaling to both HSCs155 and niche cells156 drives HSC egress from the bone marrow by downregulating the chemokine CXCL12 and upregulating metalloproteinases that degrade the adhesive extracellular matrix. This signaling is linked to the body’s circadian rhythm, which also drives clearance of aged neutrophils from the bone marrow niche147.
There is growing evidence that systemically circulating factors also influence the biology of HSCs. New studies have shown that physiological circulating estrogen157 and induced upregulation of endogenous erthyropoietin (EPO)158 can directly promote HSC proliferation or instruct HSC differentiation to specific cell fates. These molecular signals originate outside the bone marrow but are still able to regulate the fate and behavior of HSCs, raising the possibility that other endogenous hematopoietic cytokines synthesized outside the bone marrow may directly maintain the HSC pool.
Disruption of the extrinsic regulation
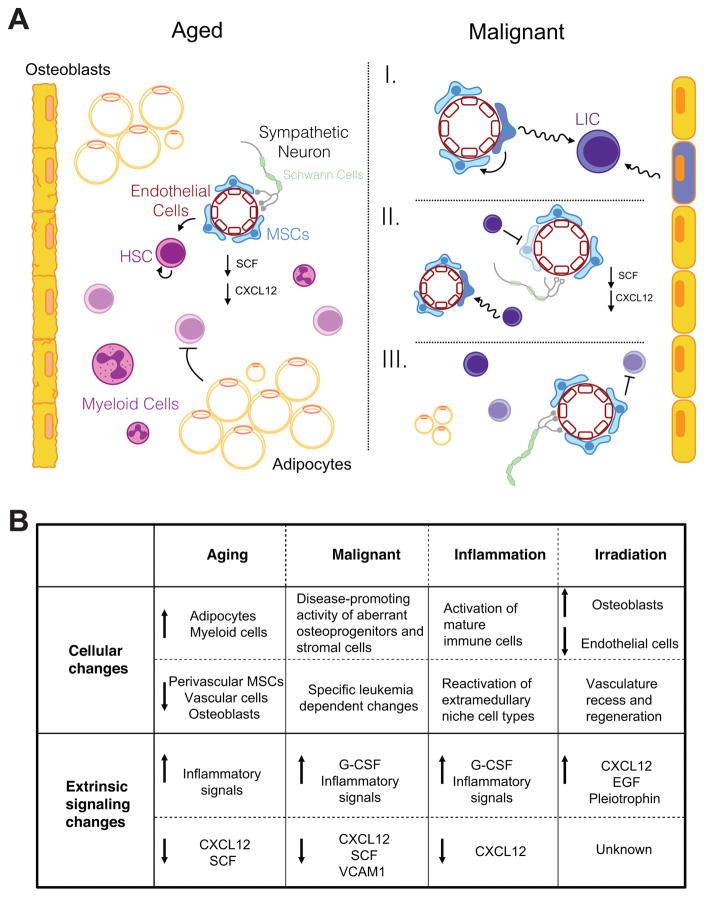
The bone marrow homeostatic niche must balance activating HSCs to replace lost progenitors while maintaining quiescent HSCs for future needs. This finite equilibrium can be disturbed in aging or other hematopoietic disorders. Aging of the hematopoietic system is associated with a decline of HSC function and hematopoiesis. This may be related to the demands of the an aging individual - to minimize oncogenic transformation at the cost of self-renewal activity159. It is evident that the extrinsic regulatory mechanisms play a role in HSC aging. Just as the HSC extrinsic regulation is important in homeostasis and aging, its dysregulation also plays a key role in coping with or even initiating pathological hematopoiesis. Below, we will discuss illustrative examples of the extrinsic regulation of HSCs during aging, hematopoietic malignancies and other stress conditions (Figure 3).
Figure 3. Disruption of extrinsic regulation of hematopoiesis.
A. The aged hematopoietic niche (left) features increased adipogenesis, decreased osteogenesis, decreased vascularity, decreased numbers of mesenchymal stromal cells (MSCs), and decreased niche signaling factors. HSCs are less functional. In states of malignancy (right), extrinsic regulation can be disrupted in many different ways. I) Aberrant niche cells can incite malignancy and leukemia-initiating cells (LIC). II) Malignant cells can inhibit normal functioning of niche cells and reduce normal niche signaling as well as induce leukemia-reinforcing phenotypes in niche cells. III) Normal niche cells can inhibit disease progression. B. Dysregulation of the niche varies across different of abnormal conditions – old age, malignancy, inflammation and irradiation. Common themes emerge, including the loss of cell types promoting critical niche signaling factors, pointing to the importance of these pathways in maintaining hematopoiesis.
Aging
The effects of aging on HSC function have been well described. Old HSCs have a decreased regenerative potential, are biased towards the myeloid lineage, and are more easily mobilized in response to cytokine cues160. Some of these phenotypes are linked to intrinsic changes within the HSC, but others have been proposed to be the result of an aging microenvironment. The effects of aging specifically on the bone marrow HSC niche has only begun to be explored. Transplantation of HSCs from young mice to old recipient mice (or vice versa) shows that at least part of the aging HSC phenotypes can be attributed to an aging environment. Young bone marrow HSCs home inefficiently to old bone marrow compared with young bone marrow, showing that there is a relevant difference in interactions between HSCs and the old environment that affects HSC engraftment161. Details on which signaling interactions differ and how engraftment is altered have yet to emerge. Similarly, old HSCs are less myeloid-biased when transplanted into young mice, suggesting that the age of the environment plays a key role in HSC differentiation lineage bias, which could be explained by an increase in the inflammatory cytokine Rantes162. Systemic age-related changes might also influence HSCs. Parabiosis experiments between old and young mice have demonstrated blood-borne factors that vary with age can influence muscle satellite stem cell and neural stem/progenitor cells function163,164. These studies suggest the possibility of systemic mechanisms for stem cell aging in general, but direct evidence for age-related systemic changes in HSC regulation is still needed. The question still remains of exactly how other age-related changes in HSCs are regulated by an old environment, both locally and systemically.
Age-related niche dysregulation may in part be explained by the increase in adipocyte content of old bone marrow, but the exact mechanisms are not yet clear. From adolescence to old age, the bone marrow progressively becomes more adipogenic with a higher fat content and decreased osteogenesis165,166. These changes are thought to be derived from altered differentiation of aged bone marrow MSCs. As described above, MSCs are a key component of the HSC niche and can differentiate into adipocytes, osteoblasts and chondrocytes within the bone marrow86,167. Old bone marrow MSCs are less proliferative, less likely to differentiate into osteoblasts, more likely to differentiate into adipocytes, and more apoptotic than young MSCs168,169. Interestingly, age-related changes in HSC frequency have been shown to be strain dependent, but in the strain most studies use, C57BL/6, HSC number increases with age170. Many studies have shown that there is an age-related decrease in HSC function, an increase in HSC number and in the adiposity of older bone marrow. However, studies of adipogenesis (in which age was not controlled for) suggest that medullary adipose tissue reduces HSC self-renewal in non-elderly C57BL/6 mice, resulting in decreased HSC number and function133,134. Furthermore, excessive adipogenesis from troglitazone, a drug that enhances adipogenesis, does not impact HSC8. Thus, it is unknown whether the correlations between adipogenesis and HSC dysfunction result directly from adipocyte-mediate effects or if they are due to indirect effects, such as a reduction in other stromal cell types. The functional differences between aged HSCs and HSCs from adipogenic models suggest that excess adipogenesis is not the only change in the old microenvironment affecting HSCs.
Other cellular components have also been examined to test whether aging leads to the loss of the normal homeostatic niche architecture. Old bone marrow has reduced vascularity and a decrease in PDGFRβ+NG2+ perivascular stromal cells, causing a decrease in key niche signals like CXCL12, and SCF117. Notch activation in endothelial cells rescued some of these phenotypes, causing an increase in capillaries and arterioles, more perivascular stromal cells, higher SCF expression, and a higher HSC number in mutant old mice than in wild-type old mice117. These studies only begin to delve into how HSC regulation is altered in an aged environment.
Role of the Niche in Hematologic Malignancies
Abnormalities in the niche are associated with hematologic malignancies. Leukemia-initiating cells (LICs) hijack the HSC mechanisms to persist and fuel the growth of leukemia. For an LIC to emerge and thrive, it must lose the restraints of HSC regulatory mechanisms keeping its growth and function in check. Some of those mechanisms are intrinsic to the LICs, but many are dependent on the niche. Whatever the inciting cause, as leukemia develops, there is ongoing crosstalk between the niche and LICs that eventually leads to further dysregulation and loss of normal hematopoiesis. Here, we will discuss some of the key features of the niche and malignant cells interactions, which have been described in more detail in other reviews101,171–173.
Live imaging of the bone marrow niche post-transplantation with a B-ALL leukemic cell line or T-ALL cells has demonstrated the stable interaction of leukemic cells with the endothelial domain, where the CXCL12-CXCR4 pathway is critical for leukemia cell engraftment18,174. However, a recent study has shown that the interaction of T-ALL cells and the niche is highly dynamic and not associated with bone marrow sub-compartments175. More investigation is needed to resolve the discrepancy. Comparing with the homeostatic bone marrow, imaging data of the malignant niche is only beginning to surface; all of these studies have been focused on transplanted leukemia models rather than de novo leukemia models. Furthermore, niche information concerning LICs is lacking. Technical improvement on malignant niche imaging is expected to lead to more exciting new insights.
Regardless of the localization of leukemic cells or LICs, an abnormal environment is important for malignancy progression172. A growing number of papers have demonstrated that the niche can be the inciting factor in developing malignancy. Myeloproliferative neoplasms (MPN) or myeloproliferation can be induced by deletion of IkBa (an inhibitor of NFkB)176, Mindbomb-1 (a notch ligand regulator) or retinoic acid receptor-γ in the bone marrow microenvironment177,178. Furthermore, conditional deletion of Rbpj (a notch pathway component) from endothelial cells also promotes myeloproliferation115. Moreover, conditional deletion of retinoblastoma protein (Rb) from both hematopoietic cells and stromal cells, causes a myeloproliferative-like disorder, but not when only deleted from myeloid progenitors or stromal cells179. In a similar fashion, conditional deletion of Dicer1 from osteoprogenitors but not more mature osteoblasts causes transplantable myelodysplasia and secondary leukemia180. Finally, Kode et al. induced acute myelogenous leukemia (AML)–like disease by constitutively activating β-catenin in osteoblasts181. These studies directly demonstrate the causal role of the niche in hematopoietic malignancies.
The interaction of leukemia cells and their niche is reciprocal. Several studies have shown that the introduction of leukemia can alter the niche, and often in ways that support the progression of leukemia and suppress normal hematopoiesis. Live imaging of mouse bone marrow transplanted with human leukemia cells showed that leukemic cells alter the stromal niche182. These abnormal niches sequester normal HSPCs and impair normal hematopoiesis182. Chronic myelogenous leukemia (CML) cells have been shown to alter key niche factors – such as decreasing CXCL12 and SCF and increasing G-CSF – that prevent HSCs from occupying their normal niche and instead promote leukemic cell development183,184. Similarly, the MPN bone marrow niche undergoes an alteration of niche signals and loss of bone marrow Schwann cells and nestin+ MSCs185. Rescuing some of the lost sympathetic tone with a selective β3 adrenergic agonist reduced the severity of disease185. Besides leukemia, the niche can also be an active participant in disease progression in other hematopoietic disorders. It has been shown that myelodysplastic syndrome (MDS)-initiating hematopoietic cells have the ability to induce neighboring MSCs to propagate a diseased microenvironment and facilitate the development of MDS186. These results demonstrate that abnormal hematopoietic cells may benefit from the changes they induce in the niche.
However, studies have also shown that the niche can act as a leukemia suppressor. Ablation of certain components of the microenvironment, such as catecholaminergic neurons, can lead to more severe disease in an aggressive MLL-AF9 AML model187. Krause et al. also illustrate this well in their work showing that activation of parathyroid hormone in osteoblasts inhibits CML progression but worsens survival in a model of AML188. Different types of hematologic malignancy may rely on and interact with the HSC niche very differently, reflecting not only the variety of ways that dysregulation of the niche may occur but also the complexity of signaling that fights to maintain hematopoiesis.
Recent understanding of how the bone marrow niche contributes to malignancy has led to the development of niche-targeted therapies with promising initial results. For example, AML cells exploit the CXCL12/CXCR4 axis for proliferation and survival, and high expression of CXCR4 on LICs correlates with worse prognosis. Inhibitors of CXCR4, most notably plerixafor, in combination with traditional chemotherapy agents have improved outcomes in AML murine models and clinical trials189. While niche-targeted agents have yet to become a part of standard treatments, research in this area is likely to grow.
Extrinsic regulation of HSCs in damage responses
Extrinsic regulatory mechanisms can also protect against infection and injury by modulating HSCs. During infection, the hematopoietic system must generate a sufficient immune response that often requires more mature hematopoietic cells from HSCs. Inflammatory signaling from Toll-like receptors (TLRs) and cytokines such as interferon-γ (IFNγ), interferon-α (IFNα), and tumor necrosis factor-α (TNFα) can activate HSCs190. These signals can be sensed directly by HSCs, but the bone marrow hematopoietic environment also plays a key role in mediating some of these immune responses191. For example, it has been shown that bacterial cell wall products from E.coli infection activate TLR and nucleotide-binding oligomerization domain-containing receptors (NOD) signaling, which induces G-CSF expression and decreases CXCL12 expression from niche endothelial cells, which in turn mobilizes HSCs to the spleen192. Similarly, IFNγ has been linked to increased HSC proliferation and mobilization to the spleen193. This effect can be mediated by MSCs expressing IL6 in response to IFNγ, which results in increased myeloid differentiation in response to infection194.
The bone marrow also responds to injury induced by irradiation. After irradiation, sinusoids regress, osteoblasts expand and megakaryocytes cluster around the endosteum and sinusoidal endothelium to promote HSC niche recovery20,22,96,97,106. Bone marrow vasculature regeneration plays a critical role in HSC recovery from irradiation. Targeting sinusoidal endothelial cells caused delay in hematopoietic recovery95. Angiopoietin-1-mediated interactions between HSCs and the bone marrow vasculature are important for optimal hematopoietic recovery after irradiation106 and cotransplantation of HSCs with endothelial cells results in faster hematopoietic recovery195. Moreover, endothelial jagged-1, pleiotrophin and EGF have been shown to be important for HSC regeneration after irradiation118,124,126.
Myelosuppression associated with chemotherapy is another stressor with clinical relevance. Similar to irradiation, recovery of the vasculature is critical for efficient hematopoietic recovery after myelosuppression. The endothelial VEGFR2 pathway is required for the restoration of hematopoiesis95. In this setting, TGF-β pathway plays an important role in dampening HSC activity196, suggesting that inhibiting this pathway could lead to better recovery after myelosuppression.
Perspectives
Significant advances have been made in the field over the last several years and it is expected that our understanding of the cellular and molecular components of extrinsic regulation of HSCs will continue to grow. This will allow us to compare and contrast the extrinsic regulatory mechanisms in different physiological and pathological settings. Interestingly, the sites of hematopoiesis are not conserved across vertebrates. For example, adult hematopoiesis occurs in the kidneys of fish and the livers of amphibians. However, in adult birds and mammals, the bone marrow is the primary hematopoietic organ. These observations suggest that extrinsic HSC supporting mechanisms are not confined to a specific organ. This is also reflected in the migration of mammalian HSCs during development and EMH. When the adult bone marrow niche is compromised in leukemia, myelofibrosis, osteopetrosis, and other medullary diseases, extramedullary sites are often reactivated to support hematopoiesis. Understanding more about the developmental and EMH niche could help us improve the responses of hematopoietic niches to disease and stress. Emerging evidence suggests that the vasculature plays important roles in creating the niche across different organs. Investigating the common and unique vascular components in these niches will shed light on potential unifying HSC niche mechanisms. For example, while the majority of HSCs are kept quiescent in the adult bone marrow niche, fetal liver HSCs actively cycle. Understanding signals from the fetal liver microenvironment that allows HSCs to expand rapidly while maintaining their self-renewal potential could help devise novel strategies to amplify HSCs.
As the cellular components are better defined, studying the regulatory mechanisms of the niche cells will likely help generate more functional HSC niches. The markers currently used for identifying HSC niche cells require further improvement. Even in the best-studied bone marrow niche, the frequencies of perivascular stromal cells and endothelial cells are at least an order of magnitude greater than that of functional HSCs. It could be that only a subset of these niche cells maintains HSCs. Like the evolvement of HSC markers, development of better markers that can be used to purify and target true niche cells will significantly improve the precision with which we can study the extrinsic regulation of HSCs, not only during homeostasis but also in hematopoietic disorders.
With the discovery of markers that can subdivide the niches, more exciting biology awaits. For example, HSCs are heterogenous in their cell cycle status, marker expression, lineage output and response to extrinsic signals197,198. This raises the question as to whether there is heterogeneity within the niches. Some recent studies have started shedding some light on the heterogeneity of the niche in regulating function of heterogenous HSCs. But more investigation is needed regarding the niche heterogeneity in the bone marrow and other hematopoietic organs under homeostasis and pathological conditions.
Most studies of HSC extrinsic regulation have been focused on the local microenvironment. However, systemic regulation is also critical. Hematopoiesis and HSC function are tightly regulated by the overall physiological demands of the body. This provides a feedback mechanism essential to maintaining just the right amount of hematopoiesis. It appears that all of the known systemic regulators are geared to push HSCs along specific differentiation pathways. The systemic factors that determine the physiological homeostatic level of HSCs have yet to be discovered.
Acknowledgments
This work is supported by the Rita Allen Foundation and the National Heart, Lung and Blood Institute (HL132074). MD and HL are supported by the Columbia Medical Scientist Training Program. We apologize to authors whose work could not be cited due to space constraints.
References
- 1.He S, Nakada D, Morrison SJ. Mechanisms of stem cell self-renewal. Annual review of cell and developmental biology. 2009;25:377–406. doi: 10.1146/annurev.cellbio.042308.113248. [DOI] [PubMed] [Google Scholar]
- 2.Losick VP, Morris LX, Fox DT, Spradling A. Drosophila stem cell niches: a decade of discovery suggests a unified view of stem cell regulation. Developmental cell. 2011;21:159–171. doi: 10.1016/j.devcel.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weissman IL, Shizuru JA. The origins of the identification and isolation of hematopoietic stem cells, and their capability to induce donor-specific transplantation tolerance and treat autoimmune diseases. Blood. 2008;112:3543–3553. doi: 10.1182/blood-2008-08-078220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 5.Ford CE, Hamerton JL, Barnes DW, Loutit JF. Cytological identification of radiation-chimaeras. Nature. 1956;177:452–454. doi: 10.1038/177452a0. [DOI] [PubMed] [Google Scholar]
- 6.Till JE, Mc CE. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiation research. 1961;14:213–222. [PubMed] [Google Scholar]
- 7.Jones RJ, et al. Characterization of mouse lymphohematopoietic stem cells lacking spleen colony-forming activity. Blood. 1996;88:487–491. [PubMed] [Google Scholar]
- 8.Jones RJ, Wagner JE, Celano P, Zicha MS, Sharkis SJ. Separation of pluripotent haematopoietic stem cells from spleen colony-forming cells. Nature. 1990;347:188–189. doi: 10.1038/347188a0. [DOI] [PubMed] [Google Scholar]
- 9.Becker AJ, Mc CE, Till JE. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 1963;197:452–454. doi: 10.1038/197452a0. [DOI] [PubMed] [Google Scholar]
- 10.Abkowitz JL, Golinelli D, Harrison DE, Guttorp P. In vivo kinetics of murine hemopoietic stem cells. Blood. 2000;96:3399–3405. [PubMed] [Google Scholar]
- 11.Kiel MJ, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 12.Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 13.Arai F, et al. Tie2/Angiopoietin-1 Signaling Regulates Hematopoietic Stem Cell Quiescence in the Bone Marrow Niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Lo Celso C, et al. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457:92–96. doi: 10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie Y, et al. Detection of functional haematopoietic stem cell niche using real-time imaging. Nature. 2009;457:97–101. doi: 10.1038/nature07639. [DOI] [PubMed] [Google Scholar]
- 16.Chen JY, et al. Hoxb5 marks long-term haematopoietic stem cells and reveals a homogenous perivascular niche. Nature. 2016;530:223–227. doi: 10.1038/nature16943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sawai CM, et al. Hematopoietic Stem Cells Are the Major Source of Multilineage Hematopoiesis in Adult Animals. Immunity. 2016;45:597–609. doi: 10.1016/j.immuni.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sipkins DA, et al. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature. 2005;435:969–973. doi: 10.1038/nature03703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Acar M, et al. Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature. 2015;526:126–130. doi: 10.1038/nature15250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bertrand JY, et al. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature. 2010;464:108–111. doi: 10.1038/nature08738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boisset JC, et al. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464:116–120. doi: 10.1038/nature08764. [DOI] [PubMed] [Google Scholar]
- 22.Kissa K, Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature. 2010;464:112–115. doi: 10.1038/nature08761. [DOI] [PubMed] [Google Scholar]
- 23.Broudy VC. Stem cell factor and hematopoiesis. Blood. 1997;90:1345–1364. [PubMed] [Google Scholar]
- 24.Calvi LM, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 26.Mendez-Ferrer S, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. nature09262 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sacchetti B, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 28.Chan CK, et al. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature. 2009;457:490–494. doi: 10.1038/nature07547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song J, et al. An in vivo model to study and manipulate the hematopoietic stem cell niche. Blood. 2010;115:2592–2600. doi: 10.1182/blood-2009-01-200071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495:231–235. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silver L, Palis J. Initiation of murine embryonic erythropoiesis: a spatial analysis. Blood. 1997;89:1154–1164. [PubMed] [Google Scholar]
- 33.Moore MA, Metcalf D. Ontogeny of the haemopoietic system: yolk sac origin of in vivo and in vitro colony forming cells in the developing mouse embryo. British journal of haematology. 1970;18:279–296. doi: 10.1111/j.1365-2141.1970.tb01443.x. [DOI] [PubMed] [Google Scholar]
- 34.Palis J, Robertson S, Kennedy M, Wall C, Keller G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development. 1999;126:5073–5084. doi: 10.1242/dev.126.22.5073. [DOI] [PubMed] [Google Scholar]
- 35.Dieterlen-Lievre F. On the origin of haemopoietic stem cells in the avian embryo: an experimental approach. J Embryol Exp Morphol. 1975;33:607–619. [PubMed] [Google Scholar]
- 36.Lassila O, Eskola J, Toivanen P, Martin C, Dieterlen-Lievre F. The origin of lymphoid stem cells studied in chick yold sac-embryo chimaeras. Nature. 1978;272:353–354. doi: 10.1038/272353a0. [DOI] [PubMed] [Google Scholar]
- 37.Cumano A, Ferraz JC, Klaine M, Di Santo JP, Godin I. Intraembryonic, but not yolk sac hematopoietic precursors, isolated before circulation, provide long-term multilineage reconstitution. Immunity. 2001;15:477–485. doi: 10.1016/s1074-7613(01)00190-x. [DOI] [PubMed] [Google Scholar]
- 38.Kumaravelu P, et al. Quantitative developmental anatomy of definitive haematopoietic stem cells/long-term repopulating units (HSC/RUs): role of the aorta-gonad-mesonephros (AGM) region and the yolk sac in colonisation of the mouse embryonic liver. Development. 2002;129:4891–4899. doi: 10.1242/dev.129.21.4891. [DOI] [PubMed] [Google Scholar]
- 39.Yoder MC, et al. Characterization of definitive lymphohematopoietic stem cells in the day 9 murine yolk sac. Immunity. 1997;7:335–344. doi: 10.1016/s1074-7613(00)80355-6. [DOI] [PubMed] [Google Scholar]
- 40.Yoder MC, Hiatt K, Mukherjee P. In vivo repopulating hematopoietic stem cells are present in the murine yolk sac at day 9.0 postcoitus. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:6776–6780. doi: 10.1073/pnas.94.13.6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samokhvalov IM, Samokhvalova NI, Nishikawa S. Cell tracing shows the contribution of the yolk sac to adult haematopoiesis. Nature. 2007;446:1056–1061. doi: 10.1038/nature05725. [DOI] [PubMed] [Google Scholar]
- 42.Adelman DM, Maltepe E, Simon MC. Multilineage embryonic hematopoiesis requires hypoxic ARNT activity. Genes & development. 1999;13:2478–2483. doi: 10.1101/gad.13.19.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Costa G, Kouskoff V, Lacaud G. Origin of blood cells and HSC production in the embryo. Trends Immunol. 2012;33:215–223. doi: 10.1016/j.it.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 44.Oostendorp RA, et al. Stromal cell lines from mouse aorta-gonads-mesonephros subregions are potent supporters of hematopoietic stem cell activity. Blood. 2002;99:1183–1189. doi: 10.1182/blood.v99.4.1183. [DOI] [PubMed] [Google Scholar]
- 45.Kusadasi N, Oostendorp RA, Koevoet WJ, Dzierzak EA, Ploemacher RE. Stromal cells from murine embryonic aorta-gonad-mesonephros region, liver and gut mesentery expand human umbilical cord blood-derived CAFC(week6) in extended long-term cultures. Leukemia. 2002;16:1782–1790. doi: 10.1038/sj.leu.2402615. [DOI] [PubMed] [Google Scholar]
- 46.Matsubara A, et al. Endomucin, a CD34-like sialomucin, marks hematopoietic stem cells throughout development. The Journal of experimental medicine. 2005;202:1483–1492. doi: 10.1084/jem.20051325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lydon NB, Druker BJ. Lessons learned from the development of imatinib. Leukemia research. 2004;28(Suppl 1):S29–38. doi: 10.1016/j.leukres.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 48.Shimoto M, Sugiyama T, Nagasawa T. Numerous niches for hematopoietic stem cells remain empty during homeostasis. Blood. 2017 doi: 10.1182/blood-2016-09-740563. [DOI] [PubMed] [Google Scholar]
- 49.Tomita Y, Sachs DH, Sykes M. Myelosuppressive conditioning is required to achieve engraftment of pluripotent stem cells contained in moderate doses of syngeneic bone marrow. Blood. 1994;83:939–948. [PubMed] [Google Scholar]
- 50.Westerhuis G, van Pel M, Toes RE, Staal FJ, Fibbe WE. Chimerism levels after stem cell transplantation are primarily determined by the ratio of donor to host stem cells. Blood. 2011;117:4400–4401. doi: 10.1182/blood-2011-01-328518. [DOI] [PubMed] [Google Scholar]
- 51.Bhattacharya D, Rossi DJ, Bryder D, Weissman IL. Purified hematopoietic stem cell engraftment of rare niches corrects severe lymphoid deficiencies without host conditioning. The Journal of experimental medicine. 2006;203:73–85. doi: 10.1084/jem.20051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burns CE, Traver D, Mayhall E, Shepard JL, Zon LI. Hematopoietic stem cell fate is established by the Notch-Runx pathway. Genes & development. 2005;19:2331–2342. doi: 10.1101/gad.1337005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumano K, et al. Notch1 but not Notch2 is essential for generating hematopoietic stem cells from endothelial cells. Immunity. 2003;18:699–711. doi: 10.1016/s1074-7613(03)00117-1. [DOI] [PubMed] [Google Scholar]
- 54.Espin-Palazon R, et al. Proinflammatory signaling regulates hematopoietic stem cell emergence. Cell. 2014;159:1070–1085. doi: 10.1016/j.cell.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Y, et al. Inflammatory signaling regulates embryonic hematopoietic stem and progenitor cell production. Genes & development. 2014;28:2597–2612. doi: 10.1101/gad.253302.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sawamiphak S, Kontarakis Z, Stainier DY. Interferon gamma signaling positively regulates hematopoietic stem cell emergence. Developmental cell. 2014;31:640–653. doi: 10.1016/j.devcel.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Orelio C, Haak E, Peeters M, Dzierzak E. Interleukin-1-mediated hematopoietic cell regulation in the aorta-gonad-mesonephros region of the mouse embryo. Blood. 2008;112:4895–4904. doi: 10.1182/blood-2007-12-123836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Robin C, et al. An unexpected role for IL-3 in the embryonic development of hematopoietic stem cells. Developmental cell. 2006;11:171–180. doi: 10.1016/j.devcel.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 59.Petit-Cocault L, Volle-Challier C, Fleury M, Peault B, Souyri M. Dual role of Mpl receptor during the establishment of definitive hematopoiesis. Development. 2007;134:3031–3040. doi: 10.1242/dev.001818. [DOI] [PubMed] [Google Scholar]
- 60.Brecher G, Ansell JD, Micklem HS, Tjio JH, Cronkite EP. Special proliferative sites are not needed for seeding and proliferation of transfused bone marrow cells in normal syngeneic mice. Proceedings of the National Academy of Sciences of the United States of America. 1982;79:5085–5087. doi: 10.1073/pnas.79.16.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nilsson SK, Dooner MS, Tiarks CY, Weier HU, Quesenberry PJ. Potential and distribution of transplanted hematopoietic stem cells in a nonablated mouse model. Blood. 1997;89:4013–4020. [PubMed] [Google Scholar]
- 62.Gekas C, Dieterlen-Lievre F, Orkin SH, Mikkola HK. The placenta is a niche for hematopoietic stem cells. Developmental cell. 2005;8:365–375. doi: 10.1016/j.devcel.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 63.Ottersbach K, Dzierzak E. The murine placenta contains hematopoietic stem cells within the vascular labyrinth region. Developmental cell. 2005;8:377–387. doi: 10.1016/j.devcel.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 64.Gekas C, et al. Hematopoietic stem cell development in the placenta. The International journal of developmental biology. 2010;54:1089–1098. doi: 10.1387/ijdb.103070cg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chhabra A, et al. Trophoblasts regulate the placental hematopoietic niche through PDGF-B signaling. Developmental cell. 2012;22:651–659. doi: 10.1016/j.devcel.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rhodes KE, et al. The emergence of hematopoietic stem cells is initiated in the placental vasculature in the absence of circulation. Cell stem cell. 2008;2:252–263. doi: 10.1016/j.stem.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Johnson GR, Moore MA. Role of stem cell migration in initiation of mouse foetal liver haemopoiesis. Nature. 1975;258:726–728. doi: 10.1038/258726a0. [DOI] [PubMed] [Google Scholar]
- 68.Morrison SJ, Hemmati HD, Wandycz AM, Weissman IL. The purification and characterization of fetal liver hematopoietic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:10302–10306. doi: 10.1073/pnas.92.22.10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Harrison DE, Zhong RK, Jordan CT, Lemischka IR, Astle CM. Relative to adult marrow, fetal liver repopulates nearly five times more effectively long-term than short-term. Exp Hematol. 1997;25:293–297. [PubMed] [Google Scholar]
- 70.Rebel VI, Miller CL, Eaves CJ, Lansdorp PM. The repopulation potential of fetal liver hematopoietic stem cells in mice exceeds that of their liver adult bone marrow counterparts. Blood. 1996;87:3500–3507. [PubMed] [Google Scholar]
- 71.Ogawa M, et al. Expression and function of c-Kit in fetal hemopoietic progenitor cells: transition from the early c-Kit-independent to the late c-Kit-dependent wave of hemopoiesis in the murine embryo. Development. 1993;117:1089–1098. doi: 10.1242/dev.117.3.1089. [DOI] [PubMed] [Google Scholar]
- 72.Ikuta K, Weissman IL. Evidence that hematopoietic stem cells express mouse c-kit but do not depend on steel factor for their generation. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:1502–1506. doi: 10.1073/pnas.89.4.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moore KA, Ema H, Lemischka IR. In vitro maintenance of highly purified, transplantable hematopoietic stem cells. Blood. 1997;89:4337–4347. [PubMed] [Google Scholar]
- 74.Tsai S, Emerson SG, Sieff CA, Nathan DG. Isolation of a human stromal cell strain secreting hemopoietic growth factors. J Cell Physiol. 1986;127:137–145. doi: 10.1002/jcp.1041270117. [DOI] [PubMed] [Google Scholar]
- 75.Chou S, Lodish HF. Fetal liver hepatic progenitors are supportive stromal cells for hematopoietic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:7799–7804. doi: 10.1073/pnas.1003586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang H, et al. The existence of epithelial-to-mesenchymal cells with the ability to support hematopoiesis in human fetal liver. Cell Biol Int. 2005;29:213–219. doi: 10.1016/j.cellbi.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 77.Chagraoui J, Lepage-Noll A, Anjo A, Uzan G, Charbord P. Fetal liver stroma consists of cells in epithelial-to-mesenchymal transition. Blood. 2003;101:2973–2982. doi: 10.1182/blood-2002-05-1341. [DOI] [PubMed] [Google Scholar]
- 78.Iwasaki H, Arai F, Kubota Y, Dahl M, Suda T. Endothelial protein C receptor-expressing hematopoietic stem cells reside in the perisinusoidal niche in fetal liver. Blood. 2010;116:544–553. doi: 10.1182/blood-2009-08-240903. blood-2009-08-240903 [pii] [DOI] [PubMed] [Google Scholar]
- 79.Khan JA, et al. Fetal liver hematopoietic stem cell niches associate with portal vessels. Science. 2016;351:176–180. doi: 10.1126/science.aad0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Christensen JL, Wright DE, Wagers AJ, Weissman IL. Circulation and chemotaxis of fetal hematopoietic stem cells. PLoS Biol. 2004;2:E75. doi: 10.1371/journal.pbio.0020075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bertrand JY, et al. Fetal spleen stroma drives macrophage commitment. Development. 2006;133:3619–3628. doi: 10.1242/dev.02510. [DOI] [PubMed] [Google Scholar]
- 82.Ara T, et al. Long-term hematopoietic stem cells require stromal cell-derived factor-1 for colonizing bone marrow during ontogeny. Immunity. 2003;19:257–267. doi: 10.1016/s1074-7613(03)00201-2. [DOI] [PubMed] [Google Scholar]
- 83.Mizoguchi T, et al. Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development. Developmental cell. 2014;29:340–349. doi: 10.1016/j.devcel.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Isern J, et al. The neural crest is a source of mesenchymal stem cells with specialized hematopoietic stem cell niche function. Elife. 2014;3:e03696. doi: 10.7554/eLife.03696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ono N, Ono W, Nagasawa T, Kronenberg HM. A subset of chondrogenic cells provides early mesenchymal progenitors in growing bones. Nat Cell Biol. 2014;16:1157–1167. doi: 10.1038/ncb3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou BO, Yue R, Murphy MM, Peyer JG, Morrison SJ. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell stem cell. 2014;15:154–168. doi: 10.1016/j.stem.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bowie MB, et al. Identification of a new intrinsically timed developmental checkpoint that reprograms key hematopoietic stem cell properties. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:5878–5882. doi: 10.1073/pnas.0700460104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim CH. Homeostatic and pathogenic extramedullary hematopoiesis. Journal of blood medicine. 2010;1:13–19. doi: 10.2147/JBM.S7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Inra CN, et al. A perisinusoidal niche for extramedullary haematopoiesis in the spleen. Nature. 2015;527:466–471. doi: 10.1038/nature15530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang X, et al. C-X-C motif chemokine 12 influences the development of extramedullary hematopoiesis in the spleens of myelofibrosis patients. Exp Hematol. 2015;43:100–109 e101. doi: 10.1016/j.exphem.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–334. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lord BI, Testa NG, Hendry JH. The relative spatial distributions of CFUs and CFUc in the normal mouse femur. Blood. 1975;46:65–72. [PubMed] [Google Scholar]
- 93.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 94.Nombela-Arrieta C, et al. Quantitative imaging of haematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment. Nature Cell Biology. 2013;15:533–543. doi: 10.1038/ncb2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hooper AT, et al. Engraftment and Reconstitution of Hematopoiesis Is Dependent on VEGFR2-Mediated Regeneration of Sinusoidal Endothelial Cells. Cell stem cell. 2009;4:263–274. doi: 10.1016/j.stem.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dominici M, et al. Restoration and reversible expansion of the osteoblastic hematopoietic stem cell niche after marrow radioablation. Blood. 2009;114:2333–2343. doi: 10.1182/blood-2008-10-183459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Olson TS, et al. Megakaryocytes promote murine osteoblastic HSC niche expansion and stem cell engraftment after radioablative conditioning. Blood. 2013;121:5238–5249. doi: 10.1182/blood-2012-10-463414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Goncalves KA, et al. Angiogenin Promotes Hematopoietic Regeneration by Dichotomously Regulating Quiescence of Stem and Progenitor Cells. Cell. 2016;166:894–906. doi: 10.1016/j.cell.2016.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Silberstein L, et al. Proximity-Based Differential Single-Cell Analysis of the Niche to Identify Stem/Progenitor Cell Regulators. Cell stem cell. 2016 doi: 10.1016/j.stem.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mendelson A, Frenette PS. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nature medicine. 2014;20:833–846. doi: 10.1038/nm.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hoggatt J, Kfoury Y, Scadden DT. Hematopoietic Stem Cell Niche in Health and Disease. Annual review of pathology. 2016;11:555–581. doi: 10.1146/annurev-pathol-012615-044414. [DOI] [PubMed] [Google Scholar]
- 102.Taichman RS, Emerson SG. Human osteoblasts support hematopoiesis through the production of granulocyte colony-stimulating factor. The Journal of experimental medicine. 1994;179:1677–1682. doi: 10.1084/jem.179.5.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sugimura R, et al. Noncanonical Wnt Signaling Maintains Hematopoietic Stem Cells in the Niche. Cell. 2012;150:351–365. doi: 10.1016/j.cell.2012.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yoshihara H, et al. Thrombopoietin/MPL Signaling Regulates Hematopoietic Stem Cell Quiescence and Interaction with the Osteoblastic Niche. Cell stem cell. 2007;1:685–697. doi: 10.1016/j.stem.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 105.Maillard I, et al. Canonical Notch Signaling Is Dispensable for the Maintenance of Adult Hematopoietic Stem Cells. Cell stem cell. 2008;2:356–366. doi: 10.1016/j.stem.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bo Zhou LD, Morrison Sean. Hematopoietic stem and progenitor cells regulate the regeneration of their niche by secreting Angiopoietin-1. ELife. 2015 doi: 10.7554/eLife.05521. doi: http://dx.doi.org/10.7554/eLife.05521. [DOI] [PMC free article] [PubMed]
- 107.Kiel MJ, Acar M, Radice GL, Morrison SJ. Hematopoietic stem cells do not depend on N-cadherin to regulate their maintenance. Cell stem cell. 2009;4:170–179. doi: 10.1016/j.stem.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bromberg O, et al. Osteoblastic N-cadherin is not required for microenvironmental support and regulation of hematopoietic stem and progenitor cells. Blood. 2012;120:303–313. doi: 10.1182/blood-2011-09-377853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Greenbaum AM, Revollo LD, Woloszynek JR, Civitelli R, Link DC. N-cadherin in osteolineage cells is not required for maintenance of hematopoietic stem cells. Blood. 2012;120:295–302. doi: 10.1182/blood-2011-09-377457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Visnjic D, et al. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103:3258–3264. doi: 10.1182/blood-2003-11-4011. [DOI] [PubMed] [Google Scholar]
- 111.Zhu J, et al. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood. 2007;109:3706–3712. doi: 10.1182/blood-2006-08-041384. [DOI] [PubMed] [Google Scholar]
- 112.Lymperi S, et al. Strontium can increase some osteoblasts without increasing hematopoietic stem cells. Blood. 2008;111:1173–1181. doi: 10.1182/blood-2007-03-082800. [DOI] [PubMed] [Google Scholar]
- 113.Calvi LM, et al. Osteoblastic expansion induced by parathyroid hormone receptor signaling in murine osteocytes is not sufficient to increase hematopoietic stem cells. Blood. 2012;119:2489–2499. doi: 10.1182/blood-2011-06-360933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Greenbaum A, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495:227–230. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang L, et al. Notch-dependent repression of miR-155 in the bone marrow niche regulates hematopoiesis in an NF-kappaB-dependent manner. Cell stem cell. 2014;15:51–65. doi: 10.1016/j.stem.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kobayashi H, et al. Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nature Cell Biology. 2010;12:1046–1056. doi: 10.1038/ncb2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kusumbe AP, et al. Age-dependent modulation of vascular niches for haematopoietic stem cells. Nature. 2016;532:380–384. doi: 10.1038/nature17638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Poulos MG, et al. Endothelial Jagged-1 is necessary for homeostatic and regenerative hematopoiesis. Cell reports. 2013;4:1022–1034. doi: 10.1016/j.celrep.2013.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Itkin T, et al. Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature. 2016;532:323–328. doi: 10.1038/nature17624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yao L, Yokota T, Xia L, Kincade PW, McEver RP. Bone marrow dysfunction in mice lacking the cytokine receptor gp130 in endothelial cells. Blood. 2005;106:4093–4101. doi: 10.1182/blood-2005-02-0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Smith-Berdan S, et al. Robo4 Cooperates with Cxcr4 to Specify Hematopoietic Stem Cell Localization to Bone Marrow Niches. Cell stem cell. 2011;8:72–83. doi: 10.1016/j.stem.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Smith-Berdan S, Nguyen A, Hong Matthew A, Forsberg EC. ROBO4-Mediated Vascular Integrity Regulates the Directionality of Hematopoietic Stem Cell Trafficking. Stem Cell Reports. 2015;4:255–268. doi: 10.1016/j.stemcr.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Himburg Heather A, et al. Pleiotrophin Regulates the Retention and Self-Renewal of Hematopoietic Stem Cells in the Bone Marrow Vascular Niche. Cell reports. 2012;2:964–975. doi: 10.1016/j.celrep.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Himburg HA, et al. Pleiotrophin regulates the expansion and regeneration of hematopoietic stem cells. Nature medicine. 2010;16:475–482. doi: 10.1038/nm.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Butler JM, et al. Endothelial Cells Are Essential for the Self-Renewal and Repopulation of Notch-Dependent Hematopoietic Stem Cells. Cell stem cell. 2010;6:251–264. doi: 10.1016/j.stem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Doan PL, et al. Epidermal growth factor regulates hematopoietic regeneration after radiation injury. Nature medicine. 2013;19:295–304. doi: 10.1038/nm.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Morikawa S, et al. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. The Journal of experimental medicine. 2009;206:2483–2496. doi: 10.1084/jem.20091046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pinho S, et al. PDGFRalpha and CD51 mark human nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. The Journal of experimental medicine. 2013;210:1351–1367. doi: 10.1084/jem.20122252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tran E, et al. Immune targeting of fibroblast activation protein triggers recognition of multipotent bone marrow stromal cells and cachexia. The Journal of experimental medicine. 2013;210:1125–1135. doi: 10.1084/jem.20130110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the Hematopoietic Stem Cell Pool by CXCL12-CXCR4 Chemokine Signaling in Bone Marrow Stromal Cell Niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 131.Omatsu Y, et al. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity. 2010;33:387–399. doi: 10.1016/j.immuni.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 132.Roberts EW, et al. Depletion of stromal cells expressing fibroblast activation protein-alpha from skeletal muscle and bone marrow results in cachexia and anemia. The Journal of experimental medicine. 2013;210:1137–1151. doi: 10.1084/jem.20122344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Omatsu Y, Seike M, Sugiyama T, Kume T, Nagasawa T. Foxc1 is a critical regulator of haematopoietic stem/progenitor cell niche formation. Nature. 2014;508:536–540. doi: 10.1038/nature13071. [DOI] [PubMed] [Google Scholar]
- 134.Naveiras O, et al. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460:259–263. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yamazaki S, et al. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell. 2011;147:1146–1158. doi: 10.1016/j.cell.2011.09.053. [DOI] [PubMed] [Google Scholar]
- 136.Bruns I, et al. Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nature medicine. 2014;20:1315–1320. doi: 10.1038/nm.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhao M, et al. Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nature medicine. 2014;20:1321–1326. doi: 10.1038/nm.3706. [DOI] [PubMed] [Google Scholar]
- 138.Winkler IG, et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 2010;116:4815–4828. doi: 10.1182/blood-2009-11-253534. [DOI] [PubMed] [Google Scholar]
- 139.Chow A, et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. The Journal of experimental medicine. 2011;208:261–271. doi: 10.1084/jem.20101688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hur J, et al. CD82/KAI1 Maintains the Dormancy of Long-Term Hematopoietic Stem Cells through Interaction with DARC-Expressing Macrophages. Cell stem cell. 2016;18:508–521. doi: 10.1016/j.stem.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 141.Fujisaki J, et al. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature. 2011;474:216–219. doi: 10.1038/nature10160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Parmar K, Mauch P, Vergilio J-A, Sackstein R, Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. PNAS. 2007;104:5431–5436. doi: 10.1073/pnas.0701152104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jang Y-Y, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007;110:3056–3063. doi: 10.1182/blood-2007-05-087759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Simsek T, et al. The Distinct Metabolic Profile of Hematopoietic Stem Cells Reflects Their Location in a Hypoxic Niche. Cell stem cell. 2010;7:380–390. doi: 10.1016/j.stem.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Takubo K, et al. Regulation of the HIF-1α Level Is Essential for Hematopoietic Stem Cells. Cell stem cell. 2010;7:391–402. doi: 10.1016/j.stem.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 146.Miharada K, et al. Cripto Regulates Hematopoietic Stem Cells as a Hypoxic-Niche-Related Factor through Cell Surface Receptor GRP78. Cell stem cell. 2011;9:330–344. doi: 10.1016/j.stem.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 147.Casanova-Acebes M, et al. Rhythmic Modulation of the Hematopoietic Niche through Neutrophil Clearance. Cell. 2013;153:1025–1035. doi: 10.1016/j.cell.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mantel CR, et al. Enhancing Hematopoietic Stem Cell Transplantation Efficacy by Mitigating Oxygen Shock. Cell. 2015;161:1553–1565. doi: 10.1016/j.cell.2015.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Spencer JA, et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. 2014;508:269–273. doi: 10.1038/nature13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Vukovic M, et al. Adult haematopoietic stem cells lacking Hif-1α self-renew normally. Blood. 2016 doi: 10.1182/blood-2015-10-677138. blood-2015-2010-677138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kunisaki Y, et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502:637–643. doi: 10.1038/nature12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang L, et al. Identification of a clonally expanding haematopoietic compartment in bone marrow. The EMBO journal. 2013;32:219–230. doi: 10.1038/emboj.2012.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lucas D, Battista M, Shi PA, Isola L, Frenette PS. Mobilized Hematopoietic Stem Cell Yield Depends on Species-Specific Circadian Timing. Cell stem cell. 2008;3:364–366. doi: 10.1016/j.stem.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 155.Spiegel A, et al. Catecholaminergic neurotransmitters regulate migration and repopulation of immature human CD34+ cells through Wnt signaling. Nature Immunology. 2007;8:1123–1131. doi: 10.1038/ni1509. [DOI] [PubMed] [Google Scholar]
- 156.Katayama Y, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 157.Nakada D, et al. Oestrogen increases haematopoietic stem-cell self-renewal in females and during pregnancy. Nature. 2014;505:555–558. doi: 10.1038/nature12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Grover A, et al. Erythropoietin guides multipotent hematopoietic progenitor cells toward an erythroid fate. The Journal of experimental medicine. 2014;211:181–188. doi: 10.1084/jem.20131189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Signer RA, Morrison SJ. Mechanisms that regulate stem cell aging and life span. Cell stem cell. 2013;12:152–165. doi: 10.1016/j.stem.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Geiger H, de Haan G, Florian MC. The ageing haematopoietic stem cell compartment. Nature Reviews Immunology. 2013;13:376–389. doi: 10.1038/nri3433. [DOI] [PubMed] [Google Scholar]
- 161.Liang Y, Van Zant G, Szilvassy SJ. Effects of aging on the homing and engraftment of murine hematopoietic stem and progenitor cells. Blood. 2005;106:1479–1487. doi: 10.1182/blood-2004-11-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ergen AV, Boles NC, Goodell MA. Rantes/Ccl5 influences hematopoietic stem cell subtypes and causes myeloid skewing. Blood. 2012;119:2500–2509. doi: 10.1182/blood-2011-11-391730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Conboy IM, et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 164.Villeda SA, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Burkhardt R, et al. Changes in trabecular bone, hematopoiesis and bone marrow vessels in aplastic anemia, primary osteoporosis, and old age: A comparative histomorphometric study. Bone. 1987;8:157–164. doi: 10.1016/8756-3282(87)90015-9. [DOI] [PubMed] [Google Scholar]
- 166.Justesen J, et al. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2:165–171. doi: 10.1023/a:1011513223894. [DOI] [PubMed] [Google Scholar]
- 167.Kolf CM, Cho E, Tuan RS. Mesenchymal stromal cells: Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Research & Therapy. 2007;9:204. doi: 10.1186/ar2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhou S, et al. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell. 2008;7:335–343. doi: 10.1111/j.1474-9726.2008.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Stenderup K, Justesen J, Clausen C, Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33:919–926. doi: 10.1016/j.bone.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 170.Haan Gd, Zant GV. Dynamic Changes in Mouse Hematopoietic Stem Cell Numbers During Aging. Blood. 1999;93:3294–3301. [PubMed] [Google Scholar]
- 171.Calvi LM, Link DC. The hematopoietic stem cell niche in homeostasis and disease. Blood. 2015;126:2443–2451. doi: 10.1182/blood-2015-07-533588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Schepers K, Campbell TB, Passegue E. Normal and Leukemic Stem Cell Niches: Insights and Therapeutic Opportunities. Cell stem cell. 2015;16:254–267. doi: 10.1016/j.stem.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sanchez-Aguilera A, Mendez-Ferrer S. The hematopoietic stem-cell niche in health and leukemia. Cellular and molecular life sciences : CMLS. 2016 doi: 10.1007/s00018-016-2306-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pitt LA, et al. CXCL12-Producing Vascular Endothelial Niches Control Acute T Cell Leukemia Maintenance. Cancer cell. 2015;27:755–768. doi: 10.1016/j.ccell.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hawkins ED, et al. T-cell acute leukaemia exhibits dynamic interactions with bone marrow microenvironments. Nature. 2016 doi: 10.1038/nature19801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rupec RA, et al. Stroma-Mediated Dysregulation of Myelopoiesis in Mice Lacking IκBα. Immunity. 2005;22:479–491. doi: 10.1016/j.immuni.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 177.Kim Y-W, et al. Defective Notch activation in microenvironment leads to myeloproliferative disease. Blood. 2008;112:4628–4638. doi: 10.1182/blood-2008-03-148999. [DOI] [PubMed] [Google Scholar]
- 178.Walkley CR, et al. A Microenvironment-Induced Myeloproliferative Syndrome Caused by Retinoic Acid Receptor γ Deficiency. Cell. 2007;129:1097–1110. doi: 10.1016/j.cell.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Walkley CR, Shea JM, Sims NA, Purton LE, Orkin SH. Rb Regulates Interactions between Hematopoietic Stem Cells and Their Bone Marrow Microenvironment. Cell. 2007;129:1081–1095. doi: 10.1016/j.cell.2007.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Raaijmakers MHGP, et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 2010;464:852–857. doi: 10.1038/nature08851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kode A, et al. Leukaemogenesis induced by an activating β-catenin mutation in osteoblasts. Nature. 2014;506:240–244. doi: 10.1038/nature12883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Colmone A, et al. Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science. 2008;322:1861–1865. doi: 10.1126/science.1164390. [DOI] [PubMed] [Google Scholar]
- 183.Zhang B, et al. Altered Microenvironmental Regulation of Leukemic and Normal Stem Cells in Chronic Myelogenous Leukemia. Cancer Cell. 2012;21:577–592. doi: 10.1016/j.ccr.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Schepers K, et al. Myeloproliferative Neoplasia Remodels the Endosteal Bone Marrow Niche into a Self-Reinforcing Leukemic Niche. Cell stem cell. 2013;13:285–299. doi: 10.1016/j.stem.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Arranz L, et al. Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature. 2014;512:78–81. doi: 10.1038/nature13383. [DOI] [PubMed] [Google Scholar]
- 186.Medyouf H, et al. Myelodysplastic Cells in Patients Reprogram Mesenchymal Stromal Cells to Establish a Transplantable Stem Cell Niche Disease Unit. Cell stem cell. 2014;14:824–837. doi: 10.1016/j.stem.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 187.Hanoun M, et al. Acute myelogenous leukemia-induced sympathetic neuropathy promotes malignancy in an altered hematopoietic stem cell niche. Cell stem cell. 2014;15:365–375. doi: 10.1016/j.stem.2014.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Krause DS, et al. Differential regulation of myeloid leukemias by the bone marrow microenvironment. Nature medicine. 2013;19:1513–1517. doi: 10.1038/nm.3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rashidi A, DiPersio JF. Targeting the leukemia–stroma interaction in acute myeloid leukemia: rationale and latest evidence. Ther Adv Hematol. 2016;7:40–51. doi: 10.1177/2040620715619307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Riether C, Schürch CM, Ochsenbein AF. Regulation of hematopoietic and leukemic stem cells by the immune system. Cell Death Differ. 2015;22:187–198. doi: 10.1038/cdd.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Essers MA, et al. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458:904–908. doi: 10.1038/nature07815. [DOI] [PubMed] [Google Scholar]
- 192.Burberry A, et al. Infection Mobilizes Hematopoietic Stem Cells through Cooperative NOD-like Receptor and Toll-like Receptor Signaling. Cell Host & Microbe. 2014;15:779–791. doi: 10.1016/j.chom.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Baldridge MT, King KY, Boles NC, Weksberg DC, Goodell MA. Quiescent haematopoietic stem cells are activated by IFN-γ in response to chronic infection. Nature. 2010;465:793–797. doi: 10.1038/nature09135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Schürch Christian M, Riether C, Ochsenbein Adrian F. Cytotoxic CD8+ T Cells Stimulate Hematopoietic Progenitors by Promoting Cytokine Release from Bone Marrow Mesenchymal Stromal Cells. Cell stem cell. 2014;14:460–472. doi: 10.1016/j.stem.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 195.Chute JP, et al. Transplantation of vascular endothelial cells mediates the hematopoietic recovery and survival of lethally irradiated mice. Blood. 2007;109:2365–2372. doi: 10.1182/blood-2006-05-022640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Brenet F, Kermani P, Spektor R, Rafii S, Scandura JM. TGFbeta restores hematopoietic homeostasis after myelosuppressive chemotherapy. The Journal of experimental medicine. 2013;210:623–639. doi: 10.1084/jem.20121610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Crisan M, Dzierzak E. The many faces of hematopoietic stem cell heterogeneity. Development. 2016;143:4571–4581. doi: 10.1242/dev.114231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Copley MR, Beer PA, Eaves CJ. Hematopoietic stem cell heterogeneity takes center stage. Cell stem cell. 2012;10:690–697. doi: 10.1016/j.stem.2012.05.006. [DOI] [PubMed] [Google Scholar]