Abstract
Maternal stress can have long-term negative consequences for offspring learning performance. However, it is unknown whether these maternal effects extend to the ability of offspring to apply previously learned information to new situations. In this study, we first demonstrate that juvenile threespine sticklebacks, Gasterosteus aculeatus, are indeed capable of generalizing an association between a colour and a food reward learned in one foraging context to a new foraging context (i.e. they can apply previously learned knowledge to a new situation). Next, we examined whether this ability to generalize was affected by maternal predator stress. We manipulated whether mothers were repeatedly chased by a model predator while yolking eggs (i.e. before spawning) and then assessed the learning performance of their juvenile offspring in groups and pairs using a colour discrimination task that associated a colour with a food reward. We found that maternal predator exposure affected the tendency of offspring to use social cues: offspring of predator-exposed mothers were faster at copying a leader’s behaviour towards the rewarded colour than offspring of unexposed mothers. However, once the colour–reward association had been learned, offspring of predator-exposed and unexposed mothers were equally able to generalize their learned association to a new foraging task. These results suggest that offspring of predator-exposed mothers might be able to overcome learning deficits caused by maternal stress by relying more on social cues.
Keywords: colour discrimination task, Gasterosteus aculeatus, generalization, maternal effects, maternal stress, predation risk, social learning, stimulus enhancement, threespine stickleback, transgenerational plasticity
Maternal effects can occur when a mother’s experiences and her reaction to these experiences influence her offspring (Bernardo, 1996; Mousseau & Fox, 1998). For example, in a diversity of taxa, mothers exposed to predation risk produce offspring with altered phenotypes compared to offspring of unexposed mothers (e.g. birds: Coslovsky & Richner, 2011; daphnia: Agrawal, Laforsch, & Tollrian, 1999; fish: McGhee, Pintor, Suhr, & Bell, 2012; mammals: Sheriff, Krebs, & Boonstra, 2009; insects: Storm & Lima, 2010; reptiles: Bestion, Teyssier, Aubret, Clobert, & Cote, 2014). There is growing appreciation of the ecological and evolutionary significance of such nongenetic transgenerational effects (reviewed in: Badyaev & Uller, 2009; Monaghan, 2008; Sheriff & Love, 2013).
Maternal stress can influence a variety of offspring behaviours, including those associated with behavioural plasticity and learning performance (reviewed in: Maccari, Krugers, Morley-Fletcher, Szyf, & Brunton, 2014; Schoech, Rensel, & Heiss, 2011; Weinstock, 2008). For example, in threespine stickleback, Gasterosteus aculeatus, adult offspring of predator-exposed mothers were slower at learning a colour discrimination task compared to offspring of unexposed mothers (Roche, McGhee, & Bell, 2012). Similar detrimental effects of maternal stress on offspring learning have also been documented in mammals and birds (reviewed in: Maccari et al., 2014; Schoech et al., 2011; Weinstock, 2008). However, it is unknown whether the consequences of maternal stress on offspring learning extend to performance in other contexts, such as the ability of offspring to apply previously learned knowledge to new situations.
If a learned association can be generalized across contexts, individuals can potentially reap even greater benefits from learning by behaving appropriately in a novel context without having to discover additional information. For example, learning about one predator can improve antipredator behaviour and survival when exposed to a different predator (Brown et al., 2011; Ferrari, Brown, Messier, & Chivers, 2009; Ferrari, Gonzalo, Messier, & Chivers, 2007; Griffin, Evans, & Blumstein, 2001; Mitchell, McCormick, Chivers, & Ferrari, 2013). Similarly, learning about particular prey items can improve foraging performance (or avoidance) when exposed to novel items (Ihalainen, Rowland, Speed, Ruxton, & Mappes, 2012; Marples, Quinlan, Thomas, & Kelly, 2007; Svádová et al., 2009). Thus, the ability to generalize learned associations could be advantageous, particularly in a seasonal and changing environment. If maternal stress affects the overall learning ability of offspring, including their ability to generalize learned associations, this could have important consequences in many contexts.
In this study, we explored how maternal stress affects offspring learning performance and their ability to generalize a learned association in threespine stickleback. Before we could explore the consequences of maternal stress on offspring learning however, we had to determine whether threespine sticklebacks are in fact capable of generalizing information they have learned in one context to a novel context. Thus, our study consisted of two separate experiments. In the first experiment, we determined whether learning a colour–reward association in a group under one set of conditions improved learning performance under a different set of conditions. In the second experiment, we examined whether a mother’s experience with predators affected their offspring’s ability to generalize a group-learned colour–reward association to a new foraging context. If maternal stress has negative consequences for overall offspring learning performance across a variety of tasks, then we would predict that offspring of predator-exposed mothers would be less able to generalize a colour–reward association compared to offspring of unexposed mothers. In both parts of the study, we interpreted a preference for the rewarded stimulus over the unrewarded stimulus as evidence for a learned colour–reward association.
EXPERIMENT 1
Can Sticklebacks Generalize a Learned Colour–Reward Association?
Methods
Juvenile collection and housing
Sixty threespine stickleback juveniles (average standard length ± SE = 20.5 ± 2.0 mm) were collected from the Navarro River, CA, U.S.A. in summer 2013. Piscivorous predators such as sculpin are present in this population and are primarily a threat to eggs and juvenile stickleback, although small adults are also vulnerable (Maccoll & Chapman, 2011; Pressley, 1981). Juveniles were transported by air to the University of Illinois and housed in groups of six in 26.5-litre tanks (N = 10 tanks, 36 × 33 × 24 cm, length × width × height) with gravel on the bottom of the tank and two plastic plants on opposite sides of the tank. These tanks are referred to as ‘initial group tanks’. The fronts of the tanks were covered with opaque plastic to minimize disturbance due to human movements in the fish room. When fish were not being trained or tested, the sides of the tanks were left uncovered and fish could see neighbours. Fish were maintained at 20.6 °C on a summer photoperiod schedule (16:8 h light:dark cycle) and water was cleaned in all tanks via a recirculating flow-through system with particulate, biological and UV filters (Aquaneering, San Diego, CA, U.S.A.). Using a clear pipette, we fed juveniles a slurry of frozen adult Artemia, mysis shrimp, bloodworms and cyclopeez once a day. The juveniles were acclimated in the laboratory for 2.5 weeks prior to any training.
Training trials
The juveniles were randomly assigned to either a colour–reward association training or a no-training treatment. The training treatment consisted of colour–reward association training in groups and subsequently in pairs, and the no-training treatment consisted of neither group nor pair training (N = 10 groups with six individuals per group; N = 30 pairs). We elected to train and test sticklebacks with other individuals rather than by themselves because pilot studies showed that juvenile sticklebacks from this population were unlikely to explore the tank when alone. Note that both treatments were handled in the same way, with the only difference being the presence or absence of coloured cups during the group and pair training periods.
Groups in the training treatment were trained to associate a coloured cup with a food reward for 7 days. We trained fish twice a day at random times to prevent individuals from associating feeding with particular times of day. Opaque screens were placed in between tanks during feeding. During a training trial, two Solo® brand coloured cups (one blue and one yellow) were submerged 1 cm into the surface of the water at the front on opposite sides of the tank (Fig. 1a). The bottom of both cups had a small opening for the release of food or water from a pipette within. The blue cup was always rewarded and the pipette within it contained chopped bloodworms, whereas the yellow cup was never rewarded and the pipette within it contained tank water. After an individual in the group oriented to the blue cup, a food reward was pipetted into the water. The individual then moved towards the blue cup to obtain the food. In contrast, when an individual in the group oriented to the yellow cup, tank water was pipetted into the water. The trial ended when three different individuals were rewarded following orientation to the rewarded cup. During 7 days of group training, we recorded how quickly the first (the leader), second and third individuals oriented to the rewarded blue cup. The total time it took three fish to orient to the rewarded cup decreased as the training progressed (day 1: 48.2 ± 6.2 s; day 7: 29.6 ± 2.1 s). Because of the challenges of recording data on several individuals within a tank simultaneously, we did not record how quickly fish oriented to the unrewarded yellow cup or how quickly they approached either cup. However, individuals usually quickly approached the blue cup after orienting, since food was released after an orient to blue. The coloured cups were removed at the end of each training trial. The side of the tank that initially contained the blue cup was determined by coin toss and then alternated between trials. Blue and yellow colours were chosen based on previous studies of learning in threespine sticklebacks showing no evidence for an inherent bias for either colour (Girvan & Braithwaite, 1998; Roche et al., 2012). Groups in the no-training treatment were not exposed to the coloured cups and did not receive colour–reward association training: they were not fed from the coloured cups and continued to be fed from the clear pipette twice a day for 7 days. Fish were not fed outside of trials.
Figure 1.
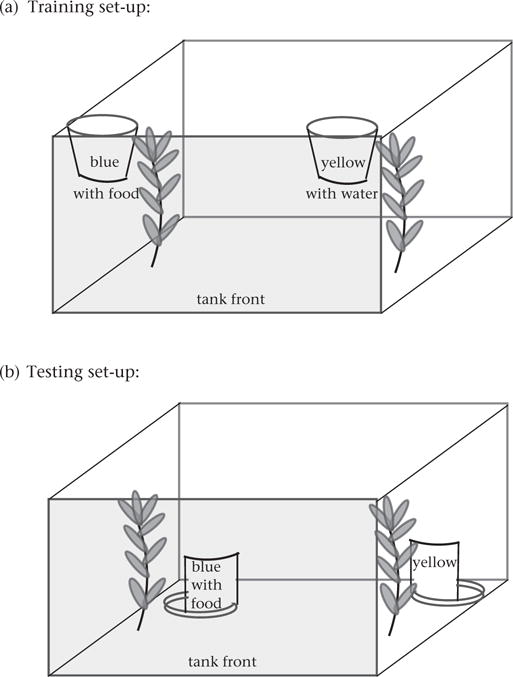
Experimental set-up during (a) training and (b) testing of threespine stickleback in a novel context. Note that the side with the rewarded blue cup was randomly determined. The sides and back of the tanks were covered with opaque plastic during the training and testing assays.
Reminder trials
After 7 days of group training (or no training, in the case of the no-training treatment), individuals were paired randomly within their initial group tank and transferred to new 26.5-litre tanks with gravel and plastic plants. Fish were observed in the new tanks in pairs. On day 8, individuals in the training treatment completed two additional training trials, which served as a ‘reminder’ that the blue cup continued to be rewarded despite the new tank and social environment (pair instead of group). Opaque screens were placed in between tanks during feeding. Before each trial, an opaque holding cylinder was lowered over both individuals and moved to the centre of the tank. As in the group trials, cups were positioned at the surface of the water at the front of the tank on opposite sides and the blue cup contained a pipette with chopped bloodworms while the yellow cup contained a pipette with water (Fig. 1a). The trial began when the opaque holding cylinder was removed. These ‘reminder’ trials lasted 10 min with food (or water) being released from the rewarded blue (or unrewarded yellow) cup whenever a fish oriented to the cup. The side of the tank that contained the blue or yellow cup was switched between trials. As in the group trials, pairs in the no-training treatment were not exposed to the coloured cups during these ‘reminder’ trials and were fed from a clear pipette. Fish were not fed outside of trials. Data from these two ‘reminder’ trials were not used in any analysis.
Test trial
After 8 days of training or no training (7 days in a group and 1 day in a pair), individuals were tested in their ‘pair’ tank on day 9. During the test trial, sticklebacks were again tested with the yellow and blue cups, but the cups were configured differently compared to the training trials so that sticklebacks had to change their strategy to receive food. The goal was to assess whether sticklebacks could apply what they had learned during training (that blue is rewarded) to a new situation where both location and access to food were novel. The coloured cups were positioned in the opposite corners at the bottom of the back of the tank (Fig. 1b) and cut to make ‘chambers’: the back half of the cup was open to allow individuals to enter into the ‘chamber’ and the front of the cup was left solid (i.e. the wall of the chamber). In the base of each cup was a glass petri dish. The petri dish in the rewarded blue chamber contained bloodworms stuck in petroleum jelly whereas the petri dish in the unrewarded yellow chamber had only petroleum jelly. Opaque screens were placed in between tanks during testing. Before each trial, an opaque holding cylinder was lowered over both individuals and moved to the centre of the tank. The testing trial began when the opaque holding cylinder was removed and lasted for 10 min.
Because of nonindependence of individuals while they were tested in pairs, we focused only on a single fish (the leader) within each assay. We recorded the latency of the first fish (leader) to begin moving, to first orient to each cup (yellow and blue) and to first approach within 5 cm of each cup (yellow and blue). Individuals who did not orient to or approach a cup within 10 min received a latency of 600 s for that cup colour. Thus, each leader had orient and approach latencies for both cup colours. Fish were not fed outside trials, so the only available food was the food reward.
Previous work has found that threespine sticklebacks are not able to find food based on smell cues alone and that they show no inherent bias for the colours blue or yellow (Girvan & Braithwaite, 1998), but our analysis of the no-training treatment can examine this directly. Specifically, if fish can find food based on chemical cues alone, then we would expect fish in our no-training treatment to show a preference for orienting to and/or approaching the rewarded cup during testing even though they had not encountered the coloured cups before. Similarly, if sticklebacks show a strong colour bias, then we would expect them to systematically prefer one colour over the other prior to training. Note, however, that food was always present in our assays; thus, we could not assess whether there is an inherent colour bias in the absence of food cues. After the test trial, we measured standard length for each individual and returned the fish to their initial group tank. We tested a total of 30 pairs, with 15 pairs from each of the training/no-training treatments.
Data analysis
To examine whether sticklebacks can apply a colour–reward association learned under one set of conditions to a different set of conditions, we compared the behaviour of fish from the training versus no-training treatments in how quickly leaders oriented to and approached either the rewarded or unrewarded cup during testing using linear mixed models. Cup colour (rewarded blue or unrewarded yellow) was included as a fixed factor that was repeated within a subject. Based on estimates of model fit (AIC), we specified unstructured covariance structure for the repeated measures of the pair within the tank. If sticklebacks can generalize a learned colour–reward association across contexts, we would expect a significant treatment*cup colour interaction where trained individuals (with a colour–reward association) would be more likely to orient and approach blue over yellow compared to untrained individuals. To ensure that any differences between treatments were not driven by differences in body size or inherent exploration tendency, we also compared latency to initially move and standard length between treatments.
Analyses were conducted with SAS™, v.9.3. All data were ln transformed after adding 1 to each value to account for zero values and model assumptions were validated by examining residuals. We specified REML estimation and estimated the degrees of freedom with the Satterthwaite method. The identity of the initial group tank (from which the pairs were taken) was included as a random factor and its significance was assessed with log likelihood tests. Unless otherwise stated, initial group tank did not significantly affect any behaviours. To get insight into the biological relevance of our comparisons, we also calculated Cohen’s d estimates for different comparisons with d = 0.8, d = 0.5 and d = 0.2 interpreted as large, medium and small effect sizes, respectively (Cohen, 1988; LeCroy & Krysik, 2007). Means ± SE are given throughout.
Results
Threespine sticklebacks could indeed generalize the colour–reward association they had learned under one set of conditions and use it in a different context: training in the colour discrimination task improved performance during testing on a modified colour discrimination task. In the test trial with modified cups of the same colours as during training, but in a new location, trained juveniles were significantly faster at both orienting to and approaching the rewarded blue colour instead of the unrewarded yellow colour compared to those without training (Table 1, Fig. 2; orienting to blue: training = 184.1 ± 51.0 s, no training = 297.0 ± 55.8 s; approaching blue: training = 311.0 ± 66.5 s, no training = 429.9 ± 47.1 s). In addition, trained juveniles were not simply more likely to orient and approach cups in general, as they would if they had associated the presence of ‘cups’ of any colour with the food reward instead of the blue cup specifically. Trained and untrained juveniles did not differ in how quickly they first oriented and approached a cup (whether yellow or blue) (orienting to any cup: training = 207.6 ± 47.9 s, no training = 240.0 ± 46.5 s; 1,28 = 0.24, P = 0.6309; approaching any cup: training = 280.3 ± 64.0 s, no training = 381.6 ± 45.8 s; F1,28 = 1.65, P = 0.2089). As expected, untrained juveniles that had not experienced the coloured cups previously and had not learned a colour–reward association did not have a colour preference (Table 1, Fig. 2). This result is consistent with previous work (Girvan & Braithwaite, 1998; Roche et al., 2012) suggesting that sticklebacks do not use the scent of bloodworms to find the reward, and also suggests that they do not have a strong bias for blue or yellow. Note that since food was always present at testing (i.e. we did not test for bias in the absence of food), we cannot rule out the possibility that behaviour might have been affected by an interaction between any colour bias and food cues. Not all leaders approached a coloured cup within a testing trial (9 of 30 leaders did not approach), but results were similar even when analyses were restricted only to those trials where leaders approached a cup (N = 21 leaders approached, with 10 from training treatment and 11 from no-training treatment; training treatment: F1,19 = 10.69, P = 0.0040; cup colour: F1,19 = 17.39, P = 0.0005; training*cup= colour: F1,19 = 7.01, P = 0.0159). Treatments did not differ significantly in the time at which the leader first started exploring the tank (training = 7.1 ± 1.3 s, no training = 6.5 ± 2.0 s, N = 30; F1,8 = 0.37, P = 0.5586), nor in fish body length (training = 18.7 ± 0.2 mm, no training = 19.4 ± 0.3 mm, N = 60; F1,8 = 1.92, P= 0.2033).
Table 1.
Ability of wild-caught threespine sticklebacks to generalize a learned colour–reward association across contexts
| Effect | df | F | P | Cohen’s d (on blue cup) |
|---|---|---|---|---|
| Latency for leader to first orient to a cup | ||||
| Previous training (yes, no) | 1, 8.2 | 0.10 | 0.7590 | 0.54 |
| Cup colour (rewarded blue, unrewarded yellow) | 1, 28 | 6.10 | 0.0199 | |
| Training*cup colour | 1, 28 | 6.92 | 0.0137 | |
| Latency for leader to approach a cup | ||||
| Previous training (yes, no) | 1, 27 | 3.51 | 0.0715 | 0.53 |
| Cup colour (rewarded blue, unrewarded yellow) | 1, 28 | 12.02 | 0.0017 | |
| Training*cup colour | 1, 28 | 4.41 | 0.0449 | |
Leaders that had received training for 8 days under one set of conditions were faster at orienting to the rewarded cup and approaching the rewarded cup compared to leaders that had not received previous training. Estimates of Cohen’s d refer to comparisons between the training treatment means towards the blue rewarded cup (N = 30).
Figure 2.
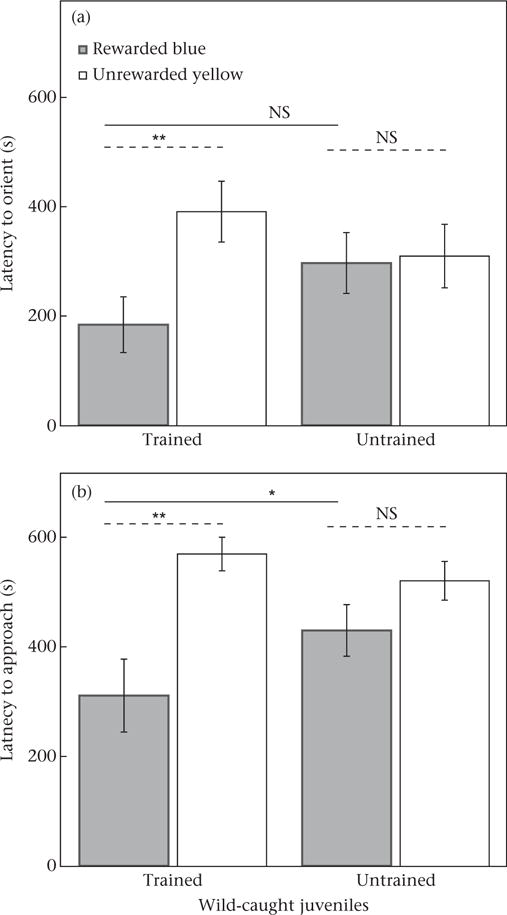
Effect of 8 days of training (7 days in a group, 1 day in a pair) under one set of conditions on subsequent testing performance under a different set of conditions. Shown are means ± SE for latency of wild-caught fish leaders to (a) first orient to and (b) first approach the rewarded blue cup and the unrewarded yellow cup (N = 30). Lines above bars indicate results of comparisons between least square means: solid lines indicate comparisons of the rewarded blue cup between training treatments, dashed lines indicate comparisons between blue and yellow cups within a training treatment (*P < 0.05; **P < 0.01).
EXPERIMENT 2
Does Maternal Predator Exposure Affect Offspring Generalization of a Colour–Reward Association?
Methods
Maternal predator exposure and offspring rearing
Fish were collected from the Navarro River, CA, in autumn 2011 (as juveniles) and spring 2012 (as adults) and transported by air to the University of Illinois. In summer 2012, females were randomly assigned to either predator-exposed or unexposed treatment tanks (37.85-litre: 53 × 33 × 24 cm). There were eight predator-exposed and eight unexposed tanks with 10 females per tank. All females were initially spine-clipped to allow individual identification within their respective treatment tank. Predator-exposed females were chased for 45 s once a day with a rubber sculpin model (10 cm length; Jewel Bait Company, Bakersfield, MO, U.S.A.) attached to a stiff wire rod at a random time per day to prevent habituation. Unexposed females were left undisturbed. All females experienced their treatments for at least 1 week, but total treatment time was variable depending on when females became gravid and males had completed nests (range 7–107 days). This is a similar protocol to what we have employed in the past (Giesing, Suski, Warner, & Bell, 2011; McGhee et al., 2012), although in this study we used a model sculpin instead of a model northern pike, Esox lucius. A pilot study suggested that exposure to the model sculpin is an effective stressor and elevates plasma cortisol levels in adults (unexposed adults: 15.5 ± 3.3 ng/ml, N = 17; adults 30 min after exposure to the sculpin model: 28.3 ± 6.0 ng/ml, N = 24; McGhee & Bell, 2012). As females became gravid, we randomly paired them with a male from the same population that had constructed a nest and we left them overnight with the male. After a successful spawn, we removed fertilized eggs from a male’s nest and reared them in small mesh cups suspended in 9.5-litre tanks (34 × 18 × 23 cm) with a couple drops of methylene blue and an airstone to prevent fungus. Females were returned to their original treatment tanks and thus were potentially reused.
After hatching, siblings were reared together in these tanks until large enough to safely handle (~3 months). Unrelated juveniles from the same maternal treatment were then combined into groups of four and transferred to new tanks. Both offspring of predator-exposed and unexposed mothers had a single encounter with a live sculpin predator for a different experiment (presented elsewhere, McGhee & Bell, n.d.). In these predator–prey assays, the four sticklebacks were in the sculpin tank for a maximum of 1 h and, in most cases, the sculpin caught none or only one of the four juveniles. The surviving juveniles were then combined according to maternal treatment and redistributed into 10 group tanks of six individuals (5 groups per maternal treatment) in 26.5-litre tanks with gravel on the bottom of the tank and two plastic plants on opposite sides of the tank. Approximately 35 days passed between the sculpin assay and the learning assays. A total of 60 juvenile offspring (standard length = 19.0 ± 1.5 mm) of approximately 6 months old were used in the study presented here. In total, 19 different predator-exposed mothers from six different female-housing tanks and 24 unexposed mothers from eight different female-housing tanks contributed offspring to these groups, and juveniles within a group tank were not full siblings.
Training trials
Offspring of both predator-exposed and unexposed mothers were trained in March 2013 to associate the blue coloured cup with a food reward. This colour–reward association training was identical to that described for the training trial in experiment 1 above (Fig. 1a) and consisted of both group (7 days; N = 10 groups, with six to seven individuals per group) and subsequent pair (1 day; N = 30 pairs) training. Over 7 days of group training, the total time it took three fish to orient to the rewarded cup (i.e. trial duration) decreased as the training progressed (day 1: 77.1 ± 13.9 s; day 7: 34.2 ± 3.7 s). During these group-training trials, we recorded how quickly the first individual (the leader) oriented to the rewarded blue cup, as well as how long it took the other fish in the same group to copy the leader’s behaviour and orient to the blue cup. The difference between the latency of the first and second fish and of the first and third fish to orient to the rewarded cup was measured as their copying time (see Day, MacDonald, Brown, Laland, & Reader, 2001). A total of 10 groups were group-trained with five groups from each maternal treatment.
Reminder trials
After 7 days of group training, individuals were paired randomly within their group tank and transferred to new pair tanks. These additional pair-training trials were identical to that described for the reminder trials in experiment 1 for the ‘training’ treatment (Fig. 1a). Again, no data were analysed from these trials.
Testing trial
After 8 days of training (7 days in a group and 1 day in a pair) for both maternal treatments, offspring of predator-exposed and unexposed mothers were tested in their pair tank on day 9. Coloured cups were modified to create chambers and presented in a novel location at the bottom of the tank and on opposite sides at the back. The testing trial was identical to that described for the testing trial in experiment 1 (Fig. 1b). One pair of offspring from the unexposed maternal treatment was excluded because it took 150 s for either fish to start moving in the tank, more than twice as long as any other pair. We tested a total of 29 pairs, with 14 pairs of offspring from unexposed mothers and 15 pairs of offspring from predator-exposed mothers.
Data analysis
Group training and use of social cues
To examine whether maternal predator exposure affected the ability of offspring to initially learn a colour–reward association, we compared maternal treatments in how quickly leaders oriented to the rewarded cup during their 7 days of group training using repeated measures mixed models with group tank as the subject repeated through time. Because we were interested in how training affected performance, we compared maternal treatments ‘before training’ (day 1) and ‘after training’ (day 7) with this aspect of time (day 1 versus day 7) as a repeated measure. Although fish were trained in groups for 7 days, we restricted our analyses to only the first and last day of training for ease at interpreting any treatment*training (i.e. time) interactions. Based on estimates of model fit (AIC), we specified unstructured covariance structure for the repeated measures of group tank.
Since individuals were trained in groups, it is possible that individuals might use social cues to find food. To examine whether maternal treatment affected the use of social cues during group training, we compared how quickly the second and third fish copied the leader and oriented towards the rewarded cup (i.e. copying time) in a similar repeated measures analysis. Note that since individuals within a group were not individually marked, the identity of leaders and followers could change between days.
Generalizing a learned colour–reward association
To examine whether maternal predator exposure affected the ability of offspring to generalize a learned colour–reward association across contexts, we compared offspring of unexposed and predator-exposed mothers in how quickly leaders oriented to and approached either the blue rewarded or yellow unrewarded cup during testing in the novel context using repeated measures mixed models. Cup colour (rewarded blue or unrewarded yellow) was included as a fixed factor that was repeated within a subject. Based on estimates of model fit (AIC), we specified unstructured covariance structure for the repeated measures of the pair within the tank. This is the same type of analysis as that used in experiment 1. If maternal predator exposure affects the ability of stickleback offspring to generalize a learned colour–reward association across contexts, we would expect a significant maternal treatment*cup colour interaction. If maternal treatment only affects how quickly offspring orient to and/or approach any cup, regardless of colour (i.e. they cannot generalize), we would expect a significant effect of maternal treatment only. If all fish, regardless of maternal treatment, learn the colour–reward association and generalize it to the novel foraging context, we would expect a significant effect of cup colour. To ensure that any differences between treatments were not driven by differences in body size or inherent exploration tendency, we also compared latency to initially move and standard length between maternal treatments. Details of all analyses (e.g. transformations, degrees of freedom, Cohen’s d, etc.) are identical to those used in experiment 1 (see Data analysis above).
Results
Group training and use of social cues
How quickly the leader first oriented to the blue rewarded cup during group training was not significantly affected by training or maternal predator exposure (Table 2, Fig. 3a). Judging from the medium to large effect size (= 0.70), prior to training, offspring of predator-exposed mothers tended to orient more quickly to the rewarded cup compared to offspring of unexposed mothers, but this was not statistically significant. However, the attentiveness of other individuals to the behaviour of the leader and how quickly they copied the leader’s orienting behaviour to the rewarded cup was affected by both training and maternal predator exposure (Table 2, Fig. 3b, c). During the training trials, other fish in the tank became faster at copying the leader and attending to the social information about orienting to the rewarded cup. In addition, offspring of predator-exposed mothers were faster at copying the leader than offspring of unexposed mothers even before any training.
Table 2.
Effect of maternal predator exposure and group training (i.e. day 1 vs day 7 of training) on the latency of leaders to orient to the rewarded cup and the speed at which the second and third fish copied the leader’s orienting behaviour
| Effect | df | F | P | Cohen’s d
|
|
|---|---|---|---|---|---|
| Day 1 | Day 7 | ||||
| Latency for leader to orient to rewarded cup | |||||
| Maternal predator exposure | 1, 8 | 1.10 | 0.3244 | 0.70 | 0.05 |
| Training (before, after) | 1, 8 | 2.49 | 0.1529 | ||
| Maternal predator exposure*training | 1, 8 | 2.32 | 0.1664 | ||
| Copying time of 2nd fish to orient (2nd fish latency minus leader latency) | |||||
| Maternal predator exposure | 1, 8 | 6.05 | 0.0394 | 2.24 | 0.62 |
| Training (before, after) | 1, 8 | 13.01 | 0.0069 | ||
| Maternal predator exposure*training | 1, 8 | 2.47 | 0.1544 | ||
| Copying time of 3rd fish to orient (3rd fish latency minus leader latency) | |||||
| Maternal predator exposure | 1, 8 | 12.66 | 0.0074 | 2.07 | 0.37 |
| Training (before, after) | 1, 8 | 12.50 | 0.0077 | ||
| Maternal predator exposure*training | 1, 8 | 6.96 | 0.0245 | ||
Estimates of Cohen’s d refer to comparisons between the maternal predator exposure treatment means before (day 1) and after (day 7) training (N = 10).
Figure 3.
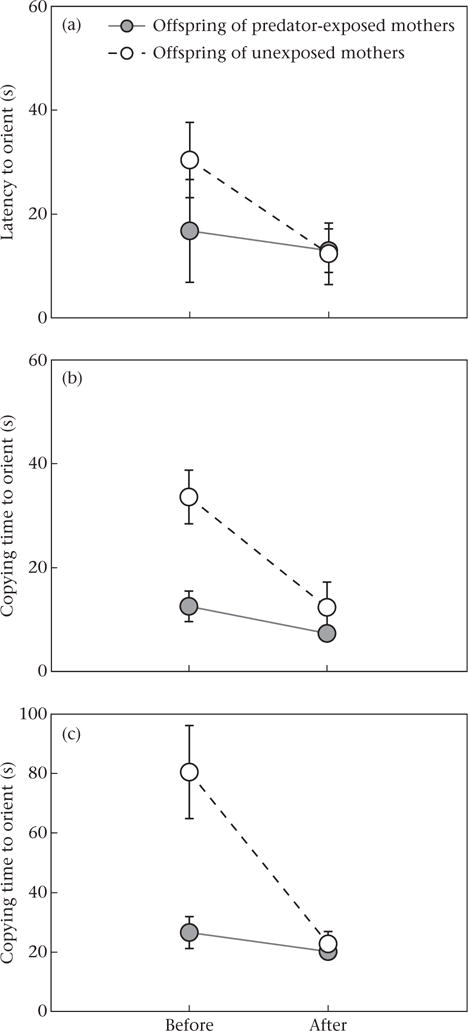
Effect of maternal predator exposure treatment on offspring behaviour and how quickly (a) the first fish (i.e. leader) oriented to the rewarded blue cup, (b) the second fish copied the leader and oriented to the rewarded cup (second fish minus leader) and (c) the third fish copied the leader and oriented to the rewarded cup (third fish minus leader) before and after 7 days of group training. Shown are group means ± SE (N = 10).
Although not an initial goal of this study, we can compare the behaviour during group training for the laboratory-reared juveniles of both maternal treatments (experiment 2) to that of our trained wild-caught juveniles (experiment 1). This comparison allows us to examine how the learning performance of laboratory-reared juveniles aligns with that of wild-caught juveniles and, thus, whether our findings reflect patterns present in natural populations. The orienting behaviour of the leader for trained wild-caught fish fell in between that of the offspring of predator-exposed and unexposed mothers before any training (orienting time on day 1: trained wild-caught juveniles = 17.8 ± 5.2 s, offspring of predator-exposed mothers = 16.8 ± 9.8 s, offspring of unexposed mothers = 30.4 ± 7.2 s; Fig. 3a). However, the attendance to social cues and the copying behaviour for trained wild-caught fish aligned more closely with that of the offspring of predator-exposed mothers compared to that of offspring of unexposed mothers. Copying times were similar for wild-caught fish and offspring of predator-exposed mothers before any training for both the second fish to follow the leader (second fish copying time on day 1: trained wild-caught juveniles = 17.2 ± 4.6 s, offspring of predator-exposed mothers = 12.6 ± 2.9 s, offspring of unexposed mothers = 33.6 ± 5.1 s; Fig. 3b) and the third fish to follow the leader (third fish copying time on day 1: trained wild-caught juveniles = 30.4 ± 2.8 s, offspring of predator-exposed mothers = 26.6 ± 5.3 s, offspring of unexposed mothers = 80.4 ± 15.6 s; Fig. 3c).
Generalizing a learned colour–reward association
Sticklebacks generalized the colour–reward association they had learned during group and pair training to the new context and they oriented towards and approached the rewarded blue cup faster than the unrewarded yellow cup (main effect of cup colour; Table 3). Maternal predator exposure did not significantly affect the offsprings’ ability to generalize and offspring of both predator-exposed and unexposed mothers behaved similarly (orienting to blue: offspring of predator-exposed mothers = 43.9 ± 10.5 s, offspring of unexposed mothers = 35.3 ± 9.8 s; approaching blue: offspring of predator-exposed mothers = 286.4 ± 67.3 s, offspring of unexposed mothers = 390.5 ± 67.3 s; Fig. 4). Judging from the medium effect size (d = 0.41), offspring of predator-exposed mothers tended to approach the rewarded blue cup more quickly compared to offspring of unexposed mothers, but this was not statistically significant (Table 3). Not all leaders approached a coloured cup within a testing trial (9 of 29 leaders did not approach), but results were similar even when we restricted the analyses to only those trials where leaders approached a cup (N = 20 leaders approached, with 11 from the maternal predator-exposed treatment and nine from the maternal unexposed treatment; maternal treatment: F1,18 = 2.48, P = 0.1327; cup colour: F1,18 = 4.47, P = 0.0488; maternal treatment*cup colour: F1,18 = 0.84, P = 0.3722). Maternal treatments did not differ significantly in how quickly the leader first started exploring the tank (offspring of predator-exposed mothers = 17.2 ± 4.3 s, offspring of unexposed mothers = 16.3 ± 5.7 s, N = 29; F1,8.19 = 0.59, P = 0.4628; random effect of group tank: χ2 = 3.9, P = 0.0483), nor in standard length of offspring (offspring of predator-exposed mothers = 20.8 ± 0.4 mm, offspring of unexposed mothers = 20.1 ± 0.4 mm, N = 58; F1,56 = 1.93, P = 0.1707).
Table 3.
Effect of maternal predator exposure on how leaders behaved towards blue (rewarded) and yellow (unrewarded) colours after 8 days of training
| Effect | df | F | P | Cohen’s d (on blue cup) |
|---|---|---|---|---|
| Latency for leader to first orient to a cup | ||||
| Maternal predator exposure | 1, 8.72 | 0.00 | 0.9814 | 0.22 |
| Cup colour (rewarded blue, unrewarded yellow) | 1, 27 | 13.59 | 0.0010 | |
| Maternal predator exposure*training | 1, 27 | 0.30 | 0.5883 | |
| Latency for leader to approach a cup | ||||
| Maternal predator exposure | 1, 27 | 2.22 | 0.1476 | 0.41 |
| Cup colour (rewarded blue, unrewarded yellow) | 1, 27 | 4.51 | 0.0430 | |
| Maternal predator exposure*training | 1, 27 | 1.06 | 0.3122 | |
Leaders generalized across contexts by orienting and approaching the rewarded blue cup faster than the unrewarded yellow cup, but this was not affected by maternal predator exposure. Cohen’s d values refer to comparisons of means between the maternal predator exposure treatments towards the blue rewarded cup (N = 29).
Figure 4.
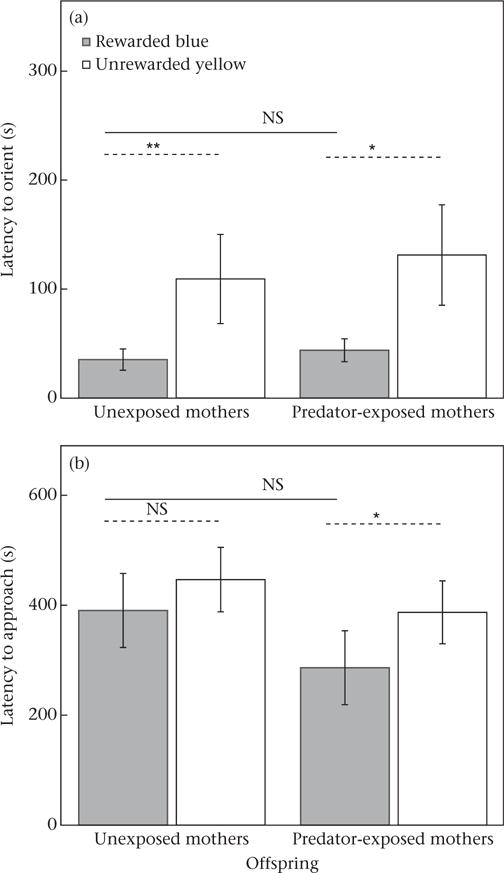
Effect of maternal predator exposure treatment on offspring testing performance in a novel context after 8 days of training (7 days in a group, 1 day in a pair). Shown are means ± SE for latency of leaders to (a) first orient to and (b) first approach the rewarded blue cup and the unrewarded yellow cup (N = 29). Lines above bars indicate results of comparisons between least square means: solid lines indicate comparisons of the rewarded blue cup between maternal treatments, dashed lines indicate comparisons between blue and yellow cups within a maternal treatment (*P < 0.05; **P < 0.01).
Ethical note
Efforts were made throughout to minimize animal stress (e.g. enrichment and shelters in all tanks, minimal handling, group and pair housing). Wild-caught fish were maintained in the laboratory for additional experiments and breeding. Laboratory-reared fish were euthanized with an overdose of the anaesthetic MS-222 after completion of the study. This study was approved by the Animal Care and Use Committee at the University of Illinois (protocol no. 12118).
DISCUSSION
The results from experiment 1 show that threespine sticklebacks are able to generalize a colour–reward association learned under one set of conditions to a new set of conditions. Trained individuals preferentially oriented to and approached the blue rewarded cup over the yellow unrewarded cup even when the stimuli were presented in a novel configuration. In contrast, untrained individuals did not prefer the blue rewarded cup over the yellow unrewarded cup. To our knowledge this is the first time that generalization of a colour–reward association across foraging tasks has been shown in fish. This is somewhat surprising considering the ability of fish to generalize learned associations between a predator and alarm cues across novel piscivorous predators (Brown et al., 2011; Ferrari et al., 2007; Mitchell et al., 2013). If previous knowledge can be used to deal with new challenges, this could have important implications for how individuals adjust behaviour under changing environmental conditions.
The results from experiment 2 show that juvenile sticklebacks oriented faster to the blue rewarded cup compared to the yellow unrewarded cup after 1 week of training, but there was no difference between offspring of predator-exposed and unexposed mothers. This result is in contrast to the pattern observed by Roche et al. (2012), where adult offspring of predator-exposed mother sticklebacks showed less improvement in locating a food reward following training compared with offspring of unexposed mothers. However, in the Roche et al. (2012) study, individuals were trained and tested alone and were unable to use social information. Since maternal predator exposure also results in offspring that shoal more closely with one another (Giesing et al., 2011), it is possible that offspring of predator-exposed mothers have increased access to social information via this tighter shoaling behaviour. Thus, some of the negative consequences of maternal predator exposure for offspring might be overcome when offspring are able to use social information from conspecifics. Closer inspection of behaviour during group training supports this hypothesis: offspring of predator-exposed mothers were faster to cue in on what the leader was doing and copy the leader by orienting towards the blue rewarded cup compared to offspring of unexposed mothers. However, because leaders were rewarded with food for orienting to the blue cup, it is also possible that the other fish in the group were modifying their behaviour based on the food stimulus rather than the leader behaviour or the cup colour stimulus. Alternatively, other fish in the group might have been simply following the movement of the leader, rather than cueing in on either the cup or food stimulus, due to their tendency to shoal, as has been previously found in threespine stickleback (untransmitted social effects: Atton, Hoppitt, Webster, Galef, & Laland, 2012). Thus, we cannot distinguish among a variety of social processes that might have directly or indirectly led to learning (e.g. local enhancement, stimulus enhancement, observational conditioning: see Hoppitt & Laland, 2008). Regardless of the initial mechanism involved in copying others, individuals were able to learn the colour–reward association and then generalize this knowledge to a new context, and in the absence of a group, during testing. Determining whether offspring of predator-exposed and unexposed mothers use different cues in forming associations and distinguishing between these different types of social learning would be an interesting area of future study.
How prenatal stress affects social learning has received little attention despite evidence that early postnatal experiences, such as maternal deprivation, can affect social learning (e.g. Levy, Melo, Galef, Madden, & Fleming, 2003; Lindeyer, Meaney, & Reader, 2013; Melo et al., 2006). An exception is a study by Boogert, Zimmer, and Spencer (2013), in which quail eggs were exposed to varying levels of the avian stress hormone corticosterone. These authors found that prenatal exposure to elevated levels of corticosterone (such as would occur due to maternal stress) increased the tendency of these individuals to use social information and imitate demonstrators in choosing a foraging patch later on (Boogert et al., 2013). Indeed, if maternal stress causes offspring to rely more on social cues, as our study and that of Boogert et al. (2013) suggest, there could be several benefits for offspring that find themselves in a stressful or dangerous environment. Attending to social cues can allow individuals to exploit novel prey and new foraging patches, as well as learn about potential predators, without having to discover the information on their own (reviewed in: Brown & Laland, 2003; Laland, 2004; Laland, Atton, & Webster, 2011). While private information is potentially more reliable than public information, it can be costly to obtain (Laland, 2004). Indeed, a greater reliance on social cues is associated with risky environments, such as high predation (Coolen, van Bergen, Day, & Laland, 2003; Webster & Laland, 2008; but see Galef & Yarkovsky, 2009). Although being group-trained clearly played a role in individuals’ ability to learn the colour–reward association, our experiments do not allow us to determine how individuals might weigh private versus public information (see Coolen et al., 2003; Laland et al., 2011). Examining how maternal predator exposure alters the reliance on social versus individual information is an obvious area for future research.
Our results suggest that once a colour–reward association has been learned (after 8 days of training), behavioural differences between maternal predator exposure treatments that are present initially in group training (this study) or after 5 days of training singly (Roche et al., 2012) are no longer detectable. Offspring of predator-exposed and unexposed mothers were equally able to generalize the colour–reward association from training to that of testing, although offspring of predator-exposed mothers tended to approach the rewarded cup slightly faster than offspring of unexposed mothers during testing. While offspring of both maternal treatments were quick to orient to the rewarded blue cup in its new location, they were much slower at approaching the cups, suggesting that this assay may have been challenging for them. Our results as well as those of Roche et al. (2012) suggest that maternal predator exposure does not prevent offspring from learning a colour–reward association but rather has subtle effects on how offspring learn.
Interestingly, the group behaviour of our laboratory-reared offspring from predator-exposed mothers aligns with the group behaviour of wild-caught juveniles from a high-predation population. Whether this behavioural similarity is due to both types of juveniles having predator-exposed mothers and/or having survived interactions with live predators is unclear, but these factors are clearly intertwined in nature. Whether personal experiences regarding predation risk are linked to later offspring learning or the tendency of offspring to use social cues regarding food (e.g. Galef & Yarkovsky, 2009; Webster & Laland, 2008) and how this combines with maternal predator exposure would be an interesting focus of future research, particularly since the background level of predation risk can alter prey learning (e.g. Chivers, McCormick, Mitchell, Ramasamy, & Ferrari, 2014).
In this study, we showed that threespine sticklebacks are capable of generalizing a colour–reward association from one foraging context to a novel foraging context. We also showed that maternal predator exposure affected the tendency for offspring to use social information in groups. Furthermore, we found that once a colour–reward association had been learned, any learning deficits due to maternal predator exposure were no longer detectable and that all fish, regardless of maternal experience, were capable of generalizing a learned colour–reward association. Thus, familiarity with a group of conspecifics (as in Atton, Galef, Hoppitt, Webster, & Laland, 2014) and differential use of social cues by offspring during the learning process might overcome any learning deficits associated with maternal predator exposure.
Acknowledgments
We thank D. Roche, A. Kephart, E. Suhr and S. Leasure for assistance with the fish in the lab, and L. Hostert, S. Pearish and M. Bensky for collecting fish from the field. We thank the Bell lab, N. Boogert, M. Mitchell, M. Schrader, M. Webster and two referees for comments that greatly improved the manuscript. We also thank the University of Illinois Office of Undergraduate Research for funding S.F. to present this work and get valuable feedback at the annual meeting of the Animal Behavior Society. This work was supported by National Science Foundation grant IOS 1121980 to A.M.B. and K.E.M. with REU support for S.F., and National Institutes of Health grant PHS R01 GM082937 to A.M.B. and M. Band.
References
- Agrawal AA, Laforsch C, Tollrian R. Transgenerational induction of defences in animals and plants. Nature. 1999;401:60–63. http://dx.doi.org/10.1038/43425. [Google Scholar]
- Atton N, Galef BJ, Hoppitt W, Webster MM, Laland KN. Familiarity affects social network structure and discovery of prey patch locations in foraging stickleback shoals. Proceedings of the Royal Society B: Biological Sciences. 2014;281:20140579. doi: 10.1098/rspb.2014.0579. http://dx.doi.org/10.1098/rspb.2014.0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atton N, Hoppitt W, Webster MM, Galef BJ, Laland KN. Information flow through threespine stickleback networks without social transmission. Proceedings of the Royal Society B: Biological Sciences. 2012;279:4272–4278. doi: 10.1098/rspb.2012.1462. http://dx.doi.org/10.1098/rspb.2012.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badyaev AV, Uller T. Parental effects in ecology and evolution: mechanisms, processes and implications. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364:1169–1177. doi: 10.1098/rstb.2008.0302. http://dx.doi.org/10.1098/rstb.2008.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo J. Maternal effects in animal ecology. American Zoologist. 1996;36:83–105. [Google Scholar]
- Bestion E, Teyssier A, Aubret F, Clobert J, Cote J. Maternal exposure to predator scents: offspring phenotypic adjustment and dispersal. Proceedings of the Royal Society B: Biological Sciences. 2014;281:20140701. doi: 10.1098/rspb.2014.0701. http://dx.doi.org/10.1098/rspb.2014.0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boogert NJ, Zimmer C, Spencer KA. Pre- and post-natal stress have opposing effects on social information use. Biology Letters. 2013;9:20121088. doi: 10.1098/rsbl.2012.1088. http://dx.doi.org/10.1098/rsbl.2012.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C, Laland KN. Social learning in fishes: a review. Fish and Fisheries. 2003;4:280–288. http://dx.doi.org/10.1046/j.1467-2979.2003.00122.x. [Google Scholar]
- Brown GE, Ferrari MCO, Malka PH, Russo S, Tressider M, Chivers DP. Generalization of predators and nonpredators by juvenile rainbow trout: learning what is and is not a threat. Animal Behaviour. 2011;81:1249–1256. http://dx.doi.org/10.1016/j.anbehav.2011.03.013. [Google Scholar]
- Chivers DP, McCormick MI, Mitchell MD, Ramasamy RA, Ferrari MCO. Background level of risk determines how prey categorize predators and non-predators. Proceedings of the Royal Society B: Biological Sciences. 2014;281:20140355. doi: 10.1098/rspb.2014.0355. http://dx.doi.org/10.1098/rspb.2014.0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. New York, NY: Academic Press; 1988. [Google Scholar]
- Coolen I, van Bergen Y, Day RL, Laland KN. Species difference in adaptive use of public information in sticklebacks. Proceedings of the Royal Society B: Biological Sciences. 2003;270:2413–2419. doi: 10.1098/rspb.2003.2525. http://dx.doi.org/10.1098/rspb.2003.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coslovsky M, Richner H. Predation risk affects offspring growth via maternal effects. Functional Ecology. 2011;25:878–888. http://dx.doi.org/10.1111/j.1365-2435.2011.01834.x. [Google Scholar]
- Day RL, MacDonald T, Brown C, Laland KN, Reader SM. Interactions between shoal size and conformity in guppy social foraging. Animal Behaviour. 2001;62:917–925. http://dx.doi.org/10.1006/anbe.2001.1820. [Google Scholar]
- Ferrari MCO, Brown GE, Messier F, Chivers DP. Threat-sensitive generalization of predator recognition by larval amphibians. Behavioral Ecology and Sociobiology. 2009;63:1369–1375. http://dx.doi.org/10.1007/s00265-009-0779-5. [Google Scholar]
- Ferrari MCO, Gonzalo A, Messier F, Chivers DP. Generalization of learned predator recognition: an experimental test and framework for future studies. Proceedings of the Royal Society B: Biological Sciences. 2007;274:1853–1859. doi: 10.1098/rspb.2007.0297. http://dx.doi.org/10.1098/rspb.2007.0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galef BG, Yarkovsky N. Further studies of reliance on socially acquired information when foraging in potentially risky situations. Animal Behaviour. 2009;77:1329–1335. http://dx.doi.org/10.1016/j.anbehav.2009.01.038. [Google Scholar]
- Giesing ER, Suski CD, Warner RE, Bell AM. Female stickleback transfer information via eggs; effects of maternal experience with predators on offspring. Proceedings of the Royal Society B: Biological Sciences. 2011;278:1753–1759. doi: 10.1098/rspb.2010.1819. http://dx.doi.org/10.1098/rspb.2010.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girvan JR, Braithwaite VA. Population differences in spatial learning in three-spined sticklebacks. Proceedings of the Royal Society B: Biological Sciences. 1998;265:913–918. http://dx.doi.org/10.1098/rspb.1998.0378. [Google Scholar]
- Griffin AS, Evans CS, Blumstein DT. Learning specificity in acquired predator recognition. Animal Behaviour. 2001;62:577–589. http://dx.doi.org/10.1006/anbe.2001.1781. [Google Scholar]
- Hoppitt W, Laland KN. Social processes influencing learning in animals: a review of the evidence. Advances in the Study of Behavior. 2008;38:105–165. http://dx.doi.org/10.1016/S0065-3454(08)00003-X. [Google Scholar]
- Ihalainen E, Rowland HM, Speed MP, Ruxton GD, Mappes J. Prey community structure affects how predators select for Mullerian mimicry. Proceedings of the Royal Society B: Biological Sciences. 2012;279:2099–2105. doi: 10.1098/rspb.2011.2360. http://dx.doi.org/10.1098/rspb.2011.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laland KN. Social learning strategies. Learning & Behavior. 2004;32:4–14. doi: 10.3758/bf03196002. [DOI] [PubMed] [Google Scholar]
- Laland KN, Atton N, Webster MM. From fish to fashion: experimental and theoretical insights into the evolution of culture. Philosophical Transactions of the Royal Society B: Biological Sciences. 2011;366:958–968. doi: 10.1098/rstb.2010.0328. http://dx.doi.org/10.1098/rstb.2010.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeCroy CW, Krysik J. Understanding and interpreting effect sizes. Social Work Research. 2007;31:243–248. [Google Scholar]
- Levy F, Melo AI, Galef BG, Jr, Madden M, Fleming AS. Complete maternal deprivation affects social, but not spatial learning in adult rats. Developmental Psychobiology. 2003;43:177–191. doi: 10.1002/dev.10131. http://dx.doi.org/10.1002/dev.10131. [DOI] [PubMed] [Google Scholar]
- Lindeyer CM, Meaney MJ, Reader SM. Early maternal care predicts reliance on social learning about food in adult rats. Developmental Psychobiology. 2013;55:168–175. doi: 10.1002/dev.21009. http://dx.doi.org/10.1002/dev.21009. [DOI] [PubMed] [Google Scholar]
- Maccari S, Krugers HJ, Morley-Fletcher S, Szyf M, Brunton PJ. The consequences of early-life adversity: neurobiological, behavioural and epigenetic adaptations. Journal of Neuroendocrinology. 2014;26:707–723. doi: 10.1111/jne.12175. http://dx.doi.org/10.1111/jne.12175. [DOI] [PubMed] [Google Scholar]
- Maccoll ADC, Chapman SM. A benthic predatory fish does not cause selection on armour traits in three-spined stickleback Gasterosteus aculeatus (Gasterosteiformes: Gasterosteidae) Biological Journal of the Linnean Society. 2011;104:877–885. http://dx.doi.org/10.1111/j.1095-8312.2011.01759.x. [Google Scholar]
- Marples NM, Quinlan M, Thomas RJ, Kelly DJ. Deactivation of dietary wariness through experience of novel food. Behavioral Ecology. 2007;18:803–810. http://dx.doi.org/10.1093/beheco/arm053. [Google Scholar]
- McGhee KE, Bell AM. Pilot experiment examining the physiological consequences of being chased by a model sculpin. 2012 Unpublished raw data. [Google Scholar]
- McGhee KE, Bell AM. Effect of maternal predator-exposure on offspring shoaling behaviour and survival. (n.d.) Manuscript in preparation. [Google Scholar]
- McGhee KE, Pintor LM, Suhr EL, Bell AM. Maternal exposure to predation risk decreases offspring antipredator behaviour and survival in threespined stickleback. Functional Ecology. 2012;26:932–940. doi: 10.1111/j.1365-2435.2012.02008.x. http://dx.doi.org/10.1111/j.1365-2435.2012.02008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo AI, Lovic V, Gonzalez A, Madden M, Sinopoli K, Fleming AS. Maternal and littermate deprivation disrupts maternal behavior and social-learning of food preference in adulthood: tactile stimulation, nest odor, and social rearing prevent these effects. Developmental Psychobiology. 2006;48:209–219. doi: 10.1002/dev.20130. http://dx.doi.org/10.1002/dev.20130. [DOI] [PubMed] [Google Scholar]
- Mitchell MD, McCormick MI, Chivers DP, Ferrari MCO. Generalization of learned predator recognition in coral reef ecosystems: how cautious are damselfish? Functional Ecology. 2013;27:299–304. http://dx.doi.org/10.1111/1365-2435.12043. [Google Scholar]
- Monaghan P. Early growth conditions, phenotypic development and environmental change. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008;363:1635–1645. doi: 10.1098/rstb.2007.0011. http://dx.doi.org/10.1098/rstb.2007.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousseau TA, Fox CW. Maternal effects as adaptations. Oxford, U.K: Oxford University Press; 1998. [Google Scholar]
- Pressley PH. Parental effort and the evolution of nest-guarding tactics in the threespine stickleback, Gasterosteus aculeatus L. Evolution. 1981;35:282–295. doi: 10.1111/j.1558-5646.1981.tb04887.x. http://dx.doi.org/10.2307/2407838. [DOI] [PubMed] [Google Scholar]
- Roche DP, McGhee KE, Bell AM. Maternal predator-exposure has lifelong consequences for offspring learning in threespined sticklebacks. Biology Letters. 2012;8:932–935. doi: 10.1098/rsbl.2012.0685. http://dx.doi.org/10.1098/rsbl.2012.0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoech SJ, Rensel MA, Heiss RS. Short and long-term effects of developmental corticosterone exposure on avian physiology, behavioral phenotype, cognition, and fitness: review. Current Zoology. 2011;57:514–530. [Google Scholar]
- Sheriff MJ, Krebs CJ, Boonstra R. The sensitive hare: sublethal effects of predator stress on reproduction in snowshoe hares. Journal of Animal Ecology. 2009;78:1249–1258. doi: 10.1111/j.1365-2656.2009.01552.x. http://dx.doi.org/10.1111/j.1365-2656.2009.01552.x. [DOI] [PubMed] [Google Scholar]
- Sheriff MJ, Love OP. Determining the adaptive potential of maternal stress. Ecology Letters. 2013;16:271–280. doi: 10.1111/ele.12042. http://dx.doi.org/10.1111/ele.12042. [DOI] [PubMed] [Google Scholar]
- Storm JJ, Lima SL. Mothers forewarn offspring about predators: a transgenerational maternal effect on behavior. American Naturalist. 2010;175:383–390. doi: 10.1086/650443. http://dx.doi.org/10.1086/650443. [DOI] [PubMed] [Google Scholar]
- Svádová K, Exnerová A, Šètys P, Landova E, Valenta J, Fučíková A, et al. Role of different colours of aposematic insects in learning, memory and generalization of naive bird predators. Animal Behaviour. 2009;77:327–336. http://dx.doi.org/10.1016/j.anbehav.2008.09.034. [Google Scholar]
- Webster MM, Laland KN. Social learning strategies and predation risk: minnows copy only when using private information would be costly. Proceedings of the Royal Society B: Biological Sciences. 2008;275:2869–2876. doi: 10.1098/rspb.2008.0817. http://dx.doi.org/10.1098/rspb.2008.0817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock M. The long-term behavioural consequences of prenatal stress. Neuroscience and Biobehavioral Reviews. 2008;32:1073–1086. doi: 10.1016/j.neubiorev.2008.03.002. http://dx.doi.org/10.1016/j.neubiorev.2008.03.002. [DOI] [PubMed] [Google Scholar]


