Abstract
Background
A medical need exists for successfully treating patients afflicted with leukemia and especially those that relapse and ultimately become refractory to front line chemotherapies. Leukemia cases are particularly high within Hispanic populations where this disease is among the most frequently occurring cancer. A possible cause is somatic mutations in Janus tyrosine kinase (Jak3). Fourteen somatic mutations have been reported in Jak3, including M511I and A573V, from patients with various forms of leukemia. While several of these Jak3 mutations have been shown to possess transforming ability in cell lines, whether these mutations are susceptible to Jak3 selective inhibitors remains less clear.
Methods
The IL-3 dependent pro-B cell line Ba/F3 was virally transduced with plasmids encoding GFP and different mutant forms of Jak3, some of which conferred IL-3 independence. Sensitivity to pre-clinical and clinical Jak3 selective inhibitors was assessed for cellular viability and growth.
Results
Two Jak3 mutations conferred IL-3 independent growth in Ba/F3 cells. However, the level of drug sensitivity varied with respect to Jak3 inhibitors NC1153, CP-690,550, and EP-009.
Conclusion
Jak3 inhibitors CP-690,550 and NC1153 showed efficacy in reducing viability of Ba/F3 cells transformed with mutant forms of Jak3, thus providing new therapeutic strategies to treat these types of cancer.
Keywords: Janus tyrosine kinase, signal transducer and activator of transcription, tyrosine kinase inhibitor, acute lymphoblastic leukemia
INTRODUCTION
Janus kinase 3 (Jak3), is a non-receptor tyrosine kinase necessary for homeostatic balance of the immune system. This is especially true in T-, B- and Natural Killer cells where Jak3 is predominantly expressed [3]. Loss of Jak3 regulation in immune cells leads to diseases including Severe Combined Immunodeficiency (SCID) [4, 5], and cancers of the blood, such as leukemia and lymphoma [6, 7]. Hematological cancers have been previously reported to be associated with Jak3 gain of function mutations [7–10]. Indeed, fourteen activating point mutations of Jak3 have been identified from screening patients with both myelogenous and lymphocytic forms of leukemia or lymphoma: G62S, I87T, P132T, Q501H, M511I, A572V, A573V, M576L, A593T, R657Q, V722I, S789P, R918C and L1017M [6–11]. Many of these mutations have been shown to be transforming in Ba/F3 cells, a murine pro B-cell cell line that is normally dependent on IL-3 for growth [7–10]. It is important to understand how this transformation occurs and if mutant Jak3 can be targeted in the treatment of leukemia.
Indeed, leukemia is the most prevalent form of cancer in Hispanics aged 0–19 years [1]. Acute Lymphocytic Leukemia (ALL) accounts for 78% of childhood leukemia cases in Hispanics and along with acute myelogenous leukemia (AML) have higher incidence rates in Hispanic over non-Hispanic white children. This is underscored by the fact that the 5-year survival rates are lower in Hispanic children than non-Hispanic white children, 89% vs 93% in ALL, and 69% vs 77% in AML, respectively [2]. Therefore, there is a critical need to understand the mechanisms involved in leukemic cell transformation and to identify novel therapeutic strategies against this disease.
We sought to determine the role of mutant Jak3 in leukemia by assessing the auto-activation ability of the above listed fourteen Jak3 mutations and their ability to activate downstream effector, STAT5B, in comparison to wild-type (WT) Jak3. We also examined the transformational ability of these Jak3 mutations, and their sensitivity to clinical and pre-clinical inhibitors. Here we provide evidence that Jak3 A573V and M511I mutations confer transformative properties and are sensitive to the FDA approved Jak3 inhibitor CP-690,550 (Tofacitinib) as well as NC1153.
In the United States, the Hispanic population is rapidly expanding, however, few studies evaluate this group and the possible uniqueness of their cancers. To better understand this type of cancer associated with this population, we examined known Jak3 mutations and their response to current therapies. Moreover, cancer biopsies from our nearly 90% Hispanic population were examined for these Jak3 mutations. While no mutations were identified in 103 patients with hematopoietic cancers, this study provides evidence that Jak3 M511I and A573V driven leukemias, while rare, are sensitive to Jak3 inhibitors and thus patients positive for these mutations can benefit from currently available Jak3 approved therapies.
MATERIALS AND METHODS
Cell Culture, Drug Treatment and Viability Assay
The human HEK293T cell line was grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10 % fetal bovine serum (FBS; Atlanta Biologicals), 2 mM L-glutamine (Corning), and 1 % penicillin/streptomycin (Corning). Murine pro-B-cells, Ba/F3 (DSMZ), and human embryonic kidney cells, Hek293, were cultured in RPMI 1640 medium containing 10 % FBS, 2 mM l-glutamine, and 1 % penicillin/streptomycin. Ba/F3 cell culture media was supplemented with 1 ng/ml murine interleukin-3 (IL-3) (Peprotech). At 48 hours post-transduction cells were washed 3 times with, and resuspended in IL-3 free media. Cells were then analyzed by FACS using a Beckman Coulter Gallios™ Flow Cytometer for GFP and propidium iodide (MP Biomedicals). Transformed Ba/F3 cells were seeded into 96 well plates at a density of 104 cells per 100 μL. They were then treated at a range of 0 to 500 nM with CP-690,550 (Selleck Chemicals), 0 to 10 μM with NC1153 [3] or 0 to 10 μM with EP-009 [4] for 24 hours at 37 °C. Cellular viability was then determined by MTS assay (Promega) according to the manufacturer’s instructions in triplicate (n=3). MTS assay and FACS analysis was performed on Ba/F3 cells grown in IL-3 free media. Cell counting was performed by using a hemocytometer (Hausser Scientific) and trypan blue (MP Biomedicals).
Plasmids and Site-directed Mutagenesis
Full length wild-type (WT) Jak3 was subcloned into the EcoRI and XhoI sites of MSCV-IRES-GFP (MIG) plasmid, a gift from Tannishtha Reya (Addgene plasmid 20672), or into the TOPO cloning site of pLenti7.3/V5-TOPO (pLenti7.3) plasmid (Invitrogen). Mutant forms of Jak3 were prepared using the QuikChange site-directed mutagenesis kit (Stratagene) according to the manufacturer’s instructions. Primers used for mutagenesis of Jak3 were as follows: M511I (forward primer 5′-CCAATACCAGCTGAGTCA GATCACACACAAGATCCCTG-3′ and reverse primer 5′-GAGGGATCTTGTGAAATGT-GATCTGACTCAGCTGG TATTGG-3′); A573V (forward primer 5′-GTCATTCCTGG AAGCAGTGAGCTTGATGAGCCAAG-3′ and reverse primer 5′-CTTGGCTCATCAAGCTCACTGCTTCCAGG AATGAC-3′). The pcDNA3.1 human Jak3 and STAT5B cDNAs (OriGene) were obtained as described previously [5, 6]. All subclones and mutations were verified by DNA sequencing at the Genomic Analysis Core Facility of the Border Biomedical Research Center at The University of Texas at El Paso.
Virus Production and Infection of Target Cells
Lentiviruses were generated by co-transfection of pLenti7.3 plasmid vectors with packaging plasmids pMD.G and pCMVΔR8.91, gifts from Manuel Llano, into Hek293T cells. Retroviruses were generated by co-transfection of MIG retroviral plasmid vectors and packaging plasmids pCL-Ampho (Addgene) and pMD.G, into Hek293T cells. Viral supernatants were collected 72 h post-transfection, centrifuged to remove cell debris, filtered through 0.45-μM filters (Thermo Scientific) and concentrated by ultracentrifugation at 124,750 x g for 2 h on a 20% sucrose cushion. Target cells were plated at 1 × 105 cells in 500 μL of RPMI 1640 culture medium in 24-well plates and infected with 50 μL of either pLenti7.3 Jak3 variants or MIG Jak3 variants.
Patient Samples, Purification of Genomic DNA and Sequencing
Qiagen DNeasy Blood & Tissue Kit was used to purify genomic DNA (gDNA) from leukemia and lymphoma primary cells, obtained from The University of Texas at El Paso tumor biorepository comprised primarily of Hispanic donors, according to manufacturer’s protocol. DNA concentration was measured using NanoDrop 3000 (Thermo Fisher Scientific, Inc., Wilmington, DE, USA), and was verified using 1 % agarose gel (Ultrapure Agarose with 1x TBE) containing 0.002% EtBr. Patient gDNA was amplified using the following primers: Exon 11 (forward primer 5′-GTTGCAGT GAGCTGAGATCG-3′ and reverse primer 5′-TCTCATGCT GAATGGTGAGG-3′); Exon 13 (forward primer 5′-TCCCGTATCAGAAAATCATGG-3′ and reverse primer 5′-GCTGGATATGGGTGAGAACC-3′). Subsequently, PCR product was sequenced using a 3130x/Genetic Analyzer (Thermo Fisher Scientific, Inc., Wilmington, DE, USA).
Transfection and Western Blot Analysis
16 hours pre-transfection Hek293 cells were seeded into 10 cm dishes to yield 90–95% confluency at the time of transfection. Cells were transfected with MIG plasmid containing mutant JAK3 or WT Jak3 with three controls, Jak3 K855A, Y981F and/or Y980F and STAT5B using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. 48 hours post-transfection cells were pelleted, lysed, clarified and either immunoprecipitated (IP) for JAK3 using anti-JAK3 polyclonal antibody as previously described [5], or whole cell lysate (WCL) saved. Samples were separated by 7.5 % SDS-PAGE and transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore) for Western blot analysis as previously described [7]. Briefly, membranes were probed overnight with mouse monoclonal 4G10 anti-phosphotyrosine (pY) antibody (EMD Millipore) or anti-pY STAT5 (Cell Signaling Technology) developed using horseradish peroxidase-conjugated goat anti-mouse IgG and visualized by enhanced chemiluminescence and X-ray film (Phenix). Membranes were stripped as previously described [5] and re-probed with Jak3 (Abcam) or STAT5 (Santa Cruz) antibodies to ensure equal protein loading.
RESULTS
Leukemia Related Jak3 Transforming Mutants are Capable of Auto-activation and Phosphorylation of Downstream Effectors
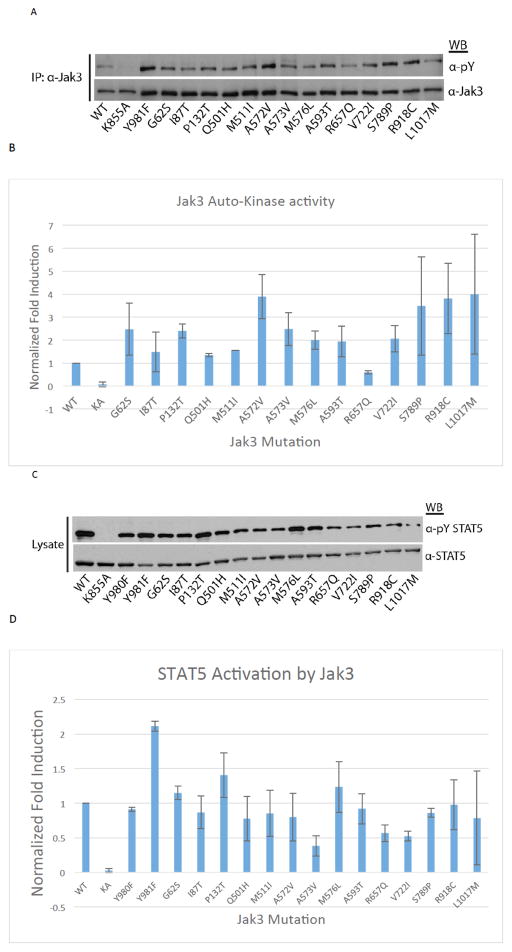
To assess whether previously identified transforming mutations within Jak3 are tyrosine phosphorylated, Hek293 cells were transfected with plasmids for either Jak3 WT, Jak3 kinase dead mutant, K855A, Jak3 hyperactive active mutant Y981F, or one of fourteen mutant Jak3 plasmids identified in human leukemia patients [8–11]. As shown in (Fig. 1A and B), all Jak3 variants displayed equal or greater tyrosine phosphorylation compared to the WT enzyme. Kinase dead Jak3 (K855A) showed no activity. Similarly, Hek293 cells were transfected as above but with the inclusion of WT STAT5B as a substrate for Jak3 kinase activity (Fig. 1C and D). All Jak3 variants were competent to induce STAT5 tyrosine phosphorylation. Together, these results confirm that the tested Jak3 mutations are catalytically active and are able to associate with and activate downstream effector proteins such as STAT5 in the absence of cytokine stimulation.
Fig. 1. Hyperactivation of Jak3 somatic mutations and STAT5B activation.
(A) Hek293 cells were transfected with Jak3 plasmids for WT, catalytically inactive mutant (K855A), hyperactive mutant (Y981F), or one of fourteen Jak3 mutations previously identified in leukemia patients as indicated. At 48 hours post-transfection, cells were lysed, clarified, immunoprecipitated for Jak3 and Western blot analysis was performed for anti-phosphotyrosine (pY) and total Jak3. (B) Representative blots of A were used to quantitate pY band intensity that was normalized to total Jak3 using densitometric analysis, n=2. (C) Hek293 cells were transfected as above with the inclusion of a STAT5B WT plasmid and Jak3 (Y980F). Cells were lysed 48 hours post-transfection and Western blot analysis was performed for pY-STAT5 and total STAT5. (D) Representative blots of C were used to quantitate pY-STAT5 band intensity that was normalized to total STAT5 using densitometric analysis, n=2.
Jak3 Mutations M511I and A573V Confer Transforming Ability in Ba/F3 Cells
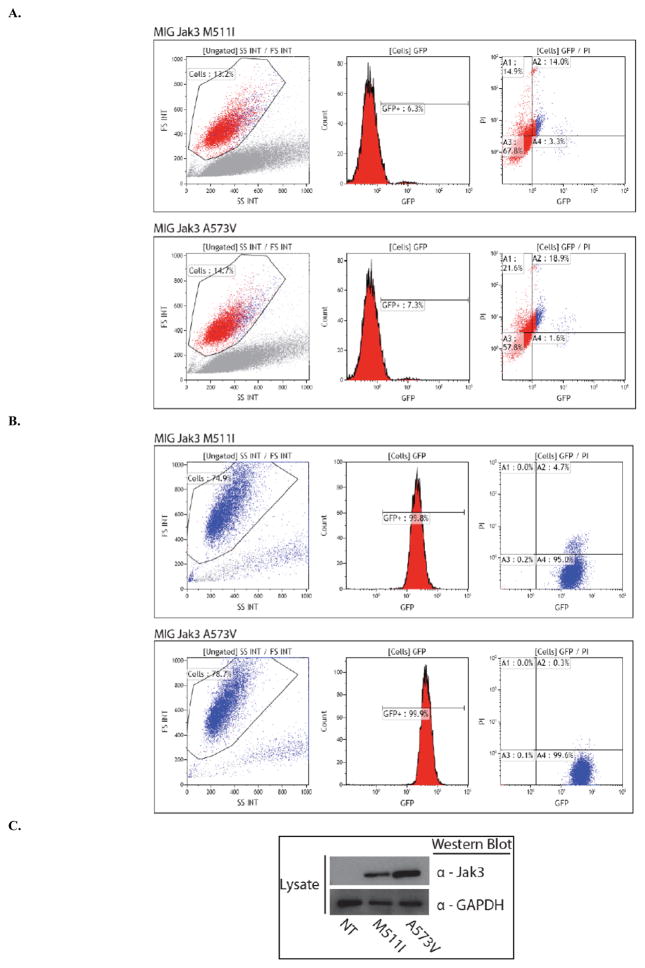
After evaluating the catalytic activity of Jak3 mutants, we sought to determine whether any of these Jak3 mutations have transforming potential. Ba/F3 cells, a murine IL-3 dependent pro-B cell line cloned from C3H mice, were transduced with either pLenti7.3 Jak3 variants or MIG Jak3 variants and 48 hours post-transduction underwent IL-3 starvation [12]. At 48 hours post IL-3 starvation, the living cell population of transduced cells was analyzed by FACS with gating on the FS/SS histogram. MIG Jak3 M511I and A573V transformed cells, grown in IL-3 free media, were evaluated for GFP expression, as an indicator of transduction efficiency, and for propidium iodide staining as an indicator of cellular viability 48 h post-transduction (Fig. 2A). Following 30 days of IL-3 withdrawal both MIG Jak3 M511I and A573V transduced Ba/F3 cells were found to be 100 % GFP positive and were greater than 75 % viable (Fig. 2B); This indicated that both cell lines had been successfully transformed into IL-3 independent cell growth. Ba/F3 transformed cells were then lysed and Western blotted for Jak3 to confirm protein expression (Fig. 2C). Viruses were generated for all 14 Jak3 mutants using the pLenti7.3 system and the MIG vector system was used to generate 10 of the mutant Jak3 viruses. Only Jak3 mutant M511I showed transforming ability using the pLenti7.3 system (data not shown), while Jak3 mutants M511I and A573V were competent to transform Ba/F3 cells using the MIG vector system (Table 1).
Fig. 2. Generation of stable IL-3 independent Jak3 positive, M511I and A573V, Ba/F3 cell lines.
(A) Ba/F3 cells were transduced with either MIG Jak3 M511I (top) or MIG Jak3 A573V (bottom). At 2 days post-IL-3 withdrawal cells were analyzed by flow cytometry for GFP expression. (B) At 30 days post-IL-3 withdrawal transduced cells were analyzed by flow cytometry for GFP expression. (C) Transduced Ba/F3 cells were lysed and Western blot analysis was performed for Jak3 and GAPDH.
Table 1.
Transforming ability of Jak3 mutations using the MSCV-IRES-GFP vector in Ba/F3 cells.
| Transforming Capability | |
|---|---|
| G62S | Not applicable |
| I87T | Not transforming |
| P132T | Not transforming |
| Q501H | Not transforming |
| M511I | Transforming |
| A572V | Not applicable |
| A573V | Transforming |
| M576L | Not applicable |
| A593T | Not transforming |
| R657Q | Not applicable |
| V722I | Not transforming |
| S789P | Not transforming |
| R918C | Not transforming |
| L1017M | Not transforming |
Jak3 Transformed Ba/F3 Cell Lines are Sensitive to Jak3 Inhibitors, NC1153 and CP-690,550
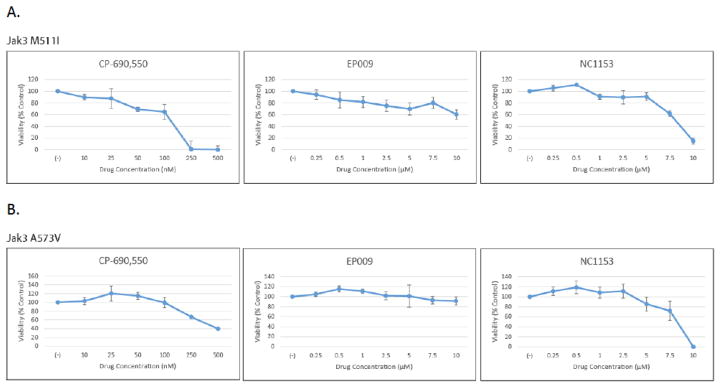
MTS assay was used to determine the sensitivity of Ba/F3 M511I and A573V cells to the following Jak3 selective inhibitors: NC1153, EP-009 and CP-690,550. The Mannich base NC1153 and its derivative, EP-009, have been shown to be selective in targeting Jak3 resulting in reduced tyrosine kinase activity and induction of apoptosis in Jak3 expressing lymphocytes [3, 4, 13]. Similarly, CP-690,550 has been shown to be an effective driver of apoptosis in a Jak3 transformed Ba/F3 model but also has inhibitory effects on Jak1 and Jak2 [14]. Ba/F3 cells transformed with M511I and A573V showed a dose dependent sensitivity to NC1153 and CP-690,550 (Fig. 3A and 3B). Neither Jak3 transforming mutant responded to EP-009 treatment (Figs. 3A and 3B).
Fig. 3. Cytotoxicity of Jak3 inhibitors on M511I and A573V Jak3 transformed Ba/F3 cells.
(A) MIG Jak3 M511I transformed Ba/F3 cells were cultured in IL-3 free media for 24 h at 37°C with increasing amounts of either NC1153 (0–10μM), EP-009 (0–10μM) or CP-690,550 (0–500 nM) and cell viability measured by MTS tetrazolium salt assay. Values represent mean absorbance (OD490–OD650 nm) normalized to vehicle (PBS or DMSO) treated control cells. Error bars represent standard deviation of n=3. (B) MIG Jak3 A573V transformed Ba/F3 cells were cultured as described above.
Common Jak3 Mutations are not Found in Hispanic Leukemia and Lymphoma Patients
Sequencing primers were generated to identify Jak3 mutations in leukemia and lymphoma patient samples from a mostly Hispanic tumor biorepository. Exons 3, 4, 11, 13, 15, 16, 18 and 19, where 12 of the 14 mutations identified are located, were sequenced using both forward and reverse primers in 103 patient samples but no new nor previously identified Jak3 mutations were found (data not shown). This is the first study that has focused on identifying mutant Jak3 in a Hispanic population and it is interesting that the incidence is so low, in stark contrast to other ethnic populations. Indeed, many studies have focused on identifying Jak3 mutations in Asian communities, predominantly Japanese with limited Singaporean, Thai, and Chinese populations, which range from as low as 8.7% to as high as 35.4% [8, 9, 11, 15, 16]. This work highlights the difference of leukemia and lymphoma mechanics between ethnic populations and that targeting Jak3 for leukemia therapy in Hispanics may not be as effective as in an Asian population.
DISCUSSION
The present study describes the transforming potential of Jak3 somatic mutations in cytokine dependent Ba/F3 cells and characterization of their response to Jak3 inhibitors. Here, we have shown that the previously identified Jak3 mutations, M511I and A573V, are transforming and are sensitive to Jak3 kinase inhibitors. Importantly, the FDA approved Jak3 inhibitor CP-690,550 is already being used in the treatment of auto-immune disorders such as rheumatoid arthritis and is undergoing clinical trials for psoriasis and ankylosing spondylitis [17, 18]. The NC1153 derivative used in this study, EP-009, is currently in the pre-clinical stages of development and has shown promise in targeting Jak3-driven tumor T-cells both in vitro and in vivo [4]. Indeed, these findings suggest that CP-690,550 might be useful in the treatment of certain hematopoietic cancers such as Jak3 driven leukemias that harbor the M511I and A573V mutations. Moreover, these data indicate a need for an FDA approved Jak3 specific inhibitor such as NC1153 which may provide greater treatment options. Whether or not current Jak3 inhibitors in phase III of clinical development would prove useful in treating these mutated forms of Jak3 remains to be evaluated.
Our studies involved the creation of a virus that could stably infect Ba/F3 cells in order to determine the transforming ability of fourteen Jak3 mutations identified in leukemia patients [8–11]. Two separate viral constructs were generated to create these mutations, plenti 7.3 and MIG. In contrast to earlier studies regarding the expression of this group of fourteen mutants [8, 10, 11, 19], in our hands, only M511I was successful at transforming Ba/F3 cells using either viral vector plenti 7.3 or MIG, while the A573V mutation was only successful when transforming employed the MIG vector (Table 1). However, these transformations were only a transient effect associated with downregulation of Jak3 expression through promotor silencing.
According to the CDC, since 2010, cancer is the number one killer of Hispanic/Latino citizens within the United States bucking the trend of heart disease across all other ethnicities. This is further highlighted by the fact that Hispanic children and young adults under 20 are at higher risk of developing and succumbing to acute lymphoblastic leukemia (ALL) in the U.S. [20–22]. Alarmingly, this trend is on the rise with Hispanic children being 1.71 times more likely to be diagnosed with ALL than non-Hispanic children [23]. In addition, these mutations were not identified in the tumor samples that were collected by our laboratory. Moreover, given the absence of any identifiable Jak3 mutant is very interesting as it suggests that the Jak3/STAT pathway may not be a critical oncogenic cascade in this population, suggesting other targets should be sought to account for these health disparity differences.
Acknowledgments
We thank the staff of the Border Biomedical Research Center Core Laboratories including the Bioinformatics Computing Core Facility, Biomolecule Analysis Core Facility (BACF), the Cytometry, Screening and Imaging (CSI) Core Facility, the Genomic Analysis Core Facility (GACF), and the Statistical Consulting Laboratory for services and facilities provided. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of NIMHD or NIH. The authors would also like to thank Dr. Manuel Llano and Angelica P. Lopez for the technical assistance generating the virus used to create stable Ba/F3 cell lines. This work was supported, in whole or in part, by grants to R.A.K. from the Lizanell and Colbert Coldwell Foundation, the Edward N. and Margaret G. Marsh Foundation, Grant 2G12MD007592 from the National Institute on Minority Health and Health Disparities, National Institutes of Health, and the RISE Graduate Scholars Program Grant 2R25GM069621-06 to G.S.M.
Footnotes
Send Orders for Reprints to reprints@benthamscience.ae
DISCLAIMER: The above article has been published in Epub (ahead of print) on the basis of the materials provided by the author. The Editorial Department reserves the right to make minor modifications for further improvement of the manuscript.
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
References
- 1.Copeland GLA, Firth R. Cancer Incidence in North America: Vol I: Combined Cancer Incidence for the United States, Canada, and North America. North American Association of Central Cancer Registries; Springfield, IL: 2015. [Google Scholar]
- 2.Bhatia S. Racial and ethnic differences in survival of children with acute lymphoblastic leukemia. Blood. 2002;100(6):1957–64. doi: 10.1182/blood-2002-02-0395. [DOI] [PubMed] [Google Scholar]
- 3.Stepkowski SM. The Mannich base NC1153 promotes long-term allograft survival and spares the recipient from multiple toxicities. J Immunol. 2005;175(7):4236–46. doi: 10.4049/jimmunol.175.7.4236. [DOI] [PubMed] [Google Scholar]
- 4.Ross JA. Inhibition of JAK3 with a novel, selective and orally active small molecule induces therapeutic response in T-cell malignancies. Leukemia: official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2014;28(4):941–4. doi: 10.1038/leu.2013.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng H. Phosphorylation of human Jak3 at tyrosines 904 and 939 positively regulates its activity. Mol Cell Biol. 2008;28(7):2271–82. doi: 10.1128/MCB.01789-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitra A. Signal transducer and activator of transcription 5b (Stat5b) serine 193 is a novel cytokine-induced phosphoregulatory site that is constitutively activated in primary hematopoietic malignancies. J Biol Chem. 2012;287(20):16596–608. doi: 10.1074/jbc.M111.319756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruiz-Medina BE, Ross JA, Kirken RA. Interleukin-2 Receptor beta Thr-450 Phosphorylation Is a Positive Regulator for Receptor Complex Stability and Activation of Signaling Molecules. J Biol Chem. 2015;290(34):20972–83. doi: 10.1074/jbc.M115.660654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiyoi H. JAK3 mutations occur in acute megakaryoblastic leukemia both in Down syndrome children and non-Down syndrome adults. 3. Vol. 21. Leukemia: official journal of the Leukemia Society of America, Leukemia Research Fund, U.K; 2007. pp. 574–6. [DOI] [PubMed] [Google Scholar]
- 9.Koo GC. Janus kinase 3-activating mutations identified in natural killer/T-cell lymphoma. Cancer Discov. 2012;2(7):591–7. doi: 10.1158/2159-8290.CD-12-0028. [DOI] [PubMed] [Google Scholar]
- 10.Mullighan CG. JAK mutations in high-risk childhood acute lymphoblastic leukemia. Proc Nat Acad Sci United States of Am. 2009;106(23):9414–8. doi: 10.1073/pnas.0811761106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamashita Y. Array-based genomic resequencing of human leukemia. Oncogene. 2010;29(25):3723–31. doi: 10.1038/onc.2010.117. [DOI] [PubMed] [Google Scholar]
- 12.Palacios R, Steinmetz M. Il-3-dependent mouse clones that express B-220 surface antigen, contain Ig genes in germline configuration, and generate B lymphocytes in vivo. Cell. 1985;41(3):727–34. doi: 10.1016/s0092-8674(85)80053-2. [DOI] [PubMed] [Google Scholar]
- 13.Nagy ZS. Uncoupling JAK3 activation induces apoptosis in human lymphoid cancer cells via regulating critical survival pathways. FEBS Lett. 2010;584(8):1515–20. doi: 10.1016/j.febslet.2010.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Degryse S. JAK3 mutants transform hematopoietic cells through JAK1 activation, causing T-cell acute lymphoblastic leukemia in a mouse model. Blood. 2014;124(20):3092–100. doi: 10.1182/blood-2014-04-566687. [DOI] [PubMed] [Google Scholar]
- 15.Sakaguchi H. Exome sequencing identifies secondary mutations of SETBP1 and JAK3 in juvenile myelomonocytic leukemia. Nat Gen. 2013;45(8):937–41. doi: 10.1038/ng.2698. [DOI] [PubMed] [Google Scholar]
- 16.Guo Y. Activated janus kinase 3 expression not by activating mutations identified in natural killer/T-cell lymphoma. Pathol Int. 2014;64(6):263–6. doi: 10.1111/pin.12166. [DOI] [PubMed] [Google Scholar]
- 17.Papp KA. Tofacitinib, an oral Janus kinase inhibitor, for the treatment of chronic plaque psoriasis: results from two, randomised, placebo-controlled, Phase 3 trials. The British J Dermatol. 2015 doi: 10.1111/bjd.14018. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka Y. Recent progress and perspective in JAK inhibitors for rheumatoid arthritis: from bench to bedside. J Biochem. 2015 doi: 10.1093/jb/mvv069. [DOI] [PubMed] [Google Scholar]
- 19.Walters DK. Activating alleles of JAK3 in acute megakaryoblastic leukemia. Cancer Cell. 2006;10(1):65–75. doi: 10.1016/j.ccr.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 20.McNeil DE. SEER update of incidence and trends in pediatric malignancies: acute lymphoblastic leukemia. Med Pediatric Oncol. 2002;39(6):554–7. doi: 10.1002/mpo.10161. [DOI] [PubMed] [Google Scholar]
- 21.Wilkinson JD. Cancer incidence among Hispanic children in the United States. Revista panamericana de salud publica = Pan. Am J Public Health. 2005;18(1):5–13. doi: 10.1590/s1020-49892005000600002. [DOI] [PubMed] [Google Scholar]
- 22.Xie Y. Trends in leukemia incidence and survival in the United States (1973–1998) Cancer. 2003;97(9):2229–35. doi: 10.1002/cncr.11316. [DOI] [PubMed] [Google Scholar]
- 23.Barrington-Trimis JL. Rising rates of acute lymphoblastic leukemia in Hispanic children: trends in incidence from 1992 to 2011. Blood. 2015;125(19):3033–4. doi: 10.1182/blood-2015-03-634006. [DOI] [PMC free article] [PubMed] [Google Scholar]