Abstract
Biomaterial-based delivery of angiogenic growth factors restores perfusion more effectively than bolus delivery methods in rodent models of peripheral vascular disease, but the same success in clinically relevant aged-animal and large-animal studies has not yet been demonstrated. These studies explore, in clinically-relevant models, a therapeutic angiogenesis strategy for the treatment of peripheral vascular disease which overcomes challenges encountered in previous clinical trials. Alginate hydrogels providing sustained release of Vascular Endothelial Growth Factor (VEGF) and Insulin-like Growth Factor-1 (IGF) were injected into ischemic hindlimbs in middle and old age mice, and in young rabbits as a test of the scalability of this local growth factor treatment. Spontaneous perfusion recovery diminished with increasing age, and only the combination of VEGF and IGF delivery from gels significantly rescued perfusion in middle age (13 month) and old age (20 month) mice. In rabbits, delivery of VEGF alone or in combination with IGF from alginate hydrogels, at a dose two orders of magnitude lower than typical doses used in past rabbit studies, enhanced perfusion recovery when given immediately after surgery, or as a treatment for chronic ischemia. Capillary density measurements and angiographic analysis demonstrated the benefit of gel delivery. These data together suggest that alginate hydrogels providing local delivery of low doses of VEGF and IGF constitutes a safe and effective treatment for hindlimb ischemia in clinically relevant animal models, supporting the potential clinical translation of this concept.
Keywords: Angiogenesis, Ischemia, Peripheral Vascular Disease
Introduction
Peripheral Artery Disease (PAD) affects nearly 30 million people, and can lead to limb amputation1,2. Therapeutic angiogenesis offers an alternative treatment strategy compared to traditional medical and surgical interventions, and consists of promoting new blood vessel formation from pre-existing vessels3. Individual angiogenic proteins, such as Vascular Endothelial Growth Factor (VEGF), have been delivered via systemic administration to drive angiogenesis. However, large clinical trials using recombinant protein delivery to date have not demonstrated a significant benefit4,5. Bolus delivery strategies are limited due to the inherent instability of these proteins in vivo (e.g., short half-life following intravenous infusion)6,7 and inadequate targeting to the tissue of interest8. Ischemic tissues require an extended time period of exposure to these factors to initiate and complete the angiogenic process9. The delivery of large protein doses to overcome the rapid degradation is constrained by the possibility of serious side effects at distant sites, and systemic injection of angiogenic proteins has largely been abandoned due to risks associated with the required large doses10. Gene and cell delivery therapies may bypass these limitations, but Phase 3 trials for plasmid and DNA delivery did not provide compelling data of patient benefit, and the efficacy of cell therapies remains to be validated in large, randomized trials10–17. Altogether, the results to date from therapeutic angiogenesis studies suggest that the efficacy of single molecule therapies is likely to be limited, and the time frame and spatial distribution of any therapeutic agent is likely to be critical to its effect8.
To address the limitations confronted in clinical trials to date, we previously developed biomaterials that allow angiogenic factors, such as VEGF, to be localized to the tissue of interest and to have improved pharmacokinetic profiles18,19. In particular, alginate gel-based hydrogels are considered biocompatible, can be modified to alter the degradation profile, and can be processed into formulations allowing minimally invasive delivery20–22. An injectable alginate gel formulation, comprised of a combination of high and low molecular weight alginate polymers, both oxidized to 1%, and mixed with VEGF, effectively restored perfusion in mouse models of hindlimb ischemia by inducing angiogenesis22,23. Additionally, incorporating Insulin-like Growth Factor (IGF) along with VEGF improved recovery of muscle strength following induction of hindlimb ischemia in this mouse model24. We have repeatedly demonstrated that the use of alginate gel materials loaded with growth factors constitutes a safe, reliable, and effective approach for the treatment of hindlimb ischemia in rodent models.
In the present study, we investigated the impact of local delivery of VEGF and IGF from injectable alginate on perfusion recovery in a young rabbit model of PAD, and in old mice, two animal models which are relevant for the clinical translation of this concept. Past studies were performed in mice of approximately 8–10 weeks of age, which are equivalent to human teenagers25. In contrast, the age of various patient cohorts in large therapeutic angiogenesis clinical trials (e.g., VIVA, TRAFFIC, RAVE, NVIFGF) ranged from 58±8 to 72±10 years old 4,26–28. Mapping of human to mouse age indicates that a 70 year old human with arteriopathy would correlate to a 500 day old mouse25. Second, humans are approximately 3000 times bigger than a mouse, raising significant concerns regarding the scaling of the gel-based delivery of angiogenic factors. Testing this system in a rabbit model provides a significant test of the scalability of the concept, as rabbits are approximately100-fold larger than mice, and only 30-fold smaller than humans. As concerns arose in human clinical trials regarding high systemic concentrations of VEGF, the dose of VEGF for this local therapy was scaled to the volume of the treatment region in the hindlimb, and is significantly reduced compared to dose scaling based on whole body mass, which is typically used for systemically administered drugs. In taking steps towards completing important preclinical development for this therapeutic angiogenesis strategy, these studies illustrate the therapeutic efficacy of VEGF and IGF delivered locally from alginate in two clinically relevant models of hindlimb ischemia.
Methods
See online supplement for extended methods
Alginate Gels
Alginate gels were prepared from a mix of high and low molecular weight ultrapure alginates (Pronova MVG #4200106 and VLVG #4200506, respectively)22. Gels were cross-linked with CaSO4 and injected approximately 1 hour after preparation.
In vivo gel degradation
Fluorescently-labeled alginate gel was injected into the hindlimb muscle of mice (n=4). Mice were imaged with an In Vivo Imaging System (Perkin Elmer) at various time points. All mouse protocols followed institutional guidelines set by the Harvard University IACUC.
Mouse Hind-limb Ischemia Model and Blood Vessel Analysis
Hindlimb ischemia was induced in female C57BL6/J mice as previously described19,22,23, and was immediately followed by an intramuscular injection of 50μl of alginate gel containing 3μg of each VEGF and IGF (R&D Systems #293-VE/CF and #291-G1, respectively), VEGF alone, IGF alone, or no growth factors (control condition), given directly into the area where the vessels were ligated (n=4–6). Mice were monitored by Laser Doppler Perfusion Imaging (LDPI), and capillary density was quantified by staining for mouse CD31 (BD Biosciences #557355)22,23 .
Rabbit Hind-limb Ischemia Model and Blood Vessel Analysis
All rabbit procedures followed institutional guidelines set by the University of Michigan IACUC. Ischemia was induced in New Zealand White rabbits (female, 2.5–3kg) by ligation of the lateral circumflex artery, and the common, superficial and deep femoral arteries. Ten 50μl injections of alginate gel, in total containing 20μg of each growth factor (VEGF+IGF, VEGF alone, or VEGF+IGF in saline) were then delivered in two rows of five evenly spaced locations surrounding the ligation site. A PDMS-based mold was used as a guide to ensure even spacing of injections and consistency between animals. Control rabbits were given no treatment. For one condition, rabbits were given no treatment immediately, and then treated with VEGF+IGF containing gels on day 30, via percutaneous injections (n=6). Rabbits were monitored by LDPI. Blood vessels were stained using an antibody to CD31 (Abcam #ab9498) and were quantified (n = 6–9 for all groups).
Angiography
Angiographies were performed on one rabbit per treatment group on day 28. Contrast medium was manually injected via catheter proximal to the branch point of the common iliac arteries. Serial images were then recorded, and the image representing the best arterial filling was chosen for analysis. The length of the hypogastric artery and all whole visible collateral branches were measured.
Statistical Analysis
All data was compared using either Student’s t-test or a One-way ANOVA with Tukey post hoc test; p<0.05 considered statistically significant.
Results
Hindlimb Ischemia Recovery Variation with Age
Spontaneous recovery in young, middle age, and old mice that underwent hindlimb ischemia surgery was monitored over time. Middle age (13 months) and old age (20 months) mice were chosen to bracket average age of patients in therapeutic angiogenesis clinical trials (70 year old human with arteriopathy is equivalent to a 17 month old mouse)25–28. Only 20% of young mice displayed any necrosis (Fig 1a). Middle age mice displayed increased necrosis, while old mice exhibited significant necrosis of the foot. Altered cytokine levels may impair regeneration, and middle age mice displayed lower levels of IGF binding proteins-1 and -9, and matrix metalloproteinase-9 compared to young animals (Fig 1b, Fig S1).
Figure 1.
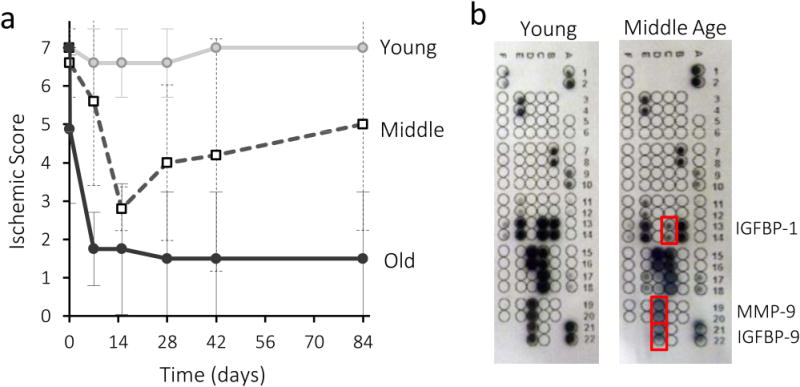
Variation in healing and serum cytokine levels with age. a) Ischemia score from young (8–10 weeks), middle age (13 months), and old mice (20 months) following creation of hindlimb ischemia at time 0. Ischemia scores represent no necrosis (7), one nail discoloration (6), two or more nail discoloration (5), one toe discoloration (4), two or more toe discoloration (3), foot necrosis (2), leg necrosis (1) and auto-amputation (0). Values represent Mean±SD (young mice, n=5; middle age and old mice, n=4–5.) b) Mouse angiogenic array of factors present in peripheral blood. Red boxes indicate reduced cytokines levels in middle aged compared to young mice.
Alginate Gels
Alginate polymers were made into an injectable formulation by combining 1% oxidized, low and high molecular weight polymers in a 75:25 ratio (Fig 2a). The viscosity of the solution was sufficiently low to allow the gel to be injected after cross-linking with CaSO4 (Fig 2b). Oxidation of the polymer backbone allows hydrolytic degradation of the polymer backbone29. The high molecular weight polymer component degraded from 233kDa to 117kDa, and the low molecular weight polymer degraded from 31kDa to 25kDa over approximately one week in physiological saline solution at 37°C. In vivo, alginate gels were lost from the injection site in mice over a few months (Fig 2c). VEGF and IGF release kinetics from these gels have been previously established22,24.
Figure 2.
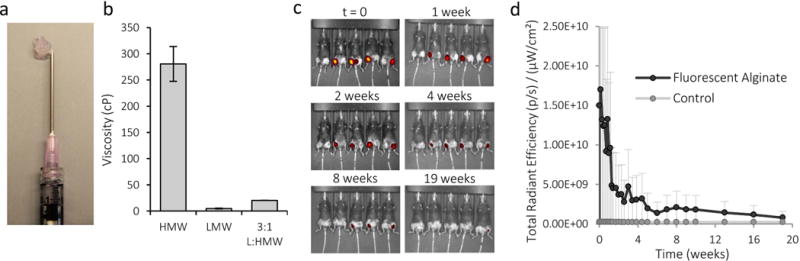
Characterization of injectable alginate. a) Photo of alginate gel after injection through a syringe. b) Viscosity of high molecular weight (HMW), low molecular weight (LMW) and the combination of high and low molecular alginates used to make the injectable formulation. c) IVIS photos of intramuscularly-injected, fluorescently-labeled alginate gels in hind-limbs at various time points. d) Quantification of fluorescence emitted from intramuscularly-injected alginate at various times. Control is unlabeled, non-fluorescent alginate. Values are Mean±SD, n=4.
Effect of VEGF/IGF Treatment in Aged Mice
As angiogenic and overall regenerative capacity decreases with age, supplementation with exogenously delivered factors may be increasingly important to improve healing outcomes. Alginate gels loaded with VEGF and IGF were tested for their ability to drive neovascularization and muscle tissue regeneration following hindlimb ischemia surgery in old animals, as this growth factor combination was previously found effective in young animals24. IGF supplementation may be especially important in aged mice, because the decreased levels of IGF binding proteins leaves IGF unprotected and results in a shortened half-life30. Old animals treated with alginate gels delivering both VEGF and IGF displayed a 2-fold increase in the regional blood flow compared to blank gels (Fig 3a), while no statistical differences were found for animals which received either VEGF or IGF alone. These trends were replicated in ischemic score measurements (Fig S2a). However, all three treatment groups showed increased capillary density compared to mice which received blank gels (Fig 3b). Further, muscle strength measurements were highest for mice treated with the combination of VEGF and IGF (Fig S2b). Increased doses of VEGF (2× and 4×) were also tested to determine if the poor outcome in old mice was simply a result of decreased sensitivity to VEGF, but higher doses did not increase blood flow (Fig S2c). Previous studies have repeatedly demonstrated no impact of the bolus delivery of these factors, at these doses, on perfusion recovery in this model22,24,31, and so were not repeated here to minimize animal use.
Figure 3.
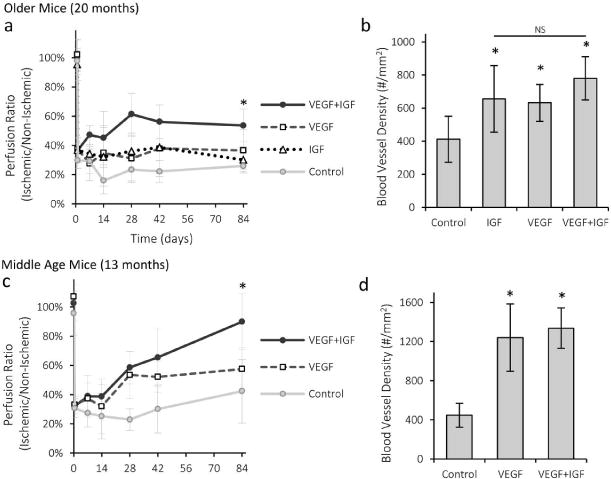
Recovery from ischemia with VEGF and IGF treatment for middle and old age mice. a–b) Quantification of regional perfusion (a) and capillary density (b) for old mice treated with VEGF+IGF gel, VEGF-only gel, IGF-only gel and control (blank) alginate gel. c–d) Quantification of regional perfusion (c) and capillary density (d) for middle age mice treated with VEGF+IGF gel, VEGF-only gel, and control (blank) alginate gel. Values are Mean±SD, n=4–6. *p < 0.05 vs control condition, N.S. indicate no statistical significant differences between conditions.
Middle age animals were next subjected to hindlimb ischemia surgery and were treated with either blank gel, gel loaded with VEGF or gel loaded with VEGF and IGF, as gels with IGF alone do not improve perfusion recovery in young24 or old animals. Mice treated with the gels containing VEGF alone and VEGF+IGF displayed a prominent increase in perfusion compared to blank gels (Fig 3c). VEGF+IGF treatment was close to pre-surgery levels (~90%), and led to greater perfusion than VEGF alone. The density of blood vessels was increased with both treatment groups (Fig 3d). Importantly, as the stability of vessels formed with angiogenic factor treatment has been raised as an issue32,33, mice were analyzed for 90 days post-treatment in these studies and a stable reperfusion was noted. While past studies have typically examined perfusion for time periods ranging from 7 to 42 days, the extended monitoring in the present studies demonstrates a stable reperfusion has been established with these factors and this mode of delivery.
Hindlimb Ischemia Treatment in Young Rabbits
Standardized processes for the alginate polymer modification and gel material fabrication were developed for the rabbit studies, and the gels and growth factors were packaged into a standardized kit in order to maintain consistency between all studies (Fig S3). Experimental conditions included control, bolus VEGF+IGF, alginate loaded with VEGF and alginate loaded with VEGF and IGF. In contrast with mice studies and in an effort to minimize the number of animals, alginate loaded IGF only gels were not used for the rabbit experiments. This condition was shown to be ineffective to restore blood profusion in murine hind-limb ischemia24. The bolus injection of VEGF and IGF was included to validate if the gel-based delivery was superior to bolus delivery in this species (not shown before). In addition, the chosen dose of VEGF and IGF (20μg each) was much lower than previous VEGF doses used for the treatment of ischemia in rabbits, mini-swine, and in human clinical trials (Table 1; details in Table S1). Gel injection into ischemic limbs did not result in any detectable systemic exposure (Fig 4a), consistent with past rodent studies 22. This contrasts to delivery approaches in past clinical studies, where large quantities of potent factors were present in the systemic circulation4,7.
Table 1.
Average and range of dosing regimens used in previous human clinical trials and large animal studies.
| Total Dose (μg) | Dose by Weight (μg/kg) | |||
|---|---|---|---|---|
| Average1 | Range | Average1 | Range | |
| Human | 1595 | (8–2960) | 18.4 | (0.1–37) |
| Mini swine | 900 | (600–1200) | 22.5 | (15–30) |
| Rabbit | 3125 | (500–10,000) | 1250 | (800–4000) |
Averages are weighted based on the number of animals or humans which received each dose of VEGF.
Figure 4.
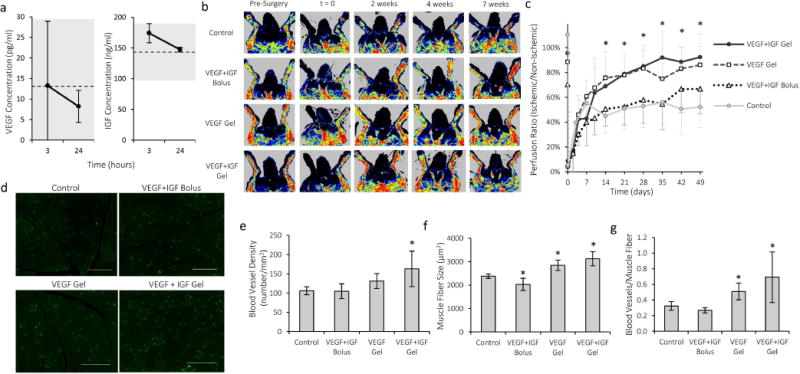
Treatment with VEGF-containing alginate gels improves regional perfusion and angiogenesis in the ischemic rabbit hindlimb. a) Serum concentration of VEGF and IGF after intramuscular injection of gels into ischemic hind-limbs of rabbits. The dotted lines represent the average serum growth factor concentration measured prior to injection, and the grey regions mark one standard deviation for the baseline levels. b) Representative LDPI images of rabbit hindlimbs treated with VEGF+IGF alginate gels, VEGF gels, a bolus of VEGF and IGF (without alginate gel), or untreated control animals. c) Quantification of perfusion recovery after hindlimb ischemia surgery (n=6–9). *p < 0.05 compared to control condition for both VEGF and VEGF+IGF. d) Fluorescent images of capillaries stained for CD31 (green) in rabbit hind-limb muscle tissue. Scale bars 200μm. e) Capillary densities in rabbit hindlimb muscle seven weeks after surgery. f) Average muscle fiber size in treated region of rabbit hindlimb. g) Number of capillaries per muscle fiber in rabbit hindlimb. Values are Mean±SD. *p < 0.05 compared to control condition.
LDPI imaging (Fig 4b) revealed that perfusion in the ischemic limb was reduced to approximately 5% immediately after surgery (Fig 4c). Without treatment, perfusion recovered tô50% within ~1 week, and then remained stable. However, immediate treatment with alginate gels (total dose of 20μg of VEGF or VEGF and IGF) led to a significant improvement in perfusion, which reached 80–90% of the level in the healthy, contralateral limb and was maintained throughout the seven week monitoring period. Quantification of capillary density at seven weeks revealed a slight increase with VEGF and a statistically significant increase with VEGF and IGF treatment, compared to the control condition (Fig 4d,e; Fig S4). Treatment with VEGF and VEGF with IGF also positively impacted muscle fiber size (Fig 4f) and blood vessels per muscle fiber (Fig 4g), with the combination demonstrating the greatest effect. Bolus delivery of VEGF and IGF was used as a control, and no impact on perfusion or capillary density was found (Fig. 4b–g).
Delayed Treatment
As a model of chronic ischemia, one rabbit cohort was alternatively treated on day 30, after perfusion stabilized at approximately 50%. Percutaneous injections were used to deliver gels into the muscle tissue surrounding the vessel ligation site, with the same factor doses as used for acute treatment (Fig 5a). The combination of VEGF and IGF increased perfusion significantly within 14 days after the injection, which was the same amount of time required for significant differences to be observed with acute treatment (Fig 5b). Perfusion subsequently increased to the same level as rabbits which were immediately treated with VEGF or VEGF and IGF. Rabbits with delayed treatment also displayed a higher capillary density (126 ± 29 blood vessels/mm2) than control rabbits.
Figure 5.
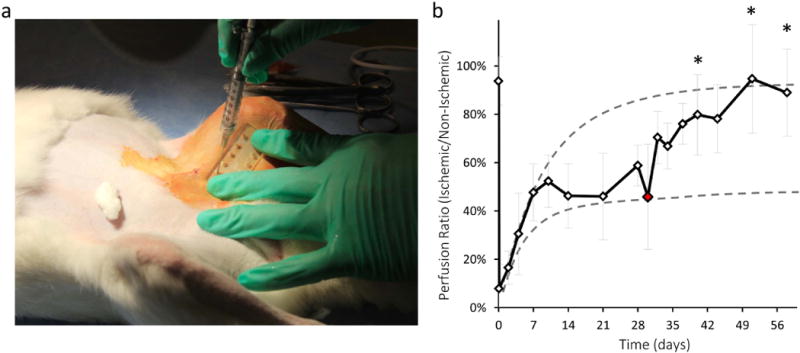
Treatment of chronic ischemia with VEGF+IGF delivery from alginate gels. a) Percutaneous gel injection procedure. b) Quantification of perfusion recovery from LDPI images following VEGF+IGF gel delivery on day 30 following surgery. Red data point indicates time of gel injection. Dotted lines represent acutely treated (upper line) and untreated control (lower line) perfusion recovery averages. Values are Mean±SD (n=6–9) *p < 0.05 compared to control measurements at the same time points.
Angiographic Observations
Angiographic imaging was performed on one animal in each of the gel treatment groups and the control group. Since no difference in perfusion was observed with bolus delivery of VEGF and IGF, that condition was not analyzed. Extensive collateral formation was found in the ischemic limb for all conditions (Fig 6a,b). Delivery of VEGF and IGF from alginate gels resulted in more extensive collateral vessels, which appeared to reconstitute the superficial femoral artery distal to the ligation site more briskly and fully (Fig 6a). The hypogastric artery appeared to be the major collateral donor vessel. Measurement of the total length of the hypogastric artery and its branches, normalized to vessel length in the contralateral limb, and the total number of collateral branches in the ischemic limb, revealed that treatment with VEGF and IGF increased the formation of angiographically-visible collateral vessels (Fig 6b, Fig S5).
Figure 6.
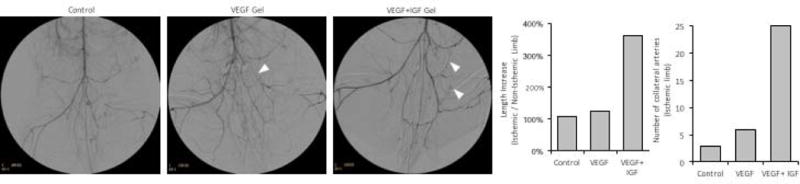
Angiography images for acutely treated rabbits. a) Digitally subtracted angiographic images of rabbits treated with a VEGF+IGF gel or a VEGF gel, and the untreated control on day 28 following surgery. Arrowheads indicate collateral arteries. b) Quantification of the length of the hypogastric artery and all visible branches, relative to the contralateral limb (n=1, see Fig S5 for example quantification). c) Number of visible collateral arteries branching from the hypogastric artery in the ischemic limb (n=1).
Discussion
These experiments demonstrate that treatment with low doses of VEGF and IGF delivered locally from injectable alginate hydrogels results in significant and stable increases in tissue perfusion in a young rabbit model of hindlimb ischemia and in aged mice. Delivery of IGF in combination with VEGF can offset the diminished response to VEGF with age. In young rabbits, immediate treatment with alginate gels loaded with VEGF and the combination of VEGF and IGF similarly improved perfusion and capillary density. Alginate gel-based delivery also effectively treated chronic ischemia, and appears to provide a safe and effective means for local angiogenic factor delivery. Old mice and young rabbits had some differences in response to the delivery of growth factors, and the effects likely relate to both the species and age differences between old mice and young rabbits. However, the robust response to alginate gel delivery of VEGF in young mice in previous studies22 suggest the differences mainly result from the age difference in this study between the two models. It is important to note, though, that delivery of VEGF and IGF from alginate gels resulted in significantly improved perfusion recovery in both of these relevant animal models. Alginate gel-based growth factor delivery led to both angiogenesis and arteriogenesis in muscles surrounding the injection site, and both likely contributed to the beneficial effects on perfusion.
Recovery from ischemia is a complex, multistep process, and the ability of individuals to recover is weakened with age34. While most angiogenesis studies using rodent models are done on young mice23,24, aged mice do not naturally recover as well as young mice, similar to the human condition. In young mice, treatment with VEGF alone effectively restores perfusion22–24, but in old mice even large doses of VEGF did not promote significant healing. This result may partially explain the poor results from human clinical trials in which VEGF was infused as a standalone therapy. The combination of VEGF and IGF gel delivery, in contrast, was found to improve perfusion, capillary density, muscle strength, and prevented significant necrosis. IGF plays a key role in embryonic and postnatal growth and metabolism35, muscle regeneration36, and in regulation of angiogenesis and vascular remodeling37,38. The aging process is associated with a significant reduction in circulating IGF levels39 possibly due to lower IGF binding protein levels30, and corresponding decreases in capillary density are also associated with age-related decreases in IGF40. Our results and past work together suggest that delivery of IGF in combination with VEGF can help to overcome healing detriments in aged individuals and restore perfusion to ischemic limbs.
A rabbit hindlimb ischemia model was also used as a rigorous test of the scalability of our alginate-based, local growth factor delivery system. Delivery of VEGF and IGF or VEGF alone from gels resulted in improved perfusion recovery, while a direct bolus injection of VEGF and IGF did not augment perfusion. The enhanced perfusion was reflected in histologic measurements of capillary density, and digitally subtracted angiograms revealed that treatment with the combination of VEGF and IGF, and to a lesser extent, VEGF alone, also led to larger and more numerous angiographically-visible collateral arteries. Reconstitution of an occluded artery by collateral arteries is thought to be clinically relevant for humans with severe PAD, further supporting the use of VEGF and IGF together for future clinical applications. While in these young rabbits VEGF performed nearly as well as the combination of VEGF and IGF, it is likely that aged rabbits would exhibit a similar diminished healing ability as was shown for mice; those studies are beyond the current scope of this report due to practical time limitations. In translating the aged mice results to aged rabbits, and later to humans, the combination of VEGF and IGF would be expected to augment perfusion and overall regeneration more effectively than using VEGF as a single molecule therapy.
Local delivery of VEGF and IGF from gels also restored perfusion to rabbit limbs that displayed stable, chronic ischemia. By day 30 following permanent ligation of the femoral artery tree, acute inflammation and immune cell infiltration were expected to have subsided41,42. While untreated animals displayed a permanent reduction in perfusion which persisted until the end of the experiment, rabbits that were treated on day 30 recovered to the same level as rabbits treated acutely. To our knowledge, this is the first demonstration of a significantly delayed therapeutic intervention following ischemic injury. This is particularly relevant to the human condition, as slow narrowing of vessels due to atherosclerotic plaque formation characterizes ischemic cardiovascular and peripheral vascular diseases, and the onset of PAD often goes unnoticed until the disease has progressed to severe stages, when patients experience classic claudication and face threats of limb amputation due to critical limb ischemia43.
Finally, the use of alginate hydrogels to deliver VEGF and IGF is more effective, and likely safer, than systemic infusions or bolus injections of growth factors. In these studies, growth factor dose was not scaled from previous successful mouse studies based on weight, but instead by the volume of the treatment region. Rabbits are 100× the size of a mouse, but a much lower dose of VEGF and IGF (~6.5× the mouse dose) effectively restored perfusion. Previous rabbit studies that employed intracoronary, intravascular or intramuscular injections to deliver a bolus of VEGF were able to augment collateral growth and capillary density, but required doses that were 25 to 500× larger than the dose used here (Table 1; details in Table S1). Scaling our rabbit dose to humans based on weight suggests that delivering ~20% of the maximum tolerated VEGF dose (determined from systemic delivery in previous human clinical trials44) should be effective when this alginate gel delivery system is utilized. These studies also demonstrate that containing the growth factors within the gel and delivering the gel directly to the target tissue leads to minimal impact in the systemic circulation, which is expected to contribute to the safety of this system. In summary, these data together suggest that local delivery of VEGF and IGF from injectable alginate hydrogels would be a viable treatment option for PAD that can address the specific limitations and overcome significant challenges faced by alternative therapeutic angiogenesis treatment strategies.
Supplementary Material
Acknowledgments
Funding Sources
This research was supported by the NIH (RO1 HL069957) and the Wyss Institute for Biologically Inspired Engineering.
Footnotes
Disclosures
None.
References
- 1.About Peripheral Artery Disease. American Heart Association. 2013 at < http://www.heart.org/HEARTORG/Conditions/More/PeripheralArteryDisease/About-Peripheral-Artery-Disease-PAD_UCM_301301_Article.jsp>.
- 2.Norgren L, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) Eur J Vasc Endovasc Surg. 2007;33:S1–S75. doi: 10.1016/j.ejvs.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 3.Isner JM, Asahara T. Angiogenesis and vasculogenesis as therapeutic strategies for postnatal neovascularization. J Clin Invest. 1999;103:1231–6. doi: 10.1172/JCI6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henry TD, et al. The VIVA Trial: Vascular Endothelial Growth Factor in Ischemia for Vascular Angiogenesis. Circulation. 2003;107:1359–1365. doi: 10.1161/01.cir.0000061911.47710.8a. [DOI] [PubMed] [Google Scholar]
- 5.Tillman B, Hardin-young J, Shannon W, Russell AJ, Parenteau NL. Meeting the Need for Regenerative Therapies : Vascular Medicine Using Detailed Incidence Data. Tissue Eng Part B. 2013;19:99–115. doi: 10.1089/ten.TEB.2011.0678. [DOI] [PubMed] [Google Scholar]
- 6.Lazarous DF, et al. Comparative effects of basic fibroblast growth factor and vascular endothelial growth factor on coronary collateral development and the arterial response to injury. Circulation. 1996;94:1074–1082. doi: 10.1161/01.cir.94.5.1074. [DOI] [PubMed] [Google Scholar]
- 7.Eppler SM, et al. A target-mediated model to describe the pharmacokinetics and hemodynamic effects of recombinant human vascular endothelial growth factor in humans. Clin Pharmacol Ther. 2002;72:20–32. doi: 10.1067/mcp.2002.126179. [DOI] [PubMed] [Google Scholar]
- 8.Zachary I, Morgan RD. Therapeutic angiogenesis for cardiovascular disease: biological context, challenges, prospects. Heart. 2011;97:181–9. doi: 10.1136/hrt.2009.180414. [DOI] [PubMed] [Google Scholar]
- 9.Yancopoulos GD, et al. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–8. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 10.Silvestre JS, Smadja DM, Lévy BI. Postischemic revascularization: from cellular and molecular mechanisms to clinical applications. Physiol Rev. 2013;93:1743–802. doi: 10.1152/physrev.00006.2013. [DOI] [PubMed] [Google Scholar]
- 11.Nikol S, et al. Therapeutic angiogenesis with intramuscular NV1FGF improves amputation-free survival in patients with critical limb ischemia. Mol Ther. 2008;16:972–8. doi: 10.1038/mt.2008.33. [DOI] [PubMed] [Google Scholar]
- 12.Powell RJ, et al. Results of a double-blind, placebo-controlled study to assess the safety of intramuscular injection of hepatocyte growth factor plasmid to improve limb perfusion in patients with critical limb ischemia. Circulation. 2008;118:58–65. doi: 10.1161/CIRCULATIONAHA.107.727347. [DOI] [PubMed] [Google Scholar]
- 13.Favaloro L, et al. High-dose plasmid-mediated VEGF gene transfer is safe in patients with severe ischemic heart disease (Genesis-I). A phase I, open-label, two-year follow-up trial. Catheter Cardiovasc Interv. 2013;82:899–906. doi: 10.1002/ccd.24555. [DOI] [PubMed] [Google Scholar]
- 14.Creager MA, et al. Effect of hypoxia-inducible factor-1alpha gene therapy on walking performance in patients with intermittent claudication. Circulation. 2011;124:1765–73. doi: 10.1161/CIRCULATIONAHA.110.009407. [DOI] [PubMed] [Google Scholar]
- 15.Belch J, et al. Effect of fibroblast growth factor NV1FGF on amputation and death: a randomised placebo-controlled trial of gene therapy in critical limb ischaemia. Lancet. 2011;377:1929–37. doi: 10.1016/S0140-6736(11)60394-2. [DOI] [PubMed] [Google Scholar]
- 16.Shimamura M, Nakagami H, Koriyama H, Morishita R. Gene therapy and cell-based therapies for therapeutic angiogenesis in peripheral artery disease. Biomed Res Int. 2013;2013:186215. doi: 10.1155/2013/186215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Botham CM, Bennett WL, Cooke JP. Clinical trials of adult stem cell therapy for peripheral artery disease. Methodist Debakey Cardiovasc J. 2013;9:201–5. doi: 10.14797/mdcj-9-4-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 19.Chen RR, et al. Integrated approach to designing growth factor delivery systems. FASEB J. 2007;21:3896–3903. doi: 10.1096/fj.06-7873com. [DOI] [PubMed] [Google Scholar]
- 20.Augst AD, Kong HJ, Mooney DJ. Alginate hydrogels as biomaterials. Macromol Biosci. 2006;6:623–633. doi: 10.1002/mabi.200600069. [DOI] [PubMed] [Google Scholar]
- 21.Boontheekul T, Kong HJ, Mooney DJ. Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials. 2005;26:2455–2465. doi: 10.1016/j.biomaterials.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 22.Silva EA, Mooney DJ. Spatiotemporal control of vascular endothelial growth factor delivery from injectable hydrogels enhances angiogenesis. J Thromb Haemost. 2007;5:590–598. doi: 10.1111/j.1538-7836.2007.02386.x. [DOI] [PubMed] [Google Scholar]
- 23.Silva EA, Mooney DJ. Effects of VEGF temporal and spatial presentation on angiogenesis. Biomaterials. 2010;31:1235–41. doi: 10.1016/j.biomaterials.2009.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borselli C, et al. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proc Natl Acad Sci U S A. 2010;107:3287–3292. doi: 10.1073/pnas.0903875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geifman N, Rubin E. The mouse age phenome knowledgebase and disease-specific inter-species age mapping. PLoS One. 2013;8:e81114. doi: 10.1371/journal.pone.0081114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rajagopalan S, et al. Regional angiogenesis with vascular endothelial growth factor in peripheral arterial disease: a phase II randomized, double-blind, controlled study of adenoviral delivery of vascular endothelial growth factor 121 in patients with disabling intermittent cl. Circulation. 2003;108:1933–8. doi: 10.1161/01.CIR.0000093398.16124.29. [DOI] [PubMed] [Google Scholar]
- 27.Lederman RJ, et al. Therapeutic angiogenesis with recombinant fibroblast growth factor-2 for intermittent claudication (the TRAFFIC study): a randomised trial. Lancet. 2002;359:2053–8. doi: 10.1016/s0140-6736(02)08937-7. [DOI] [PubMed] [Google Scholar]
- 28.Niebuhr A, et al. Long-term safety of intramuscular gene transfer of non-viral FGF1 for peripheral artery disease. Gene Ther. 2012;19:264–70. doi: 10.1038/gt.2011.85. [DOI] [PubMed] [Google Scholar]
- 29.Bouhadir KH, et al. Degradation of partially oxidized alginate and its potential application for tissue engineering. Biotechnol Prog. 2001;17:945–950. doi: 10.1021/bp010070p. [DOI] [PubMed] [Google Scholar]
- 30.Stewart CE, Bates PC, Calder TA, Woodall SM, Pell JM. Potentiation of insulin-like growth factor-I (IGF-I) activity by an antibody: supportive evidence for enhancement of IGF-I bioavailability in vivo by IGF binding proteins. Endocrinology. 1993;133:1462–5. [Google Scholar]
- 31.Cao L, Arany PR, Wang YS, Mooney DJ. Promoting angiogenesis via manipulation of VEGF responsiveness with notch signaling. Biomaterials. 2009;30:4085–4093. doi: 10.1016/j.biomaterials.2009.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–93. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 33.Dellian M, Witwer BP, Salehi HA, Yuan F, Jain RK. Quantitation and Physiological Characterization of Angiogenic Vessels in Mice: Effect of Basic Fibroblast Growth Factor, Vascular Endothelial Growth Factor/Vascular Permeability Factor, and Host Microenvironment. Am J Pathol. 1996;149:59–71. [PMC free article] [PubMed] [Google Scholar]
- 34.Rivard A, et al. Age-Dependent Impairment of Angiogenesis. Circulation. 1999;99:111–120. doi: 10.1161/01.cir.99.1.111. [DOI] [PubMed] [Google Scholar]
- 35.Gow DJ, Sester DP, Hume DA. CSF-1, IGF-1, and the control of postnatal growth and development. J Leukoc Biol. 2010;88:475–81. doi: 10.1189/jlb.0310158. [DOI] [PubMed] [Google Scholar]
- 36.Pillon NJ, Bilan PJ, Fink LN, Klip A. Cross-talk between skeletal muscle and immune cells: muscle-derived mediators and metabolic implications. Am J Physiol Endocrinol Metab. 2013;304:E453–65. doi: 10.1152/ajpendo.00553.2012. [DOI] [PubMed] [Google Scholar]
- 37.Dunn SE. Insulin-like growth factor I stimulates angiogenesis and the production of vascular endothelial growth factor. Growth Horm IGF Res. 2000;10(Suppl A):S41–2. doi: 10.1016/s1096-6374(00)90020-0. [DOI] [PubMed] [Google Scholar]
- 38.Lopez-Lopez C, LeRoith D, Torres-Aleman I. Insulin-like growth factor I is required for vessel remodeling in the adult brain. Proc Natl Acad Sci U S A. 2004;101:9833–8. doi: 10.1073/pnas.0400337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Welle S. Gene transcript profiling in aging research. Experiemental Gerontol. 2002;37:583–590. doi: 10.1016/s0531-5565(01)00231-5. [DOI] [PubMed] [Google Scholar]
- 40.Sonntag WE, Lynch CD, Cooney PT, Hutchins PM. Decreases in cerebral microvasculature with age are associated with the decline in growth hormone and insulin-like growth factor 1. Endocrinology. 1997;138:3515–20. doi: 10.1210/endo.138.8.5330. [DOI] [PubMed] [Google Scholar]
- 41.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364:656–65. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silvestre JS, Mallat Z, Tedgui A, Lévy BI. Post-ischaemic neovascularization and inflammation. Cardiovasc Res. 2008;78:242–9. doi: 10.1093/cvr/cvn027. [DOI] [PubMed] [Google Scholar]
- 43.Lau JF, Weinberg MD, Olin JW. Peripheral artery disease. Part 1: clinical evaluation and noninvasive diagnosis. Nat Rev Cardiol. 2011;8:405–18. doi: 10.1038/nrcardio.2011.66. [DOI] [PubMed] [Google Scholar]
- 44.Henry TD, et al. Intracoronary administration of recombinant human vascular endothelial growth factor to patients with coronary artery disease. Am Heart J. 2001;142:872–80. doi: 10.1067/mhj.2001.118471. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


