Abstract
The Hippo pathway is a signalling cascade conserved from Drosophila melanogaster to mammals. The mammalian core kinase components comprise MST1 and MST2, SAV1, LATS1 and LATS2 and MOB1A and MOB1B. The transcriptional co-activators YAP1 and TAZ are the downstream effectors of the Hippo pathway and regulate target gene expression. Hippo signalling has crucial roles in the control of organ size, tissue homeostasis and regeneration, and dysregulation of the Hippo pathway can lead to uncontrolled cell growth and malignant transformation. Mammalian intestine consists of a stem cell compartment as well as differentiated cells, and its ability to regenerate rapidly after injury makes it an excellent model system to study tissue homeostasis, regeneration and tumorigenesis. Several studies have established the important role of the Hippo pathway in these processes. In addition, crosstalk between Hippo and other signalling pathways provides tight, yet versatile, regulation of tissue homeostasis. In this Review, we summarize studies on the role of the Hippo pathway in the intestine on these physiological processes and the underlying mechanisms responsible, and discuss future research directions and potential therapeutic strategies targeting Hippo signalling in intestinal disease.
The mammalian gastrointestinal tract is part of the digestive system responsible for food digestion and absorption. As a result of constant exposure to pathogens and xenobiotics, intestinal epithelial cells (IECs) have a short half-life, and have therefore evolved the ability to rapidly regenerate after damage. Intestinal regeneration is precisely controlled by a myriad of cellular signalling pathways and the crosstalk between them. The Hippo pathway plays a key part in cell-fate decision, tissue homeostasis and control of organ size by regulating cell proliferation, apoptosis and differentiation1,2, and dysregulation of this pathway has been associated with many human malignancies3,4. In the past few years, studies have revealed the importance of the Hippo pathway in gastrointestinal tract physiology5. In this Review, we will focus on the role of the Hippo pathway in tissue homeostasis, regeneration and tumorigenesis in the small intestine and the colon. In addition, we will also briefly discuss the functional roles of the Hippo pathway in the liver.
Hippo pathway overview
The Hippo pathway in Drosophila melanogaster
The Hippo pathway is mostly conserved between Drosophila melanogaster and mammals6,7; it was initially identified in Drosophila during screens for genes that negatively regulate tissue growth. Loss of the serine/threonine-protein kinase warts (Wts) resulted in organ overgrowth in Drosophila wing and eye8,9. Similar genetic screens later identified that mutation of the Hippo kinase (Hpo) resulted in a similar tissue overgrowth phenotype10–14. In addition, the adaptor proteins salvador (Sav) and MOB kinase activator-like 1 (Mats) were found to be partners of Hpo and Wts, respectively, with similar mutation phenotypes15–17.
Five proteins comprise the core of the Hippo pathway: the Hpo kinase and its binding partner Sav; the Wts kinase and its binding partner Mats; and the transcriptional co-activator yorkie (Yki). Yki mediates the main functional output of the Hippo pathway and binds to the transcription factor Scalloped (Sd), which induces the expression of genes that promote cell proliferation, for example cyclin E (CycE) and the bantam microRNA (miRNA), or genes that prevent apoptosis, for example death-associated inhibitor of apoptosis (Diap1)18,19. The Hpo–Sav complex phosphorylates and activates the Wts–Mats complex10–12,14,17,20, which in turn phosphorylates and inactivates Yki by sequestering Yki in the cytoplasm, thereby negatively regulating Yki–Sd interaction and Sd-mediated gene expression21. Three studies published between 2014 and 2015 show that, in addition to Hpo, two kinases known as Happyhour (Hppy) and Misshapen (Msn) also activate the Wts–Mats complex22–24. Overexpression of Yki or deletion of Hpo, Sav, Wts or Mats leads to overgrowth phenotypes in Drosophila eye, wing, midgut and other tissues as a result of increased cell proliferation and decreased apoptosis10–12,14,25.
The Hippo pathway in mammals
The Hippo pathway in mammals consists of the core components mammalian STE20-like protein kinase 1 and 2 (MST1 and MST2, homologues of Hpo, also known as serine/threonine-protein kinase 4 and 3, respectively), salvador homologue 1 (SAV1, a homologue of Sav), large tumour suppressor 1 and 2 (LATS1 and LATS2, homologues of Wts), MOB kinase activator 1A and 1B (MOB1A and MOB1B, homologues of Mats), and the two Yki homologues Yes-associated protein 1 (YAP1, also known as transcriptional co-activator YAP1) and transcriptional co-activator with PDZ-binding motif (TAZ, also known as WW domain-containing transcription regulator protein 1, WWTR1)26. YAP1 and TAZ have overlapping functions when they are expressed in the same cells27, but also have divergent functional roles in different organs as YAP1 and TAZ knockout mice show different developmental deficiencies28. MST1 and MST2 form homodimers via C-terminal Sav/RassF/Hpo (SARAH) domains, resulting in activation loop autophosphorylation29–32. MST1 and MST2 can phosphorylate LATS1 and LATS2 at the hydrophobic motif to induce their activation. The scaffold proteins SAV1 and MOB1 are also phosphorylated and activated by MST1 and MST2; activated SAV1 and MOB1 promote LATS1 and LATS2 activity33–35. The Hppy homologues MAP4K1, MAP4K2, MAP4K3 and MAP4K5, and the Msn homologues MAP4K4, MAP4K6 and MAP4K7 act in parallel with MST1 and MST2 to phosphorylate the hydrophobic motifs of LATS1 and LATS2, thereby leading to their activation22,24. Activated LATS1 and LATS2 in turn phosphorylate YAP1 and TAZ, leading to the retention of YAP1 and TAZ in the cytoplasm by 14-3-3 family proteins. Cytoplasmic YAP1 and TAZ can undergo further phosphorylation and ubiquitylation-dependent degradation36,37, preventing their interaction with the mammalian homologues of Sd, transcriptional enhancer factor TEF-1 (also known TEA domain family member 1, TEAD1), TEAD2, TEAD3 and TEAD4. Because the YAP1–TEAD complex activates the transcription of genes involved in cell growth and survival, including CTGF, MYC and BIRC5 (also known as survivin), the degradation of YAP1 as a result of phosphorylation by LATS1 or LATS2 reduces expression of these genes38,39. Loss-of-function mutation of the Hippo kinase cascade components in mammals, such as inactivation of Merlin (also known as NF2) and deletion of LATS2 in meso-thelioma40, also leads to overgrowth phenotypes and consequently carcinogenesis in multiple tissues3,41.
The regulatory signals of the Hippo pathway
The mammalian Hippo pathway responds to a variety of extracellular signals, including cell polarity, contact inhibition, stress, mechanotransduction, cell attachment, hormones and growth factors7. More than 20 upstream regulators of the Hippo pathway42 have been discovered, many of which are involved in intestinal homeostasis and carcinogenesis (FIG. 1).
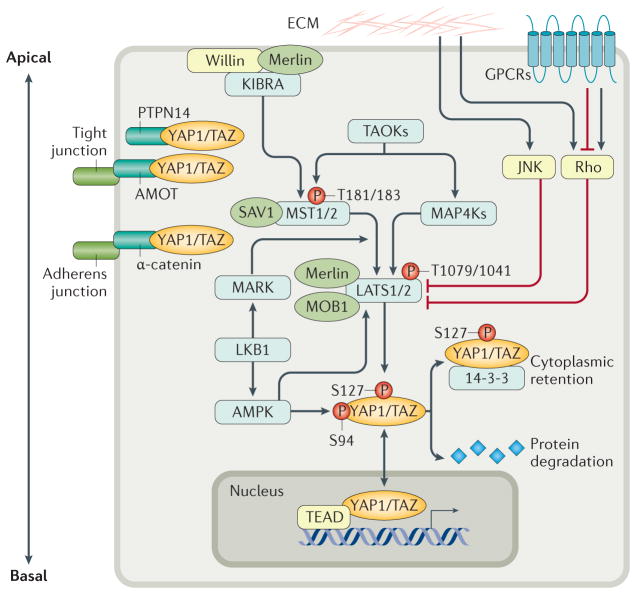
Figure 1. The Hippo pathway integrates signals to regulate the activity of YAP1 and TAZ.
The core of the Hippo pathway is the kinase cascade of MST1 and MST2 and LATS1 and LATS2. MST proteins, activated by upstream signals, phosphorylate and activate LATS proteins directly, and also activate the scaffold proteins MOB1 and SAV1. MAP4Ks also phosphorylate and activate LATS proteins. Activated LATS proteins phosphorylate YAP1 and TAZ at multiple sites, triggering 14-3-3-mediated cytoplasmic retention and protein degradation. YAP1 and TAZ function as transcriptional co-activators of TEAD proteins to induce expression of genes involved in cell proliferation and apoptosis. Merlin, Willin and KIBRA form a complex to recruit MSTs and LATS to the apical plasma membrane, activating LATS proteins. AMOT and PTPN14 sequesters YAP1 and TAZ at tight junctions, and α-catenin sequesters YAP1 and TAZ at adherens junctions, to prevent their nuclear translocation. LKB1, which has important roles in both cell polarity and cellular stress response, regulates the activity of YAP1 and TAZ through activation of its substrates MARK and AMPK, both of which activate LATS. Activated AMPK also phosphorylates YAP1 and disrupts the YAP1 TEAD complex. The Hippo pathway is further regulated by ECM stiffness and mechanotransduction through Rho GTPases or JNK. GPCRs mediate many extracellular signals to either activate or inhibit LATS via Rho GTPase. AMOT, Angiomotin; AMPK, AMP-activated protein kinase; ECM, extracellular matrix; GPCRs, G protein-coupled receptors; JNK, c-Jun N-terminal kinase; LATS, large tumour suppressor; LKB1, liver kinase B1; MAP4K, mitogen-activated protein kinase kinase kinase kinase; MARK, microtubule affinity-regulating kinase; MOB1, Mob1 homologue; MST, mammalian ste20-like kinase; P, phosphorylation of stated amino acid; PTPN14, protein tyrosine phosphatase non-receptor type 14; SAV1, salvador family WW domain-containing protein 1; TAZ, transcriptional co-activator with PDZ-binding motif; TEAD, TEA domain family member; TAOK, thousand and one amino acid protein kinase; YAP1, Yes-associated protein 1.
Polarity and cell-adherence signals
Intestinal epithelial cells, which display apical–basal polarity, are joined together by tight junctions and adherens junctions, forming the intestinal epithelium layer. Intestinal diseases are often associated with disruption of IEC polarity or epithelial junctions43. The components of apical–basal polarity complexes attenuate the transcription activities of YAP1 and TAZ. For instance, in addition to stimulating LATS kinase activity, Angiomotin (AMOT) family proteins can directly bind to YAP1 and TAZ, sequestering them at tight junctions and inhibiting their transcriptional activity44,45. Studies in Drosophila have shown that the FERM (Four-point-one protein, ezrin, radixin and moesin) domain proteins Merlin and Expanded colocalize with Kibra at the apical domain of polarized epithelial cells to promote the activation of Wts by Hpo46. Many other key regulators of the Hippo pathway, such as α-catenin, tyrosine-protein phosphatase non-receptor type 14 (also known as PTPN14), and cadherin-1 (also known as E-cadherin), are also components of junction complexes, and attenuate YAP1 and TAZ transcriptional activity by either increasing the kinase activity of LATS or directly sequestering YAP1 and TAZ at cell junctions26. A study published in 2014 showed that LKB1, which is a master regulator of the development and maintenance of cell polarity, activates the Hippo pathway in human cell lines through its substrates, the serine/threonine-protein kinase MARK protein family and Scribble49. Another LKB1 substrate, 5′-AMP-activated protein kinase (AMPK), directly phosphorylates YAP1, disrupting YAP1–TEAD interaction and inhibiting YAP1 transcriptional activity50–52. In Drosophila, the planar cell polarity proteins cadherin-related tumour suppressor (also known as Fat) and Dachsous inactivate Yki by promoting the abundance and localization of Expanded at the apical membrane, thereby stimulating Wts activity53–56.
Regulation of contact inhibition
The Hippo pathway is believed to integrate signals from the spatial organization and the physical state of neighbouring cells, and can regulate contact inhibition of cell growth by phosphorylating and inactivating YAP1, leading to inhibition of YAP1-mediated transcription36. Hippo pathway signalling is required for contact inhibition of cell growth mediated by the E-cadherin–catenin complex57. In addition, overexpression of constitutively active YAP1 leads to loss of contact inhibition in epithelial cells36. Contact inhibition has been shown to control miRNA biogenesis through the Hippo pathway. High YAP1 activity in cancer cells, in part due to loss of contact inhibition, led to a global downregulation of miRNA synthesis and potentially enhanced tumorigenesis58. However, another study reported conflicting results59, showing that YAP1 and TAZ promote processing and biogenesis of miRNA in human cells cultured at low density without contact inhibition. By contrast, cells under high density experiencing contact inhibition showed defective processing of miRNAs owing to loss of YAP1 and TAZ activities59.
Regulation of Hippo signalling by stress
As a result of its physiological role in digestion, gastrointestinal tract tissue experiences many stresses, including oxidative stress, endoplasmic reticulum stress and hypoxia. These stresses regulate YAP1 and TAZ by various mechanisms60–62. In addition, YAP1 and TAZ transcriptional activities are also controlled by mechanical signals through Rho GTPases or JNK1/2 (REFS 63–65), although the involvement of the Hippo pathway in YAP1 and TAZ regulation in this context is still under debate65,66. Mechanical forces affect many aspects of intestinal physiology, particularly by promoting the proliferation and migration of gut epithelial cells67. However, whether YAP1 and TAZ play any part in the response of IECs to mechanical signals has not been studied.
G protein-coupled receptor regulation
Extracellular signalling molecules such as hormones can regulate the Hippo pathway via G protein-coupled receptors (GPCRs)68–70. GPCR overexpression or mutations of guanine nucleotide-binding protein subunit alpha (Gα) proteins have been associated with carcinogenesis in a variety of tissues71. Many GPCRs known to promote cell growth and tumorigenesis, such as lysophosphatidic acid receptors (LPARs), sphingosine 1-phosphate receptors (S1PRs) and protease-activated receptors (PARs), are now recognized as cell surface receptors for Hippo signalling68,70, suggesting that the Hippo pathway mediates GPCR-induced tumorigenesis. Indeed, GPCRs coupled to Gα12 or Gα13, or to Gαq/11, can activate YAP1 and TAZ, leading to tumorigenesis72–74 On the other hand, Gαs activation was shown to inhibit YAP1 and TAZ68,75,76. Intestinal GPCRs respond to many nutrients and other components in food, and therefore play a crucial part in regulating intestine physiology, particularly in metabolic homeostasis and appetite77. It would be interesting to evaluate whether the Hippo pathway is regulated by GPCRs that are highly expressed in the intestine, for instance the GPCRs that respond to fatty acids (which include free fatty acid receptors and GPR120)77.
Intestinal regeneration and homeostasis
Hippo pathway in Drosophila midgut regeneration
Drosophila has been widely recognized as a powerful and convenient animal model to study cellular mechanisms of human intestinal diseases, owing to its simple structure and well-defined cell populations78. The Drosophila gut consists of foregut, midgut and hindgut79, with Drosophila midgut and hindgut corresponding to the mammalian small and large intestine, respectively. Studies in the past few years have revealed a crucial role for the Hippo pathway in Drosophila midgut regeneration after injury.
Adult Drosophila midgut homeostasis is maintained by intestinal stem cells (ISCs). Cell division of ISCs can be either symmetric or asymmetric, depending on the nutrient needs of the animal and the status of the midgut epithelial lining78. In asymmetric division, one ISC is divided into an enteroblast and the other remains an ISC. Enteroblasts can further differentiate into enteroendocrine cells and enterocytes, which constitute the epithelium layer of the midgut. Stresses, pathogens and dietary toxins can stimulate enterocytes to secrete ligands that bind the epidermal growth factor receptor (Egfr) or activate the Jak–Stat pathway, stimulating proliferation of neighbouring ISCs in a paracrine manner78.
Under normal conditions, the Hippo pathway restricts ISC proliferation by inactivating Yki. Staley et al.25 reported that Yki is activated in enterocytes upon tissue damage, leading to increased expression of Unpaired (Upd) target genes. Upd, a secreted hormone for the Jak–Stat pathway, initiates non-autonomous proliferation of ISCs through activation of Jak–Stat signalling25 (FIG. 2). Yki is activated by stress-activated protein kinase JNK upon intestinal injury; JNK phosphorylates LIM domain-containing protein jub (also known as Jub), and phosphorylated Jub in turn prevents the phosphorylation of Yki by binding to Wts80. Although Staley et al.25 did not observe a role for Yki in regulating ISC division, several other studies have demonstrated that inactivation of Hpo in ISCs also leads to ISC overproliferation as a result of Yki-mediated upregulation of downstream genes such as Cyclin E, Diap1 and bantam miRNA81–84. These studies support the role of the Hippo pathway in regulating the autonomous proliferation of ISCs. Ren et al.83 further showed that in addition to Upd, Yki also promotes the transcription of multiple Egfr ligands to promote non-autonomous proliferation of ISCs (FIG. 2). Besides JNK and Jub, several other proteins play important parts in regulating Yki activities. Pez, the Drosophila homologue of PTPN14, negatively regulates Yki-mediated ISC proliferation by binding to Kibra, thereby activating the core Hippo pathway kinase cascade85. A later study showed that Pez protein stability is negatively regulated by E3 ubiquitin-protein ligase Su(dx) (also known as Su(dx)), a member of the Nedd4 family of E3 ubiquitin ligases, and overexpression of Su(dx) in enterocytes leads to Drosophila midgut epithelium proliferation86. Notably, mammalian PTPN14 has been reported to be highly mutated in human colorectal cancers (CRCs)87. Exploring the role of PTPN14 in CRCs and whether the Hippo pathway is involved in tumori-genesis would be interesting. In addition, Yki activity in Drosophila ISCs is attenuated by Tao, a protein kinase that activates Hpo, to maintain midgut homeostasis88. Msn, which seems to activate Wts preferentially in enteroblasts in a Hpo-independent manner, is involved in controlling ISC proliferation by inhibiting Upd3 expression during intestinal regeneration23.
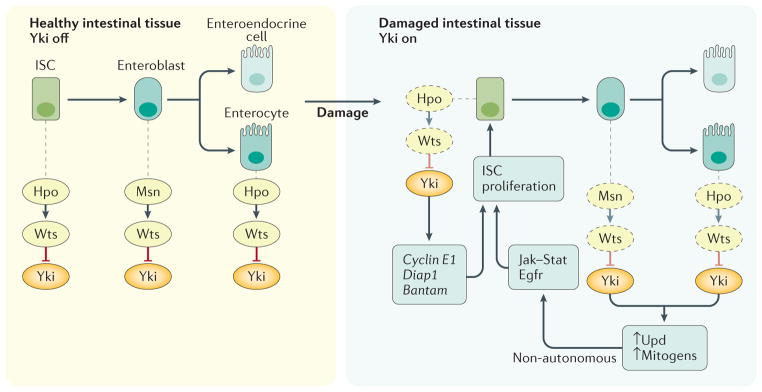
Figure 2. The Hippo pathway controls Drosophila midgut homeostasis by restricting Yki activity.
In healthy ISCs and enterocytes, the Hpo Wts kinase cascade (homologous to the MST LATS kinase cascade in mammals) suppresses Yki activity. Upon intestinal damage Wts is inactivated, in part by JNK signalling, resulting in Yki activation. Yki activation in enterocytes leads to expression and secretion of Upd protein, a Jak Stat ligand, and Egfr ligands, which promote non-autonomous ISC proliferation in a paracrine manner. Yki activation in ISCs results in expression of cyclin E1, bantam and diap1, promoting autonomous ISC proliferation and survival. In enteroblasts, Msn activates Wts in a Hpo-independent manner, and therefore negatively regulates Yki. Egfr, epidermal growth factor receptor; Hpo, Hippo; ISC, intestinal stem cell; LATS, large tumour suppressor; Msn, misshapen; MST, mammalian ste20-like kinase; Upd, unpaired; Wts, warts; Yki, transcriptional co-activator yorkie.
Downstream effectors of Yki in midgut regeneration have also been identified, and seem to be crucial in mediating the function of Yki. For instance, Yki is reported to regulate dMyc expression89. Inhibition of caspase 3 by Yki target gene Diap1 is essential for Brahma to participate in ISC proliferation90. Future studies are needed to determine whether the mammalian homologues of these Hippo pathway regulators and effectors also have important roles in mammalian intestinal homeostasis.
The Hippo pathway in mammalian intestine
Intestine cellular structure
The functional unit of the mammalian small intestine is the villus, which contains a group of dynamic and self-renewing epithelial cells91. Between the villi are invaginations of the epithelium, called the crypts of Lieberkühn. The bottom of the crypt, where ISCs reside, is the main stem cell niche. These stem cells divide to produce highly proliferative transit amplifying cells, which migrate upwards to the tip of the villi and eventually lose their progenitor identity. While moving upwards, transit amplifying cells differentiate into either absorptive enterocytes or secretory cells, which comprise goblet cells, enteroendocrine cells and Paneth cells91. Unlike the goblet cells and enteroendocrine cells, which both reside within the villi, Paneth cells migrate towards the base of the crypt and reside beside the ISCs (FIG. 3). Paneth cells are critical for stem cell maintenance as they secrete essential niche signal ligands such as epidermal growth factor (EGF), WNT3a and Notch ligand DLL4 (REF. 92). Although the cellular structure and organization of the colon is mostly similar to that of the small intestine, the colon has a flat surface of epithelia with no villi and no Paneth cells in the crypt93. A subpopulation of CD24+ cells, located adjacent to colonic stem cells in the colonic crypt, are proposed to provide the stem cell niche by functioning equivalently to Paneth cells92.
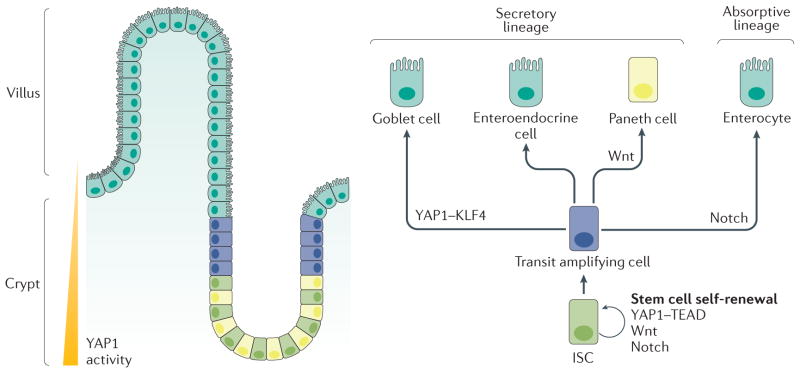
Figure 3. Mammalian intestine cellular structure and cell fate decision.
The mammalian intestinal mucosa is to form villi and crypts. YAP1 activity is highest at the bottom of the crypt, where ISCs and Paneth cells reside. The villus consists of differentiated cells. ISCs in the crypt base differentiate into transit amplifying cells, which further differentiate along a secretory lineage (into goblet cells, enteroendocrine cells and Paneth cells) or an absorptive lineage (into enterocytes). After differentiation, Paneth cells move down to the stem cell niche and reside beside ISCs at the bottom of the crypt. YAP1, Wnt signalling and Notch signalling play important parts in stem cell self-renewal and progenitor cell differentiation. YAP1 interacts with KLF4 to induce progenitor cell differentiation into goblet cells, whereas Wnt signalling is involved in Paneth cell differentiation, and high Notch signalling induces differentiation along the absorptive lineage. ISC, intestinal stem cell; KLF4, krueppel-like factor 4; TEAD, TEA domain family member; YAP1, Yes-associated protein 1.
The base of the intestinal crypt is a specialized ISC niche harbouring two distinct stem cell populations, active stem cells and reserved stem cells. Active stem cells, called crypt base columnar (CBC) cells, express Leucine-rich repeat-containing G-protein coupled receptor 5 (LGR5)94. LGR5+ cells are evenly distributed between Paneth cells and are responsible for normal epithelial homeostasis. The identity of reserved stem cells is less clear. The proteins polycomb complex protein BMI1 (also known as BMI1), homeodomain-only protein (also known as HOPX), telomerase reverse transcriptase (also known as TERT), and Leucine-rich repeats and immunoglobulin-like domains protein 1 (also known as LRIG1) were proposed as markers for +4 reserved stem cells, which reside at the fourth position from the crypt base, but all were later reported to be also highly expressed in CBC cells95. Nonetheless, it is widely accepted that reserved stem cells are responsible for repopulating the entire tissue after injury91,96. The turnover time of mouse intestinal epithelial cells is ~3–5 days91. This high rate of cellular turnover has evolved to maintain optimal intestinal function, and makes the intestine an attractive organ system for the study of cell proliferation, differentiation and regenerative medicine.
Role of the Hippo pathway in normal homeostasis
YAP1 and TAZ have been reported to maintain and promote stem cell properties in various tissues, such as the liver, skin and nervous system97. In the intestine, endogenous YAP1 protein expression is restricted to the stem cell niche at the bottom of the crypt, suggesting an association with ISC regulation98–100. Several studies have reported that high YAP1 activity can promote ISCs proliferation. Camargo et al.98 and Zhou et al.101 showed that YAP1 overexpression in the intestine leads to expansion of undifferentiated cells and the replacement of differentiated cells at the tips of the villi, similar to the phenotype observed in intestines deficient in MST1 and MST2. In addition, using the intestine-specific gene transfer technique, YAP1 and TAZ were shown to promote proliferation of stem cells and progenitor cells by binding to TEAD transcription factors, and to induce differentiation of progenitor cells into goblet cells by binding to the transcription factor Krueppel-like factor 4 (KLF4)102. Under normal homeostasis, protein kinase C zeta type (PKCζ) acts as a tumour suppressor gene, suppressing YAP1 activity through direct phosphorylation in ISCs. Loss of PKCζ in the intestine increases YAP1 nuclear localization, leading to elevated stem cell activity and tumorigenesis103. In general, YAP1 and TAZ activity promotes stem cell properties in the intestine. However, Barry et al.104 showed that intestine-specific acute induction of YAP1 expression in mice results in disruption of normal intestinal tissues, such as mislocalized Paneth cells and reduced ISC numbers, resulting in loss of cellular proliferation in crypts. This finding suggests that YAP1 has a growth-suppressive function in healthy intestine. The inconsistency of these results with those of Camargo et al.98 might be due to different experimental settings and mouse models. For example, mice with inducible systemic YAP1 overexpression were used in the study by Camargo et al.98, whereas Barry et al.104 induced YAP1 expression specifically in the IECs. IECs constantly interact with neighbouring stromal cells and immune cells, which might also regulate YAP1 by chemical or mechanical cues. Thus, the opposite proliferation phenotypes observed in the models could result from autonomous and non-autonomous effects. YAP1 cellular localization might also be different after systemic induction of expression compared with localization after intestine-specific induction. In contrast to the activation of Wnt signalling by nuclear YAP1, cytoplasmic YAP1 inhibits Wnt signalling, leading to opposite effects on progenitor cell preservation and proliferation2. Moreover, strong YAP1 induction also initiates negative feedback regulation by inducing LATS2 expression, which might exert some growth inhibitory effect105–107.
The Hippo pathway is important in maintaining normal intestine homeostasis; disrupted Hippo signalling leads to tissue overgrowth and tumorigenesis. Mice with conditional knockout of Mst1 and Mst2 specifically in IECs show a disorganized villus structure and expansion of undifferentiated cells. Dysplastic epithelia and adenomas are frequently observed in the colons of these mice101. Similarly, mice with Sav1-deficient small and large intestines show enlarged crypt structure and increased polyp formation100. Surprisingly, various reports show that depletion of YAP1 and/or TAZ protein levels in the intestine has no observable adverse phenotype under normal homeostasis, meaning that YAP1 (REFS 98,100,104) and TAZ99 might be dispensable under normal conditions. Conversely, the Hippo pathway components MST1, MST2 and SAV1 are indispensable for normal tissue homeostasis, suggesting that the Hippo pathway is constitutively active under normal conditions to restrict YAP1 and TAZ activity. Disruption of the Hippo pathway leads to hyperactivation of YAP1 and TAZ, which then causes uncontrolled stem cell proliferation and tumori-genesis100,101. Currently, only mice with intestine-specific conditional knockout of Mst1 and Mst2, Sav1, and Yap1 and/or Taz have been studied, and further investigations could provide new mechanistic insights into how other Hippo pathway components regulate intestinal tissue homeostasis.
Role of the Hippo pathway in regeneration
Although YAP1 is not required during normal intestinal homeostasis, its activity is essential for intestinal regeneration after injury. In mice, an increase in YAP1 protein level is observed 2–5 days after dextran sulfate sodium (DSS)-induced colitis and regeneration100,108. In vivo and in vitro studies show interleukin-6 receptor subunit beta (also known as gp130), a co-receptor for IL-6, activates YAP1 through Src family kinases (SFKs) upon DSS-induced intestinal colitis108. Mice with IEC-specific conditional knockout of Yap1 had a higher mortality rate and more extensive loss of the crypt compartment than wild-type mice after both groups were subjected to DSS-induced intestinal injury100. Whole-body irradiation is also widely used to study intestinal regeneration in mice. YAP1 is activated in IECs 2–4 days after irradiation109, and IEC-specific and ISC-specific Yap1 knockout mice show reduced crypt proliferation after irradiation-induced injury109. These studies suggest that YAP1 positively regulates intestinal tissue regeneration by regulating ISC proliferation. Furthermore, Gregorieff et al.109 found that YAP1 reprogrammes ISCs by transiently inactivating Wnt signalling and activating another regenerative programme, the EGFR pathway. They showed that the EGFR ligand epiregulin is upregulated in the intestines of Yap1 conditional knockout mice after regeneration of the intestinal epithelia following whole-body exposure to ionizing radiation, which might serve as a compensatory mechanism for Yap1 loss in some crypts. Indeed, they further showed that exogenous epiregulin can rescue YAP1-deficient organoid formation ex vivo109. By contrast, Barry et al.104 found that whole-body irradiation of mice with intestine-specific Yap1 conditional knockout resulted in crypt hyperplasia and overgrowth 7 days later, and suggested that Wnt signalling hyperactivation was responsible for this phenotype. In summary, functional YAP1 is important in the first few days of the regeneration phase. The hyperproliferative phase seen in Yap1-IEC-specific conditional knockout mice 7 days after injury could be the result of enhanced Wnt signalling (FIG. 4). The divergent roles of YAP1 in the regenerative response at different time points after injury might be due to the distinct cellular repair mechanisms activated at these time points. For instance, pathways involved in cell cycle and DNA repair are active 2–5 days after injury whereas pathways for tissue development might be activated at subsequent time points, 1 week or later after injury110. YAP1 and Wnt signalling work in coordination to maintain proper tissue regeneration after injuries. Mouse genetic studies clearly demonstrate a role of the Hippo pathway in normal intestine homeostasis as well as in the response to tissue injury.
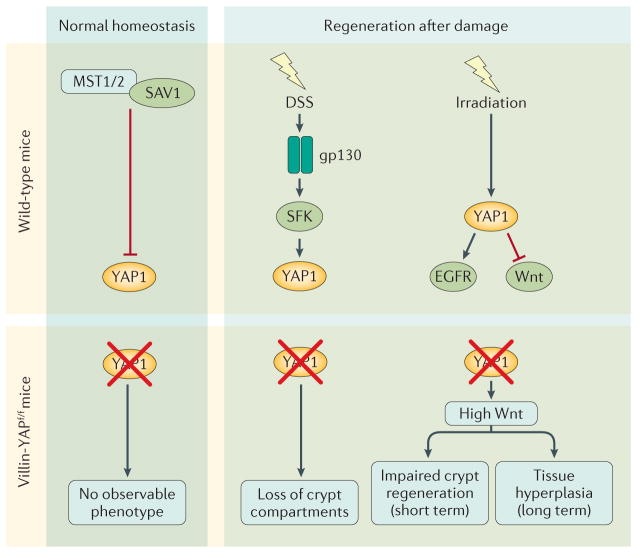
Figure 4. The Hippo pathway in intestinal homeostasis and injury-induced regeneration.
In normal homeostasis, the Hippo pathway is constitutively active to keep YAP1 activity at low levels. Mice lacking YAP1 in intestinal epithelial cells (Villin-YAPf/f mice) have no observable phenotype. During tissue regeneration induced by damage caused by DSS, YAP1 is activated by gp130 signalling to stimulate cell proliferation. Mice with Yap1 deficiency in intestinal epithelial cells have higher mortality and greater loss of crypt compartments than wild-type mice. Whole-body irradiation of mice causes YAP1 activation, which transiently inhibits Wnt and stimulates EGFR signalling to promote regeneration. Loss of YAP1 in intestinal epithelial cells leads to Wnt hyperactivation, impairing crypt regeneration shortly after injury but inducing tissue hyperplasia in the long term. DSS, dextran sulfate sodium; EGFR, epidermal growth factor receptor; gp130, interleukin-6 receptor subunit beta; SAV1, salvador family WW domain-containing protein 1; SFK, Src family kinases; YAP1, Yes-associated protein 1.
The Hippo pathway and signalling crosstalk
Intestinal epithelial homeostasis is tightly controlled and regulated by interconnected developmental pathways that keep cellular processes such as proliferation and differentiation in balance. Several conserved signalling pathways, such as the Wnt, Notch, Hedgehog and bone morphogenetic protein (BMP) pathways, have been shown to regulate mammalian intestinal homeostasis and regeneration111. Understanding how the Hippo pathway interacts with these signalling pathways can help explain the phenotypes observed in mouse models with disrupted Hippo signalling, and will be beneficial for developing therapeutic strategies (FIG. 5).
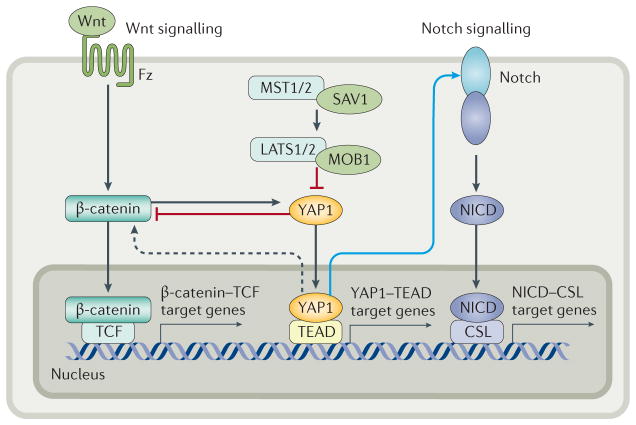
Figure 5. Crosstalk between the Hippo pathway and Wnt and Notch signalling in the intestine.
When the Hippo pathway is activated, cytoplasmic YAP1 inhibits Wnt signalling by sequestering β-catenin in the cytoplasm. When the Hippo pathway is inactive, nuclear YAP1 upregulates Wnt signalling through an unknown mechanism. In addition, Wnt activation leads to β-catenin-induced activation and upregulation of YAP1. Activated YAP1 transcriptionally upregulates expression of Notch receptors, leading to increased activation of Notch signalling and translocation of the NICD to the nucleus. Notch is considered to be a mediator of the Hippo pathway. Black arrows indicate direct activation, blue arrows indicate transcriptional regulation, dotted lines indicate processes supported by experimental evidence but without a known mechanism. CSL, (CBF1/RBPjκ/Su(H)/Lag-1) transcription factor; Fz, Frizzled; LATS, large tumour suppressor; MOB1, Mob1 homologue; MST, mammalian ste20-like kinase; NICD, Notch intracellular domain; SAV1, salvador family WW domain-containing protein 1; TCF, T-cell factor; TEAD, TEA domain family member; YAP1, Yes-associated protein 1.
Hippo and Wnt signalling
Wnt signalling is essential for intestinal tissue homeostasis and stem cell maintenance112. Canonical Wnt signalling depends on β-catenin as its key effector. β-catenin binds to the TCF and LEF families of transcription factors to activate the transcription of target genes. Nuclear localization of β-catenin, which represents active Wnt signalling, has been observed in mice in the ISCs at the base of the crypt113. Also in mice, inhibition of canonical Wnt signalling results in loss of transit amplifying cells and disrupted crypt structure, leading to defects in intestinal homeostasis and function114. Interplay between the Hippo pathway and Wnt signalling has been intensively studied in the context of stem cell maintenance and damage repair. In the mammalian intestine, YAP1 maintains the proliferation and stemness of crypt stem cells by activating Wnt signalling pathways98,101. YAP1 overexpression induces nuclear β-catenin accumulation and expression of β-catenin target genes98. In addition, the Wnt target genes and stem cell markers Lgr5 and Ascl2 are upregulated in the intestines of mice with intestine-specific conditional double knockout of Mst1 and Mst2 (REF. 101). In vitro studies show that downregulation of YAP1 in the SW480 human colon cancer cell line results in a decrease in β-catenin-dependent transcription101. These studies indicate that YAP1 activity potentiates Wnt signalling. On the other hand, several studies have shown that cytoplasmic YAP1 and TAZ inhibit canonical Wnt signalling, and several mechanisms have been proposed to explain this regulation. Firstly, cytoplasmic YAP1 and TAZ bind to segment polarity protein dishevelled homologue DVL-1 (known as DVL), a positive regulator of Wnt, and suppress its activity104,115. Secondly, β-catenin nuclear translocation is supressed by direct binding with YAP1 and TAZ116; and thirdly, YAP1 and TAZ incorporate into the β-catenin destruction complex to recruit F-box/WD repeat-containing protein 1A (also known as β-transducin repeat-containing protein, β-TrCP), which ubiquitylates β-catenin and leads to its degradation99. YAP1 overexpression in mice causes an increase in cytoplasmic YAP1 levels and downregulates intestinal β-catenin target gene expression104. Reciprocally, several studies have shown that YAP1 and TAZ can be activated by Wnt signalling, and are required for some Wnt responses in the intestine99,117,118. Notably, the non-canonical Wnt signalling pathway, which is β-catenin-independent, has also been shown to have a critical role in intestinal regeneration119. YAP1 and TAZ can be activated by noncanonical Wnt signalling, and many proteins induced by active YAP1 and TAZ can antagonize canonical Wnt signalling in vitro. This study provides a mechanism by which noncanonical Wnt signalling might antagonize canonical Wnt signalling through YAP1 (REF. 120). As a result, testing whether the effect of noncanonical Wnt signalling in intestinal regeneration is mediated by YAP1 and TAZ would be interesting. Crosstalk between Wnt and the Hippo pathway is highly complex and future investigation is needed to fully understand their interplay.
Hippo and Notch signalling
In addition to Wnt, the Notch pathway is also required to maintain ISCs. Mammals possess four Notch receptors (Notch1, Notch2, Notch3 and Notch4), each of which consists of a large extracellular ligand binding domain, a transmembrane domain and an intracellular domain. Upon ligand activation, the Notch intracellular domain (NICD) is cleaved from the receptors and translocates into the nucleus121. Nuclear NICD associates with recombining binding protein suppressor of hairless (RBPJ, also known as CSL (CBF1/RBPjκ/Su(H)/Lag-1)), to induce the expression of target genes such as the HES transcription factor family, CDKN1A (also known as p21) and MYC. Pharmacological inhibition of Notch signalling in mice results in decreased expression of the stem cell markers olfactomedin 4 (Olfm4) and Lgr5 and reduced numbers of crypt base columnar stem cells122. Besides preserving stemness, Notch signalling is able to determine cell fate during differentiation. High Notch activity promotes the differentiation of transit amplifying cells along the absorptive lineage123, whereas inhibition of Notch signalling leads to loss of stem cell properties and induces secretory lineage differentiation124. Notch signalling is also regulated by the Hippo pathway. Mice with intestine-specific conditional double knockout of Mst1 and Mst2 have intestines with increased NICD nuclear localization and upregulated Hes1 gene expression101. The expansion of undifferentiated intestinal progenitor cells resulting from YAP1 overexpression in transgenic mice is attenuated when γ-secretase inhibitors that block Notch signalling are administered98. In summary, these data suggest that YAP1 can positively regulate Notch signalling in the intestine, and that Notch is a mediator of YAP1-induced ISC expansion. The molecular mechanism by which Hippo regulates Notch in the intestine has not yet been elucidated. Nevertheless, several members of the Notch pathway, such as Notch1 and Notch2, Sox9 and Hes1, are transcriptionally upregulated in hepatocytes with YAP1 overexpression, and Notch2 was identified as a direct target gene of the YAP1–TEAD complex125. This notion might provide insights into the interplay between Hippo and Notch pathways in the intestine.
Hippo and BMP signalling
Although not in the context of intestinal cells, the existence of crosstalk between Hippo and BMP signalling or between Hippo and Hedgehog signalling has also been established. BMPs regulate cellular differentiation, apoptosis and cell growth126. Inhibition of BMP signalling by over-expressing its antagonist Noggin in the mouse intestine results in hyperproliferation of ISCs, an increased number of crypts, and eventually intestinal polyposis127,128. These data suggest that BMP signalling negatively regulates stem cell proliferation in the crypt. BMP signalling is initiated by BMP ligands binding to type I and type II serine/threonine kinase receptors. Activated receptors then phosphorylate the C-termini of SMADs (SMAD1, SMAD5, and SMAD8), which then bind to their cooperative partner SMAD4, and translocate into the nucleus126. This transcriptional complex of SMAD1, SMAD5, SMAD7 and SMAD4 is fully activated by phosphorylation at the linker region by cyclin-dependent kinase (CDK)8 and/or CDK9. In mouse embryonic stem cells, the phosphorylated SMAD1–SMAD4 complex recruits YAP1 to the enhancer region of BMP-responsive genes, resulting in BMP-induced gene expression129.
Hippo and Hedgehog signalling
Hedgehog signalling has also been implicated in intestinal homeostasis. Indian hedgehog and sonic hedgehog, two key Hedgehog signalling proteins, are expressed in mouse intestinal epithelium130. Ablation of Indian hedgehog protein in the mouse small intestine causes overexpansion of the stem cell niche and reduces stem cell differentiation along the absorptive lineage131. Although the crosstalk between Hippo and Hedgehog signalling has not been characterized in intestinal tissue, there is evidence of crosstalk between the two pathways in other tissues. For example, in medulloblastoma, cerebellar granule neuron precursors (CGNPs) are implicated as the origin of the tumours, and their proliferation depends on Sonic hedgehog signalling132,133. Activation of Sonic hedgehog in CGNPs upregulates YAP1 protein levels, and direct binding between YAP1 and insulin receptor substrate 1 (IRS1), a Sonic hedgehog effector, results in nuclear localization of YAP1. In addition, YAP1 depletion abolishes Sonic-hedgehog-induced CGNP proliferation. GLI2, a downstream effector of Sonic hedgehog signalling, is identified as a target gene of the YAP1–TEAD complex, suggesting a mechanism by which YAP1 drives tumorigenesis in medulloblastoma134. However, Indian hedgehog is the predominant Hedgehog protein expressed in the gastrointestinal tract. Understanding the crosstalk between Hippo and Hedgehog signalling in the intestine, which might be mediated by Indian hedgehog, remains an interesting question and needs further exploration. In summary, the crosstalk between Hippo and other signalling pathways is complex and might be context-dependent. The Hippo pathway has been shown to communicate with the BMP pathway and the Hedgehog pathways in other biological contexts, and one can speculate that these interactions would also be present in the intestine and contribute to tissue homeostasis.
Hippo pathway in intestinal diseases
The Hippo pathway in colon cancer
Uncontrolled tissue regeneration can lead to malignant transformation135. Dysregulation of the Hippo pathway has been observed in many cancer types41, including CRC. Genetically engineered mouse models serve as useful tools to study how the Hippo pathway influences tumorigenesis. Because global knockout of many genes encoding Hippo pathway components, such as Nf2, Sav1, Mst1, Mst2 and Lats2, results in embryonic lethality in mice, intestinal-epithelium-specific conditional knockout mice have been developed. Mice with conditional knockout of both Mst1 and Mst2 in the intestinal epithelium develop dysplastic small and large intestine and spontaneous adenomas in the colon101. The increased tumour risk might be the result of elevated YAP1 protein expression in these mice, resulting in strong activation of Wnt and Notch signalling. Intestinal-epithelium-specific Sav1-knockout mice develop colonic polyps by 13 months of age, whereas wild-type littermates do not develop polyps100. This study further showed that DSS-induced intestinal injury and repair greatly exacerbated the tumorigenicity of the Sav1-deficient crypts. This effect was YAP1-dependent, as mice with intestinal epithelium-specific knockout of both Sav1 and Yap1 had no colonic polyp formation. In addition to canonical Hippo pathway components, the NDR protein kinases have been identified as tumour suppressors upstream of YAP1. Mice with intestinal epithelium-specific knockout of both Ndr1 and Ndr2 show increased YAP1 expression level and are more sensitive to colon carcinogenesis induced by DSS or azoxymethane136. These data support the notion that dysregulation of Hippo pathway leads to tumorigenesis and that active YAP1 is oncogenic in CRCs.
Studies of samples from patients with colon cancer and human colon-cancer-derived cell lines also support the oncogenic property of YAP1 and TAZ in CRCs. A positive correlation between YAP1 protein expression level and poor prognosis has been observed in patients with CRC101,137,138, and TAZ has also been found to be a prognostic indicator for CRC outcome139,140. In human colon cancer cell lines, YAP1 knockdown by small-hairpin RNA resulted in a dramatic decrease in cell proliferation, metastasis and invasion, whereas over-expression of YAP1 resulted in an increased proliferation rate101,139. Knockdown of TAZ resulted in decreased cell proliferation, both in vitro and in xenograft mouse models141. These studies support YAP1 and TAZ as oncogenes in CRCs.
Several underlying mechanisms of Hippo-pathway-driven tumour transformation have been proposed. The Wnt–β-catenin pathway has a crucial role in initiating CRC. Loss of function mutations in the gene encoding adenomatous polyposis coli (APC) are observed in most colorectal tumours142. APC mutation increases the risk of CRC through the constitutive activation of the β-catenin–TCF4 complex in IECs143. Proteins encoded by β-catenin–TCF4 complex target genes, including matrilysin (also known as MMP-7), Myc, cyclin D1 and PPARd, have been shown to contribute to CRC tumour progression144–148. YAP1 and TAZ are activated in APC-deficient cells both in vitro and in vivo99,117,149, and mouse models with intestine-specific Apc-knockout do not show intestinal hyperplasia if TAZ or YAP1 are also knocked out in the intestines99,149. Different mechanisms have been proposed for how APC regulates the activity of YAP1 and TAZ. Azzolin et al.99 reported that YAP1 and TAZ are sequestered in the cytoplasm by the β-catenin destruction complex when Wnt signalling is not active. When Wnt is activated by APC depletion, YAP1, TAZ and β-catenin separate from the destruction complex and become active. Cai et al.149 reported that the activity of YAP1 and TAZ can also be regulated by APC via a mechanism independent of the β-catenin destruction complex. They showed that APC serves as a scaffolding protein to facilitate LATS activation. Loss of APC inactivates the Hippo pathway, leading to YAP1 and TAZ activation149. Other studies have shown that the β-catenin–TCF4 complex directly binds to the YAP1 enhancer region to promote YAP1 gene expression, thereby identifying YAP1 as a TCF4 target gene118. In addition, β-catenin can complex with YAP1 and TBX5 to induce anti-apoptotic gene expression; this complex is essential for survival of β-catenin-driven colon cancers150. Together, these results suggest that YAP1 and TAZ can be mediators downstream of APC, and that their activities contribute to colonic tumorigenesis. The dispensable nature of YAP1 and TAZ under normal conditions make them an attractive therapeutic target for treating CRC.
In addition to Wnt signalling, JNK signalling has also been implicated in inducing tumorigenesis in the intestine151,152. Several studies indicate that JNK is an upstream regulator of the Hippo pathway. In the Drosophila midgut, bleomycin-induced injury activates JNK, leading to Yki nuclear translocation. Activated Yki upregulates Upd expression and Jak–Stat signalling, which induces cellular proliferation25,153. In mammals, JNK induces YAP1 activity when cells are exposed to mechanical strain66 and DNA damage154. Mechanistically, JNK activates YAP1 through inhibition of LATS by increasing binding between LATS proteins and Ajuba (the mammalian homologue of Jub)80. Although no studies have established a connection between JNK signalling and the Hippo pathway in the context of the mammalian intestine, this mechanism could be worthy of further exploration in colorectal tumorigenesis.
The Hippo pathway in other intestinal diseases
Peutz–Jeghers syndrome (PJS) is characterized by the development of benign polyps in the gastrointestinal tract. In PJS, the tumour suppressor LKB1 is frequently mutated, leading to intestinal polyp formation155. These polyps can cause bleeding, bowel obstructions and pain. Patients with PJS are at an increased risk of developing various cancers, especially gastrointestinal tumours156,157. The polyps in these patients were observed to exhibit increased YAP1 nuclear localization, prompting the investigation of the connection between LKB1 and the Hippo pathway. As a result, LKB1 has been identified as an upstream regulator of Hippo signalling. In vitro studies show that loss of LKB1 leads to YAP1 nuclear localization in intestinal epithelial cell lines, and depleting YAP1 expression reduces cancer phenotypes, including decreasing cell proliferation rate, colony formation and tumour size49,158. In addition, LKB1 is a major positive upstream regulator of AMPK activity. Activated AMPK inhibits YAP1 through several mechanisms, including: activation of LATS proteins; direct phosphorylation of YAP1 at serine 94, disrupting YAP1 interaction with TEAD proteins; and stabilizing Angiomotin-like protein 1 (AMOTL1), which inhibits YAP1 (REFS 50,52). Loss of LKB1 might contribute to tumorigenesis by lowering AMPK activity, resulting in YAP1 hyperactivation.
IBD, including Crohn’s disease and ulcerative colitis, is a chronic idiopathic inflammatory disorder that affects the gastrointestinal tract. Appropriate intestinal epithelial regeneration is required to improve the outcomes of treatments for IBD. YAP1 regulation has been identified as a responsive mechanism for mucosal regeneration in IBD using a DSS-induced colitis mouse model100,108. IL-6 is a key modulator of the intestinal inflammatory response that can promote intestinal epithelial cell proliferation and regeneration159. As mentioned earlier, gp130 activates YAP1 upon DSS treatment. Villin-gp130Act mice, which express constitutively active gp130 exclusively in IECs, have increased YAP1 nuclear localization, and IEC-specific Yap1 depletion in gp130Act mice reverses their DSS resistance108. This study establishes a mechanism by which YAP1 is activated upon intestinal injury and provides insights into potential therapeutic strategies for IBD.
The Hippo pathway in the liver
The liver is the central organ controlling metabolism of carbohydrates, lipids and amino acids, as well as detoxification of both exogenous and endogenous compounds160. The liver is capable of rapidly renewing its parenchymal cells (hepatocytes) and non-parenchymal cells (cholangiocytes, Kupffer cells, stellate cells and sinusoidal endothelial cells) after injury to resume its physiological functions161.
Regulation of liver size by Hippo signalling
Liver regeneration and homeostasis are considered a classic model for mammalian organ size control162. The discovery that Hippo signalling is essential in the regulation of tissue homeostasis in Drosophila was followed immediately by speculation over whether the Hippo pathway similarly regulates mammalian organ size163. In mammalian models, overexpression of YAP1 in the liver leads to tissue overgrowth, and sustained over-expression of YAP1 eventually results in hepatocellular carcinogenesis39,98. In human hepatocellular carcinoma specimens, high YAP1 protein levels and increased YAP1 nuclear localization have been observed36,39,164. Because liver cancer results from uncontrolled proliferation of hepatocytes, increased YAP1 expression in human liver cancer and mouse models supports the notion that YAP1 can serve as a major driver of hepatocyte proliferation. Mouse models bearing deletion of Hippo pathway proteins, including MST1, MST2, SAV1, Merlin, LATS1 and LATS2, have been generated and characterized for the roles of these components in liver size control and carcinogenesis106,165–169 (FIG. 6). Hepatomegaly and spontaneous hepatocellular carcinogenesis were consistently observed in liver-specific Mst1 and Mst2 knockout mice165–168. Liver-specific Sav1-knockout mice exhibit more complex tumorigenesis, and display both hepatocellular carcinoma and cholangiocarcinoma165,168. SAV1-deficient mice have been speculated to be more prone to the malignant transformation of liver progenitor cells than wild-type mice, whereas hepatocytes are unaffected by loss of SAV1 (REF. 165). Similarly, liver-specific Nf2-knockout mice develop hepatocellular carcinoma and bile duct hamartoma at 1 year of age169. A study published in 2013 showed that combined liver-specific deletion of Nf2 and Sav1 causes an exaggerated liver overgrowth phenotype in mice at a very early age (8 days old), whereas deletion of either Nf2 or Sav1 alone did not result in any abnormality in liver at that age170. This result indicates the synergistic actions of Merlin and SAV1 attenuate YAP1 and TAZ activity during liver development. Consistently, mice with knockout of other Hippo pathway components, such as Mob1a and Mob1b, also induce spontaneous hepatocellular carcinogenesis171 (FIG. 6).
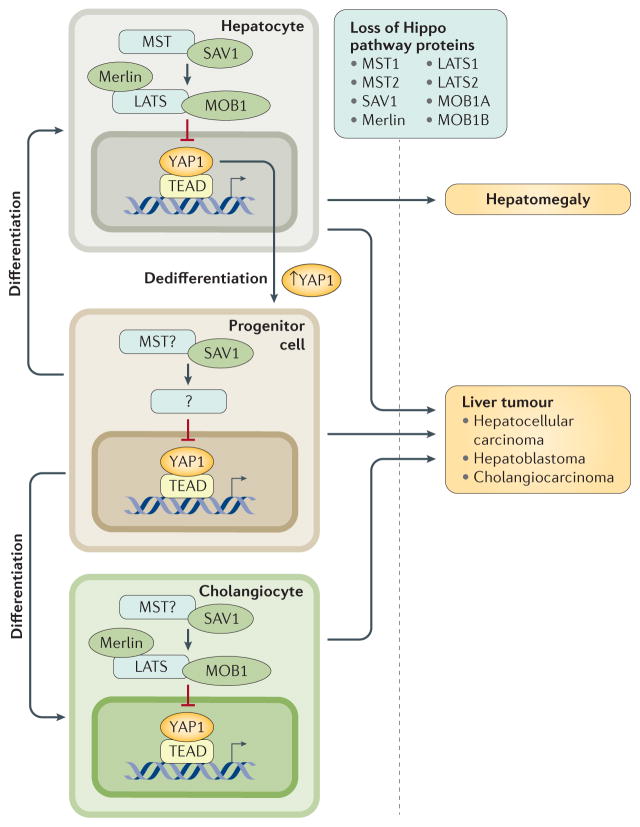
Figure 6. Hippo pathway has roles in organ size control and carcinogenesis in different types of liver cells.
Disruption of Hippo signalling by deleting core kinase components (MST1, MST2, SAV1, MOB1A, MOB1B, LATS1, LATS2 and Merlin) leads to uncontrolled proliferation of hepatocytes and bile duct epithelial cells (cholangiocytes). Transgenic mice with deficiency in Hippo pathway show spontaneous liver tumours derived from hepatocytes and/or cholangiocytes, as well as hepatomegaly caused by expansion of hepatocytes. Notably, Sav1-knockout mice exhibit expansion of hepatic progenitor cells, which is not observed in mice with deficiency of other Hippo pathway components. On the other hand, induced ectopic expression of YAP1 converts hepatocytes into progenitor or ductal-like cells. These observations indicate a unique role of SAV1 in regulating YAP1 activities in hepatic progenitor roles. LATS, large tumour suppressor; MOB1, Mob1 homologue protein; MST, mammalian ste20-like kinase; SAV1, salvador family WW domain-containing protein 1; YAP1, Yes-associated protein 1.
The role of Hippo signalling in liver development is further supported by evidence that the level of nuclear YAP1 correlates with cyclin D1 expression and hepatocyte proliferation during postnatal development in mice172. Mice with liver-specific Yap1 deficiency have smaller livers and a reduced hepatocyte proliferation rate compared with wild-type mice169. In addition, liver-specific Yap1-knockout mice show impaired bile duct formation, suggesting a critical role for YAP1 in bile duct development169. A study published in 2015 found that the Hippo pathway might be a major downstream effector of the transcription factors SOX4 and SOX9, which regulate liver and bile duct development173. Another study published in 2014 showed that the main function of the Hippo pathway in the liver is to maintain the differentiation status of hepatocytes125. The activation of YAP1 dedifferentiates adult mouse hepatocytes into progenitor or ductal-like cells. In summary, YAP1 induces cell proliferation and suppresses differentiation during liver development, and reduced Hippo pathway activity causes uncontrolled organ growth and therefore tumorigenesis.
Hippo signalling is also involved in liver repair after injury in mouse models. A few studies have implicated that the Hippo signalling regulates liver regeneration after partial hepatectomy in rats and mice174–176. In addition, studies in the past few years have demonstrated the essential role of YAP1 in liver repair after injury. Deletion of Yap1 in mice potentiates cholestatic liver injury caused by bile-duct ligation144. Similarly, mice with liver-specific knockout of Yap1 exhibit deficient liver repair after carbon-tetrachloride-induced liver injury177. Notably, this study used a mosaic knockout mouse model to show that YAP1 activation alone in a fraction of hepatocytes is not sufficient to drive growth of the liver, whereas previous reports showed an organ hyperplasia phenotype as a result of YAP1 over-expression in the entire liver39,98. A possible explanation for this finding is that relative YAP1 activation in the two models is different. In conjunction with high YAP1 activity, inflammatory signals, which are always present in the context of liver injury, are essential to induce proliferation of hepatocytes177. Upon liver injury, YAP1 is also activated in cells besides hepatocytes. In the livers of carbon-tetrachloride-treated mice and tissue samples from human fibrotic livers, activated YAP1 causes activation of hepatic stellate cells. Prolonged activation of these cells leads to liver fibrogenesis178.
Hippo signalling and human liver tumorigenesis
In the healthy adult liver, YAP1 is expressed and active in the bile duct cells, whereas it is expressed at low levels and is inactive in hepatocytes125. However, numerous immunohistochemistry and immunoblot analyses show raised YAP1 protein levels and increased nuclear localization in human liver cancer cells compared with adjacent normal tissues36,39,164,179–182. YAP1 activity, defined either as protein levels or the degree of nuclear localization, can serve as a prognostic marker for overall survival and disease-free survival of patients with hepatocellular carcinoma164,183.
YAP1 activation in liver cancer could result from many factors. The YAP1 gene was observed to be frequently amplified in human hepatocellular carcinoma tumours184. YAP1 can also be activated by carcinogenic compounds such as 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene185, which mimics the xenobiotic Phenobarbital to activate the nuclear receptor constitutive androstane receptor (CAR). The tumour-promoting role of CAR has already been implicated in liver cancer186,187. However, a direct link between YAP1 and CAR has not been clearly established. HBV is a major cause of liver cancer, and the viral HBx protein can increase hepatocyte protein levels of YAP1 and TAZ in vitro, which might contribute to tumorigenesis induced by HBV infection188,189. In addition, bile acids, which play an important regulatory part in liver injury and injuryrelated carcinogenesis190,191, also activate YAP1 to promote spontaneous liver tumorigenesis in a mouse model of hepatocellular carcinoma192.
Mechanistically, the tumour-promoting property of YAP1 in liver cancer mainly relies on TEAD-mediated gene transcription. The YAP–TEAD complex can upregulate the expression of genes directly involved in promoting cell proliferation and tissue overgrowth, for instance the Notch ligand Jagged1 and the amino acid transporters SLC38A1 and SLC7A5 (REFS 193,194). In addition, YAP–TEAD also increases expression of the microRNA miR-130a, which in turn activates YAP1 by repressing the translation of the YAP1 antagonist VGLL4, resulting in a positive feedback loop that reinforces TEAD-mediated transcription195. Using gene-set enrichment analysis of liver tissue from a mouse model of liver cancer, a study published in 2015 reported that the YAP–TEAD complex colocalizes with other transcription factors, such as E2F1, HNF4α and β-catenin, to coordinate the expression of genes that modulate hepatocyte quiescence and differentiation196. Disruption of YAP–TEAD interaction using verteporfin abolishes tumour growth in vitro and in mouse models74,197; targeting YAP–TEAD interaction could therefore be a potential therapeutic strategy for hepatocellular carcinoma.
Conclusions
The Hippo pathway has an important role in intestinal homeostasis and regeneration in both Drosophila and mammals, and its dysregulation often leads to tumorigenesis. In general, YAP1 and TAZ activity is associated with ISC maintenance and progenitor cell proliferation. Although YAP1 and TAZ might be dispensable under normal intestinal homeostasis, their activation is crucial during tissue regeneration, particularly in response to injury. Negative regulation of YAP1 and TAZ activity by upstream components of the Hippo pathway, such as MST1, MST2 and SAV1, is required to maintain normal intestine homeostasis and prevent tissue overgrowth. The Hippo pathway is integrated with other key pathways that are important for intestinal homeostasis, such as the Wnt signalling pathway. YAP1 acts either as a downstream effector and/or an inhibitor of Wnt signalling to contribute to ISC maintenance and differentiation of transit amplifying cells. The complex communications between the Hippo pathway and other pathways, especially the Wnt pathway, are still being explored and remain an important question in the gastrointestinal field. Notably, in vitro studies show the Hippo pathway can be activated by tight junctions and adherence junctions. Intestinal diseases are often associated with disruption of the intestine lining and junction structures. One can speculate that the intactness of IEC junction structure is a major signal acting via the Hippo pathway to modulate intestine homeostasis. Thus, it would be interesting to investigate the regulation of the Hippo pathway by junctions in the intestine, and how the junction structures contribute to intestinal tissue regeneration in the context of Hippo signalling pathway.
Key points.
The Hippo pathway plays an important part in intestinal homeostasis and regeneration in both Drosophila melanogaster and mammals, and its dysregulation often leads to uncontrolled tissue growth
In general, YAP1 and TAZ activity promotes intestinal stem cell properties, and overexpression of YAP1 induces stem cell expansion
YAP1 and TAZ are crucial for intestinal tissue regeneration after injury; YAP1 depletion leads to disturbed tissue formation during regeneration
Crosstalk between the Hippo pathway and other signalling pathways, such as Wnt and Notch, regulates intestinal tissue homeostasis and regeneration, although detailed mechanisms have not yet been elucidated
Dysregulation of the Hippo pathway leads to tumorigenesis; YAP1 and TAZ are oncogenic in colorectal cancers, as illustrated by data from mouse models and patient specimens
The Hippo pathway also has an important role in liver damage repair and tumorigenesis, and dysregulation of the Hippo pathway leads to uncontrolled growth in the liver
Acknowledgments
The authors apologize to colleagues who have made many important contributions to the Hippo field but whose work could not be cited because of the scope of this Review. We would like to thank K. Lin and T. Moroishi for critical reading of this manuscript. K-L.G. is supported by grants from the NIH (CA132809, EYO226116, and P30CA023100). A.W.H. is supported in part by the T32 GM007752 training grant.
Footnotes
Author contributions
All authors contributed equally to the article.
Competing interests statement
The authors declare no competing interests.
References
- 1.Barry ER, Camargo FD. The Hippo superhighway: signaling crossroads converging on the Hippo/Yap pathway in stem cells and development. Curr Opin Cell Biol. 2013;25:247–253. doi: 10.1016/j.ceb.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev. 2014;94:1287–1312. doi: 10.1152/physrev.00005.2014. [DOI] [PubMed] [Google Scholar]
- 3.Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246–257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- 4.Moroishi T, Hansen CG, Guan KL. The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer. 2015;15:73–79. doi: 10.1038/nrc3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu FX, Meng Z, Plouffe SW, Guan KL. Hippo pathway regulation of gastrointestinal tissues. Annu Rev Physiol. 2015;77:201–227. doi: 10.1146/annurev-physiol-021014-071733. [DOI] [PubMed] [Google Scholar]
- 6.Bossuyt W, et al. An evolutionary shift in the regulation of the Hippo pathway between mice and flies. Oncogene. 2014;33:1218–1228. doi: 10.1038/onc.2013.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meng Z, Moroishi T, Guan KL. Mechanisms of Hippo pathway regulation. Genes Dev. 2016;30:1–17. doi: 10.1101/gad.274027.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Justice RW, Zilian O, Woods DF, Noll M, Bryant PJ. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 1995;9:534–546. doi: 10.1101/gad.9.5.534. [DOI] [PubMed] [Google Scholar]
- 9.Xu T, Wang W, Zhang S, Stewart RA, Yu W. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development. 1995;121:1053–1063. doi: 10.1242/dev.121.4.1053. [DOI] [PubMed] [Google Scholar]
- 10.Wu S, Huang J, Dong J, Pan D. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell. 2003;114:445–456. doi: 10.1016/s0092-8674(03)00549-x. [DOI] [PubMed] [Google Scholar]
- 11.Udan RS, Kango-Singh M, Nolo R, Tao C, Halder G. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat Cell Biol. 2003;5:914–920. doi: 10.1038/ncb1050. [DOI] [PubMed] [Google Scholar]
- 12.Pantalacci S, Tapon N, Leopold P. The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nat Cell Biol. 2003;5:921–927. doi: 10.1038/ncb1051. [DOI] [PubMed] [Google Scholar]
- 13.Jia J, Zhang W, Wang B, Trinko R, Jiang J. The Drosophila Ste20 family kinase dMST functions as a tumor suppressor by restricting cell proliferation and promoting apoptosis. Genes Dev. 2003;17:2514–2519. doi: 10.1101/gad.1134003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harvey KF, Pfleger CM, Hariharan IK. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell. 2003;114:457–467. doi: 10.1016/s0092-8674(03)00557-9. [DOI] [PubMed] [Google Scholar]
- 15.Kango-Singh M, et al. Shar-pei mediates cell proliferation arrest during imaginal disc growth in Drosophila. Development. 2002;129:5719–5730. doi: 10.1242/dev.00168. [DOI] [PubMed] [Google Scholar]
- 16.Tapon N, et al. salvador promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell. 2002;110:467–478. doi: 10.1016/s0092-8674(02)00824-3. [DOI] [PubMed] [Google Scholar]
- 17.Lai ZC, et al. Control of cell proliferation and apoptosis by mob as tumor suppressor, mats. Cell. 2005;120:675–685. doi: 10.1016/j.cell.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 18.Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila homolog of YAP. Cell. 2005;122:421–434. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Thompson BJ, Cohen SM. The Hippo pathway regulates the bantam microRNA to control cell proliferation and apoptosis in Drosophila. Cell. 2006;126:767–774. doi: 10.1016/j.cell.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 20.Wei X, Shimizu T, Lai ZC. Mob as tumor suppressor is activated by Hippo kinase for growth inhibition in Drosophila. EMBO J. 2007;26:1772–1781. doi: 10.1038/sj.emboj.7601630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu S, Liu Y, Zheng Y, Dong J, Pan D. The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev Cell. 2008;14:388–398. doi: 10.1016/j.devcel.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Zheng Y, et al. Identification of Happyhour/MAP4K as alternative Hpo/Mst-like kinases in the Hippo kinase cascade. Dev Cell. 2015;34:642–655. doi: 10.1016/j.devcel.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Q, et al. The conserved misshapen–warts–Yorkie pathway acts in enteroblasts to regulate intestinal stem cells in Drosophila. Dev Cell. 2014;31:291–304. doi: 10.1016/j.devcel.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meng Z, et al. MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2 in the Hippo pathway. Nat Commun. 2015;6:8357. doi: 10.1038/ncomms9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Staley BK, Irvine KD. Warts and Yorkie mediate intestinal regeneration by influencing stem cell proliferation. Curr Biol. 2010;20:1580–1587. doi: 10.1016/j.cub.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu FX, Guan KL. The Hippo pathway: regulators and regulations. Genes Dev. 2013;27:355–371. doi: 10.1101/gad.210773.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lei QY, et al. TAZ promotes cell proliferation and epithelial–mesenchymal transition and is inhibited by the Hippo pathway. Mol Cell Biol. 2008;28:2426–2436. doi: 10.1128/MCB.01874-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varelas X. The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development. 2014;141:1614–1626. doi: 10.1242/dev.102376. [DOI] [PubMed] [Google Scholar]
- 29.Glantschnig H, Rodan GA, Reszka AA. Mapping of MST1 kinase sites of phosphorylation. Activation and autophosphorylation. J Biol Chem. 2002;277:42987–42996. doi: 10.1074/jbc.M208538200. [DOI] [PubMed] [Google Scholar]
- 30.Lee KK, Yonehara S. Phosphorylation and dimerization regulate nucleocytoplasmic shuttling of mammalian STE20-like kinase (MST) J Biol Chem. 2002;277:12351–12358. doi: 10.1074/jbc.M108138200. [DOI] [PubMed] [Google Scholar]
- 31.Creasy CL, Ambrose DM, Chernoff J. The Ste20-like protein kinase, Mst1, dimerizes and contains an inhibitory domain. J Biol Chem. 1996;271:21049–21053. doi: 10.1074/jbc.271.35.21049. [DOI] [PubMed] [Google Scholar]
- 32.Hwang E, et al. Structural insight into dimeric interaction of the SARAH domains from Mst1 and RASSF family proteins in the apoptosis pathway. Proc Natl Acad Sci USA. 2007;104:9236–9241. doi: 10.1073/pnas.0610716104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ni L, Zheng Y, Hara M, Pan D, Luo X. Structural basis for Mob1-dependent activation of the core Mst–Lats kinase cascade in Hippo signaling. Genes Dev. 2015;29:1416–1431. doi: 10.1101/gad.264929.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Callus BA, Verhagen AM, Vaux DL. Association of mammalian sterile twenty kinases, Mst1 and Mst2, with hSalvador via C-terminal coiled-coil domains, leads to its stabilization and phosphorylation. FEBS J. 2006;273:4264–4276. doi: 10.1111/j.1742-4658.2006.05427.x. [DOI] [PubMed] [Google Scholar]
- 35.Chan EH, et al. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene. 2005;24:2076–2086. doi: 10.1038/sj.onc.1208445. [DOI] [PubMed] [Google Scholar]
- 36.Zhao B, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCFβ-TRCP. Genes Dev. 2010;24:72–85. doi: 10.1101/gad.1843810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao B, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong J, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murakami H, et al. LATS2 is a tumor suppressor gene of malignant mesothelioma. Cancer Res. 2011;71:873–883. doi: 10.1158/0008-5472.CAN-10-2164. [DOI] [PubMed] [Google Scholar]
- 41.Plouffe SW, Hong AW, Guan KL. Disease implications of the Hippo/YAP pathway. Trends Mol Med. 2015;21:212–222. doi: 10.1016/j.molmed.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson R, Halder G. The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat Rev Drug Discov. 2014;13:63–79. doi: 10.1038/nrd4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schulzke JD, et al. Epithelial tight junctions in intestinal inflammation. Ann NY Acad Sci. 2009;1165:294–300. doi: 10.1111/j.1749-6632.2009.04062.x. [DOI] [PubMed] [Google Scholar]
- 44.Paramasivam M, Sarkeshik A, Yates JR, 3rd, Fernandes MJ, McCollum D. Angiomotin family proteins are novel activators of the LATS2 kinase tumor suppressor. Mol Biol Cell. 2011;22:3725–3733. doi: 10.1091/mbc.E11-04-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao B, et al. Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes Dev. 2011;25:51–63. doi: 10.1101/gad.2000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu J, et al. Kibra functions as a tumor suppressor protein that regulates Hippo signaling in conjunction with Merlin and Expanded. Dev Cell. 2010;18:288–299. doi: 10.1016/j.devcel.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Genevet A, Wehr MC, Brain R, Thompson BJ, Tapon N. Kibra is a regulator of the Salvador/Warts/Hippo signaling network. Dev Cell. 2010;18:300–308. doi: 10.1016/j.devcel.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baumgartner R, Poernbacher I, Buser N, Hafen E, Stocker H. The WW domain protein Kibra acts upstream of Hippo in Drosophila. Dev Cell. 2010;18:309–316. doi: 10.1016/j.devcel.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 49.Mohseni M, et al. A genetic screen identifies an LKB1–MARK signalling axis controlling the Hippo–YAP pathway. Nat Cell Biol. 2014;16:108–117. doi: 10.1038/ncb2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mo JS, et al. Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway. Nat Cell Biol. 2015;17:500–510. doi: 10.1038/ncb3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang W, et al. AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nat Cell Biol. 2015;17:490–499. doi: 10.1038/ncb3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DeRan M, et al. Energy stress regulates hippo–YAP signaling involving AMPK-mediated regulation of angiomotin-like 1 protein. Cell Rep. 2014;9:495–503. doi: 10.1016/j.celrep.2014.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bennett FC, Harvey KF. Fat cadherin modulates organ size in Drosophila via the Salvador/Warts/Hippo signaling pathway. Curr Biol. 2006;16:2101–2110. doi: 10.1016/j.cub.2006.09.045. [DOI] [PubMed] [Google Scholar]
- 54.Cho E, et al. Delineation of a Fat tumor suppressor pathway. Nat Genet. 2006;38:1142–1150. doi: 10.1038/ng1887. [DOI] [PubMed] [Google Scholar]
- 55.Silva E, Tsatskis Y, Gardano L, Tapon N, McNeill H. The tumor-suppressor gene fat controls tissue growth upstream of expanded in the hippo signaling pathway. Curr Biol. 2006;16:2081–2089. doi: 10.1016/j.cub.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 56.Willecke M, et al. The Fat cadherin acts through the hippo tumor-suppressor pathway to regulate tissue size. Curr Biol. 2006;16:2090–2100. doi: 10.1016/j.cub.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 57.Kim NG, Koh E, Chen X, Gumbiner BM. E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proc Natl Acad Sci USA. 2011;108:11930–11935. doi: 10.1073/pnas.1103345108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mori M, et al. Hippo signaling regulates microprocessor and links cell-density-dependent miRNA biogenesis to cancer. Cell. 2014;156:893–906. doi: 10.1016/j.cell.2013.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chaulk SG, Lattanzi VJ, Hiemer SE, Fahlman RP, Varelas X. The Hippo pathway effectors TAZ/YAP regulate dicer expression and microRNA biogenesis through Let-7. J Biol Chem. 2014;289:1886–1891. doi: 10.1074/jbc.C113.529362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shao D, et al. A functional interaction between Hippo-YAP signalling and FoxO1 mediates the oxidative stress response. Nat Commun. 2014;5:3315. doi: 10.1038/ncomms4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu H, et al. Integration of Hippo signalling and the unfolded protein response to restrain liver overgrowth and tumorigenesis. Nat Commun. 2015;6:6239. doi: 10.1038/ncomms7239. [DOI] [PubMed] [Google Scholar]
- 62.Ma B, et al. Hypoxia regulates Hippo signalling through the SIAH2 ubiquitin E3 ligase. Nat Cell Biol. 2015;17:95–103. doi: 10.1038/ncb3073. [DOI] [PubMed] [Google Scholar]
- 63.Dupont S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 64.Zhao B, et al. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 2012;26:54–68. doi: 10.1101/gad.173435.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rauskolb C, Sun S, Sun G, Pan Y, Irvine KD. Cytoskeletal tension inhibits Hippo signaling through an Ajuba–Warts complex. Cell. 2014;158:143–156. doi: 10.1016/j.cell.2014.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Codelia VA, Sun G, Irvine KD. Regulation of YAP by mechanical strain through Jnk and Hippo signaling. Curr Biol. 2014;24:2012–2017. doi: 10.1016/j.cub.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gayer CP, Basson MD. The effects of mechanical forces on intestinal physiology and pathology. Cell Signal. 2009;21:1237–1244. doi: 10.1016/j.cellsig.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu FX, et al. Regulation of the Hippo–YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu FX, et al. Protein kinase A activates the Hippo pathway to modulate cell proliferation and differentiation. Genes Dev. 2013;27:1223–1232. doi: 10.1101/gad.219402.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mo JS, Yu FX, Gong R, Brown JH, Guan KL. Regulation of the Hippo–YAP pathway by protease-activated receptors (PARs) Genes Dev. 2012;26:2138–2143. doi: 10.1101/gad.197582.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.O’Hayre M, et al. The emerging mutational landscape of G proteins and G-protein-coupled receptors in cancer. Nat Rev Cancer. 2013;13:412–424. doi: 10.1038/nrc3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu G, et al. Kaposi sarcoma-associated herpesvirus promotes tumorigenesis by modulating the Hippo pathway. Oncogene. 2014;34:3536–3546. doi: 10.1038/onc.2014.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Feng X, et al. Hippo-independent activation of YAP by the GNAQ uveal melanoma oncogene through a trio-regulated rho GTPase signaling circuitry. Cancer Cell. 2014;25:831–845. doi: 10.1016/j.ccr.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu FX, et al. Mutant Gq/11 promote uveal melanoma tumorigenesis by activating YAP. Cancer Cell. 2014;25:822–830. doi: 10.1016/j.ccr.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bao Y, et al. A cell-based assay to screen stimulators of the Hippo pathway reveals the inhibitory effect of dobutamine on the YAP-dependent gene transcription. J Biochem. 2011;150:199–208. doi: 10.1093/jb/mvr063. [DOI] [PubMed] [Google Scholar]
- 76.Iglesias-Bartolome R, et al. Inactivation of a Gαs-PKA tumour suppressor pathway in skin stem cells initiates basal-cell carcinogenesis. Nat Cell Biol. 2015;17:793–803. doi: 10.1038/ncb3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reimann F, Tolhurst G, Gribble FM. G-protein-coupled receptors in intestinal chemosensation. Cell Metab. 2012;15:421–431. doi: 10.1016/j.cmet.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 78.Pasco MY, Loudhaief R, Gallet A. The cellular homeostasis of the gut: what the Drosophila model points out. Histol Histopathol. 2015;30:277–292. doi: 10.14670/HH-30.277. [DOI] [PubMed] [Google Scholar]
- 79.Buchon N, Broderick NA, Lemaitre B. Gut homeostasis in a microbial world: insights from Drosophila melanogaster. Nat Rev Microbiol. 2013;11:615–626. doi: 10.1038/nrmicro3074. [DOI] [PubMed] [Google Scholar]
- 80.Sun G, Irvine KD. Ajuba family proteins link JNK to Hippo signaling. Sci Signal. 2013;6:ra81. doi: 10.1126/scisignal.2004324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shaw RL, et al. The Hippo pathway regulates intestinal stem cell proliferation during Drosophila adult midgut regeneration. Development. 2010;137:4147–4158. doi: 10.1242/dev.052506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Karpowicz P, Perez J, Perrimon N. The Hippo tumor suppressor pathway regulates intestinal stem cell regeneration. Development. 2010;137:4135–4145. doi: 10.1242/dev.060483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ren F, et al. Hippo signaling regulates Drosophila intestine stem cell proliferation through multiple pathways. Proc Natl Acad Sci USA. 2010;107:21064–21069. doi: 10.1073/pnas.1012759107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huang H, et al. Bantam is essential for Drosophila intestinal stem cell proliferation in response to Hippo signaling. Dev Biol. 2014;385:211–219. doi: 10.1016/j.ydbio.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 85.Poernbacher I, Baumgartner R, Marada SK, Edwards K, Stocker H. Drosophila Pez acts in Hippo signaling to restrict intestinal stem cell proliferation. Curr Biol. 2012;22:389–396. doi: 10.1016/j.cub.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 86.Wang C, et al. Suppressor of Deltex mediates Pez degradation and modulates Drosophila midgut homeostasis. Nat Commun. 2015;6:6607. doi: 10.1038/ncomms7607. [DOI] [PubMed] [Google Scholar]
- 87.Wang Z, et al. Mutational analysis of the tyrosine phosphatome in colorectal cancers. Science. 2004;304:1164–1166. doi: 10.1126/science.1096096. [DOI] [PubMed] [Google Scholar]
- 88.Huang X, et al. The sterile 20-like kinase tao controls tissue homeostasis by regulating the hippo pathway in Drosophila adult midgut. J Genet Genomics. 2014;41:429–438. doi: 10.1016/j.jgg.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 89.Ren F, et al. Drosophila Myc integrates multiple signaling pathways to regulate intestinal stem cell proliferation during midgut regeneration. Cell Res. 2013;23:1133–1146. doi: 10.1038/cr.2013.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jin Y, et al. Brahma is essential for Drosophila intestinal stem cell proliferation and regulated by Hippo signaling. eLife. 2013;2:e00999. doi: 10.7554/eLife.00999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol. 2014;15:19–33. doi: 10.1038/nrm3721. [DOI] [PubMed] [Google Scholar]
- 92.Sato T, et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 94.Barker N, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 95.Munoz J, et al. The Lgr5 intestinal stem cell signature: robust expression of proposed quiescent ‘+4’ cell markers. EMBO J. 2012;31:3079–3091. doi: 10.1038/emboj.2012.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tian H, et al. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255–259. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mo JS, Park HW, Guan KL. The Hippo signaling pathway in stem cell biology and cancer. EMBO Rep. 2014;15:642–656. doi: 10.15252/embr.201438638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Camargo FD, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 99.Azzolin L, et al. YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158:157–170. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 100.Cai J, et al. The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes Dev. 2010;24:2383–2388. doi: 10.1101/gad.1978810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhou D, et al. Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance. Proc Natl Acad Sci USA. 2011;108:E1312–E1320. doi: 10.1073/pnas.1110428108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Imajo M, Ebisuya M, Nishida E. Dual role of YAP and TAZ in renewal of the intestinal epithelium. Nat Cell Biol. 2015;17:7–19. doi: 10.1038/ncb3084. [DOI] [PubMed] [Google Scholar]
- 103.Llado V, et al. Repression of intestinal stem cell function and tumorigenesis through direct phosphorylation of β-catenin and Yap by PKCζ. Cell Rep. 2015;10:740–754. doi: 10.1016/j.celrep.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Barry ER, et al. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature. 2013;493:106–110. doi: 10.1038/nature11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Moroishi T, et al. A YAP/TAZ-induced feedback mechanism regulates Hippo pathway homeostasis. Genes Dev. 2015;29:1271–1284. doi: 10.1101/gad.262816.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen Q, et al. Homeostatic control of Hippo signaling activity revealed by an endogenous activating mutation in YAP. Genes Dev. 2015;29:1285–1297. doi: 10.1101/gad.264234.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dai X, et al. YAP activates the Hippo pathway in a negative feedback loop. Cell Res. 2015;25:1175–1178. doi: 10.1038/cr.2015.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Taniguchi K, et al. A gp130–Src–YAP module links inflammation to epithelial regeneration. Nature. 2015;519:57–U107. doi: 10.1038/nature14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gregorieff A, Liu Y, Inanlou MR, Khomchuk Y, Wrana JL. Yap-dependent reprogramming of Lgr5+ stem cells drives intestinal regeneration and cancer. Nature. 2015;526:715–718. doi: 10.1038/nature15382. [DOI] [PubMed] [Google Scholar]
- 110.Stroncek JD, Reichert WM. Indwelling Neural Implants: Strategies for Contending with the In Vivo Environment. CRC Press; 2008. [PubMed] [Google Scholar]
- 111.Vanuytsel T, Senger S, Fasano A, Shea-Donohue T. Major signaling pathways in intestinal stem cells. Biochim Biophys Acta. 2013;1830:2410–2426. doi: 10.1016/j.bbagen.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pinto D, Gregorieff A, Begthel H, Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17:1709–1713. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Batlle E, et al. β-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell. 2002;111:251–263. doi: 10.1016/s0092-8674(02)01015-2. [DOI] [PubMed] [Google Scholar]
- 114.Korinek V, et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 115.Varelas X, et al. The Hippo pathway regulates Wnt/β-catenin signaling. Dev Cell. 2010;18:579–591. doi: 10.1016/j.devcel.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 116.Imajo M, Miyatake K, Iimura A, Miyamoto A, Nishida E. A molecular mechanism that links Hippo signalling to the inhibition of Wnt/β-catenin signalling. EMBO J. 2012;31:1109–1122. doi: 10.1038/emboj.2011.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Azzolin L, et al. Role of TAZ as mediator of Wnt signaling. Cell. 2012;151:1443–1456. doi: 10.1016/j.cell.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 118.Konsavage WM, et al. Wnt/β-catenin signaling regulates Yes-associated protein (YAP) gene expression in colorectal carcinoma cells. J Biol Chem. 2012;287:11730–11739. doi: 10.1074/jbc.M111.327767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Miyoshi H, Ajima R, Luo CT, Yamaguchi TP, Stappenbeck TS. Wnt5a potentiates TGF-β signaling to promote colonic crypt regeneration after tissue injury. Science. 2012;338:108–113. doi: 10.1126/science.1223821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Park HW, et al. Alternative Wnt signaling activates YAP/TAZ. Cell. 2015;162:780–794. doi: 10.1016/j.cell.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.VanDussen KL, et al. Notch signaling modulates proliferation and differentiation of intestinal crypt base columnar stem cells. Development. 2012;139:488–497. doi: 10.1242/dev.070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sancho R, Cremona CA, Behrens A. Stem cell and progenitor fate in the mammalian intestine: Notch and lateral inhibition in homeostasis and disease. EMBO Rep. 2015;16:571–581. doi: 10.15252/embr.201540188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.van Es JH, et al. Notch/γ-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 125.Yimlamai D, et al. Hippo pathway activity influences liver cell fate. Cell. 2014;157:1324–1338. doi: 10.1016/j.cell.2014.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang RN, et al. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis. 2014;1:87–105. doi: 10.1016/j.gendis.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Haramis AP, et al. De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science. 2004;303:1684–1686. doi: 10.1126/science.1093587. [DOI] [PubMed] [Google Scholar]
- 128.He XC, et al. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-β-catenin signaling. Nat Genet. 2004;36:1117–1121. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- 129.Alarcon C, et al. Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGF-β pathways. Cell. 2009;139:757–769. doi: 10.1016/j.cell.2009.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Batts LE, Polk DB, Dubois RN, Kulessa H. Bmp signaling is required for intestinal growth and morphogenesis. Dev Dyn. 2006;235:1563–1570. doi: 10.1002/dvdy.20741. [DOI] [PubMed] [Google Scholar]
- 131.Kosinski C, et al. Indian hedgehog regulates intestinal stem cell fate through epithelial-mesenchymal interactions during development. Gastroenterology. 2010;139:893–903. doi: 10.1053/j.gastro.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Reifenberger J, et al. Missense mutations in SMOH in sporadic basal cell carcinomas of the skin and primitive neuroectodermal tumors of the central nervous system. Cancer Res. 1998;58:1798–1803. [PubMed] [Google Scholar]
- 133.Wetmore C. Sonic hedgehog in normal and neoplastic proliferation: insight gained from human tumors and animal models. Curr Opin Genet Dev. 2003;13:34–42. doi: 10.1016/s0959-437x(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 134.Fernandez LA, et al. YAP1 is amplified and up-regulated in hedgehog-associated medulloblastomas and mediates Sonic hedgehog-driven neural precursor proliferation. Genes Dev. 2009;23:2729–2741. doi: 10.1101/gad.1824509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–2114.e5. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 136.Zhang L, et al. NDR functions as a physiological YAP1 kinase in the intestinal epithelium. Curr Biol. 2015;25:296–305. doi: 10.1016/j.cub.2014.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Steinhardt AA, et al. Expression of Yes-associated protein in common solid tumors. Hum Pathol. 2008;39:1582–1589. doi: 10.1016/j.humpath.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lam-Himlin DM, et al. The hippo pathway in human upper gastrointestinal dysplasia and carcinoma: a novel oncogenic pathway. Int J Gastrointest Cancer. 2006;37:103–109. doi: 10.1007/s12029-007-0010-8. [DOI] [PubMed] [Google Scholar]
- 139.Wang L, et al. Overexpression of YAP and TAZ is an independent predictor of prognosis in colorectal cancer and related to the proliferation and metastasis of colon cancer cells. PLoS ONE. 2013;8:e65539. doi: 10.1371/journal.pone.0065539. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 140.Yuen HF, et al. TAZ expression as a prognostic indicator in colorectal cancer. PLoS ONE. 2013;8:e54211. doi: 10.1371/journal.pone.0054211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pan J, et al. Lentivirus-mediated RNA interference targeting WWTR1 in human colorectal cancer cells inhibits cell proliferation in vitro and tumor growth in vivo. Oncol Rep. 2012;28:179–185. doi: 10.3892/or.2012.1751. [DOI] [PubMed] [Google Scholar]
- 142.Powell SM, et al. APC mutations occur early during colorectal tumorigenesis. Nature. 1992;359:235–237. doi: 10.1038/359235a0. [DOI] [PubMed] [Google Scholar]
- 143.Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell. 2000;103:311–320. doi: 10.1016/s0092-8674(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 144.Brabletz T, Jung A, Dag S, Hlubek F, Kirchner T. β-catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am J Pathol. 1999;155:1033–1038. doi: 10.1016/s0002-9440(10)65204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Crawford HC, et al. The metalloproteinase matrilysin is a target of β-catenin transactivation in intestinal tumors. Oncogene. 1999;18:2883–2891. doi: 10.1038/sj.onc.1202627. [DOI] [PubMed] [Google Scholar]
- 146.He TC, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 147.Tetsu O, McCormick F. β-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 148.He TC, Chan TA, Vogelstein B, Kinzler KW. PPARδ is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell. 1999;99:335–345. doi: 10.1016/s0092-8674(00)81664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cai J, Maitra A, Anders RA, Taketo MM, Pan D. β-catenin destruction complex-independent regulation of Hippo-YAP signaling by APC in intestinal tumorigenesis. Genes Dev. 2015;29:1493–1506. doi: 10.1101/gad.264515.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rosenbluh J, et al. β-catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell. 2012;151:1457–1473. doi: 10.1016/j.cell.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sancho R, et al. JNK signalling modulates intestinal homeostasis and tumourigenesis in mice. EMBO J. 2009;28:1843–1854. doi: 10.1038/emboj.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chen F. JNK-induced apoptosis, compensatory growth, and cancer stem cells. Cancer Res. 2012;72:379–386. doi: 10.1158/0008-5472.CAN-11-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sun G, Irvine KD. Regulation of Hippo signaling by Jun kinase signaling during compensatory cell proliferation and regeneration, and in neoplastic tumors. Dev Biol. 2011;350:139–151. doi: 10.1016/j.ydbio.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tomlinson V, et al. JNK phosphorylates Yes-associated protein (YAP) to regulate apoptosis. Cell Death Dis. 2010;1:e29. doi: 10.1038/cddis.2010.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mehenni H, et al. Loss of LKB1 kinase activity in Peutz–Jeghers syndrome, and evidence for allelic and locus heterogeneity. Am J Hum Genet. 1998;63:1641–1650. doi: 10.1086/302159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Giardiello FM, et al. Increased risk of cancer in the Peutz–Jeghers syndrome. N Engl J Med. 1987;316:1511–1514. doi: 10.1056/NEJM198706113162404. [DOI] [PubMed] [Google Scholar]
- 157.Sanchez-Cespedes M. A role for LKB1 gene in human cancer beyond the Peutz–Jeghers syndrome. Oncogene. 2007;26:7825–7832. doi: 10.1038/sj.onc.1210594. [DOI] [PubMed] [Google Scholar]
- 158.Nguyen HB, Babcock JT, Wells CD, Quilliam LA. LKB1 tumor suppressor regulates AMP kinase/mTOR-independent cell growth and proliferation via the phosphorylation of Yap. Oncogene. 2013;32:4100–4109. doi: 10.1038/onc.2012.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Grivennikov S, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bechmann LP, et al. The interaction of hepatic lipid and glucose metabolism in liver diseases. J Hepatol. 2012;56:952–964. doi: 10.1016/j.jhep.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 161.Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5:836–847. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- 162.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–S53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 163.Pan D. Hippo signaling in organ size control. Genes Dev. 2007;21:886–897. doi: 10.1101/gad.1536007. [DOI] [PubMed] [Google Scholar]
- 164.Xu MZ, et al. Yes-associated protein is an independent prognostic marker in hepatocellular carcinoma. Cancer. 2009;115:4576–4585. doi: 10.1002/cncr.24495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lee KP, et al. The Hippo–Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc Natl Acad Sci USA. 2010;107:8248–8253. doi: 10.1073/pnas.0912203107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhou D, et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16:425–438. doi: 10.1016/j.ccr.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Song H, et al. Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proc Natl Acad Sci USA. 2010;107:1431–1436. doi: 10.1073/pnas.0911409107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lu L, et al. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc Natl Acad Sci USA. 2010;107:1437–1442. doi: 10.1073/pnas.0911427107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhang N, et al. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev Cell. 2010;19:27–38. doi: 10.1016/j.devcel.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yin F, et al. Spatial organization of Hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2. Cell. 2013;154:1342–1355. doi: 10.1016/j.cell.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nishio M, et al. Cancer susceptibility and embryonic lethality in Mob1a/1b double-mutant mice. J Clin Invest. 2012;122:4505–4518. doi: 10.1172/JCI63735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Septer S, et al. Yes-associated protein is involved in proliferation and differentiation during postnatal liver development. Am J Physiol Gastrointest Liver Physiol. 2012;302:G493–G503. doi: 10.1152/ajpgi.00056.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Poncy A, et al. Transcription factors SOX4 and SOX9 cooperatively control development of bile ducts. Dev Biol. 2015;404:136–148. doi: 10.1016/j.ydbio.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 174.Grijalva JL, et al. Dynamic alterations in Hippo signaling pathway and YAP activation during liver regeneration. Am J Physiol Gastrointest Liver Physiol. 2014;307:G196–G204. doi: 10.1152/ajpgi.00077.2014. [DOI] [PubMed] [Google Scholar]
- 175.Apte U, et al. Enhanced liver regeneration following changes induced by hepatocyte-specific genetic ablation of integrin-linked kinase. Hepatology. 2009;50:844–851. doi: 10.1002/hep.23059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Herr KJ, et al. Loss of α-catenin elicits a cholestatic response and impairs liver regeneration. Sci Rep. 2014;4:6835. doi: 10.1038/srep06835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Su T, et al. Two-signal requirement for growth-promoting function of Yap in hepatocytes. eLife. 2015;4:e02948. doi: 10.7554/eLife.02948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mannaerts I, et al. The Hippo pathway effector YAP controls mouse hepatic stellate cell activation. J Hepatol. 2015;63:679–688. doi: 10.1016/j.jhep.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 179.Bai H, et al. Expression of Yes-associated protein modulates Survivin expression in primary liver malignancies. Hum Pathol. 2012;43:1376–1385. doi: 10.1016/j.humpath.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Li X, et al. Co-activation of PIK3CA and Yap promotes development of hepatocellular and cholangiocellular tumors in mouse and human liver. Oncotarget. 2015;6:10102–10115. doi: 10.18632/oncotarget.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Li H, et al. Deregulation of Hippo kinase signalling in human hepatic malignancies. Liver Int. 2012;32:38–47. doi: 10.1111/j.1478-3231.2011.02646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ahn EY, Kim JS, Kim GJ, Park YN. RASSF1A-mediated regulation of AREG via the Hippo pathway in hepatocellular carcinoma. Mol Cancer Res. 2013;11:748–758. doi: 10.1158/1541-7786.MCR-12-0665. [DOI] [PubMed] [Google Scholar]
- 183.Sohn BH, et al. Inactivation of Hippo pathway is significantly associated with poor prognosis in hepatocellular carcinoma. Clin Cancer Res. 2016;22:1256–1264. doi: 10.1158/1078-0432.CCR-15-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zender L, et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253–1267. doi: 10.1016/j.cell.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kowalik MA, et al. Yes-associated protein regulation of adaptive liver enlargement and hepatocellular carcinoma development in mice. Hepatology. 2011;53:2086–2096. doi: 10.1002/hep.24289. [DOI] [PubMed] [Google Scholar]
- 186.Huang W, et al. Xenobiotic stress induces hepatomegaly and liver tumors via the nuclear receptor constitutive androstane receptor. Mol Endocrinol. 2005;19:1646–1653. doi: 10.1210/me.2004-0520. [DOI] [PubMed] [Google Scholar]
- 187.Dong B, et al. Activating CAR and β-catenin induces uncontrolled liver growth and tumorigenesis. Nat Commun. 2015;6:5944. doi: 10.1038/ncomms6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhang T, et al. Hepatitis B virus X protein modulates oncogene Yes-associated protein by CREB to promote growth of hepatoma cells. Hepatology. 2012;56:2051–2059. doi: 10.1002/hep.25899. [DOI] [PubMed] [Google Scholar]
- 189.Liu P, et al. HBV preS2 promotes the expression of TAZ via miRNA-338-3p to enhance the tumorigenesis of hepatocellular carcinoma. Oncotarget. 2015;6:29048–29059. doi: 10.18632/oncotarget.4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wang X, et al. Bile acid receptors and liver cancer. Curr Pathobiol Rep. 2013;1:29–35. doi: 10.1007/s40139-012-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Liu N, et al. Hepatocarcinogenesis in FXR−/− mice mimics human HCC progression that operates through HNF1α regulation of FXR expression. Mol Endocrinol. 2012;26:775–785. doi: 10.1210/me.2011-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Anakk S, et al. Bile acids activate YAP to promote liver carcinogenesis. Cell Rep. 2013;5:1060–1069. doi: 10.1016/j.celrep.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Park YY, et al. Yes-associated protein 1 and transcriptional coactivator with PDZ-binding motif activate the mammalian target of rapamycin complex 1 pathway by regulating amino acid transporters in hepatocellular carcinoma. Hepatology. 2015;63:159–172. doi: 10.1002/hep.28223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Tschaharganeh DF, et al. Yes-associated protein up-regulates Jagged-1 and activates the Notch pathway in human hepatocellular carcinoma. Gastroenterology. 2013;144:1530–1542.e12. doi: 10.1053/j.gastro.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Shen S, et al. A miR-130a-YAP positive feedback loop promotes organ size and tumorigenesis. Cell Res. 2015;25:997–1012. doi: 10.1038/cr.2015.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Fitamant J, et al. YAP inhibition restores hepatocyte differentiation in advanced HCC, leading to tumor regression. Cell Rep. 2015;10:1692–1707. doi: 10.1016/j.celrep.2015.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Liu-Chittenden Y, et al. Genetic and pharmacological disruption of the TEAD–YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26:1300–1305. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]