INDEX CASE
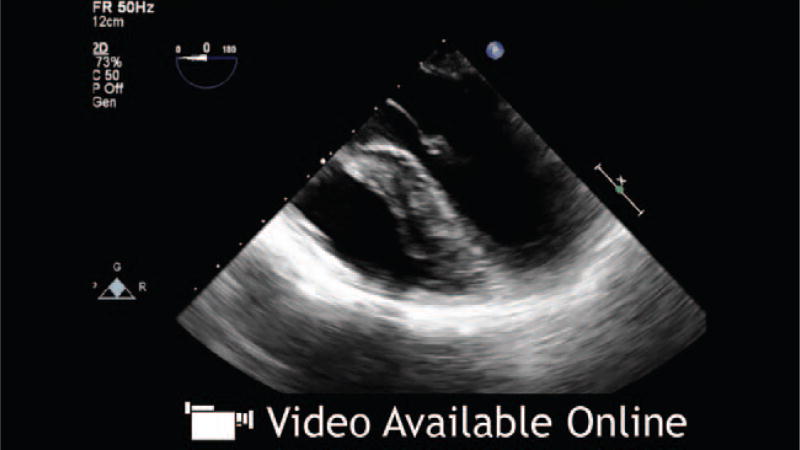
A 34-year-old woman with end-stage lung disease secondary to cystic fibrosis is undergoing bilateral lung transplantation. After reperfusion of the left-transplanted lung, transesophageal echocardiography (TEE) assessment reveals a large (45 × 4 mm) echogenic mass arising from the left upper pulmonary vein (PV) extending into the left atrium (LA).
Perioperative care of a patient undergoing lung transplantation can be challenging, given the patient’s limited cardiopulmonary reserve, and potential for hemodynamic and respiratory instability. Practice Guidelines from the American Society of Anesthesiologists and Society of Cardiovascular Anesthesiologists recommend TEE in the management of patients undergoing lung transplantation,1 particularly for assessment of hemodynamic instability and evaluation of pulmonary vasculature anastomoses.2,3 This Echo Didactics describes a focused examination for patients undergoing lung transplantation.
Pretransplant
A pretransplant TEE examination (Table 1) should include assessment of right (RV) and left ventricular function as significant changes in functional status can occur between the initial transplant eligibility evaluation and time of transplant. Furthermore, previously unidentified intracardiac shunts were shown to alter the surgical plan in 25% of patients.4
Table 1.
Pretransplant Transesophageal Echocardiographic Assessment
| Interatrial septum: PFO assessment |
|
| Right ventricle assessment |
|
| Left ventricle assessment |
|
| Pulmonary veins (view all 4 veins if possible) |
|
PFO = patent foramen ovale; ME = midesophageal; TAPSE = tricuspid annular plane systolic excursion; RIMP = right ventricle index of myocardial performance; PW = pulse wave; SAX = short axis; TG = transgastric; TR = tricuspid regurgitation; CW = continuous wave; RVSP = right ventricle systolic pressure; IVCT=Isovolumic contraction time; IVRT = isovolumic relaxation time; ET = ejection time.
RIMP = (IVCT+IVRT)/ET.
Guidelines for a complete assessment of the right heart have recently been published by the American Society of Echocardiography.5 Relevant to the patient undergoing lung transplantation, assessment of RV function includes evaluation of systolic function, size, and presence of hypertrophy. A dilated RV, diminished systolic function, reduced tricuspid annular plane systolic excursion, an increased RV index of myocardial performance, and severe tricuspid regurgitation portend difficulty in tolerating the hemodynamic changes during lung transplantation without cardiopulmonary bypass (CPB).
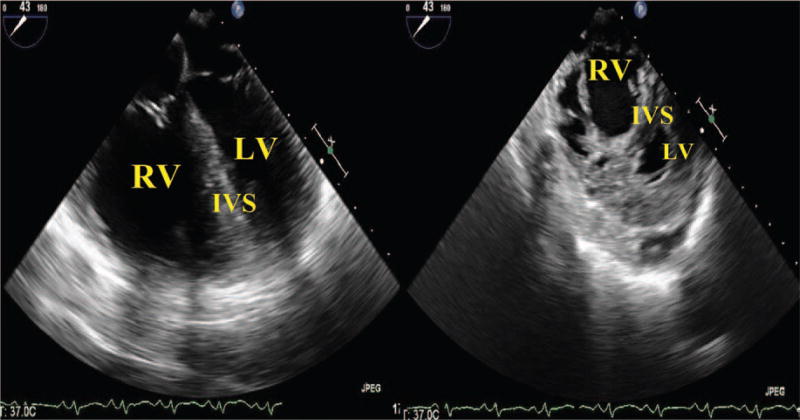
Signs of RV pressure overload from longstanding intrinsic lung disease, and elevated pulmonary arterial (PA) pressure include leftward bowing of the interatrial septum or flattening of the interventricular septum to the right during systole. RV pressure overload or acute RV volume overload (flattening of the interventricular septum during diastole) may also impair LV filling and cardiac output. This can be visualized in the midesophageal 4-chamber and transgastric midpapillary views (Fig. 1). In the presence of a patent foramen ovale (PFO) or atrial septal defect, a right to left interatrial shunt (from elevated right-sided pressures) could lead to paradoxical embolism and stroke or hypoxemia. Examination of the interatrial septum in the midesophageal bicaval view by using color-flow Doppler or agitated saline bubble injection can identify those patients with an interatrial shunt. The mere presence of a PFO does not necessarily mandate its closure that would complicate the surgery due to the need for CPB. While there are little data on the relative risk versus benefits of surgical PFO closure for patients undergoing lung transplantation, the presence of shunt-related severe hypoxemia or hemodynamic instability may justify closure.6 In the presence of a large atrial septal defect, the decrease in right-sided pressures after lung transplanation could result in severe left-to-right shunting, causing high blood flow to the transplanted lung and graft failure.4
Figure 1.
D-shaped left ventricle. Side by side comparison demonstrating severely dilated RV in a 4-chamber and transgastric view. The increase in pressure has distorted the normally round, “ doughnut-shaped”, LV and created a “D-shaped” ventricle instead. RV = right ventricle; LV = left ventricle; IVS = intraventricular septum.
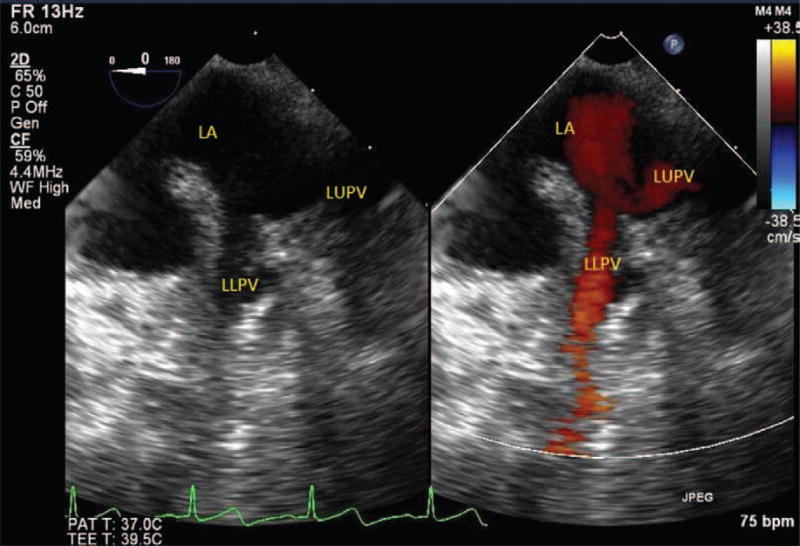
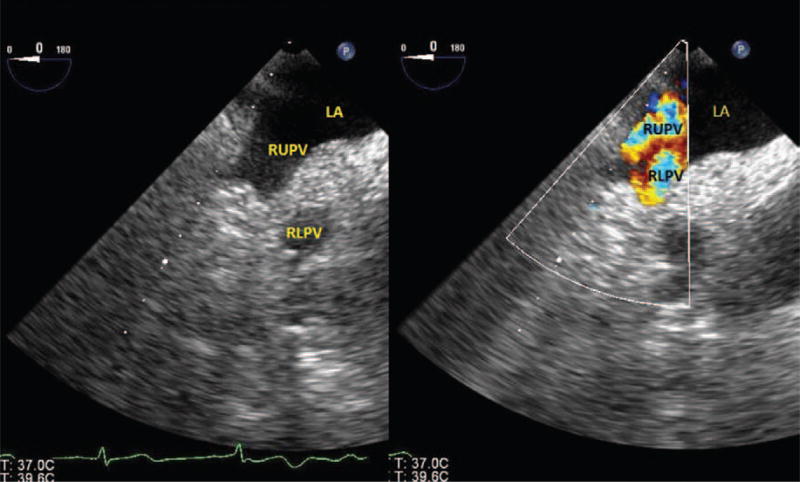
Baseline imaging and Doppler evaluation of the PVs and PA should be performed for posttransplant comparison. All 4 PVs should be interrogated; however, in practice, it can be challenging to clearly visualize all 4 veins, particularly the left lower PV. Starting from a midesophageal 4- chamber view, the left PVs can be visualized by withdrawing the probe slightly to allow the aortic valve to be visible, turning the probe to the left and retroflexing (Fig. 2 and Video 1, see Supplemental Digital Content 1, http://links.lww.com/AA/A711). The left upper PV enters the LA, just superoposterior to the LA appendage. Further insertion of the probe will reveal the left lower PV just as the LA appendage disappears from view.7 The use of color-flow Doppler will assist in identifying the PVs (Video 1, see Supplemental Digital Content 1, http://links.lww.com/AA/A711). A number of views can be used to image the right PVs. Starting from a standard midesophageal 4-chamber view and turning the probe to the right, the right upper PV can be seen entering the LA from a superior position and the right lower PV entering from an inferoposterior position (Fig. 3 and Video 2, see Supplemental Digital Content 2, http://links.lww.com/AA/A712).7 A slight flexion and/or increase in the omniplane angle to 45° to 60° may be necessary. The right PVs can also be seen by turning the probe to the right from the standard bicaval view. The right upper PV will be seen entering the LA adjacent to the superior vena cava with the right lower PV just inferior to it.7
Figure 2.
Left pulmonary veins. LUPV = left upper pulmonary vein.
Video 1.
Left pulmonary veins. To obtain the left pulmonary veins, first develop a midesophageal 4-chamber view. Then withdraw the probe slightly to allow the aortic valve to be visible. Finally, turn the probe to the left and retroflex to allow the left side pulmonary veins to come into view. The use of color-flow Doppler can help identify the pulmonary veins. Left Sup PV = left superior pulmonary vein; Left Inf PV = left inferior pulmonary vein.
Figure 3.
Right pulmonary veins. LA = left atrium; RUPV = right upper pulmonary vein; RLPV = right lower pulmonary vein.
Video 2.
Right pulmonary veins. To obtain the right pulmonary veins first develop a midesophageal 4-chamber view. Withdraw the probe slightly to visualize the aortic valve in the center of the other 4 chambers. Turn the probe towards the right and retroflex to allow the right side pulmonary veins to come into view. Right Sup PV = right superior pulmonary vein; Right Inf PV = left inferior pulmonary vein.
The main PA can be visualized from a midesophageal ascending aorta short-axis view or an upper esophageal aortic arch short-axis view.8 From the midesophageal ascending aorta short-axis view, turning of the probe to the right or left will bring the right and left PAs respectively into view. The interposition of the left mainstem bronchus makes visualization of the left PA challenging.
Intraprocedural Concerns
While the decision whether or not to initiate CPB is multifactorial (including hemodynamic, oxygenation, ventilation, and acid-base status), the use of TEE greatly assists the anesthesiologist in the decision-making process (Table 2). An analysis of RV function needs to be repeated after application of the PA cross-clamp. The additional afterload in the setting of preexisting pulmonary hypertension (present in varying degrees in nearly all lung transplant recipients) may lead to RV failure. In patients with preexisting RV hypertrophy, the RV may be able to maintain its contractility and preserve cardiac output despite the added afterload. TEE can be helpful in assessing RV response to inotropes, preload, and strategies to reduce RV afterload such as inhaled pulmonary vasodilators. In addition, the application of the PA cross-clamp can cause a previously left-to-right interatrial shunt to reverse due to the increased right-sided pressures further worsening hypoxemia. Evaluation of interatrial septum and the degree of tricuspid regurgitation should be performed.
Table 2.
Intraprocedural Transesophageal Echocardiographic Assessment
| Assess right ventricular response to pulmonary artery cross-clamping |
|
| If PFO present |
|
PFO = patent foramen ovale; TAPSE = tricuspid annular plane systolic excursion; TR = tricuspid regurgitation; PA = pulmonary arterial.
Hypotension after the release of the cross-clamp and during the immediate posttransplantation period is common. Incomplete de-airing of the PV anastomosis can lead to coronary air embolism and transient myocardial ischemia.9 Air, often seen on TEE midesophageal views as “microbubles” after release of the PV clamp, can be followed by development of regional wall motion abnormalities. The continued appearance of air emboli in the LA and ventricle despite adequate de-airing should induce reexamination of the integrity of the bronchial anastomosis.10 RV dysfunction from increased pulmonary vascular resistance (PVR) and RV compression from pleural fluid or pericardial fluid can also cause hypotension. Rarely, the RV outflow tract can become obstructed from a decrease in pulmonary pressures and sudden collapse of the cavity of a hypertrophied RV (seen on a midesophageal RV inflow–outflow view).9 Hypovolemia is also common, given a restrictive fluid strategy. TEE can also exclude unexplained hypoxia from a persistent right to left shunt, PV obstruction, or large pleural effusions.
Posttransplant
After implantation of the lung allograft, special attention must be paid to assessing the PV and PA anastomoses as even experienced surgeons report that they underestimate severity of anastomotic constriction.11 Patients with fibrotic lung disease and women, due to smaller thoracic cavities, are at higher risk for vascular anastomotic complications.12
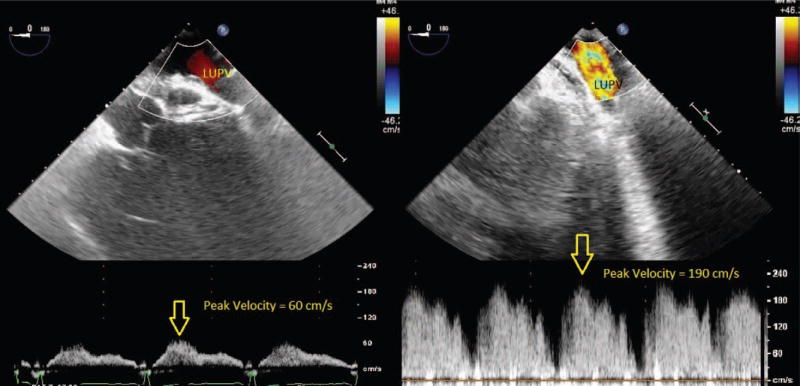
An obstructed PV flow is a serious problem after lung transplantation, leading to hypoxia, pulmonary edema, early graft failure, stroke, and death.2,13 Obstruction caused by thrombus at the anastomotic site was reported in 9% to 24% of patients.2,3,13 Other causes of obstructed PV flow include sutures, kinking, or external compression. Intraoperative TEE diagnosis offers the opportunity for safe surgical evacuation of a PV clot with no added morbidity, because there is controversy regarding the proper management when identified postoperatively.14 Assessment of the PV should be performed on all patients before leaving the operating room. In some instances, as in the index case at the beginning of this Didactic, an echogenic mass may be seen on 2-dimensional echocardiographical imaging originating from one of the PVs and extending into the LA, suggesting thrombus. Even when a mass is not visualized, Doppler should be performed to aid in the diagnosis of PV thrombus or obstuction. Expected values for both S and D waves range from 30 to 60 cm/s.15 A velocity (>100 cm/s) by spectral Doppler, turbulence, or deviation from systolic flow predominance (S>D) may be suggestive of clinically significant PV obstruction and warrants further investigation (Fig. 4).7 Atrial flow reversal is rarely seen, given that the LA is surgically reconstructed.16 Only having one of the above criteria is not sufficient for diagnosis as each variable depends on many factors including whether the procedure was uni- or bilateral as well as LA pressure. A PV diameter <0.25 cm has been described as a threshold for graft failure.2
Figure 4.
Color-flow Doppler through left upper pulmonary vein. These panels document color-flow Doppler (CFD) through the left pulmonary veins. The left panel demonstrates normal velocity flow in the patient’s native lungs. The right panel demonstrates flow in the left pulmonary veins after a left lung transplant with increased flow velocities that are suspicious for anastomotic stenosis. LUPV = left upper pulmonary vein.
High velocity flow could also indicate narrowing of the contralateral PA anastomosis, high blood volume flowing through the lung, or venous donor vasoconstriction.17 Significant left-to-right shunting through the interatrial septum will result in fusion of the S and D waves on pulsed wave Doppler.17 Arrhythmias, such as atrial fibrillation, can also distort S and D wave morphology. Alternatively, low PV velocity could be due to flow obstruction from narrowing of the atrial cuff anastomosis or low blood volume flowing through the lung due to myocardial dysfunction or relative hypovolemia.3
In the case of bilateral sequential lung transplantation performed off-CPB support, PV velocities are transiently increased in the first transplanted lung while the second lung is being implanted. Since the contralateral PA is clamped, all cardiac output is flowing across the first implanted lung, increasing the velocity. Therefore, assessment of all PVs should be repeated after both lungs are implanted to ensure that the velocity has decreased.14 In single-lung transplants, higher PV velocities in the transplanted lung as compared with the native lung are expected, given its lower PVR. The absence of this may be an early indication of a vascular complication.16
Persistent pulmonary hypertension could be linked to a narrowing of the PA anastomosis (kinking secondary to excessive vessel length or restrictive arterial suture due to a short allograft artery) or to an early thrombus. Interrogation with color and spectral Doppler should be performed. In practice, however, the anastomosis is often too distal from the main PA to allow sufficient visualization. Two small studies report conflicting success rates of either 0% or 71%.17,18 Epicardial echocardiography has been demonstrated to be of value in these situations.19 The PA anastomosis is considered normal if the minimal diameter of the lumen measures at least 75% of the proximal PA and if color Doppler illustrates nonturbulent flow through the anastomosis.3,17 No echocardiographic threshold for significant stenosis of the PA has been defined; therefore, PA catheter data suggesting pulmonary hypertension and clinical signs of early graft dysfunction are warranted before recommending surgical revision based solely on TEE findings. Despotis et al.20 demonstrated that PA catheterization can be used to differentiate PA anastomotic stenosis from increasing PVR by the surgeon gently advancing the catheter through the anastomosis to measure proximal and distal PA pressures. Table 3 summarizes the use of TEE for posttransplant assessment.
Table 3.
Posttransplant Transesophageal Echocardiographic Assessment
| Pulmonary veins (examine all 4 veins if possible) assessment |
|
| Pulmonary artery anastomosis |
|
| Right ventricle assessment |
|
| Left ventricle assessment |
|
| Flow across PFO (If exists) |
|
PFO = patent foramen ovale; UE = upper esophageal; ME = midesophageal; TAPSE = tricuspid annular plane systolic excursion; RVSP = right ventricle systolic pressure;
RIMP = (IVCT + IVRT)/ET; IVCT = isovolumic contraction time; IVRT = isovolumic relaxation time; ET = ejection time.
In conclusion, TEE has a central role for the patient undergoing lung transplantation, and the echocardiographer needs to be aware of the key aspects to focus on pretransplant, intraprocedural, and posttransplant.
Teaching Points.
The goal of the pretransplant TEE examination is to identify a PFO and to evaluate RV function with both qualitative and quantitative assessments. A dilated RV, diminished systolic function (fractional area change <35%), reduced tricuspid annular plane systolic excursion (< 16 mm), and severe tricuspid regurgitation herald difficulty in tolerating the hemodynamic changes during lung transplantation without CPB.
The decision whether or not to initiate CPB for lung transplantation depends on oxygenation, acid-base status, and hemodynamic stability. The use of TEE greatly assists the anesthesiologist in the decision-making process.
Pulsed wave and color-flow D Doppler are useful to demonstrate PV narrowing posttransplantation due to postsurgical changes, external compression, PV kinking, or thrombus. Findings that warrant concern include a PV velocity >100 cm/s, turbulent color Doppler, and a diameter <5 mm.
Imaging of the PA size and velocity at baseline and after anastomosis can be useful in excluding anastomotic strictures. Identification by TEE may be difficult. Epicardial echocardiography may be helpful in these situations.
Acknowledgments
Funding: None.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.anesthesia-analgesia.org).
The authors declare no conflicts of interest.
DISCLOSURES
Name: Adam Evans, MD.
Contribution: This author helped design and conduct of study, analyze the data, and prepare the manuscript.
Attestation: The author attests to having approved the final manuscript.
Name: Sanjay Dwarakanath, MD.
Contribution: This author analyzed the data.
Attestation: The author attests to having approved the final manuscript.
Name: Charles Hogue, MD.
Contribution: This author helped prepare the manuscript.
Attestation: The author attests to having approved the final manuscript.
Name: Marybeth Brady, MD, FASE.
Contribution: This author helped design and conduct of study, analyze the data, and prepare the manuscript.
Attestation: The author attests to having approved the final manuscript.
Name: Jeremy Poppers, MD.
Contribution: This author helped design and conduct of study, analyze the data, and prepare the manuscript.
Attestation: The author attests to having approved the final manuscript.
Name: Steven Miller, MD.
Contribution: This author helped design and conduct of study, analyze the data, and prepare the manuscript.
Attestation: The author attests to having approved the final manuscript.
Name: Menachem M. Weiner, MD.
Contribution: This author helped design and conduct of study, analyze the data, and prepare the manuscript.
Attestation: The author attests to having approved the final manuscript.
RECUSE NOTE
Dr. Charles Hogue is the Associate Editor-in-Chief for Cardiovascular Anesthesiology for the journal. This manuscript was handled by Dr. Martin J. London, Section Editor for Perioperative Echocardiography#8232;and Cardiovascular Education, and Dr. Hogue was not involved in any way with the editorial process or decision.
References
- 1.Thys DM, Brooker RF, Cahalan MK, Connis RT, Duke PG, Nickinovich DG, Reeves ST, Rozner MA, Russell IA, Streckenbach SC, Sears-Rogan P, Stewart WJ. Practice guidelines for perioperative transesophageal echocardiography. An updated report by the American Society of Anesthesiologists and the Society of Cardiovascular Anesthesiologists Task Force on Transesophageal Echocardiography. Anesthesiology. 2010;112:1084–1096. doi: 10.1097/ALN.0b013e3181c51e90. [DOI] [PubMed] [Google Scholar]
- 2.Schulman LL, Anandarangam T, Leibowitz DW, Ditullio MR, McGregor CC, Galantowicz ME, Homma S. Four-year prospective study of pulmonary venous thrombosis after lung transplantation. J Am Soc Echocardiogr. 2001;14:806–12. doi: 10.1067/mje.2001.111855. [DOI] [PubMed] [Google Scholar]
- 3.Leibowitz DW, Smith CR, Michler RE, Ginsburg M, Schulman LL, McGregor CC, Li Mandri G, Weslow RG, Di Tullio MR, Homma S. Incidence of pulmonary vein complications after lung transplantation: a prospective transesophageal echocardiographic study. J Am Coll Cardiol. 1994;24:671–5. doi: 10.1016/0735-1097(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 4.Gorcsan J, 3rd, Edwards TD, Ziady GM, Katz WE, Griffith BP. Transesophageal echocardiography to evaluate patients with severe pulmonary hypertension for lung transplantation. Ann Thorac Surg. 1995;59:717–22. doi: 10.1016/0003-4975(94)01054-4. [DOI] [PubMed] [Google Scholar]
- 5.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. quiz 786–8. [DOI] [PubMed] [Google Scholar]
- 6.Krasuski RA, Hart SA, Allen D, Qureshi A, Pettersson G, Houghtaling PL, Batizy LH, Blackstone E. Prevalence and repair of intraoperatively diagnosed patent foramen ovale and association with perioperative outcomes and long-term survival. JAMA. 2009;302:290–7. doi: 10.1001/jama.2009.1012. [DOI] [PubMed] [Google Scholar]
- 7.Cartwright BL, Jackson A, Cooper J. Intraoperative pulmonary vein examination by transesophageal echocardiography: an anatomic update and review of utility. J Cardiothorac Vasc Anesth. 2013;27:111–20. doi: 10.1053/j.jvca.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 8.Jerath A, Roscoe A, Vegas A. Normal upper esophageal transesophageal echocardiography views. Anesth Analg. 2012;115:507–10. doi: 10.1213/ANE.0b013e31825e6e79. [DOI] [PubMed] [Google Scholar]
- 9.Gorscan J, 3rd, Reddy SC, Armitage JM, Griffith BP. Acquired right ventricular outflow tract obstruction after lung transplantation: diagnosis by transesopageal echocardiography. J Am Soc Echocardiogr. 1993;6:324–326. doi: 10.1016/s0894-7317(14)80071-0. [DOI] [PubMed] [Google Scholar]
- 10.Denault AY, Couture P, McKenty S, Boudreault D, Plante F, Perron R, Babin D, Buithieu J. Perioperative use of transesophageal echocardiography by anesthesiologists: impact in noncardiac surgery and in the intensive care unit. Can J Anaesth. 2002;49:287–93. doi: 10.1007/BF03020529. [DOI] [PubMed] [Google Scholar]
- 11.Griffith BP, Magee MJ, Gonzalez IF, Houel R, Armitage JM, Hardesty RL, Hattler BG, Ferson PF, Landreneau RJ, Keenan RJ. Anastomotic pitfalls in lung transplantation. J Thorac Cardiovasc Surg. 1994;107:743–53. discussion 753–4. [PubMed] [Google Scholar]
- 12.Siddique A, Bose AK, Özalp F, Butt TA, Muse H, Morley KE, Dark JH, Parry G, Clark SC. Vascular anastomotic complications in lung transplantation: a single institution’s experience. Interact Cardiovasc Thorac Surg. 2013;17:625–31. doi: 10.1093/icvts/ivt266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McIlroy DR, Sesto AC, Buckland MR. Pulmonary vein thrombosis, lung transplantation, and intraoperative transesophageal echocardiography. J Cardiothorac Vasc Anesth. 2006;20:712–5. doi: 10.1053/j.jvca.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Myles PS, Marasco S. Misleading turbulent flow through pulmonary venous anastomoses during lung transplantation. Anesth Analg. 2008;107:1504–5. doi: 10.1213/ane.0b013e3181822e5c. [DOI] [PubMed] [Google Scholar]
- 15.Gentile F, Mantero A, Lippolis A, Ornaghi M, Azzollini M, Barbier P, Beretta L, Casazza F, Corno R, Faletra F, Giagnoni E, Gualtierotti C, Lombroso S, Mattioli R, Morabito A, Pepi M, Todd S, Pezzano A. Pulmonary venous flow velocity patterns in 143 normal subjects aged 20 to 80 years old. An echo 2D colour Doppler cooperative study. Eur Heart J. 1997;18:148–64. doi: 10.1093/oxfordjournals.eurheartj.a015097. [DOI] [PubMed] [Google Scholar]
- 16.Boyd SY, Sako EY, Trinkle JK, O’Rourke RA, Zabalgoitia M. Calculation of lung flow differential after single-lung transplantation: a transesophageal echocardiographic study. Am J Cardiol. 2001;87:1170–3. doi: 10.1016/s0002-9149(01)01488-6. [DOI] [PubMed] [Google Scholar]
- 17.Michel-Cherqui M, Brusset A, Liu N, Raffin L, Schlumberger S, Ceddaha A, Fischler M. Intraoperative transesophageal echocardiographic assessment of vascular anastomoses in lung transplantation. A report on 18 cases. Chest. 1997;111:1229–35. doi: 10.1378/chest.111.5.1229. [DOI] [PubMed] [Google Scholar]
- 18.Hausmann D, Daniel WG, Mugge A, Heublein B, Hamm M, Schafers HJ, Haverick A. Imaging of pulmonary artery and vein anastomoses by transesophageal echocardiography after lung transplantation. Circulation. 1992;86(suppl II):II-251–8. [PubMed] [Google Scholar]
- 19.Felten ML, Michel-Cherqui M, Sage E, Fischler M. Transesophageal and contact ultrasound echographic assessments of pulmonary vessels in bilateral lung transplantation. Ann Thorac Surg. 2012;93:1094–100. doi: 10.1016/j.athoracsur.2012.01.070. [DOI] [PubMed] [Google Scholar]
- 20.Despotis GJ, Karanikolas M, Triantafillou AN, Pond CG, Kirvassilis GV, Patterson GA, Cooper JD, Lappas DG. Pressure gradient across the pulmonary artery anastomosis during lung transplantation. Ann Thorac Surg. 1995;60:630–4. doi: 10.1016/0003-4975(95)00426-L. [DOI] [PubMed] [Google Scholar]