Abstract
Background
Low-grade chronic inflammation, characterized by elevations in Interleukin-6 (IL-6), is an independent risk factor for impaired mobility and slow walking speed in older adults.
Design
The ENRGISE (ENabling Reduction of low-Grade Inflammation in SEniors) Pilot study was a multicenter, double-blinded, placebo-controlled randomized pilot trial of two interventions to reduce IL-6 levels.
Setting
Five university-based research centers.
Participants
The target enrollment was 300 men and women aged ≥70 years who have an average plasma IL-6 >2.5 and <30 pg/ml across 2 measures, separated by at least one week. Participants had low to moderate physical function, defined as 1) self-reported difficulty walking ¼ mile or climbing a flight of stairs and 2) a usual walk speed <1 m/sec on a 4 m usual paced walk.
Intervention
Participants were randomized to Losartan (LO), omega-3 (ω-3) fish oil, combined LO+ω-3 or placebo. Randomization was stratified depending on eligibility for each group. A titration schedule was implemented to reach a dose that was safe and effective on IL-6 reduction. Maximal doses were 100 mg/day for LO and 2.8 g/day for ω-3 fish oil.
Measurements
IL-6, walking speed over 400 meters, physical function (Short Physical Performance Battery), other inflammatory markers, safety, tolerability, frailty domains, and maximal leg strength were measured.
Results
The ENRGISE Pilot Study was designed to determine recruitment yields, feasibility, medication tolerance and adherence, and to provide preliminary data to help justify a sample size for a more definitive randomized trial.
Conclusions
The ENRGISE Pilot Study will inform a larger subsequent trial that is expected to have important clinical and public health implications for the growing population of older adults with low-grade chronic inflammation and mobility limitations.
INTRODUCTION
The immune system has been actively studied in the aging process since the seminal work by Walford in 1969.1 Subsequently, a large body of evidence has accumulated demonstrating a chronic, low-grade elevation of inflammatory markers with increasing age.2 These markers appear to derive in part from senescent cells, which are characterized by growth arrest in response to accumulated damage. Senescent cells secrete cytokines such as IL-6, chemokines, and proteases and are resistant to apoptosis. While these senescent cells are less prone to malignant transformation, they may disrupt normal tissue function, promoting central adiposity, atherosclerotic plaque, and osteoporosis.3, 4 Moreover, age-related renal dysfunction5 and atherosclerosis6, 7 may contribute to the elevation of inflammatory markers through decreased excretion and increased production of these markers. Thus, “chronic inflammation” of aging may indicate an ongoing process of age-related damage and repair resulting a cumulative burden of disease-related damage, and cellular senescence.
While conditions such as myocardial infarction, stroke, hip fracture, and arthritis contribute to disability and inflammation, growing evidence suggests that low-grade chronic inflammation, characterized by elevations in plasma C-reactive protein (CRP), tumor necrosis factor alpha (TNF-α), and particularly interleukin-6 (IL-6),8–11 can directly impair muscle function and are independent risk factors for disability, impaired mobility, and slow walking speed.12, 13 Based on current knowledge about the detrimental effects of chronic inflammation, it seems logical that blocking the effects or reducing the sources of chronic low-grade inflammation would have clinical benefit. Alternatively, inflammatory pathways are tightly regulated so that manipulation could have adverse effects such as increased risk of infection or delayed healing. Therefore, it’s unclear whether the observed increases in inflammation with age are detrimental and/or adaptive. A randomized trial targeting inflammatory pathways directly was warranted to decide between these alternative interpretations.
The ENRGISE (ENabling Reduction of low-Grade Inflammation in SEniors) Pilot study was a randomized clinical trial (RCT) to gather preliminary data to test whether anti-inflammatory interventions improve or preserve walking ability. Older persons with mobility impairment and elevated levels of inflammation were enrolled— a population at high risk of major mobility disability.8, 9, 14 The aims of the ENRGISE Pilot study were to compare with placebo, the effects of Losartan (LO), omega-3 fish oil (ω-3) also known as n-3 long chain polyunsaturated fatty acids, and LO+ω-3 on IL-6 and walking speed in 300 older adults (70+ years of age) over 12-months of follow-up. Secondary aims were to evaluate recruitment yields, eligibility criteria, adherence, retention, tolerability, sample-size, design, and parameters affecting the cost for the main RCT focused on major mobility disability as an outcome. The study also aimed to examine intra-subject variability of IL-6, dosage, safety, and other established and novel inflammatory markers. These aims were designed to inform the feasibility of the full ENRGISE trial to assess whether targeting inflammation reduces the risk of major mobility disability. This paper provides the background, rational, conceptual basis, discussion of treatment choices, and research design of the ENRGISE-Pilot Study.
METHODS
Participants and eligibility criteria
Men and women aged 70+ years (n=300) who self-reported difficulty walking ¼ mile or climbing a flight of stairs, had a usual walking speed <1 m/sec on the 4 m walk, and had evidence of chronic low-grade inflammation (IL-6 values >2.5 pg/ml and <30 pg/ml) were enrolled. The value of IL-6 >2.5 pg/ml was chosen because of its association with risk of mobility limitation15, 16 and persons with ≥30 pg/ml were excluded because they were likely to have acute infections (urinary, respiratory or other) that did not coincide with chronic low-grade inflammation. To reduce the impact of within person variability, IL-6 levels were based on the average of 2 measures taken 1–3 weeks apart, with the first measure being >2.3 and <30 pg/ml, and the average of the two measures >2.5 and <30 pg/ml. The lower value of >2.3 pg/ml at the first measure was designed to be more inclusive of individuals with chronic low-grade inflammation accounting for day-to-day variability and inter-assay variability. Complete eligibility criteria are listed in Table 1.
Table 1.
Inclusion and exclusion criteria for the ENRGISE Pilot Study
Inclusion Criteria
|
Exclusion criteria
|
Temporary exclusion criteria
|
Participants were excluded if they reported acute infection, autoimmune disease, severe arthritis, and neurologic conditions causing low walking speed. Initially, participants were also excluded for having low vitamin D levels as required by Funding Opportunity Announcement. This requirement was dropped a third of the way through the recruitment period because of a lack of evidence for how low vitamin D levels would impact the association between inflammation and physical function. Those who took an angiotensin converting enzyme inhibitor (ACEI), angiotensin receptor blocker (ARB) or potassium sparing diuretics, were excluded from LO randomization. Those who ate >2 servings/week of fish in the past year or taking fish oil were excluded from ω-3 randomization. Recruitment targets were approximately 69% women, 20% racial minorities, and 5% Hispanic or Latino minorities, that reflected the population distribution of these subgroups in the catchment areas. The institutional review boards at each study site approved the protocol and all participants provided written informed consent. The trial is registered as clinicaltrials.gov identifier: NCT01072500.
Study measures and outcomes
A timetable of measures and assessments is listed in Table 2. IL-6 and walking speed during the 400 m walk test were identified as co-primary outcomes of the ENRGISE Pilot study. The 400 m walk test at usual pace was used to assess major mobility disability (MMD),17 defined as inability to walk ¼ mile. MMD was operationalized as the inability to complete a 400 m walk test within 15 min without sitting or help of another person or walker. In cases when the 400 m walk was not attempted, MMD was adjudicated based on objective inability to walk 4 m in <10 sec, self- or proxy- reported inability to walk across a room or medical record documentation of mobility status.17
Table 2.
Schedule of ENRGISE assessments and follow-up procedures
| Visit type | Phone scr. | Scr. visit 1 | Scr. visit 2 | Baseline. visit | Safety* | 3-Mo visit | Safety * | 6-Mo visit | Safety* | 9-Mo visit | Safety* | 12-Mo visit | Safety* | Extra visit** |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Basic eligibility screening | x | |||||||||||||
| Short consent and 4 m walk test | x | |||||||||||||
| IL-6 | x | x | x& | x& | x& | x& | ||||||||
| Medical history | x | |||||||||||||
| MMSE | x | |||||||||||||
| Safety blood tests | x | x | x | x | x* | x | x* | x | x* | x* | x | |||
| Additional biomarkers | x | x | x | x | ||||||||||
| Vital signs | x | x | x | x | x | x | x | x | x | x | x | x | ||
| Anthropometric measures | x | x | x | x | x | |||||||||
| Main Informed consent | x | |||||||||||||
| Physical performance measures^ | x | x | x | x | x | |||||||||
| Medical history & adverse events | x | x | x | x | x | x | x | x | x | x | x | |||
| Questionnaires | x | x | ||||||||||||
| Dispense study drugs | x | x | x | x | ||||||||||
| Assess compliance | x | x | x | x | ||||||||||
| Proxy interview (if needed) | x | x | x | x |
BP, and serum potassium and eGFR were measured about 1 week after the LO dose adjustment (potassium and eGFR were not measured if LO dose is reduced only for BP values).
Extra visits if there were safety concerns.
The IL-6 measure is excluded or postponed if there has been an acute illness within one month prior to the visit
Physical performance measures include: 400 m walk test, short physical performance battery, maximal grip and leg extension/flexion strength
IL-6 was prioritized over other inflammatory factors because it is a stable inflammatory marker that is less sensitive to day-to-day and diurnal variations and the marker most consistently associated with mobility limitations.15, 16 IL-6 levels were determined using a sandwich immunoassay (Human IL-6 Quantikine ELISA Kit, R & D Sytems, Minneapolis, MN, catalog #HS600B). Levels greater that 10 pg/ml were evaluated with a different assay with greater dynamic range (Human IL-6 QuantiGlo ELISA Kit, R & D Systems, catalog #Q6000B). The collection, preparation, handling and storage of biological samples followed standard operating procedure that complied with Office of Extramural Research requirements and guidelines.
Secondary outcomes included physical performance, frailty, muscle strength and inflammatory biomarkers to characterize the effect of the interventions.
Physical performance was measured on the Short Physical Performance Battery (SPPB), which is based on a timed 4 m walk, balance & chair stands tests.18
Frailty was characterized using the Fried criteria that employed self-reported exhaustion, unintentional weight loss, low energy expenditure, slow gait speed, and weak grip strength.19
Muscle strength was measured with maximal tests of isometric grip strength and isokinetic leg extension/flexion strength.17, 20 Maximal grip strength force in kilograms was measured two times using a handheld dynamometer on both hands (Jamar, Lafayette Instruments, Lafayette, IN). Maximal isokinetic leg extension/flexion torque was measured on both limbs with two trials of 5 repetitions at 60 and 180 degrees per second on an isokinetic dynamometer (Biodex Inc, Shirley, NY).
Additional inflammatory markers were measured in exploratory analyses. These included novel (sCD163, sIL2R-α, sTNF-αR1) and traditional inflammatory markers (C-reactive protein). sCD163 is a biomarker of monocyte activation that is related to CVD risk and mortality in elders.21 sIL2R -α is a marker of T cell activation that can result in accelerated loss of thymic function and adaptive immunity with chronic overexpression.22 sTNF-αR1 has emerged as a superior surrogate marker of TNF-alpha, yielding data with considerably less analytical variability23 — it is liberated from multiple cell types through the action of TNF-alpha, and therefore a biomarker of its activity.
Sample size
The sample size (N=300) was determined based on marginal comparisons (135 and 165/group) between each active intervention and placebo using a one-sided hypothesis tests at the 10% level. The goal was not to provide definitive evidence, but to rule out small effects that would have lower clinical value. For IL-6, there was 91% power to detect a difference if the difference (on the log scale) was at least 0.1625 (or a 15% difference). There was 66% power for a 10% difference and 99% power for a 20% difference. For 400 m walk speed, there was >99% power to detect >0.095 m/sec (a substantial meaningful change), and 86% power for a difference >0.038 m/sec (a small meaningful change) in walking speed. Lastly, there was 90% power to detect a difference of at least 0.99 SPPB units.
Treatment interventions and randomization
Interventions consisted of LO, ω-3, and their combination in a double-blind, placebo-controlled randomized trial. ω-3 fish oil and placebo (corn oil) were obtained from Epax, Aalesund, Norway and had identical shape, color, taste, and weight. Purity and composition of ω-3 was monitored with 13C NMR spectroscopy. LO and placebo were obtained from Almac Group, Souderton, PA. Three groups of potential participants were evaluated for the ENRGISE Pilot study. The first group consisted of potential participants who were not currently taking ω-3 fatty acids, fish oil (generic or specific, such as salmon, krill, or cod liver oil), flax, flaxseed oil or were not consuming > 2 servings of fatty fish per week (e.g. Salmon, Trout, Bluefish), but were taking either an ARB or ACE inhibitor. This group was randomized to placebo ω-3 or active ω-3. The second group consisted of potential participants who were not using an ACE inhibitor or an ARB but were taking ω-3. This group was randomized to placebo LO or to active LO. The third group consisted of people not using ω-3 or consuming other sources of polyunsaturated fatty acids as described above, an ARB, or an ACE inhibitor. This group was randomized to placebo LO/placebo ω-3, placebo LO/active ω-3, active LO/placebo ω-3, or active LO/active ω-3. Table 3 shows randomization strata and the number of participants in each group, according to baseline LO and ω-3 use. Randomization used a permuted block algorithm (with random block lengths) and was concealed via the secure web-based data management system.
Table 3.
ENRGISE randomization strata according to medication use at baseline.
| Strata according to medication use at baseline | ENRGISE Pilot N (%) Proposed |
Randomization weights | |||
|---|---|---|---|---|---|
| Placebo LO Placebo ω-3 |
LO Placebo ω-3 |
Placebo LO ω-3 |
LO ω–3 |
||
| 1 No ω-3 (ACEI/ARB ok) | 75 (25%) | 0.4* | 0 | 0.6* | 0 |
| 2 No ACEI/ARB (ω-3 ok) | 75 (25%) | 0.4** | 0.6** | 0 | 0 |
| 3 No ACEI/ARB, No ω-3 | 150 (50%) | 0.2 | 0.2 | 0.2 | 0.4 |
| Overall | 300 (100%) | 0.3 (n=90) | 0.25 (75) | 0.25 (75) | 0.2 (60) |
Did not receive placebo LO
Did not receive placebo ω-3 as the use of the corresponding drugs is permitted at baseline
Dose, titration plan and compliance
Participants randomized to the ω-3 arm began with 1.4 g/day (administered in 0.7 gram gel caps) until the 6-month follow-up visit. Each 0.7 grams of fish oil contained 400 mg eicosapentaenoic acid (EPA) and 200 mg docosahexaenoic acid (DHA). LO was obtained in 25 mg and 50 mg capsules. The LO and placebo were each encapsulated so that they were identical in shape, color, taste and weight. Participants randomized to LO began with 25 mg/day. If this dose was tolerated, based on the safety assessment that included reported symptoms and blood pressure at 1–2 weeks after the start of LO, then the dose of LO was increased to 50 mg/day. If there were no safety concerns, the dose of 50 mg/day continued until the 6-month visit. At the six month follow-up, the dose of ω-3 was increased to 2.8 g/day and LO was increased to 100 mg/day if the average of IL-6 measured at 3- and 6-month visits did not decrease by at least 40% vs. baseline (average of screening visits 1 and 2). The 40% threshold was selected based on findings of previous trials, which showed IL-6 reductions >50%.24, 25 Compliance to the interventions was monitored with both pill counts at scheduled clinic visits and participant reports. For the latter, participants were asked how many times they were not able to take the study drug and asked to rate their ability to take it during in the past month (e.g. excellent, fair, poor).
Safety measures
Safety visits consisted of a follow-up visit after initiating or increasing study drug designed to specifically assess the safety of the medication increase. They were also scheduled to follow participants who required a dose reduction because of symptoms, abnormal findings or at the Medical Safety Officer’s discretion. The safety visits were conducted within one to two weeks of a change in study medication and consisted of: 1. medical history follow-up, 2. medication inventory, 3. vitals check (blood pressure, pulse, weight & temperature), 4. safety labs relevant to the medication change, and 5. medication adherence. No drugs were dispensed at safety visits. Drug dispensing only occurred once laboratory results were obtained.
Measures to evaluate safety consisted of blood pressure, hemoglobin, serum glucose, renal function (eGFR), LDL cholesterol, and potassium at baseline and at 3, 6, and 9 months of follow-up. These measures were also collected at 1 to 2 weeks after randomization for participants randomized to LO/placebo. If serum potassium rose above 5.0mEq/L or eGFR dropped by more than 20% from baseline, LO treatment was discontinued. If blood pressure dropped to <90/50 mmHg (hypotension), or if participants experienced new symptoms of hypotension such as dizziness or pre-syncope, losartan dose was reduced or discontinued. For ω-3 fish oil, if there was a new onset of atrial fibrillation, or if hemoglobin decreased by >20%, or fasting glucose or LDL cholesterol significantly increased from a previous visit, ω-3 fish oil was discontinued. Laboratory tests and/or blood pressure were repeated whenever a dosage adjustment was made in response to an abnormal laboratory test result and/or blood pressure value. Blood testing was also repeated at the investigator or medical safety officer’s discretion.
DISCUSSION
The ENRGISE Pilot Study was designed to test ability of anti-inflammatory interventions to improve or preserve walking ability in older adults with mobility impairments. The investigators selected interventions that are widely available, safe, tolerable, acceptable, and affordable for vulnerable older persons. Additionally, to maximize the anti-inflammatory effect and potential benefit on mobility, the investigators tested both individual and combination interventions.
Choice of potential candidate drugs
Potential candidate interventions were assessed using the following criteria: (1) excellent safety records, (2) the ability to reduce elevated IL-6 levels, (3) demonstrated benefits in improving physical performance, (4) considered innovative for affecting mobility outcomes, (5) tested in similar trials acting with different but complementary biological mechanisms, and (6) broadly available at low cost. A number of candidate interventions were excluded from consideration based on the a priori criteria listed in Table 4. Under the first criterion, anti-TNF-α agents (etanercept, infliximab, adalimumab),26 anti-IL-6 agents (siltuximab),27 anti-IL1β (canakinumab – no long-term safety data available),28 and thiazolidinediones (rosiglitazone, pioglitazone)29 were excluded based on risk of infections, liver toxicity, fluid overload, CVD, fractures, or possible cancer. Chloroquine and statins were excluded due to concerns of myotoxicity and lack of effect on walking speed for statins (criteria 1 and 5).30 Corticosteroids, aspirin, non-steroidal anti-inflammatory drugs (NSAIDs), and cyclooxygenase-2 (Cox-2) inhibitors were excluded for risk of bleeding,31 gastrointestinal toxicity, and CV events for NSAIDs and COX-2 inhibitors.32 Colchicine was excluded for risk of myotoxicity33 and neuropathy (criteria 1 and 5).34 Low-dose methotrexate is potentially safe and effective in lowering IL-6, but was not acceptable as it bears the stigma of being a “dangerous” anticancer drug. Criterion 2 excluded promising interventions, such as metformin, ghrelin, lactoferrin, oxytocin, salsalate, creatine, curcuma, probiotics, and resveratrol because of lack of clinical trial evidence for reducing IL-6. Metformin, while potentially promising, showed in the Diabetes Prevention Project a small reduction in CRP (-12%),35 in part related to its modest weight-reducing effect. However other trials have shown no effect on IL-636 or CRP.37 Despite the numerous potential anti-inflammatory interventions in the literature, most were deemed to have limited public health impact for prevention due to safety and high cost.
Table 4.
Summary of selection criteria and candidate interventions
| Criteria | ||||||
|---|---|---|---|---|---|---|
| Interventions | 1. Safe, tolerable, acceptable | 2. IL-6 reduction | 3. Physical performance | 4. Innovation | 5. Mechanism | 6. Practical, affordable |
| ACEIs, ARBs | + | + | + | + | + | + |
| ω-3 | + | + | + | + | + | + |
| Anti-TNF-α, -IL6,-IL1; methotrexate thiazolidinediones | − | + | ? | + | + | ? |
| Statins, chloroquine, colchicine | − | + | − ? | + | − | + |
| Corticosteroids, aspirin, NSAIDs, cox-2 inhibitors | − | + | ? | + | + | + |
| Metformin, fosinopril, ghrelin, lactoferrin, oxytocin, salsalate, curcuma, creatine, probiotics, resveratrol | + | − ? | − ? | + | + ? | + |
+ positive evidence, − negative evidence, ? evidence lacking
Only two potential interventions met the a priori inclusion criteria. First, ACE-Is and ARBs have shown excellent safety in large hypertension and heart failure trials in older persons. Among ACE-Is, perindopril38 and enalapril39 have demonstrated reductions in IL-6. Perindopril was shown to prevent physical function and walking speed declines in older persons40 and to reduce CRP41. Regarding ARBs, most (except azilsartan) are known to reduce elevated IL-6 and were prioritized over ACE-I’s because they exhibit greater tolerability.42 A second intervention meeting a priori inclusion criteria was ω-3 fish oil 43 and lipoic acid44, which both show reduced IL-6 and CRP43 in RCTs. In older women, ω-3 improved walking speed,45 and muscle strength when supplemented with exercise.46 LO47 and ω-3,48 also have supplementary effects on vasculature, coagulation, metabolism, and skeletal muscle, all of which may benefit mobility (Figure). The combination of these interventions may provide added support regarding the relevance of their common anti-inflammatory effect on benefits of walking speed and ultimately mobility. The final decision was to prioritize LO and ω-3 (and their combination) due to established reductions of IL-6 by >40% in RCTs, excellent safety records, high tolerability, long history of trials, a shared complementary biological mechanism and their low cost (i.e. LO is 1/50 the cost of other ARBs).24, 25
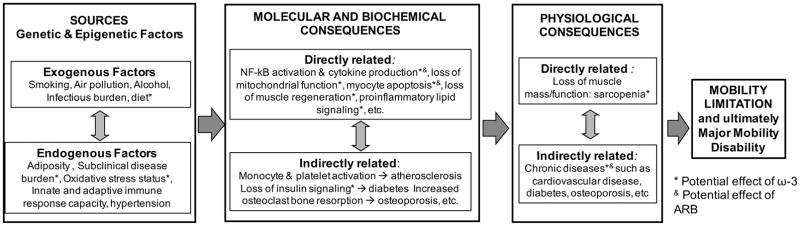
Figure.
Illustration of selected mechanisms potentially leading to progressive mobility disability that may be amenable to intervention using anti-inflammatory inventions being proposed by the ENRGISE Pilot study. The three main sources of inflammation in elders (genetic and epi genetic factors, exogenous factors and endogenous factors) combine to cause molecular and biochemical changes with important consequences, which in turn lead to physiological consequences and ultimately to mobility limitation and mortality.
Challenges and limitations
Pilot studies, by nature of their name, have inherent challenges and limitations. First, while we provide ample justification for choosing IL-6 as a marker of chronic low-grade inflammation, this marker may not provide the most robust association with the physical function outcomes. To evaluate this possibility, ENRGISE investigators assessed other markers of inflammation to compare sensitivity to change and association with the physical function outcomes of interest. The major challenges of the ENRGISE Pilot Study were identifying participants who met the IL-6 criteria while also having mobility impairments and yet were medically safe enough to participate. The joint prevalence of these criteria was largely unknown. Additionally, the MMD outcome required a large sample size to identify group differences.49 If the MMD outcome proved to be too infrequent for a larger study, the proportion of participants who changed their walking speed by a small, yet clinically meaningful amount, was an alternative primary outcome for the main trial. Another important limitation of the design was that lowering of inflammation and improved or preserved walking speed may not be directly linked to each other. The interventions tested have a myriad of biological effects that could positively affect mobility (e.g. improved blood flow with ARBs, brain function, lipid profiles etc…) and these would be challenging to mechanistically separate from their effects on inflammation in the current design.
Conclusion
The ENRGISE Pilot study design and concept had several innovative approaches. First, it targeted chronic low-grade inflammation to achieve reduced inflammation that was hypothesized to result in improved mobility. The study enrolled an older population at high risk of mobility disability, who are often excluded from large RCTs. The intervention repurposed widely available inexpensive interventions (LO and ω-3) and tested them alone and in combination to maximize their effects on inflammation and mobility. The data collected in the ENRGISE Pilot study provides a large foundation of knowledge from which to plan for a full-scale trial.
Acknowledgments
| Elements of Financial/Personal Conflicts | Manini | Anton | Beavers | Cauley | Espeland | Fielding | Kritchevsky | Leeuwenburgh | Lewis | Liu | McDermott | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Y | N | Y | N | Y | N | Y | N | Y | N | Y | N | Y | N | Y | N | Y | N | Y | N | Y | N | |
| Employment or Affiliation | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
| Grants/Funds | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
| Honoraria | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
| Speaker Forum | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
| Consultant | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
| Stocks | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
| Royalties | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
| Expert Testimony | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
| Board Member | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
| Patents | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
| Personal Relationship | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
| Elements of Financial/Personal Conflicts | Miller | Tracy | Walston | Radziszewsk | Lu | Stowe | Wu | Newman | Ambrosius | Pahor | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Y | N | Y | N | Y | N | Y | N | Y | N | Y | N | Y | N | Y | N | Y | N | Y | N | |
| Employment or Affiliation | X | X | X | X | X | X | X | X | X | X | ||||||||||
| Grants/Funds | X | X | X | X | X | X | X | X | X | X | ||||||||||
| Honoraria | X | X | X | X | X | X | X | X | X | X | ||||||||||
| Speaker Forum | X | X | X | X | X | X | X | X | X | X | ||||||||||
| Consultant | X | X | X | X | X | X | X | X | X | X | ||||||||||
| Stocks | X | X | X | X | X | X | X | X | X | X | ||||||||||
| Royalties | X | X | X | X | X | X | X | X | X | X | ||||||||||
| Expert Testimony | X | X | X | X | X | X | X | X | X | X | ||||||||||
| Board Member | X | X | X | X | X | X | X | X | X | |||||||||||
| Patents | X | X | X | X | X | X | X | X | X | X | ||||||||||
| Personal Relationship | X | X | X | X | X | X | X | X | X | X | ||||||||||
Grants/Funds explanation for all authors: The ENabling Reduction of low-Grade Inflammation in Seniors Pilot study is funded by a National Institutes of Health/National Institute on Aging Cooperative Agreement # U01 AG050499. The research is partially supported by the Claude D. Pepper Older Americans Independence Centers at the University of Florida (1 P30 AG028740), Wake Forest University (1 P30 AG21332), Tufts University (1P30AG031679) and University of Pittsburgh (P30 AG024827). Dr Roger Fielding (Tufts University) is partially supported by the US Department of Agriculture, under agreement 58-1950-0-014. The views of the authors do not reflect those of the USDA.
Sponsors role: The ENabling Reduction of low-Grade Inflammation in Seniors Pilot Study is funded by a National Institutes of Health/National Institute on Aging Cooperative Agreement U01 AG050499 and sponsored in part by the Intramural Research Program, National Institute on Aging, NIH.
Footnotes
Author Contributions: All authors: study concept, critical revisions of content and approval of final version for publication; TMM: lead the initial draft, revisions of the manuscript, the study design, and selection of outcomes; SDA: contributed to the initial manuscript draft, revisions and review; DPB: provided critical review and revisions of the manuscript; JAC: provided critical review and revisions of the manuscript; MAE: provided critical review and revisions of the manuscript; RAF: contributed to the study design, selection of outcomes, and provided critical review of manuscript; CL: provided critical review and revisions of the manuscript; KHL: provided critical review of the manuscript; CKL: provided critical review and revisions of the manuscript; MMM: provided critical review of the manuscript; MEM: provided critical review and revisions of the manuscript; RPT: provided critical review and revisions of the manuscript; JW: provided critical review and revisions of the manuscript; BR: provided critical review and revisions of the manuscript; JDL: provided critical review and revisions of the manuscript; BR: provided critical review and revisions of the manuscript; JL: contributed to the project organization, development of tables and facilitated manuscript development; CS: contributed to the study outcomes and operational procedures; SW: contributed to the statistical design, provided critical review and revisions of the manuscript; ABN: provided critical review and revisions of the manuscript; WTA: Co-Principal Investigator, secured funding and provided critical review and revisions of the manuscript; MP: Administrative Principal Investigator, responsible for the study concept, secured funding, and provided critical review and revisions of the manuscript.
Reference List
- 1.Walford R. The Immunologic theory of aging. Copenhagen; Munksgaard: 1969. [Google Scholar]
- 2.Soysal P, Stubbs B, Lucato P, et al. Inflammation and frailty in the elderly: A systematic review and meta-analysis. Ageing Res Rev. 2016;31:1–8. doi: 10.1016/j.arr.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Campisi J, Andersen JK, Kapahi P, Melov S. Cellular senescence: a link between cancer and age-related degenerative disease? Semin Cancer Biol. 2011;21:354–359. doi: 10.1016/j.semcancer.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roubenoff R. The “cytokine for gerontologists” has some company. J Gerontol A Biol Sci Med Sci. 2014;69:163–164. doi: 10.1093/gerona/glt184. [DOI] [PubMed] [Google Scholar]
- 5.Anand S, Johansen KL, Kurella TM. Aging and chronic kidney disease: the impact on physical function and cognition. J Gerontol A Biol Sci Med Sci. 2014;69:315–322. doi: 10.1093/gerona/glt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Folsom AR, Pankow JS, Tracy RP, et al. Association of C-reactive protein with markers of prevalent atherosclerotic disease. Am J Cardiol. 2001;88:112–117. doi: 10.1016/s0002-9149(01)01603-4. [DOI] [PubMed] [Google Scholar]
- 7.Libby P, Sukhova G, Lee RT, Galis ZS. Cytokines regulate vascular functions related to stability of the atherosclerotic plaque. J Cardiovasc Pharmacol. 1995;25(Suppl 2):S9–12. doi: 10.1097/00005344-199500252-00003. [DOI] [PubMed] [Google Scholar]
- 8.Sanders JL, Ding V, Arnold AM, et al. Do changes in circulating biomarkers track with each other and with functional changes in older adults? J Gerontol A Biol Sci Med Sci. 2014;69:174–181. doi: 10.1093/gerona/glt088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011;10:319–329. doi: 10.1016/j.arr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu FC, Kritchevsky SB, Liu Y, et al. Association Between Inflammatory Components and Physical Function in the Health, Aging, and Body Composition Study: A Principal Component Analysis Approach. J Gerontol A Biol Sci Med Sci. 2009;64:581–589. doi: 10.1093/gerona/glp005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brinkley TE, Leng X, Miller ME, et al. Chronic inflammation is associated with low physical function in older adults across multiple comorbidities. J Gerontol A Biol Sci Med Sci. 2009;64:455–461. doi: 10.1093/gerona/gln038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cesari M, Marzetti E, Laudisio A, et al. Interaction of HDL cholesterol concentrations on the relationship between physical function and inflammation in community-dwelling older persons. Age Ageing. 2010;39:74–80. doi: 10.1093/ageing/afp194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDermott MM, Liu K, Ferrucci L, et al. Circulating blood markers and functional impairment in peripheral arterial disease. J Am Geriatr Soc. 2008;56:1504–1510. doi: 10.1111/j.1532-5415.2008.01797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cesari M, Penninx BW, Pahor M, et al. Inflammatory markers and physical performance in older persons: the InChianti study. J Gerontol A Biol Sci Med Sci. 2004;59:242–248. doi: 10.1093/gerona/59.3.m242. [DOI] [PubMed] [Google Scholar]
- 15.Ferrucci L, Harris TB, Guralnik JM, et al. Inflammation, a novel risk factor for disability in older persons. J Am Geriatr Soc. 1999;47:639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 16.Penninx BW, Kritchevsky SB, Newman AB, et al. Inflammatory markers and incident mobility limitation in the elderly. J Am Geriatr Soc. 2004;52:1105–1113. doi: 10.1111/j.1532-5415.2004.52308.x. [DOI] [PubMed] [Google Scholar]
- 17.Fielding RA, Rejeski WJ, Blair SN, et al. The Lifestyle Interventions and Independence for Elders (LIFE) Study: Design and Methods. J Gerontol A Biol Sci Med Sci. 2011;66:1226–1237. doi: 10.1093/gerona/glr123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walston J, McBurnie MA, Newman A, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002;162:2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- 20.Manini TM, Cook SB, VanArnam T, Marko M, Ploutz-Snyder L. Evaluating task modification as an objective measure of functional limitation: repeatability and comparability. J Gerontol A Biol Sci Med Sci. 2006;61:718–725. doi: 10.1093/gerona/61.7.718. [DOI] [PubMed] [Google Scholar]
- 21.Reiner AP, Lange EM, Jenny NS, et al. Soluble CD14: genomewide association analysis and relationship to cardiovascular risk and mortality in older adults. Arterioscler Thromb Vasc Biol. 2013;33:158–164. doi: 10.1161/ATVBAHA.112.300421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olson NC, Callas PW, Hanley AJ, et al. Circulating levels of TNF-alpha are associated with impaired glucose tolerance, increased insulin resistance, and ethnicity: the Insulin Resistance Atherosclerosis Study. J Clin Endocrinol Metab. 2012;97:1032–1040. doi: 10.1210/jc.2011-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marti CN, Khan H, Mann DL, et al. Soluble tumor necrosis factor receptors and heart failure risk in older adults: Health, Aging, and Body Composition (Health ABC) Study. Circ Heart Fail. 2014;7:5–11. doi: 10.1161/CIRCHEARTFAILURE.113.000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moertl D, Hammer A, Steiner S, Hutuleac R, Vonbank K, Berger R. Dose-dependent effects of omega-3-polyunsaturated fatty acids on systolic left ventricular function, endothelial function, and markers of inflammation in chronic heart failure of nonischemic origin: a double-blind, placebo-controlled, 3-arm study. Am Heart J. 2011;161:915–919. doi: 10.1016/j.ahj.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 25.Nodari S, Triggiani M, Campia U, et al. Effects of n-3 polyunsaturated fatty acids on left ventricular function and functional capacity in patients with dilated cardiomyopathy. J Am Coll Cardiol. 2011;57:870–879. doi: 10.1016/j.jacc.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 26.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275–2285. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 27.Voorhees PM, Manges RF, Sonneveld P, et al. A phase 2 multicentre study of siltuximab, an anti-interleukin-6 monoclonal antibody, in patients with relapsed or refractory multiple myeloma. Br J Haematol. 2013;161:357–366. doi: 10.1111/bjh.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howard C, Noe A, Skerjanec A, et al. Safety and tolerability of canakinumab, an IL-1beta inhibitor, in type 2 diabetes mellitus patients: a pooled analysis of three randomised double-blind studies. Cardiovasc Diabetol. 2014;13:94. doi: 10.1186/1475-2840-13-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh S, Loke YK, Furberg CD. Long-term use of thiazolidinediones and the associated risk of pneumonia or lower respiratory tract infection: systematic review and meta-analysis. Thorax. 2011;66:383–388. doi: 10.1136/thx.2010.152777. [DOI] [PubMed] [Google Scholar]
- 30.Parker BA, Capizzi JA, Grimaldi AS, et al. Effect of statins on skeletal muscle function. Circulation. 2013;127:96–103. doi: 10.1161/CIRCULATIONAHA.112.136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuhara H, Corley DA, Nakahara F, et al. Aspirin and non-aspirin NSAIDs increase risk of colonic diverticular bleeding: a systematic review and meta-analysis. J Gastroenterol. 2014;49:992–1000. doi: 10.1007/s00535-013-0905-z. [DOI] [PubMed] [Google Scholar]
- 32.Hennekens CH, Borzak S. Cyclooxygenase-2 inhibitors and most traditional nonsteroidal anti-inflammatory drugs cause similar moderately increased risks of cardiovascular disease. J Cardiovasc Pharmacol Ther. 2008;13:41–50. doi: 10.1177/1074248407312990. [DOI] [PubMed] [Google Scholar]
- 33.Ching JK, Ju JS, Pittman SK, Margeta M, Weihl CC. Increased autophagy accelerates colchicine-induced muscle toxicity. Autophagy. 2013;9:2115–2125. doi: 10.4161/auto.26150. [DOI] [PubMed] [Google Scholar]
- 34.Kuncl RW, Duncan G, Watson D, Alderson K, Rogawski MA, Peper M. Colchicine myopathy and neuropathy. N Engl J Med. 1987;316:1562–1568. doi: 10.1056/NEJM198706183162502. [DOI] [PubMed] [Google Scholar]
- 35.Haffner S, Temprosa M, Crandall J, et al. Intensive lifestyle intervention or metformin on inflammation and coagulation in participants with impaired glucose tolerance. Diabetes. 2005;54:1566–1572. doi: 10.2337/diabetes.54.5.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erem C, Ozbas HM, Nuhoglu I, Deger O, Civan N, Ersoz HO. Comparison of effects of gliclazide, metformin and pioglitazone monotherapies on glycemic control and cardiovascular risk factors in patients with newly diagnosed uncontrolled type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2014;122:295–302. doi: 10.1055/s-0034-1370989. [DOI] [PubMed] [Google Scholar]
- 37.Kadoglou NP, Kapelouzou A, Tsanikidis H, Vitta I, Liapis CD, Sailer N. Effects of rosiglitazone/metformin fixed-dose combination therapy and metformin monotherapy on serum vaspin, adiponectin and IL-6 levels in drug-naive patients with type 2 diabetes. Exp Clin Endocrinol Diabetes. 2011;119:63–68. doi: 10.1055/s-0030-1265174. [DOI] [PubMed] [Google Scholar]
- 38.Madej A, Buldak L, Basiak M, Szkrobka W, Dulawa A, Okopien B. The effects of 1 month antihypertensive treatment with perindopril, bisoprolol or both on the ex vivo ability of monocytes to secrete inflammatory cytokines. Int J Clin Pharmacol Ther. 2009;47:686–694. doi: 10.5414/cpp47686. [DOI] [PubMed] [Google Scholar]
- 39.Trevelyan J, Brull DJ, Needham EW, Montgomery HE, Morris A, Mattu RK. Effect of enalapril and losartan on cytokines in patients with stable angina pectoris awaiting coronary artery bypass grafting and their interaction with polymorphisms in the interleukin-6 gene. Am J Cardiol. 2004;94:564–569. doi: 10.1016/j.amjcard.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 40.Sumukadas D, Witham MD, Struthers AD, McMurdo ME. Effect of perindopril on physical function in elderly people with functional impairment: a randomized controlled trial. CMAJ. 2007;177:867–874. doi: 10.1503/cmaj.061339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koz C, Baysan O, Yokusoglu M, et al. The effects of perindopril on aortic elasticity and inflammatory markers in hypertensive patients. Med Sci Monit. 2009;15:I41–I45. [PubMed] [Google Scholar]
- 42.Touyz RM, Savoia C, He Y, et al. Increased inflammatory biomarkers in hypertensive type 2 diabetic patients: improvement after angiotensin II type 1 receptor blockade. J Am Soc Hypertens. 2007;1:189–199. doi: 10.1016/j.jash.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 43.Li K, Huang T, Zheng J, Wu K, Li D. Effect of marine-derived n-3 polyunsaturated fatty acids on C-reactive protein, interleukin 6 and tumor necrosis factor alpha: a meta-analysis. PLoS ONE. 2014;9:e88103. doi: 10.1371/journal.pone.0088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sola S, Mir MQ, Cheema FA, et al. Irbesartan and lipoic acid improve endothelial function and reduce markers of inflammation in the metabolic syndrome: results of the Irbesartan and Lipoic Acid in Endothelial Dysfunction (ISLAND) study. Circulation. 2005;111:343–348. doi: 10.1161/01.CIR.0000153272.48711.B9. [DOI] [PubMed] [Google Scholar]
- 45.Hutchins-Wiese HL, Kleppinger A, Annis K, et al. The impact of supplemental n-3 long chain polyunsaturated fatty acids and dietary antioxidants on physical performance in postmenopausal women. J Nutr Health Aging. 2013;17:76–80. doi: 10.1007/s12603-012-0415-3. [DOI] [PubMed] [Google Scholar]
- 46.Rodacki CL, Rodacki AL, Pereira G, et al. Fish-oil supplementation enhances the effects of strength training in elderly women. Am J Clin Nutr. 2012;95:428–436. doi: 10.3945/ajcn.111.021915. [DOI] [PubMed] [Google Scholar]
- 47.de GM, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52:415–472. [PubMed] [Google Scholar]
- 48.Siddiqui RA, Shaikh SR, Sech LA, Yount HR, Stillwell W, Zaloga GP. Omega 3-fatty acids: health benefits and cellular mechanisms of action. Mini Rev Med Chem. 2004;4:859–871. doi: 10.2174/1389557043403431. [DOI] [PubMed] [Google Scholar]
- 49.Kwon S, Perera S, Pahor M, et al. What is a meaningful change in physical performance? findings from a clinical trial in older adults (the LIFE-P study) J Nutr Health Aging. 2009;13:538–544. doi: 10.1007/s12603-009-0104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parving HH, Brenner BM, McMurray JJ, et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med. 2012;367:2204–2213. doi: 10.1056/NEJMoa1208799. [DOI] [PubMed] [Google Scholar]