Abstract
Introduction. Case reports have emerged with identification of Gordonia bronchialis infections including sternal wound infections and foreign bodies such as central lines and shunts.
Case presentation. We present a case that demonstrates the need to consider Gordonia infection as a cause of sternal wound infection and highlights the utility of novel diagnostics to aid in the identification of unusual pathogens that can cause post-operative infections. We report here the first successful use of ceftaroline for treatment of a G. bronchialis sternal wound infection.
Conclusion. There are only case reports and in vitro assays to date to guide treatment of this infection, and we now add ceftaroline as a new drug to consider, though adequate surgical debridement is paramount.
Keywords: Gordonia bronchialis, sternal wound infection, ceftaroline, cardiac surgery
Abbreviations
CABG, coronary artery bypass grafting; CT, computed tomography.
Introduction
Sternal wound infections following coronary artery bypass grafting (CABG) are rare occurrences with incidence ranging from 1–4 % [1–3]. Variables reported to increase the risk of post-operative sternal wound infections include bilateral internal mammary artery grafting (presumably from decreased sternal blood flow), diabetes mellitus, chronic obstructive pulmonary disease, peri-operative haemodynamic instability including use of vasopressor medication, and obesity [4]. Measures to decrease surgical site infections following cardiac surgery have focused on peri-operative chlorhexidine washing and antibiotics, sternal closure techniques, glycemic control and implantable antibiotics amongst others [4]. Gordonia sternal wound infections were first described in a case series published in 1991 wherein a single circulating scrub nurse nasally colonized with Gordonia bronchialis was identified as the vector for contamination of the surgical wounds [5]. Subsequent case reports have emerged with identification of G. bronchialis infections with foreign bodies including central lines and shunts (Table 1).
Table 1. Case reports of surgical site infections due to Gordonia bronchialis.
| Type of infection (no. of cases) |
Type of procedure | Year of publication (references) |
|---|---|---|
| Sternal osteomyelitis | Sternotomy | Current case |
| Sternal wound | Sternotomy | 2016 [11] |
| Subcutaneous abscess | Needle injection | 2016 [12] |
| Peritonitis (2) | Peritoneal dialysis | 2015, 2014 [13, 14] |
| Sternal wound | Sternotomy | 2014 [15] |
| Sternal osteomyelitis | Sternotomy | 2014 [16] |
| Sternal osteomyelitis | Sternotomy | 2013 [17] |
| Sternal wound (3) | Sternotomy | 2012 [8] |
| Tibial osteomyelitis | Arthroscopy | 2012 [18] |
| Sternal wound (7) | Sternotomy | 1991 [5] |
Case report
A 69-year-old man with well controlled diabetes mellitus underwent three vessel CABG at our hospital, including internal mammary and saphenous vein grafts performed using cardiopulmonary bypass. Six weeks post-operatively he presented to an outside hospital with erythema and drainage at the cranial end of the sternotomy incision (Fig. 1a). He was found to be afebrile and haemodynamically stable. Computed tomography (CT) imaging did not identify a drainable fluid collection. Cultures of the drainage reportedly grew scant diphtheroids. Despite empiric treatment with intravenous vancomycin for one month, he noted progressive enlargement of his wound with worsening drainage and chest pain. This prompted referral to our institution, 12 weeks after the bypass surgery. The patient did not have any systemic symptoms of illness. His physical exam was notable for being afebrile with normal vital signs, and having tenderness, erythema and drainage at the cephalic end of his sternal incision (Fig. 1b).
Fig. 1.
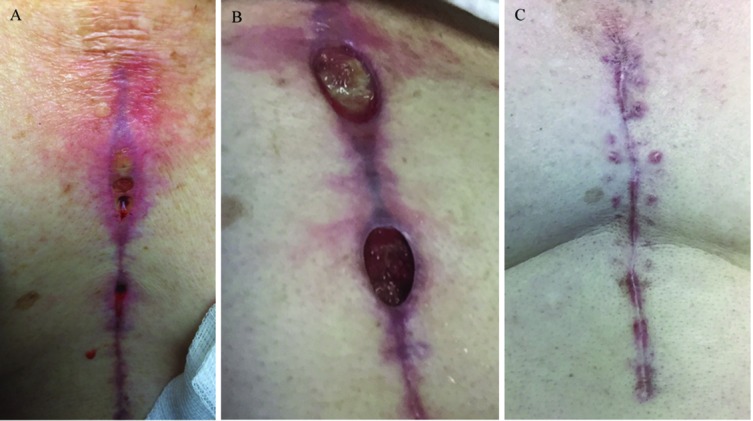
Images of the coronary artery bypass grafting wound from which G. bronchialis was isolated. (a) 8 weeks post-operative. (b) 12 weeks following initial surgery. 8 cm long sternal incisional wounds with surrounding erythema and clear drainage most prominent at cephalic location. (c) Complete resolution of incision 6 weeks post-debridement.
Further history obtained from the patient at that time revealed that he repairs industrial motorized fishing reels from around the United States. The rods often arrived covered in seaweed and barnacles. However, he did not handle the fishing gear himself. There were no other reported environmental exposures.
Investigations
Laboratory results were notable for mild elevation in CRP (25.7 mg l−1, normal ≤8 mg l−1) with normal white blood cell count (7000 cells µl−1, normal range 4500–10000 cells µl−1). CT chest with IV contrast was notable for dehiscence of the manubrium and non-fused sternum with lytic and sclerotic changes concerning for sternal osteomyelitis. Given these findings, the patient underwent debridement of the sternal wound and wire removal. Pathology from the debridement demonstrated mostly granulation tissue.
Diagnosis
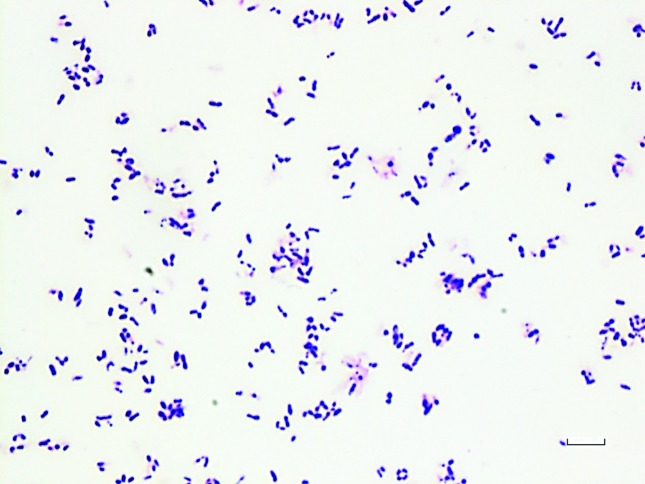
Cultures from the operating room initially grew a few colonies of methicillin-resistant Staphylococcus epidermidis (MRSE) with a vancomycin MIC of 2 µg ml−1, but after 72 h there was robust growth of a Gram-positive rod (Fig. 2) that grew on blood, chocolate and Lowenstein–Jensen media, as well as in liquid mycobacterial media (VersaTrek; ThermoFisher). The organism was catalase-positive. Further testing using Vitek2 (bioMérieux) and by API strips (bioMérieux) led to low discrimination identification of either Corynebacterium jeikeium or Microbacterium spp., Gordonia, Dietzia and Nocardia, respectively.
Fig. 2.
Gram stain made from a colony of Gordonia bronchialis isolated from a wound culture, demonstrating small pleomorphic Gram-positive rods. Bar, 10 µm.
The isolate was sent out for further analysis by mass spectrometry using the MALDI Biotyper (Bruker) and returned with probable identification of Gordonia bronchialis (score 1.83). DNA extraction and PCR-amplification of the DNA gyrase region of Gordonia spp. with a set of novel PCR primers, followed by standard Sanger sequencing, confirmed the identification with 99 % similarity to previously reported Gordonia bronchialis sequences (GenBank accession number AB438182).
Treatment
By disc diffusion testing, our G. bronchialis isolate appeared susceptible to penicillin, gentamicin, levofloxacin, minocycline, vancomycin, linezolid, tetracycline and erythromycin, although there are no established criteria for evaluating zone sizes for this organism. Given previous treatment with vancomycin without improvement, we looked for an alternative drug. Ceftaroline susceptibility was tested by Etest (bioMérieux) and the MIC was 0.19 µg ml−1, but there is not a Clinical and Laboratory Standards Institute (CLSI) breakpoint interpretation for this organism. We decided to treat this infection with ceftaroline at 600 mg every 8 h for 8 weeks, at which time the wound was closed (Fig. 1c).
Outcome and follow-up
Eight months after treatment ended the wound was still closed, inflammatory markers normalized and the patient has remained pain-free.
Discussion
Species of the genus Gordonia were previously included in the genus Rhodococcus but based on differences in 16S rRNA gene and gyrase subunit B sequences they were moved to a new genus. Species of the genus Gordonia are weakly acid-fast, aerobic, nocardioform actinomycetes that are found in soil, sewage and freshwater [6]. In the clinical microbiology laboratory they can be difficult to identify, and may be dismissed as skin or culture contaminants due to their diphtheroid-like appearance. Of note, in a large retrospective series Corynebacterium are rarely listed as a cause of sternal wound infections [7]. The case we present here appears to have been an isolated infection that presented several weeks following surgery. No subsequent cases have occurred in our hospital.
As with prior case reports (Table 1), in this case debridement was the primary modality of treatment with adjuvant antibiotics. Gordonia infections are exceedingly rare, primarily reported as surgical site infections, so there is little clinical experience that can provide guidance on optimal antibiotic therapy [8]. Antibiotics used in prior published cases included imipenem, gentamicin, ciprofloxacin, vancomycin and ceftriaxone. A study from Japan characterized 13 isolates of Gordonia bronchialis, mostly from pulmonary samples obtained between 1998 and 2008, and these showed as susceptible to carbapenems and aminoglycosides with variable susceptibility to minocycline, vancomycin and third-generation cephalosporins, though with so few isolates tested this cannot be used as a definitive guide for empiric treatment [9]. Ceftaroline is a cephalosporin developed primarily to treat methicillin-resistant Staphylococcus aureus (MRSA) infections and it is approved by the Food and Drug Administration (FDA) for skin and soft tissue infections, with or without bacteraemia. In this case, in the context of growth of MRSE and a low ceftaroline MIC for both the MRSE and the G. bronchialis, and clinical failure of prolonged vancomycin therapy, we opted to treat the patient with ceftaroline. We used a higher dose than the one that is FDA approved for skin and soft tissue infections and pneumonia, but it was within the range of what we have used to treat MRSA osteomyelitis [10]. Given the good outcome in this case and the paucity of clinical data to guide treatment, ceftaroline may be considered an alternative agent to complement adequate surgical debridement.
Funding information
This publication was made possible with help from the University of California, San Diego, Center for AIDS Research (CFAR), an NIH-funded program (P30 AI036214), which is supported by the following NIH Institutes and Centers: NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NIA, NIGMS, and NIDDK. This work was supported by the Department of Veterans Affairs San Diego and grants from the National Institutes of Health: AI118422(Smith-Immune Stimulation), AI036214 (Richman-CFAR), AI007384 (Richman-Training), AI096113 (CARE Collaboratory). The following grants also contributed support: VUMC38441 (Mehta-Vanderbilt), the James B. Pendleton Charitable Trust.
Acknowledgements
The authors acknowledge Veterans Affairs San Diego Microbiology Laboratory staff .
Conflicts of interest
The authors declare that there are no conflicts of interest.
Ethical statement
Informed consent to present de-identified patient information was obtained from the patient.
References
- 1.Filsoufi F, Castillo JG, Rahmanian PB, Broumand SR, Silvay G, et al. Epidemiology of deep sternal wound infection in cardiac surgery. J Cardiothorac Vasc Anesth. 2009;23:488–494. doi: 10.1053/j.jvca.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Kubota H, Miyata H, Motomura N, Ono M, Takamoto S, et al. Deep sternal wound infection after cardiac surgery. J Cardiothorac Surg. 2013;8:1. doi: 10.1186/1749-8090-8-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lemaignen A, Birgand G, Ghodhbane W, Alkhoder S, Lolom I, et al. Sternal wound infection after cardiac surgery: incidence and risk factors according to clinical presentation. Clin Microbiol Infect. 2015;21:674.e11–674674. doi: 10.1016/j.cmi.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 4.Sajja LR. Strategies to reduce deep sternal wound infection after bilateral internal mammary artery grafting. Int J Surg. 2015;16:171–178. doi: 10.1016/j.ijsu.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 5.Richet HM, Craven PC, Brown JM, Lasker BA, Cox CD, et al. A cluster of Rhodococcus (Gordona) bronchialis sternal-wound infections after coronary-artery bypass surgery. N Engl J Med. 1991;324:104–109. doi: 10.1056/NEJM199101103240206. [DOI] [PubMed] [Google Scholar]
- 6.Blaschke AJ, Bender J, Byington CL, Korgenski K, Daly J, et al. Gordonia species: emerging pathogens in pediatric patients that are identified by 16S ribosomal RNA gene sequencing. Clin Infect Dis. 2007;45:483–486. doi: 10.1086/520018. [DOI] [PubMed] [Google Scholar]
- 7.Si D, Rajmokan M, Lakhan P, Marquess J, Coulter C, et al. Surgical site infections following coronary artery bypass graft procedures: 10 years of surveillance data. BMC Infect Dis. 2014;14:1. doi: 10.1186/1471-2334-14-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wright SN, Gerry JS, Busowski MT, Klochko AY, McNulty SG, et al. Gordonia bronchialis sternal wound infection in 3 patients following open heart surgery: intraoperative transmission from a healthcare worker. Infect Control Hosp Epidemiol. 2012;33:1238–1241. doi: 10.1086/668441. [DOI] [PubMed] [Google Scholar]
- 9.Aoyama K, Kang Y, Yazawa K, Gonoi T, Kamei K, et al. Characterization of clinical isolates of Gordonia species in Japanese clinical samples during 1998–2008. Mycopathologia. 2009;168:175–183. doi: 10.1007/s11046-009-9213-9. [DOI] [PubMed] [Google Scholar]
- 10.Lin JC, Aung G, Thomas A, Jahng M, Johns S, et al. The use of ceftaroline fosamil in methicillin-resistant Staphylococcus aureus endocarditis and deep-seated MRSA infections: a retrospective case series of 10 patients. J Infect Chemother. 2013;19:42–49. doi: 10.1007/s10156-012-0449-9. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez-Lozano J, Pérez-Llantada E, Agüero J, Rodríguez-Fernández A, Ruiz de Alegria C, et al. Sternal wound infection caused by Gordonia bronchialis: identification by MALDI-TOF MS. JMM Case Rep. 2016;3:e005067. doi: 10.1099/jmmcr.0.005067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartolomé-Álvarez J, Sáez-Nieto JA, Escudero-Jiménez A, Barba-Rodríguez N, Galán-Ros J, et al. Cutaneous abscess due to Gordonia bronchialis: case report and literature review. Rev Esp Quimioter. 2016;29:170–173. [PubMed] [Google Scholar]
- 13.Lam JY, Wu AK, Leung WS, Cheung I, Tsang CC, et al. Gordonia species as emerging causes of continuous-ambulatory-peritoneal-dialysis-related peritonitis identified by 16S rRNA and secA1 gene sequencing and matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) J Clin Microbiol. 2015;53:671–676. doi: 10.1128/JCM.02971-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma TK, Chow KM, Kwan BC, Lee KP, Leung CB, et al. Peritoneal-dialysis related peritonitis caused by Gordonia species: report of four cases and literature review. Nephrology. 2014;19:379–383. doi: 10.1111/nep.12233. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen DB, Gupta N, Abou-Daoud A, Klekamp BG, Rhone C, et al. A polymicrobial outbreak of surgical site infections following cardiac surgery at a community hospital in Florida, 2011–2012. Am J Infect Control. 2014;42:432–435. doi: 10.1016/j.ajic.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang JH, Ji M, Hong HL, Choi SH, Kim YS, et al. Sternal osteomyelitis caused by Gordonia bronchialis after open-heart surgery. Infect Chemother. 2014;46:110. doi: 10.3947/ic.2014.46.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vasquez MA, Marne C, Villuendas MC, Arazo P. Subacute sternal osteomyelitis caused by Gordonia bronchialis after open-heart surgery. Enferm Infecc Microbiol Clin. 2013;31:559–560. doi: 10.1016/j.eimc.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 18.Siddiqui N, Toumeh A, Georgescu C. Tibial osteomyelitis caused by Gordonia bronchialis in an immunocompetent patient. J Clin Microbiol. 2012;50:3119–3121. doi: 10.1128/JCM.00563-12. [DOI] [PMC free article] [PubMed] [Google Scholar]