Abstract
The objective of this meta-analysis is to summarize the effect of exercise intervention on flow-mediated dilatation (FMD) in overweight and obese adults. We searched four electronic databases (PubMed/Medline, Scopus, and CINAHL) through June 2016 for relevant studies pertaining to the effectiveness of exercise intervention on FMD. Seventeen of the 91 studies identified met the inclusion criteria. Comprehensive Meta-Analysis software (version 3) was used to compute the standardized mean difference effect size (ES) and 95% CI using a random effects model. We calculated 34 ESs. We found that exercise intervention had medium and positive effects on FMD, with an overall ES of 0.522 (95% CI = 0.257, 0.786). Heterogeneity of ESs was observed (Qb = 239, p ≤ 0.001, I2 = 86.19), and the effect was moderated by comorbidity (Qb = 6.39, df = 1, p = 0.011). A large ES for the combination exercise, low intensity exercise, and comorbidity subgroups (ES = 0.82~1.24) was found. We conclude that while exercise intervention significantly improves FMD in overweight and obese adults, the effect may depend on the different characteristics of exercise intervention and on participants' demographics.
1. Introduction
Obesity, a chronic metabolic disorder, is strongly associated with morbidity and mortality as well as a reduced life expectancy [1]. Obesity is defined as an excessive accumulation of adipose tissue. Globally, 1.9 billion adults are overweight or obese, and this figure has more than doubled in the past two decades [2]. Epidemiological studies show that overweight and obese status in adults significantly increases the risks of numerous cardiovascular and circulatory disorders, for example, hypertension, stroke, coronary artery disease, and heart failure [1, 3]. Some of the complex, interrelated pathological states, for example, altered lipid profile and elevated blood pressure, also associated with obesity, subsequently induce insulin resistance, vascular oxidative stress, vascular endothelial dysfunction, and other debilitations [4–6].
The vascular endothelium, which is a single layer of cells lining the interior surface of blood vessels, plays a key role in vasomotor regulation mainly through the nitric oxide- (NO-) dependent signaling pathways [7]. The vascular endothelium is a sensitive structure which is susceptible to damage by certain lipids and inflammatory mediators [8]. Adipose tissue is well known to be associated with inflammatory processes, and it is also implicated in the production of reactive oxygen species (ROS) [9]. Multiple studies provide evidence that vascular endothelial function (EF) is impaired in the setting of obesity [6, 10, 11]. Endothelial dysfunction is considered to be an early precursor and common pathological feature of vascular diseases [12].
Endothelial dysfunction is commonly evaluated by flow-mediated dilatation (FMD) in human studies. The FMD is a noninvasive clinical tool that measures shear stress-mediated vasodilatory response and depends on NO bioavailability [7, 13]. One meta-analysis has reported an association between a 1% decrease in FMD and an 8% increase in the risk of future cardiovascular events [14]. While pharmacological intervention is often used to improve EF or body weight (e.g., topiramate and metformin), regular exercise training is a promising nonpharmacological option in obesity treatment [15].
Although a recent meta-analysis demonstrated the beneficial effect of exercise training on FMD in both obese and nonobese adults [16], the included studies pool data from both obese and nonobese groups, thus raising the question of whether obesity status is a confounding factor in accurate evaluation of FMD. Furthermore, the optimal intensity, modality, and duration of exercise for improving FMD are controversial [17–20]. These and similar studies also suggest there are several other potential factors confounding measurement of FMD in the setting of obesity.
To address the inconclusive findings, we conducted a meta-analysis to quantitatively evaluate the relationship between exercise training and EF in overweight and obese adults. We compared the effects of different characteristics of exercise interventions and participants' demographics on FMD.
2. Methods
2.1. Literature Search
Source of data was identified by keyword searched from four electronic databases: the PubMed/Medline, Scopus, and CINAHL. The keywords used to identify the relevant studies were “obesity”, “overweight”, “exercise”, “training”, “flow mediated dilatation”, “flow mediated dilation”, and “FMD”. Additional potential sources were identified by hand search using personal databases and a reference list of published studies.
2.2. Inclusion and Exclusion Criteria
The studies were included in the review if sufficient information was reported that allowed us to compute the standardized mean difference of FMD. Specific inclusion criteria for eligible studies were the study (1) included the value of relative FMD; (2) included exercise intervention at least 7 days; (3) considered only overweight and/or obese adults; and (4) is written in English language and published in peer-reviewed journals through June 2016. Furthermore, studies were excluded if they were purposefully designed for examining the effects of weight loss medication, antiandrogens, fertility treatments, glucocorticoids, or oral contraceptives.
2.3. Coding and Data Extraction
The two authors (YS, SJ) independently coded the identified studies using extraction sheets. The characteristics of the studies were coded for descriptive purposes and moderator analyses. Based on the procedures recommended by Lipsey and Wilson (2005), the outcome and moderator variables were extracted. The effect size (ES) of outcome variable, FMD, was computed using (a) before and after mean difference from intervention groups divided by pooled standard deviation (SD) and (b) mean difference between intervention and control groups divided by pooled SD. Also, moderator variables which may affect overall ES of FMD were coded as follows: body weight change, diet intervention, exercise duration/type/intensity, comorbidity, and baseline Body Mass Index (BMI). Exercise intensity and type were classified as low, moderate, and high intensity using the definition of the American College of Sports Medicine [21].
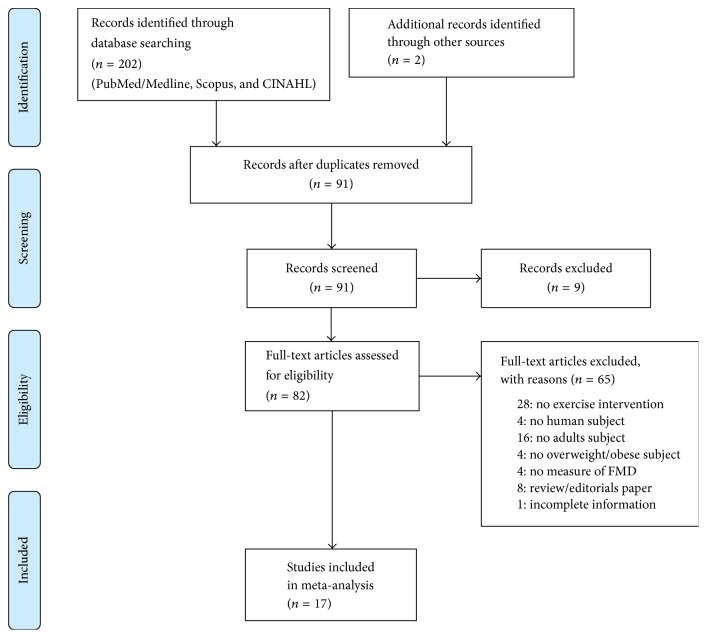
All coded data were crosschecked with authors for establishing consistency, and discrepancies were resolved by discussion. Figure 1 illustrates the schematic flow diagram of this study describing the inclusion and exclusion procedures for study selection [22].
Figure 1.
Flowchart for selection of studies.
2.4. Study Quality Rating
The methodological quality of selected studies was assessed using the Physiotherapy Evidence Database (PEDro) scale [40, 41]. This scale consists of 11 items: random allocation, concealed allocation, similarity at baseline, subject blinding, therapist blinding, assessor blinding, >85% follow-up for at least one key outcome, intention-to-treat analysis, between-group statistical comparison for at least one key outcome, and point and variability measures for at least one key outcome. The quality of studies is determined based on the average of overall scores (range = 0–10; each item, except for item 1, contributes one point) where higher scores indicate better methodological quality. The average total PEDro score is 5.0 ± 1.6 (mean ± SD) based on 27,444 records from the PEDro database on January 2017. The scoring ≥ 6/10 was considered “moderate to high” for methodological quality [42].
2.5. Data Analysis
All analyses were run in Comprehensive Meta-Analysis version-3 software with a significance level of 0.05. Because we assumed that the variety of research designs with study characteristics might affect the true ES from one study to another, a random effects model was used to estimate the overall ES and 95% confidence intervals (CIs). The measure of ES used for the present study is the standardized mean difference, Cohen's d. The ESs were evaluated based on Cohen's guideline, small (0.2), medium (0.5), and large (0.8) [43]. The Cochran's Q homogeneity statistic was used to determine the heterogeneity of the mean ESs across the groups. Moderator analyses were conducted to test the ES difference among the categorical subgroups of each moderator. The mean ES and 95 CI of each subgroup was also examined to see if an exercise intervention has an effect greater than zero. In addition, we examined the funnel plots, the Duval and Tweedie's trim and fill method, and Egger's test to detect the publication bias as all studies were published in the peer-reviewed journals in which the results are possibly subjected to publication bias.
3. Results
3.1. Search Results
Figure 1 outlines the flow diagram of the study selection process. The literature search identified 91 articles. We next reviewed the articles in full text to determine final eligibility. 17 studies ultimately met the eligibility criteria providing sufficient information for computing ESs. Some of the studies included results from separate, independent trials testing the effects of two or more exercise modalities on EF, so the 17 studies yielded a total of 34 ESs for the final meta-analysis.
3.2. Publication Bias
The funnel plot examination showed that the publication bias had little influence on our result. The studies included in the present meta-analysis were symmetrically distributed around the mean ES. The Duval and Tweedie's trim and fill method also predicted no missing study to this meta-analysis. However, the regression intercept (3.37) from Egger's test results was statistically significant (p = 0.001) that a potential publication bias for this meta-analysis should be noted for its interpretation.
3.3. Study Characteristics
The characteristics of these studies are shown in Table 1. Studies from around the world were included, including North America, Australia, South America, Europe, and Asia. They included women and men (mean age = 47.23) who are overweight or obesity with or without comorbidity. One trial used only men, 17 trials used only females, and 16 trials used both sexes. The included studies that accounted for other potential confounders such as smoking. 16 of 34 trials in 17 studies were RCTs with no exercise intervention control group. Table 2 shows the characteristics of interventions within the included studies. 71% of trials (n = 24 trials) incorporated aerobic exercise; 18% (n = 6 trials) and 11% (n = 4 trials) of trials incorporated resistance exercise and combined with aerobic and resistance exercise, respectively. Table 3 shows FMD protocol and outcomes. 23 trials in 17 studies reported fasting time (ranged from 0.5 hours to overnight fasting). Brachial artery FMD was measured in all of the included studies. 71% studies reported mean and SD of FMD percentage at preintervention and postintervention, and the rest of the studies reported amount of change or 95% confidence interval (CI). The mean and SD of FMD was 7.15% ± 3.05 (range 2.7 to 11.28%) before intervention and 8.67% ± 2.62 (range 4 to 12.9%) after intervention, and rate of change for FMD was 1.17% ± 1.63 (range −1.3 to 5%) in the treatment group. In the control group, the mean and SD of FMD was 5.69% ± 2.12 (range 2.5 to 9.9%) before intervention and 6.65% ± 2.46 (range 3.8 to 10.1%) after intervention, and rate of change for FMD was 0.61% ± 2.16 (range −0.7 to 4.9%).
Table 1.
Baseline characteristics of the included studies.
| Author and year | County | Subject characteristics of treatment group | Subject characteristics of control group | Smoker | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample size | Gender | Age | Health status | BMI | Δ weight | Sample size | Gender | Age | Health status | BMI | Δ weight | |||
| Ades et al. (2011) a [23] | USA | 23 | Both | 66 | Coronary heart disease | 32 | −8 | Not included | ||||||
| Ades et al. (2011) b [23] | USA | 15 | Both | 62 | Coronary heart disease | 33 | −2 | Not included | ||||||
| Baynard et al. (2009) a [24] | USA | 10 | Both | 52 | Metabolic syndrome | 34 | 0 | Not included | ||||||
| Baynard et al. (2009) b [24] | USA | 10 | Both | 52 | Metabolic syndrome | 34 | 0 | Not included | ||||||
| Baynard et al. (2009) c [24] | USA | 11 | Both | 53 | Healthy | 33 | 0 | Not included | ||||||
| Baynard et al. (2009) d [24] | USA | 11 | Both | 53 | Healthy | 33 | 0 | Not included | ||||||
| Bhutani et al. (2013) a [25] | USA | 18 | Both | 45 | Healthy | 35 | −6 | 25 | Both | 42 | Healthy | 35 | −3 | Not included |
| Bhutani et al. (2013) b [25] | USA | 24 | Both | 42 | Healthy | 35 | −1 | 16 | Both | 49 | Healthy | 35 | 0 | Not included |
| Blumenthal et al. (2010) [26] | USA | 49 | Both | 52 | HTN | 34 | −9 | 46 | Both | 52 | HTN | 33 | 0 | Included |
| Choo et al. (2014) a [27] | South of Korea | 39 | Female | 42 | Healthy | 29 | −2 | Included | ||||||
| Choo et al. (2014) b [27] | South of Korea | 39 | Female | 42 | Healthy | 29 | −2 | Included | ||||||
| Choo et al. (2014) c [27] | South of Korea | 39 | Female | 42 | Healthy | 29 | −2 | Included | ||||||
| Choo et al. (2014) d [27] | South of Korea | 26 | Female | 46 | Healthy | 28 | −2 | Included | ||||||
| Choo et al. (2014) e [27] | South of Korea | 26 | Female | 46 | Healthy | 28 | −2 | Included | ||||||
| Choo et al. (2014) f [27] | South of Korea | 26 | Female | 46 | Healthy | 28 | −1 | Included | ||||||
| Choo et al. (2014) g [27] | South of Korea | 27 | Female | 42 | Healthy | 29 | −1 | Included | ||||||
| Choo et al. (2014) h [27] | South of Korea | 27 | Female | 42 | Healthy | 29 | −1 | Included | ||||||
| Choo et al. (2014) i [27] | South of Korea | 27 | Female | 42 | Healthy | 29 | −1 | Included | ||||||
| Cotie et al. (2014) [28] | Canada | 20 | Female | 30 | Healthy | 32 | −6 | NR | ||||||
| Davison et al. (2008) a [29] | Australia | 13 | Both | 45 | Healthy | 34 | 1 | 11 | Both | 44 | Healthy | 35 | 2 | |
| Davison et al. (2008) b [29] | Australia | 13 | Both | 46 | Healthy | 33 | 1 | 12 | Both | 45 | Healthy | 33 | −2 | |
| Fayh et al. (2013) [30] | Brazil | 17 | Both | 31 | Healthy | 35 | −4 | 18 | Both | 32 | Healthy | 35 | −5 | Not included |
| Franklin et al. (2015) [31] | USA | 10 | Female | 30 | Healthy | 34 | −1 | 8 | Female | 31 | Healthy | 32 | 0 | Not included |
| Hamdy et al. (2003) [32] | USA | 24 | Both | 49 | Insulin resistance syndrome | 37 | −7 | Not included | ||||||
| Kwon et al. (2011) a [33] | South of Korea | 13 | Female | 56 | Type 2 diabetes | 27 | −2 | 15 | Female | 59 | Type 2 Diabetes | 27 | −1 | NR |
| Kwon et al. (2011) b [33] | South of Korea | 12 | Female | 56 | Type 2 diabetes | 27 | −1 | 15 | Female | 59 | Type 2 Diabetes | 27 | −1 | NR |
| Olson et al. (2006) [34] | USA | 15 | Female | 38 | Healthy | 28 | 2 | 15 | Female | 38 | Healthy | 28 | 0 | Not included |
| Pugh et al. (2014) [35] | UK | 13 | Both | 50 | Nonalcoholic fatty liver disease | 30 | −2 | 8 | Both | 47 | Healthy | 30 | −1 | |
| Robinson et al. (2016) [36] | USA | 10 | Both | 34 | Healthy | 32 | −3 | 9 | Both | 28 | 33 | 0 | Not included | |
| Swift et al. (2012) a [37] | USA | 68 | Female | 57 | Elevated BP | 32 | −1 | 23 | Female | 57 | Elevated BP | 32 | −1 | |
| Swift et al. (2012) b [37] | USA | 32 | Female | 56 | Elevated BP | 33 | −1 | 23 | Female | 57 | Elevated BP | 32 | −1 | |
| Swift et al. (2012) c [37] | USA | 32 | Female | 56 | Elevated BP | 31 | −1 | 23 | Female | 57 | Elevated BP | 32 | −1 | |
| Vinet et al. (2011) [38] | France | 10 | Male | 51 | Healthy | 33 | −2 | Not included | ||||||
| Wycherley et al. (2008) [39] | Australia | 13 | Both | 52 | Type 2 diabetes | 34 | −8 | 16 | Both | 53 | Type 2 Diabetes | 35 | −9 | |
Note. NR: no report.
Table 2.
Characteristics of intervention of the included studies.
| Author and year | Exercise intervention | Additional diet intervention | ||||
|---|---|---|---|---|---|---|
| Type | Duration (weeks) | Frequency of sessions (per week) | Duration of session (min) | Intensity | ||
| Ades et al. (2011) a [23] | Aerobic | 16 | 1–3 | 40–60 | Low (high-caloric-expenditure) | Yes |
| Ades et al. (2011) b [23] | Aerobic | 16 | 1–3 | 25–40 | Higher (lower-caloric-expenditure) | Yes |
| Baynard et al. (2009) a [24] | Aerobic | 10 days | 6 | 60 | 70–75% of VO2 peak | No |
| Baynard et al. (2009) b [24] | Aerobic | 10 days | 6 | 60 | 70–75% of VO2 peak | No |
| Baynard et al. (2009) c [24] | Aerobic | 10 days | 6 | 60 | 70–75% of VO2 peak | No |
| Baynard et al. (2009) d [24] | Aerobic | 10 days | 6 | 60 | 70–75% of VO2 peak | No |
| Bhutani et al. (2013) a [25] | Aerobic | 12 | 3 | 24–40 | 60–75% of HRmax | Yes |
| Bhutani et al. (2013) b [25] | Aerobic | 12 | 3 | 24–40 | 60–75% of HRmax | No |
| Blumenthal et al. (2010) [26] | Aerobic | 16 | 3 | 45 | 70–85% of HRR | Yes |
| Choo et al. (2014) a [27] | Aerobic | 12 | 3 | 60 | 50–70% of HRR | Yes |
| Choo et al. (2014) b [27] | Aerobic | 24 | 3 | 60 | 50–70% of HRR | Yes |
| Choo et al. (2014) c [27] | Aerobic | 38 | 3 | 60 | 50–70% of HRR | Yes |
| Choo et al. (2014) d [27] | Resistance | 12 | 3 | 60 | 40–60% of MS | Yes |
| Choo et al. (2014) e [27] | Resistance | 24 | 3 | 60 | 40–60% of MS | Yes |
| Choo et al. (2014) f [27] | Resistance | 38 | 3 | 60 | 40–60% of MS | Yes |
| Choo et al. (2014) g [27] | Combined | 12 | 3 | 60 (30 + 30) | 50–70% of HRR 40–60% of MS |
Yes |
| Choo et al. (2014) h [27] | Combined | 24 | 3 | 60 (30 + 30) | 50–70% of HRR 40–60% of MS |
Yes |
| Choo et al. (2014) i [27] | Combined | 38 | 3 | 60 (30 + 30) | 50–70% of HRR 40–60% of MS |
Yes |
| Cotie et al. (2014) [28] | Combined | 16 | 7 | Expend 250 kal/day | 70%/3 set 10 rep | Yes |
| Davison et al. (2008) a [29] | Aerobic | 12 | At least 1 | 45 | 75% of HRmax | Yes |
| Davison et al. (2008) b [29] | Aerobic | 12 | At least 1 | 45 | 75% of HRmax | Yes |
| Fayh et al. (2013) [30] | Aerobic | 10 | 3 | 45 | 70% of HRmax | Yes |
| Franklin et al. (2015) [31] | Circuit-based RT | 8 | 2 | 80–90% of 10 RM | No | |
| Hamdy et al. (2003) [32] | Aerobic | 24 | 3 | 30 | 60–80% of HRmax | Yes |
| Kwon et al. (2011) a [33] | Aerobic | 12 | 5 | 60 | Moderate (3.6–6 METs) | No |
| Kwon et al. (2011) b [33] | Resistance | 12 | 5 | 60 | Bands provide 1.2–3.2 kg of resistance | No |
| Olson et al. (2006) [34] | Resistance | 1 year | At least 2 | 3 sets 8–10 repetitions | No | |
| Pugh et al. (2014) [35] | Aerobic | 12 | 5 | 45 | 60% of HRR | Yes |
| Robinson et al. (2016) [36] | Aerobic | 8 | 3 | 30–45 | 75% of HRmax | No |
| Swift et al. (2012) a [37] | Aerobic | 24 | 3-4 | Expend 4 kcal/kg | 50% of VO2 peak | No |
| Swift et al. (2012) b [37] | Aerobic | 24 | 3-4 | Expend 8 kcal/kg | 50% of VO2 peak | No |
| Swift et al. (2012) c [37] | Aerobic | 24 | 3-4 | Expend 12 kcal/kg | 50% of VO2 peak | No |
| Vinet et al. (2011) [38] | Aerobic | 8 | 3 | 45 | LIPOXmaxHR ± 5 | No |
| Wycherley et al. (2008) [39] | Aerobic | 12 | 4-5 | 50–60 | 60–80% of HRmax | Yes |
Note. HRR: Heart Rate Reserve, MS: Maximum Strength, LIPOXmaxHR: maximum lipid-oxidation point.
Table 3.
FMD protocol and outcomes.
| Author anB3:H25 | Timing of measurement | Placed cuff | Treatment group | Control group | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Preintervention | Postintervention | Preintervention | Postintervention | |||||||
| mean | SD | mean | SD | mean | SD | mean | SD | |||
| Ades et al. (2011) a [23] | Fast | Brachial artery | 2.9 | 3.6 | 6.5 | 3.5 | ||||
| Ades et al. (2011) b [23] | Fast | Brachial artery | 3.6 | 4.1 | 4.9 | 3.8 | ||||
| Baynard et al. (2009) a [24] | Overnight fast | Brachial artery | 8 | 1.5 | 7.5 | 1.2 | ||||
| Baynard et al. (2009) b [24] | 0.5–1 h ingestion | Brachial artery | 6 | 1.1 | 6.3 | 1.1 | ||||
| Baynard et al. (2009) c [24] | Overnight fast | Brachial artery | 10.4 | 1.1 | 10.2 | 0.9 | ||||
| Baynard et al. (2009) d [24] | 0.5–1 h ingestion | Brachial artery | 9.8 | 0.8 | 8.5 | 0.8 | ||||
| Bhutani et al. (2013) a [25] | NR | Brachial artery | 3.8 | 1.2 | 6.4 | 0.8 | 4.8 | 1.2 | 9.7 | 1.8 |
| Bhutani et al. (2013) b [25] | NR | Brachial artery | 6.8 | 1.3 | 7.2 | 1.4 | 7 | 3.3 | 6.3 | 3 |
| Blumenthal et al. (2010) [26] | Overnight fast | Brachial artery | 4z | 1 | 3.8z | 1 | ||||
| Choo et al. (2014) a [27] | Overnight fast | Brachial artery | 11.28 | 3.5 | 11.55 | 3.8 | ||||
| Choo et al. (2014) b [27] | Overnight fast | Brachial artery | 11.28 | 3.5 | 11.08 | 4.05 | ||||
| Choo et al. (2014) c [27] | Overnight fast | Brachial artery | 11.28 | 3.5 | 10.7 | 3.75 | ||||
| Choo et al. (2014) d [27] | Overnight fast | Brachial artery | 10.32 | 3.8 | 11.22 | 4.43 | ||||
| Choo et al. (2014) e [27] | Overnight fast | Brachial artery | 10.32 | 3.8 | 10.89 | 4.33 | ||||
| Choo et al. (2014) f [27] | Overnight fast | Brachial artery | 10.32 | 3.8 | 11.54 | 4.99 | ||||
| Choo et al. (2014) g [27] | Overnight fast | Brachial artery | 11.02 | 3.49 | 11.1 | 3.4 | ||||
| Choo et al. (2014) h [27] | Overnight fast | Brachial artery | 11.02 | 3.49 | 12.41 | 4.27 | ||||
| Choo et al. (2014) i [27] | Overnight fast | Brachial artery | 11.02 | 3.49 | 11.3 | 4.04 | ||||
| Cotie et al. (2014) [28] | NR | Brachial artery | 4 | 0.5 | 6.9 | 0.6 | ||||
| Davison et al. (2008) a [29] | NR | Brachial artery | 5.37 | 0.68 | −0.4a | 0.77b | 3.65 | 1.4 | −0.3a | 0.53b |
| Davison et al. (2008) b [29] | NR | Brachial artery | 4.05 | 0.51 | 1.5a | 0.68b | 4.12 | 0.75 | 1.8a | 0.89b |
| Fayh et al. (2013) [30] | Overnight fast | Brachial artery | 8.1 | 3.6 | 10.7 | 3.6 | 9.9 | 3.4 | 10.1 | 5.8 |
| Franklin et al. (2015) [31] | NR | Brachial artery | 9.5 | 1.6 | 9.8 | 1.6 | 8.4 | 3.5 | 8 | 3.3 |
| Hamdy et al. (2003) [32] | NR | Brachial artery | 7.9 | 1 | 12.9 | 1.2 | ||||
| Kwon et al. (2011) a [33] | 10 h fast | Brachial artery | 4.3 | 1.6 | 6.4 | 1.9 | 4.7 | 1.9 | 4 | 1.9 |
| Kwon et al. (2011) b [33] | 10 h fast | Brachial artery | 4.9 | 2.5 | 5.6 | 2.8 | 4.7 | 1.9 | 4 | 1.9 |
| Olson et al. (2006) [34] | Overnight fast | Brachial artery | 6.3 | 0.2 | 6.2 | 0.1 | 6.3 | 0.2 | 6 | 0.1 |
| Pugh et al. (2014) [35] | NR | Brachial artery | 4.79 | 8.57 | (2.24–4.71)c | 5.94 | 5.32 | (−1.72–1.46)c | ||
| 3.47a | −0.13a | |||||||||
| Robinson et al. (2016) [36] | NR | Brachial artery | 8.6 | 4.8 | 7.7 | 2.79 | 9.3 | 4.2 | 9.3 | 4.1 |
| Swift et al. (2012) a [37] | Fast | Brachial artery | 4 | 2.6 | 1a | (0.29–1.76)c | 4.7 | 2.4 | −0.5a | (−1.79–0.74)c |
| Swift et al. (2012) b [37] | Fast | Brachial artery | 4.4 | 2.4 | 1.5a | (0.48–2.62)c | 4.7 | 2.4 | −0.5a | (−1.79–0.74)c |
| Swift et al. (2012) c [37] | Fast | Brachial artery | 3.7 | 2.6 | 1.2a | (0.1–2.24)c | 4.7 | 2.4 | −0.5a | (−1.79–0.74)c |
| Vinet et al. (2011) [38] | Overnight fast | Brachial artery | 2.7 | 0.4 | 4.8 | 0.5 | ||||
| Wycherley et al. (2008) [39] | Fast | Brachial artery | 4.2 | 1.2 | −0.52a | 1.06b | 2.5 | 0.9 | 0.03a | 0.26b |
Note. aΔmean; bΔSD; c95% confidence interval; zadjusted for value of preintervention; NR: no report.
3.4. Quality of Included Studies
Quality score by the PEDro scale was 7.2 ± 1.5 (ranged from 5 to 9), the median score of 8 of a maximum possible of 10 (Table 4). This score is equivalent to moderate to high quality [42]. All of the studies satisfied the following criteria: baseline comparability, intention-to-treat analysis, and mean and variability data. No study reported having blinded therapists.
Table 4.
Methodological scores by Physiotherapy Evidence Database (PEDro) scale.
| Studies | PEDro criterion | Total score | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||
| Ades et al. (2011) [23] | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 9 |
| Baynard et al. (2009) [24] | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 6 |
| Bhutani et al. (2013) [25] | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 7 |
| Blumenthal et al. (2010) [26] | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 8 |
| Choo et al. (2014) [27] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 9 |
| Cotie et al. (2014) [28] | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 5 |
| Davison et al. (2008) [29] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 9 |
| Fayh et al. (2013) [30] | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 7 |
| Franklin et al. (2015) [31] | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 8 |
| Hamdy et al. (2003) [32] | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 5 |
| Kwon et al. (2011) [33] | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 8 |
| Olson et al. (2006) [34] | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 9 |
| Pugh et al. (2014) [35] | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 7 |
| Robinson et al. (2016) [36] | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 5 |
| Swift et al. (2012) [37] | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 8 |
| Vinet et al. (2011) [38] | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 5 |
| Wycherley et al. (2008) [39] | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 8 |
|
| ||||||||||||
| Total | 16 | 12 | 12 | 17 | 3 | 0 | 4 | 9 | 17 | 16 | 17 | |
Note. Each number of PEDro criterion is represented as follows: 1: inclusion and source; 2: random allocation; 3: concealed allocation; 4: baseline comparability; 5: blinded subjects; 6: blinded therapists; 7: blinded assessors; 8: outcomes for >85%; 9: intention-to-treat analysis; 10: between-group comparisons; 11: mean and variability data.
3.5. Overall Effect Size
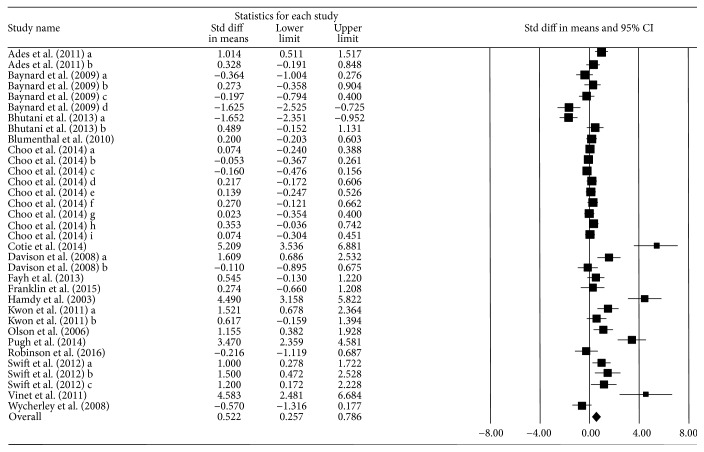
The overall mean ES was 0.522 (95% CI = 0.257, 0.786) and statistically significant. This quantitative synthesis yielded a medium and positive ES using a random effect model. This indicates that exercise training is effective in improving FMD in overweight and obese adults. There was observed to be heterogeneous (Qb = 239, p ≤ 0.001, I2 = 86.19), suggesting that moderator analyses are needed to better understand the exercise intervention effect on FMD. Figure 2 provides Forest plot with ESs of the analysis results.
Figure 2.
Forest plot illustrating effect of exercise intervention on FMD.
3.6. Moderator Analysis
The moderator analyses were performed to examine the effect of body weight change, diet intervention, exercise modality, comorbidity, and baseline BMI. The results demonstrated that only comorbidity status explained the heterogeneity of ESs (Qb = 6.39, df = 1, and p = 0.011). Table 5 shows the results of moderator analyses, which includes ESs, CIs, and Cochran's Q statistics for each moderator variables. A large ES was found in the combination exercise, low intensity exercise, and comorbidity subgroups (ES = 0.82~1.24). A moderate to large ES was found in body weight loss, with and without diet intervention, more than 12-week exercise duration, aerobic exercise, and between 30 and 34.9 baseline BMI (ES = 0.51~0.71).
Table 5.
Subgroup analysis.
| Moderator variable | n | ES | 95% CI | Q b | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Body weight change | |||||
| Increase | 7 | 0.10 | −0.50 | 0.70 | 2.545 |
| 0–2.9 kg loss | 19 | 0.61 | 0.26 | 0.97 | |
| ≥3 kg loss | 8 | 0.70 | 0.12 | 1.28 | |
| Diet intervention | |||||
| Yes | 20 | 0.51 | 0.18 | 0.85 | 0.008 |
| No | 14 | 0.54 | 0.11 | 0.97 | |
| Exercise duration | |||||
| <12 weeks | 8 | 0.09 | −0.50 | 0.68 | 2.868 |
| 12–23 weeks | 15 | 0.61 | 0.20 | 1.02 | |
| ≥24 weeks | 11 | 0.71 | 0.24 | 1.18 | |
| Exercise type | |||||
| Resistance | 6 | 0.43 | −0.22 | 1.07 | 0.601 |
| Aerobic | 24 | 0.52 | 0.18 | 0.85 | |
| Combined | 4 | 0.82 | 0.01 | 1.62 | |
| Exercise intensity | |||||
| Low | 3 | 1.24 | 0.29 | 2.19 | 2.401 |
| Moderate | 30 | 0.47 | 0.19 | 0.75 | |
| High | 1 | 0.27 | −1.38 | 1.93 | |
| Comorbidity | |||||
| No | 21 | 0.26 | −0.06 | 0.58 | 6.392∗ |
| Yes | 13 | 0.95 | 0.52 | 1.37 | |
| Baseline BMI | |||||
| 25–29.9 | 12 | 0.31 | −0.11 | 0.73 | 1.647 |
| 30–34.9 | 19 | 0.67 | 0.30 | 1.04 | |
| ≥35 | 3 | 0.66 | −0.28 | 1.61 | |
Note. ∗p < 0.05.
4. Discussion
In this meta-analysis, we found 34 trials from 17 studies including 1,045 overweight and obese adults. The meta-analysis result showed that exercise training significantly improves vascular function as measured by FMD of the brachial artery. The studies were randomized controlled trials of control and noncontrol groups of Asian and Western adult populations. Endothelial dysfunction is inherent in overweight and obese adults, and exercise training is universally accepted to ameliorate the obesity-associated endothelial dysfunction in healthy adults [16, 44]; however, more examination of the specific effects of exercise training on EF in overweight and obese population is still needed. Therefore, we combined data from each of the clinical trials to understand the relationship between exercise training and EF in overweight and obese adults.
Our results demonstrated that exercise has a moderate benefit on the improvement of FMD on overweight and obese adult populations in exercise intervention studies. When we probed moderators to examine the possible associations with ESs, we found that only comorbidity status influences the effectiveness of exercise intervention on EF. To our knowledge, we are the first to report this result. The finding could explain why exercise may not reverse the reduction of FMD attributable solely to obesity in isolation, whereas exercise may reverse the portion attributable to a comorbidity. While the explanation contrasts two previous meta-analyses [16, 45] showing that exercise is an effective method to improve FMD, they use pooled data from both obese and nonobese groups. If obesity acts on FMD in an exercise-independent way, it is possible that our finding of an FMD improvement results from the influence of exercise intervention on the portion of FMD decrement attributable to the comorbidity, rather than on the portion of FMD decrement attributable to obesity. We were unable to extrapolate the underlying mechanism of this finding, because it is beyond the scope of our study. Further research is required to examine different populations according to study characteristics (age, type of disease, stage of disease, exercise intensity, exercise type, treatment modality, etc.).
The examination of mean ES and 95 CI of each subgroup showed that ES is above medium in the subgroups with weight loss whereas there is no significant benefit of exercise intervention in the weight gain group. Although the mechanism of the effects of weight loss on FMD in overweight and obese adults requires more elucidation, numerous studies confirm the positive effects of weight loss by exercise on FMD. The beneficial effect of weight reduction by lifestyle changes, such as exercise to improve vascular function in obese adults, is strongly supported in [46], and a meta-analysis of the relationship between weight change and EF has reported a positive correlation between weight loss and an increase in FMD [45]. Therefore, it is speculated that weight reduction may be a major factor enhancing FMD in obese individuals and may depend on the method of weight reduction.
A study of the effect of surgically induced weight loss on FMD in hypertensive obese patients showed that bariatric surgery-induced weight loss improves blood pressure (BP), high-sensitivity C-reactive protein (hs-CRP), leptin, homeostasis model assessment (HOMA-IR), and abdominal fat, whereas FMD does not improve [47]. A study examining the effects of dietary weight loss on vascular function in obese men demonstrated that diet-induced weight reduction decreases aortic stiffness, total and low-density lipoprotein cholesterol, triglycerides, insulin resistance, and BP without alteration of FMD [48]. Similarly, our results demonstrate the significant benefit of exercise on FMD in overweight and obese adults regardless of diet control. Taken together, the above results indicating that exercise-mediated weight loss may improve FMD, but not diet control or surgery, suggest that exercise is a key regulator of FMD in overweight and obese adults. The notion that exercise-induced shear stress improves FMD in overweight and obese adults through an increase in the activity and expression of endothelial nitric oxide synthase (eNOS) that augments NO bioavailability also supports the suggestion [49–51].
We also found a moderate to large beneficial effect of exercise in the longer-period intervention subgroup than with 12 weeks of exercise program, and no significant benefit in the group with less than 12 weeks of intervention. We suggest that at least 12 weeks of exercise intervention may improve FMD in overweight and obese adults. Although previous reviews hypothesized that a longer duration may increase efficacy and maintain the effect on EF from the exercise intervention [16, 44], our meta-analysis provides the first quantitative evidence of the optimal exercise intervention duration to improve EF in overweight and obese adults.
As mentioned, exercise modality and intensity to improve EF remains controversial. For example, a meta-analysis of obese and nonobese adults showed that any type of exercise, including resistance, aerobic, and combined training improves EF [16]; however, another meta-analysis demonstrated that resistance exercise associates with increased arterial stiffness [18]. Our results confirmed no effect in the resistance exercise, medium to large effect in the aerobic exercise, and large effect in the combined exercise. We interpret our findings with caution, because more than 70% of included studies utilized aerobic exercises as an intervention modality. Furthermore, a recent study showed that high intensity exercise improves FMD more than moderate intensity, because higher intensity exercise causes greater shear stress resulting in more NO activation [17], even though other studies reported that high intensity exercise significantly reduces FMD [19, 20]. Our meta-analysis results showed that high intensity exercise has no effect, whereas low and moderate intensity exercise have large and medium ESs, respectively. Again, we interpret the results with caution, because a limited number of studies reported results for high (n = 1) and low (n = 3).
Moderator analysis also demonstrated that adults with a BMI 30–34.9 (level 1 obesity) have large and medium to large beneficial effect from exercise, respectively, whereas adults without a comorbidity and BMI < 30 or ≥35 have no significant benefit from exercise. Previously, Joris et al. [45] reported that the effects on FMD linearly relate to amount of weight loss in groups with obesity-related morbidities compared with healthy adults. Since this study pooled obese and nonobese adults and it did not break down groups by method of weight loss, a direct comparison with our study is not possible, because our data include only obese adults [45].
The effect of exercise on FMD may also depend on baseline BMI. Our result showed that only adults with level 1 obesity have a benefit from exercise training on FMD. A meta-analysis by Ashor et al., however, showed a greater effect of exercise on FMD in nonobese individuals than in obese individuals [16]. Again, a direct comparison is not possible. Further research should clarify the relationship between baseline obesity and exercise effect on FMD.
This study must be interpreted in the context of multiple limitations. First, the range in FMD levels in the studies is relatively small, and there is substantially less data for those with BMI > 35. Second, there were methodological limitations. FMD is well known for being operator and protocol dependent, and there was considerable variation in FMD data collection methodology [52, 53]. Therefore, further well-controlled studies are needed to draw accurate conclusions.
In summary, our meta-analysis indicates that exercise training is able to improve EF in overweight and obese adults, and that the effect of exercise may depend on the different characteristics of exercise intervention and on participants' demographics.
Disclosure
This study has been presented as the poster entitled, “Exercise and Vascular Function in Overweight and Obese Adults: A Meta-Analysis” at the American College of Sports Medicine's (ACSM's) 64th Annual Meeting and 8th World Congress on Exercise is Medicine in Denver, Colorado, USA, in 2017.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Poirier P., Giles T. D., Bray G. A., et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113(6):898–918. doi: 10.1161/circulationaha.106.171016. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. 2016, Obesity and overweight (Fact sheet ed., http://www.who.int/mediacentre/factsheets/fs311/en/
- 3.Mendis S., Davis S., Norrving B. Organizational update: the World Health Organization global status report on noncommunicable diseases 2014; one more landmark step in the combat against stroke and vascular disease. Stroke. 2015;46(5):e121–e122. doi: 10.1161/strokeaha.115.008097. [DOI] [PubMed] [Google Scholar]
- 4.Stapleton P. A., James M. E., Goodwill A. G., Frisbee J. C. Obesity and vascular dysfunction. Pathophysiology. 2008;15(2):79–89. doi: 10.1016/j.pathophys.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dimitrova R., Petkova V., Dimitrov M., Madzharov V., Nikolova I., Petkova E., et al. Obesity-relationship with vascular dysfunction. Advances in Obesity, Weight Management And Control. 2014;1:1–5. [Google Scholar]
- 6.Selthofer-Relatic K., Bosnjak I., Kibel A. Obesity related coronary microvascular dysfunction: from basic to clinical practice. Cardiology Research and Practice. 2016;2016:7. doi: 10.1155/2016/8173816.8173816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pyke K. E., Tschakovsky M. E. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. The Journal of Physiology. 2005;568(2):357–369. doi: 10.1113/jphysiol.2005.089755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruyndonckx L., Hoymans V. Y., Lemmens K., Ramet J., Vrints C. J. Childhood obesity-related endothelial dysfunction: An update on pathophysiological mechanisms and diagnostic advancements. Pediatric Research. 2016;79(6):831–837. doi: 10.1038/pr.2016.22. [DOI] [PubMed] [Google Scholar]
- 9.Hajjar D. P., Gotto A. M., Jr. Biological relevance of inflammation and oxidative stress in the pathogenesis of arterial diseases. The American Journal of Pathology. 2013;182(5):1474–1481. doi: 10.1016/j.ajpath.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mauricio M. D., Aldasoro M., Ortega J., Vila J. M. Endothelial dysfunction in morbid obesity. Current Pharmaceutical Design. 2013;19(32):5718–5729. doi: 10.2174/1381612811319320007. [DOI] [PubMed] [Google Scholar]
- 11.Phillips S. A., Mahmoud A. M., Brown M. D., Haus J. M. Exercise interventions and peripheral arterial function: implications for cardio-metabolic disease. Progress in Cardiovascular Diseases. 2015;57(5):521–534. doi: 10.1016/j.pcad.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Daiber A., Steven S., Weber A., et al. Targeting vascular (endothelial) dysfunction. British Journal of Pharmacology. 2016 doi: 10.1111/bph.13517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris R. A., Nishiyama S. K., Wray D. W., Richardson R. S. Ultrasound assessment of flow-mediated dilation. Hypertension. 2010;55(5):1075–1085. doi: 10.1161/hypertensionaha.110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inaba Y., Chen J. A., Bergmann S. R. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. International Journal of Cardiovascular Imaging. 2010;26(6):631–640. doi: 10.1007/s10554-010-9616-1. [DOI] [PubMed] [Google Scholar]
- 15.Walther C., Gielen S., Hambrecht R. The effect of exercise training on endothelial function in cardiovascular disease in humans. Exercise and Sport Sciences Reviews. 2004;32(4):129–134. doi: 10.1097/00003677-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Ashor A. W., Lara J., Siervo M., et al. Exercise modalities and endothelial function: a systematic review and dose—response meta-analysis of randomized controlled trials. Sports Medicine. 2014;45(2):279–296. doi: 10.1007/s40279-014-0272-9. [DOI] [PubMed] [Google Scholar]
- 17.Ribeiro F., Alves A. J., Duarte J. A., Oliveira J. Is exercise training an effective therapy targeting endothelial dysfunction and vascular wall inflammation? International Journal of Cardiology. 2010;141(3):214–221. doi: 10.1016/j.ijcard.2009.09.548. [DOI] [PubMed] [Google Scholar]
- 18.Miyachi M. Effects of resistance training on arterial stiffness: a meta-analysis. British Journal of Sports Medicine. 2013;47(6):393–396. doi: 10.1136/bjsports-2012-090488. [DOI] [PubMed] [Google Scholar]
- 19.Phillips S. A., Das E., Wang J., Pritchard K., Gutterman D. D. Resistance and aerobic exercise protects against acute endothelial impairment induced by a single exposure to hypertension during exertion. Journal of Applied Physiology. 2011;110(4):1013–1020. doi: 10.1152/japplphysiol.00438.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jurva J. W., Phillips S. A., Syed A. Q., et al. The effect of exertional hypertension evoked by weight lifting on vascular endothelial function. Journal of the American College of Cardiology. 2006;48(3):588–589. doi: 10.1016/j.jacc.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 21.American Collega of Sports Medicine. ACSM’S Guidelines for Exercise Testing and Prescription. Philadelphia, Pa, USA: Lippincot Williams and Wilkins; 2014. [DOI] [Google Scholar]
- 22.Moher D., Liberati A., Tetzlaff J., Altman D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. British Medical Journal. 2009;339, article b2535 doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ades P. A., Savage P. D., Lischke S., et al. The effect of weight loss and exercise training on flow-mediated dilatation in coronary heart disease: A randomized trial. Chest. 2011;140(6):1420–1427. doi: 10.1378/chest.10-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baynard T., Carhart R. L., Jr., Weinstock R. S., Ploutz-Snyder L. L., Kanaley J. A. Short-term exercise training improves aerobic capacity with no change in arterial function in obesity. European Journal of Applied Physiology. 2009;107(3):299–308. doi: 10.1007/s00421-009-1126-2. [DOI] [PubMed] [Google Scholar]
- 25.Bhutani S., Klempel M. C., Kroeger C. M., et al. Alternate day fasting with or without exercise: Effects on endothelial function and adipokines in obese humans. e-SPEN Journal. 2013;8(5):e205–e209. doi: 10.1016/j.clnme.2013.07.005. [DOI] [Google Scholar]
- 26.Blumenthal J. A., Babyak M. A., Hinderliter A. Effects of the DASH diet alone and in combination with exercise and weight loss on blood pressure and cardiovascular biomarkers in men and women with high blood pressure: the ENCORE study. Archives of Internal Medicine. 2010;170(2):126–135. doi: 10.1001/archinternmed.2009.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choo J., Lee J., Cho J.-H., Burke L. E., Sekikawa A., Jae S. Y. Effects of weight management by exercise modes on markers of subclinical atherosclerosis and cardiometabolic profile among women with abdominal obesity: A randomized controlled trial. BMC Cardiovascular Disorders. 2014;14, article no. 82 doi: 10.1186/1471-2261-14-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cotie L. M., Josse A. R., Phillips S. M., MacDonald M. J. Endothelial function increases after a 16-week diet and exercise intervention in overweight and obese young women. BioMed Research International. 2014;2014:10. doi: 10.1155/2014/327395.327395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davison K., Coates A. M., Buckley J. D., Howe P. R. C. Effect of cocoa flavanols and exercise on cardiometabolic risk factors in overweight and obese subjects. International Journal of Obesity. 2008;32(8):1289–1296. doi: 10.1038/ijo.2008.66. [DOI] [PubMed] [Google Scholar]
- 30.Fayh A. P. T., Lopes A. L., Da Silva A. M. V., Reischak-Oliveira Á., Friedman R. Effects of 5 % weight loss through diet or diet plus exercise on cardiovascular parameters of obese: A randomized clinical trial. European Journal of Nutrition. 2013;52(5):1443–1450. doi: 10.1007/s00394-012-0450-1. [DOI] [PubMed] [Google Scholar]
- 31.Franklin N. C., Robinson A. T., Bian J.-T., et al. Circuit resistance training attenuates acute exertion-induced reductions in arterial function but not inflammation in obese women. Metabolic Syndrome and Related Disorders. 2015;13(5):227–234. doi: 10.1089/met.2014.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamdy O., Ledbury S., Mullooly C., et al. Lifestyle modification improves endothelial function in obese subjects with the insulin resistance syndrome. Diabetes Care. 2003;26(7):2119–2125. doi: 10.2337/diacare.26.7.2119. [DOI] [PubMed] [Google Scholar]
- 33.Kwon H. R., Min K. W., Ahn H. J., et al. Effects of aerobic exercise vs. Resistance training on endothelial function in women with type 2 diabetes mellitus. Diabetes and Metabolism Journal. 2011;35(4):364–373. doi: 10.4093/dmj.2011.35.4.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olson T. P., Dengel D. R., Leon A. S., Schmitz K. H. Moderate resistance training and vascular health in overweight women. Medicine and Science in Sports and Exercise. 2006;38(9):1558–1564. doi: 10.1249/01.mss.0000227540.58916.0e. [DOI] [PubMed] [Google Scholar]
- 35.Pugh C. J. A., Sprung V. S., Kemp G. J., et al. Exercise training reverses endothelial dysfunction in nonalcoholic fatty liver disease. American Journal of Physiology - Heart and Circulatory Physiology. 2014;307(9):H1298–H1306. doi: 10.1152/ajpheart.00306.2014. [DOI] [PubMed] [Google Scholar]
- 36.Robinson A. T., Franklin N. C., Norkeviciute E., et al. Improved arterial flow-mediated dilation after exertion involves hydrogen peroxide in overweight and obese adults following aerobic exercise training. Journal of Hypertension. 2016;34(7):1309–1316. doi: 10.1097/HJH.0000000000000946. [DOI] [PubMed] [Google Scholar]
- 37.Swift D. L., Earnest C. P., Blair S. N., Church T. S. The effect of different doses of aerobic exercise training on endothelial function in postmenopausal women with elevated blood pressure: Results from the DREW study. British Journal of Sports Medicine. 2012;46(10):753–758. doi: 10.1136/bjsports-2011-090025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vinet A., Karpoff L., Walther G., et al. Vascular reactivity at rest and during exercise in middle-aged obese men: effects of short-term, low-intensity, exercise training. International Journal of Obesity. 2011;35(6):820–828. doi: 10.1038/ijo.2010.206. [DOI] [PubMed] [Google Scholar]
- 39.Wycherley T. P., Brinkworth G. D., Noakes M., Buckley J. D., Clifton P. M. Effect of caloric restriction with and without exercise training on oxidative stress and endothelial function in obese subjects with type 2 diabetes. Diabetes, Obesity and Metabolism. 2008;10(11):1062–1073. doi: 10.1111/j.1463-1326.2008.00863.x. [DOI] [PubMed] [Google Scholar]
- 40.Maher C. G., Sherrington C., Herbert R. D., Moseley A. M., Elkins M. Reliability of the pedro scale for rating quality of randomized controlled trials. Physical Therapy. 2003;83(8):713–721. doi: 10.1093/ptj/83.8.713. [DOI] [PubMed] [Google Scholar]
- 41.de Morton N. A. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Australian Journal of Physiotherapy. 2009;55(2):129–133. doi: 10.1016/S0004-9514(09)70043-1. [DOI] [PubMed] [Google Scholar]
- 42.Physiotherapy evidence database. PEDro statistics, http://www.pedro.org.au/english/downloads/pedro-statistics/
- 43.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd. Hillsdale, NJ, USA: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 44.Bender S. B., Laughlin M. H. Modulation of endothelial cell phenotype by physical activity: Impact on obesity-related endothelial dysfunction. American Journal of Physiology - Heart and Circulatory Physiology. 2015;309(1):H1–H8. doi: 10.1152/ajpheart.00177.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joris P. J., Zeegers M. P., Mensink R. P. Weight loss improves fasting flow-mediated vasodilation in adults: A meta-analysis of intervention studies. Atherosclerosis. 2015;239(1):21–30. doi: 10.1016/j.atherosclerosis.2014.12.056. [DOI] [PubMed] [Google Scholar]
- 46.Brook R. D. Obesity, weight loss, and vascular function. Endocrine. 2006;29(1):21–25. doi: 10.1385/ENDO:29:1:21. [DOI] [PubMed] [Google Scholar]
- 47.Flores L., Nunez I., Vidal J., et al. Endothelial function in hypertensive obese patients: 1 Year after surgically induced weight loss. Obesity Surgery. 2014;24(9):1581–1584. doi: 10.1007/s11695-014-1328-5. [DOI] [PubMed] [Google Scholar]
- 48.Joris P. J., Plat J., Kusters Y. H. A. M., et al. Diet-induced weight loss improves not only cardiometabolic risk markers but also markers of vascular function: A randomized controlled trial in abdominally obese men. American Journal of Clinical Nutrition. 2017;105(1):23–31. doi: 10.3945/ajcn.116.143552. [DOI] [PubMed] [Google Scholar]
- 49.Park Y., Booth F. W., Lee S., Laye M. J., Zhang C. H. Physical activity opposes coronary vascular dysfunction induced during high fat feeding in mice. The Journal of Physiology. 2012;590(17):4255–4268. doi: 10.1113/jphysiol.2012.234856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thijssen D. H. J., Maiorana A. J., O'Driscoll G., Cable N. T., Hopman M. T. E., Green D. J. Impact of inactivity and exercise on the vasculature in humans. European Journal of Applied Physiology. 2010;108(5):845–875. doi: 10.1007/s00421-009-1260-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tinken T. M., Thijssen D. H. J., Hopkins N., Dawson E. A., Cable N. T., Green D. J. Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension. 2010;55(2):312–318. doi: 10.1161/hypertensionaha.109.146282. [DOI] [PubMed] [Google Scholar]
- 52.Thijssen D. H. J., Black M. A., Pyke K. E., et al. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. American Journal of Physiology—Heart and Circulatory Physiology. 2011;300(1):H2–H12. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harris R. A. FMD, reproducibility, and acute exercise in the obese: Are the results confounded? European Journal of Applied Physiology. 2010;109(2):357–358. doi: 10.1007/s00421-009-1343-8. [DOI] [PubMed] [Google Scholar]