Abstract
Glutathione-S-Transferases (GSTs) have primarily been thought to be xenobiotic metabolizing enzymes that protect cells from toxic drugs and environmental electrophiles. However, in last three decades, these enzymes have emerged as the regulators of oxidative stress–induced signaling and toxicity. 4-Hydroxy-trans 2-nonenal (HNE) an end-product of lipid peroxidation, has been shown to be a major determinant of oxidative stress–induced signaling and toxicity. HNE is involved in signaling pathways, including apoptosis, proliferation, modulation of gene expression, activation of transcription factors/repressors, cell cycle arrest, and differentiation. In this article, available evidence for a major role of GSTs in the regulation of HNE-mediated cell signaling processes through modulation of the intracellular levels of HNE is discussed.
Keywords: 4-Hydroxynonenal, Glutathione-S-Transferases, Cell signaling, Oxidative stress, Cell cycle
Online Graphical Abstract
Introduction
Since the seminal discovery of 4-hydroxy-trans 2-nonenal (HNE) as an end product of lipid peroxidation by Esterbauer’s group [1,2], HNE has attracted a great deal of attention because of its potential toxicity as well as its physiological roles, particularly in cell cycle signaling [1,3–8]. HNE is the major end-product from the peroxidation of n-6-polyunstaurated fatty acids and is sufficiently stable. Due to its carbonyl group at position 1 and the reactive 2,3-unsaturated double bond that is rendered more reactive by the presence of a hydroxyl group at the position 4, HNE is believed to impart its toxicity through interactions with nucleophilic groups of cellular components including proteins, nucleic acids and phospholipids [9,10]. Initially thought to be merely a “toxic end-product,” in recent years HNE has acquired reputation as a major “second messenger” that affects cell cycle signaling in a concentration-dependent manner and has been one of the most extensively studied molecule during the past three decades with more than four thousand published studies on its multifarious effects on a cellular processes.
Glutathione S-transferases (GSTs) use HNE as a substrate [11] and it has been shown that these enzymes function as the major determinants of cellular levels of HNE by attenuating its formation during lipid peroxidation and also through its metabolism via conjugation to glutathione (GSH) [11–14]. Recent studies by us and others provide credible evidence that by modulating the intracellular levels of HNE, GSTs play a major role in the regulation of oxidative stress–induced toxicity and signaling [1, 3–8]. In this article, the role of HNE in oxidative stress-induced signaling and its regulation by GSTs is reviewed against the back drop of general mechanism of stress signaling.
HNE and Signaling
Whereas many of the initial studies on the role of HNE in signaling, e.g. stimulation of adenylate cyclase [15], phospholipase C [16,17], effects on chemo-taxis [18], and DNA synthesis [19] did not attract much attention, in recent years major roles of HNE in the cell cycle signaling including the induction of apoptosis as well as the activation of many cellular protective mechanisms in response to stress have been widely recognized and it is now considered as one of the major signaling molecules. The role of HNE in the activation of a multitude of genes, induction of apoptosis, as well as the activation of proliferative and protective mechanisms in response to reactive oxygen species (ROS), radiation, and chemical stress has been extensively covered in several reviews [1, 3–8, 20]. Some of the notable biological effects of HNE in various model systems studied by us and others are summarized in the Table-1.
Table-1.
Significant biological pathways altered by cellular HNE concentration.
| Biological effects | Model system | References |
|---|---|---|
| Differentiation and transformation | K562, HL60, HLE-B3, CCL-75, H-69, and H-226 cells. | [29–32] |
| Apoptosis | RKO, RPE, ARPE-19, K562, HL60, HepG2, H-69, H-226, HL-60, PC12, HLE-B3, and mouse embryonic fibroblast cells. | [29,30, 34, 36, 99, 106, 107, 108] |
| Pro-and anti-apoptotic signaling | HepG2, RKO, RPE, ARPE-19, HL60, K562, and SK-N-BE cells. | [25, 35, 36, 106, 109,110] |
| Cell cycle arrest | PC3, HepG2, Hep3B, and SK-N-BE cells. | [27, 37, 109] |
| Activation of p53 and p21 | RPE, ARPE-19, HepG2, Hep3B, and SK-N-BE cells. | [36,106, 109] |
| Interaction with NF-kB | RKO, H1299, VSMC, A549, HeLa, U937, macrophages, astrocytes cardiomyocytes, and bovine aortic endothelial cells. | [24, 25, 77, 93, 107, 111, 112, 113, 114] |
| Activation of HSF1 and HSPs | HepG2, RKO, HL60, K562, and HLE B-3 cells | [35, 36, 91, 110] |
| Activation of Nrf2 | PC12, HL60, K562, astrocytes, and rat epithelial type II cells. | [91, 113, 115, 116,] |
| Activation of MAPK | HBE1, K562, PC12, NT2, RPE, macrophages, and nerve cells. | [29, 97, 108, 112, 117, 118] |
| Angiogenesis | RPE cells. | [97] |
Concentration-dependent effects of HNE
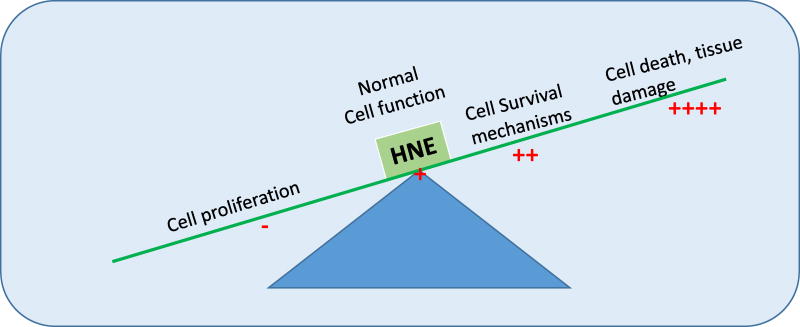
Hormetic effects of HNE on signaling (i.e. stimulatory at low cellular concentrations while inhibitory at higher concentrations) are particularly intriguing (Fig.1). At low cellular concentrations, HNE has been shown to induce cell proliferation, activation of pro-proliferative kinases, DNA and protein synthesis [21–23] while at increased concentrations, it causes inhibition of pro-proliferative mechanisms as well as of DNA and protein synthesis leading to eventual apoptotic or necrotic death [24–27]. Numerous studies suggest that HNE is a common denominator in stress signaling and there is credible evidence that HNE regulates its own cellular levels and its signaling effects through the induction of enzymes involved in its metabolism and the transport of its metabolites [28–37]. Evidence also suggests that formation of HNE is inevitable in response to even a minute stress (ROS, heat, radiation, chemical) and at the initial low levels during its generation, it seems to act as a sensor of stress signal that induces protective mechanisms [29, 30,36,37]. Primarily, these protective mechanisms include cell cycle arrest, activation of various transcription factors e.g. Nrf2, HSF, and transcription repressors e.g. DAXX to affect gene expression, activation of growth factors (e.g. EGFR, VEGFR), along with responsive pro-proliferative kinases, and induction of glutathione (GSH) synthesis, HNE specific glutathione S-transferases (GSTs) such as GSTA4-4 and RLIP76 that transports the conjugate of GSH and HNE [6,7,29–37]. Through these defense mechanisms HNE-mediated signaling seems to be geared towards providing cell protection against stress up to a certain threshold beyond which at higher levels of HNE due to sustained stress, apoptotic mechanisms are activated to protect the neighboring cells.
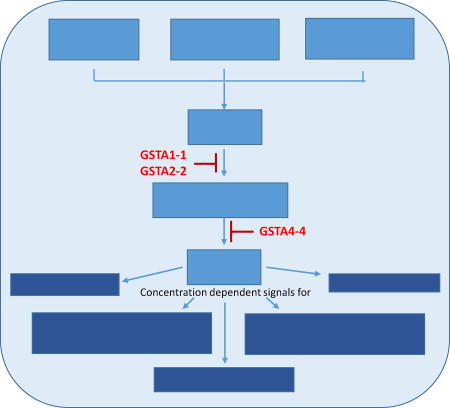
Figure-1. GSTs regulate HNE-mediated cellular homeostasis.
Under stress conditions HNE concentrations play critical role in directing cells to undergo proliferation, survival, differentiation and apoptosis. GSTs play a major role in maintaining the cellular homeostasis by adjusting the cellular concentrations of HNE.
Physiological significance of maintaining HNE homeostasis
Earlier studies have shown that low levels of exogenously added HNE in the medium promote proliferation of certain cell types while at relatively higher concentrations in the medium, HNE mediates signaling for various cellular processes, including apoptosis [22–39]. It is noteworthy that such hormetic effect of H2O2 has also been observed in studies where low levels of H2O2 promote DNA and protein synthesis while at higher concentrations it causes cell death [40–45]. Since exposure of cells to H2O2 inevitability leads to HNE formation, the hormetic effects of HNE should not be surprising. The stimulatory role of HNE at low concentrations has also been demonstrated in the cells having sub-basal levels of HNE. It has been shown that human erythroleukemia cells K562 that grow in suspension, proliferate at a much higher rate when the intracellular concentration of HNE in these cells is lowered (<one third of the basal level) through stable transfection with GSTA4-4, an isozyme with high activity for conjugating HNE to GSH [46]. As opposed to the wild-type, the GSTA4-4 transfected cells were resistant to HNE or oxidative stress-induced apoptosis and erythroid differentiation indicating a concentration dependent effect of HNE on signaling and its regulation by GSTA4-4 [46]. Studies with HLE-B3, cells that grow as attached cells and have a limited life span, further confirmed the pro-proliferative role of HNE at sub-physiological levels. Sustained low HNE levels in these cells upon stable transfection with GATA4-4 caused their transformation to suspended type cells that indefinitely grew at a much higher rate [47]. These and many other studies (5, 6, 32) underline the importance of maintaining HNE homeostasis in cells and highlight the role of GSTs in this process. Besides GSTs, other HNE metabolizing enzymes are also involved in the regulation of HNE homeostasis and signaling [48]. This article, however, primarily focuses on GSTs in the regulation of HNE- mediated signaling and the role of other enzymes may perhaps be found elsewhere in this volume.
GSTs regulate HNE homeostasis through its metabolism and by attenuating its formation
GSTs and HNE metabolism
Generation of HNE from lipid peroxidation of n-6-polyunsaturated fatty acids is an uncontrollable process depending on the ever-changing levels of endogenous ROS as well as the environmental factors such as exposure to oxidants, radiation, and drugs/xenobiotics that cause oxidative stress. Therefore, the major burden of maintaining cellular HNE homeostasis must be on enzymes that metabolize HNE and eliminate the resultant metabolites from the cells. HNE being sufficiently electrophilic can non-enzymatically interact with the major cellular thiol, GSH. However, the rate of conjugation of HNE to GSH is accelerated by more than two orders of magnitude in the presence of GSTs [11]. GSTs are a multi-gene family of proteins and at least seven of these families have been identified in mammalian tissues [49, 50]. While GST isozymes belonging to these families show a wide substrate spectrum with low catalytic efficiencies, some of the isozymes show selective preference for specific substrates with higher catalytic efficiencies [49]. Several GST isozymes with selective preference for HNE have been identified in mammalian tissues. These enzymes hGSTA4-4 (humans), mGST4-4 (mice), and rGST4-4 (rats) have been cloned and their high catalytic efficiency towards HNE has been established [51–56]. An enzyme hGST5.8 has been identified in all human tissues investigated so far [29, 56]. Even though similar to hGSTA4-4, the catalytic efficiency of hGST5.8 towards HNE is high its identity with hGSTA4-4 is not firmly established. A HNE metabolizing GST isozyme (DMGSTD1-1) has also been characterized in Drosophila Madagascars [57]. This enzyme also has high catalytic efficiency towards HNE that underscores the significance of GST mediated metabolism of HNE across the species.
In most human tissues, the bulk of GST protein is accounted for by the Alpha, Mu, and Pi class of GSTs [58–60]. In human, mouse, and rat liver the Alpha class of GSTs constitute up to 90% of total soluble protein while in some extrahepatic tissues (e.g. lung) GSTPi (P1-1) accounts for up to 95% of GST activity towards 1-chloro-2–4-dinitrobenzene which is the common substrate to measure GST activity [58–60]. The relative abundance of GSTA4-4 in tissues is only 1–2% of total GST proteins [55,56]. Thus, even though the activities of the Pi, Mu and other Alpha class of GSTs (except GSTA4-4) toward HNE are only about 1% of that displayed by GSTA4-4 [55,56], due to their high relative abundance the Mu, Pi, and other Alpha class GSTs could also contribute to the conjugation of HNE to GSH. Further quantitative studies are needed to ascertain the contribution of these enzymes in HNE metabolism.
A major fraction of cellular HNE is metabolized through its conjugation to GSH catalyzed by GSTs. The conjugate (GS-HNE) thus formed is exported from the cells through ATP-dependent transport catalyzed by RLIP76, because excessive accumulation of GS-HNE would be inhibitory to GSTs [61–69]. This mechanism is responsible for elimination of the major portion of cellular HNE as indicated by ex vivo studies with cells and also in vivo studies with GSTA4-4 and RLIP76 null mice [29,30,34,35,64,67]. This is further confirmed by studies with stressed cells that have increased HNE levels and a 3–8 fold induction of HNE-specific GST isozymes and the transporter RLIP76 [29–31]. These cells extrude GS-HNE at several fold higher rate as compared with the control cells and increased HNE levels in these stressed cells are brought down to lower than even the basal HNE levels within 30 min of resting, indicating a major role of GSTA4-4 and RLIP76 system for the elimination of HNE from the stressed cells [29–31]. HNE is also metabolized by enzymes including aldose reductase [48,70], aldehyde dehydrogenase [48], cytochrome P450 [71,72] and also in the β-oxidation pathway [73]. These enzymes also contribute to the regulation of HNE homeostasis and the role of some of these enzymes in the regulation of HNE-mediated signaling and pathogenesis has been highlighted [74–76]. In particular, the pivotal role of aldose reductase in the modulation of redox signaling via glutathionyl-1,4 dihydroxynonene (GS-DHN), which is formed by an aldose reductase-catalyzed reduction of GS-HNE has been extensively studied [77–80].
GSTs attenuate HNE formation
GSTs are multifunctional enzymes and are known to attenuate lipid peroxidation. The alpha class GSTs particularly GSTA1-1 and GSTA2-2 that constitute the bulk of total alpha class of GST protein in liver express selenium-independent glutathione peroxidase activity and can catalyze GSH-dependent reduction of lipid hydroperoxides generated during oxidative stress [12,81–84]. Since lipid hydroperoxides are the precursors of HNE, GSTs significantly attenuate HNE formation as indicated by studies showing that GSTA1-1 over expressing cells are protected against ROS-induced lipid peroxidation and apoptosis [30,32,84]. Thus, in addition to their conjugating activity, the alpha class GSTs also regulate HNE concentration, particularly in the liver. It has been shown that in liver extracts, the alpha class GSTs contribute up to 60% of the total glutathione peroxidase activity for the reduction of lipid hydroperoxides [83,84]. GSTs are primarily cytosolic enzymes and the mechanisms of GST-mediated reduction of lipid hydroperoxides located in the membranes is not completely clear. However, membrane association of GSTA4-4 has been demonstrated [85] and our unpublished studies show that in stressed cells, GSTA1-1 and GST2-2 are localized in membranes. In an isolated system, GSTA1-1 and A2-2 have been shown to catalyze GSH-dependent reduction of lipid hydroperoxides present in erythrocyte ghosts [30]. Together, these studies indicate a major role of GSTs in limiting lipid peroxidation through the reduction of lipid hydroperoxides. The role of GSTs in the regulation of metabolism of HNE and other lipid aldehydes during oxidative stress is outlined in Fig.2. Cells expressing high levels of GSTA1-1 and A2-2 showing the inhibition of various signaling effects of oxidative stress further indicate the role of GSTs in the regulation of HNE levels and stress signaling [30,31,86,87]. In addition to their catalytic activities, GSTs also act as scavengers of ligands through their ligandin type activity [88]. Even though this role of GSTs has not been investigated, it is likely that GSTs also regulate HNE levels through their ligandin type activity.
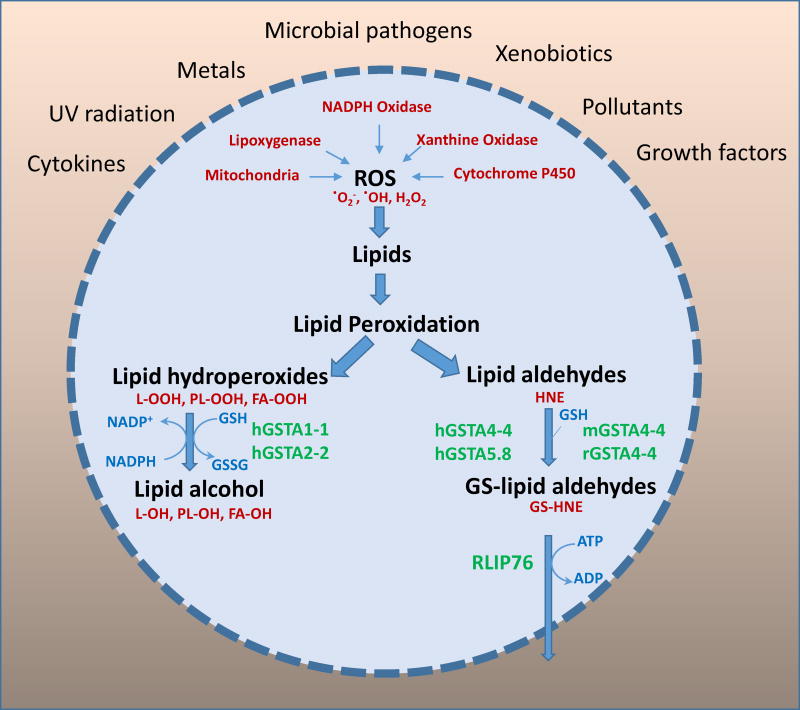
Figure-2. Significance of GSTs in the regulation of ROS-initiated lipid peroxidation - derived products under stress conditions.
Exposure of cells to external stresses such as metals, UV radiation, pollutants, pathogens, cytokines and growth factors increases cellular oxidative stress by generating reactive oxygen species via activation of endogenous factors such as NAPDH oxidase, xanthine oxidase, cytochrome P450 and mitochondrial respiration. The increased ROS causes oxidation of lipids leading to formation of lipid peroxidation products such as lipid hydroperoxides and subsequently lipid aldehydes. Human alpha class of GSTs such as hGSTA1-1 and hGSTA2-2 lipid hydroperoxides via their peroxidase activity. Human alpha class GSTs such as hGSTA4-4 and hGST5.8, and mouse and rat alpha class GSTA4-4 conjugate with cellular GSH leading to formation of glutathione-lipid aldehyde adducts and regulate the intracellular levels of toxic lipid aldehydes. Glutathione-aldehyde conjugates are primarily transported out of the cells by ATP dependent transporter, RLIP76.
Regulation of HNE-mediated signaling by GSTs
Voluminous literature is available demonstrating involvement of HNE is signaling process and many of these studies have been covered in several excellent reviews [3–8]. Majority of these studies focus on the effect of HNE on a specific target using a single cell line. Studies on the effect of HNE depletion using several human cell lines of different tissue origin as well as a regulatory role of GSTs in the various processes are worth noting here because these studies pinpoint the role of HNE in signaling in general [29,30,34–36,46,47]. The signaling role of HNE in vivo is also indicated by studies with hGSTA4-4 knockout mice where profound alterations in gene expression and transmembrane movement of transcription factors and transcriptional repressors has been demonstrated subsequent to the elevated HNE levels in these mice [8,34–36]. Several human cell lines of different tissue origin (e.g. K562, HLE-B3, H69, H226, HepG2) when transfected with GSTA4-4 grow at a faster rate because of HNE depletion [29,46,47]. As pointed out above, HLE-B3 cells stably transfected with hGSTA4-4 transform to suspended type cells and acquire unlimited life span [47]. There is a profound change to the expression of many genes in these HNE depleted transformed cells [47,89]. Likewise, human lung fibroblast cells CCL-75 are also transformed by forced expression of GSTA4-4 [47]. HNE depletion could specifically be pinpointed as the cause of this transformation because HLE-B3 cells stably transfected with hGSTP1-1 (that has negligible activity for HNE) neither underwent transformation nor showed altered gene expression [47]. Furthermore, cells transfected with a single point mutant of hGSTA4-4 (Y212F) that has <5% activity for HNE as compared to the wild-type enzyme did not undergo transformation. On the other hand, cells microinjected with the wild-type GSTA4-4 [56] did indeed undergo transformation attributing the observed transformation and altered gene expression, specifically to HNE depletion.
Massive changes in the gene expression of hGSTA4-4 transfected HLE-B3 cells were indicated by microarray studies [89] showing that more than 6000 genes in GSTA4-4 transfected cells were either up- or down-regulated by more than two-fold as compared to the empty vector transfected cells. These studies revealed that consistent with the phenotype of the transfected cells (rapidly growing cells in suspension with an unlimited life span as opposed to HLE-B3 cells that grow as attached cells with a limited life span) pro-proliferative genes were highly up-regulated while genes regulating cell cycle and cell adhesion were severely down regulated. Prominent among the pro-proliferative genes were TGF-β1, CDK2, protein kinase βII, ERK1/2 that were dramatically up-regulated [47,89]. On the other hand, the expression of regulatory genes such as P53 and Fas was severely down-regulated [47,89]. The question whether these profound changes to the gene expression were the consequences of phenotypic transformation and immortalization of cells or alternatively the changes to the gene expression led to the transformation has also been addressed. These studies showed that at least the suppression of regulatory genes P53 and Fas was preceded to transformation and suggested a major role of GSTs in regulation of HNE-mediated signaling for gene expression. Studies with cells transiently transfected with hGSTA4-4 revealed that after 48 h of transfection, when HNE was severely depleted, the expression of Fas and P53 was dramatically down-regulated as observed in the transformed cells [34–36]. However, at 240 h post-transient transfection when hGSTA4-4 expression was lost and the basal HNE level was restored, the expression of these genes returned to normal levels. No transformation was observed in these transiently transfected cells suggesting the alteration in gene expression in the transformed cells preceded the transformation and it was the cause, rather than the consequence of transformation. Possible consequences on cellular function could be envisaged from the known functions of genes that have dramatic changes in their expression subsequent to the lowering of cellular HNE levels below the ‘normal’ physiological levels. Table-2 lists some of the genes that show massive changes in their expression upon GSTA4-4 over-expression. Further detailed studies exploring the possible consequences on modulation of cellular functions subsequent to alterations in HNE homeostasis could vastly improve our understanding on the role of oxidative stress in human health and disease. Together, these studies point out a major role of GSTs in the regulation of gene expression through modulation of HNE signals that affect multifarious cellular processes.
Table-2.
Alterations in gene expression upon HNE depletion as relevant to cellular function.
| Gene | Known/suggested function | Fold change in expression |
|---|---|---|
| P53 | Tumor suppressor, apoptosis | ↓ more than 50 |
| Fas | Regulation of programmed cell death and immune system. | ↓ more than 30 |
| TGF-β1 | Cell proliferation and differentiation. | ↑3 |
| CDK2 | G1/S transition of cell cycle | ↑4 |
| PKC-β2 | Cell survival and apoptosis | ↑4 |
| Fibronectin-1 | Structural integrity | ↓ more than 60 |
| p21 | Growth arrest in response to stress, | ↓ 8 |
| c-Jun | Apoptosis and chemotaxis | ↓ 5 |
| c-Myc | Cell cycle progression and transformation | ↑ 4 |
| Connexin 43 | Gap junctions | ↓ more than 150 |
| Laminin γ1 | Wound healing and regeneration | ↓ more than 500 |
| Integrin –α6. | Cell adhesion | ↓ more than 70 |
Stress-induced toxicity and GSTs
In vitro Studies
During the past two decades, the role of HNE and GSTs in the mechanisms of stress-induced signaling and toxicity has been extensively studied by us [6,28–37,90,91] and by others [3,8,27,92,93]. Our studies reveal that over-expression of GSTA1-1 and GSTA2-2 in cells provide protection from cell death and apoptosis caused by radiation, H2O2, super oxide anions, chemicals such as doxorubicin and naphthalene [28–30, 87]. These cells are protected against apoptosis caused by lipid hydroperoxides but not against HNE [30,31,87]. Cells overexpressing hGSTA4-4 are however protected against apoptosis by above stressors as well as HNE indicating that HNE is a common denominator in stress-induced apoptosis [31,88]. Protective role of GSTs against stress (UV, ROS, naphthalene, doxorubicin) is also demonstrated in studies showing that exposure of cells to a mild transient sub-toxic stress results in a rapid induction of HNE metabolizing GST isozyme along with GS-HNE transporter RLIP76 [29]. Under these mild stress conditions, the canonical antioxidant enzymes and heat shock proteins are not induced. The mildly stressed cells having induced GSTs and RLIP76 are resistant to H2O2, UV, doxorubicin and naphthalene –induced apoptosis and are capable of transporting out GS-HNE at several fold higher rate indicating that accelerated disposal of HNE from the cells accounts for their resistance to stress toxicity [29,87]. Similar results have been obtained with several human cell lines of different tissue origins (HL-60, H-69, H-226, HLE-B3, RPE and K-562) indicating a generality of this phenomenon [29,87]. Transport of GS-HNE is crucial for conferring resistance to stress-induced apoptosis in these cells because the blockage of GS-HNE transport by coating the cells with anti-RLIP76 IgG leads to apoptosis of these cells, even in the absence of any stress [29]. These findings have major implications in cancer chemotherapy and protection against radiation poisoning [65,94–96].
It has been shown that in P53 wild-type HepG2, RPE and ARPE-19 cells, HNE induces apoptosis by activating Bax, p21, JNK and caspase-3 in the intrinsic P53-mediated pathway. HNE is also known to induce Fas-mediated apoptosis that is independent of the canonical DISCdependent pathway [6,34–36]. These studies show that HNE causes activation of Fas as well as of DAXX, a nuclear transcription suppressor. HNE causes the export of DAXX from nucleus to cytoplasm where it inhibits apoptosis as a regulatory process. In parallel, HNE promotes translocation of HSF1 and NRf2 from cytoplasm to nucleus thereby up-regulating the expression of heat shock proteins and other defense mechanisms and these effects of HNE are inhibited by GSTA4-4 over expression [Fig.3; 36,37]. HNE seems to affect the trans-nuclear movement of transcription factors as well as transcription repressors that is consistent with the observed effects of HNE on the expression of a vast multitude of genes [2–8,37,47,89]. In addition, HNE also affects signaling mediated by growth factors and tyrosine kinase receptors in a concentration– dependent manner [6,97]. The modulation of these effects of HNE by GSTA4-4 clearly indicates the specific role of HNE in these processes and above all, suggests an important role of GSTs in the regulation of oxidative stress-induced signaling because HNE is an inevitable consequence of oxidative stress and GSTs can inhibit its formation and accelerate its disposition [5,6,30,87].
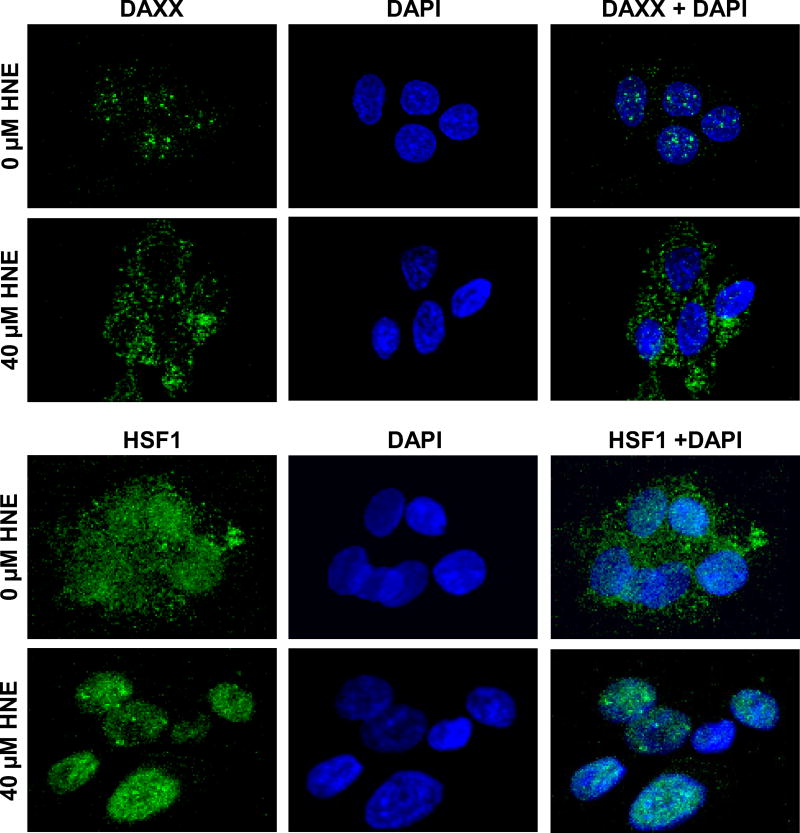
Figure-3. HNE-induced cytoplasmic and nuclear translocation of DAXX and HSF1, respectively in HepG2 cells.
Immunofluorescence staining of DAXX and HSF1 in HepG2 cells showing cytoplasmic and nuclear translocation, respectively in HNE-treated HepG2 cells. The cells were grown on glass coverslips, and untreated and treated (40 µM HNE for 2 h) cells were fixed, permeabilized, and incubated with anti-DAXX or anti-HSF1 antibody, followed by fluorescein (FITC)-conjugated secondary antibody (green). DNA was counterstained with 4’,6-diamidino-2-phenylindole (DAPI; blue). Slides were analyzed using a Zeiss LSM 510META laser-scanning fluorescence microscope. Data shows dramatic translocation of DAXX from nucleus to cytoplasm and HSF-1 from cytoplasm to nucleus upon HNE treatment. Similar data was reported in our earlier publication [36].
In vivo models
The physiological significance of HNE-mediated signaling and its regulation by GSTs is also suggested in vivo studies where the effects of HNE similar to those observed in vitro have been observed in the tissues of mGSTA4-4 null mice that have elevated HNE levels [98,99]. For example, similar to the induction of Fas, JNK, P53 and DAXX by exogenously added HNE to the cells in vitro, increased HNE levels in the GSTA4-4 null mice lead to the activation of P53, Fas, DAXX, JNK, HSF and Nrf2 in various tissues of these mice. Furthermore, P53, Nrf2 and HSF1 are accumulated in the nucleus of the tissues in these mice while DAXX is transported out of the nucleus indicating that the trans-nuclear movement of these factors is activated in vivo by HNE [6,28,34,35]. As expected, GSTA4-4 null mice are more sensitive to oxidative stress –induced toxicity due to compromised HNE metabolism [99,100]. Surprisingly, these mice are longer lived as compared to the wild-type mice. It has been suggested that longevity of these mice is due to induction of Nrf2 and HSF1 associated defense systems due to the sustained higher but apparently non-toxic HNE levels [100]. A few examples showing that GSTs play a major role in the modulation of HNE-induced signaling and toxicities are listed in Table-3.
Table-3.
Modulation of oxidative stress/HNE –induced signaling and toxicity by GSTs.
| Role of GSTs | Reference |
|---|---|
| GSTA4-4 inhibits doxorubicin/HNE-induced erythroid differentiation and apoptosis in K562 erythroleukemia cells. | [46] |
| Transfection with GSTA4-4 causes proliferation of K562 cells. | [46] |
| Stable transfection with GSTA4-4 leads to transformation and profound gene expression changes in HLE-B3 and CCL-75 cells. | [47] |
| Over-expression of GSTA1-1/2-2 inhibits H2O2, O2, radiation and naphthalene induced apoptosis in HLE-B3 cells. | [30,33,103] |
| HL-60, H-69, H-226, RPE, HLE-B3 and K-562 cells overexpressing GSTA-4-4 are resistant to H2O2, O2−, doxorubicin and radiation –induced apoptosis and toxicity. | [29,33] |
| HNE and oxidative stress –induced apoptosis in HepG2 cells inhibited by GST overexpression. | [36] |
| HNE-induced trans-nuclear movement and activation of HSF1, DAXX and P53 were inhibited by GSTA4-4 overexpression. | [28,36] |
| Fas- and P53-mediated apoptosis was inhibited by GSTA4-4 over expression. | [28,35] |
| GSTA4-4 null mice show activation of p53, Fas, JNK in their tissues. | [34–35] |
| HNE and DNA damage-induced cell cycle arrest and associated signaling in HepG2 cells were inhibited by GSTA-4-4 over expression. | [37] |
| Expression of VEGF in RPE cells regulated by GSTA4-4. | [98] |
| GSTA4-4 modifies susceptibility of skin tumor development. | [104] |
| GSTA4-4 modulates iNOS expression. | [105] |
| GSTA1-1 inhibits p53-mediated apoptosis in RPE cells. | [106] |
Role of HNE and GSTs in the regulation of cell cycle arrest and DNA repair
HNE also causes cell cycle arrest through mechanisms similar to those known for DNA damage-induced G1/G0 or G2/M cell cycle arrest and seems to be involved in the mechanisms of cell cycle arrest during DNA-damage [37,101,102]. As discussed above, HNE can simultaneously activate both pro- and anti-apoptotic singling pathways and that these are regulated by GSTA4-4 [8]. Our recent studies show that HNE induces G2/M phase cell cycle arrest during which ataxia telangiectasia mutated and Rad3 related protein (ATR)/checkpoint kinase (Chk1) signaling pathways are activated and this HNE-induced cell cycle arrest can be inhibited by the over-expression of GSTA4-4 [37]. It is generally believed that in response to DNA damage, initially the checkpoint kinases are activated leading to cell cycle arrest but when DNA damage is severe, cells undergo apoptosis [102–103]. In P53 null and wild-type HepG2 cells HNE has similar effects indicating that HNE-induced cell cycle arrest is independent of P53 [37]. Similar to DNA damage, HNE also causes G2/M cell cycle arrest via activation of CDK1-Cyclin D1 complex through the activation of ATR, Chk1 and Cdc25C pathway and the activation of p21 that is independent of P53 [37]. During HNE- induced G2/M cell cycle arrest, decreased expression of CDK1 and Cyclin D1 is observed and p21 expression is increased in the wild-type as well as in P53 null cells [37]. HNE also induces the phosphorylation of Cdc25C at serine-216 leading to its translocation from nucleus to cytoplasm where it is degraded via the ubiquitin-mediated proteasomal pathway [37]. The phosphorylation of Cdc25C is in turn regulated by ATR/Chk1 pathway. HNE also causes DNA double strand break as indicated by comet tail appearance and the phosphorylation of H2A.x histone in HNE treated cells [37]. Physiological significance of these findings is indicated by studies showing that HNE-induced DNA damage occurs in vivo because in mGSTA4-4 null mice, where HNE levels are increased, phosphorylation of H2A.x is observed in tissues [37]. These studies strongly suggest over all similarities in the mechanism of DNA damage- and HNE-induced cell cycle arrest. Since, each of the effects of HNE leading to cell cycle arrest and DNA damage are inhibited by over expression of GSTA4-4, a major role of GSTs in maintaining genomic integrity is suggested (Figure-4).
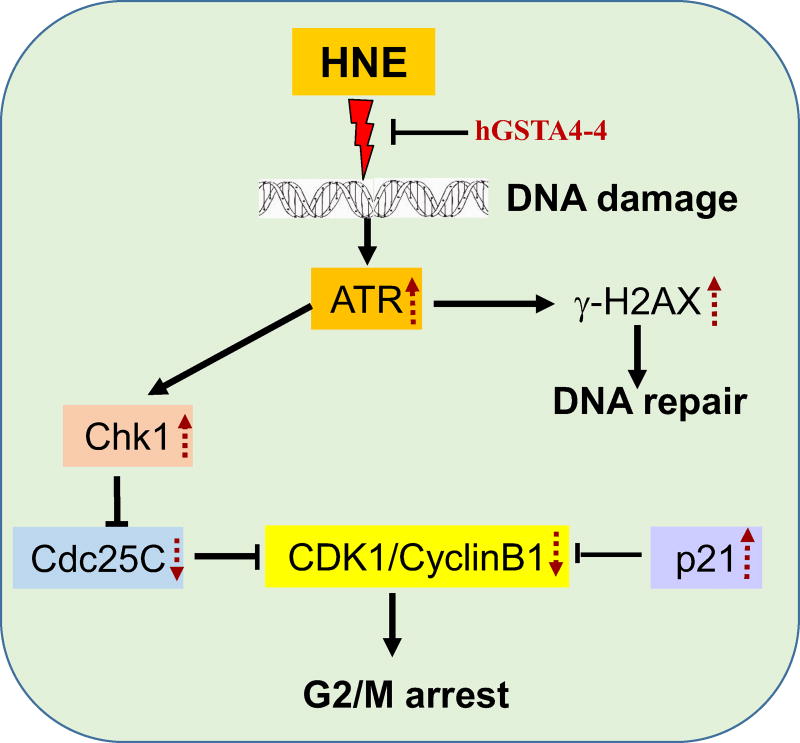
Figure -4. GSTA4-4 alters HNE –induced DNA damage and cell cycle arrest.
HNE-induced DNA damage signaling is mediated by sensor protein ATR/Chk1. Upon DNA damage, ATR activates the phosphorylation of checkpoint kinase, Chk1, which phosphorylates/inactivates Cdc25C. Chk1-mediated inactivation of Cdc25C and p53-independent activation of p21 both partially play a major role in deactivation of CDK1 kinase activity, leading to G2/M phase arrest by HNE. Simultaneously, HNE-induced phosphorylation of H2A.X at Ser-139 initiates the signal for the recruitment of DNA repair machinery. GSTA4-4 overexpression prevents HNE-induced DNA damage and cell cycle signaling events.
From the similarities in HNE and DNA damage-induced cell cycle arrest it is difficult to ascertain whether both of these pathways operate in parallel or one is preceded by the other. It is to be considered that oxidative stress causes both HNE formation and DNA damage. It may be postulated that during onset of oxidative stress, small amounts of HNE formed at the initial stage of the stress lead to DNA damage and activate defense mechanism including cell cycle arrest. This idea is consistent with the results of studies showing that even a transient rise in HNE levels in cells leads to the induction of GSTs, GSH synthesis, HSF1, Nrf2, EGFR and VEGFR [6,8]. Induction of these mechanisms along with cell cycle arrest suggests that HNE acts as a sensor of stress and activates defense mechanisms at the onset of stress but when there is overwhelming stress, increased HNE levels signal for apoptotic death. Thus GSTs function as a major regulator of cellular HNE levels and play a major role in cell cycle events.
Future perspectives
The available evidence strongly indicate that GSTs regulate HNE-mediated toxicity and signaling by either attenuating HNE formation or through its conjugate GS-HNE formation that is eventually eliminated from cells through RLIP76 -mediated transport. As detailed above, the Alpha class of GST enzymes GSTA1-1 and GSTA2-2 that prevent HNE formation and GSTA4-4 that catalyze the conjugation of HNE to GSH play an important role in this process. However, the role of other GST isozymes particularly GST P1-1 is not clear and needs to be investigated. Furthermore, the stereo chemical properties of HNE also need to be considered. HNE has a chiral center at C4 and exits as a racemic mixture of S- and R-enantiomers. Substrate specificity of various GST isozymes towards the enantiomers may provide further information on the role of other GST isozymes in the regulation of HNE-mediated signaling. The conjugation of HNE with GSH results in the formation of 2 each of R and S- diastereomers of GS-HNE. The ring closer of GS-HNE yields another chiral center and therefore formation of eight GS-HNE diastereomers is possible. Metabolites of GS-HNE such as GS-DHN formed by the aldose reductase catalyzed reduction of GS-HNE are known to be important signaling molecules [77–80]. Therefore, studies on substrate specificities of GS-HNE metabolizing enzymes such as aldose reductase and aldehyde dehydrogenase towards GSH-HNE diastereomers may also provide important information on the regulation of HNE-induced signaling.
Highlights.
HNE plays a key role in stress-mediated signaling in a concentration-dependent manner.
GSTs being the major determinants of cellular HNE levels, regulate concentration-dependent, hormetic signaling by HNE.
Hormetic signaling effects of HNE and its regulation by GSTs are crucial for cellular homeostasis during oxidative stress.
Initial low levels of HNE formed during a stress act as a sensor for the activation of multifarious cellular defense mechanisms while at sustained high cellular levels, HNE signals for cell death.
Acknowledgments
Supported by funding from NIH grants CA129383 and DK104786.
Abbreviations
- ATR
ataxia telangiectasia mutated and Rad3 related protein
- Cdc25C
cell division cycle 25C
- CDK
Cyclin-dependent kinases
- Chk1
checkpoint kinase
- DAXX
death domain-associated protein
- EGFR
Epidermal growth factor receptor
- GSH
Glutathione
- GST
Gluatathione-S-Transferase
- HSF1
heat shock factor 1
- JNK
c-Jun NH2-terminal kinase
- Nrf2
nuclear factor E2-related factor 2
- ROS
Reactive oxygen species
- HLE-B3
human lens epithelial B3
- HNE
4-Hydroxy-trans 2-nonenal
- RLIP76
76 kDa Ral-binding GTPase activating protein (Ralbp1)
- RPE
retinal pigment epithelial
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 2.Benedetti A, Comporti M, Esterbauer H. Identification of 4-hydroxynonenal as a cytotoxic product originating from the peroxidation of liver microsomal lipids. Biochim. Biophys. Acta. 1980;620:281–296. doi: 10.1016/0005-2760(80)90209-x. [DOI] [PubMed] [Google Scholar]
- 3.Dianzani MU. 4-Hydroxynonenal and cell signaling. Free Radic. Res. 1998;28:553–560. doi: 10.3109/10715769809065811. [DOI] [PubMed] [Google Scholar]
- 4.Leonarduzzi G, Arkan MC, Başağa H, Chiarpotto E, Sevanian A, Poli G. Lipid oxidation products in cell signaling. Free Radic. Biol. Med. 2000;28:1370–1378. doi: 10.1016/s0891-5849(00)00216-1. [DOI] [PubMed] [Google Scholar]
- 5.Dwivedi S, Sharma A, Patrick B, Sharma R, Awasthi YC. Role of 4-hydroxynonenal and its metabolites in signaling. Redox Rep. 2007;12:4–10. doi: 10.1179/135100007X162211. [DOI] [PubMed] [Google Scholar]
- 6.Awasthi YC, Yang Y, Tiwari NK, Patrick B, Sharma A, Li J, Awasthi S. Regulation of 4-hydroxynonenal-mediated signaling by glutathione S-transferases. Free Radic. Biol. Med. 2004;37:607–619. doi: 10.1016/j.freeradbiomed.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 7.Schaur RJ, Siems W, Bresgen N, Eckl PM. 4-Hydroxy-nonenal-A Bioactive Lipid Peroxidation Product. Biomolecules. 2015;5:2247–2337. doi: 10.3390/biom5042247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balogh LM, Atkins WM. Interactions of glutathione transferases with 4-hydroxynonenal. Drug Metab. Rev. 2011;43:165–178. doi: 10.3109/03602532.2011.558092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spitz DR, Sullivan SJ, Malcolm RR, Roberts RJ. Glutathione dependent metabolism and detoxification of 4-hydroxy-2-nonenal. Free Radic. Biol. Med. 1991;11:415–423. doi: 10.1016/0891-5849(91)90159-z. [DOI] [PubMed] [Google Scholar]
- 10.Schaur RJ. Basic aspects of the biochemical reactivity of 4-hydroxynonenal. Mol. Aspects Med. 2003;24:149–159. doi: 10.1016/s0098-2997(03)00009-8. [DOI] [PubMed] [Google Scholar]
- 11.Alin P, Danielson UH, Mannervik B. 4-Hydroxyalk-2-enals are substrates for glutathione transferase. FEBS Lett. 1985;179:267–270. doi: 10.1016/0014-5793(85)80532-9. [DOI] [PubMed] [Google Scholar]
- 12.Yang Y, Sharma R, Zimniak P, Awasthi YC. Role of alpha class glutathione S-transferases as antioxidant enzymes in rodent tissues. Toxicol. Appl. Pharmacol. 2002;182:105–115. doi: 10.1006/taap.2002.9450. [DOI] [PubMed] [Google Scholar]
- 13.Jakoby WB. The glutathione S-transferases: a group of multifunctional detoxification proteins. Adv. Enzymol. Relat. Areas Mol. Biol. 1978;46:383–414. doi: 10.1002/9780470122914.ch6. [DOI] [PubMed] [Google Scholar]
- 14.Singhal SS, Singh SP, Singhal P, Horne D, Singhal J, Awasthi S. Antioxidant role of glutathione S-transferases: 4-Hydroxynonenal, a key molecule in stress-mediated signaling. Toxicol. Appl. Pharmacol. 2015;289:361–370. doi: 10.1016/j.taap.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haenen GR, Plug HJ, Vermeulen NP, Timmerman H, Bast A. Contribution of 4-hydroxy-2,3-trans-nonenal to the reduction of beta-adrenoceptor function in the heart by oxidative stress. Life Sci. 1989;45:71–76. doi: 10.1016/0024-3205(89)90437-2. [DOI] [PubMed] [Google Scholar]
- 16.Rossi MA, Garramone A, Dianzani M. Stimulation of phospholipase C activity by 4-hydroxynonenal; influence of GTP and calcium concentration. Int. J. Tissue React. 1988;10:321–325. [PubMed] [Google Scholar]
- 17.Rossi MA, Fidale F, DiMauro C, Esterbauer H, Dianzani MU. Effect of 4-hydroxy-2,3-trans-nonenal and related aldehydes on phospholipase C activity of rat neutrophils. Int. J. Tissue React. 1993;15:201–205. [PubMed] [Google Scholar]
- 18.Curzio M, Esterbauer H, DiMauro C, Cecchini G, Dianzani MU. Chemotactic activity of the lipid peroxidation product 4-hydroxynonenal and homologous hydroxyalkenals. Biol. Chem. Hoppe. Seyler. 1986;367:321–329. doi: 10.1515/bchm3.1986.367.1.321. [DOI] [PubMed] [Google Scholar]
- 19.Poot M, Verkerk A, Koster JF, Esterbauer H, Jongkind JF. Reversible inhibition of DNA and protein synthesis by cumene hydroperoxide and 4-hydroxy-nonenal. Mech. Ageing Dev. 1988;43:1–9. doi: 10.1016/0047-6374(88)90093-0. [DOI] [PubMed] [Google Scholar]
- 20.Esterbauer H. Cytotoxicity and genotoxicity of lipid-oxidation products. Am. J. Clin. Nutr. 1993;57:779S–785S. doi: 10.1093/ajcn/57.5.779S. [DOI] [PubMed] [Google Scholar]
- 21.Zarkovic N, Ilic Z, Jurin M, Schaur RJ, Puhl H, Esterbauer H. Stimulation of HeLa cell growth by physiological concentrations of 4-hydroxynonenal. Cell Biochem. Funct. 1993;11:279–286. doi: 10.1002/cbf.290110409. [DOI] [PubMed] [Google Scholar]
- 22.Ruef J, Rao GN, Li F, Bode C, Patterson C, Bhatnagar A, Runge MS. Induction of rat aortic smooth muscle cell growth by the lipid peroxidation product 4-hydroxy-2-nonenal. Circulation. 1998;97:1071–1078. doi: 10.1161/01.cir.97.11.1071. [DOI] [PubMed] [Google Scholar]
- 23.Kakishita H, Hattori Y. Vascular smooth muscle cell activation and growth by 4-hydroxynonenal. Life Sci. 2001;69:689–697. doi: 10.1016/s0024-3205(01)01166-3. [DOI] [PubMed] [Google Scholar]
- 24.Ruef J, Moser M, Bode C, Kübler W, Runge MS. 4-hydroxynonenal induces apoptosis, NF-kappaB-activation and formation of 8-isoprostane in vascular smooth muscle cells. Basic Res. Cardiol. 2001;96:143–150. doi: 10.1007/s003950170064. [DOI] [PubMed] [Google Scholar]
- 25.Bodur C, Kutuk O, Tezil T, Basaga H. Inactivation of Bcl-2 through IκB kinase (IKK)-dependent phosphorylation mediates apoptosis upon exposure to 4-hydroxynonenal (HNE) J. Cell Physiol. 2012;227:3556–3565. doi: 10.1002/jcp.24057. [DOI] [PubMed] [Google Scholar]
- 26.Shearn CT, Fritz KS, Reigan P, Petersen DR. Modification of Akt2 by 4-hydroxynonenal inhibits insulin-dependent Akt signaling in HepG2 cells. Biochemistry. 2011;50:3984–3996. doi: 10.1021/bi200029w. [DOI] [PubMed] [Google Scholar]
- 27.Pettazzoni P, Pizzimenti S, Toaldo C, Sotomayor P, Tagliavacca L, Liu S, Wang D, Minelli R, Ellis L, Atadja P, Ciamporcero E, Dianzani MU, Barrera G, Pili R. Induction of cell cycle arrest and DNA damage by the HDAC inhibitor panobinostat (LBH589) and the lipid peroxidation end product 4-hydroxynonenal in prostate cancer cells. Free Radic. Biol. Med. 2011;50:313–322. doi: 10.1016/j.freeradbiomed.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Awasthi YC, Sharma R, Sharma A, Yadav S, Singhal SS, Chaudhary P, Awasthi S. Self-regulatory role of 4-hydroxynonenal in signaling for stress-induced programmed cell death. Free Radic. Biol. Med. 2008;45:111–118. doi: 10.1016/j.freeradbiomed.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng JZ, Sharma R, Yang Y, Singhal SS, Sharma A, Saini MK, Singh SV, Zimniak P, Awasthi S, Awasthi YC. Accelerated metabolism and exclusion of 4-hydroxynonenal through induction of RLIP76 and hGST5.8 is an early adaptive response of cells to heat and oxidative stress. J. Biol. Chem. 2001;276:41213–41223. doi: 10.1074/jbc.M106838200. [DOI] [PubMed] [Google Scholar]
- 30.Yang Y, Cheng JZ, Singhal SS, Saini M, Pandya U, Awasthi S, Awasthi YC. Role of glutathione S-transferases in protection against lipid peroxidation. Overexpression of hGSTA2-2 in K562 cells protects against hydrogen peroxide-induced apoptosis and inhibits JNK and caspase 3 activation. J. Biol. Chem. 2001;276:19220–19230. doi: 10.1074/jbc.M100551200. [DOI] [PubMed] [Google Scholar]
- 31.Awasthi YC, Sharma R, Cheng JZ, Yang Y, Sharma A, Singhal SS, Awasthi S. Role of 4-hydroxynonenal in stress-mediated apoptosis signaling. Mol. Aspects Med. 2003;24:219–230. doi: 10.1016/s0098-2997(03)00017-7. [DOI] [PubMed] [Google Scholar]
- 32.Sharma R, Yang Y, Sharma A, Awasthi S, Awasthi YC. Antioxidant role of glutathione S-transferases: protection against oxidant toxicity and regulation of stress-mediated apoptosis. Antioxid. Redox Signal. 2004;6:289–300. doi: 10.1089/152308604322899350. [DOI] [PubMed] [Google Scholar]
- 33.Yang Y, Sharma R, Sharma A, Awasthi S, Awasthi YC. Lipid peroxidation and cell cycle signaling: 4-hydroxynonenal, a key molecule in stress mediated signaling. Acta Biochim. Pol. 2003;50:319–336. [PubMed] [Google Scholar]
- 34.Li J, Sharma R, Patrick B, Sharma A, Jeyabal PV, Reddy PM, Saini MK, Dwivedi S, Dhanani S, Ansari NH, Zimniak P, Awasthi S, Awasthi YC. Regulation of CD95 (Fas) expression and Fas-mediated apoptotic signaling in HLE B-3 cells by 4-hydroxynonenal. Biochemistry. 2006;45:12253–12264. doi: 10.1021/bi060780+. [DOI] [PubMed] [Google Scholar]
- 35.Sharma R, Sharma A, Dwivedi S, Zimniak P, Awasthi S, Awasthi YC. 4-Hydroxynonenal self-limits fas-mediated DISC-independent apoptosis by promoting export of Daxx from the nucleus to the cytosol and its binding to Fas. Biochemistry. 2008;47:143–156. doi: 10.1021/bi701559f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chaudhary P, Sharma R, Sharma A, Vatsyayan R, Yadav S, Singhal SS, Rauniyar N, Prokai L, Awasthi S, Awasthi YC. Mechanisms of 4-hydroxy-2-nonenal induced pro- and anti-apoptotic signaling. Biochemistry. 2010;49:6263–6275. doi: 10.1021/bi100517x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaudhary P, Sharma R, Sahu M, Vishwanatha JK, Awasthi S, Awasthi YC. 4-Hydroxynonenal induces G2/M phase cell cycle arrest by activation of the ataxia telangiectasia mutated and Rad3-related protein (ATR)/checkpoint kinase 1 (Chk1) signaling pathway. J. Biol. Chem. 2013;288:20532–20546. doi: 10.1074/jbc.M113.467662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaillancourt F, Fahmi H, Shi Q, Lavigne P, Ranger P, Fernandes JC, Benderdour M. 4-Hydroxynonenal induces apoptosis in human osteoarthritic chondrocytes: the protective role of glutathione-S-transferase. Arthritis Res. Ther. 2008;10:R107. doi: 10.1186/ar2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zarkovic N. 4-hydroxynonenal as a bioactive marker of pathophysiological processes. Mol. Aspects Med. 2003;24:281–291. doi: 10.1016/s0098-2997(03)00023-2. [DOI] [PubMed] [Google Scholar]
- 40.Peden DB, Dailey L, DeGraff W, Mitchell JB, Lee JG, Kaliner MA, Hohman RJ. Hydrogen peroxide effects on rat mast cell function. Am. J. Physiol. 1994;267:L85–L93. doi: 10.1152/ajplung.1994.267.1.L85. [DOI] [PubMed] [Google Scholar]
- 41.Haendeler J, Tischler V, Hoffmann J, Zeiher AM, Dimmeler S. Low doses of reactive oxygen species protect endothelial cells from apoptosis by increasing thioredoxin-1 expression. FEBS Lett. 2004;577:427–433. doi: 10.1016/j.febslet.2004.10.041. [DOI] [PubMed] [Google Scholar]
- 42.Burdon RH, Alliangana D, Gill V. Hydrogen peroxide and the proliferation of BHK-21 cells. Free Radic. Res. 1995;23:471–486. doi: 10.3109/10715769509065268. [DOI] [PubMed] [Google Scholar]
- 43.Ostrovidov S, Franck P, Capiaumont J, Dousset B, Belleville F. Effects of H2O2 on the growth, secretion, and metabolism of hybridoma cells in culture. In Vitro Cell Dev. Biol. Anim. 1998;34:259–264. doi: 10.1007/s11626-998-0132-8. [DOI] [PubMed] [Google Scholar]
- 44.Patton GW, Paciga JE, Shelley SA. NR8383 alveolar macrophage toxic growth arrest by hydrogen peroxide is associated with induction of growth-arrest and DNA damage-inducible genes GADD45 and GADD153. Toxicol. Appl. Pharmacol. 1997;147:126–134. doi: 10.1006/taap.1997.8227. [DOI] [PubMed] [Google Scholar]
- 45.Chen QM, Bartholomew JC, Campisi J, Acosta M, Reagan JD, Ames BN. Molecular analysis of H2O2-induced senescent-like growth arrest in normal human fibroblasts: p53 and Rb control G1 arrest but not cell replication. Biochem. J. 1998;332:43–50. doi: 10.1042/bj3320043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng JZ, Singhal SS, Saini M, Singhal J, Piper JT, Van Kuijk FJ, Zimniak P, Awasthi YC, Awasthi S. Effects of mGST A4 transfection on 4-hydroxynonenal-mediated apoptosis and differentiation of K562 human erythroleukemia cells. Arch. Biochem. Biophys. 1999;372:29–36. doi: 10.1006/abbi.1999.1479. [DOI] [PubMed] [Google Scholar]
- 47.Sharma R, Brown D, Awasthi S, Yang Y, Sharma A, Patrick B, Saini MK, Singh SP, Zimniak P, Singh SV, Awasthi YC. Transfection with 4-hydroxynonenal-metabolizing glutathione S-transferase isozymes leads to phenotypic transformation and immortalization of adherent cells. Eur. J. Biochem. 2004;271:1690–1701. doi: 10.1111/j.1432-1033.2004.04067.x. [DOI] [PubMed] [Google Scholar]
- 48.Hartley DP, Ruth JA, Petersen DR. The hepatocellular metabolism of 4-hydroxynonenal by alcohol dehydrogenase, aldehyde dehydrogenase, and glutathione S-transferase. Arch. Biochem. Biophys. 1995;316:197–205. doi: 10.1006/abbi.1995.1028. [DOI] [PubMed] [Google Scholar]
- 49.Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit. Rev. Biochem. Mol. Biol. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- 50.Strange RC, Spiteri MA, Ramachandran S, Fryer AA. Glutathione-S-transferase family of enzymes. Mutat. Res. 2001;482:21–26. doi: 10.1016/s0027-5107(01)00206-8. [DOI] [PubMed] [Google Scholar]
- 51.Stenberg G, Ridderström M, Engström A, Pemble SE, Mannervik B. Cloning and heterologous expression of cDNA encoding class alpha rat glutathione transferase 8–8, an enzyme with high catalytic activity towards genotoxic alpha,beta-unsaturated carbonyl compounds. Biochem. J. 1992;284(Pt 2):313–319. doi: 10.1042/bj2840313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zimniak P, Singhal SS, Srivastava SK, Awasthi S, Sharma R, Hayden JB, Awasthi YC. Estimation of genomic complexity, heterologous expression, and enzymatic characterization of mouse glutathione S-transferase mGSTA4-4 (GST 5.7) J. Biol. Chem. 1994;269:992–1000. [PubMed] [Google Scholar]
- 53.Desmots F, Rauch C, Henry C, Guillouzo A, Morel F. Genomic organization, 5'-flanking region and chromosomal localization of the human glutathione transferase A4 gene. Biochem. J. 1998;336(Pt 2):437–442. doi: 10.1042/bj3360437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zimniak P, Eckles MA, Saxena M, Awasthi YC. A subgroup of class alpha glutathione S-transferases. Cloning of cDNA for mouse lung glutathione S-transferase GST 5.7. FEBS Lett. 1992;313:173–176. doi: 10.1016/0014-5793(92)81438-r. [DOI] [PubMed] [Google Scholar]
- 55.Singhal SS, Zimniak P, Awasthi S, Piper JT, He NG, Teng JI, Petersen DR, Awasthi YC. Several closely related glutathione S-transferase isozymes catalyzing conjugation of 4-hydroxynonenal are differentially expressed in human tissues. Arch. Biochem. Biophys. 1994;311:242–250. doi: 10.1006/abbi.1994.1233. [DOI] [PubMed] [Google Scholar]
- 56.Singhal SS, Zimniak P, Sharma R, Srivastava SK, Awasthi S, Awasthi YC. A novel glutathione S-transferase isozyme similar to GST 8–8 of rat and mGSTA4-4 (GST 5.7) of mouse is selectively expressed in human tissues. Biochim. Biophys. Acta. 1994;1204:279–286. doi: 10.1016/0167-4838(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 57.Singh SP, Coronella JA, Benes H, Cochrane BJ, Zimniak P. Catalytic function of Drosophila melanogaster glutathione S-transferase DmGSTS1-1 (GST-2) in conjugation of lipid peroxidation end products. Eur. J. Biochem. 2001;268:2912–2923. doi: 10.1046/j.1432-1327.2001.02179.x. [DOI] [PubMed] [Google Scholar]
- 58.Dourado DF, Fernandes PA, Ramos MJ. Mammalian cytosolic glutathione transferases. Curr. Protein Pept. Sci. 2008;9:325–337. doi: 10.2174/138920308785132677. [DOI] [PubMed] [Google Scholar]
- 59.Awasthi YC, Singh SV. Subunit structure of human and rat glutathione S-transferases. Comp. Biochem. Physiol. B. 1985;82:17–23. doi: 10.1016/0305-0491(85)90121-x. [DOI] [PubMed] [Google Scholar]
- 60.Singh SV, Dao DD, Partridge CA, Theodore C, Srivastava SK, Awasthi YC. Different forms of human liver glutathione S-transferases arise from dimeric combinations of at least four immunologically and functionally distinct subunits. Biochem. J. 1985;232:781–790. doi: 10.1042/bj2320781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Awasthi S, Sharma R, Yang Y, Singhal SS, Pikula S, Bandorowicz-Pikula J, Singh SV, Zimniak P, Awasthi YC. Transport functions and physiological significance of 76 kDa Ral-binding GTPase activating protein (RLIP76) Acta Biochim. Pol. 2002;49:855–867. [PubMed] [Google Scholar]
- 62.Sharma R, Sharma A, Yang Y, Awasthi S, Singhal SS, Zimniak P, Awasthi YC. Functional reconstitution of Ral-binding GTPase activating protein, RLIP76, in proteoliposomes catalyzing ATP-dependent transport of glutathione conjugate of 4-hydroxynonenal. Acta Biochim. Pol. 2002;49:693–701. [PubMed] [Google Scholar]
- 63.Awasthi YC, Chaudhary P, Vatsyayan R, Sharma A, Awasthi S, Sharma R. Physiological and pharmacological significance of glutathione-conjugate transport. J. Toxicol. Environ. Health B. Crit Rev. 2009;12:540–551. doi: 10.1080/10937400903358975. [DOI] [PubMed] [Google Scholar]
- 64.Singhal J, Singhal SS, Yadav S, Suzuki S, Warnke MM, Yacoub A, Dent P, Bae S, Sharma R, Awasthi YC, Armstrong DW, Awasthi S. RLIP76 in defense of radiation poisoning. Int. J. Radiat. Oncol. Biol. Phys. 2008;72:553–561. doi: 10.1016/j.ijrobp.2008.06.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Awasthi S, Singhal SS, Awasthi YC, Martin B, Woo JH, Cunningham CC, Frankel AE. RLIP76 and Cancer. Clin. Cancer Res. 2008;14:4372–4377. doi: 10.1158/1078-0432.CCR-08-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Awasthi YC, Sharma R, Yadav S, Dwivedi S, Sharma A, Awasthi S. The non-ABC drug transporter RLIP76 (RALBP-1) plays a major role in the mechanisms of drug resistance. Curr. Drug Metab. 2007;8:315–323. doi: 10.2174/138920007780655414. [DOI] [PubMed] [Google Scholar]
- 67.Awasthi S, Singhal SS, Yadav S, Singhal J, Drake K, Nadkar A, Zajac E, Wickramarachchi D, Rowe N, Yacoub A, Boor P, Dwivedi S, Dent P, Jarman WE, John B, Awasthi YC. RLIP76 is a major determinant of radiation sensitivity. Cancer Res. 2005;65:6022–6028. doi: 10.1158/0008-5472.CAN-05-0968. [DOI] [PubMed] [Google Scholar]
- 68.Awasthi S, Cheng J, Singhal SS, Saini MK, Pandya U, Pikula S, Bandorowicz-Pikula J, Singh SV, Zimniak P, Awasthi YC. Novel function of human RLIP76: ATP-dependent transport of glutathione conjugates and doxorubicin. Biochemistry. 2000;39:9327–9334. doi: 10.1021/bi992964c. [DOI] [PubMed] [Google Scholar]
- 69.Awasthi S, Cheng JZ, Singhal SS, Pandya U, Sharma R, Singh SV, Zimniak P, Awasthi YC. Functional reassembly of ATP-dependent xenobiotic transport by the N-and C-terminal domains of RLIP76 and identification of ATP binding sequences. Biochemistry. 2001;40:4159–4168. doi: 10.1021/bi002182f. [DOI] [PubMed] [Google Scholar]
- 70.Srivastava S, Chandra A, Bhatnagar A, Srivastava SK, Ansari NH. Lipid peroxidation product, 4-hydroxynonenal and its conjugate with GSH are excellent substrates of bovine lens aldose reductase. Biochem. Biophys. Res. Commun. 1995;217:741–746. doi: 10.1006/bbrc.1995.2835. [DOI] [PubMed] [Google Scholar]
- 71.Guéraud F, Alary J, Costet P, Debrauwer L, Dolo L, Pineau T, Paris A. In vivo involvement of cytochrome P450 4A family in the oxidative metabolism of the lipid peroxidation product trans-4-hydroxy-2-nonenal, using PPARalpha-deficient mice. J. Lipid Res. 1999;40:152–159. [PubMed] [Google Scholar]
- 72.Amunom I, Stephens LJ, Tamasi V, Cai J, Pierce WM, Jr, Conklin DJ, Bhatnaga A, Srivastava S, Martin MV, Guengerich FP, Prough RA. Cytochromes P450 catalyze oxidation of alpha,beta-unsaturated aldehydes. Arch. Biochem. Biophys. 2007;464:187–196. doi: 10.1016/j.abb.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Laurent A, Perdu-Durand E, Alary J, Debrauwer L, Cravedi JP. Metabolism of 4-hydroxynonenal, a cytotoxic product of lipid peroxidation. in rat precision-cut liver slices, Toxicol. Lett. 2000;114:203–214. doi: 10.1016/s0378-4274(99)00301-x. [DOI] [PubMed] [Google Scholar]
- 74.Xu X, Chai S, Wang P, Zhang C, Yang Y, Yang Y, Wang K. Aldehyde dehydrogenases and cancer stem cells. Cancer Lett. 2015;369:50–57. doi: 10.1016/j.canlet.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 75.Luo XJ, Liu B, Ma QL, Peng J. Mitochondrial aldehyde dehydrogenase, a potential drug target for protection of heart and brain from ischemia/reperfusion injury. Curr. Drug Targets. 2014;15:948–955. [PubMed] [Google Scholar]
- 76.Singh S, Brocker C, Koppaka V, Chen Y, Jackson BC, Matsumoto A, Thompson DC, Vasiliou V. Aldehyde dehydrogenases in cellular responses to oxidative/electrophilic stress. Free Radic. Biol. Med. 2013;56:89–101. doi: 10.1016/j.freeradbiomed.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ramana KV, Bhatnagar A, Srivastava S, Yadav UC, Awasthi S, Awasthi YC, Srivastava SK. Mitogenic responses of vascular smooth muscle cells to lipid peroxidation-derived aldehyde 4-hydroxy-trans-2-nonenal (HNE): role of aldose reductase-catalyzed reduction of the HNE-glutathione conjugates in regulating cell growth. J. Biol. Chem. 2006;281:17652–17660. doi: 10.1074/jbc.M600270200. [DOI] [PubMed] [Google Scholar]
- 78.Ramana KV, Fadl AA, Tammali R, Reddy AB, Chopra AK, Srivastava SK. Aldose reductase mediates the lipopolysaccharide-induced release of inflammatory mediators in RAW264.7 murine macrophages. J. Biol. Chem. 2006;281:33019–33029. doi: 10.1074/jbc.M603819200. [DOI] [PubMed] [Google Scholar]
- 79.Ramana KV, Willis MS, White MD, Horton JW, DiMaio JM, Srivastava D, Bhatnagar A, Srivastava SK. Endotoxin-induced cardiomyopathy and systemic inflammation in mice is prevented by aldose reductase inhibition. Circulation. 2006;114:1838–1846. doi: 10.1161/CIRCULATIONAHA.106.630830. [DOI] [PubMed] [Google Scholar]
- 80.Tammali R, Ramana KV, Singhal SS, Awasthi S, Srivastava SK. Aldose reductase regulates growth factor-induced cyclooxygenase-2 expression and prostaglandin E2 production in human colon cancer cells. Cancer Res. 2006;66:9705–9713. doi: 10.1158/0008-5472.CAN-06-2105. [DOI] [PubMed] [Google Scholar]
- 81.Awasthi YC, Dao DD, Saneto RP. Interrelationship between anionic and cationic forms of glutathione S-transferases of human liver. Biochem. J. 1980;191:1–10. doi: 10.1042/bj1910001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Saneto RP, Awasthi YC, Srivastava SK. Interrelationship between cationic and anionic forms of glutathione S-transferases of bovine ocular lens. Biochem. J. 1980;191:11–20. doi: 10.1042/bj1910011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao T, Singhal SS, Piper JT, Cheng J, Pandya U, Clark-Wronski J, Awasthi S, Awasthi YC. The role of human glutathione S-transferases hGSTA1-1 and hGSTA2-2 in protection against oxidative stress. Arch. Biochem. Biophys. 1999;367:216–224. doi: 10.1006/abbi.1999.1277. [DOI] [PubMed] [Google Scholar]
- 84.Yang Y, Sharma R, Cheng JZ, Saini MK, Ansari NH, Andley UP, Awasthi S, Awasthi YC. Protection of HLE B-3 cells against hydrogen peroxide- and naphthalene-induced lipid peroxidation and apoptosis by transfection with hGSTA1 and hGSTA2. Invest. Ophthalmol. Vis. Sci. 2002;43:434–445. [PubMed] [Google Scholar]
- 85.Singh SP, Janecki AJ, Srivastava SK, Awasthi S, Awasthi YC, Xia SJ, Zimniak P. Membrane association of glutathione S-transferase mGSTA4-4, an enzyme that metabolizes lipid peroxidation products. J. Biol. Chem. 2002;277:4232–4239. doi: 10.1074/jbc.M109678200. [DOI] [PubMed] [Google Scholar]
- 86.Sharma A, Patrick B, Li J, Sharma R, Jeyabal PV, Reddy PM, Awasthi S, Awasthi YC. Glutathione S-transferases as antioxidant enzymes: small cell lung cancer (H69) cells transfected with hGSTA1 resist doxorubicin-induced apoptosis. Arch. Biochem. Biophys. 2006;452:165–173. doi: 10.1016/j.abb.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 87.Yang Y, Sharma A, Sharma R, Patrick B, Singhal SS, Zimniak P, Awasthi S, Awasthi YC. Cells preconditioned with mild, transient UVA irradiation acquire resistance to oxidative stress and UVA-induced apoptosis: role of 4-hydroxynonenal in UVA-mediated signaling for apoptosis. J. Biol. Chem. 2003;278:41380–41388. doi: 10.1074/jbc.M305766200. [DOI] [PubMed] [Google Scholar]
- 88.Habig WH, Pabst MJ, Fleischner G, Gatmaitan Z, Arias IM, Jakoby WB. The identity of glutathione S-transferase B with ligandin, a major binding protein of liver. Proc. Natl. Acad. Sci. U S A. 1974;71:3879–3882. doi: 10.1073/pnas.71.10.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Patrick B, Li J, Jeyabal PV, Reddy PM, Yang Y, Sharma R, Sinha M, Luxon B, Zimniak P, Awasthi S, Awasthi YC. Depletion of 4-hydroxynonenal in hGSTA4-transfected HLE B-3 cells results in profound changes in gene expression. Biochem. Biophys. Res. Commun. 2005;334:425–432. doi: 10.1016/j.bbrc.2005.06.099. [DOI] [PubMed] [Google Scholar]
- 90.Sharma R, Sharma A, Chaudhary P, Sahu M, Jaiswal S, Awasthi S, Awasthi YC. Role of 4-hydroxynonenal in chemopreventive activities of sulforaphane. Free Radic. Biol. Med. 2012;52:2177–2185. doi: 10.1016/j.freeradbiomed.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sharma R, Sharma A, Chaudhary P, Pearce V, Vatsyayan R, Singh SV, Awasthi S, Awasthi YC. Role of lipid peroxidation in cellular responses to D,L-sulforaphane, a promising cancer chemopreventive agent. Biochemistry. 2010;49:3191–3202. doi: 10.1021/bi100104e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Forman HJ, Dickinson DA, Iles KE. HNE-signaling pathways leading to its elimination. Mol. Aspects Med. 2003;24:189–194. doi: 10.1016/s0098-2997(03)00013-x. [DOI] [PubMed] [Google Scholar]
- 93.Gargiulo S, Gamba P, Testa G, Rossin D, Biasi F, Poli G, Leonarduzzi G. Relation between TLR4/NF-κB signaling pathway activation by 27-hydroxycholesterol and 4-hydroxynonenal, and atherosclerotic plaque instability. Aging Cell. 2015;14:569–581. doi: 10.1111/acel.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang CZ, Yuan P, Xu B, Yuan L, Yang HZ, Liu X. RLIP76 expression as a prognostic marker of breast cancer. Eur. Rev. Med. Pharmacol. Sci. 2015;19:2105–2011. [PubMed] [Google Scholar]
- 95.Singhal SS, Wickramarachchi D, Yadav S, Singhal J, Leake K, Vatsyayan R, Chaudhary P, Lelsani P, Suzuki S, Yang S, Awasthi YC, Awasthi S. Glutathione-conjugate transport by RLIP76 is required for clathrin-dependent endocytosis and chemical carcinogenesis. Mol. Cancer Ther. 2011;10:16–28. doi: 10.1158/1535-7163.MCT-10-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Singhal SS, Singhal J, Yadav S, Dwivedi S, Boor PJ, Awasthi YC, Awasthi S. Regression of lung and colon cancer xenografts by depleting or inhibiting RLIP76 (Ral-binding protein 1) Cancer Res. 2007;67:4382–4389. doi: 10.1158/0008-5472.CAN-06-4124. [DOI] [PubMed] [Google Scholar]
- 97.Vatsyayan R, Chaudhary P, Sharma A, Sharma R, Rao Lelsani PC, Awasthi S, Awasthi YC. Role of 4-hydroxynonenal in epidermal growth factor receptor-mediated signaling in retinal pigment epithelial cells. Exp. Eye Res. 2011;92:147–154. doi: 10.1016/j.exer.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Engle MR, Singh SP, Czernik PJ, Gaddy D, Montague DC, Ceci JD, Yang Y, Awasthi S, Awasthi YC, Zimniak P. Physiological role of mGSTA4-4, a glutathione S-transferase metabolizing 4-hydroxynonenal: generation and analysis of mGsta4 null mouse. Toxicol. Appl. Pharmacol. 2004;194:296–308. doi: 10.1016/j.taap.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 99.McElhanon KE, Bose C, Sharma R, Wu L, Awasthi YC, Singh SP. Gsta4 Null Mouse Embryonic Fibroblasts Exhibit Enhanced Sensitivity to Oxidants: Role of 4-Hydroxynonenal in Oxidant Toxicity. Open J. Apoptosis. 2013;2:1–11. doi: 10.4236/ojapo.2013.21001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zimniak P. Relationship of electrophilic stress to aging. Free Radic. Biol. Med. 2011;51:1087–1105. doi: 10.1016/j.freeradbiomed.2011.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barrera G, Pizzimenti S, Muraca R, Barbiero G, Bonelli G, Baccino FM, Fazio VM, Dianzani MU. Effect of 4-Hydroxynonenal on cell cycle progression and expression of differentiation-associated antigens in HL-60 cells. Free Radic. Biol. Med. 1996;20:455–62. doi: 10.1016/0891-5849(95)02049-7. [DOI] [PubMed] [Google Scholar]
- 102.Goodarzi AA, Block WD, Lees-Miller SP. The role of ATM and ATR in DNA damage-induced cell cycle control. Prog. Cell Cycle Res. 2003;5:393–411. [PubMed] [Google Scholar]
- 103.Branzei D, Foiani M. Regulation of DNA repair throughout the cell cycle. Nat. Rev. Mol. Cell Biol. 2008;9:297–308. doi: 10.1038/nrm2351. [DOI] [PubMed] [Google Scholar]
- 104.Abel EL, Angel JM, Riggs PK, Langfield L, Lo HH, Person MD, Awasthi YC, Wang LE, Strom SS, Wei Q, DiGiovanni J. Evidence that Gsta4 modifies susceptibility to skin tumor development in mice and humans. J. Natl. Cancer Inst. 2010;102:1663–1675. doi: 10.1093/jnci/djq392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yang Y, Yang Y, Xu Y, Lick SD, Awasthi YC, Boor PJ. Endothelial glutathione-S-transferase A4-4 protects against oxidative stress and modulates iNOS expression through NF-kappaB translocation. Toxicol. Appl. Pharmacol. 2008;230:187–196. doi: 10.1016/j.taap.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sharma A, Sharma R, Chaudhary P, Vatsyayan R, Pearce V, Jeyabal PV, Zimniak P, Awasthi S, Awasthi YC. 4-Hydroxynonenal induces p53-mediated apoptosis in retinal pigment epithelial cells. Arch. Biochem. Biophys. 2008;480:85–94. doi: 10.1016/j.abb.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ji C, Kozak KR, Marnett LJ. κB kinase, a molecular target for inhibition by 4-hydroxy-2-nonenal. J. Biol. Chem. 2001;276:18223–18228. doi: 10.1074/jbc.M101266200. [DOI] [PubMed] [Google Scholar]
- 108.Song BJ, Soh Y, Bae M, Pie J, Wan J, Jeong K. Apoptosis of PC12 cells by 4-hydroxy-2-nonenal is mediated through selective activation of the c-Jun N-terminal protein kinase pathway. Chem. Biol. Interact. 2001;130–132:943–954. doi: 10.1016/s0009-2797(00)00247-7. [DOI] [PubMed] [Google Scholar]
- 109.Laurora S, Tamagno E, Briatore F, Bardini P, Pizzimenti S, Toaldo C, Reffo P, Costelli P, Dianzani MU, Danni O, Barrera G. 4-Hydroxynonenal modulation of p53 family gene expression in the SK-N-BE neuroblastoma cell line. Free Radic. Biol. Med. 2005;38:215–225. doi: 10.1016/j.freeradbiomed.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 110.Jacobs A, Marnett L. Heat shock factor 1 attenuates 4-hydroxynonenal-mediated apoptosis: critical role for heat shock protein 70 induction and stabilization of Bcl-XL. J. Biol. Chem. 2007;282:33412–33420. doi: 10.1074/jbc.M706799200. [DOI] [PubMed] [Google Scholar]
- 111.Wang S, Kotamraju S, Konorev E, Kalivendi S, Joseph J, Kalyanaraman B. Activation of nuclear factor-κB during doxorubicin-induced apoptosis in endothelial cells and myocytes is proapoptotic: the role of hydrogen peroxide. Biochem. J. 2002;367:729–740. doi: 10.1042/BJ20020752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lee SJ, Kim CE, Seo KW, Kim CD. HNE-induced 5-LO expression is regulated by NF-κB/ERK and Sp1/p38 MAPK pathways via EGF receptor in murine macrophages. Cardiovasc. Res. 2010;88:352–359. doi: 10.1093/cvr/cvq194. [DOI] [PubMed] [Google Scholar]
- 113.Malone P, Hernandez M. 4-Hydroxynonenal, a product of oxidative stress, leads to an antioxidant response in optic nerve head astrocytes. Exp. Eye Res. 2007;84:444–454. doi: 10.1016/j.exer.2006.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Moodie FM, Marwick JA, Anderson CS, Szulakowski P, Biswas SK, Bauter MR, Kilty I, Rahman I. Oxidative stress and cigarette smoke alter chromatin remodeling but differentially regulate NF-κB activation and proinflammatory cytokine release in alveolar epithelial cells. FASEB J. 2004;18:1897–1899. doi: 10.1096/fj.04-1506fje. [DOI] [PubMed] [Google Scholar]
- 115.Chen ZH, Saito Y, Yoshida Y, Sekine A, Noguchi N, Niki E. 4-Hydroxynonenal induces adaptive response and enhances PC12 cell tolerance primarily through induction of thioredoxin reductase 1 via activation of Nrf2. J. Biol. Chem. 2005;280:41921–41927. doi: 10.1074/jbc.M508556200. [DOI] [PubMed] [Google Scholar]
- 116.Zhang H, Liu H, Dickinson DA, Liu RM, Postlethwait EM, Laperche Y, Forman HJ. Gamma-Glutamyl transpeptidase is induced by 4-hydroxynonenal via EpRE/Nrf2 signaling in rat epithelial type II cells. Free Radic. Biol. Med. 2006;40:1281–1292. doi: 10.1016/j.freeradbiomed.2005.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dickinson DA, Iles KE, Watanabe N, Iwamoto T, Zhang H, Krzywanski DM, Forman HJ. 4-Hydroxynonenal induces glutamate cysteine ligase through JNK in HBE1 cells. Free Radic. Biol. Med. 2002;33:974. doi: 10.1016/s0891-5849(02)00991-7. [DOI] [PubMed] [Google Scholar]
- 118.Tamagno E, Parola M, Bardini P, Piccini A, Borghi R, Guglielmotto M, Santoro G, Davit A, Danni O, Smith MA, Perry G, Tabaton M. Beta-site APP cleaving enzyme up-regulation induced by 4-hydroxynonenal is mediated by stress-activated protein kinases pathways. J. Neurochem. 2005;92:628–636. doi: 10.1111/j.1471-4159.2004.02895.x. [DOI] [PubMed] [Google Scholar]