Abstract
Dementia is a frequent problem encountered in advanced stages of Parkinson disease (PD). In recent years, research has focused on the pre-dementia stages of cognitive impairment in PD, including mild cognitive impairment (MCI). Several longitudinal studies have shown that MCI is a harbinger of dementia in PD, although the course is variable, and stabilization of cognition — or even reversal to normal cognition — is not uncommon. In addition to limbic and cortical spread of Lewy pathology, several other mechanisms are likely to contribute to cognitive decline in PD, and a variety of biomarker studies, some using novel structural and functional imaging techniques, have documented in vivo brain changes associated with cognitive impairment. The evidence consistently suggests that low cerebrospinal fluid levels of amyloid-β42, a marker of comorbid Alzheimer disease (AD), predict future cognitive decline and dementia in PD. Emerging genetic evidence indicates that in addition to the APOE*ε4 allele (an established risk factor for AD), GBA mutations and SCNA mutations and triplications are associated with cognitive decline in PD, whereas the findings are mixed for MAPT polymorphisms. Cognitive enhancing medications have some effect in PD dementia, but no convincing evidence that progression from MCI to dementia can be delayed or prevented is available, although cognitive training has shown promising results.
Parkinson disease (PD) is one of the most common age-related brain disorders. PD is defined primarily as a movement disorder, with the typical symptoms being resting tremor, rigidity, bradykinesia and postural instability, and is pathologically characterized by degeneration of nigrostriatal dopaminergic neurons and the presence of Lewy bodies (misfolded α-synuclein) in the surviving neurons. In addition to the defining dopamine-related motor symptoms, however, PD is increasingly recognized as a heterogeneous multisystem disorder involving other neurotransmitter systems, such as the serotonergic, noradrenergic and cholinergic circuits. Thus, a wide variety of nonmotor symptoms (NMS) linked with these neurotransmitters are commonly observed in patients with PD. In light of this variability, subtyping of PD has been proposed, including a system based on time of onset and ongoing rate of cognitive decline1.
Cognitive decline is among the most common and important NMS, and in this article we review the current status of knowledge regarding cognitive impairment in PD. Robust evidence indicates that in comparison with age-matched groups without PD, people with PD exhibit more rapid decline in a number of cognitive domains — in particular, executive, attentional and visuospatial domains, but also memory. The full spectrum of cognitive abilities can be observed in PD, from normal cognition, through early mild subjective and objective decline (mild cognitive impairment (MCI)), to mild, moderate and even severe PD dementia (PDD). Studies from the 1990s onwards convincingly demonstrated a much higher cumulative risk of dementia in people with PD than in the general population, and systematic reviews showed that the point prevalence of dementia was 25–30%. Several long-term longitudinal studies have indicated that the majority of patients with PD will develop dementia if they survive for more than 10 years after diagnosis. On the basis of numerous, varied studies, we now know that dementia in PD has important adverse implications for functioning, quality of life, caregiver burden, and health-related costs2.
The timing, profile and rate of cognitive decline vary widely among individuals with PD, so identifying and predicting future cognitive decline in this population is crucial for researchers and clinicians alike. Identification of clinical and biological markers that can predict which patients are at increased risk of early and rapid cognitive decline is important for communicating the prognosis and managing patients clinically and, thus, is a focus of this article. Established demographic and clinical risk factors include increasing age and more severe parkinsonism, in particular, non-tremor features2. Here, we focus on cognitive and biomarker features as potential predictors of cognitive decline in PD.
Cognitive syndromes in PD
Subjective cognitive decline
In recent years, interest has focused on subjective cognitive decline (SCD), in which cognitive impairments are noted by the patient, family members or health personnel, but cognitive test performance is in the normal range. In the general population, SCD is associated with an increased risk of future cognitive decline, that is, progression to MCI or dementia, including Alzheimer disease (AD). Relatively little is known about SCD in PD, and there are no established criteria for this syndrome. No reliable method of capturing SCD in PD yet exists, possibly owing partly to the confounding effects of motor symptoms and NMS. Nevertheless, SCD has been reported in patients with PD, and might be a harbinger of further cognitive decline in this population3.
MCI and the risk of PD dementia
The two most common cognitive syndromes in patients with PD, PDD and PD-MCI, were operationally defined in diagnostic and assessment guidelines from the International Parkinson and Movement Disorder Society (MDS)4,5. In PDD, but not in PD-MCI, the cognitive deficits are severe enough to impair daily life (for example, social and occupational functioning, and personal care), independently of the impairment ascribable to motor or autonomic symptoms.
Among PD patients without dementia, approximately 25–30% have MCI, which is evident at the time of diagnosis in 10–20% of patients2. Presence of MCI is associated with a shorter time to progression to a dementia diagnosis, although considerable variability is observed, with some patients remaining stable and some even reverting to normal cognition. For example, in one study of patients with early PD6, over 20% of those with MCI reverted to normal cognition after 1 year, although persistent MCI was associated with a much lower remission rate.
Early studies indicated that the mean time to dementia after PD diagnosis was approximately 10 years. This figure is supported by more recent studies, including some that monitored patients from the time of PD diagnosis (TABLE 1), which reported dementia prevalence of 15–20% after 5 years and 46% at 10 years7,8. However, lower dementia rates (5% after 4 years) have been reported elsewhere9. One study that selected only PD patients with normal cognition reported that nearly 50% had developed cognitive decline after 6 years10. Some studies11 suggest that cortical posterior cognitive deficits (that is, memory and language impairment), but not frontally based dysfunction, indicate a higher risk of dementia, leading to the ‘dual syndrome hypothesis’ of cognition in PD12. Discrepancies between studies are likely to be attributable to a variety of factors, including differences in case selection, whether duration was measured from onset of symptoms or diagnosis, use of different criteria for PD-MCI and PDD, and loss to follow-up.
Table 1.
Development of MCI and dementia from time of PD diagnosis: longitudinal studies
| Study | N | Duration (years) |
MCI at baseline (%) |
MCI at follow-up (%) |
Dementia during study (%) |
Comments | MCI and risk of dementia |
Refs |
|---|---|---|---|---|---|---|---|---|
| Pigott, K. et al. (2015) | 141 | 4.4 | 0 | 47.4* | 28 | Normal cognition at baseline | – | 10 |
| PARKWEST: Pedersen, K. F. et al. (2013) | 182 | 5 | 20.3 | – | 19.2 | – | RR 39.2, P < 0.001 | 6 |
| Amsterdam: Broeders, M. et al. (2013) | 123 | 5 | 35 | 50‡ | 13.8 | – | “All patients who progressed to PD dementia had MCI at a previous assessment” | 213 |
| PPMI: Weintraub, D et al. (unpublished work) | 423 | 3 | 10 | 15–21§ | 3–6§ | – | – | – |
| CamPaIGN: Williams-Gray, C. H. et al. (2013) | 142 | 10 | 364 | 62 at 3 years‖ | 46 (17 at 5years) | – | Reduced semantic fluency (HR 3.05) and pentagon copying (HR 2.55) | 8 |
| Napoli: Santangelo, G. et al. (2015) | 76 | 4 | 32.9 | 38.2 | 5.4 | 50% MCI at 4 years | – | 9 |
| NYPUM: Domellöf, M. E. et al. (2015) | 147 | 5 | 42.6 | – | 27.6 | – | MCI at baseline: 6.5 × increased risk of dementia | 214 |
| Sydney: Hely, M. A. et al. (2008) | 136 | 20 | – | – | 83 | MCI criteria not available at study start | – | 215 |
Any cognitive impairment.
Of those without dementia.
Using different criteria.
Cognitive impairment rather than MCI. MCI, mild cognitive impairment; N, number of participants; PD, Parkinson disease.
Clinical challenges
Cognitive decline in PD is a continuous process affecting nearly all patients over time, and the demarcations between the four cognitive groups — cognitively normal, SCD, PD-MCI and PDD— are not strict. The distribution of patients in these four groups varies markedly among different studies, depending on factors including the case selection procedures, the criteria applied, the study design (cross-sectional or longitudinal), and the cognitive measurement procedures utilized. A recent study, which attempted to define the optimal criteria for PD-MCI, found that impairment (>1.5 SD below the normative mean) on two tests within the same cognitive domain — rather than across different domains — was the best predictor of progression to dementia during the next 4 years13. As highlighted above, the separation between MCI and dementia hinges on whether the functional impact of cognitive impairment is ‘severe enough to impair daily life’, a criterion that is difficult to operationalize and requires an element of clinical judgement.
Another clinical challenge is the distinction between PDD and dementia with Lewy bodies (DLB), owing to their clinical and pathological overlap. The ‘1-year rule’, in which PDD is defined as dementia that occurs at least 1 year after onset of PD motor symptoms, whereas dementia occurring before, simultaneously with, or within the first year of onset of parkinsonism is classified as DLB, is often difficult to apply, as a substantial proportion of patients lie within the grey zone, and categorization is usually performed retrospectively. In addition, prodromal PD and DLB symptoms overlap; for example, REM sleep behaviour disorder (RBD) can evolve into either PD or DLB. Recent evidence indicates that cognitive impairment can be present in prodromal PD, further blurring the distinction between PD and DLB14. The recently revised MDS clinical diagnostic criteria for PD propose that PD can be diagnosed regardless of when dementia occurs in relation to parkinsonism onset, and in cases where parkinsonism subsequently develops in a patient with dementia, the diagnosis ‘PD (DLB subtype)’ is recommended15. The relationship between PD and DLB requires further exploration. For example, recent findings indicate several nonmotor subtypes of PD, including cognitive and non-cognitive forms, which can occur in early untreated motor disease in late-onset PD, as well as in early-onset PD16,17. From a practical perspective, an algorithm in which premotor NMS such as RBD are channelled towards potential transformation to motor PD, including cognitive phenotypes, has been proposed17.
Visual hallucinations and dementia risk
Several longitudinal studies have identified visual hallucinations and illusions as risk factors for cognitive decline and dementia in PD, with the time frame depending on the study design. In one study, a history of visual hallucinations at baseline was found to increase the risk of dementia at 8 years (OR 3.1, 95% CI 1.6–6.2)18, and another group found that baseline visual hallucinations (OR 10.2, 90% CI 2.4–44.0) or illusions (OR 8.2, 90% CI 2.4–28.4) increased the risk of dementia at 4–5 years19. The association between visual hallucinations and dementia that has been observed in prospective studies might reflect progression of cognitive dysfunction, which seems to be present before or coincident with hallucination onset20.
Mechanisms
A variety of mechanisms, in addition to the classic nigrostriatal α-synuclein misfolding and dopaminergic neuronal loss, contribute to the brain changes associated with PD (BOX 1). PD is now recognized to involve multisystem, multipeptide neurodegeneration, with non-dopaminergic degeneration having a crucial role.
Box 1 | Mechanisms of cognitive decline.
The following mechanisms are proposed to contribute to cognitive decline in Parkinson disease:
Protein misfolding (α-synuclein, amyloid and tau)
Neurotransmitter activity
Synaptic dysfunction and loss
Neuroinflammation and diabetes
Mitochondrial dysfunction and retrograde signalling
Microglial and astroglial changes
Genetics
Epigenetics
Adenosine receptor activation
Cerebral network disruption
Compared with the motor symptoms, little is known about the mechanisms underlying cognitive decline in PD, and several key questions remain unresolved. First, is cognitive decline merely a result of more severe and widespread involvement of primary PD neuropathophysiology? Second, are some of the PD-related mechanisms particularly relevant for cognitive decline? Last, is cognitive impairment related to regional involvement or specific mechanisms?
Information on the mechanisms underlying cognitive decline in PD has come from a variety of sources. In addition to postmortem studies, in vivo studies, including clinicopathological studies and biomarker studies involving electrophysiological, imaging, electrophysiology and biofluid analyses, and genetic studies, have all contributed to an increased understanding. However, animal models for PD-related cognitive deficits have been difficult to develop.
Evidence from pathological studies
The pathological contributions to dementia in PD have been studied in some detail, and have been reviewed elsewhere21. Good evidence from postmortem studies indicates that limbic and cortical Lewy body pathology is the main pathological correlate of dementia in PD. In most cases, α-synuclein pathology seems to spread from sites in the lower brainstem or olfactory bulb — or even extracranially from the gut or other areas innervated by the vagus nucleus1 — to the midbrain, forebrain and limbic structures and, finally, neocortical regions22.
In addition, there is strong evidence that the extent of amyloid plaque pathology is a significant contributor to dementia in up to one-third of patients with PD), whereas the role of tau-related pathology is less clear. Importantly, the three proteinopathies in combination seem to have an additive effect over and above the effect of any single pathology23,24. This finding is in line with basic neuroscience studies, which show an interaction between the processing of these three proteins. For example, transgenic mice with double pathology (that is, overexpression of α-synuclein on a background of amyloid-β (Aβ) or tau pathology) show more severe Lewy body pathology than mice overexpressing α-synuclein pathology only25. Clinically, these effects manifest as earlier and more rapid cognitive decline in patients with combined Lewy body and amyloid pathology26. The roles of other pathologies, including cerebrovascular disease, hippocampal sclerosis and cerebral amyloid angiopathy, are also beginning to be explored.
Synaptic pathology and cognition
The structural pathologies described above are relevant, but only partially explain the variance in cognitive decline in patients with PD. A better understanding of the disease substrate is needed for targeted drug discovery and to enable better monitoring of disease progression. Changes in synaptic function followed by synaptic loss are likely to be early and key events in neurodegenerative diseases: in AD, loss of synapses was found to be more robustly correlated with cognitive decline than was morphological pathology27. Less is known regarding the role of synaptic dysfunction in cognition in PD, but synaptic alterations have been demonstrated (reviewed elsewhere28).
In PD, mounting evidence indicates that the initial damage in dopaminergicnigral neurons occurs at the synapse, with subsequent retrograde axonal damage and, finally, somatic degeneration, leading to the typical motor symptoms of PD (reviewed elsewhere29). Similar mechanisms might underlie limbic and cortical involvement in PD. These events probably involve α-synuclein, which is mainly located synaptically and regulates synaptic homeostasis, vesicles and neurotransmitter release, and initially aggregates at the synaptic terminal during PD pathogenesis30. Of note, several other proteins implicated in PD — in particular, PINK1, Parkin, leucine-rich repeat serine/threonine-protein kinase 2 (LRRK2) and DJ1 — are also involved in synaptic regulation. We have found that reduced neocortical levels of ZnT3, a marker of synaptic plasticity, and two key synaptic proteins, neurogranin and SNAP25 (REFS 31,32), are associated with cognition in PD. These findings suggest that cortical synaptic changes lead to cognitive decline in PD, consistent with imaging findings (see below), and preliminary work indicates that synaptic changes are also reflected in the cerebrospinal fluid (CSF). Neurogranin levels were found to be increased in the CSF in AD33, probably due to increased intracellular sequestration, and similar findings have been reported — and linked with cognitive decline — in PD34. Thus, CSF levels of synaptic proteins are potential biomarkers of future cognitive decline.
Neurotransmitters
Convincing evidence is available that mesolimbic and mesocortical dopaminergic activity is associated with cognitive functioning. The association between dopaminergic drugs and cognition is complex, however, and antiparkinson drugs can improve, worsen or have no influence on cognition35. In addition, a number of non-dopaminergic transmitter systems are affected in PD, and are likely to contribute to cognitive impairment21. For example, good evidence from postmortem and imaging studies indicates that the cholinergic system is affected relatively early in PD and contributes to the cognitive decline. Interestingly, whereas Lewy body and amyloid plaque pathologies were associated with earlier onset of dementia, cholinergic deficits were more pronounced in individuals with dementia occurring later in the disease course26,36. These observations provide a rationale for the positive effects of cholinesterase inhibitors in PD (see Management section below), as well as the worsening cognition associated with the use of medications with anticholinergic activity37.
Non-dopaminergic monoaminergic nuclei such as the noradrenergic locus coeruleus and the serotonergic raphe nuclei, with their long and widespread mesocortical connections, may affect cognition due to their influence on the activity of synaptic networks. This hypothesis was supported in a recent study, which showed that the ability of atomoxetine-mediated noradrenergic or citalopram-mediated serotonergic reuptake inhibition to improve response inhibition — a frontal executive task relevant to impulsivity — in patients with PD could be predicted by differences in brain activation, as measured by functional imaging38. In the serotonin system, several receptors are associated with cognition, including the 5-HT1B receptor, which was found to be downregulated in early PD in a recent PET study using a novel ligand, but was not associated with executive or attentional functioning39.
Striatal GABAergic neurons express adenosine A2A receptors, which have become a drug target to improve motor functioning in PD. These receptors are also located in the thalamus and neocortex, and some evidence indicates that increased receptor activity is associated with worsening cognition. Adenosine A2A antagonists may increase dopamine activity in the prefrontal cortex, and preliminary evidence suggests that they can improve cognition — in particular, working memory — in PD40,41.
Mitochondrial activity
Mitochondrial dysfunction occurs in PD, but little is known regarding its potential role in cognitive decline. However, mitochondrial pathology seems to contribute to cognitive decline in AD, and a recent postmortem study showed that deficiency in mitochondrial complex 1 activity and reduced mitochondrial DNA levels in the prefrontal cortex were more pronounced in PDD than in PD without dementia42. Mitochondrial activity is particularly high at the synapse, and is crucial to synaptic activity. A relationship between α-synuclein and mitochondrial activities at the level of synapses has been demonstrated, but their causal relationship needs to be further explored. Therapeutic approaches targeting mitochondrial activity, including complex 1 deficiency, are being developed, and manipulation of mitochondrial retrograde signalling has shown promising results in animal studies43. The role of mitochondrial dysfunction in cognitive decline in PD needs to be further explored.
Inflammation and neurotrophic factors
Neuroinflammation is relevant for both AD and PD, and might have important implications for cognitive decline in PD44, with a potential for novel treatment targets. Increased microglial activation is thought to lead to cell death in AD and PDD45, and inflammation markers represent possible prognostic biomarkers. Interestingly, CSF levels of cytokines are found to be associated with cognitive impairment in PD46 and, thus, represent possible biomarkers (see below).
Findings from many different sources convincingly demonstrate a link between diabetes, insulin resistance and PD, possibly via mechanisms involving neuro-inflammation and mitochondrial dysfunction47. A recent imaging study in a cohort of 36 patients, 12 of whom had diabetes, reported an association between diabetes, grey matter loss and cognitive impairment in PD48, indicating a possible role for antidiabetic drugs in the treatment or prevention of cognitive decline in PD, as has been suggested in AD.
Neurotrophic factors are crucial for neuronal plasticity and, thus, learning and other cognitive functions. A longitudinal study showed that cognitive impairment in PD was associated with reduced levels of growth factors, such as brain-derived neurotrophic factor and epidermal growth factor, in CSF49 and plasma50.
Summary
In addition to α-synuclein, tau and amyloid pathologies, a number of other mechanisms, including different neurotransmitter systems, early synaptic changes, inflammation, and mitochondrial dysfunction, are likely to contribute to cognitive decline in PD. The roles of these as well as other potentially relevant mechanisms for cognitive impairment, such as the unfolded protein response51, the ubiquitin–proteasome system52, and increased neurogenesis (for example, in response to neurotrophic factors)53, need to be explored further (see Management section below).
Genetic contributions
The link between risk genes for cognitive impairment and decline in PD has been the subject of several detailed reviews2,21,54. BOX 2 provides a summary of genes examined to date, and the discussion below focuses on those with the largest bodies of evidence.
Box 2 | Potential genetic risk factors for cognitive impairment in PD.
GBA (glucosylceramidase)
Mutations are associated with greater cognitive impairment and risk of dementia in patients with Parkinson disease (PD)70–75.
MAPT (microtubule-associated protein tau)
Mixed findings for cognitive impairment: the H1 haplotype is associated with an increased risk of dementia and modulation of temporal-parietal activation, as measured by functional MRI (fMRI)8, 68, 79, 80, 84, 85, 198,199.
APOE (apolipoprotein E)
The APOE*ε4 allele was associated with cognition in most (but not all) studies: evidence of distinct word list learning and semantic verbal fluency deficits; mixed evidence for dementia risk; associated with modulation of temporal-parietal activation, as measured by fMRI68,78–81,84,200
LRRK2 (leucine-rich repeat serine/threonine-protein kinase 2)
No association in the majority of studies, but some larger studies reported a reduced prevalence of cognitive impairment and dementia in carriers of LRRK2 mutations55–63,201,202.
SNCA (α-synuclein)
One study found no association between the rs356219 polymorphism and cognition in sporadic PD, but multiplications and disease-causing mutations are associated with cognitive decline and dementia in monogenic PD64,65,68,203.
COMT (catechol O methyltransferase)
Mixed findings for cognition; some evidence that the Met allele is associated with impairment on frontal-dependent tasks; no evidence for dementia risk; associated with modulation of frontal networks7,11,79,80,84,204,205.
BDNF (brain-derived neurotrophic factor)
Mixed findings: one of three studies reported an association between cognitive impairment and the Gly196Ala polymorphism206–208.
UBQLN1 (ubiquilin-1)
No association with cognitive impairment209.
FMR1 (fragile X mental retardation protein 1)
No association with cognitive impairment210.
Immune/inflammatory genes: IL10, IL17A, IL-18, IFNG
Mixed findings: IL10 was associated with lower risk of cognitive impairment and IL17A was associated with greater risk in one study211, but not in a subsequent study212.
Genetic variants that cause monogenic forms of PD have been extensively investigated with respect to their effects on the clinical presentation of PD, including cognition and susceptibility to dementia. The LRRK2 Gly2019Ser substitution is the most commonly studied mutation, and has been shown in the majority of studies to either have no effect on cognition or to be protective55–63. However, the sample sizes of the studies were restricted by the rarity of LRRK2 mutations, so an association with cognitive impairment cannot be ruled out. Moreover, limited longitudinal follow-up, along with assessment tools that lack the sensitivity to detect subtle changes, might mask true associations. α-Synuclein gene (SNCA) mutations are another major cause of autosomal dominant PD and, in contrast to LRRK2 mutations, most seem to have deleterious effects on cognition, with the exception of duplications, which produce a clinical presentation similar to sporadic PD64–67.
Polymorphisms in SNCA also confer a risk of sporadic PD, but examination of one risk variant (rs356219) in a large study failed to find any evidence of a relationship with cognition68. However, the recent identification of a haplotype in intron 4 of the gene and its association with PDD raises the possibility that SNCA-related mechanisms are associated with cognition in sporadic PD, warranting additional studies in longitudinally assessed cohorts69.
Most other research in sporadic PD has largely focused on four candidate genes: glucosylceramidase (GBA), microtubule-associated protein tau (MAPT), apolipoprotein E (APOE) and catechol-O-methyltransferase (COMT). Of these, GBA has the strongest evidence base for association with cognitive measures: heterozygous mutations in this gene have been reliably shown to increase the risk of dementia70–74 and less severe impairments in frontal cognitive domains75. GBA is a highly polymorphic gene, and two large independent studies found that the type of mutation modulates the relationship with cognitive decline, furthering the possibility of disease subtyping on the basis of genetic status76,77.
The strong association between APOE and AD has led to extensive investigation of the relationship between AD risk alleles and cognition in PD. Tentative conclusions were drawn from a large meta-analysis showing a relationship with dementia78, and some68,79,80 — but not all81,82 — subsequent studies have reported effects on cognition, as measured by a variety of neuropsychological tests. These findings point to potential genetic overlap between AD and PD, a position strengthened by recent research leveraging PD genome-wide association study data, which found evidence of genetic pleiotropy at the MAPT locus83. Whether these findings provide a mechanistic explanation specifically for cognitive impairment in PD remains to be fully established.
Regional and cognitive domain-specific alterations in cortical activation associated with MAPT, APOE and COMT variants have been reported in two neuroimaging studies84,85. These findings complement those from neuropsychological studies, and might also go some way towards explaining the inconsistent results. In particular, although neuropsychological associations may not always be detected owing to low statistical power or lack of sensitivity of the measures used, evidence is accumulating that genetic variation drives mechanistic alterations that are consistent with different patterns of cognitive deficits. These findings warrant clinical studies with larger sample sizes, conducted with cognitive batteries specific to the neural correlates of the genes of interest.
Despite some compelling findings, experiments designed to examine the effects of single genes can only capture a small proportion of the variance of complex traits such as cognition. Genome-wide association studies go some way towards addressing this issue by simultaneously genotyping markers across the genome. One such study conducted in PD cognition found no genome-wide significant hits but, with only 443 cases, was underpowered86. The application of genome-wide methods in other diseases across neurology and psychiatry has generated promising findings83,87, but much larger data sets are needed in PD. The availability of such samples — and, consequently, the potential to conduct more powerful genetic analyses — is increasing.
Biomarkers of cognitive decline in PD
Cerebrospinal fluid biomarkers
The variety of CSF-based markers in PD, and the biological factors that influence their levels in the CSF, was recently reviewed88. The most commonly studied markers have been the AD markers Aβ42 (a marker of Aβ pathology), total tau (t-tau, a marker of neurodegeneration) and phosphorylated tau (p-tau, a marker of tau pathology), and the PD marker α-synuclein. In a comprehensive review of biomarkers and cognition in PD, Lin and Wu89 identified 18 CSF studies. The most consistent finding was an association between reduced CSF Aβ42 levels and cognitive impairment, which was reported in 14 of 15 studies. By contrast, findings for t-tau and p-tau were inconsistent, with some studies reporting an association between increased CSF levels and cognitive impairment, but most reporting no associations. Subsequently, several more studies were published, including longitudinal studies reporting an association between CSF markers and future cognitive decline (TABLE 2) — a particularly relevant question for clinicians. In AD, CSF markers can accurately predict which patients with MCI will develop AD dementia in the near future, and CSF markers to predict accelerated cognitive decline in PD are an important priority. Two cross-sectional studies reported an association between low CSF Aβ42 levels and cognitive impairment in PD90,91. In addition, four longitudinal studies reported that reduced Aβ42 predicted more rapid cognitive decline92–95, confirming previous findings96–98 (TABLE 2). DeNoPa, a multimodal biomarker study in de novo PD99, found no link between PD progression and CSF markers, but the results could have been confounded by the low number of participants agreeing to serial CSF sampling, variations in CSF sample preparation, and the demographics of the cohort.
Table 2.
Predictive effects of CSF markers on cognitive decline in PD: longitudinal studies
| Study | N | Baseline MMSE score |
Disease duration (years) |
Duration of follow-up (years) |
CSF markers studied (in addition to total tau, phosphorylated tau and Aβ42) |
Outcome measures | Results | Refs |
|---|---|---|---|---|---|---|---|---|
| Compta, Y. et al. | 27 | 28 | 10 | 1.5 | – | Dementia | Low Aβ42 predicted dementia | 97 |
| Siderowf, A. et al. | 45 | DRS score: 133 | 11 | 1.5 | – | DRS score | Low Aβ42 predicted more rapid decline on DRS | 96 |
| Parnetti, L. et al. | 44 | 27 | 3 | 3 | α-Synuclein (total, oligomer) | MMSE and Montreal Cognitive Assessment score | Low Aβ42 predicted more rapid decline | 98 |
| Alves, G. et al. | 104 | 28 | De novo | 5 | – | Dementia | Low Aβ predicted early dementia | 94 |
| Bäckström, D. C. et al. | 99 | 29 | 1.4 | 5–9 | α-Synuclein total, NFL, H-FABP | Dementia | Low Aβ42, NFL and H-FABP predicted dementia | 92 |
| Hall, S. et al. | 42 | 29 | 7 | 2 | α-Synuclein total, NFL | Dementia | Low Aβ predicted memory decline, high α-synuclein predicted reduced cognitive speed | 93 |
| Terrelonge, M. et al. | 341 | – | 0.6 | 2 | α-Synuclein total | Four domains: memory, visuospatial, working memory–executive function, and attention processing speed | Low Aβ42 predicted cognitive impairment at follow-up | 95 |
| Stewart, T. et al. | 403 | 28.9 | 2.1 | 1.8 | α-Synuclein total | Tests of verbal memory, cognitive processing speed, and visuospatial working memory | Lower α-synuclein predicted better preservation of cognitive function | 101 |
Aβ, amyloid-β; CSF, cerebrospinal fluid; DRS, Dementia Rating Scale; H-FABP, fatty acid-binding protein, heart; MMSE, Mini-Mental State Examination; N, number of participants; NFL, neurofilament light chain protein; PD, Parkinson disease.
Several studies of CSF levels of total α-synuclein have been published. Most reported low values in PD, as confirmed by a recent meta-analysis100, but the association with cognition continues to be inconsistent, with both low90 and high93,101 values found to be associated with cognitive decline in different studies. The discrepant findings might be explained by differences in disease stage. CSF α-synuclein levels may increase with disease stage, and the association between high levels and cognitive impairment was found in more advanced disease but not early disease stages. In one study of CSF biomarker change with time, Hall et al.102 found that levels of t-tau, p-tau, total α-synuclein, neurofilament and YKL-40 (a marker of inflammation), but not Aβ42, increased over a 2-year period and were associated with worsening cognition, and the increase was particularly marked in patients with longer disease duration. These findings are consistent with reports that α-synuclein levels increase with more severe neurodegeneration. However, the DeNoPa study reported no change in α-synuclein or other CSF markers during a 24-month study period99. In addition to total α-synuclein, post-translationally modified forms of α-synuclein, including phosphorylated, nitrated and ubiquitylated forms, as well as oligomers, have been identified, and show some promise as markers of disease progression in PD103–105, but further research is needed to confirm their diagnostic and prognostic utility as markers for cognitive impairment in PD.
Neuroimaging
Over the past decade, structural, functional and molecular neuroimaging techniques such as MRI106 and PET107–109 have considerably advanced our understanding of the complex mechanisms underlying the development of cognitive impairment in PD106,109. Two large, ongoing 5-year observational biomarker studies, PPMI and COPPADIS, are using a range of imaging assessments, and are expected to yield important information on the utility of MRI to detect brain pathology in PD patients with cognitive impairment110. In such patients, PET imaging has provided in vivo evidence for the interplay between several pathological processes, including degeneration of subcortical cholinergic and dopaminergic projections, microglial activation, and neocortical pathology associated with misfolded protein deposition or vascular pathology45,107,111. Also, functional polymorphisms in the COMT gene, which influence dopamine storage, might contribute to cognitive deficits in PD112.
MRI measures of cortical and subcortical volume loss
Structural MRI can localize differences in regional cortical and subcortical tissue volume between groups of individuals. In PD patients without a formal diagnosis of PD-MCI or PDD, loss of tissue volume in frontal and parietal cortices has been associated with worse performance in decision-making, facial expression recognition, visual memory and executive function113–115. Studies in patients with PD-MCI have demonstrated a pattern of cortical volume loss in posterior, parietal and frontal cortices, and atrophy in the hippocampus, that correlates with memory deficits116–118. Longitudinal assessments of cortical thickness and subcortical volumes in patients with PD-MCI have indicated progression of cortical thinning in temporal, occipital, parietal and frontal cortices, and further loss of hippocampal volume that is associated with cognitive decline117,119. Retrospective analysis of structural MRI scans has demonstrated that in PD patients without cognitive impairment, this technique can predict conversion to PD-MCI status at 6 months on the basis of baseline hippocampal atrophy120, at 18 months on the basis of baseline temporal cortex thinning117, and at 2 years on the basis of baseline prefrontal thinning, and insular and caudate volume loss121. At the time when a PDD diagnosis is established, cortical thinning becomes more severe in parietal, occipital, temporal and frontal cortices, and the volume loss in the hippocampus is substantial, including atrophy of the parahippocampus, insula and cingulate gyrus, which is associated with further decline of cognitive functions122–125. The pattern of cortical thinning has also been suggested to differentiate PDD, which is characterized by predominant frontal cortex thinning, from DLB, which is characterized by predominant thinning of the parietal and occipital cortices126.
MRI-based structural and functional connectivity
Diffusion tensor imaging measures the magnitude (mean diffusivity) and direction (fractional anisotropy) of water molecule flow in brain tissue. Neurodegenerative disorders are characterized by damage to white matter tracts (structural connectivity) that results in increased mean diffusivity and decreased fractional anisotropy. In PD patients without a formal diagnosis of PD-MCI or PDD, increased mean diffusivity in the hippocampus, and in frontal and parietal white matter tracts, is associated with worse performance in terms of verbal and visuospatial memory, semantic fluency and other executive functions115,127–129. Also, decreased hippocampal fractional anisotropy correlates with measures of global cognitive decline129.
Resting state functional MRI (fMRI) measures blood-oxygen-level dependent (BOLD) signal in the brain without an externally prompted task, and is useful to explore the functional organization of networks (functional connectivity) in the human brain. PD patients without a formal diagnosis of PD-MCI or PDD exhibited progressive loss of resting state functional connectivity over a period of 3 years in multiple brain regions — especially the posterior parts of the brain — which correlated with decreasing cognitive per-formance130. Studies have demonstrated that cognitive decline in PDD is associated with disruption of corticostriatal and frontal cortex functional connectivity131,132. As seen with the pattern of cortical thinning on structural MRI, the pattern of functional connectivity disruption can differentiate PDD, which is characterized by predominant frontal cortex disruption, from DLB, which is characterized by predominant disruption of parietal and occipital cortices126. Further studies are needed to replicate these findings, and specifically to pinpoint the structural and functional brain networks that underlie cognitive decline in patients with PD.
Perfusion imaging
Arterial spin labelling (ASL) is an MRI-based technique that allows quantification of altered cerebral blood flow. ASL studies have demonstrated patterns of hypoperfusion in patients with PDD compared with cognitively normal PD patients133. The pattern of brain hypoperfusion in PDD largely overlaps with the hypoperfusion observed in the posterior cingulategyrus, precuneus and occipital regions in patients with AD, but differs in the temporal lobes (AD < PDD) and right frontal cortex (PDD < AD)134. ASL is a promising technique that merits additional study in patients with PD and cognitive impairment.
Dopaminergic molecular imaging
Studies involving dopamine decarboxylase and vesicular monoamine transporter 2 PET imaging have demonstrated decline in regional mesolimbic and mesocortical monoaminergic capacity in patients with PDD135,136. These patients show only subtle loss of monoaminergic capacity in the anterior cingulate, ventral striatum and caudate, which can be detected at a single-voxel level135. Visual or semi-quantitative assessment of dopamine transporters by PET or single-photon emission CT (SPECT) imaging reveals nigrostriatal degeneration in patients with PDD, and reduced caudate dopamine transporter uptake has been associated with impairment of executive functions137,138. However, dopaminergic molecular imaging can detect only subtle differences between PDD and PD, and no differences between PDD and DLB have been reported to date.
PET imaging of glucose metabolism
Studies using PET to image glucose metabolism have demonstrated metabolic decreases in the parietal, temporal, cingulate and frontal cortices in PD-MCI and PDD139–141. PDD and DLB are characterized by similar patterns of glucose metabolic changes142. When PDD and AD were compared, however, patients with PDD showed greater metabolic reduction in the visual cortex and relative preservation of metabolism in the medial temporal cortex139,140,142. The use of more sophisticated PET analysis has allowed the identification of PD-related metabolic brain networks underlying cognitive dysfunction in PD, including smaller areas, detected at the single-voxel level, in the occipital, parietal and frontal cortices143. However, even with advanced methods, discrimination between PDD and DLB has been difficult in view of the aforementioned similarities in metabolic network patterns, with the exception that metabolic decrease in the anterior cingulate is greater in patients with DLB than in those with PDD144.
Cholinergic PET molecular imaging
The cholinergic system has a key role in functional and structural remodelling of the cortical circuits that underlie cognitive processing145. Cholinergic PET imaging studies seem to be consistent with postmortem evidence suggesting that loss of cholinergic function, in the forebrain, is associated with the manifestation of PDD36,146–148. PET molecular imaging of acetylcholinesterase (AChE) activity has indicated that cholinergic denervation of the cerebral cortex is an early phenomenon in the course of PD, and is more widespread and profound in patients with PDD36,146. AChE PET imaging studies have demonstrated mean cortical AChE loss of 20–30% in patients with PDD, compared with 11–13% in patients with PD36,147–149. In PDD, loss of cholinergic function is evident in the temporal, frontal and medial occipital cortices, and in the thalamus36,147,150. A similar pattern is seen in DLB136,150, and to a lesser extent in AD148; however, thalamic cholinergic denervation is not observed in AD150. Cholinergic PET might also be sensitive to subclinical dementia. In PD patients without a diagnosis of cognitive impairment, lower cortical AChE PET activity was associated with reduced cognitive performance scores for attention, memory and executive functions145,148,151. A number of molecular targets related to the cholinergic system can be evaluated with PET, and further studies are needed to understand cholinergic system involvement in the development of cognitive impairment in PD.
Amyloid-β and tau PET in PDD
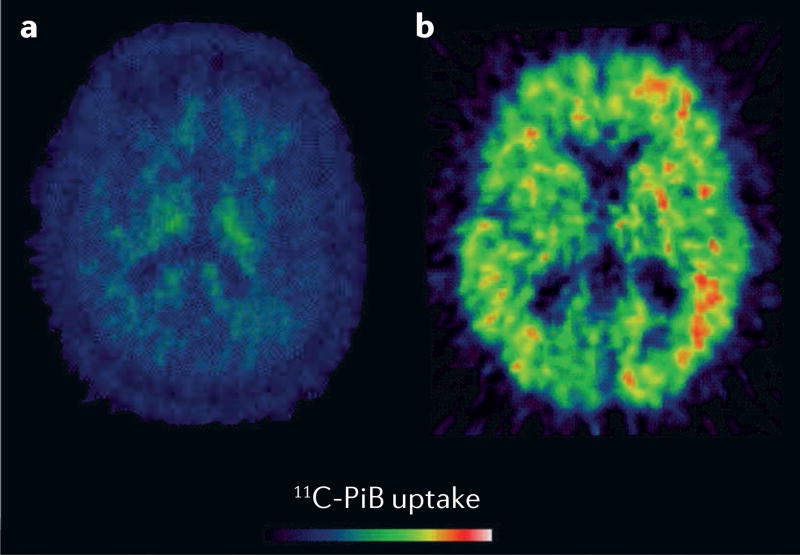
PET imaging of Aβ plaques in vivo has demonstrated that most AD cases and a subset of DLB cases show increased cortical Aβ retention152. Aβ PET imaging (FIG. 1) indicates that cortical and striatal Aβ pathology is relatively infrequent (incidence 15–20%) in patients with PDD152–155, but is sometimes uncovered by histopathology at post mortem156. Compared with cortical β-amyloidopathy alone, the combined presence of striatal and cortical Aβ plaques was associated with worse cognitive impairment in PD157. Aβ retention in patients with PD-MCI predicted an increased risk of future cognitive decline158.
Figure 1. Amyloid-β PET scans in Parkinson disease dementia.
Axial slices from two patients with Parkinson disease dementia, showing a | absence and b | presence of amyloid-β binding. 11C-PiB, 11C-labelled Pittsburgh compound B.
A recent tau PET study has shown increased signal in the inferior temporal gyrus and precuneus in patients with PPD, which was associated with heightened cognitive impairment. These findings suggest that tau cortical aggregates are present in patients with PDD, and that tau PET data could be a marker of cognitive impairment159.
As regards future research, application of novel neuroimaging techniques, including molecular imaging of α-synuclein, and of mitochondrial and non-TSPO (translocator protein) neuroinflammatory targets, could enhance our understanding of the mechanisms underlying cognitive impairment in PD, and accelerate the development of novel therapeutics.
Electrophysiology
Several studies, using a range of EEG and cognitive measures, have reported associations between slowing of the EEG in PD and cognitive deficits. The findings include associations between executive dysfunction160 or Mini-Mental State Examination (MMSE) score161 and spectral ratio (the ratio of fast to slow activity), between performance deficits in attention, executive function and memory and increased delta power162 or decreased EEG median frequency163, and between clinical ratings of background EEG slowing and combined MMSE and clock drawing test scores164. An increase in low-frequency (delta and theta) EEG spectral power distinguishes PDD from PD and AD165.
Indices of EEG slowing are predictive of the transition to dementia at 5 years (HR 13)166. Peak frequency magnetoencephalogram (MEG) slowing is also predictive of the transition to dementia (HR 3.9 at 7 years)130; however, unlike in EEG studies, greater MEG predictive power was found at higher, beta band frequencies (HR 5.2). Interestingly, combining MEG and cognitive markers improved the prognostic power over and above the single markers, with a very high hazard ratio (>27)130. A recent review of evoked potential studies in PD has suggested that a delayed (but not decreased-amplitude) P3b component in classic oddball tasks is a trait-like marker of PD cognitive impairment and dementia167. The review also noted that the mismatch negativity component of pre-attentive deviance detection tasks might be reduced more in PDD than in AD or DLB167.
Combination of biomarkers
As described above, the mechanisms underlying cognitive decline in PD are heterogeneous and, thus, the various biomarker modes show different yet connected brain changes. Creation of multiple models, by combining different types of markers, may provide complementary information and improve the prognostic accuracy for cognitive decline in PD. CSF or blood-based markers can inform the biochemical changes underlying volume reductions identified in imaging studies, as shown with some success in AD168.
Compta and colleagues reported interesting associations between structural imaging and CSF protein changes. In a cross-sectional study, high t-tau and p-tau and low Aβ42 values were associated with reduced grey matter volume in patients with PDD, but not in PD and non-PD controls169. Similar findings were reported by a different group170. In a subsequent longitudinal study, low CSF Aβ42 was associated with cortical thinning, and a combination of biomarker pathologies was a stronger predictor of cognitive decline and dementia than was a single biomarker pathology97. Finally, low CSF α-synuclein was associated with frontal cortical thinning in PD patients without dementia and in a group with idiopathic RBD, whereas in patients with PDD, cortical atrophy was associated with increased total CSF α-synuclein and t-tau89.
In two studies combining CSF biomarkers and SPECT striatal dopamine transporter uptake in early PD, reduced striatal uptake was associated with low CSF levels of t-tau and p-tau171 but not α-synuclein172. In an fMRI study, activity in motor-related networks correlated with CSF total α-synuclein, indicating that α-synuclein contributes to motor-related resting state networks in PD. In PD patients without dementia, CSF α-synuclein was associated with the dorsal attentional network, suggesting that α-synuclein is also involved in cognition. No associations between resting state fMRI networks and CSF Aβ were found173.
Taken together, these studies support the hypothesis that the cognition-related volume loss in PD is related to three key pathologies: α-synuclein, tau and amyloid. This idea is consistent with postmortem studies demonstrating that amyloid, tau and α-synuclein pathologies contribute to neuronal loss and cognitive decline from relatively early disease stages.
Management
Antidementia drugs for PDD
Robust evidence is available to support the use of cholinesterase inhibitors to treat dementia in PD174. The best evidence came from the EXPRESS study of rivastigmine175, and supportive data for donepezil were reported in the EDON study176. Memantine was tested in two medium-sized studies including both PDD and DLB cohorts177,178; only one of these studies found an effect in the PDD group177. Effect sizes were relatively small; for example, the difference in MMSE scores between patients treated with rivastigmine and placebo was 1.0 (REF. 175), with an SD of 3.8 in the treated group, indicating a Cohen’s d effect size between 0.25 and 0.30. A meta-analysis174 indicated that antidementia drugs did not increase mortality, and the drop-out rates did not differ between donepezil, rivastigmine, memantine and placebo groups. Compared with placebo, rivastigmine — but not donepezil or memantine — was associated with an increased incidence of adverse events. Preservation of frontoparietal and default mode cholinergic networks was recently reported to be associated with a positive response to cholinergic agents179, and might enable the identification of patients who are likely to benefit from this treatment.
Potential treatments for PD-MCI
As yet, no disease-modifying treatments with effects on cognition in PD are available, and there is no robust evidence that progression to dementia can be impeded. Epidemiological studies indicate that green tea and coffee can influence the risk of PD and, thus, a potential preventive role has been explored, although the effects on cognitive decline are unclear180,181.
In the first trial in PD-MCI, rivastigmine patch offered some benefit, although the primary outcome measure — clinical impression of change — was not significantly affected182. However, significant effects were observed on one of the secondary outcomes, a scale measuring everyday cognitive activities. Of note, the study had a crossover design, and included only 26 patients. Further studies of rivastigmine or other cholinesterase inhibitors for PD-MCI are warranted.
Theoretical and preliminary empirical evidence supports the hypothesis that rasagiline, a selective monoamine oxidase B inhibitor with effects on motor symptoms in PD, also has a positive cognitive effect. In a relatively large, placebo-controlled, randomized trial over 24 weeks, however, rasagiline did not significantly improve cognitive functioning in patients with PD-MCI183.
In a study focusing on depression, atomoxetine, a selective noradrenaline reuptake inhibitor, was associated with some cognitive benefit in patients with PD184. On a relatively insensitive screening instrument such as the MMSE, the placebo group worsened by 0.5 points whereas the atomoxetine group improved by 0.8 points, a statistically significant difference. Further support for the use of atomoxetine or other noradrenergic agents was provided in a single-dose, placebo-controlled study, which showed that 40 mg atomoxetine improved selected tests of decision-making and attention in PD185. We are not aware of any systematic studies of serotonergic agents in the context of cognition in PD, but vortioxetine, a novel agent that acts on a number of serotonergic receptors in addition to inhibiting serotonin reuptake, has produced cognitive improvement in elderly patients with depression186 and is a candidate for trials in PD. Various studies are ongoing, including trials of 5-HT6 receptor antagonists and donepezil for PD-MCI. 5-HT6 receptor antagonists might exert effects on cognition and mood owing to enhancement of cholinergic, glutamatergic, noradrenergic and dopaminergic neurotransmission, and the SYNAPSE study is exploring the effects of these agents in PDD.
Disease-modifying agents that slow disease progression in PD, including delaying the onset of dementia, are an urgent unmet need. The most interesting approaches include passive and active immunotherapies targeting Aβ, tau and α-synuclein, which might be particularly relevant for diseases such as PD that are characterized by accumulation and prion-like propagation of toxic protein aggregates187. Other potential strategies include drugs addressing mitochondrial dysfunction, anti-inflammatory agents, GBA-active agents, stimulation of neurogenesis, and neurotrophic factors53. As amyloid pathology has a prominent role in dementia development in PD, strategies to reduce Aβ toxicity — for example, by influencing clusterin-associated pathways188 — might be relevant in this context. Interestingly, apomorphine, which is used to treat motor complications in PD, has been shown in animal studies to reduce Aβ accumulation and toxicity, possibly via antioxidative mechanisms189. In a recent postmortem study, PD patients without dementia who had received apomorphine treatment showed reduced Aβ deposition compared with untreated patients190, and in a comparative open-label trial, apomorphine infusion was associated with improvement of several NMS191. Thus, placebo-controlled clinical trials to test whether apomorphine can delay dementia onset in PD are warranted.
Non-pharmacological approaches
Cognitive training
Studies employing systematic cognitive training to improve cognition have been conducted in AD and, more recently, in PD. A review and meta-analysis192 based on seven studies involving 272 patients with PD (MMSE scores 27–29) showed small but statistically significant improvements in working memory, processing speed and executive functioning after cognitive training. Large multicentre studies are needed to confirm these encouraging findings.
Physical exercise
Aerobic physical exercise has a range of beneficial effects on the brain, with potential relevance to cognition. A review based on seven studies concluded that physical exercise can improve motor symptoms and several NMS in PD193, possibly by improving perfusion, or by promoting growth hormone release or angiogenesis. In one relatively large study, 51 PD patients without dementia were randomly assigned to a progressive strengthening exercise training programme or to a simpler programme focusing on stretching, balance, breathing and non-progressive strengthening, administered twice a week for 24 months194. No differences between the groups were observed, although both groups showed evidence of improvement of attention and working memory after 24 months. Larger studies with carefully designed control conditions are needed to test whether physical exercise can improve cognition in PD.
Neurostimulation
Deep brain stimulation of the subthalamic nucleus is an established practice in PD. However, cognition might be adversely affected in the immediate postoperative period and beyond, with accelerated decline of executive functions being reported195. By contrast, there is some preliminary evidence of positive cognitive effects after stimulation of the cholinergic nucleus basalis of Meynert in patients with AD196. Given the probable contribution of cholinergic deficits to cognitive impairment in PD, this approach might be relevant in the PD context197, and studies are ongoing.
Conclusions
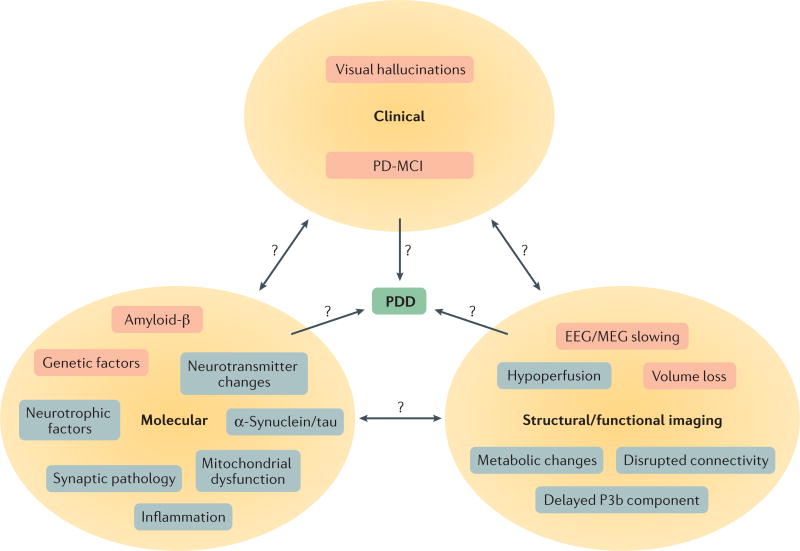
In addition to the well-known high risk of dementia in the later stages of PD, recent research has established the high frequencies of SCD and MCI as harbingers of dementia in PD. Cognitive impairment might occur very early in the disease course, even before the onset of motor symptoms in the prodromal state. A variety of mechanisms contributing to cognitive decline in PD are gradually being revealed: in addition to α-synuclein toxicity, the potential contributions of other pathologies, mitochondrial disturbances, inflammatory changes and genetic factors are currently under investigation (FIG. 2). Related to these changes, a number of imaging, electrophysiological and CSF-based bio-markers have already been shown to be associated with cognitive impairment in PD, and studies consistently show that reduced CSF Aβ42 concentrations predict more rapid cognitive decline. However, following the demonstration of efficacy of a cholinesterase inhibitor for the treatment of PDD more than 10 years ago, no additional convincing evidence has emerged regarding the treatment or prevention of PD-MCI and PDD. Therapies for PDD remain a key avenue for future investigations, and several promising candidates are now being explored.
Figure 2. Overview of risk factors for Parkinson disease dementia.
Three different categories of risk factors for Parkinson disease dementia (PDD) — clinical, molecular, and structural/functional imaging — are illustrated. Factors with evidence from longitudinal studies are shaded pink, and factors with evidence from cross-sectional studies are shaded grey. Interrelationships between factor categories (bidirectional arrows) and temporal relationships between cross-sectional factors and PDD (unidirectional arrows) remain unclear. MCI, mild cognitive impairment; MEG, magnetoencephalogram.
Key points.
The full spectrum of cognitive impairment, from subjective cognitive decline to dementia, has been observed in Parkinson disease (PD)
Mild cognitive impairment in PD usually progresses to dementia, but can be stable and even revert in some patients
The aetiology of cognitive impairment in PD has not been fully elucidated, but limbic and cortical Lewy body pathology seems to be the main cause
Amyloid plaque pathology also contributes to cognitive decline in PD, and amyloid pathology detected by cerebrospinal fluid analysis and imaging can predict subsequent dementia
Other probable mechanisms include genetics, synaptic pathology, neurotransmitter changes and inflammation
Cholinesterase inhibitors have symptomatic effects, but no disease-modifying treatments are available to reduce the risk of dementia in PD
Acknowledgments
The authors thank Dr Michael Haworth for help in preparing the final manuscript. The authors would like to thank the National Institute for Health Research (NIHR) Mental Health Biomedical Research Centre and Dementia Unit at South London and Maudsley NHS Foundation Trust and Institute of Psychiatry, Psychology and Neuroscience, King’s College London, UK. D.A. is a Royal Society Wolfson Research Merit Award Holder and would like to thank the Wolfson Foundation and the Royal Society for their support. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
D.A. has received research support and/or honoraria from Astra-Zeneca, H. Lundbeck, Novartis Pharmaceuticals and GE Health, and serves as a paid consultant for H. Lundbeck and Axovant. K.R.C. has consulted and served on advisory boards for Britannia, AbbVie, Neuronova, Mundipharma and UCB, and has also served on advisory boards for Synapsus and Medtronic. He has received honoraria from Boehringer Ingelheim, GlaxoSmithKline, AbbVie, Britannia, UCB, Mundipharma, Otsuka and Zambon, and grants from Boehringer Ingelheim, GlaxoSmithKline, Britannia, AbbVie, UCB and Neuronova. He holds intellectual property rights for the KPP scale and the PDSS, and receives royalties for the books Non-Motor Symptoms of Parkinson’s Disease and Fastfacts: Parkinson’s Disease. C.B. declares grants and personal fees from Lundbeck and Acadia, and personal fees from Roche, Orion, GlaxoSmithKline, Otusaka, Heptares and Lilly.
Footnotes
Competing interests statement
The other authors declare no competing interests.
References
- 1.Sauerbier A, Jenner P, Todorova A, Chaudhuri KR. Non motor subtypes and Parkinson’s disease. Parkinsonism Relat. Disord. 2016;22(Suppl. 1):S41–S46. doi: 10.1016/j.parkreldis.2015.09.027. [DOI] [PubMed] [Google Scholar]
- 2.Svenningsson P, Westman E, Ballard C, Aarsland D. Cognitive impairment in patients with Parkinson’s disease: diagnosis, biomarkers, and treatment. Lancet Neurol. 2012;11:697–707. doi: 10.1016/S1474-4422(12)70152-7. [DOI] [PubMed] [Google Scholar]
- 3.Erro R, et al. Do subjective memory complaints herald the onset of mild cognitive impairment in Parkinson disease? J. Geriatr. Psychiatry Neurol. 2014;27:276–281. doi: 10.1177/0891988714532015. [DOI] [PubMed] [Google Scholar]
- 4.Emre M, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov. Disord. 2007;22:1689–1707. doi: 10.1002/mds.21507. [DOI] [PubMed] [Google Scholar]
- 5.Litvan I, et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov. Disord. 2012;27:349–356. doi: 10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pedersen KF, Larsen JP, Tysnes OB, Alves G. Prognosis of mild cognitive impairment in early Parkinson disease: the Norwegian ParkWest study. JAMA Neurol. 2013;70:580–586. doi: 10.1001/jamaneurol.2013.2110. [DOI] [PubMed] [Google Scholar]
- 7.Williams-Gray CH, et al. The distinct cognitive syndromes of Parkinson’s disease: 5 year follow-up of the CamPaIGN cohort. Brain. 2009;132:2958–2969. doi: 10.1093/brain/awp245. [DOI] [PubMed] [Google Scholar]
- 8.Williams-Gray CH, et al. The CamPaIGN study of Parkinson’s disease: 10-year outlook in an incident population-based cohort. J. Neurol. Neurosurg. Psychiatry. 2013;84:1258–1264. doi: 10.1136/jnnp-2013-305277. [DOI] [PubMed] [Google Scholar]
- 9.Santangelo G, et al. Mild cognitive impairment in newly diagnosed Parkinson’s disease: a longitudinal prospective study. Parkinsonism Relat. Disord. 2015;21:1219–1226. doi: 10.1016/j.parkreldis.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 10.Pigott K, et al. Longitudinal study of normal cognition in Parkinson disease. Neurology. 2015;85:1276–1282. doi: 10.1212/WNL.0000000000002001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams-Gray CH, Hampshire A, Robbins TW, Owen AM, Barker RA. Catechol O-methyltransferase Val1 58 Met genotype influences frontoparietal activity during planning in patients with Parkinson’s disease. J. Neurosci. 2007;27:4832–4838. doi: 10.1523/JNEUROSCI.0774-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kehagia AA, Barker RA, Robbins TW. Cognitive impairment in Parkinson’s disease: the dual syndrome hypothesis. Neurodegener. Dis. 2013;11:79–92. doi: 10.1159/000341998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wood K-L, et al. Different PD-MCI criteria and risk of dementia in Parkinson’s disease: 4-year longitudinal study. NPJ Parkinsons Dis. 2016;2:15027. doi: 10.1038/npjparkd.2015.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chahine LM, et al. Cognition in individuals at risk for Parkinson’s: Parkinson associated risk syndrome (PARS) study findings. Mov. Disord. 2016;31:86–94. doi: 10.1002/mds.26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Postuma RB, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015;30:1591–1601. doi: 10.1002/mds.26424. [DOI] [PubMed] [Google Scholar]
- 16.Marras C, Chaudhuri KR. Nonmotor features of Parkinson’s disease subtypes. Mov. Disord. 2016;31:1095–1102. doi: 10.1002/mds.26510. [DOI] [PubMed] [Google Scholar]
- 17.Chaudhuri KR, Sauerbier A. Parkinson disease: unravelling the nonmotor mysteries of Parkinson disease. Nat. Rev. Neurol. 2016;12:10–11. doi: 10.1038/nrneurol.2015.236. [DOI] [PubMed] [Google Scholar]
- 18.Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sorensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch. Neurol. 2003;60:387–392. doi: 10.1001/archneur.60.3.387. [DOI] [PubMed] [Google Scholar]
- 19.Anang JB, et al. Predictors of dementia in Parkinson disease: a prospective cohort study. Neurology. 2014;83:1253–1260. doi: 10.1212/WNL.0000000000000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ffytche DH, et al. Risk factors for early psychosis in PD: insights from the Parkinson’s Progression Markers Initiative. J. Neurol. Neurosurg. Psychiatry. doi: 10.1136/jnnp-2016-314832. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halliday GM, Leverenz JB, Schneider JS, Adler CH. The neurobiological basis of cognitive impairment in Parkinson’s disease. Mov. Disord. 2014;29:634–650. doi: 10.1002/mds.25857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braak H, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 23.Compta Y, et al. Lewy- and Alzheimer-type pathologies in Parkinson’s disease dementia: which is more important? Brain. 2011;134:1493–1505. doi: 10.1093/brain/awr031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howlett DR, et al. Regional multiple pathology scores are associated with cognitive decline in Lewy body dementias. Brain Pathol. 2015;25:401–408. doi: 10.1111/bpa.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irwin DJ, Lee VM, Trojanowski JQ. Parkinson’s disease dementia: convergence of α-synuclein, tau and amyloid-β pathologies. Nat. Rev. Neurosci. 2013;14:626–636. doi: 10.1038/nrn3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ballard C, et al. Differences in neuropathologic characteristics across the Lewy body dementia spectrum. Neurology. 2006;67:1931–1934. doi: 10.1212/01.wnl.0000249130.63615.cc. [DOI] [PubMed] [Google Scholar]
- 27.Terry RD, et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 28.Pienaar IS, Burn D, Morris C, Dexter D. Synaptic protein alterations in Parkinson’s disease. Mol. Neurobiol. 2012;45:126–143. doi: 10.1007/s12035-011-8226-9. [DOI] [PubMed] [Google Scholar]
- 29.Bellucci A, et al. Review: Parkinson’s disease: from synaptic loss to connectome dysfunction. Neuropathol. Appl. Neurobiol. 2016;42:77–94. doi: 10.1111/nan.12297. [DOI] [PubMed] [Google Scholar]
- 30.Schulz-Schaeffer WJ. The synaptic pathology of α-synuclein aggregation in dementia with Lewy bodies, Parkinson’s disease and Parkinson’s disease dementia. Acta Neuropathol. 2010;120:131–143. doi: 10.1007/s00401-010-0711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitfield DR, et al. Assessment of ZnT3 and PSD95 protein levels in Lewy body dementias and Alzheimer’s disease: association with cognitive impairment. Neurobiol. Aging. 2014;35:2836–2844. doi: 10.1016/j.neurobiolaging.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 32.Bereczki E, et al. Synaptic proteins predict cognitive decline in Alzheimer’s disease and Lewy body dementia. Alzheimers Dement. 2016;12:1149–1158. doi: 10.1016/j.jalz.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 33.Wellington H, et al. Increased CSF neurogranin concentration is specific to Alzheimer disease. Neurology. 2016;86:829–835. doi: 10.1212/WNL.0000000000002423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bereczki E, et al. Synaptic proteins in CSF relate to Parkinson`s disease stage markers. NPJ Parkinsons Dis. 2017;3:7. doi: 10.1038/s41531-017-0008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kulisevsky J. Role of dopamine in learning and memory: implications for the treatment of cognitive dysfunction in patients with Parkinson’s disease. Drugs Aging. 2000;16:365–379. doi: 10.2165/00002512-200016050-00006. [DOI] [PubMed] [Google Scholar]
- 36.Shimada H, et al. Mapping of brain acetylcholinesterase alterations in Lewy body disease by PET. Neurology. 2009;73:273–278. doi: 10.1212/WNL.0b013e3181ab2b58. [DOI] [PubMed] [Google Scholar]
- 37.Ehrt U, Broich K, Larsen JP, Ballard C, Aarsland D. Use of drugs with anticholinergic effect and impact on cognition in Parkinson’s disease: a cohort study. J. Neurol. Neurosurg. Psychiatry. 2010;81:160–165. doi: 10.1136/jnnp.2009.186239. [DOI] [PubMed] [Google Scholar]
- 38.Ye Z, et al. Predicting beneficial effects of atomoxetine and citalopram on response inhibition in Parkinson’s disease with clinical and neuroimaging measures. Hum. Brain Mapp. 2016;37:1026–1037. doi: 10.1002/hbm.23087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Varrone A, et al. 5-HT1B receptor imaging and cognition: a positron emission tomography study in control subjects and Parkinson’s disease patients. Synapse. 2015;69:365–374. doi: 10.1002/syn.21823. [DOI] [PubMed] [Google Scholar]
- 40.Vorovenci RJ, Antonini A. The efficacy of oral adenosine A2A antagonist istradefylline for the treatment of moderate to severe Parkinson’s disease. Expert Rev. Neurother. 2015;15:1383–1390. doi: 10.1586/14737175.2015.1113131. [DOI] [PubMed] [Google Scholar]
- 41.Ko WK, et al. An evaluation of istradefylline treatment on Parkinsonian motor and cognitive deficits in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridi ne (MPTP)-treated macaque models. Neuropharmacology. 2016;110:48–58. doi: 10.1016/j.neuropharm.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 42.Gatt AP, et al. Dementia in Parkinson’s disease is associated with enhanced mitochondrial complex I deficiency. Mov. Disord. 2016;31:352–359. doi: 10.1002/mds.26513. [DOI] [PubMed] [Google Scholar]
- 43.Cagin U, et al. Mitochondrial retrograde signaling regulates neuronal function. Proc. Natl Acad. Sci. USA. 2015;112:E6000–E6009. doi: 10.1073/pnas.1505036112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rocha NP, et al. Plasma levels of soluble tumor necrosis factor receptors are associated with cognitive performance in Parkinson’s disease. Mov. Disord. 2014;29:527–531. doi: 10.1002/mds.25752. [DOI] [PubMed] [Google Scholar]
- 45.Fan Z, et al. Influence of microglial activation on neuronal function in Alzheimer’s and Parkinson’s disease dementia. Alzheimers Dement. 2015;11:608–621. doi: 10.1016/j.jalz.2014.06.016. e7. [DOI] [PubMed] [Google Scholar]
- 46.Lindqvist D, et al. Cerebrospinal fluid inflammatory markers in Parkinson’s disease — associations with depression, fatigue, and cognitive impairment. Brain Behav. Immun. 2013;33:183–189. doi: 10.1016/j.bbi.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 47.Aviles-Olmos I, Limousin P, Lees A, Foltynie T. Parkinson’s disease, insulin resistance and novel agents of neuroprotection. Brain. 2013;136:374–384. doi: 10.1093/brain/aws009. [DOI] [PubMed] [Google Scholar]
- 48.Petrou M, et al. Diabetes, gray matter loss, and cognition in the setting of Parkinson disease. Acad. Radiol. 2016;23:577–581. doi: 10.1016/j.acra.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leverenz JB, et al. Cerebrospinal fluid biomarkers and cognitive performance in non-demented patients with Parkinson’s disease. Parkinsonism Relat. Disord. 2011;17:61–64. doi: 10.1016/j.parkreldis.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lim NS, et al. Plasma EGF and cognitive decline in Parkinson’s disease and Alzheimer’s disease. Ann. Clin. Transl. Neurol. 2016;3:346–355. doi: 10.1002/acn3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baek JH, et al. Unfolded protein response is activated in Lewy body dementias. Neuropathol. Appl. Neurobiol. 2015;42:352–365. doi: 10.1111/nan.12260. [DOI] [PubMed] [Google Scholar]
- 52.Bajic N, Jenner P, Ballard CG, Francis PT. Proteasome inhibition leads to early loss of synaptic proteins in neuronal culture. J. Neural Transm. (Vienna) 2012;119:1467–1476. doi: 10.1007/s00702-012-0816-9. [DOI] [PubMed] [Google Scholar]
- 53.Paul G, et al. Safety and tolerability of intracerebroventricular PDGF-BB in Parkinson’s disease patients. J. Clin. Invest. 2015;125:1339–1346. doi: 10.1172/JCI79635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Collins LM, Williams-Gray CH. The genetic basis of cognitive impairment and dementia in Parkinson’s disease. Front. Psychiatry. 2016;7:89. doi: 10.3389/fpsyt.2016.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Healy DG, et al. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson’s disease: a case-control study. Lancet Neurol. 2008;7:583–590. doi: 10.1016/S1474-4422(08)70117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Srivatsal S, et al. Cognitive profile of LRRK2-related Parkinson’s disease. Mov. Disord. 2015;30:728–733. doi: 10.1002/mds.26161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shanker V, et al. Mood and cognition in leucine-rich repeat kinase 2 G2019S Parkinson’s disease. Mov. Disord. 2011;26:1875–1880. doi: 10.1002/mds.23746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ben Sassi S, et al. Cognitive dysfunction in Tunisian LRRK2 associated Parkinson’s disease. Parkinsonism Relat. Disord. 2012;18:243–246. doi: 10.1016/j.parkreldis.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 59.Belarbi S, et al. LRRK2 G2019S mutation in Parkinson’s disease: a neuropsychological and neuropsychiatric study in a large Algerian cohort. Parkinsonism Relat. Disord. 2010;16:676–679. doi: 10.1016/j.parkreldis.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 60.Nichols WC, et al. Genetic screening for a single common LRRK2 mutation in familial Parkinson’s disease. Lancet. 2005;365:410–412. doi: 10.1016/S0140-6736(05)17828-3. [DOI] [PubMed] [Google Scholar]
- 61.Goldwurm S, et al. LRRK2 G2019S mutation and Parkinson’s disease: a clinical, neuropsychological and neuropsychiatric study in a large Italian sample. Parkinsonism Relat. Disord. 2006;12:410–419. doi: 10.1016/j.parkreldis.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 62.Alcalay RN, et al. Self-report of cognitive impairment and mini-mental state examination performance in PRKN, LRRK2, and GBA carriers with early onset Parkinson’s disease. J. Clin. Exp. Neuropsychol. 2010;32:775–779. doi: 10.1080/13803390903521018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aasly JO, et al. Clinical features of LRRK2-associated Parkinson’s disease in central Norway. Ann. Neurol. 2005;57:762–765. doi: 10.1002/ana.20456. [DOI] [PubMed] [Google Scholar]
- 64.Chartier-Harlin MC, et al. α-Synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet. 2004;364:1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- 65.Somme JH, et al. Initial neuropsychological impairments in patients with the E46K mutation of the α-synuclein gene (PARK 1) J. Neurol. Sci. 2011;310:86–89. doi: 10.1016/j.jns.2011.07.047. [DOI] [PubMed] [Google Scholar]
- 66.Seidel K, et al. First appraisal of brain pathology owing to A30P mutant alpha-synuclein. Ann. Neurol. 2010;67:684–689. doi: 10.1002/ana.21966. [DOI] [PubMed] [Google Scholar]
- 67.Puschmann A, et al. A Swedish family with de novoα-synuclein A53T mutation: evidence for early cortical dysfunction. Parkinsonism Relat. Disord. 2009;15:627–632. doi: 10.1016/j.parkreldis.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mata IFAPOE, et al. MAPT, and SNCA genes and cognitive performance in Parkinson disease. JAMA Neurol. 2014;71:1405–1412. doi: 10.1001/jamaneurol.2014.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guella I, et al. α-Synuclein genetic variability: a biomarker for dementia in Parkinson disease. Ann. Neurol. 2016;79:991–999. doi: 10.1002/ana.24664. [DOI] [PubMed] [Google Scholar]
- 70.Alcalay RN, et al. Cognitive performance of GBA mutation carriers with earlyonset PD: the CORE-PD study. Neurology. 2012;78:1434–1440. doi: 10.1212/WNL.0b013e318253d54b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Setó-Salvia N, et al. Glucocerebrosidase mutations confer a greater risk of dementia during Parkinson’s disease course. Mov. Disord. 2012;27:393–399. doi: 10.1002/mds.24045. [DOI] [PubMed] [Google Scholar]
- 72.Winder-Rhodes SE, et al. Glucocerebrosidase mutations influence the natural history of Parkinson’s disease in a community-based incident cohort. Brain. 2013;136:392–399. doi: 10.1093/brain/aws318. [DOI] [PubMed] [Google Scholar]
- 73.Chahine LM, et al. Clinical and biochemical differences in patients having Parkinson disease with versus without GBA mutations. JAMA Neurol. 2013;70:852–858. doi: 10.1001/jamaneurol.2013.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oeda T, et al. Impact of glucocerebrosidase mutations on motor and nonmotor complications in Parkinson’s disease. Neurobiol. Aging. 2015;36:3306–3313. doi: 10.1016/j.neurobiolaging.2015.08.027. [DOI] [PubMed] [Google Scholar]
- 75.Mata IF, et al. GBA variants are associated with a distinct pattern of cognitive deficits in Parkinson’s disease. Mov. Disord. 2016;31:95–102. doi: 10.1002/mds.26359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu G, et al. Specifically neuropathic Gaucher’s mutations accelerate cognitive decline in Parkinson’s. Ann. Neurol. 2016;80:674–685. doi: 10.1002/ana.24781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cilia R, et al. Survival and dementia in GBA-associated Parkinson’s disease: the mutation matters. Ann. Neurol. 2016;80:662–673. doi: 10.1002/ana.24777. [DOI] [PubMed] [Google Scholar]
- 78.Williams-Gray CH, et al. Apolipoprotein E genotype as a risk factor for susceptibility to and dementia in Parkinson’s disease. J. Neurol. 2009;256:493–498. doi: 10.1007/s00415-009-0119-8. [DOI] [PubMed] [Google Scholar]
- 79.Morley JF, et al. Genetic influences on cognitive decline in Parkinson’s disease. Mov. Disord. 2012;27:512–518. doi: 10.1002/mds.24946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Paul KCAPOE, et al. MAPT, and COMT and Parkinson’s disease susceptibility and cognitive symptom progression. J. Parkinsons Dis. 2016;6:349–359. doi: 10.3233/JPD-150762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mengel D, et al. Apolipoprotein E ε4 does not affect cognitive performance in patients with Parkinson’s disease. Parkinsonism Relat. Disord. 2016;29:112–116. doi: 10.1016/j.parkreldis.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 82.Federoff M, Jimenez-Rolando B, Nalls MA, Singleton AB. A large study reveals no association between APOE and Parkinson’s disease. Neurobiol. Dis. 2012;46:389–392. doi: 10.1016/j.nbd.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Desikan RS, et al. Genetic overlap between Alzheimer’s disease and Parkinson’s disease at the MAPT locus. Mol. Psychiatry. 2015;20:1588–1595. doi: 10.1038/mp.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nombela C, et al. Genetic impact on cognition and brain function in newly diagnosed Parkinson’s disease: ICICLE-PD study. Brain. 2014;137:2743–2758. doi: 10.1093/brain/awu201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Winder-Rhodes SE, et al. Association between MAPT haplotype and memory function in patients with Parkinson’s disease and healthy aging individuals. Neurobiol. Aging. 2015;36:1519–1528. doi: 10.1016/j.neurobiolaging.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chung SJ, et al. Genomic determinants of motor and cognitive outcomes in Parkinson’s disease. Parkinsonism Relat. Disord. 2012;18:881–886. doi: 10.1016/j.parkreldis.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Andreassen OA, et al. Genetic pleiotropy between multiple sclerosis and schizophrenia but not bipolar disorder: differential involvement of immune-related gene loci. Mol. Psychiatry. 2015;20:207–214. doi: 10.1038/mp.2013.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mollenhauer B, et al. Biological confounders for the values of cerebrospinal fluid proteins in Parkinson’s disease and related disorders. J. Neurochem. 2016;139(Suppl. 1):290–317. doi: 10.1111/jnc.13390. [DOI] [PubMed] [Google Scholar]
- 89.Lin CH, Wu RM. Biomarkers of cognitive decline in Parkinson’s disease. Parkinsonism Relat. Disord. 2015;21:431–443. doi: 10.1016/j.parkreldis.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 90.Skogseth RE, et al. Associations between cerebrospinal fluid biomarkers and cognition in early untreated Parkinson’s disease. J. Parkinsons Dis. 2015;5:783–792. doi: 10.3233/JPD-150682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stav AL, et al. Amyloid-β and α-synuclein cerebrospinal fluid biomarkers and cognition in early Parkinson’s disease. Parkinsonism Relat. Disord. 2015;21:758–764. doi: 10.1016/j.parkreldis.2015.04.027. [DOI] [PubMed] [Google Scholar]
- 92.Backstrom DC, et al. Cerebrospinal fluid patterns and the risk of future dementia in early, incident Parkinson disease. JAMA Neurol. 2015;72:1175–1182. doi: 10.1001/jamaneurol.2015.1449. [DOI] [PubMed] [Google Scholar]
- 93.Hall S, et al. CSF biomarkers and clinical progression of Parkinson disease. Neurology. 2015;84:57–63. doi: 10.1212/WNL.0000000000001098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Alves G, et al. CSF Aβ42 predicts early-onset dementia in Parkinson disease. Neurology. 2014;82:1784–1790. doi: 10.1212/WNL.0000000000000425. [DOI] [PubMed] [Google Scholar]
- 95.Terrelonge M, Marder KS, Weintraub D, Alcalay RN. CSF β-amyloid 1–42 predicts progression to cognitive impairment in newly diagnosed Parkinson disease. J. Mol. Neurosci. 2016;58:88–92. doi: 10.1007/s12031-015-0647-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Siderowf A, et al. CSF amyloid β 1–42 predicts cognitive decline in Parkinson disease. Neurology. 2010;75:1055–1061. doi: 10.1212/WNL.0b013e3181f39a78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Compta Y, et al. Combined dementia-risk biomarkers in Parkinson’s disease: a prospective longitudinal study. Parkinsonism Relat. Disord. 2013;19:717–724. doi: 10.1016/j.parkreldis.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 98.Parnetti L, et al. Differential role of CSF alpha-synuclein species, tau, and Aβ42 in Parkinson’s disease. Front. Aging Neurosci. 2014;6:53. doi: 10.3389/fnagi.2014.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mollenhauer B, et al. Monitoring of 30 marker candidates in early Parkinson disease as progression markers. Neurology. 2016;87:168–177. doi: 10.1212/WNL.0000000000002651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhou B, Wen M, Yu W-F, Zhang C-L, Jiao L. The diagnostic and differential diagnosis utility of cerebrospinal fluid α-synuclein levels in Parkinson’s disease: a meta-analysis. Parkinsons Dis. 2015;2015:567386. doi: 10.1155/2015/567386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stewart T, et al. Cerebrospinal fluid α-synuclein predicts cognitive decline in Parkinson disease progression in the DATATOP cohort. Am. J. Pathol. 2014;184:966–975. doi: 10.1016/j.ajpath.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hall S, et al. Longitudinal measurements of cerebrospinal fluid biomarkers in Parkinson’s disease. Mov. Disord. 2016;31:898–905. doi: 10.1002/mds.26578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ohrfelt A, et al. Identification of novel α-synuclein isoforms in human brain tissue by using an online nanoLC-ESI-FTICR-MS method. Neurochem. Res. 2011;36:2029–2042. doi: 10.1007/s11064-011-0527-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hansson O, et al. Levels of cerebrospinal fluid α-synuclein oligomers are increased in Parkinson’s disease with dementia and dementia with Lewy bodies compared to Alzheimer’s disease. Alzheimers Res. Ther. 2014;6:25. doi: 10.1186/alzrt255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Simonsen AH, et al. The utility of α-synuclein as biofluid marker in neurodegenerative diseases: a systematic review of the literature. Biomark. Med. 2016;10:19–34. doi: 10.2217/BMM.14.105. [DOI] [PubMed] [Google Scholar]
- 106.Rocchi L, Niccolini F, Politis M. Recent imaging advances in neurology. J. Neurol. 2015;262:2182–2194. doi: 10.1007/s00415-015-7711-x. [DOI] [PubMed] [Google Scholar]
- 107.Niccolini F, Su P, Politis M. Dopamine receptor mapping with PET imaging in Parkinson’s disease. J. Neurol. 2014;261:2251–2263. doi: 10.1007/s00415-014-7302-2. [DOI] [PubMed] [Google Scholar]
- 108.Loane C, Politis M. Positron emission tomography neuroimaging in Parkinson’s disease. Am. J. Transl Res. 2011;3:323–341. [PMC free article] [PubMed] [Google Scholar]
- 109.Politis M. Neuroimaging in Parkinson disease: from research setting to clinical practice. Nat. Rev. Neurol. 2014;10:708–722. doi: 10.1038/nrneurol.2014.205. [DOI] [PubMed] [Google Scholar]
- 110.Santos-Garcia D, et al. COPPADIS-2015 (COhort of Patients with PArkinson’s DIsease in Spain, 2015), a global — clinical evaluations, serum biomarkers, genetic studies and neuroimaging prospective, multicenter, non-interventional, long-term study on Parkinson’s disease progression. BMC Neurol. 2016;16:26. doi: 10.1186/s12883-016-0548-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Politis M, Su P, Piccini P. Imaging of microglia in patients with neurodegenerative disorders. Front. Pharmacol. 2012;3:96. doi: 10.3389/fphar.2012.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wu K, et al. The catechol-O-methyltransferase Val158Met polymorphism modulates fronto-cortical dopamine turnover in early Parkinson’s disease: a PET study. Brain. 2012;135:2449–2457. doi: 10.1093/brain/aws157. [DOI] [PubMed] [Google Scholar]
- 113.Ibarretxe-Bilbao N, et al. Neuroanatomical correlates of impaired decision-making and facial emotion recognition in early Parkinson’s disease. Eur. J. Neurosci. 2009;30:1162–1171. doi: 10.1111/j.1460-9568.2009.06892.x. [DOI] [PubMed] [Google Scholar]
- 114.Ellfolk U, et al. Brain volumetric correlates of memory in early Parkinson’s disease. J. Parkinsons Dis. 2013;3:593–601. doi: 10.3233/JPD-130276. [DOI] [PubMed] [Google Scholar]
- 115.Duncan GW, et al. Gray and white matter imaging: a biomarker for cognitive impairment in early Parkinson’s disease? Mov. Disord. 2016;31:103–110. doi: 10.1002/mds.26312. [DOI] [PubMed] [Google Scholar]
- 116.Yildiz D, et al. Impaired cognitive performance and hippocampal atrophy in Parkinson disease. Turk. J. Med. Sci. 2015;45:1173–1177. doi: 10.3906/sag-1408-68. [DOI] [PubMed] [Google Scholar]
- 117.Mak E, et al. Baseline and longitudinal grey matter changes in newly diagnosed Parkinson’s disease: ICICLE-PD study. Brain. 2015;138:2974–2986. doi: 10.1093/brain/awv211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pereira JB, et al. Aberrant cerebral network topology and mild cognitive impairment in early Parkinson’s disease. Hum. Brain Mapp. 2015;36:2980–2995. doi: 10.1002/hbm.22822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hanganu A, et al. Mild cognitive impairment is linked with faster rate of cortical thinning in patients with Parkinson’s disease longitudinally. Brain. 2014;137:1120–1129. doi: 10.1093/brain/awu036. [DOI] [PubMed] [Google Scholar]
- 120.Kandiah N, et al. Hippocampal volume and white matter disease in the prediction of dementia in Parkinson’s disease. Parkinsonism Relat. Disord. 2014;20:1203–1208. doi: 10.1016/j.parkreldis.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 121.Lee JE, et al. Exploratory analysis of neuropsychological and neuroanatomical correlates of progressive mild cognitive impairment in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry. 2014;85:7–16. doi: 10.1136/jnnp-2013-305062. [DOI] [PubMed] [Google Scholar]
- 122.Rektorova I, et al. Grey matter changes in cognitively impaired Parkinson’s disease patients. PLoS ONE. 2014;9:e85595. doi: 10.1371/journal.pone.0085595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hwang KS, et al. Mapping cortical atrophy in Parkinson’s disease patients with dementia. J. Parkinsons Dis. 2013;3:69–76. doi: 10.3233/JPD-120151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zarei M, et al. Cortical thinning is associated with disease stages and dementia in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry. 2013;84:875–881. doi: 10.1136/jnnp-2012-304126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pagonabarraga J, et al. Pattern of regional cortical thinning associated with cognitive deterioration in Parkinson’s disease. PLoS ONE. 2013;8:e54980. doi: 10.1371/journal.pone.0054980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Borroni B, et al. Structural and functional imaging study in dementia with Lewy bodies and Parkinson’s disease dementia. Parkinsonism Relat. Disord. 2015;21:1049–1055. doi: 10.1016/j.parkreldis.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 127.Carlesimo GA, et al. Hippocampal abnormalities and memory deficits in Parkinson disease: a multimodal imaging study. Neurology. 2012;78:1939–1945. doi: 10.1212/WNL.0b013e318259e1c5. [DOI] [PubMed] [Google Scholar]
- 128.Agosta F, et al. Mild cognitive impairment in Parkinson’s disease is associated with a distributed pattern of brain white matter damage. Hum. Brain Mapp. 2014;35:1921–1929. doi: 10.1002/hbm.22302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen B, Fan GG, Liu H, Wang S. Changes in anatomical and functional connectivity of Parkinson’s disease patients according to cognitive status. Eur. J. Radiol. 2015;84:1318–1324. doi: 10.1016/j.ejrad.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 130.Olde Dubbelink KT, et al. Functional connectivity and cognitive decline over 3 years in Parkinson disease. Neurology. 2014;83:2046–2053. doi: 10.1212/WNL.0000000000001020. [DOI] [PubMed] [Google Scholar]
- 131.Seibert TM, Murphy EA, Kaestner EJ, Brewer JB. Interregional correlations in Parkinson disease and Parkinson-related dementia with resting functional MR imaging. Radiology. 2012;263:226–234. doi: 10.1148/radiol.12111280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rektorova I, Krajcovicova L, Marecek R, Mikl M. Default mode network and extrastriate visual resting state network in patients with Parkinson’s disease dementia. Neurodegener. Dis. 2012;10:232–237. doi: 10.1159/000334765. [DOI] [PubMed] [Google Scholar]
- 133.Lin WC, et al. Dopaminergic therapy modulates cortical perfusion in Parkinson disease with and without dementia according to arterial spin labeled perfusion magnetic resonance imaging. Medicine (Baltimore) 2016;95:e2206. doi: 10.1097/MD.0000000000002206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Le Heron CJ, et al. Comparing cerebral perfusion in Alzheimer’s disease and Parkinson’s disease dementia: an ASL-MRI study. J. Cereb. Blood Flow Metab. 2014;34:964–970. doi: 10.1038/jcbfm.2014.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ito K, et al. Striatal and extrastriatal dysfunction in Parkinson’s disease with dementia: a 6-[18F]fluoro-L-dopa PET study. Brain. 2002;125:1358–1365. doi: 10.1093/brain/awf134. [DOI] [PubMed] [Google Scholar]
- 136.Klein JC, et al. Neurotransmitter changes in dementia with Lewy bodies and Parkinson disease dementia in vivo. Neurology. 2010;74:885–892. doi: 10.1212/WNL.0b013e3181d55f61. [DOI] [PubMed] [Google Scholar]
- 137.Marquie M, et al. Striatal and extrastriatal dopamine transporter levels relate to cognition in Lewy body diseases: an 11C altropane positron emission tomography study. Alzheimers Res. Ther. 2014;6:52. doi: 10.1186/s13195-014-0052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Song IU, Kim YD, Cho HJ, Chung SW, Chung YA. An FP-CIT PET comparison of the differences in dopaminergic neuronal loss between idiopathic Parkinson disease with dementia and without dementia. Alzheimer Dis. Assoc. Disord. 2013;27:51–55. doi: 10.1097/WAD.0b013e31824acd84. [DOI] [PubMed] [Google Scholar]
- 139.Vander Borght T, et al. Cerebral metabolic differences in Parkinson’s and Alzheimer’s diseases matched for dementia severity. J. Nucl. Med. 1997;38:797–802. [PubMed] [Google Scholar]
- 140.Gonzalez-Redondo R, et al. Grey matter hypometabolism and atrophy in Parkinson’s disease with cognitive impairment: a two-step process. Brain. 2014;137:2356–2367. doi: 10.1093/brain/awu159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pappata S, et al. Mild cognitive impairment in drug-naive patients with PD is associated with cerebral hypometabolism. Neurology. 2011;77:1357–1362. doi: 10.1212/WNL.0b013e3182315259. [DOI] [PubMed] [Google Scholar]
- 142.Minoshima S, et al. Alzheimer’s disease versus dementia with Lewy bodies: cerebral metabolic distinction with autopsy confirmation. Ann. Neurol. 2001;50:358–365. doi: 10.1002/ana.1133. [DOI] [PubMed] [Google Scholar]
- 143.Huang C, et al. Changes in network activity with the progression of Parkinson’s disease. Brain. 2007;130:1834–1846. doi: 10.1093/brain/awm086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yong SW, Yoon JK, An YS, Lee PH. A comparison of cerebral glucose metabolism in Parkinson’s disease, Parkinson’s disease dementia and dementia with Lewy bodies. Eur. J. Neurol. 2007;14:1357–1362. doi: 10.1111/j.1468-1331.2007.01977.x. [DOI] [PubMed] [Google Scholar]
- 145.Roy R, Niccolini F, Pagano G, Politis M. Cholinergic imaging in dementia spectrum disorders. Eur. J. Nucl. Med. Mol. Imaging. 2016;43:1376–1386. doi: 10.1007/s00259-016-3349-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shinotoh H, et al. Positron emission tomographic measurement of acetylcholinesterase activity reveals differential loss of ascending cholinergic systems in Parkinson’s disease and progressive supranuclear palsy. Ann. Neurol. 1999;46:62–69. [PubMed] [Google Scholar]
- 147.Hilker R, et al. Dementia in Parkinson disease: functional imaging of cholinergic and dopaminergic pathways. Neurology. 2005;65:1716–1722. doi: 10.1212/01.wnl.0000191154.78131.f6. [DOI] [PubMed] [Google Scholar]
- 148.Bohnen NI, et al. Cortical cholinergic function is more severely affected in parkinsonian dementia than in Alzheimer disease: an in vivo positron emission tomographic study. Arch. Neurol. 2003;60:1745–1748. doi: 10.1001/archneur.60.12.1745. [DOI] [PubMed] [Google Scholar]
- 149.Hiraoka K, et al. Cholinergic deficit and response to donepezil therapy in Parkinson’s disease with dementia. Eur. Neurol. 2012;68:137–143. doi: 10.1159/000338774. [DOI] [PubMed] [Google Scholar]
- 150.Kotagal V, Muller ML, Kaufer DI, Koeppe RA, Bohnen NI. Thalamic cholinergic innervation is spared in Alzheimer disease compared to parkinsonian disorders. Neurosci. Lett. 2012;514:169–172. doi: 10.1016/j.neulet.2012.02.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bohnen NI, et al. Heterogeneity of cholinergic denervation in Parkinson’s disease without dementia. J. Cereb. Blood Flow Metab. 2012;32:1609–1617. doi: 10.1038/jcbfm.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Edison P, et al. Amyloid load in Parkinson’s disease dementia and Lewy body dementia measured with [11C]PIB positron emission tomography. J. Neurol. Neurosurg. Psychiatry. 2008;79:1331–1338. doi: 10.1136/jnnp.2007.127878. [DOI] [PubMed] [Google Scholar]
- 153.Petrou M, et al. Aβ-amyloid deposition in patients with Parkinson disease at risk for development of dementia. Neurology. 2012;79:1161–1167. doi: 10.1212/WNL.0b013e3182698d4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Maetzler W, et al. [11C]PIB binding in Parkinson’s disease dementia. Neuroimage. 2008;39:1027–1033. doi: 10.1016/j.neuroimage.2007.09.072. [DOI] [PubMed] [Google Scholar]
- 155.Petrou M, et al. Amyloid deposition in Parkinson’s disease and cognitive impairment: a systematic review. Mov. Disord. 2015;30:928–935. doi: 10.1002/mds.26191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Akhtar RS, et al. Amyloid-beta positron emission tomography imaging of Alzheimer’s pathology in Parkinson’s disease dementia. Mov. Disord. Clin. Pract. 2016;3:367–375. doi: 10.1002/mdc3.12290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Shah N, et al. Striatal and cortical β-amyloidopathy and cognition in Parkinson’s disease. Mov. Disord. 2016;31:111–117. doi: 10.1002/mds.26369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gomperts SN, et al. Amyloid is linked to cognitive decline in patients with Parkinson disease without dementia. Neurology. 2013;80:85–91. doi: 10.1212/WNL.0b013e31827b1a07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gomperts SN, et al. Tau positron emission tomographic imaging in the Lewy body diseases. JAMA Neurol. 2016;73:1334–1341. doi: 10.1001/jamaneurol.2016.3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kamei S, Morita A, Serizawa K, Mizutani T, Hirayanagi K. Quantitative EEG analysis of executive dysfunction in Parkinson disease. J. Clin. Neurophysiol. 2010;27:193–197. doi: 10.1097/WNP.0b013e3181dd4fdb. [DOI] [PubMed] [Google Scholar]
- 161.Morita A, Kamei S, Mizutani T. Relationship between slowing of the EEG and cognitive impairment in Parkinson disease. J. Clin. Neurophysiol. 2011;28:384–387. doi: 10.1097/WNP.0b013e3182273211. [DOI] [PubMed] [Google Scholar]
- 162.Caviness JN, et al. Longitudinal EEG changes correlate with cognitive measure deterioration in Parkinson’s disease. J. Parkinsons Dis. 2015;5:117–124. doi: 10.3233/JPD-140480. [DOI] [PubMed] [Google Scholar]
- 163.Zimmermann R, et al. Correlation of EEG slowing with cognitive domains in nondemented patients with Parkinson’s disease. Dement. Geriatr. Cogn. Disord. 2015;39:207–214. doi: 10.1159/000370110. [DOI] [PubMed] [Google Scholar]
- 164.Schlede N, et al. Clinical EEG in cognitively impaired patients with Parkinson’s disease. J. Neurol. Sci. 2011;310:75–78. doi: 10.1016/j.jns.2011.05.034. [DOI] [PubMed] [Google Scholar]
- 165.Fonseca LC, Tedrus GM, Carvas PN, Machado EC. Comparison of quantitative EEG between patients with Alzheimer’s disease and those with Parkinson’s disease dementia. Clin. Neurophysiol. 2013;124:1970–1974. doi: 10.1016/j.clinph.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 166.Klassen BT, et al. Quantitative EEG as a predictive biomarker for Parkinson disease dementia. Neurology. 2011;77:118–124. doi: 10.1212/WNL.0b013e318224af8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Seer C, Lange F, Georgiev D, Jahanshahi M, Kopp B. Event-related potentials and cognition in Parkinson’s disease: an integrative review. Neurosci. Biobehav. Rev. 2016;71:691–714. doi: 10.1016/j.neubiorev.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 168.Zhang D, et al. Multimodal classification of Alzheimer’s disease and mild cognitive impairment. Neuroimage. 2011;55:856–867. doi: 10.1016/j.neuroimage.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Compta Y, et al. Grey matter volume correlates of cerebrospinal markers of Alzheimer-pathology in Parkinson’s disease and related dementia Parkinsonism Relat. Disord. 2012;18:941–947. doi: 10.1016/j.parkreldis.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 170.Beyer MK, et al. Cerebrospinal fluid Aβ levels correlate with structural brain changes in Parkinson’s disease. Mov. Disord. 2013;28:302–310. doi: 10.1002/mds.25282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chiaravalloti A, et al. Do CSF levels of t-Tau, p-Tau and β1–42 amyloid correlate with dopaminergic system impairment in patients with a clinical diagnosis of Parkinson disease? A 123I-FP-CIT study in the early stages of the disease. Eur. J. Nucl. Med. Mol. Imaging. 2014;41:2137–2143. doi: 10.1007/s00259-014-2841-4. [DOI] [PubMed] [Google Scholar]
- 172.van Dijk KD, et al. Reduced α-synuclein levels in cerebrospinal fluid in Parkinson’s disease are unrelated to clinical and imaging measures of disease severity. Eur. J. Neurol. 2014;21:388–394. doi: 10.1111/ene.12176. [DOI] [PubMed] [Google Scholar]
- 173.Campbell MC, et al. CSF proteins and resting-state functional connectivity in Parkinson disease. Neurology. 2015;84:2413–2421. doi: 10.1212/WNL.0000000000001681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang HF, et al. Efficacy and safety of cholinesterase inhibitors and memantine in cognitive impairment in Parkinson’s disease, Parkinson’s disease dementia, and dementia with Lewy bodies: systematic review with meta-analysis and trial sequential analysis. J. Neurol. Neurosurg. Psychiatry. 2015;86:135–143. doi: 10.1136/jnnp-2014-307659. [DOI] [PubMed] [Google Scholar]
- 175.Emre M, et al. Rivastigmine for dementia associated with Parkinson’s disease. N. Engl. J. Med. 2004;351:2509–2518. doi: 10.1056/NEJMoa041470. [DOI] [PubMed] [Google Scholar]
- 176.Dubois B, et al. Donepezil in Parkinson’s disease dementia: a randomized, double-blind efficacy and safety study. Mov. Disord. 2012;27:1230–1238. doi: 10.1002/mds.25098. [DOI] [PubMed] [Google Scholar]
- 177.Aarsland D, et al. Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol. 2009;8:613–618. doi: 10.1016/S1474-4422(09)70146-2. [DOI] [PubMed] [Google Scholar]
- 178.Emre M, et al. Memantine for patients with Parkinson’s disease dementia or dementia with Lewy bodies: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010;9:969–977. doi: 10.1016/S1474-4422(10)70194-0. [DOI] [PubMed] [Google Scholar]
- 179.Colloby SJ, et al. Cholinergic and perfusion brain networks in Parkinson disease dementia. Neurology. 2016;87:178–185. doi: 10.1212/WNL.0000000000002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Jurado-Coronel JC, et al. Implication of green tea as a possible therapeutic approach for Parkinson disease. CNS Neurol. Disord. Drug Targets. 2016;15:292–300. doi: 10.2174/1871527315666160202125519. [DOI] [PubMed] [Google Scholar]
- 181.Postuma RB, et al. Caffeine for treatment of Parkinson disease: a randomized controlled trial. Neurology. 2012;79:651–658. doi: 10.1212/WNL.0b013e318263570d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mamikonyan E, Xie SX, Melvin E, Weintraub D. Rivastigmine for mild cognitive impairment in Parkinson disease: a placebo-controlled study. Mov. Disord. 2015;30:912–918. doi: 10.1002/mds.26236. [DOI] [PubMed] [Google Scholar]
- 183.Weintraub D, et al. Rasagiline for mild cognitive impairment in Parkinson’s disease: a placebo-controlled trial. Mov. Disord. 2016;31:709–714. doi: 10.1002/mds.26617. [DOI] [PubMed] [Google Scholar]
- 184.Weintraub D, et al. Atomoxetine for depression and other neuropsychiatric symptoms in Parkinson disease. Neurology. 2010;75:448–455. doi: 10.1212/WNL.0b013e3181ebdd79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kehagia AA, et al. Targeting impulsivity in Parkinson’s disease using atomoxetine. Brain. 2014;137:1986–1997. doi: 10.1093/brain/awu117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Katona C, Hansen T, Olsen CK. A randomized, double-blind, placebo-controlled, duloxetine-referenced, fixed-dose study comparing the efficacy and safety of Lu AA21004 in elderly patients with major depressive disorder. Int. Clin. Psychopharmacol. 2012;27:215–223. doi: 10.1097/YIC.0b013e3283542457. [DOI] [PubMed] [Google Scholar]
- 187.Valera E, Spencer B, Masliah E. Immunotherapeutic approaches targeting amyloid-β, α-synuclein, and tau for the treatment of neurodegenerative disorders. Neurotherapeutics. 2016;13:179–189. doi: 10.1007/s13311-015-0397-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Killick R, et al. Clusterin regulates β-amyloid toxicity via Dickkopf-1-driven induction of the wnt-PCP-JNK pathway. Mol. Psychiatry. 2014;19:88–98. doi: 10.1038/mp.2012.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Himeno E, et al. Apomorphine treatment in Alzheimer mice promoting amyloid-β degradation. Ann. Neurol. 2011;69:248–256. doi: 10.1002/ana.22319. [DOI] [PubMed] [Google Scholar]
- 190.Yarnall AJ, et al. Apomorphine: a potential modifier of amyloid deposition in Parkinson’s disease? Mov. Disord. 2016;31:668–675. doi: 10.1002/mds.26422. [DOI] [PubMed] [Google Scholar]
- 191.Martinez-Martin P, et al. EuroInf: a multicenter comparative observational study of apomorphine and levodopa infusion in Parkinson’s disease. Mov. Disord. 2015;30:510–516. doi: 10.1002/mds.26067. [DOI] [PubMed] [Google Scholar]
- 192.Leung IH, et al. Cognitive training in Parkinson disease: a systematic review and meta-analysis. Neurology. 2015;85:1843–1851. doi: 10.1212/WNL.0000000000002145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Reynolds GO, Otto MW, Ellis TD, Cronin-Golomb A. The therapeutic potential of exercise to improve mood, cognition, and sleep in Parkinson’s disease. Mov. Disord. 2016;31:23–38. doi: 10.1002/mds.26484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.David FJ, et al. Exercise improves cognition in Parkinson’s disease: the PRET-PD randomized, clinical trial. Mov. Disord. 2015;30:1657–1663. doi: 10.1002/mds.26291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Klingelhoefer L, Samuel M, Chaudhuri KR, Ashkan K. An update of the impact of deep brain stimulation on non motor symptoms in Parkinson’s disease. J. Parkinsons Dis. 2014;4:289–300. doi: 10.3233/JPD-130273. [DOI] [PubMed] [Google Scholar]
- 196.Kuhn J, et al. Deep brain stimulation of the nucleus basalis of Meynert in Alzheimer’s dementia. Mol. Psychiatry. 2015;20:353–360. doi: 10.1038/mp.2014.32. [DOI] [PubMed] [Google Scholar]
- 197.Gratwicke J, et al. The nucleus basalis of Meynert: a new target for deep brain stimulation in dementia? Neurosci. Biobehav. Rev. 2013;37:2676–2688. doi: 10.1016/j.neubiorev.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 198.Seto-Salvia N, et al. Dementia risk in Parkinson disease: disentangling the role of MAPT haplotypes. Arch. Neurol. 2011;68:359–364. doi: 10.1001/archneurol.2011.17. [DOI] [PubMed] [Google Scholar]
- 199.Goris A, et al. Tau and α-synuclein in susceptibility to, and dementia in, Parkinson’s disease. Ann. Neurol. 2007;62:145–153. doi: 10.1002/ana.21192. [DOI] [PubMed] [Google Scholar]
- 200.Kurz MW, et al. APOE alleles in Parkinson disease and their relationship to cognitive decline: a population-based, longitudinal study. J. Geriatr. Psychiatry Neurol. 2009;22:166–170. doi: 10.1177/0891988709332945. [DOI] [PubMed] [Google Scholar]
- 201.Marras C, et al. Motor and nonmotor heterogeneity of LRRK2-related and idiopathic Parkinson’s disease. Mov. Disord. 2016;31:1192–1202. doi: 10.1002/mds.26614. [DOI] [PubMed] [Google Scholar]
- 202.Thaler A, et al. Lower cognitive performance in healthy G2019S LRRK2 mutation carriers. Neurology. 2012;79:1027–1032. doi: 10.1212/WNL.0b013e3182684646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Farrer M, et al. Comparison of kindreds with parkinsonism and α-synuclein genomic multiplications. Ann. Neurol. 2004;55:174–179. doi: 10.1002/ana.10846. [DOI] [PubMed] [Google Scholar]
- 204.Foltynie T, et al. Planning ability in Parkinson’s disease is influenced by the COMT val158met polymorphism. Mov. Disord. 2004;19:885–891. doi: 10.1002/mds.20118. [DOI] [PubMed] [Google Scholar]
- 205.Williams-Gray CH, Hampshire A, Barker RA, Owen AM. Attentional control in Parkinson’s disease is dependent on COMT val158met genotype. Brain. 2008;131:397–408. doi: 10.1093/brain/awm313. [DOI] [PubMed] [Google Scholar]
- 206.Svetel M, et al. No association between brain-derived neurotrophic factor G196A polymorphism and clinical features of Parkinson’s disease. Eur. Neurol. 2013;70:257–262. doi: 10.1159/000352033. [DOI] [PubMed] [Google Scholar]
- 207.Gao L, et al. Brain-derived neurotrophic factor G196A polymorphism and clinical features in Parkinson’s disease. Acta Neurol. Scand. 2010;122:41–45. doi: 10.1111/j.1600-0404.2009.01253.x. [DOI] [PubMed] [Google Scholar]
- 208.Białecka M, et al. BDNF G196A (Val66Met) polymorphism associated with cognitive impairment in Parkinson’s disease. Neurosci. Lett. 2014;561:86–90. doi: 10.1016/j.neulet.2013.12.051. [DOI] [PubMed] [Google Scholar]
- 209.Arias-Vasquez A, et al. Relationship of the Ubiquilin 1 gene with Alzheimer’s and Parkinson’s disease and cognitive function. Neurosci. Lett. 2007;424:1–5. doi: 10.1016/j.neulet.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 210.Kurz MW, et al. FMR1 alleles in Parkinson’s disease: relation to cognitive decline and hallucinations, a longitudinal study. J. Geriatr. Psychiatry Neurol. 2007;20:89–92. doi: 10.1177/0891988706297737. [DOI] [PubMed] [Google Scholar]
- 211.Nie K, et al. Polymorphisms in immune/inflammatory cytokine genes are related to Parkinson’s disease with cognitive impairment in the Han Chinese population. Neurosci. Lett. 2013;541:111–115. doi: 10.1016/j.neulet.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 212.Liu Z, et al. Lack of association between IL-10 and IL-18 gene promoter polymorphisms and Parkinson’s disease with cognitive impairment in a Chinese population. Sci. Rep. 2016;6:19021. doi: 10.1038/srep19021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Broeders M, et al. Evolution of mild cognitive impairment in Parkinson disease. Neurology. 2013;81:346–352. doi: 10.1212/WNL.0b013e31829c5c86. [DOI] [PubMed] [Google Scholar]
- 214.Domellof ME, Ekman U, Forsgren L, Elgh E. Cognitive function in the early phase of Parkinson’s disease, a five-year follow-up. Acta Neurol. Scand. 2015;132:79–88. doi: 10.1111/ane.12375. [DOI] [PubMed] [Google Scholar]
- 215.Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov. Disord. 2008;23:837–844. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]