Abstract
A 24-week feeding experiment was conducted to assess whether males and females of Oreochromis niloticus exhibit differences in their hematological responses and organosomatic indices to dietary AFB1 contamination. Triplicate groups of O. niloticus (initial body weight: 24.1 ± 0.6 g) were fed with four diets (Diets 1 to 4) containing 0, 20, 200, and 2,000 μg AFB1 kg−1. A significant decrease (P < 0.05) in hemoglobin (Hb), red blood cells (RBC), and hematocrit (Hct) was observed in AFB1 exposure groups, with the lowest levels recorded in the 2000 μg AFB1 kg−1 treatment. A significant increase in mean white blood cells (WBC), neutrophils, and lymphocytes was observed in AFB1 exposure groups. No sex-related differences in RBC, WBC, lymphocytes, monocytes, and neutrophils levels were observed. However, hemoglobin and hematocrit values for female O. niloticus were significantly lower than those for male O. niloticus. Organosomatic indices showed that the relative liver, kidney, and spleen weights were significantly higher (P < 0.05) in the AFB1 supplemented group than in the control group. However, the effect of aflatoxin on organosomatic indices does not depend on sex but rather depends on the dose of aflatoxin in the diet. These results provide useful information for monitoring changes in the health status of male and female O. niloticus.
1. Introduction
Oreochromis niloticus are currently the main cultured fish species in East Africa (accounting for 75%), and they contribute the bulk of fish, yielding approximately 3,400 tons (69.9%) [1]. In O. niloticus, diets are predominantly formulated using high levels of grain and plant protein and are at high risk of being contaminated by aflatoxin [2]. Aflatoxin (AF), a potent mycotoxin produced by Aspergillus flavus and Aspergillus parasiticus, is a top challenge in aquaculture production due to the increasing use of vegetable sources in fish feeds [3]. Aflatoxin contamination in fish causes anemia, hemorrhaging, liver damage, weight reduction, vulnerability to secondary infectious diseases, and a slow decrease in reared fish stock quality, thus representing a significant challenge in aquaculture [4, 5].
Information on various infections of visceral organs, necrosis, inflammation, and the presence of stress factors is generally provided by hematological parameters [6, 7]. These parameters are associated with the blood and blood-forming organs [8]. Blood acts as a pathological reflector of the status of animals exposed to the toxicant and other conditions; animals with good blood composition likely show better performance [9, 10]. The study of the physiological and hematological characteristics of cultured fish species is an essential tool in the advancement of aquaculture systems, especially with respect to the detection of healthy versus infected or stressed fish [11, 12]. In addition, Ronald and Bruce [13] described organosomatic indices as the proportion of organs to body weight; an organ measured in relation to body mass can be directly connected to toxic effects by a chemical to the target organ [14]. The most commonly used organosomatic indices are the gonadosomatic index (GSI), spleen somatic index (RSI), kidney somatic index (KSI), and hepatosomatic index (HSI) [15, 16]. These organosomatic indices may provide more specific information related to the function of the selected organ [15].
The toxic effect of aflatoxin in animals depends on the dose in the feeds and duration of toxin exposure, as well as the sex and age [17–19]. Sex-related differences in aflatoxin tolerance have been reported in pigs, broilers, and rats and these studies reported that males are more susceptible to aflatoxin than their female counterparts [20–22]. Despite the vast number of studies on the effects of aflatoxin on hematology and organosomatic indices of different species of fish, no study investigated whether males and females of O. niloticus exhibit differences in hematological responses and organosomatic indices to dietary AFB1 contamination. Therefore, this study evaluates sex-related differences in hematological responses and organosomatic indices of O. niloticus exposed to an aflatoxin B1-contaminated diet.
2. Materials and Methods
2.1. Experimental Fish and Procedure
A total of 300 O. niloticus weighing 24.1 ± 0.6 g were obtained from the National Aquaculture Research and Development Centre (NARDTC), Sagana, Kenya. Fish were acclimatized to the trial condition and fed with the control diet for 15 days. A total of 300 O. niloticus were divided into twelve equal groups of 25 fish. Each treatment was divided into 3 replicates of 25 fish each and kept in cages. Fish were fed to apparent repletion twice a day at 0900 and 1500 hr. To minimize feed wastage, pellets were slowly fed to the fish. The experiment was performed in 12 cages (1 m3) placed in three different ponds (40 × 20 m).
During the experiment, the water quality parameters were as follows: temperature 24.2–26.8°C, pH 7.5–7.65, dissolved oxygen 4.6–6.3 mg L−1, total alkalinity 146.65–225.95 mg L−1, and salinity 0.03–0.05. The trial lasted for 24 weeks. Females and males in each experiment unit were checked monthly to determine the number, weight, length, and any physiological changes. Twenty fish from each cage were sampled randomly after 1 day of food deprivation near the end of the experiment.
2.2. Experimental Diets
A pure crystalline powder of AFB1 was purchased from the Libios Chemical Company, Rue Edmond Michelet, France. AFB1 (5 mg) was dissolved in 100 mL of methanol to form a stock solution containing 0.05 mg AFB1 mL−1 of methanol. Four experimental diets (Diets 1 to 4) were formulated to contain 0, 20, 200, and 2000 μg AFB1 kg−1 diets. The control diet was Diet 1, without supplementary chemical AFB1.
All diets were processed with the aid of a meat grinder with approximately 32% moisture and pelleted, followed by 4 hr of drying in an air flow oven at 60°C until the moisture content was less than 10%. The dry pellets (5 mm diameter) were stored at −20°C until fed. Table 1 shows the composition and nutrient content of the diets.
Table 1.
Ingredients and nutrient composition of the basal diet of O. niloticus.
| Ingredients∗ | Content (g/100 g diet) |
|---|---|
| Soybean meal | 30 |
| Shrimp meal | 17.9 |
| Wheat bran | 5 |
| Sunflower seedcake | 40.57 |
| Sunflower oil | 2 |
| Cassava flour | 1.5 |
| Vitamin premix1 | 1.06 |
| Starch | 1.97 |
| Chemical analysis (% or kJ g −1 in dry matter) | |
| Crude protein | 30.83 |
| Ether extract | 5.93 |
| Ash | 5.93 |
| NFE | 51.56 |
| GE (Kcal/g) | 441.89 |
NFE: nitrogen-free extract; GE: gross energy. 1Vitamin premix (mg kg−1 diet): vitamin A, 18 MIU; vitamin D3, 4 MIU; vitamin E, 6.5 g; vitamin B2, 3.5 g; vitamin K3, 2 g; nicotinic acid, 17 g; pantothenic acid, 7 g; folic acid, 0.4 g; vitamin B1, 1.5 g; vitamin B6, 2.5 g; vitamin C, 12 g; magnesium, 6 g; potassium, 7.5 g; sodium, 20 g; citric acid, 18 g. ∗Experimental doses of AFB1 were 0, 20, 200, and 2,000 μg kg−1; measured levels were 0.68, 22.5, 235.4, and 2246.5 μg AFB1 kg−1, respectively.
The given toxin levels were finally cross-checked using an Enzyme-Linked Immunosorbent Assay (ELISA) (BioTek Instruments, Inc., VT, USA).
2.3. Relative Organ Weight
At the end of the 24-week experiment, the fish were carefully netted to minimize stress, and the fish was measured (total length) with an ichthyometer (±1 cm precision) and weighed. After this, the fish were anesthetized with sodium bicarbonate and sacrificed. The liver, spleen, and kidney were carefully removed and weighed.
The organosomatic indices of the liver, spleen, and kidney were calculated for the twenty fish according to Dogan and Can [23] to obtain the ratios of organ weight to body weight for the fish.
2.4. Blood Parameters Analysis
Blood specimens were taken from the caudal vein in heparinized tubes and centrifuged at 3,500 g for 10 min; serum samples were separated and stored at −20°C until analysis. The analyzed serum parameters such as alanine aminotransferase (ALT) were determined using an automatic analyzer (Beckman Coulter DU @ 530, automatic biochemical analyzer, USA).
Hemoglobin (Hb) concentration was evaluated by Sahli's hemometer [24] and hematocrit (Hct) concentration was determined using the microhematocrit method [25]. Red blood cells (RBC) and white blood cells (WBC) were counted using the improved Neubauer hemocytometer [25], and for differential leucocyte counts, freshly prepared blood smears were stained with modified Giemsa's stain [26] and observed under a microscope. The mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC) were calculated as stated by Mahfouz and Sherif [27].
2.5. Histopathological Procedure
From each treatment, ten fish were sacrificed to acquire liver samples for histology and tissue analysis. Bouin's solution was used to store samples for the first 24 hr, and then samples were stored in 70% ethanol. Tissue samples were dehydrated in a graded ethanol series and embedded in paraffin before being processed. Tissue blocks were subsequently sectioned and stained with hematoxylin and eosin [28] and then examined under a microscope for common and/or significant lesions and associated histopathological changes of aflatoxicosis [29].
2.6. Data Analysis
Data analyses were performed using a two-way ANOVA for the main effects of aflatoxin, sex, and their interaction using the General Linear Model procedure of SPSS (version 16.0). Significant means (P < 0.05) were separated using Tukey's Test. Data are presented as the means ± SEM.
3. Results
3.1. Relative Organ Weight and Physiology Response
Data for internal organs indices of the tested O. niloticus are in Table 2. Aflatoxin B1 significantly increased the HSI, KSI, and SSI (P < 0.05). The HSI, KSI, and SSI in the control group had the lowest values (0.88 g, 0.15 g, and 0.05 g, resp.) compared to Diet 4 (2.97, 0.42, and 0.34 g, resp.). The HSI and KSI significantly increased in Diets 2, 3, and 4 but SSI increased in Diets 3 and 4. The HSI, KSI, and SSI revealed no sex differences in response to aflatoxin B1 (Table 2).
Table 2.
Mean ± SE of relative organ weights (g organ per 100 g body weight), white blood cells, differential counts, and liver enzymes (e.g., alanine aminotransferase (ALT)) of male and female O. niloticus exposed to different levels of AFB1 for 24 weeks.
| Diet∗ | Sex† | HSI | KSI | SSI | WBC 103 | Monocytes% | Neutrophils% | Lymphocytes% | ALT (UL−1) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 0.88 ± 0.03a | 0.15 ± 0.002a | 0.05 ± 0.001a | 19.30 ± 0.28a | 3.56 ± 0.003a | 0.37 ± 0.001a | 10.82 ± 0.2a | 41.96 ± 0.08a |
| 2 | 1.42 ± 0.02b | 0.24 ± 0.001b | 0.07 ± 0.001a | 20.29 ± 0.24a | 3.05 ± 0.005b | 0.26 ± 0.008b | 16.50 ± 0.01b | 42.56 ± 0.06a | |
| 3 | 1.94 ± 0.05c | 0.33 ± 0.003c | 0.11 ± 0.002b | 28.26 ± 0.85b | 0.52 ± 0.004c | 2.01 ± 0.01c | 29.21 ± 0.18c | 46.82 ± 0.05b | |
| 4 | 2.97 ± 0.04d | 0.42 ± 0.005d | 0.34 ± 0.12c | 37.97 ± 0.08c | 0.37 ± 0.003d | 3.97 ± 0.005d | 30.75 ± 0.03cd | 50.50 ± 0.08c | |
|
| |||||||||
| 1 | M | 0.84 ± 0.03a | 0.15 ± 0.002a | 0.05 ± 0.001a | 19.45 ± 0.29a | 3.58 ± 0.03a | 0.38 ± 0.001a | 10.95 ± 0.02a | 41.99 ± 0.06a |
| 2 | 1.40 ± 0.02b | 0.24 ± 0.001b | 0.07 ± 0.001a | 20.47 ± 0.25a | 3.06 ± 0.05b | 0.28 ± 0.006b | 16.70 ± 0.01b | 42.60 ± 0.08a | |
| 3 | 1.89 ± 0.05c | 0.34 ± 0.003c | 0.11 ± 0.02b | 28.45 ± 0.85b | 0.54 ± 0.03c | 2.02 ± 0.01c | 29.36 ± 0.10c | 46.84 ± 0.08b | |
| 4 | 2.94 ± 0.04d | 0.42 ± 0.004d | 0.33 ± 0.13c | 38.16 ± 0.08c | 0.38 ± 0.03d | 3.98 ± 0.004d | 30.83 ± 0.06d | 50.69 ± 0.05c | |
|
| |||||||||
| ANOVA | |||||||||
| Aflatoxin∗ sex | 0.97 | 1 | 1 | 1.00 | 0.99 | 0.99 | 0.98 | 0.68 | |
| Sex | 0.10 | 0.62 | 0.98 | 0.62 | 0.19 | 0.33 | 0.18 | 0.25 | |
| Aflatoxin (dose) | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | |
†M: male O. niloticus; F: female O. niloticus with n = 10 (10 individual fish per treatment (10 males and 10 females) were measured); ∗diet 1: 0 μg kg−1 (control); diet 2: 20 μg kg−1; diet 3: 200 μg kg−1; diet 4: 2000 μg kg−1. ∗Means followed by similar letters do not differ significantly (P < 0.05).
Aflatoxin B1 also significantly increased the activity of the serum ALT liver enzyme (P < 0.05). The control group showed a low activity of the ALT liver enzyme (41.96 UL−1) compared to that in Diet 4 (50.69 UL−1). Serum ALT revealed no sex differences in response to AFB1.
3.2. Hematological Characteristics
The inclusion of different aflatoxin levels in the diet significantly (P < 0.05) affected RBC in both sexes, with the highest value (2.96) in the control group compared to AFB1 supplemented diets (1.98, 1.43, and 1.01 for Diets 2, 3, and 4, resp.) in Table 3.
Table 3.
Mean ± SE of hematological parameters of male and female O. niloticus exposed to different levels of AFB1 for 24 weeks.
| Diet∗ | Sex† | Length (cm) | RBC ×106 | Hb (g dL−1) | HCT% | MCV (μm3) | MCH (pg) | MCHC% |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 21.67 ± 0.76a | 2.96 ± 0.01a | 8.02 ± 0.07a | 38.50 ± 0.6a | 129.8 ± 1.75a | 27.06 ± 0.15a | 25.13 ± 0.62b |
| 2 | 20.27 ± 0.43b | 1.98 ± 0.80b | 7.52 ± 0.04b | 30.00 ± 0.7b | 151.1 ± 3.50b | 37.89 ± 0.25b | 20.85 ± 0.17a | |
| 3 | 18.92 ± 0.13c | 1.43 ± 0.02c | 4.05 ± 0.06c | 24.50 ± 0.5c | 170.4 ± 3.20c | 28.16 ± 0.32c | 16.56 ± 0.49c | |
| 4 | 17.05 ± 0.34d | 1.01 ± 0.67d | 3.55 ± 0.02d | 20.25 ± 0.4d | 198.7 ± 4.80d | 34.83 ± 0.22d | 17.56 ± 0.39c | |
|
| ||||||||
| 1 | M | 22.22 ± 0.86a | 2.97 ± 0.01a | 8.15 ± 0.09a | 40.50 ± 0.65a | 136.5 ± 1.73a | 27.46 ± 0.23a | 20.13 ± 0.12a |
| 2 | 20.12 ± 0.45b | 1.99 ± 0.85b | 7.72 ± 0.05b | 32.00 ± 0.70b | 161.0 ± 3.52b | 38.86 ± 0.25b | 24.18 ± 0.56b | |
| 3 | 18.37 ± 0.05c | 1.44 ± 0.02c | 4.27 ± 0.04c | 26.50 ± 0.50c | 184.1 ± 3.30c | 29.69 ± 0.29c | 16.15 ± 0.39c | |
| 4 | 17.22 ± 0.38d | 1.02 ± 0.67d | 3.67 ± 0.05d | 21.50 ± 0.50d | 210.8 ± 4.40d | 36.02 ± 0.27d | 17.11 ± 0.34c | |
|
| ||||||||
| ANOVA | ||||||||
| Aflatoxin∗ sex | 0.18 | 1.00 | 0.77 | 0.89 | 0.76 | 0.19 | 0.980 | |
| Sex | 0.97 | 0.79 | <0.05 | <0.05 | <0.05 | <0.05 | >0.05 | |
| Aflatoxin (dose) | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | |
†M: male O. niloticus; F: female O. niloticus with n = 10 (10 individual fish per treatment (10 males and 10 females) were measured); ∗diet 1: 0 μg kg−1 (control); diet 2: 20 μg kg−1; diet 3: 200 μg kg−1; diet 4: 2000 μg kg−1. ∗Means followed by similar letters do not differ significantly (P < 0.05).
There were significantly decreased Hct and Hb in both sexes of O. niloticus on Diets 2, 3, and 4 compared to controls (Table 3). Hct decreased by 30.00, 24.50, and 20.25% (for Diets 2, 3, and 4, resp.) compared to the control group value (38.50%) in Table 3. However, MCV and MCH of male and female fish were significantly (P < 0.05) higher in the AFB1 supplemented diet than in the control diet. The highest MCV was recorded in fish fed with Diet 4 (198.7 μm3), while the lowest was recorded in the control group (129.8 μm3). There was no significant difference in RBC and MCHC between sexes. The statistical comparison between male and female O. niloticus revealed a significant difference for Hb (P < 0.05), Hct (P < 0.05), MCV (P < 0.05), and MCH (P < 0.05) in Table 3. Female O. niloticus fed with Diets 2, 3, and 4 recorded lower Hb (7.52, 4.05, and 3.55 gdL−1) than male O. niloticus (7.72, 4.27, and 3.67 gdL−1).
The total WBC counts of fish of both sexes from Diet 3 were higher than in the control group due to an increase in lymphocytes and neutrophils. Absolute monocyte counts of fish fed with the AFB1 supplemented diets were much lower than in the control group (Table 2). There were no significant sex differences (P > 0.05) in WBC, lymphocytes, neutrophils, and monocytes among all treatments. The hematocrit values presented a strong positive correlation with the length of the fish (P < 0.05, r = 0.939). As the aflatoxin dose increases, the fish length significantly decreases (Table 3).
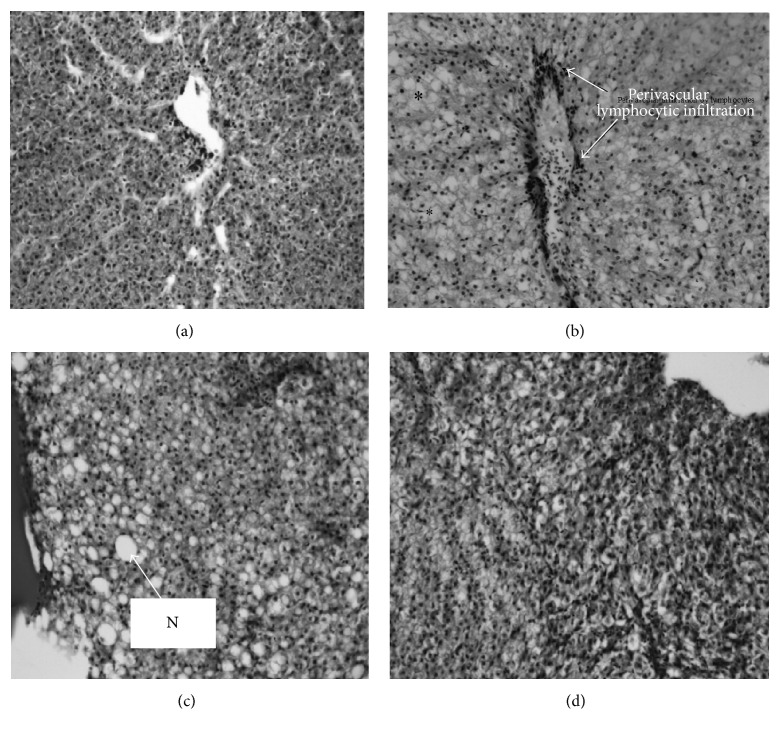
3.3. Histopathology
Histopathological examinations revealed that fish liver from the control treatment had normal hepatocytes arranged in trabecula forming a centripetal sinusoid (Figure 1(a)). However, the trabecular arrangement of hepatocytes was less apparent in fish fed with Diet 2, with the presence of multifocal lymphocytic infiltration including the perivascular region (Figure 1(b)). Hepatocytes showed hemorrhagic and fatty necrosis, as evidenced by vacuolization and hemorrhage (Figure 1(c)). Some cells were granular in appearance, with enlarged nuclei indicative of necrosis (Figures 1(c) and 1(d)). The severity of liver pathology varied with low and high doses of aflatoxin B1. In the liver with low-dose treatments, there were early signs of necrosis, as indicated by cell vacuolization with normal nuclei (Figure 1(c)). In the livers with a high dose, there was late-stage necrosis indicated by enlarged cell nuclei, karyolysis, and cytoplasmic disintegration (Figure 1(d)).
Figure 1.
Histology of a liver in O. niloticus fed with Diet 1 (a), Diet 2 (b), Diet 3 (c), and Diet 4 (d) for 24 weeks. H&E; bar = 400 μm. ∗N: fatty necrosis.
4. Discussion
In this study, we evaluated the sex-related differences in the hematological responses and organosomatic indices of O. niloticus exposed to aflatoxin B1-contaminated diet and found differences among the sexes.
In the present study, RBC, Hct, Hb, and MCHC levels decreased significantly (P < 0.05) in the high AFB1 exposed group compared with the control group. In the AFB1 exposed group, O. niloticus suffered from anemic conditions as revealed by a marked decrease in RBC and Hb. The diminished RBC, Hct, and hemoglobin may be due to factors such as inhibited protein synthesis, erythropoiesis, hemosynthesis, and osmoregulatory dysfunction owing to repressed activities of several enzymes involved in heme biosynthesis [27] and hematopoietic cellular defects of aflatoxin [30]. Our results corroborate the studies of Mahfouz and Sherif [27] and Jantrarotai and Lovell [31], who observed a decrease in hemoglobin concentration and erythrocyte counts in O. niloticus and channel catfish exposed to AFB1 diets.
The present study also assessed whether male or female O. niloticus exhibit differences in their hematological response when exposed to AFB1 diet. Because of the wide use of male monosex tilapia, sex-based differences in the hematological parameters should be determined if male O. niloticus are more resistant when exposed to AFB1 diet. According to our results, no sex-related differences in RBC and MCHC levels were observed. However, hemoglobin and hematocrit values for males were significantly higher than for female O. niloticus. We suspect that the differences may not be because of the toxicity effect of AFB1 but due to the higher metabolic rate of males compared to females or a sex-differential oxygen requirement and this requires further investigation.
A higher MCV is an indicator of macrocytic anemia, while a reduced MCHC is indicative of anemic conditions due to iron deficiency [32, 33]. Similar to our results, a higher MCV and lower MCHC were observed in O. niloticus exposed to AFB1 compared to the control group. Our results were also supported by the findings of Ewuola et al. [34] and Weaver et al. [35] who found an increase in MCV and a decrease in MCHC of goat and pig fed with an AFB1-contaminated diet. Gabriel et al. [36] reported that there is a relationship between blood hematocrit and body length: longer fish have higher hematocrit in C. carpio. This implies that rapid increases in mean body weight and length and blood volume are accompanied by adequate erythropoiesis [37]. This is in agreement with our findings that O. niloticus exposed to AFB1 were shorter and hence had lower Hct.
In the present study, we found that WBC, lymphocytes, monocytes, and neutrophils were influenced by the AFB1 dose and not the sex of O. niloticus. Significantly, greater WBC and lymphocytes were observed in aflatoxin exposed groups, which may be attributed to the immunosuppressive effect of aflatoxin on fish. These results are in contrast with those of Mahfouz, [38] who reported that WBC, lymphocytes, and neutrophils were decreased in O. niloticus fed with an AFB1 supplemented diet. However, our results are in accordance with those of Quist et al. [39] and Jantrarotai and Lovell [31], who observed increased WBC and lymphocytes in wild turkey pouts and channel catfish fed with an AFB1 supplemented diet. WBC increased with infection, first cooperating to recognize the pathogen as an invader and destroy it. There was an increase in WBC during infection and allergic responses in the host [40]. Therefore, aflatoxin might enhance the invasion of pathogens in tested animals, giving way to opportunistic infections and a subsequent increase in WBC [40].
In this study, the hepatosomatic index (HSI), kidney somatic index (KSI), and spleen somatic index (SSI) disclosed no sex differences in response to aflatoxin B1. These results are in agreement with the previous study of Bryden et al. [20], who found that the hepatosomatic index of male and female chicken fed with AFB1 does not depend on sex. The HSI, KSI, and SSI of O. niloticus (both sexes) exposed to AFB1 supplemented diets were found to be significantly higher than in the control group. These findings are in concurrence with past studies where AFB1 resulted in an increase in HSI and KSI [41–45]. Additionally, serum ALT shows no sex differences in response to aflatoxin B1. The levels of ALT were found to be significantly higher in AFB1 exposed fish. Similarly, previous reports show that AFB1 resulted in an increase in HSI and increased activities of plasma ALT [41–43]. Elevation of ALT activity in the serum is an indication of cellular damage and liver tissue necrosis following the exposure of O. niloticus to AFB1 [46]. This is confirmed by our histological results, in which fatty necrosis, hemorrhagic necrosis, and enlarged cell nuclei with vacuolized cytoplasm were identified as the most prevalent histological characteristics in fish fed with AFB1 diet. These observations are comparable with previous histopathological findings by Zychowski et al. [47] and Baptista et al. [48] who reported hepatocellular lipid deposition, hemorrhagic necrosis, and enlarged cell nuclei with vacuolized cytoplasm in the livers of rats and O. niloticus fed with an aflatoxin diet.
5. Conclusion
The hematological and organosomatic indices measured in the present study are useful for monitoring the effects of aflatoxin on fish. This work concluded that aflatoxin is highly toxic to both male and female O. niloticus. Elevation of ALT activity in the serum and HSI for both male and female O. niloticus appears to reflect histopathological alterations in the liver of the AFB1 exposed fish. Aflatoxin increases the WBC and lymphocytes and reduces RBC, Hct, and Hb of both male and female O. niloticus. However, hemoglobin and hematocrit values were lower in female O. niloticus than in male O. niloticus. Further studies to determine the mechanism of action of AFB1 in female and male O. Niloticus hematological system are recommended.
Acknowledgments
This research was supported by the Organization for Women in Science for Developing World (OWSD) and the Tanzania Commission for Science (COSTECH). The authors are grateful to the National Aquaculture Research and Development Centre (NARDTC), Sagana, Kenya, for the provision of ponds, cages, and fingerlings. The assistance of Mr. Hesbon Odongo of the Physiology Laboratory, Ms. Ann Owiti of the Centre for Biotechnology, and Mr. Peter Thimbu of the Parasitology Laboratory at the University of Nairobi is acknowledged. The authors are also grateful to Mr. Peter Muruka, Ms. Sally Mwarumba, Ms. Florence Mbugua, Mr. Elijah Gichana, and Mr. Nathan Chuma, who assisted in the field and laboratory work at the NARDTC.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this manuscript.
References
- 1.Ngugi C. C., Manyala J. O. Food and Agriculture Organization (FAO) Rome, Italy: 2004. Aquaculture extension in Sub-Saharan Africa. [Google Scholar]
- 2.Abbas H. K., Weaver M. A., Zablotowicz R. M., Horn B. W., Shier W. T. Relationships between aflatoxin production and sclerotia formation among isolates of Aspergillus section Flavi from the Mississippi Delta. European Journal of Plant Pathology. 2005;112(3):283–287. doi: 10.1007/s10658-004-4888-8. [DOI] [Google Scholar]
- 3.Spring P., Fegan D. F., Lyons T. P., Jacques K. A. Mycotoxins - a rising threat to aquaculture. In Nutritional biotechnology in the feed and food industries. Proceedings of Alltechs 21st Annual Symposium; 2005; Alltech UK: Lexington, Kentucky, USA. pp. 323–331. [Google Scholar]
- 4.Santacroce M. P., Conversano M. C., Casalino E., et al. Aflatoxins in aquatic species: Metabolism, toxicity and perspectives. Reviews in Fish Biology and Fisheries. 2008;18(1):99–130. doi: 10.1007/s11160-007-9064-8. [DOI] [Google Scholar]
- 5.Ortatatli M., Ciftci M. K., Tuzcu M., Kaya A. The effects of aflatoxin on the reproductive system of roosters. Research in Veterinary Science. 2002;72(1):29–36. doi: 10.1053/rvsc.2001.0516. [DOI] [PubMed] [Google Scholar]
- 6.Jurcik R., Suvegova K., Hanusova E., Massanyi P., Ryban L., Chrenek P. Evaluation of haematological, biochemical and histopathological parameters of transgenic rabbits. Journal of Veterinary Medicine Series A: Physiology Pathology Clinical Medicine. 2007;54(9):527–531. doi: 10.1111/j.1439-0442.2007.00976.x. [DOI] [PubMed] [Google Scholar]
- 7.Betancourt-Alonso M. A., Orihuela A., Aguirre V., Vázquez R., Flores-Pérez I. Changes in behavioural and physiological parameters associated with Taenia pisiformis infection in rabbits (Oryctolagus cuniculus) that may improve early detection of sick rabbits. World Rabbit Science. 2011;19(1):21–30. doi: 10.4995/wrs.2011.801. [DOI] [Google Scholar]
- 8.Waugh A., Grant A. Ross And Wilson Anatomy And Physiology in Health And Illness. 11th. Vol. 28. Churchill Livingstone Elsevier; 2014. [DOI] [Google Scholar]
- 9.Acharya G., Mohanty P. K. Haematological and Serum Biochemical Parameters in different Sexes of Walking Cat Fish, Clarias batrachus (Linnaeus, 1758) International Journal of Scientific Research. 2014;3:1914–1917. [Google Scholar]
- 10.Etim N., Williams M., Akpabio U., Offiong E. Haematological parameters and factors affecting their values. Agricultural Science. 2014;2(1):37–47. doi: 10.12735/as.v2i1p37. [DOI] [Google Scholar]
- 11.Ranzani-Paiva M. J., Rodrigues E. L., Veiga M. L., Eiras A. C., Campos B. E. Differential leukocyte counts in “dourado”, Salminus maxillosus Valenciennes, 1840, from the Mogi-Guaçu River, Pirassununga, SP. Brazilian journal of biology = Revista brasleira de biologia. 2003;63(3):517–525. doi: 10.1590/S1519-69842003000300018. [DOI] [PubMed] [Google Scholar]
- 12.O'neal C. C., Hawke J. P., Weirich C. R. Evaluation of low levels of salinity on hematological parameters and health status of channel catfish reared in multiple-crop ponds. Journal of Aquatic Animal Health. 2006;18(1):1–10. doi: 10.1577/H05-005.1. [DOI] [Google Scholar]
- 13.Ronald W. G., Bruce A. B. Organosomic indices and an autopsy based assessment as indicators of health condition of fish. American Fisheries Society. 1990:93–108. [Google Scholar]
- 14.Di Giulio R. T., Hinton D. E. The toxicology of fishes. Food and Chemical Toxicology. 2008;29:p. 1096. [Google Scholar]
- 15.Martin-Diaz M. L., Tuberty S. R., McKenney C. L., Sales D., Del Valls T. A. Effects of cadmium and zinc on Procambarus clarkii: Simulation of the Aznalcollar mining spill. Ciencias Marinas. 2005;31:197–202. doi: 10.7773/cm.v31i12.96. [DOI] [Google Scholar]
- 16.Ariweriokuma S., Akinrotimi O., Gabriel U. Effects of cypermethrin on condition factor and organosomatic indices of clarias gariepinus. Journal of Agriculture and Social Research. 2011;11:67–72. [Google Scholar]
- 17.Deng S. X., Tian L.-X., Liu F.-J., et al. Toxic effects and residue of aflatoxin B1 in tilapia (Oreochromis niloticus × O. aureus) during long-term dietary exposure. Aquaculture. 2010;307(3-4):233–240. doi: 10.1016/j.aquaculture.2010.07.029. [DOI] [Google Scholar]
- 18.Cote L. M., Beasley V. R., Bratich P. M., Swanson S. P., Shivaprasad H. L., Buck W. B. Sex-related reduced weight gains in growing swine fed diets containing deoxynivalenol. Journal of Animal Science. 1985;61(4):942–950. doi: 10.2527/jas1985.614942x. [DOI] [PubMed] [Google Scholar]
- 19.Dortant P. M., Peters-Volleberg G. W. M., Van Loveren H., Marquardt R. R., Speijers G. J. A. Age-related differences in the toxicity of ochratoxin A in female rats. Food and Chemical Toxicology. 2001;39(1):55–65. doi: 10.1016/S0278-6915(00)00107-1. [DOI] [PubMed] [Google Scholar]
- 20.Bryden W. L., Cumming R. B., Lloyd A. B. Sex and strain responses to aflatoxin B1 in the chicken. Avian Pathology. 1980;9(4):539–550. doi: 10.1080/03079458008418441. [DOI] [PubMed] [Google Scholar]
- 21.Andretta I., Kipper M., Lehnen C. R., Hauschild L., Vale M. M., Lovatto P. A. Meta-analytical study of productive and nutritional interactions of mycotoxins in growing pigs. Animal. 2012;6(9):1476–1482. doi: 10.1017/S1751731111002278. [DOI] [PubMed] [Google Scholar]
- 22.Gurtoo H. L., Motycka L. Effect of sex difference on the in vitro and in vivo metabolism of aflatoxin B1 by the rat. Cancer Research. 1976;36(12):4663–4671. [PubMed] [Google Scholar]
- 23.Dogan D., Can C. Hematological, biochemical, and behavioral responses of Oncorhynchus mykiss to dimethoate. Fish Physiology and Biochemistry. 2011;37(4):951–958. doi: 10.1007/s10695-011-9492-1. [DOI] [PubMed] [Google Scholar]
- 24.Basmacioglu H., Oguz H., Ergul M., Col R., Birdane Y. O. Effect of dietary esterified glucomannan on performance, serum biochemistry and haematology in broilers exposed to aflatoxin. Czech Journal of Animal Science. 2005;50(1):31–39. [Google Scholar]
- 25.Bain B. J., Lewis S. M., Shirley M., Dacie J. V., John V. Dacie And Lewis Practical Haematology. 11th. Elsevier Churchill Livingstone; 2012. [Google Scholar]
- 26.Dönmez N., Dönmez H. H., Keskin E., Kısadere İ. Effects of aflatoxin on some haematological parameters and protective effectiveness of esterified glucomannan in Merino rams. The Scientific World Journal. 2012;2012:p. 342468. doi: 10.1100/2012/342468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahfouz M. E., Sherif A. H. A multiparameter investigation into adverse effects of aflatoxin on Oreochromis niloticus health status. The Journal of Basic & Applied Zoology. 2015;71:48–59. doi: 10.1016/j.jobaz.2015.04.008. [DOI] [Google Scholar]
- 28.Suvarna S. K., Layton (Histologist) C., Bancroft J. D. Bancrofts Theory And Practice of Histological Techniques. 7th. Churchill Livingstone Elsevier; 2013. [Google Scholar]
- 29.Chávez-Sánchez M. C., Martínez Palacios C. A., Osorio Moreno I. Pathological effects of feeding young oreochromis niloticus diets supplemented with different levels of aflatoxin B1. Aquaculture. 1994;127(1):49–60. doi: 10.1016/0044-8486(94)90191-0. [DOI] [Google Scholar]
- 30.Van Vleet J. F., Ferrans V. J. Etiologic factors and pathologic alterations in selenium-vitamin E deficiency and excess in animals and humans. Biological Trace Element Research. 1992;33(1-3):1–21. doi: 10.1007/BF02783988. [DOI] [PubMed] [Google Scholar]
- 31.Jantrarotai W., Lovell R. T. Subchronic toxicity of dietary aflatoxin B1 to channel catfish. Journal of Aquatic Animal Health. 1990;2:248–254. doi: 10.1577/1548-8667(1990)002<0248:STODAB>2.3.CO;2. [DOI] [Google Scholar]
- 32.Tripathi M. K., Mondal D., Karim S. A. Growth, haematology, blood constituents and immunological status of lambs fed graded levels of animal feed grade damaged wheat as substitute of maize. Journal of Animal Physiology and Animal Nutrition. 2007;92(1):75–85. doi: 10.1111/j.1439-0396.2007.00712.x. [DOI] [PubMed] [Google Scholar]
- 33.Radostits O. M., Done S. H. Veterinary medicine: a textbook of the diseases of cattle, sheep, pigs, goats, and horses. Elsevier Saunders; 2007. [Google Scholar]
- 34.Ewuola E., Bello, Jimoh O. A., Jagun A. T. Haematological response of growing west african dwarf goats to micro doses of dietary aflatoxins. Mycotoxicology Society of Nigeria. 2014;1(3-4):182–187. [Google Scholar]
- 35.Weaver A. C., Todd See M., Hansen J. A., et al. The use of feed additives to reduce the effects of aflatoxin and deoxynivalenol on pig growth, organ health and immune status during chronic exposure. Toxins. 2013;5(7):1261–1281. doi: 10.3390/toxins5071261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gabriel U. U., Akinrotimi O. A., Eseimokumo F. Haematological responses of wild nile tilapia oreochromis niloticus after acclimation to captivity. Jordan Journal of Biological Sciences. 2011;4:225–230. [Google Scholar]
- 37.Svetina A., Matašin Ž., Tofant A., Vučemilo M., Fijan N. Haematology and some blood chemical parameters of young carp till the age of three years. Acta Veterinaria Hungarica. 2002;50(4):459–467. doi: 10.1556/AVet.50.2002.4.8. [DOI] [PubMed] [Google Scholar]
- 38.Mahfouz M. E. Ameliorative effect of curcumin on aflatoxin B1-induced changes in liver gene expression of Oreochromis niloticus. Molecular Biology. 2015;49(2):275–286. doi: 10.1134/S0026893315020089. [DOI] [PubMed] [Google Scholar]
- 39.Quist C. F., Bounous D. I., Kilburn J. V., Nettles V. F., Wyatt R. D. The effect of dietary aflatoxin on wild turkey poults. Journal of Wildlife Diseases. 2000;36(3):436–444. doi: 10.7589/0090-3558-36.3.436. [DOI] [PubMed] [Google Scholar]
- 40.Willey J. M., Sherwood L. M., Woolverton C. J. Applied environmental microbiology. in Prescotts Principles of Microbiology. 2009 [Google Scholar]
- 41.Tessari E. N. C., Oliveira C. A. F., Cardoso A. L. S. P., Ledoux D. R., Rottinghaus G. E. Effects of aflatoxin B1 and fumonisin B1 on body weight, antibody titres and histology of broiler chicks. British Poultry Science. 2006;47(3):357–364. doi: 10.1080/00071660600756071. [DOI] [PubMed] [Google Scholar]
- 42.Harvey R. B., Edrington T. S., Kubena L. F., Elissalde M. H., Rottinghaus G. E. Influence of aflatoxin and fumonisin B1-containing culture material on growing barrows. American Journal of Veterinary Research. 1995;56(12):1668–1672. [PubMed] [Google Scholar]
- 43.Huang Y., Han D., Xiao X., et al. Effect of dietary aflatoxin B1 on growth, fecundity and tissue accumulation in gibel carp during the stage of gonad development. Aquaculture. 2014;428-429:236–242. doi: 10.1016/j.aquaculture.2014.03.010. [DOI] [Google Scholar]
- 44.Mehrim A., Abdelhamid A., Abou-Shousha A. Nutritious attempts to detoxify aflatoxic diets of tilapia fish: 2-Clinical, biochemical and histological parameters. Arabian Journal of Chemistry. 2006 [Google Scholar]
- 45.Abdelhamid A. M., Abdelkhalek A. E., Mehrm A. I., Khalil F. F. An attempt to alleviate aflatoxicosis on Nile tilapia fish by dietary supplementations with chicken-hatchery by-products (egg shells) and shrimp processing wastes (shrimp shells) 2-On clinical, blood and histological parameters. Journal of Agricultural Science Mansoura University. 2004;29:6175–6196. [Google Scholar]
- 46.Gül Ş., Belge-Kurutaş E., Yildiz E., Şahan A., Doran F. Pollution correlated modifications of liver antioxidant systems and histopathology of fish (Cyprinidae) living in seyhan dam lake, turkey. Environment International. 2004;30(5):605–609. doi: 10.1016/S0160-4120(03)00059-X. [DOI] [PubMed] [Google Scholar]
- 47.Zychowski K. E., Pohlenz C., Mays T., et al. The effect of NovaSil dietary supplementation on the growth and health performance of Nile tilapia (Oreochromis niloticus) fed aflatoxin-B1 contaminated feed. Aquaculture. 2013;376-379:117–123. doi: 10.1016/j.aquaculture.2012.11.020. [DOI] [Google Scholar]
- 48.Baptista A. S., Abdalla A. L., Aguiar C. L., et al. Utilization of diets amended with yeast and amino acids for the control of aflatoxicosis. World Journal of Microbiology and Biotechnology. 2008;24(11):2547–2554. doi: 10.1007/s11274-008-9776-5. [DOI] [Google Scholar]