Abstract
Background
Morphometric measurements of systemic atherosclerosis and direct quantification of visceral fat are only possible using materials from autopsy studies. However, the few autopsy studies that have investigated the association of visceral fat with atherosclerosis had small sample sizes and focused on coronary arteries of young or middle-aged White subjects. We aimed to investigate the association of pericardial fat (PF) and abdominal visceral fat (AVF) with atherosclerosis in the aorta, coronary, carotid, and cerebral arteries in a large autopsy study.
Materials and methods
We evaluated deceased subjects aged 30 years or above. We dissected and weighted the PF and the AVF and evaluated the atherosclerotic burden in the aorta, as well as the carotid, coronary, and cerebral arteries using morphometric measurements. We also investigated the interaction of PF and AVF with age regarding the atherosclerotic burden.
Results
The mean age of the 240 included subjects was 64.8±15.3 years, and 63% was male. Greater PF was associated with a higher degree of aortic atherosclerosis after adjusting for confounding variables (coefficient = 4.39, 95% CI = 0.83; 7.94, p = 0.02). Greater AVF was associated with a higher coronary stenosis index (coefficient = 1.49, 95% CI = 0.15; 2.83, p = 0.03) and a greater number of coronary plaques (coefficient = 0.71, 95% CI = 0.24; 1.19, p = 0.003). We did not find an association of PF or AVF with carotid or cerebral atherosclerotic burden. We found a significant interaction of AVF (coefficient = -0.08; 95% CI = -0.14; -0.02, p = 0.009) and PF (coefficient = -0.87, 95% CI = -1.70; -0.04, p = 0.04) with age regarding carotid artery atherosclerotic burden.
Conclusions
Greater AVF was associated with greater atherosclerotic burden and extent in coronary arteries, while greater PF correlated with a higher degree of atherosclerosis in the aorta.
Introduction
Since 1980, the prevalence of obesity has more than doubled worldwide. In 2014, 39% of adults were overweight and 13% were obese across the globe [1]. Obesity may be related to atherosclerosis by a complex process that may involve a chronic inflammatory state, insulin resistance, dyslipidemia, and hypertension [2]. Previous epidemiological studies showed an association of coronary artery atherosclerosis with epicardial [3], pericardial (PF) [4–6], and abdominal visceral fat (AVF) [7–9]. PF consists of epicardial and paracardial fat, which is located between the visceral pericardium and the myocardium, and outside of the parietal pericardium, respectively [10]. The association between subclinical atherosclerosis as measured by carotid artery intima-media thickness (CIMT) and AVF [11] or epicardial fat [12] has also been described. Aortic atherosclerosis is associated with epicardial fat thickness, but PF was not evaluated [13]. Cerebral artery plaque volume is associated with AVF [14]. Despite such evidence, all of these studies used imaging methods [3–6, 11–15], which quantified visceral fat and atherosclerosis through indirect measurements.
Autopsy studies are the gold standard for evaluating the association of visceral fat with atherosclerosis [16], allowing the direct measurement of atherosclerosis and the exact quantification of visceral fat [17]. However, the few autopsy studies that have investigated the association between visceral fat and atherosclerosis restricted their analyses to coronary arteries [7, 8, 18–20]. Moreover, the majority of the studies evaluated White young and middle-aged adults [7, 8, 20, 21]. Furthermore, evidence on the influence of age on the association of PF and AVF with systemic atherosclerosis is scarce [11]. Therefore, in the present study we investigated the association of PF and AVF with the severity of atherosclerosis in multiple arterial sites (aorta, coronary, carotid, and cerebral arteries) in a large autopsy study.
Materials and methods
This study was conducted at the Sao Paulo Autopsy Service from University of Sao Paulo (Brazil). It was approved by the Ethics Committee in Research from University of Sao Paulo Medical School and complied with the 1975 Declaration of Helsinki. The deceased’s next of kin (NOK) was informed about this study, was invited to participate, and signed a written informed consent form. In the city of Sao Paulo, autopsy is compulsory for individuals whose cause of natural death is unclear [22]. Further details about Sao Paulo Autopsy Service and this study can be found elsewhere [23]. During 2011 to 2014, we included participants aged 30 years or above. The exclusion criteria were as follows: (1) the NOK provided inconsistent information during the clinical interview; (2) the NOK had less than weekly contact with the deceased; (3) the NOK was unable to participate due to emotional suffering; (4) subjects who had lost 10% or more of regular weight during the six months prior to death; (5) arteries or visceral fat was retained at autopsy by the pathologist; (6) subjects with post mortem interval ≥ 24 hours; and (7) subjects with signs of body autolysis according to the Crossley criteria [24].
Clinical assessment
Information about the subject’s sociodemographic data (age, sex, race, years of education, marital status, and socioeconomic status [25]) and cardiovascular risk factors [hypertension, diabetes mellitus, dyslipidemia, coronary artery disease (CAD), heart failure, stroke, smoking, alcohol use, and physical inactivity] were collected from the deceased’s NOK through a semi-structured clinical interview [23, 26].
Measurement of visceral fat
The heart together with the PF was washed in running water to remove clots; then, fixed in 70% alcohol by immersion for at least 24 hours, dissected, and weighed. Omental, mesenteric, mesocolon, and perirenal fat were dissected after the autopsy and weighed using a calibrated electronic scale. To avoid measurement error, we were especially careful to tare the scale before using it. The measurements were expressed in grams. AVF was determined by the sum of the omental, mesenteric, mesocolon, and perirenal fat.
Atherosclerosis evaluation
We dissected the following:
The aorta from the ascending to the abdominal segment before the iliac bifurcation;
The common and internal carotid arteries;
The coronary arteries, including the left main, left anterior descending and right coronary artery as well as the circumflex artery; and
The cerebral arteries (e.g., basilar, posterior, posterior communicating, middle, anterior, anterior communicating, and internal carotid arteries proximal to the circle of Willis).
All arteries were washed in running water to remove clots and fixed in 70% alcohol by immersion for 24 hours. Gelatin was injected inside the vessel lumen of the carotid, coronary, and cerebral arteries to prevent artery flatness. The arteries were then stored in 10% formalin. Subsequently, the carotid and coronary arteries were cut cross-sectionally at 5-mm intervals [27], and the cerebral arteries were cut at 3-mm intervals. We photographed the largest atheroma plaque in each artery using a stereomicroscope (Nikon® SMZ 1000, Nikon Inst., Tokyo, Japan). The areas delineated by the outer vessel wall and by the lumen were measured using the image software ImageJ® (Fig 1). The stenosis index was calculated by subtracting the lumen area from the outer area, dividing the difference by the outer area, and multiplying the result by 100 [28]. We used the mean stenosis index of all measured sections in each vessel bed (i.e., coronary, carotid, and cerebral arteries). We also counted the number of atherosclerotic plaques in the cerebral and coronary arteries as a measurement of atherosclerotic disease extent.
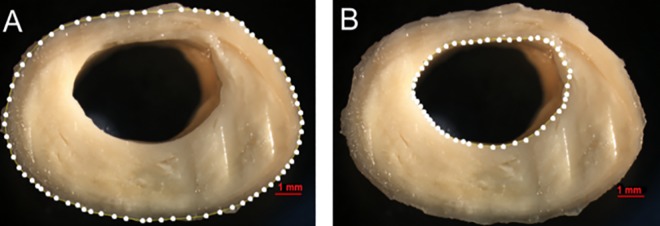
Fig 1. Calculation of the stenosis index in the carotid artery.
(A) Area limited by the outer wall of the vessel. (B) Lumen area. A similar method was used to evaluate coronary and cerebral arteries.
We assessed inter-rater reliability of stenosis index measurements in arterial segments. We randomly selected 164 segments, and two blinded independent raters measured the stenosis index. We calculated the intraclass correlation coefficient (ICC) using two-way mixed-effects model [29]. The inter-rater reliability was excellent with an ICC of 0.962 (95%CI = 0.948; 0.972).
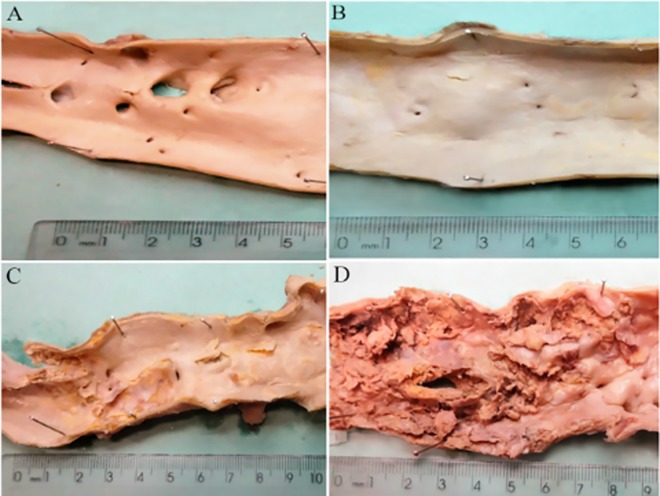
The aorta was opened longitudinally to investigate the severity of atherosclerosis and the presence of confluent lesions. Atherosclerosis in the aorta was classified as grade 1 (plaques were not confluent, and there were no ulcerations and protrusions); grade 2 (confluent areas or/and an area of ulceration with minimal protrusion); and grade 3 (confluent plaques, multifocal ulcerations, or protrusions) (Fig 2) [30].
Fig 2. Evaluation of the severity of atherosclerosis in the aorta.
(A) Absence of atherosclerosis. (B) Grade 1, non-confluent plaques without ulcerations and protrusions. (C) Grade 2, confluent areas or/and an area of ulceration with minimal protrusion. (D) Grade 3, confluent plaques with multifocal ulcerations or protrusions.
Statistical analysis
The sample size of 165 subjects was estimated based on previous studies [19] with a power of 90%, an alpha of 5%, and an effect size of 0.24 for the correlation between anterior epicardial fat surface and the score of coronary stenosis in a two sided-test. However, we opted to include 240 subjects to investigate the effect modification by age.
We defined the dependent variables as the stenosis indexes in carotid, coronary, and cerebral arteries (continuous variables); the number of atherosclerotic plaques in the coronary and cerebral arteries (discrete variables); and the severity of atherosclerosis in the aorta (ordinal variable). The independent variables were the PF and AVF weights (continuous variables). The sample characteristics were described with measurements of central tendency and dispersion for quantitative variables or proportions for qualitative variables.
The association of visceral fat with the stenosis indexes and the number of plaques in the coronary, carotid, and cerebral arteries was assessed using linear regression models. The association of visceral fat with the severity of atherosclerosis in the aorta was assessed using ordinal logistic regression. We adjusted all models for height [31–34], which was used as a measure of the participant’s size. We adjusted the multivariate models for age, sex, smoking status, alcohol use, physical inactivity, hypertension, and diabetes mellitus. We also evaluated the possibility of interaction [11] between age and visceral fat by creating an interaction term of age with AVF and PF and testing it in regression models in coronary, carotid and cerebral arteries adjusted for the same set of variables described above. The alpha level was set at 0.05 in two sided-tests. We used Stata/MP 13 (StataCorp LP, College Station, Texas, USA) for the statistical analyses.
Results
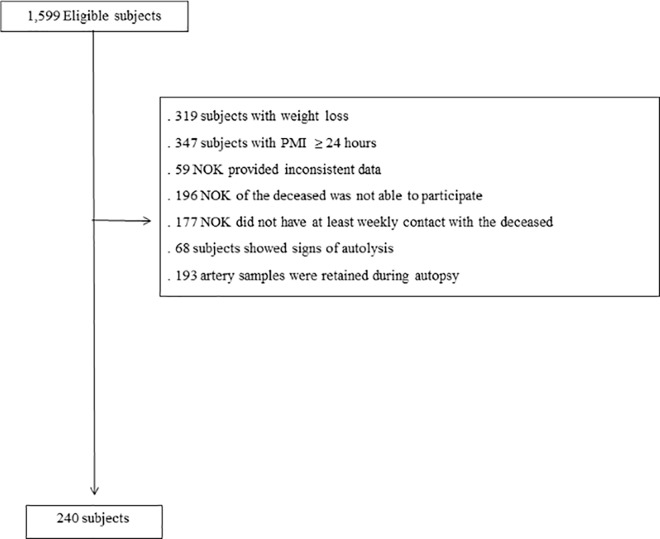
Among 1,599 eligible subjects during the study period, 240 met the criteria for this study (Fig 3). The mean age of the subjects was 64.8±15.3 years (range = 30–98), 151 (63%) were male, 147 (61%) were White, and 197 (82%) of the NOK had daily contact with the deceased. The main cause of death was cardiovascular related 116 (48%) (Table 1). The mean weight of the AVF was 2,040±1,250 g, and the mean weight of the PF was 160±80 g. The mean stenosis index was 77.8±11.0% for coronary arteries, 64.1±0.4% for carotid arteries, and 52.8±0.5% for cerebral arteries. The mean number of plaques was 6.3±3.8 in coronary arteries; and 9.3±5.6 in cerebral arteries. Regarding the severity of aortic atherosclerosis, degrees 2 and 3 were most prevalent (40% each one), and no subject was devoid of atherosclerosis.
Fig 3. Flow chart of study population.
NOK = next of kin; and PMI = post mortem interval.
Table 1. Characteristics of the sample (n = 240).
| Variable | Mean (standard deviation) or n (%) |
|---|---|
| Age (years) | 64.8 (15.3) |
| Male | 151 (62.9) |
| White | 147 (61.2) |
| Married | 122 (50.8) |
| Education (years), median (interquartile range) | 4 (4) |
| Socioeconomic status (classes) a | |
| . Upper | 93 (38.8) |
| . Middle | 129 (53.7) |
| . Lower | 18 (7.5) |
| Daily contact of the next of kin with the deceased | 197 (82.1) |
| Cardiovascular cause of death | 116 (48.3) |
| Post mortem interval (hours) | 14.7 (3.4) |
| Hypertension | 163 (67.9) |
| Diabetes mellitus | 67 (27.9) |
| Coronary artery disease | 67 (27.9) |
| Heart failure | 55 (22.9) |
| Dyslipidemia | 36 (15.0) |
| Stroke | 25 (10.4) |
| Smoking | |
| . Never | 78 (32.5) |
| . Current | 90 (37.5) |
| . Former | 72 (30.0) |
| Alcohol use | |
| . Never | 85 (35.4) |
| . Current | 100 (41.7) |
| . Former | 54 (22.5) |
| Physical inactivity | 149 (62.1) |
a Socioeconomic status was defined according to gross family annual income in US$: Upper social class: ≥6,783; Middle: 3,256 to 6,782; Lower: ≤ 3,255 (1 dollar = 3.3 BRL)
Association between visceral fat and aortic atherosclerosis
AVF was not associated with the severity of aortic atherosclerosis (p = 0.17), but greater PF was associated with the severity of aortic atherosclerosis in the multivariate analysis (coefficient = 4.39, 95%CI = 0.83; 7.94, p = 0.02) (Table 2).
Table 2. Association of visceral fat with the degree and extension of atherosclerosis (n = 240).
| Arteries | Model 1a | Model 2b | ||||
|---|---|---|---|---|---|---|
| Coef | 95% CI | p | Coef | 95% CI | p | |
| Stenosis index or degree of atherosclerosis | ||||||
| Aortac | ||||||
| AVF | 0.20 | 0.001; 0.40 | 0.05 | 0.18 | -0.07; 0.44 | 0.17 |
| PF | 5.65 | 2.61; 8.69 | <0.0001 | 4.39 | 0.83; 7.94 | 0.02 |
| Coronaryd | ||||||
| AVF | 1.16 | -0.01; 2.33 | 0.05 | 1.49 | 0.15; 2.83 | 0.03 |
| PF | 21.69 | 4.92; 38.47 | 0.01 | 17.07 | -0.85; 34.99 | 0.06 |
| Carotidd | ||||||
| AVF | -0.10 | -0.88; 0.68 | 0.81 | -0.23 | -1.15; 0.68 | 0.63 |
| PF | 0.60 | -11.15; 12.35 | 0.92 | -4.13 | -16.78; 8.51 | 0.52 |
| Cerebrald | ||||||
| AVF | 0.38 | -0.51; 1.27 | 0.40 | 0.32 | -0.69; 1.34 | 0.53 |
| PF | 4.90 | -7.99; 17.80 | 0.45 | 1.49 | -12.08; 15.07 | 0.83 |
| Number of plaques | ||||||
| Coronaryd | ||||||
| AVF | 0.69 | 0.28; 1.10 | 0.001 | 0.71 | 0.24; 1.19 | 0.003 |
| PF | 8.54 | 2.60; 14.48 | 0.005 | 6.25 | -0.13; 12.63 | 0.05 |
| Cerebrald | ||||||
| AVF | 0.48 | -0.13; 1.10 | 0.12 | 0.41 | -0.26; 1.08 | 0.23 |
| PF | 7.06 | -1.69; 15.81 | 0.11 | 0.96 | -7.96; 9.88 | 0.83 |
Coef = coefficient; CI = confidence interval; AVF = abdominal visceral fat; PF = Pericardial fat
a Model 1: Adjusted for height
b Model 2: Adjusted for height, age, sex, smoking, alcohol use, physical inactivity, hypertension, and diabetes mellitus
c Ordered logistic regression
d Linear regression
Association between visceral fat and coronary artery atherosclerosis
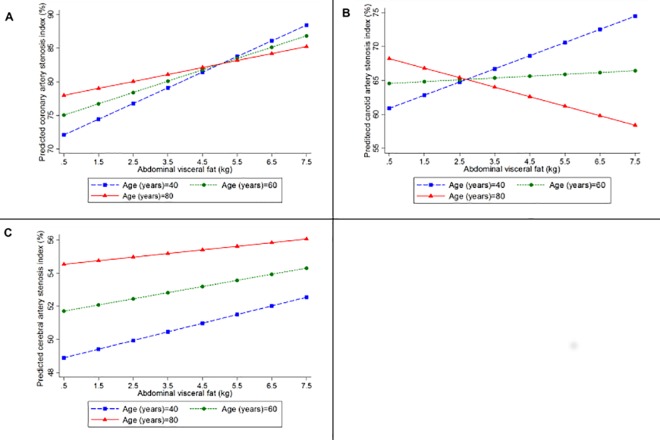
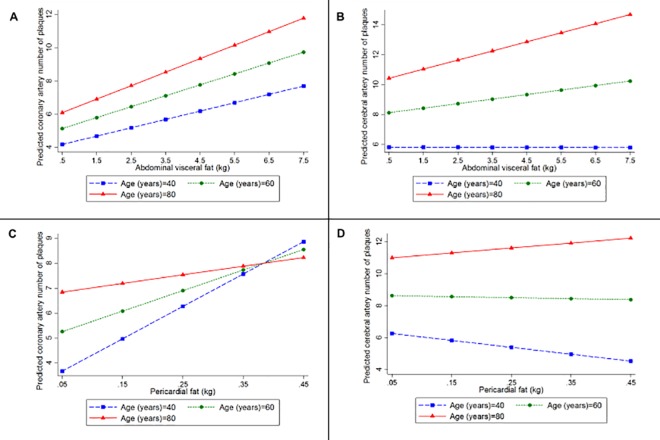
A greater amount of AVF was associated with a higher stenosis index (coefficient = 1.49, 95%CI = 0.15; 2.83, p = 0.03) and a greater number of plaques in coronary arteries (coefficient = 0.71, 95%CI = 0.24; 1.19, p = 0.003) in the multivariate analyses (Table 2). However, there was no interaction of AVF and age regarding the atherosclerotic burden (p = 0.47) (Fig 4A), nor on the extent of atherosclerosis (p = 0.68) in coronary arteries (Fig 5A) (Table 3). Despite the lack of statistical significance, a trend was noted for the association of PF with coronary atherosclerosis as measured by the stenosis index (p = 0.06) and with the number of atherosclerotic plaques (p = 0.05) (Table 2). We did not observe any interaction between PF and age on coronary atherosclerotic burden (p = 0.53) (Fig 6A), nor on its extent (p = 0.28) (Fig 5C) (Table 3).
Fig 4.
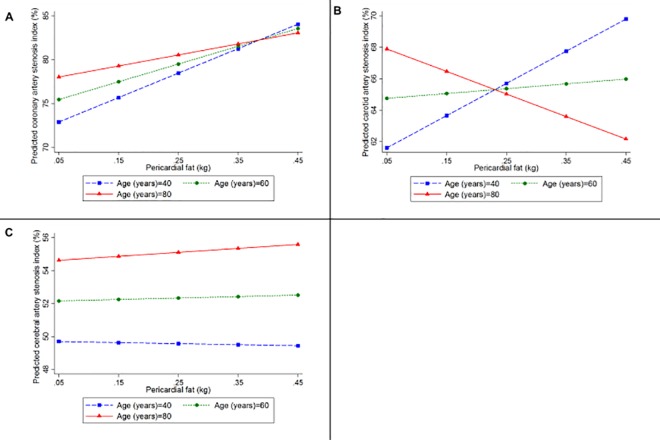
Predicted values of the stenosis index in (A) coronary, (B) carotid, and (C) cerebral arteries, according to the amount of abdominal visceral fat calculated for participants with 40 (blue line), 60 (green line), and 80 (red line) years old, using linear regression models adjusted for height, age, sex, smoking status, alcohol use, physical inactivity, hypertension, and diabetes mellitus, and including an interaction between age and abdominal visceral fat.
Fig 5.
Predicted values of the number of plaques in (A) coronary and (B) cerebral arteries according to the amount of abdominal visceral fat, and in the (C) coronary and (D) cerebral arteries according to the amount of pericardial fat for participants with 40 (blue line), 60 (green line), and 80 (red line) years old, using linear regression models adjusted for height, age, sex, smoking status, alcohol use, physical inactivity, hypertension, and diabetes mellitus, and including an interaction between age and abdominal visceral fat or pericardial fat.
Table 3. Association between visceral fat and atherosclerosis in different arterial sites, considering an interaction term between visceral fat and age (n = 240).
| Arteries | Model 1a | Model 2b | ||||
|---|---|---|---|---|---|---|
| Coef | 95% CI | p | Coef | 95% CI | p | |
| Stenosis index | ||||||
| Coronary | ||||||
| AVF | 5.03 | -0.42; 10.48 | 0.07 | 3.63 | -2.35; 9.60 | 0.23 |
| Age | 0.25 | 0.08; 0.43 | 0.005 | 0.16 | -0.03; 0.36 | 0.10 |
| AVF * age | -0.06 | -0.14; 0.02 | 0.13 | -0.03 | -0.12; 0.05 | 0.47 |
| PF | 52.64 | -26.00; 131.29 | 0.19 | 43.18 | -40.15; 126.52 | 0.31 |
| Age | 0.21 | 0.01; 0.40 | 0.03 | 0.15 | -0.06; 0.35 | 0.16 |
| PF * age | -0.54 | -1.68; 0.60 | 0.35 | -0.38 | -1.58; 0.81 | 0.53 |
| Carotid | ||||||
| AVF | 5.30 | - 1.55; 9.05 | 0.01 | 5.29 | 1.12; 9.45 | 0.01 |
| Age | 0.24 | 0.12; 0.36 | <0.0001 | 0.22 | 0.09; 0.36 | 0.001 |
| AVF * age | -0.08 | -0.14; -0.03 | 0.003 | -0.08 | -0.14; -0.02 | 0.009 |
| PF | 54.14 | -0.44; 108.71 | 0.05 | 55.33 | -2.92; 113.59 | 0.06 |
| Age | 0.22 | 0.08; 0.35 | 0.002 | 0.20 | 0.06; 0.34 | 0.01 |
| PF * age | -0.85 | -1.64; -0.06 | 0.03 | -0.87 | -1.70; -0.04 | 0.04 |
| Cerebral | ||||||
| AVF | 0.47 | -3.65; 4.59 | 0.82 | 0.83 | -3.71; 5.36 | 0.72 |
| Age | 0.16 | 0.03; 0.29 | 0.01 | 0.14 | -0.003; 0.29 | 0.06 |
| AVF * age | -0.005 | -0.07; 0.05 | 0.85 | -0.01 | -0.07; 0.06 | 0.82 |
| PF | -17.46 | -76.79; 41.87 | 0.56 | -3.67 | -66.78; 59.43 | 0.91 |
| Age | 0.12 | -0.02; 0.26 | 0.10 | 0.12 | -0.03; 0.27 | 0.13 |
| PF * age | 0.24 | -0.62; 1.10 | 0.58 | 0.07 | -0.83; 0.98 | 0.87 |
| Number of plaques | ||||||
| Coronary | ||||||
| AVF | 0.85 | -1.18; 2.88 | 0.41 | 0.19 | -2.09; 2.48 | 0.87 |
| Age | 0.07 | 0.004; 0.13 | 0.04 | 0.04 | -0.03; 0.11 | 0.23 |
| AVF * age | -0.004 | -0.03; 0.02 | 0.79 | 0.01 | -0.03; 0.04 | 0.68 |
| PF | 21.94 | -5.90; 49.77 | 0.12 | 22.49 | -7.63; 52.62 | 0.14 |
| Age | 0.09 | 0.02; 0.16 | 0.01 | 0.09 | 0.02; 0.17 | 0.02 |
| PF * age | -0.23 | -0.63; 0.17 | 0.26 | -0.24 | -0.67; 0.19 | 0.28 |
| Cerebral | ||||||
| AVF | -0.50 | -3.35; 2.34 | 0.73 | -0.61 | -3.81; 2.58 | 0.70 |
| Age | 0.14 | 0.05; 0.23 | 0.001 | 0.11 | 0.01; 0.21 | 0.03 |
| AVF * age | 0.01 | -0.03; 0.05 | 0.64 | 0.01 | -0.03; 0.06 | 0.52 |
| PF | -11.02 | -49.36; 27.31 | 0.57 | -11.70 | -53.02; 29.62 | 0.58 |
| Age | 0.13 | 0.04; 0.23 | 0.005 | 0.11 | 0.01; 0.21 | 0.04 |
| PF * age | 0.17 | -0.38; 0.73 | 0.53 | 0.18 | -0.40; 0.77 | 0.54 |
Coef = coefficient; CI = confidence interval; AVF = abdominal visceral fat; PF = Pericardial fat
aModel 1: Linear regression model, adjusted for height and age, including an interaction term between visceral fat and age
bModel 2: Linear regression model, adjusted for height, age, sex, smoking status, alcohol use, physical inactivity, hypertension, and diabetes mellitus, including an interaction term between visceral fat and age
Fig 6.
Predicted values of the stenosis index in (A) coronary, (B) carotid, and (C) cerebral arteries, according to the amount of pericardial fat calculated for participants with 40 (blue line), 60 (green line), and 80 (red line) years old, using linear regression models adjusted for height, age, sex, smoking status, alcohol use, physical inactivity, hypertension, and diabetes mellitus, and including an interaction between age and pericardial fat.
Association between visceral fat and carotid artery atherosclerosis
AVF (p = 0.63) and PF (p = 0.52) were not associated with the carotid artery stenosis index (Table 2) in multivariate analysis. However, we observed an interaction of age with both AVF (coefficient = -0.08; 95%CI = -0.14; -0.02, p = 0.009) (Fig 4B) and PF (coefficient = -0.87; 95%CI = -1.70; -0.04, p = 0.04) (Fig 6B) (Table 3). While middle-aged adults showed a worse atherosclerotic burden in carotid arteries with increases in AVF and PF, we observed an inverse association of carotid artery atherosclerotic burden with the AVF and PF weight in the oldest subjects.
Association between visceral fat and cerebral artery atherosclerosis
AVF was not associated with the cerebral artery stenosis index (p = 0.53) nor with the number of cerebral artery atherosclerotic plaques after adjusting for confounding factors (p = 0.23) (Table 2). We did not observe an interaction between AVF and age on cerebral artery atherosclerotic burden (p = 0.82) (Fig 4C) nor on its disease extent (p = 0.52) (Fig 5B) (Table 3). Similarly, multivariate analysis showed that PF was also not associated with the stenosis index in cerebral arteries (p = 0.83) nor with the number of plaques (p = 0.83) (Table 2). The interaction between age and PF on atherosclerotic burden (p = 0.87) (Fig 6C) and on its extent (p = 0.54) (Fig 5D) was not significant (Table 3).
Discussion
Our study has demonstrated that the association between visceral fat and atherosclerosis is highly variable depending on the location of visceral fat and the vascular bed. While PF was associated with atherosclerotic burden in the aorta and marginally associated with coronary artery, AVF was associated with coronary artery atherosclerosis. On the other hand, visceral fat was not associated with atherosclerosis in the cerebral and carotid arteries. Interestingly, the effect of visceral fat on carotid artery atherosclerotic burden seems to be modified by age.
Visceral fat seems to have local and systemic effects on atherosclerosis pathophysiology [35–37]. Among the systemic inflammatory effects, macrophage infiltration was found in the AVF in obese individuals [35]. These cells are involved in the production of adipokines, which are related to metabolic syndrome [38], and increased cardiovascular risk [39]. Previous imaging studies have shown that larger deposits of AVF were associated with higher calcification scores in the abdominal aorta [40, 41], which contradicts our current findings. However, some important methodological differences need to be highlighted. First, we examined the whole aorta; and second, we evaluated the confluence, ulceration, and protrusion of the plaques, including non-calcified plaque components that are not represented by the calcium score [30]. On the other hand, we found that AVF was indeed associated with the burden and extent of coronary artery atherosclerosis, which corroborates previous imaging studies [9, 42]. However, these studies did not evaluate the atherosclerosis burden as a continuous variable, but as a categorical variable. Kortelainen and Sarkioja were the first to use autopsy material, and they also found an association between AVF and coronary narrowing [7, 8]. However, in one study [20], they did not find an association between AVF and the extent of coronary atherosclerosis; and in another study, they did not evaluate disease extent [7]. Moreover, their samples were restricted to middle-age White subjects [7, 8, 20]. Our results extend this knowledge to a larger and ethnically diverse sample with a wider age range. Interestingly, no association was noted between AVF and carotid or cerebral artery atherosclerosis. This finding contrasts with previous imaging-based studies, which found an association between AVF and CIMT [11], as well as with total cerebral artery plaque volume; however, that study was limited by a small sample size of 25 subjects, which only allowed for univariate analysis [14]. In another study, AVF was associated with stenosis or occlusion in cerebral arteries; however, stenosis was evaluated as a categorical variable using the cut-off of ≥70%, and the participants were Asian and aged 40 years or older [43].
On the other hand, the effect of visceral fat on atherosclerosis may not be fully explained by systemic effects. In fact, prior data suggest that epicardial fat can locally contribute to atherosclerosis by lipotoxicity, cytokine secretion, and the increased production of hemostatic factors [44], thereby inducing an inflammatory response that may play a role in coronary atherogenesis [37, 45]. Previous studies have already demonstrated that the effect of epicardial fat may extend to aortic atherosclerosis as measured by the percentage of obstruction by computed tomography [13]. In our study, we observed an association between PF and the severity of aorta atherosclerosis. However, PF was marginally associated with the burden and extent of coronary artery atherosclerosis. Most of the previous imaging studies evaluated only the epicardial fat [19, 46] or the epicardial and paracardial fat separately [18], and few studies evaluated the PF itself [5, 6]. Moreover, epicardial and paracardial fat were estimated indirectly using computed tomography [5, 6, 18, 46] or computerized photographs of the heart to quantify the epicardial fat thickness and area [19]. In addition, analyses were adjusted only for body mass index [18] or age [7] in some studies, while we adjusted our regression models for a more comprehensive set of possible confounding factors. Moreover, coronary artery atherosclerosis was evaluated macroscopically in some autopsy studies [18, 19], instead of using more robust morphometric measurements such as the ones that we performed. Finally, differences in age and race composition could also explain the marginal association between PF and coronary artery atherosclerosis in our study. Since we compared AVF with PF in the same individuals under the same conditions, and adjusting for the same set of confounding factors, our results suggest AVF seems to have more effect in coronary artery atherosclerosis than PF. Therefore, coronary artery atherosclerosis may be more influenced by systemic than local effects, while aorta seems to be more influenced by local effects. We did not find an association between PF and carotid or cerebral artery atherosclerosis. In contrast, previous studies have found an association between CIMT and epicardial fat [12, 15], but data regarding cerebral arteries were not found in the literature.
We were also able to demonstrate the effect modification of age on both the association of AVF and PF with carotid artery atherosclerosis. While we found a positive association of visceral fat with carotid artery atherosclerosis in younger individuals, this association became negative in older adults. Although this might be related to survival bias, one may speculate whether the effect might be variable across different age groups. Greater AVF and PF were related to higher coronary atherosclerosis burden [5–9, 18–20, 46]. Nevertheless, since CAD may develop before carotid atherosclerosis [47], individuals with greater AVF and PF may die earlier from CAD, leading to a reverse association between visceral fat and carotid atherosclerosis in older individuals; such a phenomenon is known as the “obesity paradox” that has been described in other chronic diseases [48]. However, longitudinal studies with multiple measurements of visceral fat and carotid artery atherosclerosis across the lifespan would be necessary to confirm this finding. We did not observe an interaction of age with AVF and PF regarding atherosclerosis in the coronary and cerebral arteries. To our knowledge, these interactions were not evaluated in previous studies.
The results of our study should be interpreted with consideration of some limitations. First, since this was a cross-sectional study, we could not establish the causal relationship between visceral adiposity and systemic atherosclerosis. Moreover, information about cardiovascular risk factors was collected post mortem. Previous studies from our group showed good reliability of the data collected from the NOK [26, 49], and we excluded participants with limited contact with the NOK. Additionally, it is important to highlight that the main measurements (visceral fat and atherosclerosis) were not affected by the lack of follow-up since they were measured directly. On the other hand, our study has several strengths. We used morphometric measurements of atherosclerosis rather than using estimated atherosclerosis quantification [7, 8, 18, 19]. Similarly, we used direct measurements to quantify the visceral fat instead of imaging methods [5, 6, 12–15, 18, 19, 46]. Another strength was the larger sample size compared to previous autopsy studies that assessed a maximum of 116 individuals [7, 8, 18–20], allowing us to test the interaction between age and visceral fat. Furthermore, the mean post mortem interval of the participants was short compared with other studies [19] in that the autopsy occurred within 48 hours after death. Finally, to the best of our knowledge, this is the first autopsy study to investigate the association of AVF and PF with systemic atherosclerosis in addition to coronary artery atherosclerosis. Future research should be directed towards the assessment of the atherosclerotic plaque composition and the fibrous cap thickness.
Conclusion
We found that AVF was associated with coronary artery atherosclerosis. Only PF was related to the severity of atherosclerosis in the aorta and marginally associated with coronary atherosclerosis. We also found an interaction of age with AVF and PF regarding the carotid artery stenosis index. Understanding the association between specific visceral fat deposits and systemic atherosclerosis is important for the identification of individuals at higher risk in order to promote preventive actions and reduce atherosclerotic events.
Acknowledgments
We thank the Sao Paulo Autopsy Service, Laboratory of Cardiovascular Pathology, and Pathophysiology in Aging Lab staff. We are very grateful to the NOK who agreed to participate in this study.
Data Availability
Data contain patient-sensitive material and therefore are available to qualified researchers who have IRB approval after agreeing to a material transfer agreement. To request these data, please contact Ms. Marta Crocci at gerolab@gmail.com. Once approved, data are available via http://www2.fm.usp.br/gerolab/mostrahp.php?origem=gerolab&xcod=PLOS%20Medicine&dequem=Paper%20datasets.
Funding Statement
AN was supported by a scholarship from the CAPES Foundation, Ministry of Education of Brazil (1074888) (http://www.capes.gov.br/). DSF-I was supported by a scholarship from the FAPESP (Sao Paulo Research Foundation, 2013/ 12290-3) (http://www.fapesp.br/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.WHO. Obesity and overweight: World Health Organization; 2016 [cited 2016 July]. Available from: http://www.who.int/mediacentre/factsheets/fs311/en/.
- 2.Bastien M, Poirier P, Lemieux I, Després JP. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog Cardiovasc Dis. 2014;56(4):369–81. 10.1016/j.pcad.2013.10.016. doi: 10.1016/j.pcad.2013.10.016 . [DOI] [PubMed] [Google Scholar]
- 3.Jeong JW, Jeong MH, Yun KH, Oh SK, Park EM, Kim YK, et al. Echocardiographic epicardial fat thickness and coronary artery disease. Circ J. 2007;71(4):536–9. . [DOI] [PubMed] [Google Scholar]
- 4.Ding J, Hsu FC, Harris TB, Liu Y, Kritchevsky SB, Szklo M, et al. The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA). The American journal of clinical nutrition. 2009;90(3):499–504. Epub 2009/07/03. 10.3945/ajcn.2008.27358 [pii]. doi: 10.3945/ajcn.2008.27358 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taguchi R, Takasu J, Itani Y, Yamamoto R, Yokoyama K, Watanabe S, et al. Pericardial fat accumulation in men as a risk factor for coronary artery disease. Atherosclerosis. 2001;157(1):203–9. Epub 2001/06/28. . [DOI] [PubMed] [Google Scholar]
- 6.Greif M, Becker A, von Ziegler F, Lebherz C, Lehrke M, Broedl UC, et al. Pericardial adipose tissue determined by dual source CT is a risk factor for coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29(5):781–6. 10.1161/ATVBAHA.108.180653. doi: 10.1161/ATVBAHA.108.180653 . [DOI] [PubMed] [Google Scholar]
- 7.Kortelainen ML, Sarkioja T. Coronary atherosclerosis and myocardial hypertrophy in relation to body fat distribution in healthy women: an autopsy study on 33 violent deaths. Int J Obes Relat Metab Disord. 1997;21(1):43–9. Epub 1997/01/01. . [DOI] [PubMed] [Google Scholar]
- 8.Kortelainen ML, Sarkioja T. Extent and composition of coronary lesions in relation to fat distribution in women younger than 50 years of age. Arterioscler Thromb Vasc Biol. 1999;19(3):695–9. Epub 1999/03/12. . [DOI] [PubMed] [Google Scholar]
- 9.Ohashi N, Yamamoto H, Horiguchi J, Kitagawa T, Kunita E, Utsunomiya H, et al. Association between visceral adipose tissue area and coronary plaque morphology assessed by CT angiography. JACC Cardiovasc Imaging. 2010;3(9):908–17. 10.1016/j.jcmg.2010.06.014. doi: 10.1016/j.jcmg.2010.06.014 . [DOI] [PubMed] [Google Scholar]
- 10.Bertaso AG, Bertol D, Duncan BB, Foppa M. Epicardial fat: definition, measurements and systematic review of main outcomes. Arq Bras Cardiol. 2013;101(1):e18–28. 10.5935/abc.20130138. doi: 10.5935/abc.20130138 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lear SA, Humphries KH, Kohli S, Frohlich JJ, Birmingham CL, Mancini GB. Visceral adipose tissue, a potential risk factor for carotid atherosclerosis: results of the Multicultural Community Health Assessment Trial (M-CHAT). Stroke. 2007;38(9):2422–9. 10.1161/STROKEAHA.107.484113. doi: 10.1161/STROKEAHA.107.484113 . [DOI] [PubMed] [Google Scholar]
- 12.Kocaman SA, Baysan O, Çetin M, Kayhan Altuner T, Polat Ocaklı E, Durakoğlugil ME, et al. An increase in epicardial adipose tissue is strongly associated with carotid-intima media thickness and atherosclerotic plaque, but LDL only with the plaque. Anatol J Cardiol. 2016. 10.14744/AnatolJCardiol.2016.6885. doi: 10.14744/AnatolJCardiol.2016.6885 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yorgun H, Canpolat U, Hazırolan T, Sunman H, Ateş AH, Gürses KM, et al. Epicardial adipose tissue thickness predicts descending thoracic aorta atherosclerosis shown by multidetector computed tomography. Int J Cardiovasc Imaging. 2012;28(4):911–9. 10.1007/s10554-011-9899-x. doi: 10.1007/s10554-011-9899-x . [DOI] [PubMed] [Google Scholar]
- 14.Karcher HS, Holzwarth R, Mueller HP, Ludolph AC, Huber R, Kassubek J, et al. Body fat distribution as a risk factor for cerebrovascular disease: an MRI-based body fat quantification study. Cerebrovasc Dis. 2013;35(4):341–8. 10.1159/000348703. doi: 10.1159/000348703 . [DOI] [PubMed] [Google Scholar]
- 15.Natale F, Tedesco MA, Mocerino R, de Simone V, Di Marco GM, Aronne L, et al. Visceral adiposity and arterial stiffness: echocardiographic epicardial fat thickness reflects, better than waist circumference, carotid arterial stiffness in a large population of hypertensives. Eur J Echocardiogr. 2009;10(4):549–55. Epub 2009/02/13. 10.1093/ejechocard/jep002. doi: 10.1093/ejechocard/jep002 . [DOI] [PubMed] [Google Scholar]
- 16.Rabkin SW. Epicardial fat: properties, function and relationship to obesity. Obes Rev. 2007;8(3):253–61. 10.1111/j.1467-789X.2006.00293.x. doi: 10.1111/j.1467-789X.2006.00293.x . [DOI] [PubMed] [Google Scholar]
- 17.van der Kooy K, Seidell JC. Techniques for the measurement of visceral fat: a practical guide. Int J Obes Relat Metab Disord. 1993;17(4):187–96. Epub 1993/04/01. . [PubMed] [Google Scholar]
- 18.Sequeira DI, Ebert LC, Flach PM, Ruder TD, Thali MJ, Ampanozi G. The correlation of epicardial adipose tissue on postmortem CT with coronary artery stenosis as determined by autopsy. Forensic Sci Med Pathol. 2015;11(2):186–92. 10.1007/s12024-015-9659-7. doi: 10.1007/s12024-015-9659-7 . [DOI] [PubMed] [Google Scholar]
- 19.Silaghi A, Piercecchi-Marti MD, Grino M, Leonetti G, Alessi MC, Clement K, et al. Epicardial adipose tissue extent: relationship with age, body fat distribution, and coronaropathy. Obesity (Silver Spring). 2008;16(11):2424–30. 10.1038/oby.2008.379. doi: 10.1038/oby.2008.379 . [DOI] [PubMed] [Google Scholar]
- 20.Kortelainen ML, Sarkioja T. Extent and composition of coronary lesions and degree of cardiac hypertrophy in relation to abdominal fatness in men under 40 years of age. Arterioscler Thromb Vasc Biol. 1997;17(3):574–9. Epub 1997/03/01. . [DOI] [PubMed] [Google Scholar]
- 21.Kortelainen ML, Sarkioja T. Visceral fat and coronary pathology in male adolescents. Int J Obes Relat Metab Disord. 2001;25(2):228–32. Epub 2001/06/19. 10.1038/sj.ijo.0801466. doi: 10.1038/sj.ijo.0801466 . [DOI] [PubMed] [Google Scholar]
- 22.Grinberg LT, Ferretti RE, Farfel JM, Leite R, Pasqualucci CA, Rosemberg S, et al. Brain bank of the Brazilian aging brain study group—a milestone reached and more than 1,600 collected brains. Cell Tissue Bank. 2007;8(2):151–62. Epub 2006/11/01. 10.1007/s10561-006-9022-z. doi: 10.1007/s10561-006-9022-z . [DOI] [PubMed] [Google Scholar]
- 23.Nishizawa A, Suemoto CK, Farias DS, Campos FM, da Silva KC, Cuelho A, et al. Association between adiposity and systemic atherosclerosis: a protocol of a cross-sectional autopsy study. Open Heart. 2016;3(2):e000433 10.1136/openhrt-2016-000433. doi: 10.1136/openhrt-2016-000433 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crossley RP. Crossley Checklist—A system for determination of the time of death The Police Chief. 1974;41(3):65–8, 85. [Google Scholar]
- 25.ABEP: Associação Brasileira de Empresas de Pesquisa. 2013 [cited 2014 10 Nov]. Available from: http://www.abep.org.
- 26.Ferretti REL, Damin AE, Brucki SMD, Morillo LS, Perroco TR, Campora F, et al. Post-mortem diagnosis of dementia by informant interview. Dement Neuropsychol. 2010;4(2):138–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suemoto CK, Nitrini R, Grinberg LT, Ferretti RE, Farfel JM, Leite RE, et al. Atherosclerosis and dementia: a cross-sectional study with pathological analysis of the carotid arteries. Stroke. 2011;42(12):3614–5. 10.1161/STROKEAHA.111.628156. doi: 10.1161/STROKEAHA.111.628156 . [DOI] [PubMed] [Google Scholar]
- 28.Roher AE, Esh C, Kokjohn TA, Kalback W, Luehrs DC, Seward JD, et al. Circle of willis atherosclerosis is a risk factor for sporadic Alzheimer's disease. Arterioscler Thromb Vasc Biol. 2003;23(11):2055–62. Epub 2003/09/27. 10.1161/01.atv.0000095973.42032.44. doi: 10.1161/01.ATV.0000095973.42032.44 . [DOI] [PubMed] [Google Scholar]
- 29.Koo TK, Li MY. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med. 2016;15(2):155–63. Epub 2016/03/31. 10.1016/j.jcm.2016.02.012. doi: 10.1016/j.jcm.2016.02.012 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dolan H, Crain B, Troncoso J, Resnick SM, Zonderman AB, Obrien RJ. Atherosclerosis, dementia, and Alzheimer disease in the Baltimore Longitudinal Study of Aging cohort. Ann Neurol. 2010;68(2):231–40. Epub 2010/08/10. 10.1002/ana.22055. doi: 10.1002/ana.22055 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuipers AL, Zmuda JM, Carr JJ, Terry JG, Nair S, Cvejkus R, et al. Association of ectopic fat with abdominal aorto-illiac and coronary artery calcification in african ancestry men. Atherosclerosis. 2017;263:198–204. Epub 2017/06/16. 10.1016/j.atherosclerosis.2017.06.030. doi: 10.1016/j.atherosclerosis.2017.06.030 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inomoto A, Fukuda R, Deguchi Phn J, Kato G, Kanzaki Rpt R, Hiroshige Rpt K, et al. The association between the body composition and lifestyle affecting pulmonary function in Japanese workers. J Phys Ther Sci. 2016;28(10):2883–9. Epub 2016/10/28. 10.1589/jpts.28.2883. doi: 10.1589/jpts.28.2883 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seo JA, Cho H, Eun CR, Yoo HJ, Kim SG, Choi KM, et al. Association between visceral obesity and sarcopenia and vitamin D deficiency in older Koreans: the Ansan Geriatric Study. J Am Geriatr Soc. 2012;60(4):700–6. Epub 2012/02/08. 10.1111/j.1532-5415.2012.03887.x. doi: 10.1111/j.1532-5415.2012.03887.x . [DOI] [PubMed] [Google Scholar]
- 34.Berg RM, Wallaschofski H, Nauck M, Rettig R, Markus MR, Laqua R, et al. Positive Association Between Adipose Tissue and Bone Stiffness. Calcif Tissue Int. 2015;97(1):40–9. Epub 2015/05/01. 10.1007/s00223-015-0008-3. doi: 10.1007/s00223-015-0008-3 . [DOI] [PubMed] [Google Scholar]
- 35.Wu FZ, Wu CC, Kuo PL, Wu MT. Differential impacts of cardiac and abdominal ectopic fat deposits on cardiometabolic risk stratification. BMC Cardiovasc Disord. 2016;16:20 Epub 2016/01/22. 10.1186/s12872-016-0195-5. doi: 10.1186/s12872-016-0195-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117(5):605–13. 10.1161/CIRCULATIONAHA.107.743062. doi: 10.1161/CIRCULATIONAHA.107.743062 . [DOI] [PubMed] [Google Scholar]
- 37.Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108(20):2460–6. Epub 2003/10/27. 10.1161/01.CIR.0000099542.57313.C5. doi: 10.1161/01.CIR.0000099542.57313.C5 . [DOI] [PubMed] [Google Scholar]
- 38.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116(1):39–48. Epub 2007/06/18. 10.1161/CIRCULATIONAHA.106.675355. doi: 10.1161/CIRCULATIONAHA.106.675355 . [DOI] [PubMed] [Google Scholar]
- 39.Liu J, Fox CS, Hickson D, Bidulescu A, Carr JJ, Taylor HA. Fatty liver, abdominal visceral fat, and cardiometabolic risk factors: the Jackson Heart Study. Arterioscler Thromb Vasc Biol. 2011;31(11):2715–22. 10.1161/ATVBAHA.111.234062. doi: 10.1161/ATVBAHA.111.234062 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Efe D, Aygün F, Acar T, Yildiz M, Gemici K. Investigation of relation between visceral and subcutaneous abdominal fat volumes and calcified aortic plaques via multislice computed tomography. Vascular. 2015;23(4):396–402. Epub 2014/09/22. 10.1177/1708538114552012. doi: 10.1177/1708538114552012 . [DOI] [PubMed] [Google Scholar]
- 41.Liu J, Musani SK, Bidulescu A, Carr JJ, Wilson JG, Taylor HA, et al. Fatty liver, abdominal adipose tissue and atherosclerotic calcification in African Americans: the Jackson Heart Study. Atherosclerosis. 2012;224(2):521–5. Epub 2012/08/10. 10.1016/j.atherosclerosis.2012.07.042. doi: 10.1016/j.atherosclerosis.2012.07.042 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marques MD, Santos RD, Parga JR, Rocha-Filho JA, Quaglia LA, Miname MH, et al. Relation between visceral fat and coronary artery disease evaluated by multidetector computed tomography. Atherosclerosis. 2010;209(2):481–6. 10.1016/j.atherosclerosis.2009.10.023. doi: 10.1016/j.atherosclerosis.2009.10.023 . [DOI] [PubMed] [Google Scholar]
- 43.Higuchi S, Kabeya Y, Kato K. Visceral-to-subcutaneous fat ratio is independently related to small and large cerebrovascular lesions even in healthy subjects. Atherosclerosis. 2017;259:41–5. Epub 2017/03/02. 10.1016/j.atherosclerosis.2017.03.001. doi: 10.1016/j.atherosclerosis.2017.03.001 . [DOI] [PubMed] [Google Scholar]
- 44.Lim S, Meigs JB. Links between ectopic fat and vascular disease in humans. Arterioscler Thromb Vasc Biol. 2014;34(9):1820–6. 10.1161/ATVBAHA.114.303035. doi: 10.1161/ATVBAHA.114.303035 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirata Y, Kurobe H, Akaike M, Chikugo F, Hori T, Bando Y, et al. Enhanced inflammation in epicardial fat in patients with coronary artery disease. Int Heart J. 2011;52(3):139–42. . [DOI] [PubMed] [Google Scholar]
- 46.Bettencourt N, Toschke AM, Leite D, Rocha J, Carvalho M, Sampaio F, et al. Epicardial adipose tissue is an independent predictor of coronary atherosclerotic burden. Int J Cardiol. 2012;158(1):26–32. 10.1016/j.ijcard.2010.12.085. doi: 10.1016/j.ijcard.2010.12.085 . [DOI] [PubMed] [Google Scholar]
- 47.Solberg LA, McGarry PA, Moossy J, Tejada C, Loken AC, Robertson WB, et al. Distribution of cerebral atherosclerosis by geographic location, race, and sex. Lab Invest. 1968;18(5):604–12. . [PubMed] [Google Scholar]
- 48.Lajous M, Banack HR, Kaufman JS, Hernán MA. Should patients with chronic disease be told to gain weight? The obesity paradox and selection bias. Am J Med. 2015;128(4):334–6. Epub 2014/11/22. 10.1016/j.amjmed.2014.10.043. doi: 10.1016/j.amjmed.2014.10.043 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Apolinario D, Brucki SM, Ferretti RE, Farfel JM, Magaldi RM, Busse AL, et al. Estimating premorbid cognitive abilities in low-educated populations. PLoS One. 2013;8(3):e60084 Epub 2013/04/05. 10.1371/journal.pone.0060084. doi: 10.1371/journal.pone.0060084 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data contain patient-sensitive material and therefore are available to qualified researchers who have IRB approval after agreeing to a material transfer agreement. To request these data, please contact Ms. Marta Crocci at gerolab@gmail.com. Once approved, data are available via http://www2.fm.usp.br/gerolab/mostrahp.php?origem=gerolab&xcod=PLOS%20Medicine&dequem=Paper%20datasets.