Abstract
Adolescence is hypothesized to be a critical period for the maturation of self-regulatory capacities, including those that depend on interoceptive sensitivity, but the neural basis of interoceptive regulation in adolescence is unknown. We used functional magnetic resonance imaging and psychophysiology to study interoceptive regulation in healthy adolescent females. Participants regulated their gut activities in response to a virtual roller coaster by deep breathing aided by visually monitoring their online electrogastrogram (EGG) activity through a virtual thermometer (i.e., gut biofeedback), or without biofeedback. Analyses focused on the insula, given its putative role in interoception. The bilateral posterior insula showed increased activation in the no-biofeedback compared to biofeedback condition, suggesting that the participants relied more on interoceptive input when exteroceptive feedback was unavailable. The bilateral dorsal anterior insula showed activation linearly associated with age during both induction and regulation, and its activation during regulation correlated positively with change of EGG in the tachygastria frequency band from induction to regulation. Induction-related activation in the bilateral ventral anterior insula was nonlinearly associated with age and peaked at mid-adolescence. These results implicate different developmental trajectories of distinct sub-regions of the insula in interoceptive processes, with implications for competing neurobiological theories of female adolescent development.
Keywords: adolescence, functional magnetic resonance imaging, insula, interoception, self regulation
Interoception is a general term encompassing the perception of sensations from the viscera (e.g., gut motility, heart palpitations). Interoceptive processes map the current physiological state of the body and form a critical foundation of self-awareness (Craig, 2002). Awareness of visceral change helps signal to an individual any deviations from homeostasis and the corresponding need to up- or down-regulate the viscera to regain a steady state (Craig, 2008). We refer to this set of bioregulatory processes as interoceptive regulation. The perception and regulation of interoceptive signals are critical constituents of self-awareness and behavioral control.
Adolescence -- the bridge between childhood and adulthood that begins with pubertal maturation -- is one of the most sensitive periods for the emergence of self-awareness, and perhaps correspondingly, in changes in the elaboration of interoceptive states. Behaviors dependent on interoceptive processes, such as eating and drinking, become more contextually constrained and socially moderated in adolescence. In addition, psychiatric disorders with prominent somatic features (e.g., panic disorder) tend to emerge during this developmental period. Yet, despite its importance, interoception has not been widely incorporated into neurocognitive models of adolescent development. Traditional dual-systems models of self-regulation in adolescence emphasize that prefrontal cortical development associated with executive control peaks later in adolescence relative to sensory-limbic networks subserving emotion, cognition, and social behavior (e.g., Casey et al., 2011). Other evidence indicates that brain regions mediating socioaffective processing and reward valuation specifically show a non-linear developmental trajectory peaking in mid-adolescence (Crone & Dahl, 2012; Pfeifer & Allen, 2012). Both models indicate that mid-adolescence which corresponds to roughly to ages 13 to 17 (Crone & Dahl, 2012) may be a particularly crucial developmental window for the contextualization of interoceptive processes by social and affective factors, whereas other executive control influences over interoception may peak later in development. One possible contribution to these myriad neurodevelopmental changes is that there is increased binding of interoceptive state with context, and thus adolescents can increasingly appreciate the cause and effect relationship among environmental contributions to visceral change (i.e., knowing what makes them feel better or worse and using that information to guide their behavior).
Understanding the role of interoception in adolescent development may benefit from investigation of the neuronal basis of interoception. The insular cortex is hypothesized to be critical for the elaboration of visceral states and their contextual regulation (Craig, 2010). The insula receives inputs from afferent fibers that carry information concerning the internal state of the body (Craig, 2002). Craig (2010) proposed that the posterior insula codes lower-level basic interoceptive, gustatory, and somatosensory information, and that as one progresses to the posterior insula, this low-level sensory information is linked with higher- level cognitive and affective networks that impart salience and meaning to these basic interoceptive signals (Simmons et al., 2014). For instance, objective intensity of graded cooling stimuli correlates with activation of the contralateral dorsal middle/posterior insula, whereas subjective ratings of thermal intensity correlates with activation of the right ipsilateral anterior insula (Craig et al., 2000). Several recent studies have further parcellated the human insula using meta-analysis, analysis of gray matter structure, and cluster analysis of resting-state connectivity (Cauda et al., 2011; Chang et al., 2012; Deen, Pitskel & Pelphrey, 2011; Kelly et al., 2012). These studies have reached the consensus that the insula can be functionally parcellated into a posterior part and two anterior parts. The posterior insula is functionally connected to mostly sensorimotor areas and the posterior dorsal anterior cingulate cortex, and it is often activated in tasks involving action, perception, and interoception. The dorsal anterior insula (which includes parts of the mid-insula) is functionally connected to a control network (Dosenbach et al., 2007) including the anterior dorsal anterior cingulate cortex, pre-supplementary motor area, supramarginal gyrus, and inferior frontal gyrus, and it is often involved in tasks related to cognitive regulation. The ventral anterior insula is functionally connected to the ventral anterior cingulate cortex and limbic regions, including the amygdala and ventral tegmental area, and it is often involved in emotion processing. Thus, the posterior insula appears to be a key region for perception of interoceptive feelings, whereas the anterior insula appears to be critical for contextual integration of interoceptive input, association of interoception and emotion, and cognitive regulation of the integrated interoceptive representations. Craig (2010) summarized this description by stating that in the anterior insula, interoceptive feelings are contextualized – in regard to value, time, and circumstance, such that an individual has a coherent “global emotional moment” – arguably the essence of self-awareness. Despite these advances in characterizing insula function, the neural mechanisms of voluntary interoceptive regulation have not been well characterized, and no study, to our knowledge, has investigated the neurodevelopmental trajectory of interoceptive regulation in adolescence.
Here we wish to integrate neurodevelopmental models of adolescent development with the functional and anatomical parcellation of insula circuitry to predict trajectories of change related to interoceptive capacities. Because components of the insula are hypothesized to play different roles in interoception, different aspects of interoceptive capacities may develop across different phases of adolescence. On one hand, the dorsal anterior insula, given its hypothesized role in cognitive regulation of interoception, may show a linear correlation with age during interoceptive regulation, consistent with dual-systems models of executive control in adolescence. The ventral anterior insula, given its hypothesized role in binding of interoceptive sensations with emotional salience, may show a quadratic pattern peaking at mid-adolescence, consistent with recent developmental models of socioemotional integration and contextualization. Finally, the posterior insula, given its hypothesized role in perception of visceral states, should mature early, along with exteroceptive sensory regions (vision, hearing, etc.), and exhibit little noticeable change across adolescence. These considerations, in combination, would imply a developmental shift towards increasing elaboration of the meaning of interoceptive signals and their regulation.
In this study, we investigated the ability of adolescent females to perceive and regulate interoceptive signals and to contextually integrate those interoceptive signals with visual feedback. We were interested in various aspects of interoception, including induction of interoceptive experience and regulation of interoceptive experience with or without contextual information. Modeled after a biofeedback task developed by Critchley and colleagues (2001), we induced alterations in interoceptive responses in the stomach (queasy ‘gut feelings’) by having participants “ride” a virtual roller coaster in a first-person (egocentric) 2-D perspective while undergoing functional magnetic resonance imaging (fMRI). Sequences of roller coaster clips were altered with periods of instructed regulation, during which the participants adjusted their gut feelings through deep, diaphragmatic breathing. To investigate the influence of contextual information on interoceptive regulation, physiological biofeedback was used in half of the regulation trials, in which individuals regulated interoceptive sensations with the aid of contextual feedback: biofeedback information was provided visually in the form of a thermometer indexing online gut activity measured by electrogastrogram (EGG). In the other half of the regulation trials, participants relied solely on interoceptive cues to guide their regulatory breathing.
We hypothesized that participants would successfully regulate their physiological response across both conditions, although they might be more capable of doing it when visually-presented biofeedback information is available. Given that feedback is provided visually in the biofeedback condition, we hypothesized relatively greater fMRI activity in the visual cortex in this condition relative to the no-biofeedback condition. In contrast, the no-biofeedback condition should engage the posterior insula to a greater extent, given that interoceptive input from the viscera is the sole source of information for regulation. Because different theoretical accounts of socioemotional processing in adolescence postulate different developmental trajectories in the relevant brain regions, we included both age and age-squared effects in our regression models. We predicted that activity in the dorsal anterior insula, which is associated with cognitive control, would show a linear correlation with age particularly during interoceptive regulation, consistent with dual-systems models of executive control in adolescence. We further predicted that this region’s activity would correlate with interoceptive indices of regulatory success, measured as a change in EGG power from induction to regulation. Finally, consistent with neural models of socioemotional processing in adolescence, we predicted that activity in the ventral anterior insula during the induction phase would show a non-linear correlation with age, peaking at mid-adolescence, under the assumption that affect would be more salient during the induction phase while participants are freely riding the virtual roller coaster without attempting to regulate their interoceptive response. If supported, these results would provide important insights into how various sectors of the insula mediate interoceptive processes that contribute to self-regulation dynamically across adolescence.
Materials and Methods
Recruitment
This report is part of a larger laboratory and fMRI study examining insular structure and function in adolescent anorexia nervosa, and the relationship of this function to self-regulatory capacities across adolescence. We recruited our study sample by screening all adolescents who visited the pediatric primary care of a Southeastern academic medical center for either sickness or non-sickness routine check. Practice nurses approached adolescents and their caregivers to inquire if they were interested in learning more about a study of “Gut Feelings.” If interested, a study recruiter then informed the dyad about the study, and, if interested, completed a consent for screening and a screening for inclusion and exclusion criteria. All subjects were screened for the presence of substance use and abuse and were excluded if they endorsed regular use. Control participants were screened for the absence of mental health symptoms using questions used to predict diagnostic status from a prior population cohort study of child and adolescent psychopathology. Children who scored above the screen cut-off were excluded from further participation but were given a small prize. The demographic composition of the primary care practice selected mimicked that of the surrounding county. In this way, we intended to ensure that our control sample reflected the demographics of the surrounding county. Clinical information was gathered from medical record abstraction and adolescent and parent report. Only data from healthy participants are reported here. Due to the overarching focus on anorexia nervosa, only female participants participated in this study.
Participants
Thirty-seven healthy right-handed females participated in the study. All participants were between 10 and 20 years old, with a mean age of 15.53 ± 3.10 s.d. yrs. The participants completed a self-report measure of Tanner Stage: the Physical Self-Assessment Scale. Based on results from this scale, 95% of our sample was post menarche. Eleven participants were not included in fMRI analysis for the following reasons: five participants had incomplete psychophysical data, four participants had incomplete fMRI data, and two participants had excessive head movements during fMRI scans. One participant had missing respiration data but was still included in fMRI and EGG analyses. Thus, data analyses included 26 participants, with a mean age of 14.73 ± 2.96 s.d. yrs. The excluded participants did not differ from the included participants in age (p = .296). Handedness was assessed both by self-report and parental report.
For individuals below the age of 18 years old, informed assent was obtained from the adolescents and informed consent from caregivers. Written informed consent was acquired directly from individuals 18 or older. The participants received monetary payment for completion of the study at a rate of $20 per hour. Monetary compensation was split between the participant and the guardian, and the participant received an additional prize bag as an incentive for completing the series of tasks as part of a larger research protocol (e.g., a Silly Band for each hour on task). The research was approved by the Duke University Medical Center Institutional Review Board.
Materials and Procedure
Participants were required to fast for two hours prior to the study. Prior the MRI, all participants were ask to complete a standardized snack to control for EGG activity due to food consumption. Prior to the experimental task in the MRI scanner, participants attended a laboratory session in which they were trained in the regulation task. Participants were given an explanation of the regulation instructions. The participants were trained in deep, diaphragmatic breathing as a regulation strategy to help calm the stomach during the regulation phase of each trial. Using a designated script for “belly breathing,” adolescents were trained to breathe in through their nose, feel their belly rise, breathe out through their nose, and feel their belly fall. Research assistants ensured that participants were trained to proficiency, i.e., when they could see the diaphragm but not the chest expanding with each breath. The participants were also introduced to the virtual thermometer display to be used in the MRI scanner for biofeedback trials, which illustrated how the level of activity in the gut corresponds to the height of the mercury in the thermometer. As a validity check, participants were asked whether they understood task instructions, whether they thought the task was easy, and how well they think they performed on the task. Instructions were repeated until the participant indicated she understood the task. As a partial validity check, participants were debriefed following the laboratory task and asked how hard the task was, how easy the instructions were to follow, and how good they perceived themselves at the task: 87% found the task easy or very easy; 87% thought they were good or very good at the task; and 92% said they understood task instructions, 7% said maybe, and 1% said no. We feel that this debriefing information provides reasonably good evidence of task compliance in this adolescent sample.
On the day of the MRI scan, participants were instructed to not eat for 2 hours prior to the scan. All participants were then required to consume 100% of a small, standardized snack to control for effects on the EGG due to food digestion. Participants first underwent a brief (< 20 min) mock scanning session using head-motion tracking to help acclimate them to the scanner environment and to train them to keep their heads still. At the beginning of the fMRI session, participants first rested for 5 min and then practiced biofeedback regulation by viewing a visual thermometer display for 2 min. The experimental stimuli were projected to the participants through a mirror mounted on the radiofrequency coil cage. The experimental task during fMRI scans required the participants to regulate their interoceptive response, specifically their ‘gut feelings’, following provocation of the gut by riding segments of a virtual roller coaster in a first-person, 2-D movie format (NoLimits 1 – Roller Coaster Simulator by Ole Lange; http://www.nolimitscoaster.com/index.php). Each experimental trial consisted of two stages: induction and regulation. During the induction stage, the participants “rode” a virtual roller coaster segment that lasted 56 sec, followed by a 3.5-sec period during which the participants rated their gut feelings using a four-point likert-type scale with visual indicators (‘−−’, ‘−’, ‘+’, and ‘++’) for increasing levels of activity. After a 12-sec interval, the regulation stage began and lasted 2 min. These long induction and regulation stages ensured sufficient duration for EGG recording of gut activity, which has a slow time course (see “Physiological Data Acquisition and Analysis” below).
During the regulation stage, the participants were instructed to regulate their gut response under one of two conditions: biofeedback and no-biofeedback. In the biofeedback condition, a virtual thermometer was shown on the screen, whose level represented gastric activity measured by EGG in real-time. In the no-biofeedback condition, a virtual thermometer was also shown on the screen, but its level remained fixed. In both conditions, the participants were instructed to use deep, diaphragmatic breathing to help calm the stomach. After the regulation stage, the participants again were instructed to rate their gut feelings on a four-point scale during a 3.5-sec period1.
Three functional runs were collected for each participant. Each run consisted of an 18-sec baseline period with visual fixation, two experimental trials (one induction-biofeedback combination and one induction-nobiofeedback combination) with a 10-sec interval between the offset of the first trial and the onset of the second trial, and a 6-sec fixation period. The order of regulation conditions was counterbalanced across participants, such that participants with an even subject number performed a “biofeedback-no biofeedback” sequence in each run, and that participants with an odd subject number performed a “no biofeedback-biofeedback” sequence in each run.
Physiological Data Acquisition and Analysis
EGG was used to measure the participants’ gut motility during both induction and regulation stages, as well as to provide information for real-time feedback of gut motility in the biofeedback regulation trials. To verify that the deep breathing instructions were followed by research participants, respiration was assessed via a transducer that measured change in circumference of the chest. All physiological measures were recorded via a psychophysiological monitoring system (BIOPAC Systems, Goleta, CA).
Preprocessing of all psychophysiological data were performed using MATLAB (The MathWorks Inc., Natick, MA). The average respiration tidal volume and spectral analyses of EGG data were calculated for rest, induction, and regulation epochs. The EGG time series was mean-centered, linearly detrended, and tapered using a hamming window. The preprocessed EGG data were then converted to frequency domain using a fast Fourier transform, in which spectral densities were acquired in 0.33 cpm bins in the frequency range of 0.33 to 9.66 cpm. The percent total power for the bradygastria, normogastria, and tachygastria frequency bands were calculated using the following formulae (Harrison et al., 2010): bradygastria = (0.33 – 2.33 cpm power)/(0.33 – 9.66 cpm power), normogastria = (2.66 – 3.66 cpm power)/(0.33 – 9.66 cpm power), and tachygastria = (4.00 – 9.66 cpm power)/(0.33 – 9.66 cpm power).
Increases of EGG power in the bradygastria and tachygastria frequency bands have been associated with abnormal gut feelings (Hu et al., 1989; Jednak et al., 1999; Meissner, Muth, & Herbert, 2011; Stern et al., 1985; Williamson, Thomas, & Stern, 2003) and can thus be used as indices for individual’s abnormal gut motility.
Statistical analyses of physiological data were performed using R (R Core Team, 2013). Data normality was examined using Shapiro-Wilk tests implemented by the R function shapiro.test. The respiration tidal volume and EGG data at each frequency band and experimental stage were normally distributed.
MRI Data Acquisition and Preprocessing
MRI scans were conducted on a 3 Tesla General Electric MR 750 system with 50-mT/m gradients and an 8-channel head coil for parallel imaging (General Electric, Waukesha, Wisconsin, USA). T1-weighted structural scans were acquired using a 3D FSPGR BRAVO pulse sequence (TR = 7.58 ms; TE = 2.936 ms; flip angle = 12°; image matrix = 256 × 256; voxel size = 1 × 1 × 1 mm3; 206 contiguous axial slices). T2*-weighted functional scans were acquired using a SENSE spiral-in pulse sequence along the axial plane (TR = 2000 ms; TE = 30 ms; flip angle = 70°; image matrix = 64 × 128; voxel size = 3.8 × 3.8 × 3.8 mm3; 34 contiguous axial slices).
Preprocessing and statistical analysis of MRI data were performed using the Statistical Parametric Mapping 8 software (SPM8; Wellcome Trust Center for Neuroimaging). Functional images were spatially realigned to correct for head motion artifacts, coregistered to T1-weighted structural images, normalized to the Montreal Neurologic Institute (MNI) space using high-dimensional warping estimated from T1-weighted structural images implemented in the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm), smoothed using a 4-mm full-width half-maximum (FWHM) Gaussian kernel, and temporally filtered using a 128-s high-pass filter. The first five images of each run were excluded to focus analysis on images acquired after the magnet achieved steady-state equilibrium.
FMRI Data Analysis
Statistical analysis was performed using a random-effects general linear model approach. In the first-level analysis, the biofeedback and no-biofeedback regulation stages were modeled separately using two finite impulse response (FIR) predictor sets, each of which included 60 δ functions covering a time window of 120 seconds. The FIR approach was used because previous studies showed that the FIR approach captured more variance than approaches using other basis sets (Henson, Rugg, & Friston, 2001). The induction stage was modeled as a covariate by convolving the canonical hemodynamic response (HDR) function implemented in SPM8 with a boxcar function covering a 56-second time window that is equal to the duration of the roller coaster movies. A baseline predictor was included in the design matrix as a covariate by convolving the HDR function with a boxcar function covering the first 8 seconds in each functional run (after the first five images were excluded to achieve steady-state equilibrium). Head motion was modeled as six nuisance covariates, three for translations and three for rotations. No global normalization was used. Serial correlations between volumes due to noise and unmodelled neural activity were corrected using an autoregressive AR (1) model implemented in SPM8.
Contrasts of Induction – Baseline, Regulation – Baseline, Regulation – Induction, and Biofeedback – No-biofeedback were calculated for each subject. Group-level random-effect analyses were then performed for each contrast. To assess the developmental trajectory of interoceptive regulation, age was transformed to Z scores (age-Z), and both age-Z and its square were used in all random-effect analyses as covariates to evaluate linear changes across adolescence as well as peak changes in mid-adolescence. Given our strong a priori hypotheses regarding the insula (Craig, 2002), our main outcome measure was a targeted region-of-interest (ROI) analysis on the insula. For the ROI analysis, a bilateral insular mask was generated from the Wake Forest University PickAtlas toolbox (Tzourio-Mazoyer et al., 2002) and was used in all random-effect analyses. Statistical thresholds for the ROI analysis were calculated by estimating the false positive rate using Monte Carlo simulation (Forman et al., 1995). Using AlphaSim implemented in the REST toolbox (Song et al., 2011), a simulation of 1000 iterations produced a threshold of cluster size = 26 voxels (uncorrected voxel-level threshold at p < .05) to fulfill a corrected false positive rate of p < .05. Other brain regions were queried using a whole-brain exploratory analyses. For this analysis, thresholds for statistical inference were calculated to correct for multiple comparisons by using a false discovery rate (FDR; Benjamini & Hochberg, 1995; Genovese, Lazar, & Nichols, 2002) of q < .05 and cluster size > 5 voxels.
Results
Physiological Results
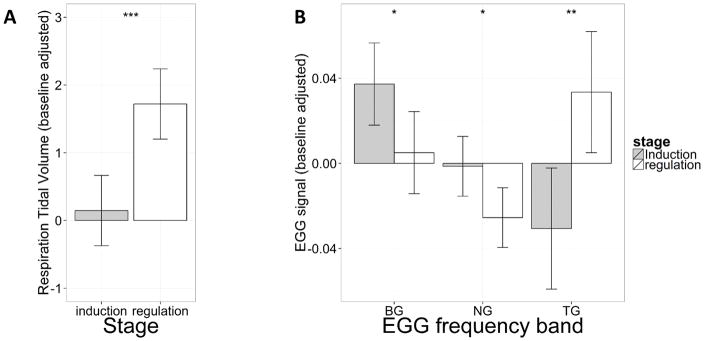
Both respiration and EGG data showed significant differences between induction and regulation stages. A one-way repeated measures ANOVA using respiratory tidal volume as the dependent variable and experimental stage (induction, regulation) as the within-subject factor showed a significant effect of experimental stage (F(1, 24) = 19.58, p = .00018), which implicates higher tidal volume in the regulation stage compared to that in the induction stage. This result confirms that the participants were actively engaged in deep breathing to regulate their gut motility. Post-hoc analyses showed no significant difference in respiratory tidal volumes between biofeedback and no-biofeedback conditions, suggesting similar compliance across feedback conditions. No age effect was found.
EGG data were analyzed separately for the bradygastria (BG), normogastria (NG), and tachygastria (TG) frequency bands. One-way repeated measures ANOVAs using EGG signal in the three frequency bands as the dependent variables and experimental stage (induction, regulation) as the within-subject factor showed significant effects of experimental stage in EGG signal in all three frequency bands (BG, F(1, 25) = 5.91, p = .023; TG, F(1, 25) = 10.77, p = .0030; NG, F(1, 25) = 6.27, p = .019). These effects remained significant after FDR correction for multiple comparisons (Benjamin & Hochberg, 1995). EGG signal from induction to regulation decreased in the BG and NG frequency band and increased in the TG frequency band. We interpret the increase in TG as a compensatory (regulatory) response to the increased BG power in the induction phase. Post-hoc analyses showed no significant difference of EGG signals between the biofeedback and no-biofeedback conditions, indicating equivalent interoceptive modulation across feedback conditions. EGG signal did not show any linear or quadratic relationship with age.
FMRI Results
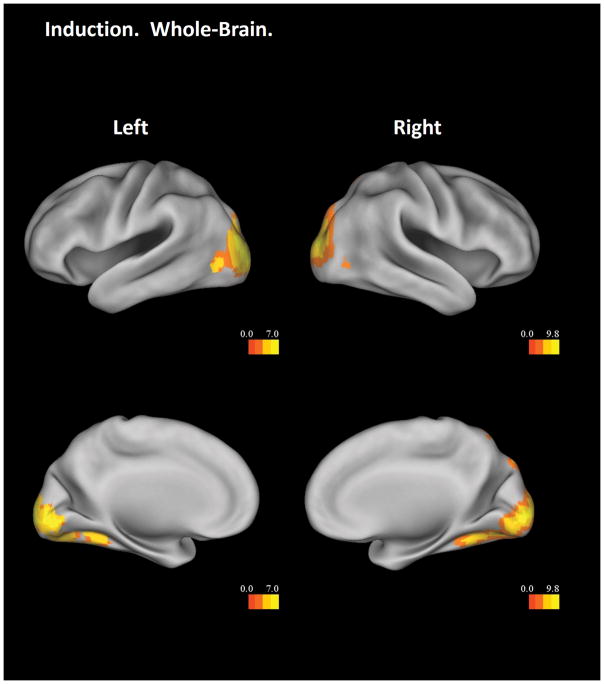
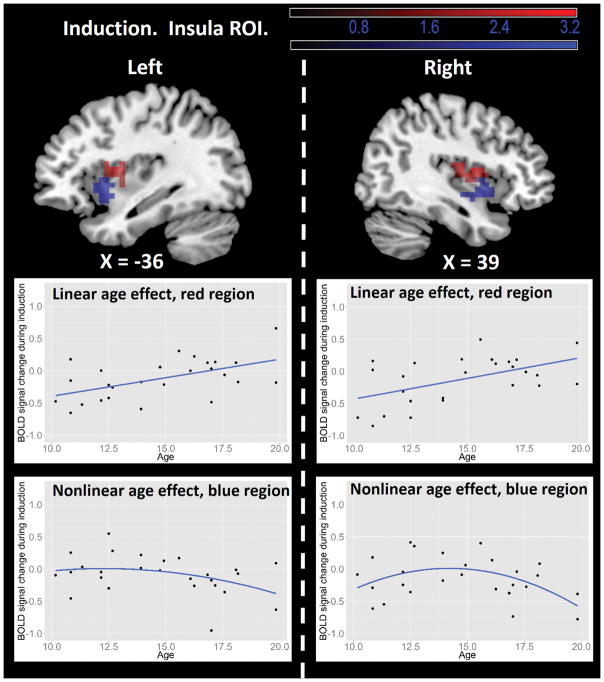
The Induction – Baseline contrast showed significant main effects in the bilateral occipital cortex and right superior parietal lobule, independent of age (Figure 2). The insular ROI analysis revealed two age effects. First, a region in the bilateral dorsal anterior insula showed a significant linear age effect, such that older participants had more activation during induction (Figure 3, top panel red color and middle panel). Second, the bilateral ventral anterior insula showed significant non-linear age effect, such that participants in mid-adolescence showed the highest activation during induction (Figure 3, top panel green color and bottom panel).
Figure 2.
Whole-brain results from the Induction – Baseline contrast. Threshold is set at a voxel-wise level of p < .05, FDR corrected. Only clusters with more than 5 voxels are displayed. Results are visualized on an inflated PALS-B12 fiducial atlas (Van Essen 2005; Van Essen and Dierker 2007) provided by the Caret software (http://www.nitrc.org/projects/caret/; Van Essen et al. 2001).
Figure 3.
Insular ROI results from the Regulation – Baseline contrast. Top panel: insula regions showing age effects. In both left and right hemispheres, the dorsal anterior insula (red regions) showed linear age effects, whereas the ventral anterior insula (blue regions) showed nonlinear age effects. The statistical threshold is set at a voxel-wise level of p < .05 and cluster size > 26. The dashed line separates results of left and right hemispheres. Middle panel: illustration of linear age effects in the dorsal anterior insula (red regions). Bottom panel: illustration of nonlinear age effects in the ventral anterior insula (blue regions). All scatter plots used average BOLD signal from the corresponding functional clusters and are for illustrative purposes only. Results are visualized on the Colin anatomical template in MNI space using MRIcron software (http://www.mricro.com/mricron/).
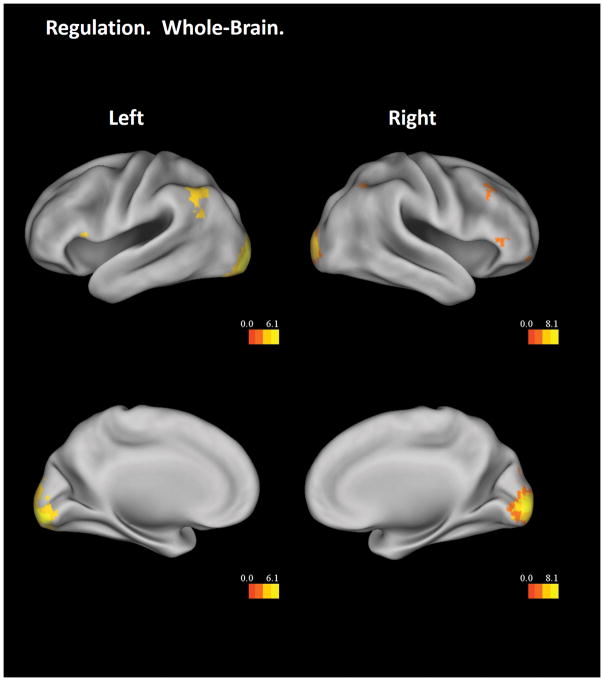
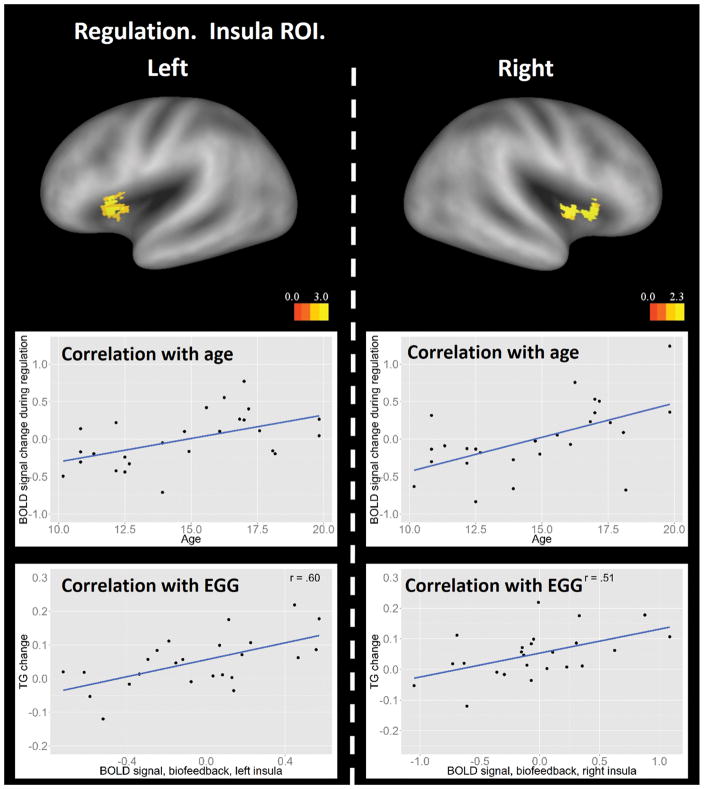
The Regulation – Baseline contrast, collapsing results across regulation types, showed significant main effects in the whole-brain analysis in the bilateral occipital cortex, bilateral angular gyrus, bilateral inferior frontal cortex, right middle frontal cortex, and the precuneus, all independent of participant age (Figure 4). The insula ROI analysis revealed a significant positive linear effect with age in the bilateral dorsal anterior insula (Figure 5, top and middle panels), similar to the result in the induction stage. Importantly, activation in this insular region showed positive correlations with changes of EGG power in the tachygastria frequency band from induction to regulation across participants (left insula, Pearson’s r = .47, p = .019; right insula, Pearson’s r = .34, p = .085), which implicates a functional role of the dorsal anterior insula in EGG regulation. Correlations with EGG power in the bradygastria and normogastria frequency bands failed to reach significance (bradygastria, left insula, Pearson’s r = −.17, p = .43; right insula, Pearson’s r = −.12, p = .59; normogastria, left insula, Pearson’s r = 0.15, p = .48; right insula, Pearson’s r = 0.23, p = .28).
Figure 4.
Whole-brain results from the Regulation – Baseline contrast. The statistical threshold is set at a voxel-wise level of p < .05, FDR corrected. Only clusters with more than 5 voxels are displayed. Results are visualized on an inflated PALS-B12 fiducial atlas (Van Essen 2005; Van Essen and Dierker 2007) provided by the Caret software (http://www.nitrc.org/projects/caret/; Van Essen et al. 2001).
Figure 5.
Insular ROI results from the Regulation – Baseline contrast. Top panel: insula regions showing linear age effects. The statistical threshold is set at a voxel-wise level of p < .05 and cluster size > 26. The dashed line separates results of left and right hemispheres. Results are visualized on an inflated PALS-B12 fiducial atlas (Van Essen 2005; Van Essen and Dierker, 2007) provided by the Caret software (http://www.nitrc.org/projects/caret/; Van Essen et al. 2001). Middle panel: illustration of the linear correlations between age and BOLD activity in the left and right dorsal anterior insula averaged across biofeedback and no-biofeedback conditions. Scatter plots in the middle panel used average BOLD signal from the corresponding functional clusters and are for illustrative purpose only. Bottom panel: correlation between change of EGG signal in the tachygastria frequency band from induction to regulation and BOLD signal in the left and right dorsal anterior insula in the biofeedback condition.
Further examination revealed that correlations between activation in the dorsal anterior insula and changes of EGG power in the tachygastria frequency band were driven by the Biofeedback condition (left insula, Pearson’s r = .60, p = .0018; right insula, Pearson’s r = .51, p = .010; Figure 5, bottom panel), and that correlations in the No-biofeedback condition were not significant (left insula, Pearson’s r = .25, p = .24; right insula, Pearson’s r = .13, p = .53). Correlations between dorsal anterior insula activation and changes in power within the tachygastria frequency band was greater in the Biofeedback compared to the No-feedback condition (left insula, p = .000021; right insula, p = .000038; Lee and Preacher 2013).
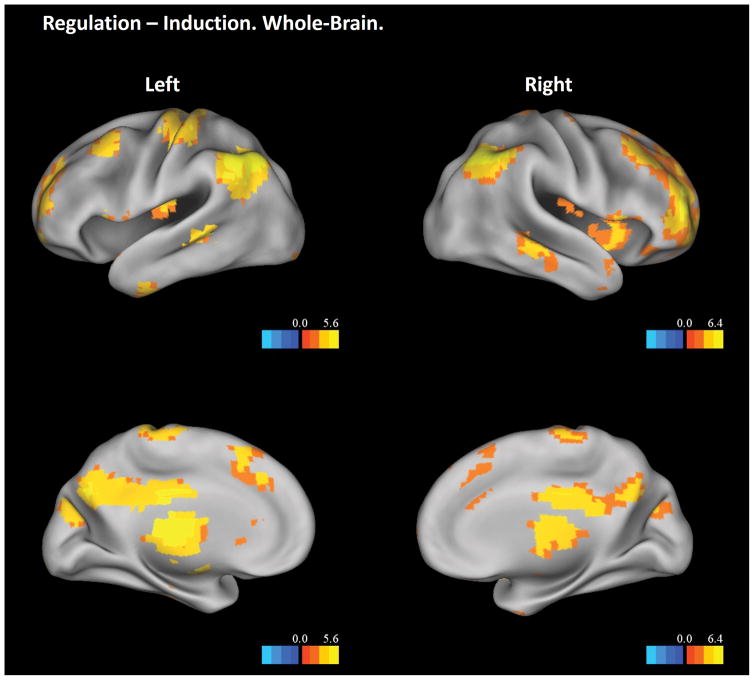
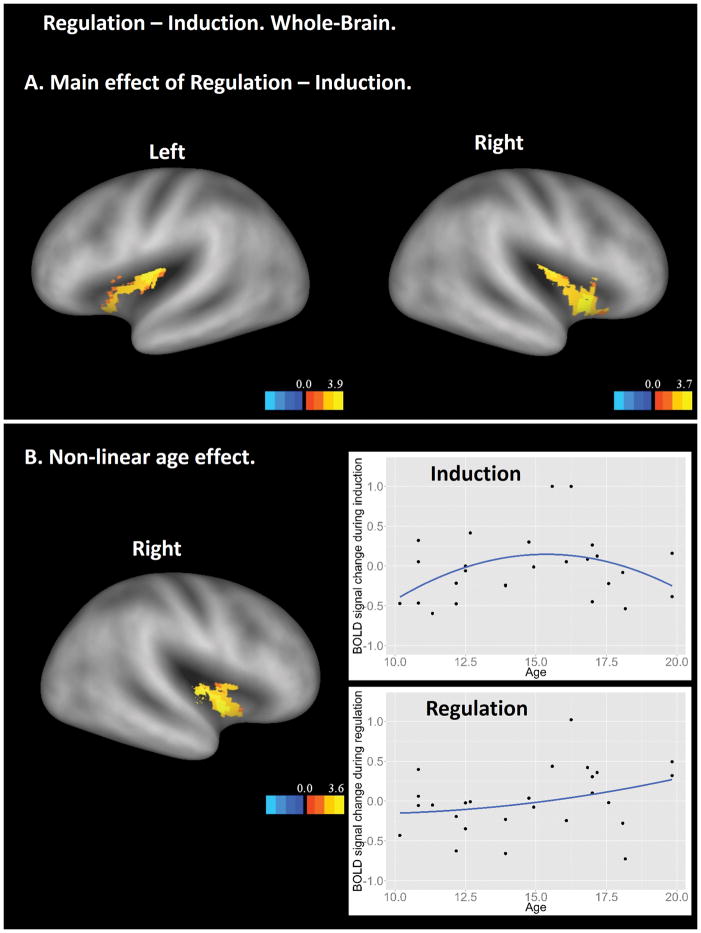
The Regulation – Induction contrast showed significant main effects in a wide range of brain regions independent of participant age (Figure 6). Many of these brain regions were the same as those shown in the Regulation – Baseline contrast, including the bilateral occipital cortex, bilateral angular gyrus, bilateral inferior frontal cortex, right middle frontal cortex, and the precuneus. Additional brain regions included the dorsal medial prefrontal cortex, mid- and posterior cingulate, thalamus, cerebellum, middle temporal gyrus, hippocampus, and insula. The insula ROI analysis revealed two findings (Figure 7). First, both anterior and posterior insula showed significantly higher activation in the regulation stage than in the induction stage (Figure 7A), suggesting different levels of visceral awareness in these two stages. Second, an anterior portion of the insula showed interaction between age-squared and experiment stage (Figure 7B). Further examination showed that the interaction was driven by greater activations in the anterior insula for mid-adolescents during the induction but not the regulation stage, which is consistent with the results in other lower-level contrast (Figure 3, bottom panels).
Figure 6.
Whole-brain results from the Regulation – Induction contrast. The statistical threshold is set at a voxel-wise level of p < .05, FDR corrected. Only clusters with more than 5 voxels are displayed. Results are visualized on an inflated PALS-B12 fiducial atlas (Van Essen 2005; Van Essen and Dierker 2007) provided by the Caret software (http://www.nitrc.org/projects/caret/; Van Essen et al. 2001).
Figure 7.
Insular ROI results from the Regulation – Induction contrast. A. Bilateral insula showing significant higher activation in regulation than in induction. B. Right anterior insula showing interaction between age-square and experiment stage. The scatter plots used average BOLD signal from the corresponding functional clusters and are for illustrative purpose only. In both A and B, results are visualized on an inflated PALS-B12 fiducial atlas (Van Essen 2005; Van Essen and Dierker 2007) provided by the Caret software (http://www.nitrc.org/projects/caret/; Van Essen et al. 2001).
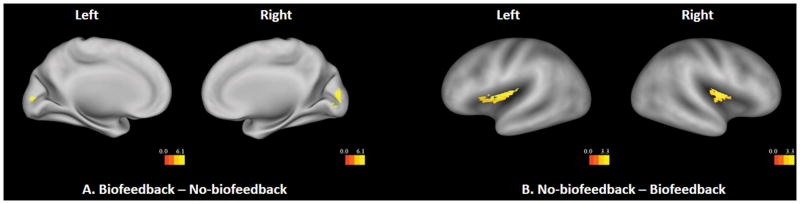
The Biofeedback – No-biofeedback regulation contrast in the whole-brain analysis showed a significant main effect in the primary visual cortex, independent of participant age (Figure 8A). This activation was most likely induced by sensory processing of the visual thermometer that actively monitored real-time gut activity in the biofeedback condition. In the No-biofeedback – Biofeedback contrast, the insula ROI analysis revealed a significant main effect in the bilateral posterior insula (Figure 8B), suggesting that the posterior insula was more actively recruited to regulate the interoceptive response when externally-guided biofeedback cues were unavailable. No significant interactions were found with age or age-squared in these analyses.
Figure 8.
Differences between biofeedback and no-biofeedback regulation. A: Whole-brain results from the contrast of Biofeedback – No-biofeedback. The statistical threshold is set at a voxel-wise level of p < .05, FDR corrected. Only clusters with more than 5 voxels are displayed. B: Insular ROI results from the contrast of No-biofeedback – Biofeedback. The statistical threshold is set at a voxel-wise level of p < .05 and cluster size > 26. In both A and B, results are visualized on an inflated PALS-B12 fiducial atlas (Van Essen 2005; Van Essen and Dierker 2007) provided by the Caret software (http://www.nitrc.org/projects/caret/; Van Essen et al. 2001).
Discussion
Adolescence is a key transitional developmental period for socioemotional and self-regulatory capacities. Despite the importance of interoception in self-awareness, affective judgments, and intuitive decision-making, little is known about the neural basis of interoceptive regulation across this age period. The present study addressed this gap by developing a virtual reality-based roller coaster induction method to induce changes in gut feelings in adolescents. Participants were taught deep breathing-based regulatory strategies to alter their gut motility, and those strategies were implemented either with visually-guided EGG biofeedback or without it (pure interoception-based regulation). As such, this task attempted to parallel functionally one of challenges of adolescent development: integrating their internal visceral experience with context (biofeedback condition) or tuning inward and altering internal sensation based on their own felt experience. Neurocognitive models of adolescent development were integrated with knowledge of insula function to make predictions about the developmental trajectories of processes that support interoceptive regulation. The results provide new insights into the abilities of adolescents to modulate their visceral responses, and show how the insula mediates inductive and regulatory components of interoception as a function of adolescent age. Importantly, the findings show that interoceptive capacities in adolescence are multifaceted - components of interoception are associated with different developmental trajectories, which are subserved by distinct sub-regions in the insular cortex. The implications of the findings with respect to the psychophysiology of interoception, parcellation of insula function, and neurocognitive models of adolescent development are discussed below.
Psychophysiology of interoceptive induction and regulation
Interoceptive induction and regulation were monitored using physiological measures of respiration and stomach motility, which clearly showed that adolescents were capable of implementing the deep breathing strategy to modulate their gut activity, irrespective of age. Respiratory tidal volume increased from induction to regulation, confirming that deep, diaphragmatic breathing was effectively adopted. The EGG signals also showed interesting patterns of change. EGG power in the bradygastria frequency band increased during induction (compared to rest) and returned to rest level in regulation, whereas EGG power in the tachygastria frequency band decreased during induction and increased to above-rest levels during regulation. These results suggest that increased power in the bradygastric range was induced by the virtual roller coaster experience, which was successfully regulated and was accompanied by a compensatory increase in the tachygastric power band. Additionally, power in the normogastric frequency band showed a significant decrease from induction to regulation, which might be related to increases of power in the tachygastria frequency band during regulation.
Changes in EGG power in the bradygastria and tachygastria frequency bands have been associated with various sources and symptoms of stomach provocation. In a series of studies, Stern and colleagues proposed that increase of EGG power in the tachygastria frequency band is related to stomach dysrhythmia induced by motion sickness (Hu et al., 1989; Stern et al., 1985; Williamson, Thomas, & Stern, 2003). This result, however, was not replicated in other studies, most likely due to various confounding factors and significant individual differences in tachygastria and motion sickness (reviewed in Cheung & Vaitkus, 1998). On the other hand, increase of EGG power in the bradygastria frequency band has been associated with stomach provocation as well, such as disgust induced by viewing pictures with negative valence (Meissner, Muth, & Herbert, 2011), first-trimester nausea in pregnant women (Jednak et al., 1999), and motion sickness in cats (Lang, Sarna, & Shaker, 1999). Thus, correspondence between EGG power in the bradygastria/tachygastria frequency bands and sources of stomach dysrhythmia remains elusive. The present study supports the latter evidence in that bradygastria was induced during a virtual roller coaster ride. In sum, the dynamics of EGG power in the bradygastria and tachygastria bands in this study reflect active, successful regulation of stomach activity by voluntary diaphragmmatic breathing across adolescence.
The induction and regulation of gut feelings may be interpreted in the context of the polyvagal theory (Porges, 2007). The polyvagal theory proposes that the human autonomic nervous system includes three phylogenetically ordered subsystems: myelinated vagus system, sympathetic-adrenal system, and unmeyelinated vagus system. These subsystems each correspond to distinct behavioral functions: social communication (e.g., facial expression), mobilization (e.g., fight-flight), and immobilization (e.g., freezing), respectively. These subsystems are in evolutionarily new-old order, and evolutionarily old subsystems are inhibited by new subsystems and are only active when evolutionarily new subsystems temporarily become less available. To protect against a dangerous or disturbing environment, such as when viewing the roller coaster movies, the myelinated vagus system may activate lower-level systems and cause nausea. During regulation, higher-level systems regain functionality, and the deep breathing exercise might facilitate effective regulation of the nausea feeling by elevating awareness of viscera state (Farmer et al., 2015). That said, we did not intend to test the polyvagal theory in this study, and thus future work is needed to directly test these hypotheses.
Role of the insula and other brain regions to interoceptive induction and regulation
The human insula has been functionally parcellated into hierarchically organized sub-regions, which is consistent with the anatomical parcellation of monkey insula based on cytoarchitectonical organizations (Mesulam & Mufson, 1982). Although there are several proposed functional divisions, one common scheme is that the posterior insula is primarily a somatosensory region important for interoceptive perception; the dorsal anterior insula, which includes parts of the mid-insula, is involved in cognitive control and regulation; and the ventral anterior insula is involved in emotion processing (Cauda et al., 2011; Chang et al., 2013; Kelly et al., 2012; Deen, Pitskel, & Pelphrey, 2011). The proposed functions of these insular sub-regions are consistent with results from the insular ROI analysis in this study.
First, the ROI analysis showed that during interoceptive regulation, the no-biofeedback condition elicited more activation in the bilateral posterior insula compared to the biofeedback condition (Figure 8A), suggesting that the posterior insula was more active when visual feedback of interoceptive activity was unavailable. Given that the posterior insula is known to track objective intensity of interoceptive inputs (Craig, 2002), its higher activation in the no-biofeedback condition suggests that the participants relied more on these objective interoceptive markers to guide regulation in the absence of exteroceptive feedback. This role of the posterior insula was unchanged across adolescent age, which may dovetail with the earlier structural maturation of this region, consistent with early maturation of exteroceptive sensory processing areas (Gogtay et al., 2004).
Second, activity in the dorsal anterior insula showed a positive linear correlation with age during both induction and regulation across regulation types (top and middle panels of Figure 3 and 5). The dorsal anterior insula has been shown to functionally connect to executive control regions and to be important for regulating emotional responses (Chang et al., 2012; Deen, Pitskel, & Pelphery, 2010; Kelly et al., 2012). Importantly, activation of this brain region during biofeedback regulation was positively correlated with changes of EGG power in the tachygastria frequency band from induction to regulation (Figure 5, bottom panel), indicating a higher reliance on the dorsal anterior insula in regulating stomach motility later in adolescence. The additional dorsal anterior insula activity during induction, which also showed a linear age effect, may be due to uninstructed regulatory efforts, or other cognitive control processes, when ‘riding’ the virtual roller coaster. Because a low-level baseline was used, there are several processes that could contribute to these effects in the insula. However, the EGG correlations provide strong support for a regulatory function. Future studies will need to determine the relative contributions of this brain region to induction vs. regulation, or to voluntary vs. incidental regulation.
Although both biofeedback and no-biofeedback regulation showed similar EGG and fMRI activation patterns during induction and regulation, only biofeedback regulation showed significant correlation between changes of EGG power in the tachygastria frequency band and BOLD activity in the dorsal anterior insula. This finding may suggest integration of contextual information, such as the biofeedback information derived from real-time EGG signals, with interoceptive regulation.
Third, brain activity in the ventral anterior insula showed a quadratic developmental pattern and exhibited the highest activation in mid-adolescence during interoceptive induction (Figure 3, bottom panel). Non-linear relationships as a function of adolescent age have been associated with several socioemotional factors, including emotional arousal and sensation seeking (Martin et al., 2002; Steinberg, 2005). Future research should examine whether individual differences in sensation-seeking during mid-adolescence moderates the amplification of interoceptive sensations during a provocation. Recent neuroimaging studies have emphasized activation changes in limbic and social cognitive brain regions that mediate these and related behaviors, such as trustworthiness evaluation (Kragel et al., 2014), that peak in mid-adolescence (reviewed in Crone & Dahl, 2012; Pfeifer & Anderson, 2012). The dynamic shift in ventral anterior insular processing observed in the present study is a novel contribution to this literature and may contribute to heightened socioemotional vulnerability during this critical age range.
Other frontoparietal brain regions also contributed to interoceptive regulation across age and biofeedback conditions. These regions included the bilateral inferior frontal cortex, right middle frontal cortex, bilateral angular gyrus, and the precuneus (Figure 4). The inferior frontal cortex was shown to interact with multiple subcortical regions in emotion regulation (Ochsner et al., 2002; Wager et al., 2008), especially for the reappraisal of emotional aspects of stimuli (Ochsner & Gross, 2005; Winecoff et al., 2009). The middle frontal cortex is involved in multiple aspects of executive control and appears to indirectly modulate limbic regions during emotion regulation (Ochsner & Gross, 2005). Similarly, the angular gyrus was shown to activate during reappraisal of emotional stimuli (Goldin et al., 2008). In this study, we extend these findings to show that these brain regions are also involved in interoceptive regulation, which is likely due to the close relationship between emotion and interoception (Craig, 2008). Finally, the precuneus is associated with the default mode of brain processing and elevated self-consciousness, as when people are asked to answer self-referencing questions (Johnson et al., 2002). Thus, the precuneus may also contribute to participants’ self-awareness or self-monitoring during interoceptive regulation. Further studies are warranted to elucidate the functional contribution of each of these brain structures to interoceptive regulation.
Given that a low-level fixation baseline was used, the main effects of the “Induction – Baseline” and “Regulation – Baseline” contrasts were associated with a host of factors. For example, the main effect of the “Induction – Baseline” contrast may be evoked by visual complexity, perspective taking, emotional content, etc. Thus, future work is needed to use higher-level baselines to examine both interoceptive induction and regulation.
Implications for neurobiological theories of adolescent development
Two overarching theories have been postulated regarding neurocognitive development in adolescence. One set of theories emphasizes the late maturation of prefrontal cortex for the executive control of behavior. More recently, Casey and colleagues (e.g., Casey et al., 2008; Casey et al., 2011) have modified this traditional model to emphasize differential maturation rates for subcortical vs. cortical structures, such that striatal and limbic regions involved in reward and affective processing mature at an earlier rate than prefrontal control regions, thus creating an imbalance between subcortical vs. cortical processing during decision-making until late adolescence. In contrast to these theories, which emphasize monotonically increasing developmental trajectories, more recent theories focused on socioemotional processing have implicated an inverted-U shaped relationship with adolescent age (Crone & Dahl, 2012; Pfeifer & Allen, 2012). In these latter theories, social and emotional processes are postulated to have a peak contribution to behavior in mid-adolescence due in part to maturation of social and affective networks during this particular time period.
Given that the insula serves as a key hub to interface between emotional processing and attentional and executive control (Fichtenholtz & LaBar, 2012), it serves as an interesting test case for these theories. As discussed above, knowledge of insula parcellation from prior work aligns the dorsal anterior insula with executive control processes, and results from the present study indicate a linear relationship of this region with adolescent age during both induction and regulation, and a greater reliance on this region during biofeedback-based regulation in late adolescence. This pattern is consistent with theories emphasizing late maturation of executive control regions. At the same time, data from the ventral anterior insula, which, as discussed above, is more aligned with emotional processing, peaked in mid-adolescence during the roller coaster induction. This pattern is consistent with theories emphasizing mid-adolescent changes in brain regions subserving socioemotional functions. Thus, maturation processes in the insula linked to distinct executive and emotional functions follow different developmental trajectories and provide complementary support for both classes of theories.
Implications for emotion regulation
Deep diaphragmatic breathing is an example of a response-focused emotion regulation strategy (Gross, 1998). The preponderance of neuroscientific studies on emotion regulation have focused on cognitive reappraisal as an antecedent-focused emotion regulation strategy that serves to prepare the organism to deal with upcoming emotional events before a full-blown reaction occurs (Buhle et al., 2013). In contrast, there has been little research on response-focused emotion regulation strategies, except for work on expressive suppression, which is an ineffective regulatory strategy (e.g., Levesque et al., 2003; Hayes et al., 2010). Here we show that deep breathing exercises are an effective regulatory strategy to alter gut responses induced by a virtual roller coaster induction in adolescence, both combined with visual biofeedback of ongoing EGG activity and as a pure interoceptive regulatory skill. Future studies can determine whether this strategy is similarly effective for regulating other aspects of emotion and visceral experience in adolescence. In addition to the insula, interoceptive regulation recruited multiple frontoparietal regions throughout adolescence that are commonly reported in the adult emotion regulation literature using other strategies (Buhle et al., 2013).
Conclusion
This study constituted a novel investigation of interoceptive regulation in adolescence using a combined psychophysiological-fMRI approach with a biofeedback component and a virtual reality-based induction method. Sub-regions in the insula showed distinct developmental profiles, which is consistent with insular parcellation studies identifying three functional subdivisions of the insula. The distinct profiles further support current neurodevelopmental theories that adolescence is characterized by linear development of cognitive control ability and non-linear development of interoceptive and socioemotional processing. Because the current study focused on females, future work should be conducted to extend the findings to males, taking into consideration different pubertal onsets as a function of sex.
Figure 1.
Physiological results. A: Respiration tidal volumes in induction and regulation. B: Relative power of electrogastrogram (EGG) signal in different frequency bands in induction and regulation. The x axis denotes different EGG frequency bands: BG – bradygastria, NG – normogastria, TG – tachygastria. Both respiration tidal volume and relative EGG power are adjusted by subtracting resting signal from the induction/regulation signal. The error bars represent 95% repeated-measure confidence intervals (Loftus and Masson 1994; Morey 2005). Significance results obtained from paired t-tests are overlaid on the plots (* represents p < .05; ** represents p < .005; *** represents p < .0005).
Research Highlights.
Sub-regions of the insula showed different developmental trajectories in interoceptive processes, which provides important insights for competing neurobiological theories of adolescent development.
Activation in the ventral anterior insula showed a quadratic pattern with age and peaked at mid-adolescence during interoceptive induction.
Activation in the dorsal anterior insula was linearly associated with age in adolescence during interoceptive induction and regulation.
Activation in the dorsal anterior insula during interoceptive regulation was correlated with the change of electrogastrogram signal in the tachygastria frequency band from interoceptive induction to regulation.
Acknowledgments
The authors wish to thank Matthew Fecteau for assistance with psychophysiological recording and analysis. This work was supported by NIH RC1 MH088678 and a Duke Institute for Brain Sciences Research Incubator Award to N.L.Z. and K.S.L.
Footnotes
Behavioral ratings were also obtained after the regulation stage. These ratings, however, were unusable due to subject non-compliance and/or technical issues (only 3 valid ratings were recorded), and were therefore not used in the analysis.
References
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995:289–300. [Google Scholar]
- Buhle JT, Silvers Ja, Wager TD, Lopez R, Onyemekwu C, Kober H, Weber J, Ochsner KN. Cognitive Reappraisal of Emotion: A Meta-Analysis of Human Neuroimaging Studies. Cerebral Cortex. 2013;24:2981–2990. doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett S, Sebastian C, Cohen Kadosh K, Blakemore SJ. The social brain in adolescence: evidence from functional magnetic resonance imaging and behavioural studies. Neuroscience & Biobehavioral Reviews. 2011;35:1654–1664. doi: 10.1016/j.neubiorev.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA. The adolescent brain. Annals of the New York Academy of Sciences. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Somerville LH. Braking and Accelerating of the Adolescent Brain. Journal of Research on Adolescence. 2011;21:21–33. doi: 10.1111/j.1532-7795.2010.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nature Reviews Neuroscience. 2012;13:636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Cheung B, Vaitkus P. Perspectives of electrogastrography and motion sickness. Brain Research Bulletin. 1998;47:421–431. doi: 10.1016/S0361-9230(98)00095-1. [DOI] [PubMed] [Google Scholar]
- Chitkara DK, Van Tilburg M, Whitehead WE, Talley NJ. Teaching diaphragmatic breathing for rumination syndrome. The American Journal of Gastroenterology. 2006;101:2449–2452. doi: 10.1111/j.1572-0241.2006.00801.x. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD, Chen K, Bandy D, Reiman EM. Thermosensory activation of insular cortex. Nature Neuroscience. 2000;3:184–190. doi: 10.1038/72131. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Melmed RN, Featherstone E, Mathias CJ, Dolan RJ. Brain activity during biofeedback relaxation: A functional neuroimaging investigation. Brain. 2001;124:1003–1012. doi: 10.1093/brain/124.5.1003. [DOI] [PubMed] [Google Scholar]
- Cullen KR, Gee DG, Klimes-Dougan B, Gabbay V, Hulvershorn L, Mueller BA, Camchong J, Bell CJ, Houri A, Kumra S, Lim KO, Castellanos FX, Milham MP. A preliminary study of functional connectivity in comorbid adolescent depression. Neuroscience Letters. 2009;460:227–231. doi: 10.1016/j.neulet.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR. Descartes’ error: emotion, reason, and the human brain. New York, NY: Putnam Books; 1994. [Google Scholar]
- Dunn BD, Galton HC, Morgan R, Evans D, Oliver C, Meyer M, Cusack R, Lawrence AD, Dalgleish T. Listening to your heart. How interoception shapes emotion experience and intuitive decision making. Psychological Science. 2010;21:1835–1844. doi: 10.1177/0956797610389191. [DOI] [PubMed] [Google Scholar]
- Ernst M, Fudge JL. A developmental neurobiological model of motivated behavior: Anatomy, connectivity and ontogeny of the triadic nodes. Neuroscience and Biobehavioral Reviews. 2009;33:367–382. doi: 10.1016/j.neubiorev.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer AD, Ban VF, Coen SJ, Sanger GJ, Barker GJ, Gresty MA, Giampietro VP, Williams SC, Webb DL, Hellström PM, Andrews PLR, Aziz Q. Visually induced nausea causes characteristic changes in cerebral, autonomic and endocrine function in humans. The Journal of Physiology. 2015;593:1183–1196. doi: 10.1113/jphysiol.2014.284240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassino S, Piero A, Garmaglia C, Abbate-Daga G. Interoceptive awareness in anorexia nervosa, bulimia nervosa, and obesity. Psychopathology. 2004;37:168–174. doi: 10.1159/000079420. [DOI] [PubMed] [Google Scholar]
- Fichtenholtz HM, LaBar KS. Emotional influences on visuospatial attention. In: Mangun GR, editor. Neuroscience of Attention: Attentional Control and Selection. New York: Oxford University Press; 2012. pp. 250–266. [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, III, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: Reappraisal and suppression of negative emotion. Biological Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ. Antecedent- and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology. 1998;74:224–237. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Harrison NA, Gray MA, Gianaros PJ, Critchley HD. The embodiment of emotional feelings in the brain. The Journal of Neuroscience. 2010;30:12878–12884. doi: 10.1523/JNEUROSCI.1725-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JP, Morey RA, Petty CM, Seth S, Smoski MJ, McCarthy G, LaBar KS. Staying cool when things get hot: Emotion regulation modulates neural mechanisms of memory encoding. Frontiers in Human Neuroscience. 2010;4:230. doi: 10.3389/fnhum.2010.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson R, Rugg MD, Friston KJ. The choice of basis functions in event-related fMRI. Neuroimage. 2001;13:149–149. [Google Scholar]
- Hu S, Stern R, Vasey M, Koch K. Motion sickness and gastric myoelectric activity as a function of speed of rotation of a circular vection drum. Aviation, Space, and Environmental Medicine. 1989;60:411–414. [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex — Developmental changes and effects of aging. Brain Research. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Johnson S, Baxter L, Wilder L. Neural correlates of self-reflection. Brain. 2002;125:1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Fudge JL, Paulus M. New insights into symptoms and neurocircuit function of anorexia nervosa. Nature Reviews Neuroscience. 2009;10:573–584. doi: 10.1038/nrn2682. [DOI] [PubMed] [Google Scholar]
- Kragel PA, Zucker NL, Covington VE, LaBar KS. Developmental trajectories of cortical-subcortical interactions underlying the evaluation of trust in adolescence. Social, Cognitive, and Affective Neuroscience. 2014 doi: 10.1093/scan/nsu050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang IM, Sarna SK, Shaker R. Gastrointestinal motor and myoelectric correlates of motion sickness. The American Journal of Physiology. 1999;277(3 Pt 1):G642–652. doi: 10.1152/ajpgi.1999.277.3.G642. [DOI] [PubMed] [Google Scholar]
- Lee IA, Preacher KJ. Calculation for the test of the difference between two dependent correlations with one variable in common [Computer software] 2013 Available from http://quantpsy.org.
- Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neuroscience and Biobehavioral Reviews. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Levesque J, Eugene F, Joanette Y, Paquette V, Mensour B, Beaudoin G, et al. Neural circuitry underlying voluntary suppression of sadness. Biological Psychiatry. 2003;53:502–510. doi: 10.1016/s0006-3223(02)01817-6. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Wandell BA. Interpreting the BOLD signal. Annual Review of Physiology. 2004;66:735–769. doi: 10.1146/annurev.physiol.66.082602.092845. [DOI] [PubMed] [Google Scholar]
- Loftus GR, Masson ME. Using confidence intervals in within-subject designs. Psychonomic Bulletin & Review. 1994;1:476–90. doi: 10.3758/BF03210951. [DOI] [PubMed] [Google Scholar]
- Martin CA, Kelly TH, Rayens MK, Brogli BR, Brenzel A, Smith WJ, Omar HA. Sensation seeking, puberty, and nicotine, alcohol, and marijuana use in adolescence. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:1495–1502. doi: 10.1097/00004583-200212000-00022. [DOI] [PubMed] [Google Scholar]
- Morey R. Confidence intervals from normalized data: A correction to Cousineau (2005) Tutorial in Quantitative Methods for Psychology. 2008;4:61–64. [Google Scholar]
- Paulus MP, Stein MB. Interoception in anxiety and depression. Brain Structure & Function. 2010;214:451–463. doi: 10.1007/s00429-010-0258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Allen NB. Arrested development? Reconsidering dual-systems models of brain development in adolescence and disorders. Trends in Cognitive Sciences. 2012;16:322–329. doi: 10.1016/j.tics.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. URL http://www.R-project.org/ [Google Scholar]
- Steinberg L. Cognitive and affective development in adolescence. Trends in Cognitive Sciences. 2005;9:69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Stern RM, Koch KL, Leibowitz HW, Lindblad IM, Shupert CL, Stewart WR. Tachygastria and motion sickness. Aviation, Space, and Environmental Medicine. 1985;56:1074–1077. [PubMed] [Google Scholar]
- Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, He Y, Yan CG, Zang YF. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PloS One. 2011;6:e25031. doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Van Essen DC. A population-average, landmark- and surface-based (PALS) atlas of human cerebral cortex. NeuroImage. 2005;28:635–662. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Dickson J, Harwell J, Hanlon D, Anderson CH, Drury HA. An integrated software system for surface-based analyses of cerebral cortex. Journal of American Medican Informatics Association. 2001;8:443–459. doi: 10.1136/jamia.2001.0080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Dierker D. Surface-based and probabilistic atlases of primate cerebral cortex. Neuron. 2007;56:209–225. doi: 10.1016/j.neuron.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Vohs KD, Baumeister RF, editors. Handbook of self-regulation: Research, theory, and applications. Guilford Press; 2011. [Google Scholar]
- Winecoff A, LaBar KS, Madden D, Cabeza R, Huettel SA. Cognitive and neural contributors to emotion regulation in aging. Social Cognitive and Affective Neuroscience. 2011;6:165–176. doi: 10.1093/scan/nsq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson MJ, Thomas MJ, Stern RM. The contribution of expectations to motion sickness symptoms and gastric activity. Journal of Psychosomatic Research. 2004;56:721–726. doi: 10.1016/S0022-3999(03)00130-2. [DOI] [PubMed] [Google Scholar]
- Zaki J, Davis JI, Ochsner KN. Overlapping activity in anterior insula during interoception and emotional experience. NeuroImage. 2012;62:493–499. doi: 10.1016/j.neuroimage.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]