Abstract
Since the discovery of the KATP channel in 1983, numerous studies have revealed its physiological functions. The KATP channel is expressed in various organs, including the pancreas, brain and skeletal muscles. It functions as a “metabolic sensor” that converts the metabolic status to electrical activity. In pancreatic beta-cells, the KATP channel regulates the secretion of insulin by sensing a change in the blood glucose level and thus maintains glucose homeostasis. In 2004, heterozygous gain-of-function mutations in the KCNJ11 gene, which encodes the Kir6.2 subunit of the KATP channel, were found to cause neonatal diabetes. In some mutations, diabetes is accompanied by severe neurological symptoms [developmental delay, epilepsy, neonatal diabetes (DEND) syndrome]. This review focuses on mutations of Kir6.2, the pore-forming subunit and sulfonylurea receptor (SUR) 1, the regulatory subunit of the KATP channel, which cause neonatal diabetes/DEND syndrome and also discusses the findings of the pathological mechanisms that are associated with neonatal diabetes, and its neurological features.
Keywords: neonatal diabetes, DEND syndrome, KATP channel, sulphonylurea
The Structure and Physiological Roles of the KATP Channel
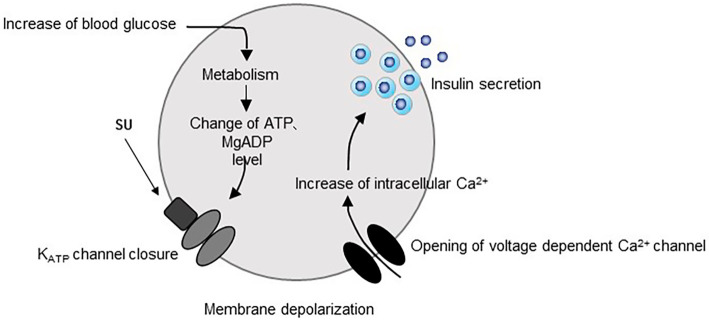
The glucose-dependent insulin secretion is initiated by an increase in the plasma glucose concentration, which enhances the glucose metabolism in beta-cells. This enhanced glucose metabolism leads to an increase in the intracellular concentration of adenosine triphosphate (ATP). This leads to the closure of the KATP channel, which in turn induces membrane depolarization and triggers the opening of the voltage dependent Ca2+ channel, which stimulates the release of insulin (1,2) (Fig. 1). However, the detailed mechanisms that are involved in glucose-dependent insulin secretion and the regulation of the KATP channel are more complicated than the above mechanism.
Figure 1.
The mechanism of insulin secretion.
The electrophysiological findings of KATP channel property confused researchers in the early days of KATP channel research. From the early electrophysiological studies, it was clear that an increased ATP concentration inhibited the KATP channel, but the measurement of the ATP concentration in intact beta-cells revealed that the intracellular ATP concentration was so high, even under low glucose conditions (2-4 mM), that the KATP channel should be permanently closed (3,4). However, this discrepancy was later explained by the finding that MgADP can activate the KATP channel (5,6). At low glucose concentrations, the activation of the KATP channel by MgADP was found to be more dominant, while at high glucose concentrations the inhibitory effects of ATP were found to become more dominant as the intracellular ATP concentration increased. The regulation of KATP channel activity was therefore revealed to result from a balance between the inhibitory effects of intracellular ATP and the activation of the KATP channel by intracellular MgADP.
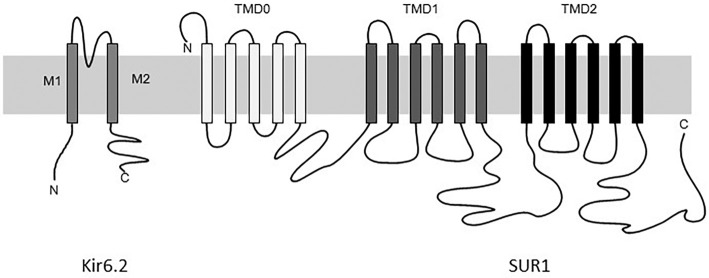
When the KATP channel was cloned in 1995, it was found to be a complex of two different proteins: pore forming subunit Kir6.2, a member of the inwardly rectifying potassium channel family; and sulfonylurea receptor (SUR) 1, a member of the ABCC family, and an ATP-binding cassette transporter (7,8) (Fig. 2). Four Kir6.2 subunits compose a channel pore and four SUR1 subunits surrounding the Kir6.2 channel pore compose a 4:4 octameric complex (9). Each Kir6.2 and SUR1 subunit possesses a endoplasmic reticulum retention motif and since Kir6.2 and SUR1 mask each other's motifs, none of the subunits can reach the cell membrane alone (10). The binding site for ATP lies on the Kir6.2 subunit while the MgADP binding site lies on the SUR1 subunit (11-14).
Figure 2.
A schematic illustration of the KATP channel. Kir6.2 has two transmembrane domains and an inner loop that controls the K+ flux. SUR1 subunit consists of three transmembrane domains (TMD 0-2) and contains two nucleotide binding domains (NBD1 and 2).
The Discovery of Neonatal Diabetes due to Gain-of-function Mutations in the KATP Channel
It is well known that KATP channel mutations can cause congenital hyperinsulinism. The underlying mechanisms of congenital hyperinsulinism include a total loss of the KATP channel in the plasma membrane or the impairment of KATP channel activation by MgADP, which modulates the closed state of the KATP channel, resulting in permanent membrane depolarization and insulin secretion (15-18).
On the other hand, the existence of genetic variance in residue 23 of Kir6.2 (E23K) has been reported to be common in patients with type 2 diabetes (the precise mechanism by which E23K contributes to the development of diabetes remains unclear) (19-21). In 2000, a gain-of-function mutation of the KATP channel was found to cause glucose intolerance in a mouse model (22). This report indicated the possibility that gain-of-function mutations in the KATP channel may decrease insulin secretion, leading to the development of diabetes in humans.
In 2004, the first case of neonatal diabetes with a gain-of-function mutation in the KATP channel was reported (23). The prevalence of neonatal diabetes is estimated to be 1 in 250,000. It is characterized by the onset of diabetes within 6 months after birth (24-29). Nearly half of the cases of neonatal diabetes are caused by Kir6.2 (KCNJ11) and SUR1 (ABCC8) mutations (23-32). Approximately 31% of the cases are due to KCNJ11 mutations, while 13% are due to ABCC8 mutations (33). Although the majority of these patients develop diabetes, only approximately 20% of patients with KATP channel mutations develop neurological symptoms. Neonatal diabetes is classified into the following four subtypes based on the severity of the symptoms: transient hyperglycemia (transient neonatal diabetes: TNDM); permanent hyperglycemia (permanent neonatal diabetes: PNDM); the presence of severe neurological symptoms such as developmental delay, epilepsy and muscle weakness (DEND syndrome); and DEND syndrome symptoms without epilepsy (intermediate DEND syndrome: iDEND) (24-28).
Kir6.2 Mutations and the Pathophysiology of Neonatal Diabetes
The reported mutations of Kir6.2 are mostly dominant heterozygous (one homozygous mutation has been reported), whereas those in SUR1 are either dominant or recessively inherited (29,32,34).
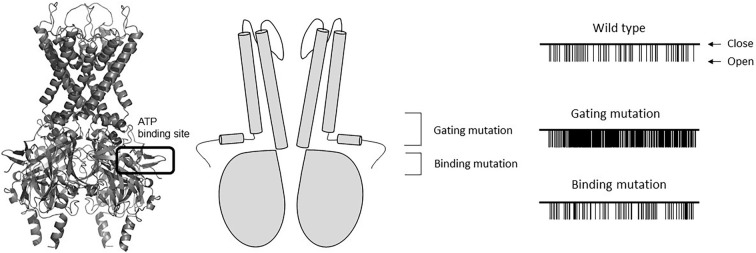
The functional studies of neonatal diabetes-causing Kir6.2 mutations have shown that these mutations reduce the ability of ATP to inhibit the KATP channel (23,25-30). These tiny changes in the KATP channel activity, which occur due to a small shift in ATP sensitivity, can alter the beta-cell electrical activity and insulin secretion to an extent that causes diabetes (34). The molecular mechanism underlying this reduced ATP sensitivity depends on the location of the Kir6.2 mutation (Fig. 3).
Figure 3.
A molecular model of Kir6.2 (left) and a simplified illustration of Kir6.2 (middle). ATP docks to its binding site. A schematic illustration of single KATP channel currents recorded at -60mV from inside-out patches with wild type or mutant Kir6.2 are shown on the right. Note that with the gating mutation, the Po increases in comparison to the wild-type channels. The increase in the Po is not observed with the binding mutation.
Mutations located in the predicted ATP binding site are considered to directly impair the binding of ATP to Kir6.2 (binding mutations). Mutations located in the regions involved in channel gating are considered to indirectly reduce the inhibition of channel activity by ATP (gating mutations). Binding mutations do not usually affect the gating property of the KATP channel. In single channel recordings of the KATP channel with binding mutations, the fraction of time that channels spend in the open state (open probability: Po) is similar to that of the wild-type KATP channel (25,35-37). On the other hand, when KATP channel gating mutations are present, the Po is increased in comparison to the wild-type KATP channel, which indicates that these mutations shift the channel gating toward the open state. The shift of channel gating toward the open state will ultimately reduce the inhibition of the channel by ATP (37-40).
Although there are no correlations between the underlying mechanism (gating or binding) and the severity of patient symptoms, there is a clear correlation between the degree of ATP sensitivity and the severity of the disease. In comparison to the fraction of the remaining KATP channel current under 3 mM MgATP (which corresponds to the physiological concentration of intracellular ATP), the mutations that cause DEND syndrome cause larger remaining KATP channel currents than those that cause iDEND (41). Similarly, the mutations that cause iDEND are associated with larger remaining KATP channel currents in comparison to PNDM or TNDM (41).
SUR1 Mutations and the Pathophysiology of Neonatal Diabetes
The mechanism through which SUR1 mutations induce neonatal diabetes/DEND syndrome is complicated and poorly understood. SUR1 is composed of three transmembrane domains (TMDs), which are linked by a cytosolic linker region (CL3) and two nucleotide-binding domains (NBDs), NBD1 and NBD2. TMD 1 and 2 contain six transmembrane helices, while TMD0 contains five transmembrane helices (7,32) (Fig. 2).
Each NBD contains Walker A and Walker B motifs, which are required for nucleotide binding. NBD1 and 2 dimerize to undergo MgATP binding, and NBD2 hydrolyses MgATP to MgADP and stimulates the KATP channel activity.
To date, more than 60 mutations that induce neonatal diabetes have been identified in SUR1. Most patients with SUR mutations only have diabetes; however, approximately 30% of patients with SUR mutation are reported to have neurological features (32). There are two possible mechanisms through which SUR1 mutations can induce an increase in the KATP channel activity. The first mechanism is through the actions of Kir6.2, such as reducing the binding of ATP to Kir6.2 or stabilizing the open state of the channel by impairing the gating mechanism of Kir6.2 (42). The second possible mechanism involves the enhancement of the activating effect of MgADP (43-48). How SUR1 is coupled to the total KATP channel activity remains unclear. However, because very recent studies have clarified the structure of the KATP channel in high resolution, it is expected that the details of the interaction of Kir6.2 and SUR1 will be revealed (47,48).
The Neurological Features of KATP Channel Mutations Causing DEND Syndrome
The mechanism underlying the induction of hyperglycemia by KATP channel mutations has been well investigated. However, the mechanisms underlying the development of the neurological features that are seen in patients with DEND syndrome are less understood (49). The KATP channel is expressed in multiple types of neurons in the brain. Although numerous studies have reported on the KATP channel and the brain function, the details of the physiological contribution of the brain KATP channel remain to be elucidated. However, recent studies have clarified the physiological contribution of the brain KATP channel to some extent. The neurons in the hypothalamus are known to sense changes in glucose concentrations through the KATP channel, which ultimately regulates the food intake and glucose metabolism (49-52). It has also been reported that the KATP channel contributes to the protection of neurons against stressful conditions such as brain infarction and hypoglycemia (53).
However, these functions, which are already known, cannot fully explain the neurological symptoms of DEND syndrome. It is hypothesized that the underlying mechanism through which DEND syndrome patients develop epilepsy involves the deactivation of the inhibitory neurons, which is induced by the opening of the KATP channel.
As previously mentioned, the neurological symptoms of patients with DEND syndrome were originally considered to be associated with developmental delay, epilepsy and muscle weakness. However, recent reports suggest the existence of psychiatric disorders. Some patients with causative mutations of DEND syndrome are reported to show signs of attention deficit hyperactivity disorder (ADHD), autism or sleeping disorder (54,55). In a mouse model, mice with neural tissue with DEND-causing V59M mutations displayed hyperactivity, increased exploratory behavior and reduced anxiety, which is consistent with the symptoms of ADHD and autism (56). The precise mechanism underlying the development of these psychiatric disorders remains unclear. Because these psychiatric symptoms have a severe impact on both the patient and the patient's family, an integrated and collaborative approach to clinical care is required.
Implications for Therapy
Before the discovery of KATP channel mutations, neonatal diabetes was considered to be a rare form of type 1 diabetes, and was therefore treated with insulin. However, numerous basic and clinical studies on mutations have revealed that many patients with neonatal diabetes due to KATP channel mutations can be treated with sulphonylurea drugs, which bind to both Kir6.2 and SUR1 subunits (57,58).
The low affinity-binding site in Kir6.2 is blocked by high concentrations of the sulphonylurea drugs and has no clinical relevance. The primary effect of the drug is mediated by the high affinity-binding site on SUR1 (59,60). Clinically, sulphonylureas bind to the high affinity-binding site and induce the closure of the KATP channel by suppressing the activating effect of MgADP and unmasking the inhibiting effect of ATP on Kir6.2 (59)
Studies of the KATP channel mutations using the Xenopus oocyte expression system have revealed that many (if not all) mutations were found to be closed to some extent by sulphonylurea drugs (27,35,37,38,58). Based on these findings, more than 90% (87.5% in Japan) of patients with neonatal diabetes were successfully switched from insulin injection treatment to oral sulphonylurea (glibenclamide) therapy (57,58,61). Because a high dose of glibenclamide is required for the treatment of neonatal diabetes, the risk of inducing hypoglycemia should be considered. However, clinical studies have shown that sulphonylurea therapy induces fewer fluctuations in blood glucose, and a marked decrease in HbA1c levels in comparison to insulin injection therapy (57).
It is suggested that sulphonylureas can also improve the neurological features of patients with DEND syndrome (26). Hashimoto et al. reported the successful improvement of the neurological features in patients with T293N and R50P mutations, which are associated with DEND syndrome (61). However, the patients' neurological symptoms did not completely recover after treatment with sulphonylureas. This may be because the concentration of sulphonylurea is not sufficient to close the KATP channel in the brain. Whether sulphonylureas can cross the blood brain barrier (BBB) remains unclear. A previous study showed that when glibenclamide was administered peripherally in mice, the drug concentration in the cerebral spinal fluid was not high enough to block the mutated KATP channel, which affected the neural electrical activity (62). However, an increase in the blood flow in the cerebellum was observed in patients with DEND syndrome after they switched to sulphonylurea therapy (63). This shows that sulphonylureas have an effect on the brain, and that the improvement of the motor function by the initiation of sulphonylurea therapy may be explained by the improved cerebellum function; however, further study is required to test this hypothesis.
Generally, sulphonylurea treatment is more effective when it is started at an early stage of life. Age and prolonged poor glycemic control seem to be important predictors of responsiveness to sulphonylureas in neonatal diabetic patients. Effective transfer is less likely in elderly patients with poor glycemic control (64-66). Because insulin therapy does not improve the neurological symptoms of DEND syndrome, an early-stage diagnosis is critical, as it enables sulphonylurea therapy to be initiated as quickly as possible.
Minor side effects of high-dose sulphonylurea treatment have been reported in patients with neonatal diabetes. Transient diarrhea was reported by some patients after switching to sulphonylurea treatment (67). Furthermore, approximately 7.5% of sulphonylurea-treated patients experienced tooth discoloration (68). The severity of discoloration varies from a mild color change to a loss of enamel. The mechanism responsible for this symptom is unclear but thought possibly to be related to the direct exposure of the teeth to high doses of the drug, because the patients with discoloration either chewed the drug or took it in solution (68).
Conclusion
The increased activity of the KATP channel due to mutations leads to reduced insulin secretion and the development of neonatal diabetes. Some mutations cause severe neurological symptoms. Recent studies have shown that many patients with neonatal diabetes and KATP channel mutations can be effectively treated using sulphonylurea drugs. Functional studies on KATP channel mutations provide novel therapeutic options for patients with neonatal diabetes; thus, it is important to make a precise diagnosis at an early stage of the disease.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The author (KS) wishes to thank Prof. Frances Ashcroft (Oxford University, UK) for providing the opportunity to study KATP channels at Oxford University for 8 years with warm and generous support. The author would also like to dedicate this paper to the memory of the late Prof. Shigeji Matsumoto (Nippon Dental University, Japan) who provided support and encouragement to the author for 20 years.
References
- 1.Ashcroft FM. ATP-sensitive potassium channelopathies: focus on insulin secretion. J Clin Invest 115: 2047-2058, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature 440: 470-476, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Ashcroft FM, Kakei M. ATP-sensitive K-channels: modulation by ATP and Mg2+ ions. J Physiol 416: 349-367, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cook DL, Hales CN. Intracellular ATP directly blocks K+ channels in pancreatic β-cells. Nature 311: 271-273, 1984. [DOI] [PubMed] [Google Scholar]
- 5.Dunne MJ, Peterson OH. Intracellular ADP activates ATP-sensitive K+ channels in insulin secreting cell line. FEBS Lett 208: 52-62, 1986. [DOI] [PubMed] [Google Scholar]
- 6.Kakei M, Kelly RP, Ashcroft SJH, Ashcroft FM. The ATP-sensitivity of K+ channels in rat pancreatic β-cells is modulated by ADP. FEBS Lett 208: 63-66, 1986. [DOI] [PubMed] [Google Scholar]
- 7.Inagaki N, Gonoi T, Clement JP, et al. . Reconstruction of IKATP: An inward rectifier subunit plus the sulfonylurea receptor. Science 270: 1166-1170, 1995. [DOI] [PubMed] [Google Scholar]
- 8.Sakura H, Ammala C, Smith PA, Gribble FM, Ashcroft FM. Cloning and functional expression of the cDNA encoding novel ATP-sensitive potassium channel subunit expressed in pancreatic beta cells, brain, heart and skeletal muscle. FEBS Lett 377: 338-344, 1995. [DOI] [PubMed] [Google Scholar]
- 9.Inagaki N, Gonoi T, Seino S. Subunit stoichiometry of the pancreatic beta-cell ATP-sensitive K+ channel. FEBS Lett 409: 232-236, 1997. [DOI] [PubMed] [Google Scholar]
- 10.Zerangue N, Schwappach B, Jan YN, Jan LY. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane KATP channels. Neuron 22: 537-548, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Gribble FM, Tucker SJ, Ashcroft FM. The essential role of the Walker A motifs of SUR1 in KATP channel activation by Mg-ADP and diazoxide. EMBO J 16: 1145-1152, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nichols CG, Shyng SL, Nestrorowicz A. Adenosine diphosphate as an intracellular regulator of insulin secretion. Science 272: 1785-1787, 1996. [DOI] [PubMed] [Google Scholar]
- 13.Tucker SJ, Gribble FM, Zhao C, Trapp S, Ashcroft FM. Truncation of Kir6.2 produces ATP-sensitive K+ channels in the absence of the sulphonylurea receptor. Nature 387: 179-183, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Mikhailov MV, Campbell JD, de Wet H, et al. . 3-D structural and functional characterization of the purified KATP channel complex Kir6.2-SUR1. EMBO J 24: 4166-4175, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunne MJ, Cosgrove KE, Shepherd RM, Aynsley-Greeen A, Lindley KJ. Hyperinsulinism in infancy: from basic science to clinical disease. Physiol Rev 84: 239-275, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Nestrowicz A, Inagaki N, Gonoi T, et al. . A nonsense mutation in the inward rectifier potassium channel gene, Kir6.2, is associated with familial hyperinsulinism. Diabetes 46: 1743-1748, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Thomas PM, Cote GJ, Wohllk N, et al. . Mutations in the sulfonylurea receptor gene in familial persistent hyperinsulinemic hypoglycemia of infancy. Science 268: 426-429, 1995. [DOI] [PubMed] [Google Scholar]
- 18.Shimomura K, Tusa M, Iberl M, et al. . A mouse model of human hyperinsulinism produced by the E1506K mutation in the sulphonylurea receptor SUR1. Diabetes 62: 3797-3806, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gloyn AL, Weedon MN, Owen KR, et al. . Large scale association studies of variants in genes encoding the pancreatic b-cell KATP channel subunits Kir6.2 (KCNJ11) and SUR1 (ABCC8) confirm that the KCNJ11 E23K variant is associated with type 2 diabetes. Diabetes 52: 568-572, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Sakura H, Wat N, Horton V, Millns H, Turner RC, Ashcroft FM. Sequence variations in the human Kir6.2 gene, a subunit of the beta-cell ATP-sensitive K-channel: no association with NIDDM in while Caucasian subjects or evidence of abnormal function when expressed in vitro. Diabetologia 39: 1233-1236, 1996. [DOI] [PubMed] [Google Scholar]
- 21.Zeggini E, Weedon MN, Lindgren CM, et al. . Replication of genome-wide association signals in UK samples reveals loci for type 2 diabetes. Science 316: 1336-1341, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koster JC, Marshall BA, Ensor N, Corbett JA, Nichols CG. Targeted overactivity of beta cell KATP channels induces profound neonatal diabetes. Cell 100: 645-654, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Gloyn AL, Pearson ER, Antcliff JF, et al. . Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N Eng J Med 350: 1838-1849, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Hattersley AT, Ashcroft FM. Activating mutations in Kir6.2 and neonatal diabetes: new clinical syndromes, new scientific insights, and new therapy. Diabetes 54: 2503-2513, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Shimomura K. The KATP channel and neonatal diabetes. Endocr J 56: 165-175, 2009. [DOI] [PubMed] [Google Scholar]
- 26.Shimomura K, Hoster F, de Wet H, et al. . A novel mutation causing DEND syndrome: a treatable channelopathy of pancreas and brain. Neurology 69: 1342-1349, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Shimomura K, de Nanclares GP, Foutinou C, Caimari M, Castano L, Ashcroft FM. The first clinical case of a mutation at residue K185 of Kir6.2 (KCNJ11): a major ATP binding residue. Diabet Med 27: 225-229, 2010. [DOI] [PubMed] [Google Scholar]
- 28.Flanagan SE, Patch FM, Mackay DJ, et al. . Mutations in ATP-sensitive K+ channel genes cause transient neonatal diabetes and permanent diabetes in childhood or adulthood. Diabetes 56: 1930-1937, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanakatti Shanker R, Pihoker C, Dolan LM, et al. . SEARCH for Diabetes in Youth Study Group. Parmanent neonatal diabetes mellitus: prevalence and genetic diagnosis in the SEARCH for diabetes youth study. Pedatr Diabetes 14: 174-180, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edghill EL, Gloyn Al, Gillespie KM, et al. . Activating mutations in the KCNJ11 gene encoding the ATP-sensitive K+ channel subunit Kir6.2 are rare in clinically defined type 1 diabetes before 2 years. Diabetes 53: 2998-3001, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Proks P, Shimomura K, Craig TJ, Girard CA, Ashcroft FM. Mechanism of action of a sulphonylurea receptor SUR1 mutation (F132L) that cause DEND syndrome. Hum Mol Genet 16: 2011-2019, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Proks P. Neonatal diabetes caused by activating mutations in the sulphonylurea receptor. Diabetes Metab J 37: 157-164, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edghill EL, Flanagan SE, Ellard S. Permanent neonatal diabetes due to activating mutation in ABCC8 and KCNJ11. Rev Endocr Metab Disord 11: 193-198, 2010. [DOI] [PubMed] [Google Scholar]
- 34.Vedovato N, Cliff E, Proks P, et al. . Neonatal diabetes caused by a homozygous KCNJ11 mutation demonstrates that tiny changes in ATP sensitivity markedly affect diabetes risk. Diabetolodia 59: 1430-1436, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimomura K, Girard CA, Proks P, et al. . Mutations at the same residue (R50) of Kir6.2 (KCNJ11) that cause neonatal diabetes produce different functional effects. Diabetes 55: 1705-1712, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Masia R, Koster JC, Tumini S, et al. . An ATP binding mutation (G334D) in KCNJ11 is associated with sulfonylurea-insensitive form of developmental delay, epilepsy and neonatal diabetes. Diabetes 56: 328-336, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Proks P, Antcliff JF, Lippiat J, Gloyn A, Hattersley AT, Ashcroft FM. Molecular basis of Kir6.2 mutations associated with neonatal diabetes or neonatal diabetes plus neurological features. Proc Natl Acad Sci USA 101: 17539-17544, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Girard C, Shimomura K, Proks P, et al. . Functional analysis of six Kir6.2 (KCNJ11) mutations causing neonatal diabetes. Pflugers Arch 453: 323-332, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Proks P, Girard C, Baevre H, Njolstad PR, Ashcroft FM. Functional effects of mutations at F35 in the NH2 terminus of Kir6.2 (KCNJ11) causing neonatal diabetes and response to sulphonylurea therapy. Diabetes 55: 1731-1737, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Proks P, Girard C, Haider S, et al. . A gating mutation at the internal mouth of the Kir6.2 pore is associated with DEND syndrome. EMBO Rep 6: 470-475, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ashcroft FM. ATP-sensitive K+ channels and disease: from molecule to malady. Am J Physiol Endocrinol Metab 293: E880-E889, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Lin YW, Akrouh A, Hsu Y, Hughes N, Nichols CG, De Leon DD. Compound heterozygous mutations in the SUR1 (ABCC8) subunit of pancreatic KATP channels cause neontals diabetes by perturbing the coupling between Kir6.2 and SUR1 subunits. Channels 6: 133-138, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Babenko AP, Polak M, Cave H, et al. . Activating mutations in the ABCC8 gene in neonatal diabetes mellitus. N Eng J Med 355: 155-166, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Zhou Q, Garin I, Castano L, et al. . Neonatal diabetes caused by mutations in sulfonylurea receptor 1: interplay between expression and Mg-nucleotide gating defects of ATP-sensitive potassium channels. J Clin Endocrinol Metab 95: E473-E478, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Wet H, Rees MG, Shimomura K, et al. . Increased ATP activity produced by mutations at arginine-1380 in nucleotide-binding domain 2 of ABCC8 causes neonatal diabetes. Proc Natl Acad Sci USA 104: 18988-18992, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ortiz D, Voyvodic P, Gossack L, Quast U, Bryan J. Two neonatal diabetes mutations on transmembrane helix 15 of SUR1 increase affinity for ATP and ADP at nucleotide binding domain 2. J Biol Chem 287: 17985-17995, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin GM, Yoshioka C, Rex EA, et al. . Cryo-EM structure of ATP-sensitive potassium channel illuminates mechanisms of assembly and gating. Elife 2017. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li N, Wu JX, Ding D, Cheng J, Gao N, Chen L. Structure of a pancreatic ATP-sensitive potassium channel. Cell 168: 101-110, 2017. [DOI] [PubMed] [Google Scholar]
- 49.Clark RH, Mctaggart JS, Webster R, et al. . Muscle dysfunction caused by KATP channel mutation in neonatal diabetes is neuronal in origin. Science 329: 458-461, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miki T, Seino S. Roles of KATP channels as metabolic sensors in acute metabolic changes. J Mol Cell Cardiol 38: 917-925, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Wang R, Liu X, Hentges ST, et al. . The regulation of glucose-excited neurons in the hypothalamic arcuate nucleus by glucose and feeding-relevant peptides. Diabetes 53: 1959-1965, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Miki T, Liss B, Minami K, et al. . ATP-sensitive K+ channels in the hypothalamus are essential for the maintenance of glucose homeostasis. Nat Neurosci 4: 507-512, 2001. [DOI] [PubMed] [Google Scholar]
- 53.Yamada K, Ji JJ, Yuan H, et al. . Protective role of ATP-sensitive potassium channels in hypoxia-induced generalized seizure. Science 292: 1543-1546, 2001. [DOI] [PubMed] [Google Scholar]
- 54.Bowman P, Broadbridge E, Knight BA, et al. . Educational and psychological aspects psychiatric morbidity in children with KCNJ11 neonatal diabetes. Diabet Med 33: 1387-1391, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Landmeier KA, Lanning M, Carmody D, Greeley SA, Msall ME. ADHD, learning difficulties and sleep disturbances associated with KCNJ11-related neonatal diabetes. Pedatr Diabetes 2016. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lahmann C, Clark RH, Iberl M, Ashcroft FM. A mutation causing increased KATP channel activity leads to reduced anxiety in mice. Physiol Behav 129: 79-84, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pearson ER, Fletcher I, Njolstad PR, et al. . Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Eng J Med 355: 467-477, 2006. [DOI] [PubMed] [Google Scholar]
- 58.Babiker T, Vedovato N, Patel K, et al. . Successful transfer to sulphonylureas in KCNJ11 neonatal diabetes is determined by the mutation and duration of diabetes. Diabetokogia 59: 1162-1166, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Proks P, de Wet H, Ashcroft FM. Molecular mechanism of sulphonylurea block of KATP channels carrying mutations that impair ATP inhibition and cause neonatal diabetes. Diabetes 62: 3909-3919, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Campbell JD, Proks P, Lippiat JD, Sansom MSP, Ashcroft FM. Identification of a functionally important negatively charged residue within the second catalytic site of the SUR1 nucleotide-binding domains. Diabetes 53: S123-S127, 2004. [DOI] [PubMed] [Google Scholar]
- 61.Hashimoto Y, Dateki S, Hirose M, et al. . Molecular and clinical features of KATP-channel neonatal diabetes mellitus in Japan. Pediatr Diabetes 2016. (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 62.Lahmann C, Kramer HB, Ashcroft FM. Systemic administration of glibenclamide fails to achieve therapeutic levels in the brain and cerebrospinal fluid of rodents. PLoS One 10: e0134476, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fendler W, Drozdz I, Pietrzak I, et al. . Switching to sulphonylureas in children with iDEND syndrome caused by KCNJ11 mutations results in improves cerebellar perfusion. Diabetes Care 36: 2311-2316, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lau E, Correia C, Freitas P, et al. . Permanent neonatal diabetes by a new mutation in KCNJ11: unsuccessful switch of sulfonylurea. Arch Endocrinol Metab 59: 559-561, 2015. [DOI] [PubMed] [Google Scholar]
- 65.Stoy J, Greeley SA, Paz VP, et al. . United States National Diabetes Working Group. Diagnosis and treatment of neonatal diabetes: a United States experience. Pediatr Diabetes 9: 450-459, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wambach JA, Marshall BA, Koster JC, White NH, Nichols CG. Successful sulfonylurea treatment of an insulin-naïve neonate with diabetes mellitus due to a KCNJ11 mutation. Pediatr Diabetes 11: 286-288, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Codner E, Flanagan S, Ellard S, Garcia H, Hattersley AT. High-dose glibenclamide can replace insulin therapy despite transitory diarrhea in early-onset diabetes caused by novel R201L Kir6.2 mutation. Diabetes Care 28: 758-759, 2005. [DOI] [PubMed] [Google Scholar]
- 68.Kumagaguru J, Stoy J, Flanagan SE, et al. . Tooth discoloration in patient with neonatal diabetes after transfer onto glibenclamide. Diabetes Care 32: 1428-1430, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]