Abstract
Background
Earlier age of pubertal maturation in females is associated with increased risk for mental health problems in adolescence, compared with on-time or later maturation. However, most investigations of pubertal timing and mental health consider risk for individual disorders and fail to account for comorbidity. A latent-modeling approach using a large, nationally representative sample could better explain the transdiagnostic nature of the consequences of early-onset puberty.
Methods
Data on age of menarche and mental disorders were drawn from a population-representative sample of adolescents (n=4925), ages 13–17. Confirmatory factor analysis was used to fit four latent disorder categories: distress, eating, and externalizing, and fear disorders. Timing of menarche included those with earlier (age <=10, age 11) and later age of onset (age 13, 14+), relative to those with average timing of menarche (age 12). Associations between timing of menarche and latent disorders were estimated in a structural equation model (SEM), adjusted for age, income, race, parent marital status, BMI, and childhood adversity.
Results
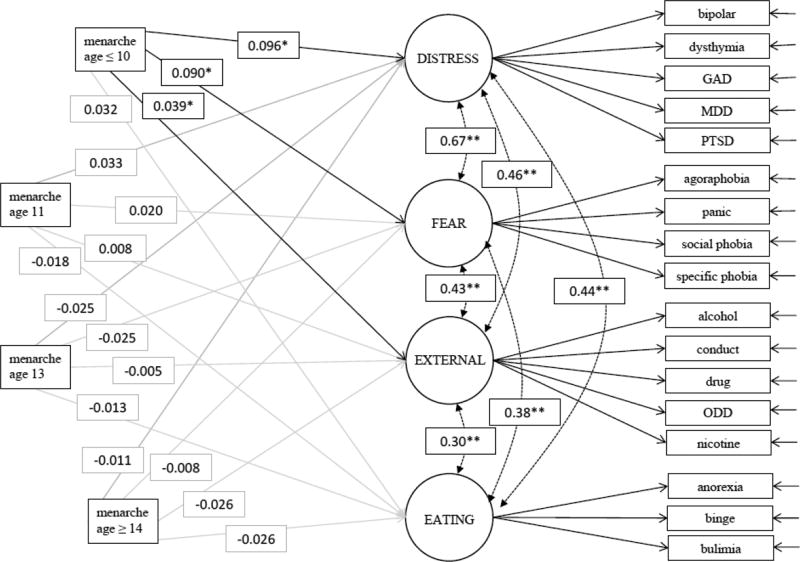
The measurement model evidenced acceptable fit (CFI= 0.91; RMSEA= 0.02). Onset of menarche before age 11 was significantly associated with distress disorders (coefficient=0.096; p<.0001), fear disorders (coefficient=0.09; p<.0001), and externalizing disorders (coefficient=0.039; p=0.049) as compared to on-time or late menarche. No residual associations of early menarche with individual disorders over and above the latent disorders were observed.
Conclusion
The latent modeling approach illuminated meaningful transdiagnostic psychiatric associations with early timing of menarche. Biological processes initiated at puberty can influence cognitive and affective processes as well as social relationships for adolescents. Under developmentally normative conditions, these changes may be adaptive. However, for those out of sync with their peers, researchers and clinicians should recognize the potential for these processes to influence liability to a broad array of psychopathological consequences in adolescence.
Keywords: Puberty, Psychopathology, Comorbidity, Structural Equation Modeling
1. Introduction
Puberty is a complex developmental process that involves a host of biological, psychological and social changes. Although puberty reflects changes across multiple systems, one specific and reliable marker of pubertal stage for females is menarche, or the onset of the menstrual cycle. Menarche represents a discrete and meaningful event that has been shown to be a good marker of the overall maturation process [1]. It is noteworthy that the onset of many psychiatric disorders in the population first begin during the pubertal transition, particularly in adolescent females [2]–[5], suggesting a role for puberty-related changes in the etiology of psychiatric disorders. In particular, adolescent girls begin to exhibit significantly greater rates of internalizing disorders relative to adolescent boys during the pubertal transition [6].
Two general theoretical explanations for the increased risk of psychopathology that emerges around puberty have been proposed (see [7] for a review). First, increases in production and circulation of sex-hormones (e.g., DHEA, DHEA-S, estradiol, testosterone, progesterone) may directly influence brain function [8]. Changes in the levels of these hormones influence neurotransmitters such as dopamine and serotonin, and the development of both the prefrontal cortex and limbic structures. Changes in these neural systems may underlie disruptions in sleep, concentration, appetite, and sensation-seeking processes, which may increase the risk of disorder incidence, severity, or chronicity [9]–[11]. Alternatively, the salient effects of puberty may be due to the psychological stress from changes in body morphology, including secondary sex characteristics, but also increased appearance of acne, weight gain, and voice changes [12]. Physical manifestation of these changes may increase exposure to new social norms, environments, and expectations from peers and adults regarding maturity which may increase levels of psychosocial stress [13] and traumatic experiences, such as unwanted sexual experiences [14], ultimately increasing risk of psychiatric disorders [15]. These increased risks may be especially high for females who experience earlier pubertal maturation than peers [12], [16], [17]. Much of the empirical research into the psychiatric consequences of puberty has focused on timing of menarche [18]. For girls, there is consistent evidence that early age of menarche is associated with increased risk of many psychiatric disorders [19], such as depression [20], [21], anxiety [22], [23], eating [24], and externalizing disorders [25].
Despite the extensive body of literature on psychiatric disorders and timing of menarche, there remain three unresolved areas of inquiry. First, while several studies have examined multiple psychiatric disorders that may result from non-normative timing of menarche [15], [26], [27], fewer have considered the comorbidities among psychiatric disorders [15]. This type of consideration may highlight an underlying dimensional structure of multiple disorders resulting from non-normative timing of menarche. An identification of a broader psychopathological structure would facilitate better understanding of the role of early menarche as a risk factor for liability to psychopathology more broadly as opposed to specific individual disorders [16]. Further, this approach may be useful for characterizing the most salient general features of comorbid disorders, including shared risk factors, pathological processes, and illness course [28]. Second, investigations of timing of menarche nearly always consider the psychiatric consequences of early onset of menarche, but the effects of late menarche in females are less frequently considered. Where late timing of menarche has been considered, the effects have been less consistent [12]. Nevertheless, considering the psychiatric consequences of late menarche in females may help to test and strengthen the hypotheses related to the social consequences of early menarche. For example, if the experience of early maturation causes an increased exposure to risky environments, females with later menarche onset than average should report an equal or lower risk of psychiatric disorders than those who report an average onset of menarche. Alternately, if the negative consequences of early maturation are due to experiencing morphological changes that are out of sync with same-aged peers, then girls who reach menarche later than average (and thus look physically different than same-age peers) would have a risk of psychiatric disorders that is also greater than those with earlier than average menarche. Third, most studies utilize data from small samples with potentially limited generalizability. Although these studies allow for an investigation of differences in timing among a specific cohort (e.g., within particular schools [15] or communities [11]), population-representative sample are uniquely capable of identifying the transdiagnostic risks from early or late onset of puberty at the population level.
The current study examined the association between the age of onset of menarche and a broad array of psychiatric disorders in a large, population-representative sample of adolescent girls. Because both the timing of and social reactions to puberty are quite different for boys and girls [26], [29], the current study was limited to girls. We examined these relationships using structural equation modeling approach, as a way to better understand the transdiagnostic psychiatric risk posed by initiating puberty earlier or later than average.
2. Methods
2.1 Data source
Data were from the National Comorbidity Survey Adolescent Supplement (NCS-A). The NCS-A data were collected during a single wave interview of a United States population-representative sample of adolescents age 13–18 from 2001–2004 [30]. The study was designed to estimate prevalence of a broad range of psychiatric disorders in adolescence, as well as a detailed survey of the demographic correlates and risk factors for disorders [31]. Data were weighted based on the 2000 Census frequencies, to yield population-representative estimates. Further study details can be found elsewhere [32], [33]. The entire NCS-A sample consisted of 10,148 respondents, including 5183 female adolescents (51.1%). Girls with missing responses for their age of menarche (n=83; 1.6%) and those who had not yet initiated menarche were excluded (n=163; 3.2%). The final analytic sample size included 4937 respondents, of which 64.7% were non-Hispanic White, 16.5% were non-Hispanic Black, 13.9% were Hispanic, 5.0% specified ‘other’ racial or ethnic backgrounds.
Study participants received $50 for participation. Written informed consent from adults and assent from adolescents was obtained, in accordance to the procedures approved by Human Subjects Committees of Harvard Medical School and the University of Michigan. The Institutional Review Board of Columbia University approved the present analysis.
2.2 Measures
2.2.1 Psychiatric disorders
Seventeen adolescent psychiatric disorders were considered as individual outcomes in survival analytic models, and were used to estimate the latent measurement model. They included: bipolar disorder (including types I, II, and subthreshold), dysthymia, general anxiety disorder (GAD), major depressive disorder (MDD), post-traumatic stress disorder (PTSD), agoraphobia, panic disorder, social phobia, specific phobia, anorexia, binge eating, bulimia, conduct disorder, oppositional defiant disorder (ODD), alcohol abuse, with or without dependence, drug abuse, with or without dependence, and nicotine dependence. ODD and major depression were diagnosed based on the symptom report of parent or child respondents, to increase diagnostic reliability [34], [35]. Disorders were defined using an adolescent version of the Composite International Diagnostic Interview for DSM-IV [30], [33]. Typical CIDI diagnostic thresholds were lowered in order to include complete assessments of comorbid symptoms and sub-threshold cases [30]. Multiple disorder subtypes were assigned only if there was clear evidence for a temporal distinction between the manifestations of multiple types. Children and parents who met diagnostic thresholds were subsequently asked the age that their symptoms began.
To create the latent outcomes, we ran an exploratory factor analysis to identify latent domains from the 17 separate disorders. The results of our EFA were compared to previously published empirical investigations [36] and current guidance regarding trans-diagnostic dimensional structure to determine the consistency and validity of our groupings [37]. We did not include attention deficit/ hyperactivity disorder and separation anxiety disorder, because the median age of onset was younger than 12 years and thus most disorders would be expected to onset before puberty, thus bypassing the risk period coinciding with onset of menarche [36]. Our final model included four latent domains, including distress (dysthymia, GAD, MDD, and PTSD), fear (agoraphobia, bi-polar, panic, social phobia, specific phobia), externalizing (alcohol abuse/dependence, drug abuse/dependence, and nicotine dependence, conduct, oppositional defiance), and eating (anorexia, binge, bulimia) disorders. The factor model evidenced good fit with the sample data (CFI= 0.99; RMSEA= 0.019).
2.2.2 Timing of menarche onset
All female respondents were asked the age at which they had their first period. Responses ranged from 6–18 years old. Ages were grouped into a five-level categorical variable of the timing of menarche onset. The reference group was set as age 12, corresponding to the median age of menarche in the United States at the time of the survey [38] and to the mean (12.1) and median (11.6) age of menarche onset in the analytic sample. Four age groups were compared to the reference: those with earlier (age <=10, age 11) and those with a later age of onset (age 13, 14+). These groups were selected in order to investigate specific and potentially non-linear differences in the association between age of menarche and latent disorders, while maintaining adequate sample sizes within each level to avoid statistical imprecision. As a sensitivity analysis, we considered three other categorizations of menarche timing, including as a continuous variable, a three-level categorical variable with age 11–13 as the reference, and a three-level categorical variable with age 10–14 as the reference. Exposure levels were modeled using a set of indicator variables.
2.2.3 Covariates
Models were also adjusted for a series of potential confounders of the associations of interest. Categorical or dichotomous variables included: age (13–14, 15–16, 17–18 years old), parent income (<1.5, 1.5 ≤ 3, 3 ≤ 6, 6+ times the poverty level), race (non-Hispanic Black, non-Hispanic White, Hispanic, other race), parent marital status (married, separated/divorced, never married), exposure to childhood trauma (yes/no) based on a trauma checklist administered in the CIDI, and exposure to other adversities including poverty, low parental education, and neglect [39]. BMI was modeled as a continuous variable, standardized according to established procedures for adolescents [40], [41], using the SAS macro available here: https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm. The distribution of all model covariates and associations with age of menarche can be found in supplementary table 1.
2.3 Data analysis
Two separate methods were used to examine the relationship between age of menarche and psychopathology. First, single disorders were considered using a survival analysis models, and second, latent disorder groups were considered using structural equation methods. Using Cox proportional hazards models, we examined temporal pathways between age of menarche and age of psychiatric disorders. More specifically, models tested whether the rate of disorder differed at each age of menarche, compared to the reference age. The outcome in these models included only individuals whose age of menarche preceded their age of disorder. Adolescents without the focal disorder of interest were censored at their current age. In all models, unadjusted and adjusted hazard ratios between the onset of menarche and individual psychiatric disorders were estimated in SAS 9.4 [42].
Second, the structural equation model (SEM) was fit to estimate the associations between age of menarche onset and each latent disorder. Because of the difficulty assigning age of onset for multiple individual disorders that comprised latent groups, lifetime diagnoses were used regardless of whether menarche began before or after the disorder onset. This analysis contained two-sub steps; first, an EFA was used to identify the measurement model of latent disorders and assess goodness-of-fit. Second, we regressed the independent variable and model covariates onto these latent disorders. Levels of the independent variable were allowed to correlate. Model parameters were estimated using weighted least squares means and variances (WLSMV) methods, in order to handle categorical variables with non-normally distributed errors and missing data [43]. For both steps, nested models were compared in order to identify the best-fitting model. Modification indices were considered as a method to improve the marginal model fit [44], and to identify direct effects for associations between the age of menarche onset and individual disorders in addition to the SEM path coefficients. All latent models were created in Mplus 7.0 [45].
Goodness-of-fit in the measurement model and the SEM was evaluated using the Root Mean Square Error of Approximation (RMSEA) and its 90% confidence interval (CI), and the Comparative Fit Index (CFI). Based on previous recommendations [46], [47], we set cutoffs for acceptable fit at RMSEA < .10 and CFI > .90, and cutoffs for good fit at RMSEA < .05 and CFI > .95. Factor correlation was estimated using Pearson’s r coefficient.
3. Results
3.1 Descriptive statistics
Overall, 35.7% of the analytic sample reported initiating menarche at the age of 12. Among those who were earlier than age 12, 9.2% reported an onset younger than 11, and 18.2% reported onset at age 11. Among those who were later than age 12, 25.8% reported onset at age 13, and 11.1% reported an onset of age 14 or older. Specific phobia was most commonly reported disorder (23.0%), while anorexia was the least frequently reported disorder (0.4%). The age of menarche onset was significantly associated with endorsement of bipolar, dysthymia, major depression, social phobia, specific phobia, bulimia, and nicotine dependence. Table 1 shows the prevalence of all disorders within each menarche onset group.
Table 1.
Frequency of disorders by age of menarche onset (N; %)
| Age of menarche onset (N; %) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤10 | 11 | 12 | 13 | ≥14 | p | ||||||
| Total (n=4937) | 450 | 9.2 | 932 | 18.2 | 1714 | 35.7 | 1304 | 25.8 | 525 | 11.1 | <0.001 |
| Distress disorders | |||||||||||
| MDD | 125 | 26.9 | 172 | 19.1 | 278 | 17.0 | 195 | 15.1 | 94 | 16.5 | 0.001 |
| Bipolar | 50 | 11.5 | 62 | 6.9 | 103 | 6.5 | 92 | 6.0 | 30 | 4.7 | 0.029 |
| Dysthymia | 40 | 9.2 | 47 | 5.2 | 68 | 3.9 | 48 | 3.3 | 25 | 7.0 | 0.001 |
| GAD | 25 | 5.5 | 35 | 4.1 | 66 | 4.2 | 51 | 4.2 | 25 | 5.2 | 0.903 |
| PTSD | 54 | 17.3 | 80 | 15.6 | 112 | 13.3 | 81 | 10.9 | 36 | 11.3 | 0.317 |
| Eating | |||||||||||
| Anorexia | 1 | 0.0 | 0 | -- | 5 | 0.2 | 8 | 0.6 | 4 | 0.4 | - |
| Binge | 36 | 7.2 | 57 | 4.9 | 103 | 5.7 | 79 | 7.1 | 28 | 4.5 | 0.314 |
| Bulimia | 12 | 3.2 | 11 | 0.5 | 26 | 1.6 | 16 | 1.1 | 6 | 0.6 | 0.003 |
| External | |||||||||||
| Alcohol | 32 | 6.7 | 50 | 6.1 | 84 | 5.5 | 79 | 5.9 | 38 | 6.8 | 0.935 |
| Conduct | 25 | 5.5 | 38 | 4.2 | 74 | 5.3 | 58 | 3.6 | 27 | 6.3 | 0.366 |
| Drug | 39 | 11.2 | 64 | 6.8 | 109 | 7.6 | 100 | 7.9 | 43 | 10.6 | 0.309 |
| ODD | 57 | 12.7 | 95 | 9.1 | 180 | 11.0 | 126 | 9.5 | 38 | 9.1 | 0.617 |
| Nicotine | 53 | 12.3 | 68 | 8.6 | 106 | 6.2 | 99 | 7.5 | 35 | 7.1 | 0.034 |
| Fear disorders | |||||||||||
| Agoraphobia | 28 | 7.0 | 41 | 3.5 | 61 | 4.6 | 50 | 2.9 | 18 | 2.5 | 0.051 |
| Panic | 19 | 4.5 | 30 | 3.1 | 45 | 2.8 | 30 | 2.0 | 10 | 2.0 | 0.330 |
| Social phobia | 81 | 19.4 | 109 | 11.4 | 184 | 12.1 | 140 | 8.8 | 56 | 9.0 | 0.002 |
| Specific phobia | 141 | 31.3 | 226 | 20.6 | 391 | 24.1 | 268 | 19.0 | 122 | 20.2 | 0.004 |
GAD=general anxiety disorder, MDD=major depressive disorder, PTSD=Post-traumatic stress disorder, ODD=oppositional defiant disorder.
3.2 Cox proportional hazards models
After adjusting for covariates, those who reported an onset of menarche before age 11 had significantly greater rates of bipolar disorder (HR=3.9; 95% CI= 1.1–14.2), dysthymia (HR=2.3; 95% CI= 1.0–5.2), major depression (HR=1.8; 95% CI= 1.2–2.7), social phobia (HR=2.7; 95% CI= 1.4–5.3), specific phobia (HR=4.9; 95% CI= 2.0–12.0), and nicotine dependence, compared to those who initiated menarche at age 12. Those with an onset of menarche at age 11 had significantly greater rates of bipolar disorder (HR=4.4; 95% CI= 1.2–16.8), dysthymia (HR=3.5; 95% CI= 1.6–7.6), and major depression (HR=1.7; 95% CI= 1.0–2.6), and nicotine dependence (HR=1.9; 95% CI 1.2–2.9).
Compared to females with average age of menarche, those who reported menarche onset at age 14 or older had a significantly lower rate of major depression (HR=0.3; 95% CI= 0.2–0.6), panic disorder (HR=0.1; 95% CI= 0.01–0.3), and social phobia (HR=2; 95% CI= 0.1–0.7). The full model results can be found in table 2. Unadjusted model results can be found in supplementary table 2.
Table 2.
Adjusted hazard ratios for the adjusted rate of disorder by age of menarche
| Age of menarche onset (HR; 95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|
| ≤10 | 11 | 13 | ≥14 | |||||
| Distress disorders | ||||||||
| Bipolar | 3.9 | 1.1 – 14.2 | 4.4 | 1.2 – 16.8 | 1.9 | 0.5 – 7.0 | 0.6 | 0.1 – 3.5 |
| Dysthymia | 2.3 | 1.0 – 5.2 | 3.5 | 1.6 – 7.6 | 1.2 | 0.5 – 3.1 | 1.6 | 0.5 – 5.7 |
| GAD | 1.0 | 0.5 – 2.2 | 1.2 | 0.4 – 3.8 | 1.0 | 0.5 – 2.2 | 0.4 | 0.1 – 1.4 |
| Major depression | 1.8 | 1.2 – 2.7 | 1.7 | 1.0 – 2.6 | 0.7 | 0.4 – 1.1 | 0.3 | 0.2 – 0.6 |
| PTSD | 1.6 | 0.7 – 3.6 | 1.4 | 0.7 – 2.7 | 0.8 | 0.4 – 1.5 | 0.4 | 0.1 – 1.2 |
| Fear disorders | ||||||||
| Agoraphobia | 2.2 | 0.9 – 5.5 | 0.7 | 0.2 – 2.2 | 0.4 | 0.1 – 1.5 | 0.3 | 0.1 – 1.5 |
| Panic | 1.1 | 0.5 – 2.4 | 0.7 | 0.4 – 1.6 | 0.5 | 0.2 – 1.3 | 0.1 | 0.01 – 0.3 |
| Social phobia | 2.7 | 1.4 – 5.3 | 1.0 | 0.5 – 1.7 | 0.6 | 0.3 – 1.2 | 0.2 | 0.1 – 0.7 |
| Specific phobia | 4.9 | 2.0 – 12 | 1.4 | 0.5 – 4.0 | 1.0 | 0.3 – 3.5 | 0.2 | 0.02 – 1.7 |
| Eating disorders | ||||||||
| Anorexia | 0.2 | 0.01 – 2.5 | -- | - | 1.7 | 0.3 – 10.2 | 1.2 | 0.2 – 6.0 |
| Binge | 1.0 | 0.6 – 1.5 | 0.9 | 0.5 – 1.4 | 1.2 | 0.8 – 1.9 | 0.6 | 0.3 – 1.2 |
| Bulimia | 1.0 | 0.3 – 3.1 | 0.2 | 0.1 – 1.1 | 0.5 | 0.2 – 1.0 | 0.3 | 0.1 – 1.1 |
| Externalizing disorders | ||||||||
| Alcohol | 0.7 | 0.4 – 1.3 | 1.1 | 0.6 – 2.1 | 0.9 | 0.5 – 1.6 | 1.0 | 0.5 – 1.7 |
| Conduct | 0.5 | 0.2 – 1.5 | 0.6 | 0.2 – 1.6 | 0.7 | 0.3 – 1.6 | 0.6 | 0.1 – 2.4 |
| Drug | 1.0 | 0.6 – 1.8 | 0.9 | 0.6 – 1.5 | 1.0 | 0.6 – 1.6 | 1.2 | 0.9 – 1.7 |
| Nicotine | 1.7 | 1.1 – 2.7 | 1.9 | 1.2 – 2.9 | 1.4 | 0.9 – 2.1 | 1.2 | 0.7 – 2.1 |
| ODD | 0.6 | 0.3 – 1.3 | 0.9 | 0.5 – 1.8 | 0.6 | 0.3 – 1.4 | 0.3 | 0.1 – 1.2 |
Reference group for all hazard ratios is menarche onset at age 12 Models adjusted for BMI, parent income, age, race/ethnicity, number of parents in the household, exposure to or experience of childhood threat and deprivation.
GAD=general anxiety disorder, MDD=major depressive disorder, PTSD=Post-traumatic stress disorder, ODD=oppositional defiant disorder.
3.3 Structural Equation Model
The final measurement model evidenced acceptable fit with the sample data (CFI= 0.91; RMSEA= 0.022). Individual item loadings ranged between 0.42–0.92 and were of similar magnitude within each factor (see supplementary table 3 for all factor loadings). Onset of menarche before age 11 was significantly associated with distress disorders (coefficient=0.096; p<.0001), fear disorders (coefficient=0.09; p<.0001), and marginally significantly associated with externalizing disorders (coefficient=0.039; p=0.05). No other pathways reached statistical significance. The full structural equation model results are presented in figure 1. The results of the sensitivity analysis were similar when grouping age of menarche with three-levels (reference=11–13) and as a continuous variable. As a three-level variable (reference=10–14), no pathways reached statistical significance. See supplementary table 4 for the model fit indices and SEM parameters among all exposure modeling strategies.
Figure 1. Structural equation model of path coefficients from age of menarche to latent psychiatric disorder groups.
n=4937. Adjusted for BMI, parent income, age, race/ethnicity, number of parents in the household, sexual activity, exposure to or experience of childhood threat and deprivation. GAD=general anxiety disorder, MDD=major depressive disorder, ODD=oppositional defiant disorder. Latent factors and exposure indicator variables were allowed to correlate with each other (p<0.01 for all correlations). *p-value <0.05, **p-value<0.01
4. Discussion
This study utilized a latent modeling approach to investigate risk for psychiatric disorders associated with timing of menarche in a population-representative sample. Elevated risk for subsequent onset of fear, distress, and externalizing disorders was observed among girls who initiated menarche earlier than average. The latent approach allowed for the investigation of a trans-diagnostic understanding of adolescent psychopathology, which led to several important findings. First, early menarche was significantly associated with the latent externalizing domain, but only nicotine dependence in the survival models for any individual externalizing disorder. Grouping conceptually related disorders not only increases the statistical power to identify significant associations, but may uncover a more general risk of externalizing disorders resulting from important developmental processes. Across all domains, we found no evidence that early menarche was associated with individual disorders over and above the effects on latent disorders. This suggests that early menarche influences latent liability to broad classes of psychopathology, rather than elevating risk for particular disorders. Identifying the specific cognitive, affective, and social processes reflected in this latent liability is an important goal for future research.
The results of this study are similar to one of the few existing studies examining concurrent risk of multiple disorders among those with early menarche [15]. In a longitudinal study of 496 adolescent girls, Stice et al [48] identified a significant risk for depression and substance abuse (including alcohol, tobacco, marijuana, stimulants, inhalants, and hallucinogens), among girls who initiated menarche earlier than average, and found no significant risk for subsequent eating disorders (including anorexia, bulimia, and binge disorder). This study also identified a nearly 3-fold increased risk of comorbid depression and substance disorder. In the present study, the covariance between distress and externalizing disorders was statistically significant (coefficient=0.46; p<.0001), suggesting that the domains may share comorbid risk.
What might explain these transdiagnostic associations? The biological processes set in motion by puberty may lead to a broad range of psychopathology, consistent with our transdiagnostic findings. Exposure to sex hormones during pubertal development is argued to open a period of plasticity in cognitive and affective neural circuits that may underlie both adaptive developmental changes and increased vulnerability to psychopathology [49]–[52]. Prominent developmental changes occur during adolescence in brain regions such as prefrontal cortex and limbic structures, including the amygdala and ventral striatum, in both humans and animals that may contribute to behavioral changes that occur during adolescence as well as risk for psychopathology [53]–[61]. Sex hormones influence sex-specific neural development in both pre-natal and early post-natal stages, demonstrating organizational effects early in development [62], [63]. The increasing exposure to sex-hormones that begins at the onset of puberty is posited to have not only organizational, but also activational effects on the developing brain [63]. Thus, the rise in sex hormones at the onset of puberty both permanently reorganizes and temporarily activates neural circuits implicated in reproductive behaviors, attention, sensory experiences, motivation, and social behaviors [49]. This period of organization and activation is posited to occur through hormone-mediated neurogenesis, synaptogenesis, apoptosis, synaptic pruning and myelination in sex and region specific ways [64]. However, the timing of pubertal development, and in turn, levels of sex hormones, and their effects on brain development has not been well-characterized. There is evidence to suggest that girls who experience an earlier timing of puberty onset not only have higher levels of sex hormones at an earlier age, but also exhibit heightened levels of sex hormones into their 20s or 30s [26]. Studies have found some evidence of a psychopathological risk of early hormonal changes, independent of the effects of physical changes [11], [65]. Overall, this evidence has been small in effect size [12] and inconsistent, as other studies have concluded that physical changes are more important [66].
The social consequences of early menarche may have similarly broad risks of psychopathology. Menarche corresponds to the development of secondary sex characteristics, which may lead others to perceive females who experience early menarche as older than their chronological age [67]. Affiliation with older peers may lead to increased opportunity or pressure to partake in risky social behaviors, such as alcohol and drug use [68]. Habituation to substance use may increase the risk of developing substance use and other behavioral disorders [69], potentially mediated by an increase in risk taking and sensation-seeking behavior [70]. However, the most commonly identified increase in psychopathology associated with the onset of puberty is the increase in depression and anxiety in adolescent girls [13], [71]. Risk for internalizing disorders has been linked to both an increase in psychosocial stressors and interpersonal conflict among peers [72], including unwanted sexual contact [14]. The impact of these experiences may be exacerbated by coping strategies such as rumination [73], which are common among adolescent girls [74]. Additional changes to body morphology, such as acne or increased adipose tissue, serve as additional sources of potential negative social interactions and expectations, psychosocial stress, and consequent disorder. These consequences may mediate the pathway between early timing of menarche and a broad array of psychiatric disorders, through disorder-specific or more general forms of psychosocial stress. Future investigations should test these and other social and behavioral mechanisms that may mediate these relationships.
We also considered the possibility that the reported associations may be based on alternative causes, such as experiences of childhood adversity [75], [76]. The mental health consequences of a broad set of negative childhood exposures, including physical and sexual abuse to institutional rearing and chronic poverty, are well-known [77], [78]. These exposures may also lead to different developmental trajectories, such as the delay or acceleration of menarche onset [79], [80]. Similarly, given that age of menarche is highly heritable, it is plausible that girls who experience early timing of menarche may have mothers who also experienced early timing of menarche, and in turn, had a child at an earlier age, experienced more financial hardship and thus, exposed their child to more adverse environmental experiences [26]. We did attempt to address this possibility, however, by adjusting all models for the reported exposure to childhood trauma, neglect, and measures of household socio-economic status. Model coefficients were generally attenuated but still significant after adjustment for these covariates, suggesting that they do not fully explain our findings.
From the adjusted survival models, we also identified protective associations between late age of menarche and major depression, panic disorder, and social phobia. This seems to provide support for social theories of the mental health effects of pubertal timing, as girls who mature later may be less likely to face exposure to the psychosocial stress and behavioral expectations that accompany the physical changes due to puberty, compared to those who initiate puberty earlier. Indeed, girls with later than average timing of menarche report later age of initiation of sexual activity [81], and fewer sexual relationships throughout adolescence [82]. More generally, late maturation appears to be protective against poor body image, low self-esteem, and depressive symptoms in girls [83]. It is also possible that these estimates represent the effects of selection bias. Late maturing girls are a highly-selected group, and exposures that delay menarche onset may also be protective against mental disorders. Theories interpreting these results through biological processes would necessarily be complex, requiring both endocrine and neural measures to understand the effects of pubertal timing on biological maturation. Future research is necessary to assess biological processes mediating the association between early menarche and psychopathology.
There are several limitations in this study that suggest important future research directions. First, the study data are cross-sectional, which limits the ability to assign temporality and may introduce recall bias, differentially by disorder status. However, as data are from an adolescent sample, the period of recall was relatively short which we expect to mitigate any significant effects of recall bias. In the measurement model, temporality was not considered and therefore we cannot draw conclusions regarding causation from the estimated path coefficients. Second, the study used only one measure of puberty, although menarche is considered an excellent marker of the overall maturation process [1]. Nonetheless, future analyses will include measures of morphological changes (e.g., Tanner staging [84]), which will allow for a more comprehensive analysis of the biological, physical, and social causes and consequences of pubertal timing. Similarly, examining the consequences of early adrenarche, a period of rising levels of androgen hormones which promote the growth of pubic hair and change in sweat composition that produces adult body odor, will help to disentangle the potential contributions of the effects of hormones on detrimental psychological outcomes of early puberty [85]. Also, including other measures of puberty will allow for the inclusion of adolescent males into the analytic sample, which is also critical to the understanding a broader range of psychiatric consequences of non-normative pubertal timing. There is some evidence that early maturing boys show poorer psychosocial functioning and greater use of tobacco, while late maturing boys are at greater risk for behavior and substance use disorders [86], [87]. Related, more detailed data measuring age of menarche in months and years might provide further insights into these relationships. In this study, age of menarche was recorded as the calendar year of onset, and alternative approaches may be able to incorporate year and month of onset or define exposure levels using the standard deviation of the age distribution. Third, due to the limited age range of the current sample, we were unable to examine whether the psychiatric consequences of non-normative menarche may extend into adulthood. Evidence of longer-term risk of early puberty remains unclear. One study found that the elevated risk of externalizing disorders conferred during adolescence attenuated by the end of adolescence [88], while other studies found that depression disorders persisted into adulthood [17], [71]. Prospective studies following adolescents into adulthood would be ideal to investigate the longer-term impact of non-normative adolescent maturation. Also, although we did compare the associations among both individual and latent disorders in separate analyses, it is possible that disorders at both levels are meaningful [89]. Future studies might examine the potentially hierarchical nature of disorders to determine the extent to which the associations we report here for individual disorders are completely explained by the variance in the latent disorders [90], adapting recently developed methods [91]. Finally, it is important to consider that these relationships may differ in meaningful ways among adolescent girls. There is evidence that psychiatric risk of early and late menarche differs significantly between non-Hispanic White, non-Hispanic Black, and Hispanic girls, for certain disorders [92]. Greater exploration of how these associations may differ within groups of females, based on other important characteristics like race and ethnicity is warranted.
In conclusion, this analysis suggests that the timing of the biological processes initiated at puberty have a meaningful influence on latent liability to fear, distress, and externalizing use disorders. Characterizing these associations from a transdiagnostic perspective may be a more flexible and informative than through traditional diagnostic definitions [37]. Under developmentally normative conditions, the physical and psychosocial changes of puberty may be adaptive. However, in the context of early puberty, these processes appear to increase the risk of a broad array of psychopathological consequences. Not only do psychiatric disorders constitute a significant burden of disease among adolescents [93], many have been linked to reduced fluid intelligence [94], educational, and occupational outcomes [88], and therefore represent a health priority for long-term success and well-being especially among girls who experience early puberty.
Supplementary Material
Acknowledgments
Funding was provided by the National Institute of Mental Health (5T32MH1304343, JMP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None.
References
- 1.Livson N, McNeill D. The accuracy of recalled age of menarche. Hum. Biol. 1962;34(3):218–221. [PubMed] [Google Scholar]
- 2.Steiner M, Dunn E, Born L. Hormones and mood: from menarche to menopause and beyond. J. Affect. Disord. 2003 Mar;74(1):67–83. doi: 10.1016/s0165-0327(02)00432-9. [DOI] [PubMed] [Google Scholar]
- 3.Seeman MV. Psychopathology in women and men: focus on female hormones. Am. J. Psychiatry. 1997 doi: 10.1176/ajp.154.12.1641. [DOI] [PubMed] [Google Scholar]
- 4.Nottelmann ED, Inoff-Germain G, Susman EJ, Chrousos GP. Hormones and behavior at puberty. 1990 [Google Scholar]
- 5.Susman EJ, et al. The relation of relative hormonal levels and physical development and social-emotional behavior in young adolescents. J. Youth Adolesc. 1985;14(3):245–264. doi: 10.1007/BF02090322. [DOI] [PubMed] [Google Scholar]
- 6.Kessler RC. Epidemiology of women and depression. J. Affect. Disord. 2003;74(1):5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- 7.Rudolph KD. Handbook of developmental psychopathology. Springer; 2014. Puberty as a developmental context of risk for psychopathology; pp. 331–354. [Google Scholar]
- 8.Plotsky PM, Owens MJ, Nemeroff CB. Psychoneuroendocrinology of depression: hypothalamic-pituitary-adrenal axis. Psychiatr. Clin. North Am. 1998;21(2):293–307. doi: 10.1016/s0193-953x(05)70006-x. [DOI] [PubMed] [Google Scholar]
- 9.Young E, Korszun a. Sex, trauma, stress hormones and depression. Mol. Psychiatry. 2010 Jan;15(1):23–8. doi: 10.1038/mp.2009.94. [DOI] [PubMed] [Google Scholar]
- 10.Stice E, Barrera M, Jr, Chassin L. Prospective differential prediction of adolescent alcohol use and problem use: examining the mechanisms of effect. J. Abnorm. Psychol. 1998;107(4):616. doi: 10.1037//0021-843x.107.4.616. [DOI] [PubMed] [Google Scholar]
- 11.Angold a, Costello EJ, Erkanli a, Worthman CM. Pubertal changes in hormone levels and depression in girls. Psychol. Med. 1999;29:1043–1053. doi: 10.1017/s0033291799008946. [DOI] [PubMed] [Google Scholar]
- 12.Graber Ja. Pubertal timing and the development of psychopathology in adolescence and beyond. Horm. Behav. 2013 Jul;64(2):262–9. doi: 10.1016/j.yhbeh.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Ge X, Conger RD, Elder GH. Coming of age too early: Pubertal influences on girls’ vulnerability to psychological distress. Child Dev. 1996;67(6):3386–3400. [PubMed] [Google Scholar]
- 14.Vicary JR, Klingaman LR, Harkness WL. Risk factors associated with date rape and sexual assault of adolescent girls. J. Adolesc. 1995;18(3):289. [Google Scholar]
- 15.Stice E, Presnell K, Bearman SK. Relation of early menarche to depression, eating disorders, substance abuse, and comorbid psychopathology among adolescent girls. Dev. Psychol. 2001;37(5):608. doi: 10.1037//0012-1649.37.5.608. [DOI] [PubMed] [Google Scholar]
- 16.Graber Julia A, Lewinsohn Peter M, Seeley John R, Brooks-Gunn Jeanne. Is psychopathology associated with the timing of pubertal development? J. Am. Acad. Child Adolesc. Psychiatry. 1997;36(12):1768–1776. doi: 10.1097/00004583-199712000-00026. [DOI] [PubMed] [Google Scholar]
- 17.Graber Ja, Seeley JR, Brooks-Gunn J, Lewinsohn PM. Is pubertal timing associated with psychopathology in young adulthood. J. Am. Acad. Child Adolesc. Psychiatry. 2004 Jun;43(6):718–26. doi: 10.1097/01.chi.0000120022.14101.11. [DOI] [PubMed] [Google Scholar]
- 18.Graber JA, Petersen AC, Brooks-Gunn J. Pubertal processes: Methods, measures, and models. 1996 [Google Scholar]
- 19.Resnick MD, et al. Protecting adolescents from harm: findings from the National Longitudinal Study on Adolescent Health. Jama. 1997;278(10):823–832. doi: 10.1001/jama.278.10.823. [DOI] [PubMed] [Google Scholar]
- 20.Born L, Shea A, Steiner M. The roots of depression in adolescent girls: is menarche the key? Curr. Psychiatry Rep. 2002 Dec;4(6):449–60. doi: 10.1007/s11920-002-0073-y. [DOI] [PubMed] [Google Scholar]
- 21.Kaltiala-Heino R, Kosunen E, Rimpelä M. Pubertal timing, sexual behaviour and self-reported depression in middle adolescence. J. Adolesc. 2003 Oct;26(5):531–545. doi: 10.1016/s0140-1971(03)00053-8. [DOI] [PubMed] [Google Scholar]
- 22.Natsuaki MN, Leve LD, Mendle J. Going through the rites of passage: timing and transition of menarche, childhood sexual abuse, and anxiety symptoms in girls. J. Youth Adolesc. 2011 Oct;40(10):1357–70. doi: 10.1007/s10964-010-9622-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reardon LE, Leen-Feldner EW, Hayward C. A critical review of the empirical literature on the relation between anxiety and puberty. Clin. Psychol. Rev. 2009;29(1):1–23. doi: 10.1016/j.cpr.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Killen JD, et al. Is puberty a risk factor for eating disorders? Am. J. Dis. Child. 1992;146(3):323–325. doi: 10.1001/archpedi.1992.02160150063023. [DOI] [PubMed] [Google Scholar]
- 25.Dick DM, Rose RJ, Viken RJ, Kaprio J. Pubertal timing and substance use: associations between and within families across late adolescence. Dev. Psychol. 2000;36(2):180. [PubMed] [Google Scholar]
- 26.Mendle J, Turkheimer E, Emery RE. Detrimental Psychological Outcomes Associated with Early Pubertal Timing in Adolescent Girls. Dev. Rev. DR. 2007 Jun;27(2):151–171. doi: 10.1016/j.dr.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Negriff S, Susman EJ. Pubertal Timing, Depression, and Externalizing Problems: A Framework, Review, and Examination of Gender Differences. J. Res. Adolesc. 2011 Sep;21(3):717–746. [Google Scholar]
- 28.Krueger RF, Markon KE. Reinterpreting comorbidity: A model-based approach to understanding and classifying psychopathology. Annu Rev Clin Psychol. 2006;2:111–133. doi: 10.1146/annurev.clinpsy.2.022305.095213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mendle J, Ferrero J. Detrimental psychological outcomes associated with pubertal timing in adolescent boys. Dev. Rev. 2012;32(1):49–66. doi: 10.1016/j.dr.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merikangas K, Avenevoli S, Costello J, Koretz D, Kessler RC. National comorbidity survey replication adolescent supplement (NCS-A): I. Background and measures. J. Am. Acad. Child Adolesc. Psychiatry. 2009;48(4):367–9. doi: 10.1097/CHI.0b013e31819996f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kessler RC, et al. National comorbidity survey replication adolescent supplement (NCS-A): II. Overview and design. J. Am. Acad. Child Adolesc. Psychiatry. 2009;48(4):380–385. doi: 10.1097/CHI.0b013e3181999705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kessler RC, et al. Design and field procedures in the US National Comorbidity Survey Replication Adolescent Supplement (NCS-A) Int. J. Methods Psychiatr. Res. 2009;18(2):69–83. doi: 10.1002/mpr.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kessler RC, et al. National comorbidity survey replication adolescent supplement (NCS-A): III. Concordance of DSM-IV/CIDI diagnoses with clinical reassessments. J. Am. Acad. Child Adolesc. Psychiatry. 2009;48(4):386–399. doi: 10.1097/CHI.0b013e31819a1cbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cantwell DP, Lewinsohn PM, Rohde P, Seeley JR. Correspondence between adolescent report and parent report of psychiatric diagnostic data. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36(5):610–619. doi: 10.1097/00004583-199705000-00011. [DOI] [PubMed] [Google Scholar]
- 35.Grills AE, Ollendick TH. Issues in parent-child agreement: the case of structured diagnostic interviews. Clin. Child Fam. Psychol. Rev. 2002;5(1):57–83. doi: 10.1023/a:1014573708569. [DOI] [PubMed] [Google Scholar]
- 36.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry. 2005 Jun;62(6):617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kotov R. The Hierarchical Taxonomy of Psychopathology (HiTOP): A Dimensional Alternative to Traditional Nosologies. J. Abnorm. Psychol. 1965. 2017 Mar; doi: 10.1037/abn0000258. [DOI] [PubMed] [Google Scholar]
- 38.Anderson SE, Dallal GE, Must A. Relative weight and race influence average age at menarche: results from two nationally representative surveys of US girls studied 25 years apart. Pediatrics. 2003;111(4):844–850. doi: 10.1542/peds.111.4.844. [DOI] [PubMed] [Google Scholar]
- 39.McLaughlin KA, Sheridan MA, Lambert HK. Childhood adversity and neural development: Deprivation and threat as distinct dimensions of early experience. Neurosci. Biobehav. Rev. 2014;47:578–591. doi: 10.1016/j.neubiorev.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cole T, Faith M, Pietrobelli A, Heo M. What is the best measure of adiposity change in growing children: BMI, BMI%, BMI z-score or BMI centile? Eur. J. Clin. Nutr. 2005;59(3):419–425. doi: 10.1038/sj.ejcn.1602090. [DOI] [PubMed] [Google Scholar]
- 41.Kakinami L, Henderson M, Chiolero A, Cole TJ, Paradis G. Identifying the best body mass index metric to assess adiposity change in children. Arch. Dis. Child. 2014 doi: 10.1136/archdischild-2013-305163. p. archdischild-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.SAS Institute Inc. Base SAS 9.4 procedures guide: statistical procedures. 2. Cary, NC: SAS Institute, Inc; 2013. [Google Scholar]
- 43.Brown T. Confirmatory factor analysis for applied researchers. 2006 [Google Scholar]
- 44.Steiger JH. Structural model evaluation and modification: An interval estimation approach. Multivar. Behav. Res. 1990;25(2):173–180. doi: 10.1207/s15327906mbr2502_4. [DOI] [PubMed] [Google Scholar]
- 45.Muthén LK, Muthén BO. Mplus: Statistical analysis with latent variables: User’s guide. Muthén & Muthén Los Angeles; 2013. [Google Scholar]
- 46.Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct. Equ. Model. Multidiscip. J. 1999;6(1):1–55. [Google Scholar]
- 47.Wall MM, Amemiya Y. Estimation for polynomial structural equation models. J. Am. Stat. Assoc. 2000;95(451):929–940. [Google Scholar]
- 48.Stice E, Ragan J, Randall P. Prospective relations between social support and depression: differential direction of effects for parent and peer support? J. Abnorm. Psychol. 2004;113(1):155. doi: 10.1037/0021-843X.113.1.155. [DOI] [PubMed] [Google Scholar]
- 49.Blakemore S-J, Burnett S, Dahl RE. The role of puberty in the developing adolescent brain. Hum. Brain Mapp. 2010 Jun;31(6):926–33. doi: 10.1002/hbm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ladouceur C, Peper J, Crone E, Dahl R. White matter development in adolescence: the influence of puberty and implications for affective disorders. Dev. Cogn. 2012;2(1):36–54. doi: 10.1016/j.dcn.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schulz KM, Sisk CL. The organizing actions of adolescent gonadal steroid hormones on brain and behavioral development. Neurosci. Biobehav. Rev. 2016;70:148–158. doi: 10.1016/j.neubiorev.2016.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Romeo RD. Puberty: A period of both organizational and activational effects of steroid hormones on neurobehavioural development. J. Neuroendocrinol. 2003;15(12):1185–1192. doi: 10.1111/j.1365-2826.2003.01106.x. [DOI] [PubMed] [Google Scholar]
- 53.Goddings A-L, Mills KL, Clasen LS, Giedd JN, Viner RM, Blakemore S-J. The influence of puberty on subcortical brain development. NeuroImage. 2014 Mar;88:242–51. doi: 10.1016/j.neuroimage.2013.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herting MM, Gautam P, Spielberg JM, Dahl RE, Sowell ER. A Longitudinal Study: Changes in Cortical Thickness and Surface Area during Pubertal Maturation. Plos One. 2015;10(3):e0119774. doi: 10.1371/journal.pone.0119774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moore WE, Pfeifer JH, Masten CL, Mazziotta JC, Iacoboni M, Dapretto M. Facing puberty: associations between pubertal development and neural responses to affective facial displays. Soc. Cogn. Affect. Neurosci. 2012 Jan;7(1):35–43. doi: 10.1093/scan/nsr066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spielberg JM, et al. Pubertal testosterone influences threat-related amygdala-orbitofrontal cortex coupling. Soc. Cogn. Affect. Neurosci. 2014 May; doi: 10.1093/scan/nsu062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spielberg JM, Olino TM, Forbes EE, Dahl RE. Exciting fear in adolescence: Does pubertal development alter threat processing? Dev. Cogn. Neurosci. 2014 Apr;8:86–95. doi: 10.1016/j.dcn.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Forbes EE, Phillips ML, Silk JS, Ryan ND, Dahl RE. Neural systems of threat processing in adolescents: role of pubertal maturation and relation to measures of negative affect. Dev. Neuropsychol. 2011 Jan;36(4):429–52. doi: 10.1080/87565641.2010.550178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pfeifer JH, et al. Longitudinal change in the neural bases of adolescent social self-evaluations: effects of age and pubertal development. J. Neurosci. Off. J. Soc. Neurosci. 2013 Apr;33(17):7415–9. doi: 10.1523/JNEUROSCI.4074-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Motta-Mena NV, Scherf KS. Pubertal development shapes perception of complex facial expressions. Dev. Sci. 2016 Jun;:1–10. doi: 10.1111/desc.12451. [DOI] [PubMed] [Google Scholar]
- 61.Ladouceur CD. Neural systems supporting cognitive-affective interactions in adolescence: the role of puberty and implications for affective disorders. Front. Integr. Neurosci. 2012 Aug;Jan;6:1–11. doi: 10.3389/fnint.2012.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sisk C, Zehr J. Pubertal hormones organize the adolescent brain and behavior. Front. Neuroendocrinol. 2005;26:163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 63.Schulz KM, Molenda-Figueira Ha, Sisk CL. Back to the future: The organizational–activational hypothesis adapted to puberty and adolescence. Horm. Behav. 2009 May;55(5):597–604. doi: 10.1016/j.yhbeh.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ahmed EI, Zehr JL, Schulz KM, Lorenz BH, DonCarlos LL, Sisk CL. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat. Neurosci. 2008;11(9):995–997. doi: 10.1038/nn.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Warren MP, Brooks-Gunn J. Mood and behavior at adolescence: evidence for hormonal factors. J. Clin. Endocrinol. Metab. 1989 Jul;69(1):77–83. doi: 10.1210/jcem-69-1-77. [DOI] [PubMed] [Google Scholar]
- 66.Paikoff RL, Brooks-Gunn J, Warren MP. Effects of girls’ hormonal status on depressive and aggressive symptoms over the course of one year. J. Youth Adolesc. 1991;20(2):191–215. doi: 10.1007/BF01537608. [DOI] [PubMed] [Google Scholar]
- 67.Patton GC, McMorris BJ, Toumbourou JW, Hemphill SA, Donath S, Catalano RF. Puberty and the onset of substance use and abuse. Pediatrics. 2004;114(3):e300–e306. doi: 10.1542/peds.2003-0626-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bachman JG, Johnston LD, O’Malley PM, Schulenberg J. Transitions in drug use during late adolescence and young adulthood. 1996 [Google Scholar]
- 69.Wilson DM, et al. Timing and rate of sexual maturation and the onset of cigarette and alcohol use among teenage girls. Arch. Pediatr. Adolesc. Med. 1994;148(8):789–795. doi: 10.1001/archpedi.1994.02170080019004. [DOI] [PubMed] [Google Scholar]
- 70.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 71.Copeland W, Shanahan L, Miller S, Costello EJ, Angold A, Maughan B. Outcomes of Early Pubertal Timing in Young Women: A Prospective Population-Based Study. Am. J. Psychiatry. 2010 Oct;167(10):1218–1225. doi: 10.1176/appi.ajp.2010.09081190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hankin BL, Mermelstein R, Roesch L. Sex differences in adolescent depression: Stress exposure and reactivity models. Child Dev. 2007;78(1):279–295. doi: 10.1111/j.1467-8624.2007.00997.x. [DOI] [PubMed] [Google Scholar]
- 73.Nolen-Hoeksema S. Sex differences in unipolar depression: evidence and theory. Psychol. Bull. 1987;101(2):259. [PubMed] [Google Scholar]
- 74.Johnson DP, Whisman MA. Gender differences in rumination: A meta-analysis. Personal. Individ. Differ. 2013;55(4):367–374. doi: 10.1016/j.paid.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mensah FK, Bayer JK, Wake M, Carlin JB, Allen NB, Patton GC. Early puberty and childhood social and behavioral adjustment. J. Adolesc. Health. 2013;53(1):118–124. doi: 10.1016/j.jadohealth.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 76.Henrichs KL, McCauley HL, Miller E, Styne DM, Saito N, Breslau J. Early menarche and childhood adversities in a nationally representative sample. Int. J. Pediatr. Endocrinol. 2014;2014(1):1. doi: 10.1186/1687-9856-2014-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cohen P, Brown J, Smailes E. Child abuse and neglect and the development of mental disorders in the general population. Dev. Psychopathol. 2001;13(4):981–999. [PubMed] [Google Scholar]
- 78.McLaughlin KA, Greif Green J, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC. Childhood Adversities and First Onset of Psychiatric Disorders in a National Sample of US Adolescents. Arch. Gen. Psychiatry. 2012 Nov;69(11):1151. doi: 10.1001/archgenpsychiatry.2011.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mustanski BS, Viken RJ, Kaprio J, Pulkkinen L, Rose RJ. Genetic and environmental influences on pubertal development: longitudinal data from Finnish twins at ages 11 and 14. Dev. Psychol. 2004;40(6):1188. doi: 10.1037/0012-1649.40.6.1188. [DOI] [PubMed] [Google Scholar]
- 80.Ellis BJ. Timing of Pubertal Maturation in Girls: An Integrated Life History Approach. Psychol. Bull. 2004;130(6):920–958. doi: 10.1037/0033-2909.130.6.920. [DOI] [PubMed] [Google Scholar]
- 81.Johansson T, Ritzén E. Abnormalities in puberty. Vol. 8. Karger Publishers; 2005. Very long-term follow-up of girls with early and late menarche; pp. 126–136. [DOI] [PubMed] [Google Scholar]
- 82.Flannery DJ, Rowe DC, Gulley BL. Impact of Pubertal Status, Timing, and Age on Adolescent Sexual Experience and Delinquency. J. Adolesc. Res. 1993 Jan;8(1):21–40. [Google Scholar]
- 83.Brooks-Gunn J. How stressful is the transition to adolescence for girls. Adolesc. Stress Causes Consequences. 1991:131–149. [Google Scholar]
- 84.Tanner JM, Whitehouse RH. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch. Dis. Child. 1976;51(3):170–179. doi: 10.1136/adc.51.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Byrne ML, Whittle S, Vijayakumar N, Dennison M, Simmons JG, Allen NB. A systematic review of adrenarche as a sensitive period in neurobiological development and mental health. Dev. Cogn. Neurosci. 2016 Dec; doi: 10.1016/j.dcn.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Graber JA, Seeley JR, Brooks-Gunn J, Lewinsohn PM. Is pubertal timing associated with psychopathology in young adulthood? J. Am. Acad. Child Adolesc. Psychiatry. 2004;43(6):718–726. doi: 10.1097/01.chi.0000120022.14101.11. [DOI] [PubMed] [Google Scholar]
- 87.Andersson T, Magnusson D. Biological maturation in adolescence and the development of drinking habits and alcohol abuse among young males: A prospective longitudinal study. J. Youth Adolesc. 1990;19(1):33–41. doi: 10.1007/BF01539443. [DOI] [PubMed] [Google Scholar]
- 88.Stattin H, Magnusson D. Pubertal maturation in female development. Lawrence Erlbaum Associates, Inc; 1990. [Google Scholar]
- 89.Markon KE, Krueger RF, Watson D. Delineating the structure of normal and abnormal personality: an integrative hierarchical approach. 2005 doi: 10.1037/0022-3514.88.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.South SC, Krueger RF, Iacono WG. Understanding general and specific connections between psychopathology and marital distress: a model based approach. J. Abnorm. Psychol. 2011;120(4):935. doi: 10.1037/a0025417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Goldberg LR. Doing it all bass-ackwards: The development of hierarchical factor structures from the top down. J. Res. Personal. 2006;40(4):347–358. [Google Scholar]
- 92.Hayward C, Gotlib IH, Schraedley PK, Litt IF. Ethnic differences in the association between pubertal status and symptoms of depression in adolescent girls. J. Adolesc. Health. 1999;25:143–149. doi: 10.1016/s1054-139x(99)00048-8. [DOI] [PubMed] [Google Scholar]
- 93.Gore FM, et al. Global burden of disease in young people aged 10–24 years: a systematic analysis. The Lancet. 2011;377(9783):2093–2102. doi: 10.1016/S0140-6736(11)60512-6. [DOI] [PubMed] [Google Scholar]
- 94.Keyes KM, Platt J, Kaufman AS, McLaughlin KA. Association of fluid intelligence and psychiatric disorders in a population-representative sample of us adolescents. JAMA Psychiatry. 2016 doi: 10.1001/jamapsychiatry.2016.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.