Abstract
Focal adhesions are specialized sites within the cell where clustered integrin receptors interact with the extracellular matrix on the outside of cells and with the actin cytoskeleton on the inside. They provide strong adhesion to the matrix and transmit mechanical tension generated within cells across the plasma membrane to the external environment. Additionally, they act as scaffolds for many signaling pathways triggered by integrin engagement or mechanical force exerted on cells. Here I describe my personal perspective on focal adhesion research which I have witnessed since the initial discovery and description of focal adhesions as electron dense regions of the ventral plasma nearly half a century ago.
Keywords: Focal adhesions, adhesion plaques, focal complexes, nascent adhesions, RhoA, FAK, stress fibers, cell migration
Graphical abstract
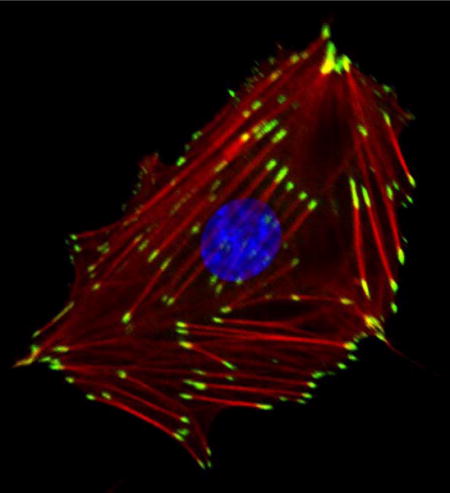
A REF52 fibroblast, plated on a collagen-coated glass coverslip, reveals focal adhesions stained for vinculin (green), F-actin (red) showing stress fibers, and the nucleus labeled in blue. In this Discovery-in-Context Review, Keith Burridge provides a personal account on focal adhesion research since its initial discovery and description nearly half a century ago.
During my first weeks as a graduate student in the autumn of 1971, I went to a lecture by Michael Abercrombie at the Cambridge Philosophical Society. With hindsight, this seems like an unlikely venue for a memorable research presentation, but Abercrombie had been invited to share his recent work investigating cell migration in tissue culture. He showed a series of light and electron microscopic images of migrating fibroblasts, and several of the images stand out in my memory. Some were electron micrographs (EMs) illustrating lamellipodia at the front of migrating cells, but the most memorable images revealed bundles of filaments approaching and possibly inserting into dense plaques on the ventral plasma membrane. These regions came close to the underlying substratum. The filaments were not identified, but Abercrombie speculated they might be actin, which had only recently been identified in non-muscle cells. The plaque structure providing attachment of filaments was discussed and a similarity noted to the zonula adherens and macula adherens of epithelial cells, structures already believed to function in cell adhesion and filament anchoring. These images appear in the paper by Abercrombie et al. [1], although now when I look at them again they are less impressive than my memory of them as presented in his talk. Little did I anticipate that much of my own career would be directed towards studying these structures which we now refer to as focal adhesions (FAs). I have witnessed research on FAs progress from being a small and obscure corner of the cytoskeletal field to becoming a huge field in itself. It is a field that includes multiple signaling pathways that influence cell growth and behavior in many ways. Some of these pathways are initiated in response to engagement of the extracellular matrix (ECM), whereas others are triggered by mechanical tension or in response to the rigidity or other physical characteristics of the matrix. FAs are also sites where mechanical force generated within the cell is transmitted to the surrounding ECM, influencing its organization but also contributing to cell migration. In this brief review, I hope to give my personal perspective on the early history of FA research and to highlight some of the key developments that have driven this field.
Early microscopy studies
In Abercrombie’s classic paper, he referred to the electron dense regions on the ventral plasma membrane as “plaques” and from this the term “adhesion plaque” became widely used to describe these structures. A few years after Abercrombie’s study, Izzard and Lochner applied Interference Reflection Microscopy (IRM) to investigate how close the underside of fibroblasts comes to the glass coverslip on which they are moving [2]. With IRM the more closely apposed the membrane is to the glass, the darker the resulting image. The darkest structures, which they termed “focal contacts”, had an elongated oval shape about 2–10 μm in length, and 0.25 to 0.5 μm wide. They calculated that the plasma membrane at focal contacts was separated by 10–15 nm from the substratum [2]. Heath and Dunn combined both EM and IRM to show that adhesion plaques and focal contacts were the same structures [3]. They used the term “focal adhesion” once in the summary of their paper and this was subsequently adopted by Couchman and Rees [4] who were studying cardiac fibroblasts migrating out of heart explants. These authors made the interesting observation that during the initial period of rapid migration the fibroblasts displayed few if any FAs; however, as cell migration slowed down, FAs developed. It is often forgotten that cell migration does not require FAs. Indeed, many cells migrate effectively without them; in these cells adhesion to the ECM is still occurring via integrins but adhesion is too transient and unstable to permit large clusters of integrins to develop into identifiable FAs. In their study, Couchman and Rees made a distinction between FAs and focal contacts, with the former term being used for the darkest structures seen by IRM. Not only were focal contacts less dark, but they were more transient than FAs. They also concluded that both structures were equivalently close to the substratum but they interpreted FAs being darker because of increased protein recruitment leading to a higher refractive index. Subsequent work has indeed supported the idea that as adhesions mature more proteins are recruited to these sites.
For about a decade the terms focal adhesion, adhesion plaque, and focal contact were used synonymously by many labs. Gradually, the term focal adhesion became the most common term used to describe these sites of tight adhesion to the ECM and the other terms are now less commonly used. However, the idea that many newly formed adhesions are less stable, with many disassembling and only a few maturing to become the relatively stable structures, was important. Nobes and Hall introduced the term “focal complex” to differentiate the smaller, more transient adhesions, only some of which mature into FAs [5]. Although focal complexes are smaller than FAs, they are still large macromolecular arrays that have developed from still smaller adhesions, for which the term “nascent adhesion” is now frequently used. In general, the terminology and temporal sequence of nascent adhesions progressing to focal complexes and then maturing to FAs has been widely adopted. Finally, the term “fibrillar adhesion” was developed to describe adhesions made to extracellular matrix (ECM) fibrils, such as fibronectin [6]. Fibrillar adhesions often grow out of FAs and typically involve a subset of integrins (α5β1) and are enriched in specific components such as tensin. These terms describing the different types of matrix adhesion are valuable but in reality there must exist a continuum of adhesions reflecting the integrins expressed by different cell types and distinct conditions in which the adhesions develop.
Fluorescence microscopy ushers in a revolution
Although EM and IRM were important in the initial discovery of FAs, it has been immunofluorescence microscopy that has driven the field more than any other technique. In the mid 1970s a revolution in cell biology was initiated by the application of immunofluorescence to visualize actin in cells in culture [7]. There was a rush to generate antibodies against many muscle proteins so as to investigate their existence and distribution in fibroblasts and other cell types. As a student in Dennis Bray’s lab, my primary focus was on non-muscle myosins, but I was also interested in how actin filaments attach to membranes. One of the few proteins known to be involved in attachment of F-actin to any structure was α-actinin, which had been implicated in attaching F-actin to striated muscle Z-discs. This prompted me to develop an antibody to look for α-actinin in non-muscle cells. Arriving at Cold Spring Harbor laboratory for my postdoc, I discovered that Elias Lazarides, a graduate student in Jim Watson’s group, had similarly developed an antibody against α-actinin. Rather than competing, we decided to pool our results and publish together [8]. We noted that α-actinin was prominently localized along stress fibers with a periodic distribution reminiscent of its localization in muscle myofibrils. Significantly, it was also concentrated in plaques at the ends of actin filament bundles (i.e. in FAs, although this term had not yet been coined). We speculated that these sites corresponded to the dense structures involved in adhesion described by Abercrombie and colleagues and that α-actinin might serve to attach actin to the plasma membrane at these regions.
The seminal breakthrough in the field came a few years later with Benny Geiger’s discovery of vinculin [9]. With striking images he showed that vinculin was a protein uniquely concentrated in FAs. Unlike α-actinin, it was not distributed along stress fibers [9]. Geiger discovered vinculin as a contaminant while purifying α-actinin from chicken gizzard smooth muscle. Rather than discarding this contaminant, he went on to purify and generate an antibody against the protein. These gave the eye-catching images of FAs. I recall my own chagrin at failing to be the first to publish on vinculin because at Cold Spring Harbor, Jim Feramisco and I had similarly stumbled onto this previously unknown protein while developing a purification of α-actinin from chicken gizzards. Sensing no rush, our characterization of this “new” protein had remained as a back-burner project. We had begun to immunize rabbits with vinculin, when in the summer of 1979 Benny Geiger came to a cytoskeletal conference at Cold Spring Harbor and electrified us and most of the attendees with his images of vinculin in FAs. Accelerating our own effort on this protein, Feramisco exploited his expertise at microinjection, introducing a fluorescently-derivatized version of the protein into cells. The results were striking as we observed fluorescent vinculin beautifully targeting FAs but also co-aligning with fibronectin fibrils on the cell surface [10].
Geiger’s discovery of vinculin lifted FA research out of obscurity and pushed FAs to center stage in both the cytoskeleton and adhesion fields. At the personal level, our failure to be first with vinculin motivated my research for several years. I no longer hesitated about generating antibodies against purified proteins for localization studies. Indeed, it became my strategy to pursue and purify “new” proteins from smooth muscle, which turned out to be a rich source of FA components, and to generate antibodies against these proteins to probe their localization in fibroblasts. This approach resulted in our discovery of talin, another prominent FA component [11]. Even with talin, however, we later discovered that the protein had previously been isolated from human platelets, although in that work it had not been shown to be a FA component [12]. We went on to demonstrate that talin interacts with vinculin [13], and then, collaborating with the labs of Rick Horwitz and Clayton Buck, we showed that isolated integrins could bind talin [14]. Notably, in subsequent work talin was discovered to be critical for activating integrins, thereby promoting their association with the ECM [15]. Given that both talin and vinculin bind actin, the interaction of talin with integrins provided a long sought link between the cytoskeletal components of FAs and the plasma membrane. Integrins had been discovered a few years earlier by multiple groups investigating cell adhesion in several different cellular contexts, ranging from platelets and leukocytes to myoblasts and fibroblasts. The work converged to identify integrins as a family of transmembrane cell adhesion proteins, potentially connecting the ECM on the outside of the cell to the actin cytoskeleton on the inside [16]. The localization of these ECM receptors revealed them to be concentrated in FAs [17, 18]. Integrins are not the only transmembrane proteins in FAs, but they are the dominant ones. Indeed, FAs can be defined as regions of tightly clustered integrins, providing strong adhesion to the underlying matrix, while simultaneously serving as sites for attachment of stress fibers, as well as being platforms on which many signaling molecules assemble. A representative image of a fibroblast adhering to a collagen-coated glass coverslip revealing FAs, prominently stained with an anti-vinculin antibody, is shown in Figure 1.
Figure 1.
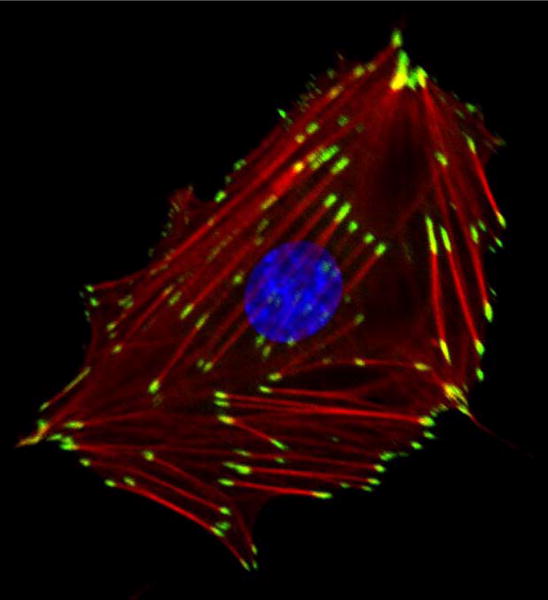
A REF52 fibroblast plated on a collagen-coated glass coverslip, reveals focal adhesions stained for vinculin (green), F-actin (red), showing stress fibers and the nucleus labeled in blue. Image was kindly provided by David Graham.
Over the years, a vast number of additional components have been discovered in FAs and this has given rise to ever more complicated models of FA structure. With hindsight it is amusing to look at the evolution of these models as more proteins and interactions were incorporated into diagrams of FA organization. An early model that I drew from ~1984 is shown in Figure 2A. At that time, integrins had not yet been identified and no transmembrane FA proteins were known. Twelve years later, not only were integrins prominent, but many more proteins and associations had been identified. More elaborate models could be constructed (Figure 2B). Progress in light microscopy has culminated with the application of interferometric photoactivated localization microscopy (iPALM), a form of super-resolution light microscopy, to determine the relative 3D organization of major FA components by Clare Waterman’s group (Figure 3) [19]. Mapping the distribution of 9 different components through the thickness of the FA revealed three distinct layers [19]. Closest to the membrane, where integrin cytoplasmic tails emerge, they have defined an “integrin signaling layer” where FAK and paxillin are located. Above this, there is a “force transduction layer” containing talin and vinculin. Farthest from the membrane, an “actin-regulatory layer” contains zyxin, VASP, α-actinin and actin filaments. More than just assigning the relative locations of these proteins to different strata within FAs, the authors were able to determine the orientation of talin. By selectively labeling either the N- or C-terminus, they revealed that this elongated molecule is oriented with its N-terminal domain close to integrins and its C-terminus extending into the actin-regulatory layer. Notably, this latter domain contains an actin-binding site, whereas the N-terminal FERM domain binds to integrins. Finding α-actinin in the actin-regulatory layer, separated by a significant distance from the integrin cytoplasmic domains, argues against it contributing to the membrane attachment of stress fibers. This is interesting because some years earlier we had discovered that α-actinin, like talin, can interact with integrins [20]. Although the spatial separation of α-actinin from the plasma membrane in the iPALM images indicates that it cannot be serving an attachment function in these FAs, nevertheless, evidence has been presented elsewhere that α-actinin may play an attachment role in FAs based on β3 integrins [21].
Figure 2.
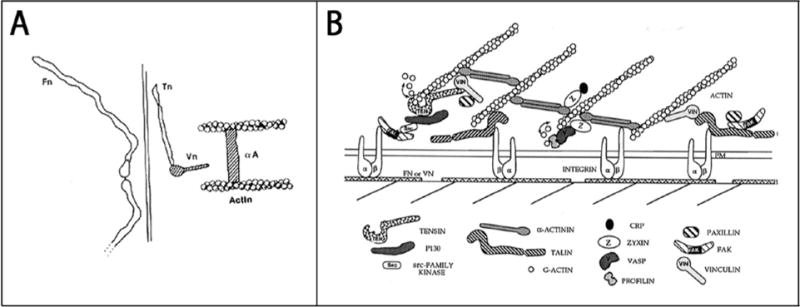
Early models of focal adhesion structure. In A, a diagram of a FA is shown that I made in ~1984. It is noteworthy both for how few components are represented and the lack of any transmembrane proteins. By 1996, the time of the model shown in B, many more proteins had been identified and a more complex FA architecture is illustrated. This figure is reproduced from Burridge and Chrzanowska-Wodnicka [40].
Figure 3.
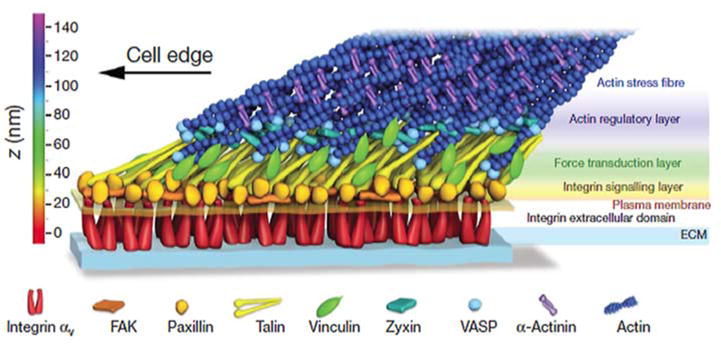
A schematic model of focal adhesion molecular architecture based on iPALM analysis. Figure reproduced with permission from Kanchanawong et al. [19].
Signaling
Whereas the 1980s were a time for discovering structural proteins within FAs and establishing some of the critical interactions, the 1990s revealed that FAs are major sites of signal transduction. Much of the interest in signaling emanating from FAs was driven by the discovery of the focal adhesion kinase (FAK) [22, 23], together with the observation made by many of us that integrin clustering or engagement with ECM triggers tyrosine phosphorylation of multiple FA proteins. FAK and Src family kinases acting in FAs have been implicated in diverse signaling pathways. These pathways in turn influence cell growth and survival, cell migration, as well as the assembly and disassembly of FAs themselves.
Coinciding with the work on FAK and tyrosine phosphorylation in FAs, Ridley and Hall made the key discovery in 1992 that active RhoA drives the assembly of FAs and stress fibers [24]. The initial interpretation was that this was due to stimulating tyrosine phosphorylation. Indeed, tyrosine phosphorylation was seen to be elevated in response to RhoA activation, but whether this drove assembly or was downstream of assembly was not determined at that time. A different model came from the discovery that RhoA activates myosin [25, 26] and that myosin activity contributes to the assembly of FAs and stress fibers [25]. Not only does RhoA promote assembly of FAs, but integrin engagement also regulates RhoA activity in a complex manner. Initial engagement is associated with decreased RhoA activity but over time, as cells spread and develop FAs, GEFs become activated and RhoA activity is stimulated [27, 28].
Mechanotransduction
It has been known for a long time that cells respond to mechanical forces. Many signaling pathways that are stimulated by integrin engagement are also activated by, or even require mechanical force. Mechanical tension exerted on cells affects their growth and survival, but also their differentiation [29]. Research into mechanotransduction has intensified in the past two decades, with much of the interest in this area being directed toward FAs [30]. Many lines have converged on this research area. For example, not only are internally-generated forces exerted on FAs [31], but FA assembly is affected by the physical state and mechanical properties of the external environment. On soft substrata, FA formation is greatly reduced or inhibited all together [32]. It is striking that FAs are seen most clearly in cells cultured on rigid glass or plastic coverslips. Mechanical tension has been implicated in the growth of FAs [33] and recruitment of proteins to these structures. It is relevant that RhoA activity is elevated on rigid substrata [34, 35] and that tension on integrins leads to activation of RhoA [36], suggesting a positive feedback loop that most likely contributes to the robust assembly of FAs on rigid surfaces. RhoA-stimulated tension can affect the strength of bonds between interacting proteins and it can expose cryptic binding sites within proteins. Investigating how RhoA-mediated contractility can promote fibronectin matrix assembly, we discovered that tension on fibronectin exposes a cryptic site involved in assembly [37]. Similarly, with respect to mechanical tension promoting assembly of components at the cytoplasmic face of FAs, Michael Sheetz’ group has shown that stretching talin exposes multiple cryptic vinculin binding sites that are buried within talin [38]. Given the prominence of talin and vinculin in FAs, this tension-induced interaction is likely key to FA assembly and growth. It will be interesting to learn how many other protein interactions within FAs are similarly regulated in this way by mechanical force.
Future directions
The field of FA research appears to be in a robust and exciting phase. With the influx of physicists, mathematicians and biomedical engineers into the field, perhaps there are even parallels with the origins of molecular biology in the 50s and 60s. It has been argued that the revolution in molecular biology came about as a result of the infusion of crystallographers and physicists into biology. Their efforts led to elucidating the structure of DNA and continued with pursuit of the genetic code. With respect to FAs, new techniques such as super-resolution light microscopy and tension-based measurements have introduced a more quantitative perspective. Now the strength of bonds mediating the interactions of key FA components is being considered, along with how mechanical forces affect these associations. When I look at the field I believe I get a glimpse of the future from recent work using super-resolution microscopy [19, 39]. One of the intriguing results from the iPALM analysis of FAs was that, whereas most of the proteins examined were confined to one of three layers, vinculin was detected in all 3 strata. Investigating the basis for this distribution, Case and coworkers demonstrated that vinculin in the membrane proximal layer was recruited to this region in an inactive closed state via binding to phosphorylated paxillin [39]. As FAs matured in response to tension, they were able to show that vinculin was activated and transitioned to higher levels in these FAs, likely through binding to the tension-exposed, cryptic sites in talin, mentioned above [38]. The ability to visualize the relative positions, interactions and activities of various structural and signaling components within FAs as these mature or disassemble will surely lead to a greater understanding of how these structures are regulated and how they, in turn, signal to other activities within cells.
Acknowledgments
For much of the 40 years I have studied focal adhesions my research has been supported by NIH grant GM029860, for which I am very grateful. I am particularly indebted to all the members of my lab, past and present, as well as to collaborators and colleagues in the field who have contributed so much and made this such an enjoyable and stimulating journey.
Abbreviations
- FA
Focal Adhesion
- EMs
electron micrographs
- ECM
Extracellular matrix
- IRM
Interference reflection microscopy
- iPALM
Interferometric photoactivated localization microscopy
- FAK
Focal Adhesion Kinase
- GEFs
Guanine nucleotide exchange factors
References
- 1.Abercrombie M, Heaysman JE, Pegrum SM. The locomotion of fibroblasts in culture. IV. Electron microscopy of the leading lamella. Experimental Cell Research. 1971;67:359–367. doi: 10.1016/0014-4827(71)90420-4. [DOI] [PubMed] [Google Scholar]
- 2.Izzard CS, Lochner LR. Cell-to-substrate contacts in living fibroblasts: an interference reflexion study with an evaluation of the technique. Journal of Cell Science. 1976;21:129–159. doi: 10.1242/jcs.21.1.129. [DOI] [PubMed] [Google Scholar]
- 3.Heath JP, Dunn GA. Cell to substratum contacts of chick fibroblasts and their relation to the microfilament system. A correlated interference-reflexion and high-voltage electron-microscope study. J Cell Sci. 1978;29:197–212. doi: 10.1242/jcs.29.1.197. [DOI] [PubMed] [Google Scholar]
- 4.Couchman JR, Rees DA. The behaviour of fibroblasts migrating from chick heart explants: changes in adhesion, locomotion and growth, and in the distribution of actomyosin and fibronectin. J Cell Sci. 1979;39:149–165. doi: 10.1242/jcs.39.1.149. [DOI] [PubMed] [Google Scholar]
- 5.Nobes CD, Hall A. Rho, Rac, and Cdc42 GTPases Regulate the Assembly of Multimolecular Focal Complexes Associated with Actin Stress Fibers, Lamellipodia, and Filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 6.Zamir E, Katz BZ, Aota S, Yamada KM, Geiger B, Kam Z. Molecular diversity of cell-matrix adhesions. Journal of Cell Science. 1999;112:1655–1669. doi: 10.1242/jcs.112.11.1655. [DOI] [PubMed] [Google Scholar]
- 7.Lazarides E, Weber K. Actin antibody: the specific visualization of actin filaments in non-muscle cells. Proc Natl Acad Sci U S A. 1974;71:2268–2272. doi: 10.1073/pnas.71.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lazarides E, Burridge K. Alpha-actinin: immunofluorescent localization of a muscle structural protein in nonmuscle cells. Cell. 1975;6:289–298. doi: 10.1016/0092-8674(75)90180-4. [DOI] [PubMed] [Google Scholar]
- 9.Geiger B. 130K Protein from Chicken Gizzard - Its Localization at the Termini of Microfilament Bundles in Cultured Chicken-Cells. Cell. 1979;18:193–205. doi: 10.1016/0092-8674(79)90368-4. [DOI] [PubMed] [Google Scholar]
- 10.Burridge K, Feramisco JR. Microinjection and localization of a 130K protein in living fibroblasts: a relationship to actin and fibronectin. Cell. 1980;19:587–595. doi: 10.1016/s0092-8674(80)80035-3. [DOI] [PubMed] [Google Scholar]
- 11.Burridge K, Connell L. A new protein of adhesion plaques and ruffling membranes. Journal of Cell Biology. 1983;97:359–367. doi: 10.1083/jcb.97.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collier NC, Wang K. Purification and properties of human platelet P235. A high molecular weight protein substrate of endogenous calcium-activated protease(s) J Biol Chem. 1982;257:6937–6943. [PubMed] [Google Scholar]
- 13.Burridge K, Mangeat P. An Interaction Between Vinculin and Talin. Nature. 1984;308:744–746. doi: 10.1038/308744a0. [DOI] [PubMed] [Google Scholar]
- 14.Horwitz A, Duggan K, Buck C, Beckerle MC, Burridge K. Interaction of Plasma-Membrane Fibronectin Receptor with Talin - A Transmembrane Linkage. Nature. 1986;320:531–533. doi: 10.1038/320531a0. [DOI] [PubMed] [Google Scholar]
- 15.Calderwood DA, Campbell ID, Critchley DR. Talins and kindlins: partners in integrin-mediated adhesion. Nat Rev Mol Cell Biol. 2013;14:503–517. doi: 10.1038/nrm3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hynes RO. Integrins - A Family of Cell-Surface Receptors. Cell. 1987;48:549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- 17.Damsky CH, Knudsen KA, Bradley D, Buck CA, Horwitz AF. Distribution of the cell substratum attachment (CSAT) antigen on myogenic and fibroblastic cells in culture. J Cell Biol. 1985;100:1528–1539. doi: 10.1083/jcb.100.5.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen WT, Hasegawa E, Hasegawa T, Weinstock C, Yamada KM. Development of Cell-Surface Linkage Complexes in Cultured Fibroblasts. Journal of Cell Biology. 1985;100:1103–1114. doi: 10.1083/jcb.100.4.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanchanawong P, Shtengel G, Pasapera AM, Ramko EB, Davidson MW, Hess HF, Waterman CM. Nanoscale architecture of integrin-based cell adhesions. Nature. 2010;468:580–584. doi: 10.1038/nature09621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Otey CA, Pavalko FM, Burridge K. An interaction between alpha-actinin and the beta 1 integrin subunit in vitro. Journal of Cell Biology. 1990;111:721–729. doi: 10.1083/jcb.111.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roca-Cusachs P, del Rio A, Puklin-Faucher E, Gauthier NC, Biais N, Sheetz MP. Integrin-dependent force transmission to the extracellular matrix by alpha-actinin triggers adhesion maturation. Proc Natl Acad Sci U S A. 2013;110:E1361–1370. doi: 10.1073/pnas.1220723110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schaller MD, Borgman CA, Cobb BS, Vines RR, Reynolds AB, Parsons JT. Pp125Fak, A Structurally Distinctive Protein-Tyrosine Kinase Associated with Focal Adhesions. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanks SK, Calalb MB, Harper MC, Patel SK. Focal adhesion protein-tyrosine kinase phosphorylated in response to cell attachment to fibronectin. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:8487–8491. doi: 10.1073/pnas.89.18.8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ridley AJ, Hall A. The Small GTP-Binding Protein Rho Regulates the Assembly of Focal Adhesions and Actin Stress Fibers in Response to Growth- Factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 25.Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. Journal of Cell Biology. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng JH, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-Associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 27.Dubash AD, Wennerberg K, Garcia-Mata R, Menold MM, Arthur WT, Burridge K. A novel role for Lsc/p115 RhoGEF and LARG in regulating RhoA activity downstream of adhesion to fibronectin. J Cell Sci. 2007;120:3989–3998. doi: 10.1242/jcs.003806. [DOI] [PubMed] [Google Scholar]
- 28.Lim Y, Lim ST, Tomar A, Gardel M, Bernard-Trifilo JA, Chen XL, Uryu SA, Canete-Soler R, Zhai J, Lin H, Schlaepfer WW, Nalbant P, Bokoch G, Ilic D, Waterman-Storer C, Schlaepfer DD. PyK2 and FAK connections to p190Rho guanine nucleotide exchange factor regulate RhoA activity, focal adhesion formation, and cell motility. J Cell Biol. 2008;180:187–203. doi: 10.1083/jcb.200708194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 30.Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- 31.Grashoff C, Hoffman BD, Brenner MD, Zhou R, Parsons M, Yang MT, McLean MA, Sligar SG, Chen CS, Ha T, Schwartz MA. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature. 2010;466:263–266. doi: 10.1038/nature09198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pelham RJ, Wang YL. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riveline D, Zamir E, Balaban NQ, Schwarz US, Ishizaki T, Narumiya S, Kam Z, Geiger B, Bershadsky AD. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. Journal of Cell Biology. 2001;153:1175–1186. doi: 10.1083/jcb.153.6.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wozniak MA, Desai R, Solski PA, Der CJ, Keely PJ. ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. Journal of Cell Biology. 2003;163:583–595. doi: 10.1083/jcb.200305010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 36.Guilluy C, Swaminathan V, Garcia-Mata R, O’Brien ET, Superfine R, Burridge K. The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat Cell Biol. 2011;13:722–727. doi: 10.1038/ncb2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhong C, Chrzanowska-Wodnicka M, Brown J, Shaub A, Belkin AM, Burridge K. Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. Journal of Cell Biology. 1998;141:539–551. doi: 10.1083/jcb.141.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP. Stretching single talin rod molecules activates vinculin binding. Science. 2009;323:638–641. doi: 10.1126/science.1162912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Case LB, Baird MA, Shtengel G, Campbell SL, Hess HF, Davidson MW, Waterman CM. Molecular mechanism of vinculin activation and nanoscale spatial organization in focal adhesions. Nat Cell Biol. 2015;17:880–892. doi: 10.1038/ncb3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. [Review] [385 refs] Annual Review of Cell and Developmental Biology. 1996;12:463–518. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]


