Abstract
Background and Purpose
The relationship between tumor-node-metastasis (TNM) stage and patterns of failure in limited-stage small cell lung cancer (LS-SCLC) remains unclear. We hypothesized that TNM stage predicts brain metastasis risk, and could inform the use of prophylactic cranial irradiation.
Material and Methods
We reviewed 283 patients with stage I–IIIB SCLC. Competing-risks regression was used to analyze local, distant, and brain failure. Multivariate analysis was used to evaluate the effect of treatment and clinical factors on failure and OS.
Results
Patients with stage I or II SCLC (35% of cohort) had significantly better survival and lower risk of distant and brain metastasis, compared with stage III patients. The 5-year cumulative incidence of brain metastasis for stage I/II and III were 12% and 26%, respectively. Stage had no correlation with local failure. On multivariate analysis, stage was independently prognostic for survival, distant metastasis risk, and brain metastasis risk.
Conclusions
TNM staging predicts likelihood of distant metastasis, brain metastasis, and survival in LS-SCLC. This supports the routine use of TNM staging in clinical practice. The lower risk of brain metastasis in stage I and II SCLC suggests that prophylactic cranial irradiation could play a more limited role in treatment of early-stage disease.
Keywords: Small cell lung cancer, radiotherapy, prophylactic cranial irradiation, staging
Introduction
Small cell lung cancer (SCLC) accounts for 15% of lung cancers diagnosed in the United States. Patients with SCLC have traditionally been divided into only two groups: limited-stage (LS) and extensive stage. Although the incidence of SCLC has been declining, it remains a highly lethal malignancy, with only 6% of patients surviving 5 years. [1] However, 25% of patients with LS-SCLC experience long-term survival if treated with a combination of chemotherapy and thoracic and cranial radiation. [2]
LS-SCLC was originally defined as disease confined to a single hemithorax that can be encompassed in a single radiation portal. This simple yet clinically useful definition was introduced by the Veterans Administration Lung Study Group for use in clinical trials in the 1950s. [3] The definition of LS-SCLC has since been expanded to include disease with contralateral hilar, mediastinal, and/or supraclavicular adenopathy. This is concordant with the tumor-node-metastasis (TNM) staging system commonly used for non-small cell lung cancer, which is now also recommended for SCLC. [4]
However, TNM staging has not been widely adopted in the clinical management of LS-SCLC. First, and most importantly, there is little evidence that treatment paradigms should be adjusted on the basis of TNM stage for patients with LS-SCLC. Second, pathologic staging information is lacking for most patients with SCLC, as most are treated with definitive radiotherapy as local therapy, rather than surgery, after needle biopsy. Third, few patients with LS-SCLC present with stage I or II disease—most have stage III disease on presentation. [4] Finally, early-stage SCLC is thought to carry a high risk of occult metastatic disease; therefore, chemotherapy is recommended in addition to thoracic radiotherapy for even the earliest presentations of LS-SCLC. [5]
Prophylactic cranial irradiation (PCI) is also recommended for patients with SCLC on the grounds that occult micrometastases in the brain are both common and insufficiently treated by chemotherapy, owing to the impermeability of the blood-brain barrier. However, despite a proven survival benefit in randomized trials, [6, 7] PCI is often declined by patients and caregivers because of a fear of long-term neurocognitive dysfunction.
It is not known whether the risk of brain metastasis is associated with extent of LS-SCLC at diagnosis and, if so, whether patients with more-limited intrathoracic disease are less likely to benefit from PCI.
We therefore undertook a retrospective review to determine whether TNM staging of LS-SCLC is associated with overall survival (OS) and patterns of local and distant recurrence.
Materials and Methods
Patient Selection
The Institutional Review Board approved this study. Patient confidentiality was maintained in accordance with the Health Insurance Portability and Accountability Act. Registries of radiotherapy and surgery were queried to identify patients with SCLC who were treated with curative intent. The radiation oncology database included patients treated from 1999 to 2013; the thoracic surgery database included patients treated from 1993 to 2013.
For all patients, histologic diagnosis of bronchogenic small cell carcinoma was confirmed at the time of diagnosis and treatment by institutional pathologic review. Patients with components of both NSCLC and SCLC were included if the SCLC component was the dominant one, and the patient was treated according to SCLC rather than NSCLC paradigms (for example, using hyperfractionated radiation therapy, platinum/etoposide chemotherapy, and/or prophylactic cranial irradiation). All patients underwent evaluation for extent of disease with body CT and/or PET scan and brain CT or MRI. LS disease was defined as clinical or pathologic stage IIIB or lower (T3N3M0 or lower) and was reclassified according to the AJCC 7th edition criteria. For clinically staged patients, clinical N stage was based on the official radiographic reports. All staging PET and/or CT studies are performed or formally reviewed at our institution, and all nodes described as having at least 50% probability of malignant involvement were deemed positive for the purposes of reclassification. Pathologic nodal assessment was rarely performed for clinically staged patients. Surgical patients were staged pathologically, unless they had received induction chemotherapy, in which case the initial clinical stage was used.
Data Collection
Patient factors collected included age, sex, Karnofsky performance status, presence of admixed non-small cell carcinoma, and maximum standardized uptake value (for those who received pretreatment PET scans). Details of treatment, including chemotherapy, surgery, and thoracic and cranial radiation, were collected. In general, patients were monitored using chest CT scans every 3–6 months after treatment. Surveillance brain imaging, however, was not regularly performed, and brain imaging after completion of initial therapy was generally performed for symptoms or for restaging workup when disease recurrence was suspected. There was no difference in the intensity or nature of clinical or radiographic followup based on TNM stage. Brain failure was defined as evidence of brain metastasis on brain MRI or CT. Local failure was defined as radiographic or clinical evidence of disease progression within the ipsilateral hemithorax. Distant failure was defined as radiographic evidence of extrathoracic metastasis, excluding brain metastasis.
Statistics
Descriptive summaries of all patient factors were used, including frequencies, percentages, medians, and ranges. Four endpoints of interest were chosen: local failure, distant metastasis, brain metastasis, and OS. Follow-up was calculated from the date of pathologic diagnosis. Competing-risks methods were used to analyze local failure, distant metastasis, and brain metastasis, using a cumulative incidence function to estimate the probability of the event, with death treated as a competing event. Differences in cumulative incidence between groups were assessed using Gray’s method for univariate analyses. The Fine and Gray method was used for multivariate analyses. OS was estimated using Kaplan-Meier methods and Cox proportional hazards models. Patients who did not experience the event of interest were censored at the date of the last available follow-up. Multivariable analysis was performed as follows: all variables with P<0.05 on univariate analysis were included in a model for each endpoint. Patients were analyzed according to the following AJCC stage groups: stage I, II, IIIA, and IIIB. Statistical significance for all analyses was 2-sided and used a 5% significance level (P<0.05). Statistical analyses were performed using R (version 3.2.0; R Development Core Team), with the “survival” and “cmprsk” packages.
Results
We excluded 48 patients who underwent local therapy despite having extensive-stage disease (n=48). We excluded two patients who received thoracic radiotherapy for recurrent disease, and three patients who had mixed NSCLC/SCLC with predominant NSCLC component and were treated using NSCLC protocols. After exclusions, 283 patients met the study criteria and were included in the analysis. Because of the observed similarity of outcomes between stages I and II and between stages IIIA and IIIB, these categories were combined. For example, the hazard ratio for risk of brain metastasis between Stage I and Stage II was 1.00 (95% CI 0.30–3.31). Patient characteristics are described in Table 1. Sixty-five percent of patients had stage IIIA or IIIB SCLC. Seventy-six percent of patients received definitive radiotherapy; the remainder underwent surgical resection with or without adjuvant radiotherapy. Patients treated with definitive radiation (n=214) received either twice-daily radiation (n=116; median dose, 4500 cGy; range, 3000–4500 cGy) or once-daily radiation (n=98; median dose, 5400 cGy; range, 2340–7000 cGy). Chemotherapy was administered to 93% of patients—all regimens were platinum based and nearly all included etoposide. Patients treated with definitive surgical resection (n=69) mostly underwent lobectomy (n=44). The remainder underwent segmentectomy (n=13), wedge resection (n=10), or pneumonectomy (n=2). Most patients (15 of 22) who received adjuvant radiation after surgery had N2 or N3 disease; there were multiple other clinicopathologic factors influencing the recommendation for adjuvant radiation in the remaining patients. The median follow-up was 21.4 months and was not significantly different for Stage I/II patients vs. Stage III patients.
Table 1.
Patient characteristics (N=283)
| Characteristic | No. | % |
|---|---|---|
| Stage | ||
| IA | 31 | 11.0 |
| IB | 15 | 5.3 |
| IIA | 38 | 13.4 |
| IIB | 15 | 5.3 |
| IIIA | 130 | 45.9 |
| IIIB | 54 | 19.1 |
| Sex | ||
| Male | 127 | 44.9 |
| Female | 156 | 55.1 |
| KPS | ||
| <80 | 42 | 14.8 |
| ≥80 | 241 | 85.2 |
| Chemotherapy given | ||
| Whole cohort | 264 | 93.3 |
| Stage I/II patients (n=89) | 84 | 94.4 |
| Stage III patients (n=184) | 180 | 97.8 |
| PCI given | ||
| Whole cohort | 116 | 41.0 |
| Stage I/II patients (n=89) | 34 | 38.2 |
| Stage III patients (n=184) | 82 | 44.6 |
| Local therapy | ||
| Radiation therapy | 214 | 75.6 |
| Surgery | 47 | 16.6 |
| Surgery + radiation therapy | 22 | 7.8 |
| Histologic diagnosis | ||
| Small cell lung cancer | 266 | 94.0 |
| Mixed | 17 | 6.0 |
KPS, Karnofsky performance status; PCI, Prophylactic cranial irradiation.
Survival
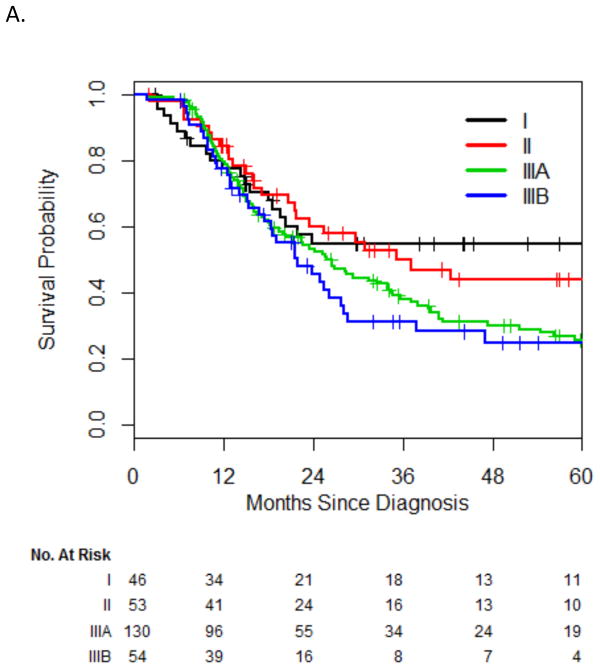
Median survival, including all 283 patients, was 26 months (95% CI, 22–34 months), with a 2-year survival of 53% and a 5-year survival of 33%. On univariate analysis, both stage IIIA (HR, 1.74; 95% CI, 1.07–2.80) and stage IIIB (HR, 1.95; 95% CI, 1.13–3.37) were significantly associated with worse survival, compared with stage I. There was no significant difference between stage I and II (HR, 1.15; 95% CI, 0.64–2.04) or between stage IIIA and IIIB (P=0.47, log-rank test) (Fig 1). Therefore, we grouped stage I/II and stage IIIA/B together for the purpose of multivariate analysis. The HR for death for stage III vs. stage I/II was 1.66 (95% CI, 1.19–2.330). OS at 2 years was 51% for stage III and 58% for stage I or II; OS diverged more sharply at 5 years (25% vs. 49%, respectively). Additionally, on univariate analysis, chemotherapy and PCI were significantly associated with improved survival; they were therefore incorporated into the multivariate model. On multivariate analysis, TNM stage, chemotherapy, and PCI remained independently prognostic for OS (Table 2).
Fig. 1.
Overall survival by stage (A) and grouped stage (B).
Table 2.
Univariate and multivariate analyses for overall survival
| Factor | HR | 95% CI | P |
|---|---|---|---|
| Univariate | |||
| Stage III vs. I/II | 1.66 | 1.19–2.33 | 0.003 |
| Age (continuous) | 1.01 | 0.99–1.03 | 0.23 |
| Sex (M vs. F) | 1.24 | 0.92–1.67 | 0.16 |
| KPS (≥80 vs. <80) | 0.75 | 0.50–1.11 | 0.15 |
| Chemotherapy (Yes vs. No) | 0.52 | 0.30–0.90 | 0.019 |
| Histologic diagnosis (SCLC vs. mixed) | 1.15 | 0.60–2.20 | 0.67 |
| SUVmax (continuous) | 1.02 | 0.99–1.05 | 0.21 |
| PCI given (Yes vs. No) | 0.69 | 0.51–0.94 | 0.019 |
| Multivariate | |||
| Stage III vs. I/II | 1.97 | 1.38–2.80 | <0.001 |
| Chemotherapy (Yes vs. No) | 0.45 | 0.25–0.81 | 0.008 |
| PCI given (Yes vs. No) | 0.67 | 0.49–0.92 | 0.014 |
KPS, Karnofsky performance status; PCI, prophylactic cranial irradiation; SCLC, small cell lung cancer; SUVmax, maximum standardized uptake value.
Distant Metastasis
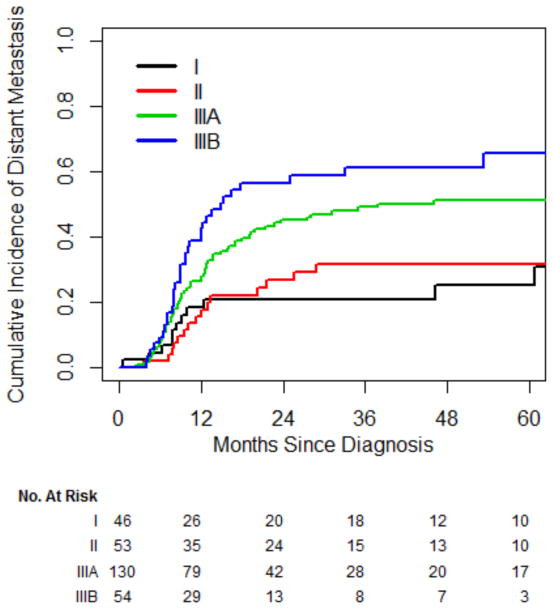
In total, 120 patients (42%) developed distant metastasis. On univariate analysis, only stage was significantly associated with distant metastasis. Nineteen patients (7%) did not receive chemotherapy; chemotherapy was not associated with distant metastasis (P=0.15). The rate of developing metastasis was not significantly different between stage I and II (HR, 1.14; 95% CI, 0.52–2.50), whereas both stage IIIA (HR, 2.16; 95% CI, 1.12–4.15) and stage IIIB (HR, 3.17; 95% CI, 1.58–6.35) were associated with a significantly higher risk of distant metastases, compared with stage I (Fig 2). The HR for distant metastases for stage III vs. stage I/II was 2.25 (95% CI, 1.46–3.47), which corresponds to a 2-year cumulative incidence of 48% vs. 24% and a 5-year cumulative incidence of 55% vs. 28%.
Fig. 2.
Cumulative incidence of distant metastasis.
Local Failure
Sixty-one patients (22%) experienced local failure. The 2- and 5-year cumulative incidence of local failure were 16% and 22%, respectively. No factors analyzed, including stage, were significantly correlated with risk of local failure. Patients with stage IIIA SCLC experienced local failure more often than patients with stage I SCLC (HR, 2.16; 95% CI, 0.92–5.10; P=0.079) (See supplemental figure).
Brain Metastasis
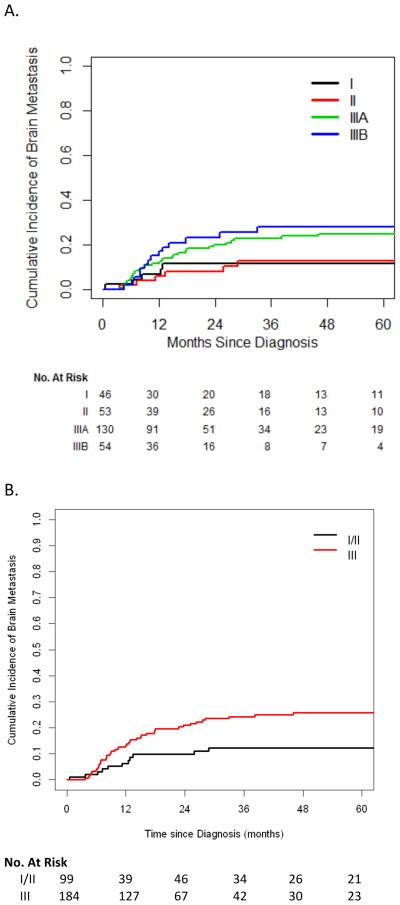
Overall, 55 patients (19%) developed brain metastasis (2-year cumulative incidence, 17%). The cumulative incidence of brain metastasis, for stage III vs. I/II SCLC, was 21% vs. 10% at 2 years and 26% vs. 12% at 5 years.
On univariate analysis, stage III was significantly associated with risk of brain metastasis (HR, 2.20; 95% CI, 1.13–4.26; P=0.020, vs. stage I/II) (Fig 3); only stage was significantly associated with brain metastasis. PCI was associated with a slightly lower risk of brain metastasis (HR, 0.81; 95% CI, 0.48–1.39), but this association was not statistically significant (P=0.45). Because we assumed that selection bias played a role in whether patients received chemotherapy or PCI, we still included those factors in addition to stage in a multivariate model (Table 3). In this model, stage remained the only factor independently associated with brain metastasis (HR, 2.09; 95% CI, 1.08–4.04; P=0.028).
Fig. 3.
Cumulative incidence of brain metastasis by stage (A) and grouped stage (B).
Discussion
The current treatment guidelines recommend PCI for all patients with LS-SCLC with a response to initial therapy, regardless of TNM stage. [5] This is due, in part, to the assumption that SCLC is essentially a systemic disease, regardless of macroscopic disease burden. Because of the diminished ability of cytotoxic chemotherapy to cross the blood-brain barrier, PCI has been part of the standard-of-care treatment for LS-SCLC ever since randomized data showed a significant survival benefit following PCI in patients with extensive-stage SCLC who had a complete response to chemotherapy. [8] In addition, a prospective trial has since established that PCI improves survival in patients with extensive-stage SCLC. [9] However, whole-brain radiotherapy is associated with a risk of neurocognitive deficits, such as impairment in short-term memory. [10] More precise prediction of the risk of brain metastasis in patients with LS-SCLC would therefore be of significant clinical utility, as low-risk patients could potentially avoid the potential toxicity of PCI with little or no detriment to survival, and higher-risk patients would have more assurance that PCI significantly improves their prognosis, justifying the risks of treatment.
LS-SCLC usually presents in a stage III pattern; therefore, stage I or II disease is rarely encountered clinically or analyzed separately from stage III in the literature. However, the IASLC, in preparation for the release of the 7th edition of the TNM staging system, showed that TNM stage correlates with survival for patients with both clinically and pathologically staged SCLC. [4, 11] With the advent of more-sensitive staging tools (such as FDG-PET imaging and brain MRI) and the widespread use of lung cancer screening, an increasing number of patients with well-characterized stage I or II SCLC are now being seen; therefore, it is increasingly relevant to ask whether patients with stage I/II SCLC have the same natural history as those with stage III SCLC. Little is known, however, about the correlation between TNM stage and patterns of failure in patients with LS-SCLC, such as the risk of distant metastasis, locoregional failure, and, in particular, brain metastasis.
A handful of reports have analyzed the relationship between TNM stage and risk of brain metastasis after surgical resection of SCLC. [12–14] In one series from China, the investigators reported that the incidence of brain metastases was only 6% in patients with stage I SCLC, compared with 29% in patients with stage II–III SCLC. They concluded that patients with stage I SCLC may not require PCI after surgical resection. These series, while suggestive, may not be entirely generalizable to standard practice for patients with LS-SCLC, which usually entails definitive thoracic radiotherapy, rather than surgical resection. This preference for definitive radiotherapy over surgery is based, in part, on the findings of historical randomized trials, [15] as well as the fact that many patients with LS-SCLC have anatomically unresectable disease. A recent report from MD Anderson Cancer Center reported that patients with larger primary tumors (≥5cm) had higher risk of brain metastasis, which is broadly consistent with our findings; however, they did not analyze patients according to TNM stage group. [16]
By analyzing a large cohort of patients with LS-SCLC, most of whom were treated with thoracic chemoradiation, the current study extends the previous work that analyzed the relationship between TNM stage and brain metastasis in patients with surgically treated SCLC. In particular, to our knowledge, this is the first report to analyze patterns of failure in patients with LS-SCLC treated with definitive radiotherapy. Our study confirms the IASLC’s findings that TNM stage is correlated with OS in clinically staged patients. That report, however, did not provide information on patterns of failure. [4] We found that increasing TNM stage correlated with increasing risk of distant metastasis, reinforcing the notion that distant failure is the primary mode of recurrence and death in patients with SCLC. Stage was a powerful independent predictor of distant failure in this cohort: stage III SCLC had more than twice the risk of metastasis than stage I or II SCLC. Nevertheless, because a quarter of patients with stage I or II SCLC still developed distant metastasis by 2 years, we find little reason to question the consensus that all patients with SCLC should receive chemotherapy regardless of TNM stage.
Whereas survival, distant metastasis, and brain metastasis were correlated with stage, local failure rates were not. Local progression was a relatively infrequent mode of failure in this cohort, and our 22% crude rate of local failure compares favorably with the 36% reported in the practice-defining Turrisi trial—although it should be noted that the Turrisi trial considered the absence of a complete intrathoracic response to be a local failure. [2]
Our most novel and clinically salient results are those indicating that the risk of brain metastasis is significantly lower for stage I or II SCLC than stage III SCLC. In contrast to the surgical series discussed above, which suggested that only patients with stage I SCLC were candidates for avoiding PCI, we found that patients with stage I and stage II SCLC had similar risks of brain metastasis and that both groups were significantly less likely to develop brain metastasis than patients with stage III SCLC. Stage I SCLC is an uncommon presentation, but patients with stage I or II SCLC made up one-third of the cases in this large series of consecutive patients treated at our institution.
The routine use of staging PET and the increasing use of CT screening in current or former smokers (on the basis of the results of a recent landmark trial [17]) make it even more likely that patients with SCLC will be diagnosed and treated for earlier stages of disease. Because patients with stage I and II SCLC are also the most likely to become long-term survivors, they may have the most to lose from permanent neurocognitive impairment resulting from brain radiation. However, their relatively good prognosis also makes it crucial to offer them any therapy that significantly reduces their risk of recurrence. This study provides support for the notion that withholding PCI may not significantly harm their prognosis. A recent report also indicates that the likelihood that patients will opt for PCI is dependent on the anticipated magnitude of the reduction in brain metastasis risk, making our data relevant to individualized patient decision-making. [18]
Our study has the limitations inherent in any retrospective study, and particular caution must be used when using retrospective data to question the use of an intervention such as PCI, which otherwise is part of the standard of care on account of its putative survival benefit. It should also be noted that Stage I/II patients, due to their lower risk of disease recurrence in general, were less likely to undergo brain imaging as part of restaging for suspected or confirmed extracranial disease recurrence, and therefore the difference in brain metastasis rate may partially be due to a lower likelihood of documenting asymptomatic brain metastasis. Attention must also be paid to the significant heterogeneity in treatments within our study cohort: we included patients treated with surgery plus or minus radiation as well as patients treated with and without PCI. The use of PCI in particular potentially clouds our ability to assess the underlying risk of brain metastasis. However, we attempted to adjust for the effect of PCI by use of multivariate modeling, and we found that stage was a predictor of brain metastasis independently of whether PCI was given. Our study also included patients treated during a relatively long timeframe, during which standards for staging and treatment may have changed. However, we only included patients who had at least a CT scan of the body and head, and note that the standard of care for treatment of LS-SCLC (platinum-based chemotherapy and thoracic radiotherapy) has not changed significantly over these years either.
PCI was not significantly associated with a lower risk of brain metastasis in our cohort as a whole—this is surprising, and we do not have a clear explanation for this. PCI was nevertheless associated with improved OS, which likely represents selection bias, as PCI is usually offered only to patients with good performance status and response to initial therapy. We speculate that the lack of a clear effect of PCI on the risk of brain metastasis may be related to our significantly lower overall rates of brain metastasis, compared with historical data. This, in turn, may reflect stage migration from the routine use of screening brain MRI—that is, patients with what was previously considered clinically occult brain metastasis are now imaged with MRI and excluded from analyses of patients with LS-SCLC. Our population accurately represents contemporary practice, as brain MRI has been adopted as a routine part of the initial staging of all patients with SCLC.
A randomized trial is needed to prove or disprove the benefit of PCI in patients with stage I or II SCLC, but such a trial is unlikely. Until additional data, ideally prospective, are available that confirm the validity of a more tailored approach to selecting patients with LS-SCLC for PCI, the current standard of care—that all patients with LS-SCLC are offered PCI as long as they have responded to initial therapy—will remain. Though retrospective data generally should not overturn standards of care based on prospective data, we suggest at a minimum that our findings support the continued use of TNM staging rather than the historical limited-vs. extensive-stage dichotomy, so that future retrospective and prospective studies can inform this question of the necessity of PCI for all TNM categories. We note that the relatively infrequent use of PCI in our practice suggests that patients and practitioners are already questioning whether the benefits of PCI outweigh the risks for all presentations of LS-SCLC. It is generally accepted that PCI reduces the relative risk of brain metastasis; however, in patient subgroups where the baseline risk is sufficiently low, the absolute benefit of PCI may not be compelling enough to warrant its routine use. Our observation that the cumulative incidence of brain metastasis is only approximately 10% for patients with stage I or II SCLC—and that this is significantly lower than that for patients with stage III SCLC—provides a basis for further study and scrutiny of the necessity for PCI in patients with earlier-stage LS-SCLC.
Supplementary Material
Acknowledgments
Funding: This work was supported by the National Institutes of Health Core Grant P30 CA008748.
The authors would like to thank David Sewell for his editorial support.
Footnotes
Conflict of Interest Statement: All conflicts are unrelated to the current work. Abraham Wu receives research funding from CivaTech Oncology. Robert Downey receives research funding from Cantel Medical and Protea Biosciences. M. Catherine Pietanza is currently employed by Merck, has received honoraria and served in consulting/advisory role with Celgene, Abbvie, Novartis, Clovis Oncology, and Bristol-Myers Squibb, and has served on a speakers’ bureau for PER. Lee Krug is currently employed by Bristol Myers-Squibb. Charles Rudin has served in a consulting/advisory role with Abbvie, AVEO, Boehringer Ingelheim, GlaxoSmithKline, Merck, Celgene, Novartis, and Bristol-Myers Squibb, and has received research funding from Biomarin. Andreas Rimner has received honoraria from Bristol-Myers Squibb, served in consulting/advisory role with GE Healthcare and Varian Medical Systems, and received research funding from Varian Medical Systems and Boehringer Ingelheim.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006;24:4539–44. doi: 10.1200/JCO.2005.04.4859. [DOI] [PubMed] [Google Scholar]
- 2.Turrisi AT, 3rd, Kim K, Blum R, Sause WT, Livingston RB, Komaki R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. The New England journal of medicine. 1999;340:265–71. doi: 10.1056/NEJM199901283400403. [DOI] [PubMed] [Google Scholar]
- 3.Zelen M. Keynote address on biostatistics and data retrieval. Cancer Chemother Rep 3. 1973;4:31–42. [PubMed] [Google Scholar]
- 4.Shepherd FA, Crowley J, Van Houtte P, Postmus PE, Carney D, Chansky K, et al. The International Association for the Study of Lung Cancer lung cancer staging project: proposals regarding the clinical staging of small cell lung cancer in the forthcoming (seventh) edition of the tumor, node, metastasis classification for lung cancer. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2007;2:1067–77. doi: 10.1097/JTO.0b013e31815bdc0d. [DOI] [PubMed] [Google Scholar]
- 5.Kalemkerian GP, Akerley W, Bogner P, Borghaei H, Chow LQ, Downey RJ, et al. Small cell lung cancer. J Natl Compr Canc Netw. 2013;11:78–98. doi: 10.6004/jnccn.2013.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giuliani M, Sun A, Bezjak A, Ma C, Le LW, Brade A, et al. Utilization of prophylactic cranial irradiation in patients with limited stage small cell lung carcinoma. Cancer. 2010;116:5694–9. doi: 10.1002/cncr.25341. [DOI] [PubMed] [Google Scholar]
- 7.Péchoux CL, Sun A, Slotman BJ, De Ruysscher D, Belderbos J, Gore EM. Prophylactic cranial irradiation for patients with lung cancer. The Lancet Oncology. 2016;17:e277–e93. doi: 10.1016/S1470-2045(16)30065-1. [DOI] [PubMed] [Google Scholar]
- 8.Auperin A, Arriagada R, Pignon JP, Le Pechoux C, Gregor A, Stephens RJ, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. The New England journal of medicine. 1999;341:476–84. doi: 10.1056/NEJM199908123410703. [DOI] [PubMed] [Google Scholar]
- 9.Slotman B, Faivre-Finn C, Kramer G, Rankin E, Snee M, Hatton M, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. The New England journal of medicine. 2007;357:664–72. doi: 10.1056/NEJMoa071780. [DOI] [PubMed] [Google Scholar]
- 10.Sun A, Bae K, Gore EM, Movsas B, Wong SJ, Meyers CA, et al. Phase III trial of prophylactic cranial irradiation compared with observation in patients with locally advanced non-small-cell lung cancer: neurocognitive and quality-of-life analysis. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011;29:279–86. doi: 10.1200/JCO.2010.29.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vallieres E, Shepherd FA, Crowley J, Van Houtte P, Postmus PE, Carney D, et al. The IASLC Lung Cancer Staging Project: proposals regarding the relevance of TNM in the pathologic staging of small cell lung cancer in the forthcoming (seventh) edition of the TNM classification for lung cancer. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2009;4:1049–59. doi: 10.1097/JTO.0b013e3181b27799. [DOI] [PubMed] [Google Scholar]
- 12.Gong L, Wang QI, Zhao L, Yuan Z, Li R, Wang P. Factors affecting the risk of brain metastasis in small cell lung cancer with surgery: is prophylactic cranial irradiation necessary for stage I–III disease? International journal of radiation oncology, biology, physics. 2013;85:196–200. doi: 10.1016/j.ijrobp.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 13.Ogawa S, Horio Y, Yatabe Y, Fukui T, Ito S, Hasegawa Y, et al. Patterns of recurrence and outcome in patients with surgically resected small cell lung cancer. International journal of clinical oncology. 2012;17:218–24. doi: 10.1007/s10147-011-0277-4. [DOI] [PubMed] [Google Scholar]
- 14.Zhu H, Bi Y, Han A, Luo J, Li M, Shi F, et al. Risk factors for brain metastases in completely resected small cell lung cancer: a retrospective study to identify patients most likely to benefit from prophylactic cranial irradiation. Radiat Oncol. 2014;9:216. doi: 10.1186/1748-717X-9-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox W, Scadding JG. Medical Research Council comparative trial of surgery and radiotherapy for primary treatment of small-celled or oat-celled carcinoma of bronchus. Ten-year follow-up. Lancet. 1973;2:63–5. doi: 10.1016/s0140-6736(73)93260-1. [DOI] [PubMed] [Google Scholar]
- 16.Farooqi AS, Holliday EB, Allen PK, Wei X, Cox JD, Komaki R. Prophylactic cranial irradiation after definitive chemoradiotherapy for limited-stage small cell lung cancer: Do all patients benefit? Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2017;122:307–12. doi: 10.1016/j.radonc.2016.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Lung Screening Trial Research T. Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. The New England journal of medicine. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lehman M, Gorayski P, Watson S, Edeling D, Jackson J, Whitty J. Patient preferences regarding prophylactic cranial irradiation: A discrete choice experiment. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2016;121:225–31. doi: 10.1016/j.radonc.2016.09.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.