Abstract
Nonalcoholic fatty liver disease (NAFLD) is a spectrum of pathologies associated with fat accumulation in the liver. NAFLD is the most common cause of liver disease in the United States, affecting up to a third of the general population. It is commonly associated with features of metabolic syndrome, particularly insulin resistance. NAFLD shares the basic pathogenic mechanisms with obesity and insulin resistance, such as mitochondrial, oxidative and endoplasmic reticulum stress. Lipoxygenases catalyze the conversion of poly-unsaturated fatty acids in the plasma membrane—mainly arachidonic acid and linoleic acid—to produce oxidized pro-inflammatory lipid intermediates. 12-Lipoxygenase (12-LOX) has been studied extensively in setting of inflammation and insulin resistance. As insulin resistance is closely associated with development of NAFLD, the role of 12-LOX in pathogenesis of NAFLD has received increasing attention in recent years. In this review we discuss the role of 12-LOX in NAFLD pathogenesis and its potential role in emerging new therapeutics.
Keywords: Non-alcoholic fatty liver disease, Fatty liver, Lipoxygenase, Oxidative stress
Introduction
Nonalcoholic fatty liver disease (NAFLD) is a clinicopathologic spectrum of liver pathologies associated with excessive accumulation of fat in the liver (Browning et al., 2004; Neuschwander-Tetri & Caldwell, 2003). This spectrum is continuous but can be graded based on pathological features; in increasing severity, these are: bland steatosis, steatohepatitis, fibrosis and cirrhosis. (Matteoni et al., 1999). NAFLD affects 31% of the US population, and is strongly correlated with the high incidence of obesity in Western cultures (Browning et al).
Simple hepatic steatosis or non-alcoholic fatty liver (NAFL) is a largely benign and reversible condition defined by an excess accumulation of lipid droplets in the liver (Burt, Mutton, & Day, 1998). However, when non-alcoholic hepatic fat accumulation is associated with a significant inflammatory reaction—seen as lobular inflammation and cellular ballooning injury on histopathology —the pathology is considered nonalcoholic steatohepatitis (NASH) (Ludwig, McGill, & Lindor, 1997). An estimated 20–33% of individuals with NAFL patients show evidence of NASH on histopathology (Williams et al., 2011). Further progression of the disease in the setting of ongoing inflammation results in fibrosis (Ludwig et al., 1997) and eventually occurs in 20% of individuals with NASH. (Matteoni et al., 1999) Individuals with NASH progress to fibrosis and cirrhosis at a rate of 7–10% annually.(Argo, Northup, Al-Osaimi, & Caldwell, 2009; Harrison, 2003; Mishra & Younossi, 2012). Annual incidence of hepatocellular cancer and liver related death in patients with NASH related cirrhosis is around 2.6% and 1.4–3% respectively. (Sanyal et al., 2006)
The prevalence of NAFL reaches up to 90% in the obese population, and more than half of these show evidence of NASH based on histopathology. (Spaulding, Trainer, & Janiec, 2003). Furthermore, NAFLD is commonly associated with features of type 2 diabetes and metabolic syndrome. For instance, among individuals with type 2 diabetes mellitus (T2D) up to 70% have NAFL, and NASH is evident in ~67% of those biopsied (Matteoni et al., 1999). Reciprocally, T2D is seen in 30% of patients with NAFLD (Loomba et al., 2012). This strong association of NAFLD with metabolic syndrome, suggests that mechanisms may be shared between these pathologies, in particular the conditions of maladaptive inflammation and insulin resistance observed in both. One biochemical pathway that is likely to be relevant but has not yet been extensively studied in NAFLD, is the eicosanoid generating lipoxygenases pathway. In this review, we summarize the current understanding of the pathogenesis of NAFLD, introduce the pertinent mechanisms by which 12-LOX could play a part in NAFLD pathogenesis, and discuss current and potential new therapeutic approaches.
NAFLD Pathogenesis
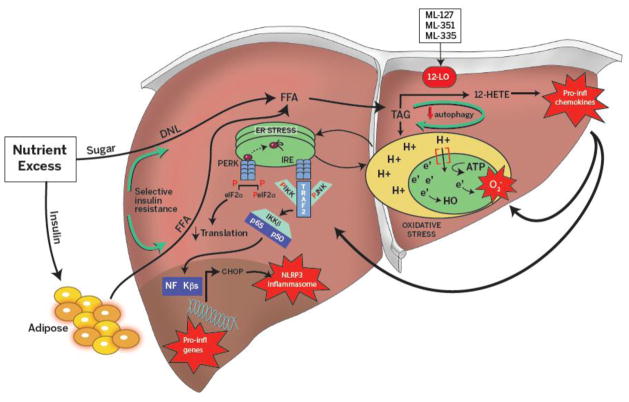
NAFLD is a complex disease, and accordingly its etiology involves multiple interacting factors, such as nutrient excess, obesity and metabolic syndrome (Assay et al., 2000; Beymer, 2003; Leite, Salles, Araujo, Villela-Nogueira, & Cardoso, 2009; Prashanth et al., 2009). In such “overfed” states, free fatty acids (FFAs) are directed to adipose tissue where they are converted into triglycerides under the control of the insulin signaling pathway. However, with chronic over-nutrition and obesity the presence of low grade inflammation in adipose tissues drives the development of peripheral insulin resistance, creating a state of relative insulin deficiency (Hirosumi et al., 2002). Under these conditions, lipolysis is no longer inhibited in adipocytes by insulin, leading to an increase in circulating FFAs (Samuel & Shulman, 2012); these FFAs in turn are sequestered by the liver for lipogenesis. Furthermore, in states of insulin resistance gluconeogenesis is uninhibited, while enhancing de novo lipogenesis. This is referred to as selective insulin resistance, as in normal conditions insulin inhibits gluconeogenesis while promoting de novo lipogenesis (Figure 1). Moreover, locally generated lipid products from cells in the liver (hepatocytes, invading immune cells) may also contribute substrate for lipogenesis. Together, the above mentioned dysfunctions drive the accumulation of triglycerides as lipid droplets in the liver, which upon exceeding 5% of the hepatocytes on histopathology is clinically defined as nonalcoholic fatty liver (NAFL), or bland steatosis (Burt et al., 1998). A considerable percentage of these patients (20–30%) develop hepatic inflammation and progress to nonalcoholic steatohepatitis (NASH) (Ludwig et al., 1997). Although the transition from NAFL to NASH generally occurs in the setting of obesity and insulin resistance, the triggering events and downstream mechanism of progression are not yet completely understood; however it is likely that progression requires two hits that lead to the disruption of distinct molecular pathways (Day & James, 1998). Traditionally it has been hypothesized that a first hit results in development of simple steatosis, while a second hit results in progression from simple steatosis to steatohepatitis (Day & James, 1998). In recent years, a consensus has been emerging that the first of these hits encompasses insulin resistance, continued nutrient excess, and impaired autophagy that lead to steatosis, and that the second of these hits encompasses oxidative stress, ER stress, impaired autophagy, altered intestinal microbiome and intestinal translocation that allow progression to steatohepatitis (Buzzetti, Pinzani, & Tsochatzis, 2016). The hepatocyte alone is not responsible for the spectrum of molecular disorders leading to steatohepatitis, and other cells such as adipocytes and hepatic dendritic cells, NK-T cells, CD4 and CD8 T cells likely contribute (He et al., 2017; Heier et al., 2017; Walker & Lemon, 2016). In the rest of this section, we review three interconnected molecular pathways in hepatocytes—autophagy, ER stress and oxidative stress —that have been implicated in NAFLD progression and introduce 12-LOX pathway which we believe plays an important role in the pathogenesis of NAFLD. Figure 1 provides an overview of the pathogenesis of NAFLD described in this paper.
Figure 1. Overview of pathogenesis of nonalcoholic fatty liver disease.
Nutrient excess leads to insulin resistance and low grade inflammation at the level of adipose tissue. This leads to the increased circulatory free fatty acids (FFA), that can be accessed by the liver. Insulin in steady state promotes de-novo lipogenesis and inhibits gluconeogenesis. But in setting of peripheral insulin resistance, insulin selectively dis-inhibits gluconeogenesis and continues to promote de novo lipogenesis, thus compounding lipid accumulation in the liver. Increased triglyceride accumulation downregulates autophagy and perpetuates triglyceride accumulation. Increased triglyceride accumulation in liver increases demand on electron transport chain in the mitochondria, generating free radical species and leading to oxidative stress. This eventually increases demand on protein folding in the endoplasmic reticulum with ensuing unfolded protein response, where transcription of inflammatory genes, which perpetuates inflammation in setting of nutrient excess. 12-Lipoxygenase (12-LOX) acts on membrane lipids (arachidonic acid) to produce oxidized lipid products such as, 12-hydroxyeicosatetranoic acid (12-HETE) which have chemokine affect, thus amplifying inflammation in the setting of fatty liver. 12LOX inhibitors (ML127, ML351, and ML355) aid in alleviating inflammatory response by reducing oxidative end product production.
Autophagy
Autophagy is a critical cellular mechanism that regulates intracellular recycling and energy homeostasis through the orderly degradation of cellular components. The three pathways of autophagy (macroautophagy, microautophagy, and chaperone-mediated autophagy) have all been described in the liver. In macroautophagy, large cytosolic regions are sequestered within double membrane autophagosome vesicles. ATG7 is an important protein for autophagosome formation and when this pathway is blocked, as in Atg7−/− mice, damaged organelles and altered proteins accumulate even under basal conditions in hepatocytes (Komatsu et al., 2005). In microautophagy, direct lysosomal engulfment of the cytoplasmic cargo occurs through membrane invagination. Chaperone-mediated autophagy is a more selective pathway that relies on the recognition of specific amino acid motifs by the chaperone Hsp70, that directs the delivery of specific proteins to lysosome. Upregulation of chaperone-mediated autophagy occurs as a response to cellular stress including nutrient deprivation and oxidative stress (Kiffin, Christian, Knecht, & Cuervo, 2004).
Autophagy has been shown to regulate lipid metabolism. Under conditions of starvation, autophagy is induced leading to lipolysis and free fatty acid production, which provides an additional source of energy (Singh et al., 2009). Notably, this process of utilizing lipids as a source of energy is hindered during nutrient excess as shown in the RALA255-10G hepatocyte cell line treated with the fatty acid oleate. Knockdown of Atg5 (a gene important in autophagy) caused fat accumulation after treatment with fatty acid oleate showing the importance of autophagy during nutrient excess (Singh et al., 2009). Nutrient excess has also been shown to inhibit autophagy in the liver of high fat diet (HFD)-fed mice. Conversely, treatment of the LO2 hepatocyte cell line with ω-3 fatty acids decreased cellular lipid accumulation partly by increasing autophagic flux and downregulation of lipogenesis genes (Chen et al., 2015). This finding suggests that in the setting of excess fat accumulation in the liver, autophagy is down regulated, leading to an additional increase of lipid accumulation in liver. Furthermore, these data indicate that not only over nutrition per se, but also diet composition, is crucial to driving NAFLD pathogenesis.
Autophagy also plays a role in controlling inflammation through regulatory interactions with inflammatory signaling pathways by removing endogenous inflammasome activators and through effects on the release of cytokines and immune mediators (Deretic, Saito, & Akira, 2013). Atg5−/− mice infected with mycobacterium resulted in macrophages that hypersecrete IL-1α and IL-17, resulting in a pro-inflammatory state (Castillo et al., 2012). Greater cell death was seen in Atg5−/− macrophages compared to wild type under conditions of oxidative and ER stress in setting of atherosclerosis (Liao et al., 2012).
Endoplasmic Reticulum Stress
Nutrient overabundance places increased demand on the endoplasmic reticulum (ER) to synthesize the additional proteins that are required to process excess fat and to package it with lipoproteins for transport throughout the body. When increased protein synthesis demands exceed the capacity of the ER, unfolded proteins accumulate in the lumen, triggering the unfolded protein response (UPR). The protein folding chaperone BiP (binding immunoglobulin protein) normally binds to and suppresses the activation of ER stress sensors in a steady state. However, as unfolded proteins mount, BiP migrates from these “folding” sensors to facilitate protein folding. This dissociation permits activation of each of the sensor pathways—PERK (double stranded RNA-dependent protein kinase-like ER kinase), IRE-α (Inositol requiring element-1α), and ATF6 (activating transcription factor 6)—initiating the UPR. Chronic activation of the UPR generates a maladaptive state of ER stress. In fact, saturated fats such as palmitate have been shown to alter ER membrane integrity by saturating the phosphatidyl choline and triacylglycerol content in ER membranes. (Borradaile et al., 2006)
ER stress is closely tied to the induction of inflammatory pathways. For example, ER stress activates the transcription factor NF-κB through PERK and IREα pathways by overcoming the constitutively expressed Iκβ (inhibitor of NF-κB). This frees NF-κB to translocate to the nucleus, thereby activating transcription of pro-inflammatory genes. Ob/ob mice, which develop steatosis due to a mutation in leptin, have been used to study fatty liver. However, these mice do not develop NASH until a second insult occurs, such as treatment with lipopolysaccharide (LPS). LPS-treated ob/ob mice develop hepatic inflammation through increased expression of pro-inflammatory IFN-γ and decreased expression of anti-inflammatory IL-10 (Yang, Lin, Lane, Clemens, & Diehl, 1997). Hepatic activation of ER stress pathway proteins, such as XBP1s, p-eIF2α and ATF4, and the downstream mediator CHOP are also increased in LPS-treated ob/ob mice. Furthermore, these mice showed increased Bcl-2 and Bcl-XL protein expression, consistent with the promotion of apoptosis with an increased CHOP expression. Lastly, ER stress in hepatocytes has been shown to activate inflammasomes—proinflammatory multiprotein complexes of the innate immune system that regulate activation of caspase-1 in response to infectious microbes or host proteins. This was demonstrated in ob/ob mice, which showed elevated mRNA levels of many inflammasome components upon LPS treatment (Lebeaupin et al., 2015).
Oxidative Stress
Oxidative stress is believed to play an important role in progression from steatosis to steatohepatitis. The metabolism of excess nutrients in hepatocytes places a high demand on the electron transport chain in the mitochondria, resulting in free radical generation, damage to cellular proteins, and increased oxidative stress. Furthermore, the increased demand for electron transport chain proteins feeds ER stress. ER stress feeds back to increase oxidative stress as free oxygen radicals are generated at the time of disulfide bond formation during protein folding (Zhang & Kaufman, 2008).
Levels of fatty acid oxidation have been shown to be elevated in the liver of obese individuals while fatty acid uptake and esterification remain similar to lean subjects (Iozzo et al., 2010). This increase correlated with insulin resistance. Interestingly this increase in fatty acid oxidation was not accompanied by mitochondrial respiratory chain (MRC) activity. When liver biopsy specimens of patients with NASH were compared to healthy controls, long chain acyl carnitine/carnitine ratio was increased, while MRC complexes I through IV were lower, suggesting that mitochondrial respiratory chain lags behind fatty acid oxidation, thereby increasing oxidative stress in the hepatocytes (Pérez-Carreras et al., 2003). Numerous studies explored and found evidence of oxidative stress in patients with NAFLD. Lipid peroxidation products such as malondialdehyde (MDA), hydroxynonenol (HNE), oxidized LDL (ox-LDL), thiobarbituric acid-reacting substances (TBARS) were found to be higher in plasma of patients with NASH compared to patients with steatosis alone, suggesting that oxidative stress could have contributed to progression from steatosis to NASH (Chalasani, Deeg, & Crabb, 2004). Also, HNE and 8-hydroxy deoxyguoanosine staining is significantly higher in liver tissue of patients with NASH compared to those with steatosis alone (Seki et al., 2002). Intensity of staining in the livers with 3-Nitrotyrosine, a lipid peroxidation product was also found to be highest in subjects with NASH, high in those with steatosis alone compared to healthy subjects (Sanyal et al., 2001). Studies have also explored using serum thioredoxin as a non-invasive marker of NASH as it was significantly elevated in patients with NASH compared to steatosis or healthy controls (Sumida, Niki, Naito, & Yoshikawa, 2013).
Lipoxygenases
A potential new pathway linking the three molecular mechanisms described with NAFLD is the lipoxygenase (LOX) pathway. Lipidomics analysis compared two mouse models of NAFLD, wild-type mice on a high fat diet (HFD) and ob/ob mice on a HFD. The study demonstrated that the enrichment of triacyl glycerol and 18:1 fatty acids are the most prominent difference compared with wild-type on regular chow (Hall et al., 2017). This finding suggests that abundance of lipid species, and perhaps their metabolism, may be important in the development of NAFLD. LOX enzymes catalyze the conversion of polyunsaturated fatty acids in the plasma membrane—mainly arachidonic acid and linoleic acid—to produce oxidized pro-inflammatory intermediates (Powell & Rokach, 2015; Tersey et al., 2015). LOXs are classified based on the carbon atom (5, 12 or 15) on arachidonic acid that is the target for oxygenation and stereo-selectivity (R or S enantiomer). Humans and mice have three homologues: 5-LOX, 12-LOX and 15-LOX, which produce 5-(S)-hydroxyeicosatetraenoic acid (5-HETE), 12-HETE, and 15-HETE from arachidonic acid, respectively (Tersey et al., 2015). However, since the mouse 15-LOX enzyme produces predominantly 12-HETE (in a 6:1 ratio over 15-HETE), this orthologue is also commonly known as 12/15-LOX. Henceforth in this review, we will use the term 12-LOX to refer to the mouse 12/15-LOX enzyme, as it is functionally equivalent to human 12-LOX and produces the majority of 12-HETE in mice. Listed in Table 1 are the major lipoxygenases, the gene encoding them, and their major lipid products.
Table 1.
Major lipoxygenases, their genes, and major products in humans and mice.
| Gene | Enzyme | Product | |
|---|---|---|---|
| Mouse | Alox15 | 12/15-LOX (or commonly 12-LOX) | 12-HETE:15-HETE (6:1) |
| Alox12 | 12-LOX | 12-HETE | |
| Human | Alox15 | 15-LOX | 15-HETE |
| Alox12 | 12-LOX | 12-HETE |
12-LOX in the pancreatic islet
Whereas 12-LOX has been studied extensively during the inflammatory response in tissues such as islets, adipocytes and macrophages, its function in the liver is not as well understood. Mice harboring deletion of the gene encoding 12-LOX (Alox15) appear phenotypically normal, however they exhibit resistance to glucose intolerance when challenged with a high fat diet (HFD) (Nunemaker et al., 2008). HFD-fed Alox15−/− mice also exhibit reduced insulin resistance and reduced macrophage infiltration within the adipocytes compared to control mice (Nunemaker et al., 2008). Whereas these studies showed the global importance of 12-LOX in the stress response to HFD-feeding, several different tissue-specific knockout models have been studied to differentiate the role of 12-LOX in various tissues/organs. 12-LOX is detected at low levels in human and mouse islets and treatment of human islets with pro-inflammatory cytokines results in an increase in both 12-LOX activity and protein levels (Chen, Yang, Smith, Carter, & Nadler, 2005). Inhibition of 12-LOX, either genetically or chemically, reverses islet β-cell dysfunction as seen in the presence of pro-inflammatory cytokines and restores normal insulin secretion, alluding to the crucial role played by 12-LOX in preserving insulin secretion during stress (Ma et al., 2017; Tersey et al., 2014). Treatment of human islets with 12-HETE shows a similar reduction in insulin secretion and β-cell dysfunction as seen with pro-inflammatory cytokines, further supporting the role of 12-HETE in islet stress. HFD-fed mice exhibited ER stress (shown by activation of CHOP) and oxidative stress (shown by activation of 4-HNE) in their islets and genetic deletion of Alox15 in the pancreas of mice results in decreased ER and oxidative stress as well as improved metabolic health (Tersey et al., 2014, 2015). This effect of 12-LOX appears to be mediated by p38MAPK, as islets treated with 12-HETE show increased phosphorylation of p38MAPK and Alox15 knockdown in mouse islets demonstrated decreased phosphorylated p38MAK (Ma et al., 2010).
12-LOX in macrophages and adipose tissue
Recruitment of CD11b+, F4/80+macrophages and elevated protein levels of inflammatory markers such as IL-1β, IL-6, IL-10, IFN-γ, Cxcl1 and TNF-α were seen in adipose tissue of control mice but not in Alox15−/− mice fed a HFD. This observation suggests a crucial role of 12-LOX in obesity and related inflammatory states (Sears et al., 2009). 3T3-L1 adipocytes treated with 12-HETE showed increased expression of inflammatory genes IL-6, TNF-α, MCP-1, IL-12p40 and reduced expression of anti-inflammatory adiponectin. These changes are mediated via janus kinase (JNK) phosphorylation, with subsequent phosphorylation of IRS-1(Ser) and impaired phosphorylation of IRS-1(Tyr) and protein kinase B phosphorylation (Chakrabarti, Cole, Wen, Keller, & Nadler, 2009). Likewise, 12-LOX expression in visceral adipose tissue of patients with T2D correlated with an increase in IL-6 and IL-12 cytokines (Lieb et al., 2014). Similarly, 12-HETE treatment of mouse macrophage cell lines (J773A.1) induced IL-6 and TNF-α production. Over-expression of 12-LOX also resulted in higher production of IL-6 and TNF-α. It appears that this action is at least partly mediated by p38MAPK and JNK (Wen et al., 2007). IL-12 production by macrophages upon IFN-γ stimulation is mediated through 12-LOX (Middleton, Rubinstein, & Pure, 2006). HFD-induced expression of TNF-α in adipose tissue was attenuated in adipocyte-specific Alox15−/− mice. In addition, macrophage infiltration of adipose tissue was also reduced in adipocyte-specific Alox15−/− mice fed HFD (Cole, Morris, Grzesik, Leone, & Nadler, 2012). It is of interest to note that in an adoptive transfer model of type 1 diabetes, splenocytes of Alox15−/− mice congenic on the non-obese diabetic (NOD) background are not able to transfer disease, whereas splenocytes from control NOD mice transfer disease at 100% within two months (Green-Mitchell et al., 2013). This role of 12-LOX in macrophages also has implications in atherosclerosis, as mice reconstituted with bone-marrow from Alox15/Apoe double-knockout mice displayed reduced atherosclerotic plaque size compared to bone-marrow from control Apoe−/− mice (Huo et al., 2004). 12-LOX deficiency has also been shown to decrease HFD-induced atherosclerotic plaques in aorta of Ldlr−/− mice (an atherosclerotic mouse model) without changing the composition of lipids in the plaque (George et al., 2001). In fact, 12-LOX has been shown to be a major player in the onset of diabetic cardiomyopathy in the streptozotocin (STZ) model of diabetes (single high dose), where increased expression of 12-LOX in cardiomyocytes was seen after exposure to high plasma glucose levels followed by deterioration in cardiac function; this deterioration was mitigated in Alox15−/− animals (Suzuki et al., 2015).
Lipoxygenases in liver
A few studies have begun to investigate the role of lipoxygenases in the liver. Metabolomics in patients with NASH demonstrated increase in products of lipoxygenase pathway, including 5-HETE, 8-HETE, 11-HETE and 15-HETE compared to healthy patients and those with steatosis alone. There was no increase in products of cyclooxygenase pathway (Puri et al., 2009). In a Methionine choline-deficient mouse model of non-alcoholic steatohepatitis, liquid chromatography and mass spectroscopy of serum demonstrated significant elevation of 12-HETE, linoleic and oleic acids along with bile acids, tauro-B muricholate and taurocholate compared to mice supplemented with methionine and choline (Tanaka, Matsubara, Krausz, Patterson, & Gonzalez, 2012). In addition, MCD fed mice showed increased gene expression of Alox12, an alternate gene whose product also produces 12-HETE. Recent study in HFD-fed wild-type mice show a significant increase in 12-HETE. These mice also demonstrated an increase in 15-HETE, 5-HETE and 11-HETE. Livers of patients with NASH who demonstrated higher histologic inflammation score had increased 15-HETE levels. (Hall et al., 2016) In clinical trials using pentoxyfylline, a methyl xanthine derivative with anti-inflammatory properties partly mediated by suppressing TNF-α gene transcription, subjects who responded to pentoxyfylline with improvement in lobular inflammation on histology demonstrated a decrease in plasma 12-HETE levels (Zein et al., 2012). Both 12-LOX and 5-LOX are expressed in normal mouse hepatocytes and upon liver damage via acetaminophen the transcript and expression levels of both are increased (Suciu et al., 2016). Likewise, upon HFD-feeding, 12-HETE and 5-HETE is increased compared chow-fed control mice (Lazic et al., 2014a). 5-LOX has been shown to be elevated in the liver of ob/ob mice and 5-LOX inhibition downregulated genes involved in hepatic fatty acid uptake and acyl-CoA oxidase expression, restored hepatic microsomal triglyceride transfer protein activity and hepatic VLDL-triglyceride and Apo-B secretion, suggesting a steatogenic role of 5-LOX. (Lopez-Parra et al., 2008). Also, in the Apoe−/− mouse, 5-LOX deficiency protected mice from macrophage infiltration in the liver with decreased hepatic expression of pro-inflammation cytokines (IL-18 and MCP-1) (Martínez-Clemente, Clària, & Titos, 2011). Along similar lines, whole-body genetic knockout of Alox15 in HFD-fed mice resulted in decreased hepatic steatosis, decreased macrophage infiltration, decreased mRNA expression levels of proinflammatory cytokine genes in the liver (IFN-γ, TNF-α and IL-10), and decreased immune cell chemoattractants (Cxcl2/3) (Lazic et al., 2014b). The liver includes multiple different cell types such as hepatocytes, cholangiocytes, Kupffer cells and stellate cells. While these studies suggest that LOXs (and in particular 12-LOX) play an important role in the pathogenesis of NAFLD in mice, the specific cell types have yet to be clarified, as studies were performed in whole animal genetic deletions. Arachidonic acid metabolism appears to interact with cholesterol transport. Products of cholesterol metabolism, bile acids have also found to have a role in pathogenesis of NAFLD. Methionine choline deficient mouse model of NASH were found to have elevated taurocholate and tauro-β-cholate were found to be elevated in addition to 12-HETE compared to control mice. (Tanaka, Matsubara, Krausz, Patterson, & Gonzalez, 2012) Reverse cholesterol transport by acetyl salicylic acid is mediated by diverting arachidonic acid metabolism from cyclooxygenase enzyme pathway to 5 lipoxygenases thereby generating leukotrienes and lipoxins from 15-HETE, which have been shown to induce Abcb11 at a post translational level. (Demetz et al., 2014)
Available treatment modalities for individuals with NAFLD
There are several treatment modalities currently used or in clinical trials for individuals with NAFLD. The most widely recommended treatment is a lifestyle modification plan. As little as 5% weight loss has been shown to improve NASH histology in a pilot study of 23 overweight/obese subjects with biopsy-proven NASH (Huang et al., 2005). In another study, 31 overweight/obese individuals with biopsy-proven NASH were randomized to intensive lifestyle therapy or structured education. The patients in the intensive arm lost significantly more weight which led to improvement in steatosis, necrosis and inflammation (Promrat et al., 2010). A more recent study documented improvement in all histologic features of NAFLD with weight loss, including fibrosis. (Vilar-Gomez et al., 2015) Several studies have examined weight loss via bariatric surgery and have found improvement in hepatic steatosis, inflammation, and fibrosis (Furuya et al., 2007; Popov, 2015). Additionally, exercise alone without any dietary intervention has been shown to decrease hepatic liver lipids and improve overall metabolic health (Golabi et al., 2016; St. George et al., 2009). Hepatic staining of malondialdehyde and Cyp2E1 protein content decreased with surgical weight loss in obese subjects with NAFLD (Bell et al., 2010). A major limitation of lifestyle modification in the treatment of NASH is patient adherence, which can be as low as 30% (Martin, Williams, Haskard, & DiMatteo, 2005).
The next most common treatment is insulin-sensitizing agents. Several different insulin-sensitizing agents have been used to treat steatohepatitis with varying degrees of success. Although initial proof-of-concept studies have shown that metformin may be associated with histologic and biochemical improvement in NASH, subsequent larger studies failed to demonstrate histological benefit for metformin in patients with NASH (Haukeland et al., 2009; Lavine et al., 2011; Loomba et al., 2009; Nair, Diehl, Wiseman, Farr, & Perrillo, 2004). Pioglitazone, another insulin-sensitizing agent, resulted in decreased inflammation and resolution of steatohepatitis when administered for 12–24 months in non-diabetic subjects with NASH. Both PPAR-γ and PPAR-α agonistic effects of pioglitazone are believed to aid in its effect on NASH (Aithal et al., 2008). However, pioglitazone is associated with higher rates of congestive heart failure and this concern has limited its widespread use in treatment of NASH. Likewise, while vitamin E (α-tocopherol) at a dose of 800 IU/day has been shown to improve NASH histology in non-diabetic adults with biopsy-proven NASH (Sanyal et al., 2010), data regarding association of high-dose vitamin E with prostate cancer has to be cautiously considered and discussed with patients before long-term use (Bjelakovic, Nikolova, Gluud, Simonetti, & Gluud, 2007; Klein et al., 2011; Miller et al., 2005). Analysis in vitro showed that α-tocopherol reduces lipoxygenase dependent peroxidation of pig liver phosphatidylcholine micelles (Hirofumi Arai, Akihiko Nagao, & Kozo Takama, 1995).
Several other agents are also being actively studied for NAFLD. A multicenter clinical trial showed that 6-ethylchenodeoxycholic acid (obeticholic acid), an activator of farsenoid X nuclear receptor (FXR), significantly improved steatohepatitis histopathology. Contrary to expectations, subjects treated with obeticholic acid also witnessed worsening HOMA-IR and an increase in mean total cholesterol and LDL fraction and a decrease in HDL fraction (Neuschwander-Tetri et al., 2015). The long-acting glucagon-like-peptide-1 agonist Liraglutide was shown to resolve NASH without progression of fibrosis in a significant number of subjects compared to placebo in a recently-published phase 2 trial (LEAN trial) (Armstrong et al., 2016). In addition, the Liraglutide group showed significant decreases in hemoglobin A1C, absolute weight, BMI and increase in HDL cholesterol fraction, thus aiding cardiovascular risk optimization in this patient cohort (Armstrong et al., 2016).
Based on both the limitations of the current available treatments and the molecular mechanisms of NAFLD, there are compelling reasons to study novel therapeutic interventions based on the 12-LOX/12-HETE signaling pathway. Protection of HFD-fed Alox15−/− mice from ER stress and inflammation described above suggests that interventions that inhibit 12-LOX with small molecule inhibitors, such as ML127, ML351 and ML355 (Kenyon et al., 2011; D. Luci et al., 2010; D. K. Luci et al., 2014; Ma et al., 2017) would serve to protect these animals from steatohepatitis. Further studies are needed to confirm the potential of these proposed interventions, particularly given the possibility of off-target effects and non-tissue-specific effects of small molecule drugs of this nature.
Animal Models of NAFLD
Human research has greatly shaped our understanding of non-alcoholic fatty liver disease, but several limitations exist in studying the disease processes in humans, such as variations in environmental exposure, pre-existing genetic risk factors, racial and ethnic differences in disease presentation, and need for multiple invasive procedures among others. Research in animals enables us to circumvent several of these issues. An ideal animal model for NAFLD must replicate human disease closely, by exhibiting fatty liver associated with inflammation in an environment of nutrient excess, preferably associated with features of metabolic syndrome such as obesity and insulin resistance. The animal model should be easy to breed and maintain in the lab environment in addition to achieving the desired disease phenotype in a reasonable timeframe. Several animal models are available to study NAFLD; each one presenting both advantages and limitations. Currently, both mice and pigs have been used in NAFLD research, though rodent studies are far more common. NAFLD occurs naturally in mice, but it can also be induced more reproducibly through genetic alterations/mutations. Alternatively, NAFLD can be induced in animals by feeding mice diets with high fat or carbohydrate content, such that 60% of caloric count is derived from fat alone and/or cholesterol or simple carbohydrates. Simple carbohydrates, such as glucose and fructose generate abundant levels of glycerol-3-phosphate, which can be used in triglyceride synthesis. Moreover, as fructokinase is not regulated by insulin, excessive fructose can lead to unregulated production of acetyl-Co-A, which is in turn converted to triglycerides (Jegatheesan & De Bandt, 2017). Diets deficient in essential nutrients can also result in fatty liver. Listed in Table 1 are the various rodent models currently used to study NAFLD and their advantages and limitations.
Ossabaw pigs, a breed of pig originally found on an island off the state of Georgia in the United States, acquired a “thrifty gene” to adapt to seasonable variability of food availability. These pigs develop steatosis and steatohepatitis when fed a diet high in fats. However, they are not widely used in research due to cumbersome nature of breeding and maintaining these animals in laboratory setting (Lee et al., 2009).
In light of the limitations of rodent and pig models of NAFLD, there is a need for new animal models that address these shortcomings. Zebrafish is being explored as a potential animal model to study NAFLD. The similarity of zebrafish hepatopancreaticobiliary anatomy to humans and presence of orthologues to most human genes in zebrafish, including synteny make zebrafish an attractive animal model for hepatopancreaticobiliary disease. In addition, genetic tractability and transgenic feasibility allow for development of desired animal models with expression of interested study pathways. Rapid external development of transparent zebrafish embryo allows for study of effect of gene expression patterns on embryonic development in a time efficient manner. All the above features lend zebrafish to study of hepatopancreaticobiliary disease (Schlegel, 2012).
Conclusion
In this review, we summarize the putative role of oxidative stress and ER stress in the development of nonalcoholic steatohepatitis and identify the 12-LOX as an under-recognized, albeit important, contributor to the pathogenesis of NAFLD. It is as yet unclear whether current approaches to therapy (weight loss, thiazolidinediones, GLP-1 receptor agonists, FXR agonists) might operate in part through the alteration of 12-LOX activity. As such, recently developed inhibitors of 12-LOX (Kenyon et al., 2011; D. Luci et al., 2010; D. K. Luci et al., 2014; Ma et al., 2017) may represent a novel approach to the treatment and/or prevention of NAFLD, and such inhibitors may augment current approaches to treatment.
Table 2.
Animal Models of nonalcoholic fatty liver disease
| Model | Mechanisms | Salient Features | References |
|---|---|---|---|
| Dietary Deficiency | |||
|
| |||
| Methionine choline deficient | Impaired VLDL secretion from liver | Features of steatohepatitis in 10 weeks But in presence of weight loss and improved insulin sensitivity |
(Caballero et al., 2010; Oz, Chen, & Neuman, 2008) |
| Conjugated Linoleic Acid (CLA) | Diet with transfat conjugated with linoleic acid | Features of steatohepatitis with mild peri-sinusoidal fibrosis with insulin resistance and near universal HCC development But in presence of weight loss and improved insulin sensitivity |
(Fujita et al., 2010) |
| Choline-def L-AA (CDAA) | Diet deficient in choline; containining only L-amino acids | Develops fibrosing NASH, cirrhosis and HCC. But in presence of weight loss, improved insulin sensitivity and increasing adiponectin levels |
(de Lima et al., 2008) |
|
| |||
| Dietary Excess | |||
|
| |||
| High Fat Diet | > 60% fat calories | Steatosis with minimal and variable inflammation and fibrosis associated with obesity, insulin resistance and dyslipidemia. | (Tetri, Basaranoglu, Brunt, Yerian, & Neuschwander-Tetri, 2008) |
| Western Diet | 45% saturated and trans fats High cholesterol | Steatohepatitis w/ballooning and variable fibrosis But takes up to 20 weeks |
(Kohli et al., 2010) |
| Atherogenic diet | 1.25% cholesterol and 0.5% cholate | Steatohepatitis with ballooning and fibrosis But occurs in setting of weight loss and improved insulin sensitivity and takes up to 24 weeks |
(Charlton et al., 2011) |
|
| |||
| Genetic Models | |||
|
| |||
| ob/ob | Mutation in leptin | Steatosis, Steatohepatitis develops after 2nd hit in obese, hyperphagic, inactive animals that show insulin resistance and dyslipidemia But resistant to fibrosis |
(Ingalls, Dickie, & Snell, 1950; Zhang et al., 1994) |
| db/db | Mutation in leptin receptor | Steatohepatitis after 2nd hit in obese animals with insulin resistance | (Chen et al., 1996; Hummel, Dickie, & Coleman, 1966) |
| ApoE KO | Absence of ApoE protein, a ligand of the LD receptor | Steatohepatitis after 2nd hit in animals with increased LDL, total cholesterol and triglycerides and atherosclerosis Model of dyslipidemia |
(Schierwagen et al., 2015) |
| aP2-nSREBP-1c transgenic | Over-exp of SREBP-1c in adipose tissue | Steatohepatitis with mild fibrosis in animals with increased Glu; decreased adiponectin Model of lipodystrophy |
(Shimano et al., 1996) |
| MAT1A KO | Absence of methionine adenosyltranfer ase (impaired anti-oxidant defense) | Steatohepatitis without fibrosis with high susceptibility to tumors But no evidence of metabolic syndrome, |
(Lu et al., 2001) |
Article Highlights.
Nonalcoholic fatty liver disease is associated with obesity and affects more than 30% of the US population.
The molecular pathogenesis of nonalcoholic fatty liver disease involves endoplasmic reticulum stress, oxidative stress, and autophagy
12-Lipoxygenase produces products that exacerbate the molecular stress pathways leading to nonalcoholic fatty liver disease
Multiple animal models of nonalcoholic fatty liver disease serve as preclinical models for testing of potential therapies for nonalcoholic liver disease
Acknowledgments
Research in the Mirmira laboratory is funded by grants R01 DK060581, R01 DK105588, and UC4 DK104166 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aithal GP, Thomas JA, Kaye PV, Lawson A, Ryder SD, Spendlove I, … Webber J. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 2008;135(4):1176–1184. doi: 10.1053/j.gastro.2008.06.047. https://doi.org/10.1053/j.gastro.2008.06.047. [DOI] [PubMed] [Google Scholar]
- Argo CK, Northup PG, Al-Osaimi AMS, Caldwell SH. Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. Journal of Hepatology. 2009;51(2):371–379. doi: 10.1016/j.jhep.2009.03.019. https://doi.org/10.1016/j.jhep.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, … Newsome PN. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387(10019):679–690. doi: 10.1016/S0140-6736(15)00803-X. https://doi.org/10.1016/S0140-6736(15)00803-X. [DOI] [PubMed] [Google Scholar]
- Assay N, Kaita K, Mymin D, Levy C, Rosser B, Minuk G. Fatty infiltration of liver in hyperlipidemic patients. Digestive Diseases and Sciences. 2000;45(10):1929–1934. doi: 10.1023/a:1005661516165. [DOI] [PubMed] [Google Scholar]
- Bell LN, Temm CJ, Saxena R, Vuppalanchi R, Schauer P, Rabinovitz M, … Mattar SG. Bariatric Surgery-Induced Weight Loss Reduces Hepatic Lipid Peroxidation Levels and Affects Hepatic Cytochrome P-450 Protein Content. Annals of Surgery. 2010;251(6):1041–1048. doi: 10.1097/SLA.0b013e3181dbb572. https://doi.org/10.1097/SLA.0b013e3181dbb572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beymer K. Prevalence and Predictors of Asymptomatic Liver Disease in Patients Undergoing Gastric Bypass Surgery. Archives of Surgery. 2003;138(11):1240–1244. doi: 10.1001/archsurg.138.11.1240. [DOI] [PubMed] [Google Scholar]
- Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297(8):842–857. doi: 10.1001/jama.297.8.842. https://doi.org/10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- Borradaile NM, Han X, Harp JD, Gale SE, Ory DS, Schaffer JE. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. Journal of Lipid Research. 2006;47(12):2726–2737. doi: 10.1194/jlr.M600299-JLR200. https://doi.org/10.1194/jlr.M600299-JLR200. [DOI] [PubMed] [Google Scholar]
- Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, … Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology. 2004;40(6):1387–1395. doi: 10.1002/hep.20466. https://doi.org/10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- Burt A, Mutton A, Day C. Diagnosis and interpretation of steatosis and steatohepatitis. Seminars in Diagnostic Pathology. 1998;15(4):246–58. [PubMed] [Google Scholar]
- Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD) Metabolism. 2016;65(8):1038–1048. doi: 10.1016/j.metabol.2015.12.012. https://doi.org/10.1016/j.metabol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- Caballero F, Fernandez A, Matias N, Martinez L, Fucho R, Elena M, … Garcia-Ruiz C. Specific contribution of methionine and choline in nutritional nonalcoholic steatohepatitis: Impact on mitochondrial s-adenosyl-l-methionine and GSH. Journal of Biological Chemistry. 2010;285(24):18528–18536. doi: 10.1074/jbc.M109.099333. https://doi.org/10.1074/jbc.M109.099333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo EF, Dekonenko A, Arko-Mensah J, Mandell MA, Dupont N, Jiang S, … Deretic V. Autophagy protects against active tuberculosis by suppressing bacterial burden and inflammation. Proceedings of the National Academy of Sciences USA. 2012;109(46):E3168–E3176. doi: 10.1073/pnas.1210500109. https://doi.org/10.1073/pnas.1210500109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti SK, Cole BK, Wen Y, Keller SR, Nadler JL. 12/15-Lipoxygenase Products Induce Inflammation and Impair Insulin Signaling in 3T3-L1 Adipocytes. Obesity. 2009;17(9):1657–1663. doi: 10.1038/oby.2009.192. https://doi.org/10.1038/oby.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalasani N, Deeg MA, Crabb DW. Systemic levels of lipid peroxidation and its metabolic and dietary correlates in patients with nonalcoholic steatohepatitis. The American Journal of Gastroenterology. 2004;99(8):1497–1502. doi: 10.1111/j.1572-0241.2004.30159.x. https://doi.org/10.1111/j.1572-0241.2004.30159.x. [DOI] [PubMed] [Google Scholar]
- Charlton M, Krishnan A, Viker K, Sanderson S, Cazanave S, McConico A, … Gores G. Fast food diet mouse: novel small animal model of NASH with ballooning, progressive fibrosis, and high physiological fidelity to the human condition. AJP: Gastrointestinal and Liver Physiology. 2011;301(5):825–834. doi: 10.1152/ajpgi.00145.2011. https://doi.org/10.1152/ajpgi.00145.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, … Ellis SJ, et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84(3):491–495. doi: 10.1016/s0092-8674(00)81294-5. Retrieved from http://www.sciencedirect.com/science/article/pii/S0092867400812945. [DOI] [PubMed] [Google Scholar]
- Chen M, Yang ZD, Smith KM, Carter JD, Nadler JL. Activation of 12-lipoxygenase in proinflammatory cytokine-mediated beta cell toxicity. Diabetologia. 2005;48:486–495. doi: 10.1007/s00125-005-1673-y. [DOI] [PubMed] [Google Scholar]
- Chen Y, Xu C, Yan T, Yu C, Li Y. ω-3 Fatty acids reverse lipotoxity through induction of autophagy in nonalcoholic fatty liver disease. Nutrition. 2015;31(11–12):1423–1429. e2. doi: 10.1016/j.nut.2015.05.022. https://doi.org/10.1016/j.nut.2015.05.022. [DOI] [PubMed] [Google Scholar]
- Cole BK, Morris MA, Grzesik WJ, Leone KA, Nadler JL. Adipose Tissue-Specific Deletion of 12/15-Lipoxygenase Protects Mice from the Consequences of a High-Fat Diet. Mediators of Inflammation. 2012;2012 doi: 10.1155/2012/851798. https://doi.org/10.1155/2012/851798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day CP, James OF. Hepatic steatosis: innocent bystander or guilty party? Hepatology (Baltimore, Md) 1998;27(6):1463–1466. doi: 10.1002/hep.510270601. https://doi.org/10.1002/hep.510270601. [DOI] [PubMed] [Google Scholar]
- de Lima VMR, Oliveira CPMS, Alves VAF, Chammas MC, Oliveira EP, Stefano JT, … Caldwell SH. A rodent model of NASH with cirrhosis, oval cell proliferation and hepatocellular carcinoma. Journal of Hepatology. 2008;49(6):1055–1061. doi: 10.1016/j.jhep.2008.07.024. https://doi.org/10.1016/j.jhep.2008.07.024. [DOI] [PubMed] [Google Scholar]
- Demetz E, Schroll A, Auer K, Heim C, Patsch JR, Eller P, … Tancevski I. The Arachidonic Acid Metabolome Serves as a Conserved Regulator of Cholesterol Metabolism. Cell Metabolism. 2014;20(5):787–798. doi: 10.1016/j.cmet.2014.09.004. https://doi.org/10.1016/j.cmet.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic V, Saito T, Akira S. Autophagy in infection, inflammation and immunity. Nature Reviews Immunology. 2013;13(10):722–737. doi: 10.1038/nri3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita K, Nozaki Y, Yoneda M, Wada K, Takahashi H, Kirikoshi H, … Nakajima A. Nitric Oxide Plays a Crucial Role in the Development/Progression of Nonalcoholic Steatohepatitis in the Choline-Deficient, l-Amino Acid-Defined Diet-Fed Rat Model. Alcoholism: Clinical and Experimental Research. 2010;34:S18–S24. doi: 10.1111/j.1530-0277.2008.00756.x. https://doi.org/10.1111/j.1530-0277.2008.00756.x. [DOI] [PubMed] [Google Scholar]
- Furuya CKJ, Oliveira CPD, Mello ESD, Faintuch J, Raskovski A, Matsuda M, … Carrilho FJ. Effects of bariatric surgery on nonalcoholic fatty liver disease: preliminary findings after 2 years. Journal of Gastroenterology and Hepatology. 2007;22(4):510–514. doi: 10.1111/j.1440-1746.2007.04833.x. [DOI] [PubMed] [Google Scholar]
- George J, Afek A, Shaish A, Levkovitz H, Bloom N, Cyrus T, … Harats D. 12/15-lipoxygenase gene disruption attenuates atherogenesis in LDL receptor–deficient mice. Circulation. 2001;104(14):1646–1650. doi: 10.1161/hc3901.095772. Retrieved from http://circ.ahajournals.org/content/104/14/1646.short. [DOI] [PubMed] [Google Scholar]
- Golabi P, Locklear CT, Austin P, Afdhal S, Byrns M, Gerber L, Younossi ZM. Effectiveness of exercise in hepatic fat mobilization in non-alcoholic fatty liver disease: Systematic review. World Journal of Gastroenterology. 2016;22(27):6318–6327. doi: 10.3748/wjg.v22.i27.6318. https://doi.org/10.3748/wjg.v22.i27.6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green-Mitchell SM, Tersey SA, Cole BK, Ma K, Kuhn NS, Cunningham TD, … Morris MA. Deletion of 12/15-Lipoxygenase Alters Macrophage and Islet Function in NOD-Alox15null Mice, Leading to Protection against Type 1 Diabetes Development. PLoS ONE. 2013;8(2):e56763. doi: 10.1371/journal.pone.0056763. https://doi.org/10.1371/journal.pone.0056763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall Z, Bond NJ, Ashmore T, Sanders F, Ament Z, Wang X, … Griffin JL. Lipid zonation and phospholipid remodeling in nonalcoholic fatty liver disease. Hepatology. 2017;65(4):1165–1180. doi: 10.1002/hep.28953. https://doi.org/10.1002/hep.28953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall Z, Bond NJ, Ashmore T, Sanders F, Ament Z, … Wang X, et al. Lipid zonation and phospholipid remodeling in non-alcoholic fatty liver disease. Hepatology. 2016;65(4):1165–1180. doi: 10.1002/hep.28953. Retrieved from http://onlinelibrary.wiley.com/doi/10.1002/hep.28953/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S. The natural history of nonalcoholic fatty liver disease: a clinical histopathological study. The American Journal of Gastroenterology. 2003;98(9):2042–2047. doi: 10.1111/j.1572-0241.2003.07659.x. https://doi.org/10.1016/S0002-9270(03)00622-1. [DOI] [PubMed] [Google Scholar]
- Haukeland JW, Konopski Z, Eggesbø HB, von Volkmann HL, Raschpichler G, Bjøro K, … Birkeland K. Metformin in patients with non-alcoholic fatty liver disease: A randomized, controlled trial. Scandinavian Journal of Gastroenterology. 2009;44(7):853–860. doi: 10.1080/00365520902845268. https://doi.org/10.1080/00365520902845268. [DOI] [PubMed] [Google Scholar]
- He B, Wu L, Xie W, Shao Y, Jiang J, Zhao Z, … Cui D. The imbalance of Th17/Treg cells is involved in the progression of nonalcoholic fatty liver disease in mice. BMC Immunology. 2017;18(1) doi: 10.1186/s12865-017-0215-y. https://doi.org/10.1186/s12865-017-0215-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heier EC, Meier A, Julich-Haertel H, Djudjaj S, Rau M, Tschernig T, … Lukacs-Kornek V. Murine CD103+ dendritic cells protect against steatosis progression towards steatohepatitis. Journal of Hepatology. 2017;66(6):1241–1250. doi: 10.1016/j.jhep.2017.01.008. Retrieved from http://www.sciencedirect.com/science/article/pii/S0168827817300120. [DOI] [PubMed] [Google Scholar]
- Arai Hirofumi, Nagao Akihiko, Takama Kozo. Effect of d-cz-Tocopherol Analogues on Lipoxygenase-Dependent Peroxidation of Phospholipid-Bile Salt Micelles. Lipids. 1995;30(2):135–140. doi: 10.1007/BF02538266. [DOI] [PubMed] [Google Scholar]
- Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal TK, Maeda K, … Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420(21):333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- Huang MA, Greenson JK, Chao C, Anderson L, Peterman D, Jacobson J, … Conjeevaram HS. One-year intense nutritional counseling results in histological improvement in patients with non-alcoholic steatohepatitis: a pilot study. The American Journal of Gastroenterology. 2005;100(5):1072–1081. doi: 10.1111/j.1572-0241.2005.41334.x. https://doi.org/10.1111/j.1572-0241.2005.41334.x. [DOI] [PubMed] [Google Scholar]
- Hummel KP, Dickie MM, Coleman DL. Diabetes, a new mutation in the mouse. Science (New York, NY) 1966;153(3740):1127–1128. doi: 10.1126/science.153.3740.1127. [DOI] [PubMed] [Google Scholar]
- Huo Y, Zhao L, Hyman MC, Shashkin P, Harry BL, Burcin T, … Ley K. Critical Role of Macrophage 12/15-Lipoxygenase for Atherosclerosis in Apolipoprotein E-Deficient Mice. Circulation. 2004;110(14):2024–2031. doi: 10.1161/01.CIR.0000143628.37680.F6. https://doi.org/10.1161/01.CIR.0000143628.37680.F6. [DOI] [PubMed] [Google Scholar]
- Ingalls A, Dickie M, Snell G. Obese, a new mutation in the house mouse. Journal of Heredity. 1950;41(12):317–318. doi: 10.1093/oxfordjournals.jhered.a106073. [DOI] [PubMed] [Google Scholar]
- Iozzo P, Bucci M, Roivainen A, Någren K, Järvisalo MJ, Kiss J, … Nuutila P. Fatty Acid Metabolism in the Liver, Measured by Positron Emission Tomography, Is Increased in Obese Individuals. Gastroenterology. 2010;139(3):846–856. doi: 10.1053/j.gastro.2010.05.039. https://doi.org/10.1053/j.gastro.2010.05.039. [DOI] [PubMed] [Google Scholar]
- Jegatheesan P, De Bandt J-P. Fructose and NAFLD: The Multifaceted Aspects of Fructose Metabolism. Nutrients. 2017;9(3) https://doi.org/10.3390/nu9030230. [Google Scholar]
- Kenyon V, Rai G, Jadhav A, Schultz L, Armstrong M, Jameson JB, … Holman TR. Discovery of potent and selective inhibitors of human platelet-type 12- lipoxygenase. Journal of Medicinal Chemistry. 2011;54(15):5485–5497. doi: 10.1021/jm2005089. https://doi.org/10.1021/jm2005089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiffin R, Christian C, Knecht E, Cuervo AM. Activation of chaperone-mediated autophagy during oxidative stress. Molecular Biology of the Cell. 2004;15(11):4829–4840. doi: 10.1091/mbc.E04-06-0477. Retrieved from http://www.molbiolcell.org/content/15/11/4829.short. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein EA, Thompson IM, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, … Baker LH. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2011;306(14):1549–1556. doi: 10.1001/jama.2011.1437. https://doi.org/10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli R, Kirby M, Xanthakos SA, Softic S, Feldstein AE, Saxena V, … Seeley RJ. High-fructose, medium chain trans fat diet induces liver fibrosis and elevates plasma coenzyme Q9 in a novel murine model of obesity and nonalcoholic steatohepatitis. Hepatology. 2010;52(3):934–944. doi: 10.1002/hep.23797. https://doi.org/10.1002/hep.23797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, … Chiba T. Impairment of starvation-induced and constitutive autophagy in Atg7 -deficient mice. The Journal of Cell Biology. 2005;169(3):425–434. doi: 10.1083/jcb.200412022. https://doi.org/10.1083/jcb.200412022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, … Rosenthal P, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305(16):1659–1668. doi: 10.1001/jama.2011.520. Retrieved from http://jamanetwork.com/journals/jama/fullarticle/899427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazic M, Inzaugarat ME, Povero D, Zhao IC, Chen M, Nalbandian M, … Sears DD. Reduced Dietary Omega-6 to Omega-3 Fatty Acid Ratio and 12/15-Lipoxygenase Deficiency Are Protective against Chronic High Fat Diet-Induced Steatohepatitis. PLoS ONE. 2014a;9(9):e107658. doi: 10.1371/journal.pone.0107658. https://doi.org/10.1371/journal.pone.0107658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazic M, Inzaugarat ME, Povero D, Zhao IC, Chen M, Nalbandian M, … Sears DD. Reduced Dietary Omega-6 to Omega-3 Fatty Acid Ratio and 12/15-Lipoxygenase Deficiency Are Protective against Chronic High Fat Diet-Induced Steatohepatitis. PLoS ONE. 2014b;9(9):e107658. doi: 10.1371/journal.pone.0107658. https://doi.org/10.1371/journal.pone.0107658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeaupin C, Proics E, de Bieville CHD, Rousseau D, Bonnafous S, Patouraux S, … Bailly-Maitre B. ER stress induces NLRP3 inflammasome activation and hepatocyte death. Cell Death and Disease. 2015;6(9):e1879. doi: 10.1038/cddis.2015.248. https://doi.org/10.1038/cddis.2015.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L, Alloosh M, Saxena R, Van Alstine W, Watkins BA, Klaunig JE, … Chalasani N. Nutritional model of steatohepatitis and metabolic syndrome in the Ossabaw miniature swine. Hepatology. 2009;50(1):56–67. doi: 10.1002/hep.22904. https://doi.org/10.1002/hep.22904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite NC, Salles GF, Araujo AL, Villela-Nogueira CA, Cardoso CR. Prevalence and associated factors of non-alcoholic fatty liver disease in patients with type-2 diabetesmellitus. Liver International. 2009;29(1):113–119. doi: 10.1111/j.1478-3231.2008.01718.x. [DOI] [PubMed] [Google Scholar]
- Liao X, Sluimer JC, Wang Y, Subramanian M, Brown K, Pattison JS, … Tabas I. Macrophage Autophagy Plays a Protective Role in Advanced Atherosclerosis. Cell Metabolism. 2012;15(4):545–553. doi: 10.1016/j.cmet.2012.01.022. https://doi.org/10.1016/j.cmet.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieb DC, Brotman JJ, Hatcher MA, Aye MS, Cole BK, Haynes BA, … Dobrian AD. Adipose Tissue 12/15 Lipoxygenase Pathway in Human Obesity and Diabetes. The Journal of Clinical Endocrinology & Metabolism. 2014;99(9):E1713–E1720. doi: 10.1210/jc.2013-4461. https://doi.org/10.1210/jc.2013-4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomba R, Abraham M, Unalp A, Wilson L, Lavine J, Doo E … the Nonalcoholic Steatohepatitis Clinical Research Network. Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology. 2012;56(3):943–951. doi: 10.1002/hep.25772. https://doi.org/10.1002/hep.25772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomba R, Lutchman G, Kleiner DE, Ricks M, Feld JJ, Borg BB, … Hoofnagle JH. Clinical trial: pilot study of metformin for the treatment of non-alcoholic steatohepatitis. Alimentary Pharmacology & Therapeutics. 2009;29(2):172–182. doi: 10.1111/j.1365-2036.2008.03869.x. https://doi.org/10.1111/j.1365-2036.2008.03869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Parra M, Titos E, Horrillo R, Ferre N, Gonzalez-Periz A, Martinez-Clemente M, … Claria J. Regulatory effects of arachidonate 5-lipoxygenase on hepatic microsomal TG transfer protein activity and VLDL-triglyceride and apoB secretion in obese mice. The Journal of Lipid Research. 2008;49(12):2513–2523. doi: 10.1194/jlr.M800101-JLR200. https://doi.org/10.1194/jlr.M800101-JLR200. [DOI] [PubMed] [Google Scholar]
- Lu SC, Alvarez L, Huang ZZ, Chen L, An W, Corrales FJ, … Mato JM. Methionine adenosyltransferase 1A knockout mice are predisposed to liver injury and exhibit increased expression of genes involved in proliferation. Proceedings of the National Academy of Sciences USA. 2001;98(10):5560–5565. doi: 10.1073/pnas.091016398. Retrieved from http://www.pnas.org/content/98/10/5560.short. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luci D, Jameson JB, Yasgar A, Diaz G, Joshi N, Kantz A, … Maloney DJ. Probe Reports from the NIH Molecular Libraries Program. Bethesda (MD): National Center for Biotechnology Information (US); 2010. Discovery of ML355, a Potent and Selective Inhibitor of Human 12-Lipoxygenase. Retrieved from http://www.ncbi.nlm.nih.gov/books/NBK259188/ [PubMed] [Google Scholar]
- Luci DK, Jameson JB, Yasgar A, Diaz G, Joshi N, Kantz A, … Maloney DJ. Synthesis and structure-activity relationship studies of 4-((2-hydroxy-3-methoxybenzyl)amino)benzenesulfonamide derivatives as potent and selective inhibitors of 12-lipoxygenase. Journal of Medicinal Chemistry. 2014;57(2):495–506. doi: 10.1021/jm4016476. https://doi.org/10.1021/jm4016476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig J, McGill DB, Lindor KD. Review: nonalcoholic steatohepatitis. Journal of Gastroenterology and Hepatology. 1997;12(5):398–403. doi: 10.1111/j.1440-1746.1997.tb00450.x. Retrieved from http://onlinelibrary.wiley.com/doi/10.1111/j.1440-1746.1997.tb00450.x/abstract. [DOI] [PubMed] [Google Scholar]
- Ma K, Nunemaker CS, Wu R, Chakrabarti SK, Taylor-Fishwick DA, Nadler JL. 12-Lipoxygenase Products Reduce Insulin Secretion and β-Cell Viability in Human Islets. The Journal of Clinical Endocrinology & Metabolism. 2010;95(2):887–893. doi: 10.1210/jc.2009-1102. https://doi.org/10.1210/jc.2009-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma K, Park SH, Lindsey G, Laura J, Tatvam B, Weaver JR, … Nadler JL. 12-lipoxygenase inhibitor improves functions of cytokine-treated human islets and type 2 diabetic islets. The Journal of Clinical Endocrinology and Metabolism. 2017 doi: 10.1210/jc.2017-00267. In Press. https://doi.org/10.1210/jc.2017-00267. [DOI] [PMC free article] [PubMed]
- Martin LR, Williams SL, Haskard KB, DiMatteo MR. The challenge of patient adherence. Therapeutics and Clinical Risk Management. 2005;1(3):189–199. Retrieved from http://core.ac.uk/download/pdf/4131473.pdf. [PMC free article] [PubMed] [Google Scholar]
- Martínez-Clemente M, Clària J, Titos E. The 5-lipoxygenase/leukotriene pathway in obesity, insulin resistance, and fatty liver disease. Current Opinion in Clinical Nutrition and Metabolic Care. 2011;14(4):347–353. doi: 10.1097/MCO.0b013e32834777fa. https://doi.org/10.1097/MCO.0b013e32834777fa. [DOI] [PubMed] [Google Scholar]
- Matteoni C, Younossi Z, Gramlich T, Boparai N, Liu Y, Mccullough A. Nonalcoholic fatty liver disease: A spectrum of clinical and pathological severity. Gastroenterology. 1999;116(6):1413–1419. doi: 10.1016/s0016-5085(99)70506-8. https://doi.org/10.1016/S0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- Middleton MK, Rubinstein T, Pure E. Cellular and Molecular Mechanisms of the Selective Regulation of IL-12 Production by 12/15-Lipoxygenase. The Journal of Immunology. 2006;176(1):265–274. doi: 10.4049/jimmunol.176.1.265. https://doi.org/10.4049/jimmunol.176.1.265. [DOI] [PubMed] [Google Scholar]
- Miller ER, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Annals of Internal Medicine. 2005;142(1):37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- Mishra A, Younossi ZM. Epidemiology and Natural History of Non-alcoholic Fatty Liver Disease. Journal of Clinical and Experimental Hepatology. 2012;2(2):135–144. doi: 10.1016/S0973-6883(12)60102-9. https://doi.org/10.1016/S0973-6883(12)60102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair S, Diehl AM, Wiseman M, Farr GH, Perrillo RP. Metformin in the treatment of non-alcoholic steatohepatitis: a pilot open label trial. Alimentary Pharmacology and Therapeutics. 2004;20(1):23–28. doi: 10.1111/j.1365-2036.2004.02025.x. https://doi.org/10.1111/j.1365-2036.2004.02025.x. [DOI] [PubMed] [Google Scholar]
- Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37(5):1202–1219. doi: 10.1053/jhep.2003.50193. https://doi.org/10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF … NASH Clinical Research Network. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385(9972):956–965. doi: 10.1016/S0140-6736(14)61933-4. https://doi.org/10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunemaker CS, Chen M, Pei H, Kimble SD, Keller SR, Carter JD, … Nadler JL. 12-Lipoxygenase-knockout mice are resistant to inflammatory effects of obesity induced by western diet. AJP: Endocrinology and Metabolism. 2008;295(5):E1065–E1075. doi: 10.1152/ajpendo.90371.2008. https://doi.org/10.1152/ajpendo.90371.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oz HS, Chen TS, Neuman M. Methionine deficiency and hepatic injury in a dietary steatohepatitis model. Digestive Diseases and Sciences. 2008;53(3):767–776. doi: 10.1007/s10620-007-9900-7. Retrieved from http://link.springer.com/article/10.1007/s10620-007-9900-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Carreras M, Del Hoyo P, Martín MA, Rubio JC, Martín A, Castellano G, … Solis-Herruzo JA. Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis. Hepatology. 2003;38(4):999–1007. doi: 10.1053/jhep.2003.50398. https://doi.org/10.1053/jhep.2003.50398. [DOI] [PubMed] [Google Scholar]
- Popov V. Treatment of Nonalcoholic Fatty Liver Disease: The Role of Medical, Surgical, and Endoscopic Weight Loss. Journal of Clinical and Translational Hepatology. 2015;3(3):230–238. doi: 10.14218/JCTH.2015.00019. https://doi.org/10.14218/JCTH.2015.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell WS, Rokach J. Biosynthesis, biological effects, and receptors of hydroxyeicosatetraenoic acids (HETEs) and oxoeicosatetraenoic acids (oxo-ETEs) derived from arachidonic acid. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2015;1851(4):340–355. doi: 10.1016/j.bbalip.2014.10.008. https://doi.org/10.1016/j.bbalip.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prashanth M, Ganesh H, Vima M, John M, Bandgar T, Joshi SR, … Shah N. Prevalence of Nonalcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Mellitus. JAPI. 2009;57:205–210. [PubMed] [Google Scholar]
- Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, … Wing RR. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51(1):121–129. doi: 10.1002/hep.23276. https://doi.org/10.1002/hep.23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri P, Wiest MM, Cheung O, Mirshahi F, Sargeant C, … Min HK, et al. The plasma lipidomic signature of nonalcoholic steatohepatitis. Hepatology. 2009;50(6):1827–1838. doi: 10.1002/hep.23229. Retrieved from http://onlinelibrary.wiley.com/doi/10.1002/hep.23229/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel VT, Shulman GI. Mechanisms for Insulin Resistance: Common Threads and Missing Links. Cell. 2012;148(5):852–871. doi: 10.1016/j.cell.2012.02.017. https://doi.org/10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal AJ, Banas C, Sargeant C, Luketic VA, Sterling RK, Stravitz RT, … Mills AS. Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology. 2006;43(4):682–689. doi: 10.1002/hep.21103. https://doi.org/10.1002/hep.21103. [DOI] [PubMed] [Google Scholar]
- Sanyal AJ, Campbell–Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, … Clore JN. Nonalcoholic steatohepatitis: Association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120(5):1183–1192. doi: 10.1053/gast.2001.23256. https://doi.org/10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM … NASH CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. The New England Journal of Medicine. 2010;362(18):1675–1685. doi: 10.1056/NEJMoa0907929. https://doi.org/10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schierwagen R, Maybüchen L, Zimmer S, Hittatiya K, Bäck C, Klein S, … Trebicka J. Seven weeks of Western diet in apolipoprotein-E-deficient mice induce metabolic syndrome and non-alcoholic steatohepatitis with liver fibrosis. Scientific Reports. 2015;5(1) doi: 10.1038/srep12931. Article number 12931. https://doi.org/10.1038/srep12931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel A. Studying non-alcoholic fatty liver disease with zebrafish: a confluence of optics, genetics, and physiology. Cellular and Molecular Life Sciences. 2012;69(23):3953–3961. doi: 10.1007/s00018-012-1037-y. https://doi.org/10.1007/s00018-012-1037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears DD, Miles PD, Chapman J, Ofrecio JM, Almazan F, Thapar D, Miller YI. 12/15-Lipoxygenase Is Required for the Early Onset of High Fat Diet-Induced Adipose Tissue Inflammation and Insulin Resistance in Mice. PLoS ONE. 2009;4(9):e7250. doi: 10.1371/journal.pone.0007250. https://doi.org/10.1371/journal.pone.0007250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki S, Kitada T, Yamada T, Sakaguchi H, Nakatani K, Wakasa K. In situ detection of lipid peroxidation and oxidative DNA damage in non-alcoholic fatty liver diseases. Journal of Hepatology. 2002;37(1):56–62. doi: 10.1016/s0168-8278(02)00073-9. [DOI] [PubMed] [Google Scholar]
- Shimano H, Horton JD, Hammer RE, Shimomura I, Brown MS, Goldstein JL. Overproduction of cholesterol and fatty acids causes massive liver enlargement in transgenic mice expressing truncated SREBP-1a. Journal of Clinical Investigation. 1996;98(7):1575–1584. doi: 10.1172/JCI118951. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC507590/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, … Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009;458(7242):1131–1135. doi: 10.1038/nature07976. https://doi.org/10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaulding L, Trainer T, Janiec D. Prevalence of non-alcoholic steatohepatitis in morbidly obese subjects undergoing gastric bypass. Obesity Surgery. 2003;13(3):347–349. doi: 10.1381/096089203765887633. https://doi.org/10.1381/096089203765887633. [DOI] [PubMed] [Google Scholar]
- St George A, Bauman A, Johnston A, Farrell G, Chey T, George J. Independent effects of physical activity in patients with nonalcoholic fatty liver disease. Hepatology. 2009;50(1):68–76. doi: 10.1002/hep.22940. https://doi.org/10.1002/hep.22940. [DOI] [PubMed] [Google Scholar]
- Suciu M, Gruia AT, Nica DV, Azghadi SM, Mic AA, Mic FA. Data on expression of lipoxygenases-5 and-12 in the normal and acetaminophen-damaged liver. Data in Brief. 2016;7:1199–1203. doi: 10.1016/j.dib.2016.03.079. Retrieved from http://www.sciencedirect.com/science/article/pii/S2352340916301871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumida Y, Niki E, Naito Y, Yoshikawa T. Involvement of free radicals and oxidative stress in NAFLD/NASH. Free Radical Research. 2013;47(11):869–880. doi: 10.3109/10715762.2013.837577. https://doi.org/10.3109/10715762.2013.837577. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Kayama Y, Sakamoto M, Iuchi H, Shimizu I, Yoshino T, … Utsunomiya K. Arachidonate 12/15-Lipoxygenase–Induced Inflammation and Oxidative Stress Are Involved in the Development of Diabetic Cardiomyopathy. Diabetes. 2015;64(2):618–630. doi: 10.2337/db13-1896. https://doi.org/10.2337/db13-1896. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Matsubara T, Krausz KW, Patterson AD, Gonzalez FJ. Disruption of phospholipid and bile acid homeostasis in mice with nonalcoholic steatohepatitis. Hepatology. 2012;56(1):118–129. doi: 10.1002/hep.25630. Retrieved from http://onlinelibrary.wiley.com/doi/10.1002/hep.25630/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tersey SA, Bolanis E, Holman TR, Maloney DJ, Nadler JL, Mirmira RG. Minireview: 12-Lipoxygenase and Islet β-Cell Dysfunction in Diabetes. Molecular Endocrinology. 2015;29(6):791–800. doi: 10.1210/me.2015-1041. https://doi.org/10.1210/me.2015-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tersey SA, Maier B, Nishiki Y, Maganti AV, Nadler JL, Mirmira RG. 12-Lipoxygenase Promotes Obesity-Induced Oxidative Stress in Pancreatic Islets. Molecular and Cellular Biology. 2014;34(19):3735–3745. doi: 10.1128/MCB.00157-14. https://doi.org/10.1128/MCB.00157-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetri LH, Basaranoglu M, Brunt EM, Yerian LM, Neuschwander-Tetri BA. Severe NAFLD with hepatic necroinflammatory changes in mice fed trans fats and a high-fructose corn syrup equivalent. AJP: Gastrointestinal and Liver Physiology. 2008;295(5):G987–G995. doi: 10.1152/ajpgi.90272.2008. https://doi.org/10.1152/ajpgi.90272.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, … Romero-Gomez M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology. 2015;149(2):367–378. e5. doi: 10.1053/j.gastro.2015.04.005. https://doi.org/10.1053/j.gastro.2015.04.005. [DOI] [PubMed] [Google Scholar]
- Walker CM, Lemon SM. Getting the Skinny on CD4 + T Cell Survival in Fatty Livers. Immunity. 2016;44(4):725–727. doi: 10.1016/j.immuni.2016.04.001. https://doi.org/10.1016/j.immuni.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y, Gu J, Chakrabarti SK, Aylor K, Marshall J, Takahashi Y, … Nadler JL. The Role of 12/15-Lipoxygenase in the Expression of Interleukin-6 and Tumor Necrosis Factor-α in Macrophages. Endocrinology. 2007;148(3):1313–1322. doi: 10.1210/en.2006-0665. https://doi.org/10.1210/en.2006-0665. [DOI] [PubMed] [Google Scholar]
- Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, … Harrison SA. Prevalence of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis Among a Largely Middle-Aged Population Utilizing Ultrasound and Liver Biopsy: A Prospective Study. Gastroenterology. 2011;140(1):124–131. doi: 10.1053/j.gastro.2010.09.038. https://doi.org/10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- Yang SQ, Lin HZ, Lane MD, Clemens M, Diehl AM. Obesity increases sensitivity to endotoxin liver injury: implications for the pathogenesis of steatohepatitis. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(6):2557–2562. doi: 10.1073/pnas.94.6.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zein CO, Lopez R, Fu X, Kirwan JP, Yerian LM, McCullough AJ, … Feldstein AE. Pentoxifylline decreases oxidized lipid products in nonalcoholic steatohepatitis: New evidence on the potential therapeutic mechanism. Hepatology. 2012;56(4):1291–1299. doi: 10.1002/hep.25778. https://doi.org/10.1002/hep.25778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454(7203):455–462. doi: 10.1038/nature07203. https://doi.org/10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. https://doi.org/10.1038/372425a0. [DOI] [PubMed] [Google Scholar]