Abstract
AIM
To investigate the factors affecting diagnostic delay and outcomes of diagnostic delay in inflammatory bowel disease (IBD)
METHODS
We retrospectively studied 165 patients with Crohn’s disease (CD) and 130 patients with ulcerative colitis (UC) who were diagnosed and had follow up durations > 6 mo at Korea University Ansan Hospital from January 2000 to December 2015. A diagnostic delay was defined as the time interval between the first symptom onset and IBD diagnosis in which the 76th to 100th percentiles of patients were diagnosed.
RESULTS
The median diagnostic time interval was 6.2 and 2.4 mo in the patients with CD and UC, respectively. Among the initial symptoms, perianal discomfort before di-agnosis (OR = 10.2, 95%CI: 1.93-54.3, P = 0.006) was associated with diagnostic delays in patients with CD; however, no clinical factor was associated with diagnostic delays in patients with UC. Diagnostic delays, stricturing type, and penetrating type were associated with increased intestinal surgery risks in CD (OR = 2.54, 95%CI: 1.06-6.09; OR = 4.44, 95%CI: 1.67-11.8; OR = 3.79, 95%CI: 1.14-12.6, respectively). In UC, a diagnostic delay was the only factor associated increased intestinal surgery risks (OR = 6.81, 95%CI: 1.12-41.4).
CONCLUSION
A diagnostic delay was associated with poor outcomes, such as increased intestinal surgery risks in patients with CD and UC.
Keywords: Diagnostic delay, Intestinal surgery, Inflammatory bowel disease, Crohn’s disease, Ulcerative colitis
Core tip: As the manifestations of inflammatory bowel disease (IBD) were nonspecific, the diagnosis is often established following considerable delay. There have been few reports about diagnostic delay associated with poor outcomes in Asian IBD patients. We aimed to investigate the factor affecting diagnostic delay and its effect in Korean IBD patients. In present study, a diagnostic delay was significantly associated with poor outcomes, such as increased IBD related intestinal surgery risks in patients with Crohn’s disease and ulcerative colitis. Therefore, it is important for the improvement of clinical outcomes in IBD patients to early diagnose and manage adequately.
INTRODUCTION
Inflammatory bowel disease (IBD) represented by Crohn’s disease (CD) and ulcerative colitis (UC) is characterized by chronic inflammation in all or part of digestive tract without definite cause[1]. Persisting and relapsing inflammation causes not only functional dysfunction in the gut, but also structural destruction of bowel such as stenosis, fistula formation and perforation[2]. Recent studies have reported that an early intensive control of inflammation with immunosuppressive agents or biological agents improved the prognosis of newly diagnosed patients with IBD, which emphasized the importance of early treatment following early diagnosis[3-5].
IBD has a waxing and waning course with as-ymptomatic remission period and with episodes of di-sease where patients present with symptoms, such as hematochezia, fever, and abdominal pain[6]. Because symptoms were not specific and not constant over time and the findings of diagnostic studies overlapped with those of other disease, such as tuberculosis and connective tissue disease, the diagnosis is often established following a considerable delay[7-9]. Indeed, in the Swiss IBD cohort study, the median duration of diagnostic delay period in CD patients was 9 mo, and about 25% of them had a duration of > 24 mo from symptom to diagnosis[9]. The median diagnostic delay period in patients with UC was 4 mo, which was shorter than that in patients with CD. Further, in the French CD cohort with 364 patients, about 40% of patients had a duration of > 12 mo from symptom to diagnosis[10]. A diagnostic delay was associated with poor clinical outcomes in patients with CD, such as an increased risk of bowel stenosis, fistula and abscess formation, and intestinal surgery[11,12]. However, no study has reported the association between the clinical outcomes and diagnostic delays in patients with UC.
Unlike in Western countries, there are few st-udies on diagnostic delays associated with IBD in Asia. Considering the significant differences in the epidemiological and clinical features of IBD according to ethnicities and environmental factors, diagnostic delays and the associated factors may differ according to countries[13,14]. Therefore, we aimed to investigate the clinical factors and outcomes associated with diagnostic delays in Korean patients with CD and UC.
MATERIALS AND METHODS
Subjects
This study included 177 patients with CD and 143 patients with UC who were definitely diagnosed at Korea University Ansan Hospital from January 1, 2000 to December 31, 2015. The CD and UC diagnoses were confirmed based on the clinical, endoscopic, radiological and histological findings. The patients younger than 18 years at the time of diagnosis, those who had a follow-up duration of < 6 mo, and those who had incomplete medical records were excluded from this study. In the present study, 25 patients with IBD were excluded because they were under 18 years old (CD 3), had insufficient medical records (CD 3, UC 7), or had a follow up duration time of < 6 mo (CD 5, UC 6). The remaining 165 patients with CD and 130 patients with UC were analyzed in the present study
This study was approved by the Institutional Review Board of Korea University Ansan Hospital (AS16206) and conducted in accordance with the Declaration of Helsinki. The need for informed consent was waived in view of the retrospective observational design of the study.
Methods
The clinical and demographic data such as age at diagnosis, sex, smoking status, the first IBD related symptom, family history of IBD and prescribed medications were collected from each patient’s medical records.
The diagnostic time interval was the duration from the first symptom to the diagnosis of IBD. This di-agnostic time interval included patient-dependent delay (time interval from first symptoms to the physician visit) and physician-dependent delay (time interval from first physician visit to IBD diagnosis). Because there are no established criteria to define diagnostic delays, it was defined based on the time interval in which the 76th to 100th percentile of the patients were diagnosed. Follow-up duration was defined as the time from the date of first diagnosis of IBD to the date of the last follow up.
Disease extent and behavior at the initial diagnosis in CD and UC were classified in accordance with the Montreal classification[15]. Disease severity was evaluated using the C-reactive protein level at diagnosis. In UC, the Mayo score was additionally used for severity assessment. Data on the prescribed medications for IBD, including 5-aminosalicylic acid, systemic corticosteroids, immunomodulator and anti-tumor necrosis factor alpha (anti-TNFα) agents were also obtained from medical records.
To assess the clinical outcomes associated with a diagnostic delay, the following outcomes were measured: the number and type of intra-abdominal surgery, number and type of any surgery including perianal surgical procedures, number and date of hospitalization, and time duration from diagnosis to immunomodulator or anti-TNFα administration. Frequent admission was defined as two or more hospitalizations during the follow-up period.
Statistical analysis
The values for the continuous variables are expressed as mean ± (SD) or medians ± (SE). Categorical or discrete variables are presented as percentages. The groups were compared using the Student t-test or χ2 test. Logistic regression was used to calculate the odds ratios (ORs) with 95% (CIs) for evaluating the risk factors for a long diagnostic delay. The covariates used in our multivariate analyses included variables with a significant result on the univariate analysis in addition to variables associated with diagnostic delays in previous studies and clinical experience. The Kaplan-Meier method was used to evaluate the association between diagnostic delays and clinical outcomes, such as IBD-related surgery and admission. The difference between the groups according to diagnostic time interval was analyzed using the log-rank test. The Cox proportional hazard regression analysis was performed to evaluate the association of long diagnostic delays with the clinical outcomes; the result was expressed as hazards ratio with 95%CI. The statistical methods of this study were reviewed by biostatistician from Korea University Ansan Hospital. And all statistical analyses were performed using the Statistical Package for the Social Science statistical software (version 18; SPSS-IBM, Chicago, IL, United States) or R version 3.02 (R Foundation for Statistical Computing, Vienna, Austria). All P < 0.05 was considered statistically significant.
RESULTS
The mean age at diagnosis was 28.2 ± 13.8 years in patients with CD and 38.8 ± 15.6 years in patients with UC. Male sex was predominant in both patients with CD and UC. Abdominal pain (51.5%) was the most prevalent symptom among the chief complaints in patients with CD and hematochezia (66.9%) in patients with UC. Regarding the disease location at diagnosis, the ileocolic area (L3, 51.5%) was the most common in CD and proctitis (40.3%) was the most prevalent in UC. The median diagnostic time interval was 6.2 mo in the patients with CD and 2.4 mo in the patients with UC. In the present study, the diagnostic time interval of ≥ 21.4 mo and 6.2 mo were defined as long diagnostic delays in CD and UC, respectively, based on the highest quartile cut off limit.
Tables 1 and 2 showed the baseline characteristics of the patients with CD and UC stratified by presence of a long diagnostic delay. Although age, sex, smoking status, IBD family history, and disease location were not different between the diagnostic delayed group and non-delayed group, the disease behavior type was significantly different; the stenosis type was especially more prevalent in the CD delayed group (P = 0.021). However, there was no significant baseline characteristic difference, except for the follow-up duration in UC.
Table 1.
Baseline characteristics of Crohn’s disease patients n (%)
| Total (n = 165) | Delayed (n = 41) | Non-delayed (n = 124) | P value | |
| Age, yr (± SD) | 28.2 ( ± 1.1) | 29.2 ( ± 12.7) | 27.9 ( ± 14.2) | 0.269 |
| Age ≥ 40 | 27 (16.4) | 5 (12.2) | 22 (17.7) | 0.405 |
| Male, | 126 (76.4) | 33 (80.5) | 93 (75.0) | 0.473 |
| Family history, | 6 (3.6) | 0 (0) | 6 (4.8) | 0.249 |
| Symptom to diagnosis1, d | 185 (45.0-642.5) | 1108 (810-2331) | 79.5 (32.0-253.5) | 0.000 |
| Symptom to visit1, d | 57.0 (14.0-255.8) | 739 (98.8-996) | 33.5 (10.8-109.8) | 0.000 |
| Visit to diagnosis1, d | 20.0 (4.0-139.0) | 150 (5.8-1188) | 14.0 (4.0-85.0) | 0.002 |
| Smoking, | 41 (24.8) | 12 (29.3) | 29 (23.4) | 0.450 |
| Chief complain | 0.241 | |||
| Diarrhea | 29 (17.6) | 4 (9.8) | 25 (20.2) | |
| GI bleeding | 20 (12.1) | 4 (9.8) | 16 (12.9) | |
| Perianal discomfort | 25 (15.2) | 10 (24.4) | 15 (12.1) | |
| Abdominal pain | 85 (51.5) | 22 (53.7) | 63 (50.8) | |
| Others | 6 (3.6) | 1 (2.4) | 5 (4.0) | |
| Location, | 0.688 | |||
| L1 | 39 (23.6) | 12 (29.3) | 27 (21.8) | |
| L2 | 18 (10.9) | 3 (7.3) | 15 (12.1) | |
| L3 | 85 (51.5) | 20 (48.8) | 65 (52.4) | |
| L4 | 23 (13.9) | 6 (14.6) | 17 (13.7) | |
| Behavior, | 0.021 | |||
| B1 | 116 (70.3) | 23 (56.1) | 93 (75.0) | |
| B2 | 32 (19.4) | 14 (34.1) | 18 (14.5) | |
| B3 | 17 (10.3) | 4 (9.8) | 13 (10.5) | |
| Perianal disease | 31 (18.8) | 5 (12.2) | 26 (21.0) | 0.213 |
| CRP at diagnosis, (± SD) | 4.17 (± 7.76) | 3.73 (± 4.35) | 4.33 (± 8.68) | 0.677 |
The duration was expressed as median (interquartile range); CRP: C-reactive protein.
Table 2.
Baseline characteristics of ulcerative colitis patients n (%)
| Total (n = 130) | Delayed (n = 32) | Non-delayed (n = 98) | P value | |
| Age at diagnosis (± SD) | 38.9 ± 15.5 | 36.9 ± 13.2 | 39.6 ± 16.1 | 0.395 |
| Male, | 71 (54.6) | 17 (53.1) | 54 (55.1) | 0.845 |
| Family history | 7 (5.4) | 2 (6.3) | 5 (5.1) | 0.803 |
| Symptom to diagnosis1, d | 73.0 (35.0-186.5) | 635 (360.5-1219) | 50.5 (31.0-90.0) | 0.000 |
| Symptom to visit1, d | 57.5 | 409.5 | 37.0 | 0.000 |
| (27.0-147.8) | (197.3-1183) | (15.8-70.0) | ||
| Visit to diagnosis1, d | 7.00 (3.0-17.0) | 10.0 (3.0-28.5) | 6.00 (3.0-16.3) | 0.215 |
| Smoking | 26 (20.2) | 6 (18.8) | 20 (20.6) | 0.819 |
| Chief complaints | 0.55 | |||
| Hematochezia | 87 (66.9) | 20 (62.5) | 67 (68.4) | |
| Diarrhea | 31 (23.8) | 10 (31.3) | 21 (21.4) | |
| Abdominal pain | 9 (6.9) | 2 (6.3) | 7 (7.1) | |
| Others | 3 (2.3) | 0 (0) | 3 (3.1) | |
| Location | 0.594 | |||
| Proctitis | 52 (40.3) | 12 (38.7) | 40 (40.8) | |
| Left sided | 37 (28.7) | 11 (35.5) | 26 (26.5) | |
| Pancolitis | 40 (31.0) | 8 (25.8) | 32 (32.7) | |
| Mayo score at diagnosis | 5.60 ± 1.98 | 5.38 ± 1.95 | 5.67 ± 1.99 | 0.460 |
| Severity2 | 0.748 | |||
| Remission (0-2) | 2 (1.5) | 1 (3.1) | 1 (1.0) | |
| Mild (3-5) | 53 (40.8) | 14 (43.8) | 39 (39.8) | |
| Moderate (6-10) | 74 (56.9) | 17 (53.1) | 57 (58.2) | |
| Severe (11-12) | 1 (0.8) | 0 (0) | 1 (1.0) | |
| CRP at diagnosis, (± SD) | 1.82 ± 3.88 | 1.85 ± 3.96 | 1.82 ± 3.88 | 0.970 |
The duration was expressed as median (interquartile range);
The severity was classified according to the Mayo score.
The factors associated with a diagnostic delay in the patients with CD and UC are summarized in Tables 3 and 4. In the patients with CD, perianal discomfort was the only clinical factor associated with diagnostic delays (OR 10.23, 95%CI: 1.93-54.37). However, there was no clinical factor associated with significant diagnostic delays in the patients with UC.
Table 3.
Factors associated with a long diagnostic delay in Crohn’s disease
| Variable | n | OR (95%CI) | P value |
| Age | - | 1.00 (0.96-1.03) | 0.787 |
| Male | 126 | 0.72 (0.23-2.26) | 0.575 |
| Smoking | 41 | 2.26 (0.85-5.98) | 0.101 |
| Chief complaint | |||
| Diarrhea | 29 | Ref. | - |
| GI bleeding | 20 | 1.94 (0.37-10.1) | 0.432 |
| Perianal discomfort | 25 | 10.23 (1.93-54.37) | 0.006 |
| Abdominal pain | 85 | 2.20 (0.64-7.62) | 0.213 |
| Others | 6 | 1.13 (0.09-14.38) | 0.925 |
| Location | |||
| L1 | 39 | Ref. | - |
| L2 | 18 | 0.71 (0.14-3.67) | 0.684 |
| L3 | 85 | 0.77 (0.28-2.11) | 0.613 |
| L4 | 23 | 0.66 (0.18-2.43) | 0.533 |
| Behavior | |||
| B1 | 116 | Ref. | |
| B2 | 32 | 2.33 (0.90-6.04) | 0.081 |
| B3 | 17 | 0.49 (0.12-1.97) | 0.312 |
| Perianal disease | 31 | 0.28 (0.07-1.17) | 0.080 |
| CRP at diagnosis | - | 0.98 (0.91-1.05) | 0.572 |
GI: Gastrointestinal; OR: Odds ratio; CRP: C-reactive protein; CI: Confidence interval.
Table 4.
Factors associated with a long diagnostic delay in ulcerative colitis
| Variable | n | OR (95%CI) | P value |
| Age | - | 0.99 (0.96-1.02) | 0.484 |
| Male | 71 | 0.78 (0.30-2.04) | 0.607 |
| Smoking | 26 | 1.26 (0.33-4.87) | 0.733 |
| IBD family history | 7 | 1.40 (0.24-8.07) | 0.709 |
| Chief complaint | |||
| Hematochezia | 87 | Ref. | - |
| Diarrhea | 31 | 1.34 (0.49-3.65) | 0.566 |
| Abdominal pain | 9 | 1.03 (0.18-5.92) | 0.975 |
| Others | 3 | Not assessed | - |
| Location | |||
| Proctitis | 39 | Ref. | - |
| Left sided | 18 | 1.45 (0.48-4.39) | 0.513 |
| Left sided | 85 | 0.90 (0.28-2.92) | 0.860 |
| Severity1 | |||
| Mild | 53 | Ref. | |
| Moderate to severe | 75 | 0.78 (0.29-2.13) | 0.634 |
The severity was classified according to the Mayo score. IBD: Inflammatory bowel disease; OR: Odds ratio; CI: Confidence interval.
Medication history during the follow-up period in the patients with IBD is shown in Supplementary Table 1. There was no difference in the types and frequencies of prescribed medications between the diagnostic delay and non-diagnostic delay groups in patients with CD and UC. However, the median time interval from diagnosis to anti-TNFα administration was significantly shorter in the non-diagnostic delay group of the patients with CD than in the diagnostic delay group. In the patients with UC, the time interval from diagnosis to systemic steroid, immunomodulator and anti-TNFα administration was not different between groups.
After diagnosis, 28 (17.0%) patients with CD underwent intestinal surgeries due to CD related problems, such as uncontrolled internal fistula, stenosis, or abdominal abscess and 43 (26.1%) underwent any surgical treatments including perianal surgery due to perianal abscess or fistula. Among the patients with UC, 6 (4.6%) underwent intestinal surgeries due to uncontrolled inflammation and UC-related colon cancer. Figure 1 show that a long diagnostic delay was associated with an increased risk of intestinal surgery in the patients with CD and UC, which was analyzed using Kaplan-Meier method. Tables 5 and 6 show the clinical risk factors associated with intestinal or any surgery in the patients with IBD. In patients with CD, a long diagnostic delay (OR = 2.54, 95%CI: 1.06-6.09) was a significant risk factor for intestinal surgery, but not for any surgery. Further, stricturing and penetrating types were significantly associated with intestinal and any surgery. However, the diagnostic delay was the only clinical risk factor associated with intestinal surgery in UC (OR = 6.81, 95%CI: 1.12-41.4).
Figure 1.
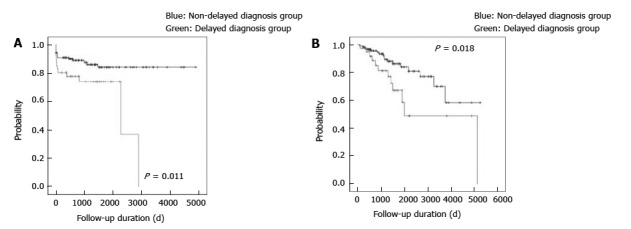
Surgery-free survival according to the presence of a long diagnostic delay in the patients with inflammatory bowel disease. A: Intestinal surgery in the patients with CD; B: Intestinal surgery in the patients with UC. IBD: Inflammatory bowel disease; CD: Crohn’s disease; UC: Ulcerative colitis.
Table 5.
Risk factors associated with Crohn’s disease related surgery
|
Intestinal surgery |
Any surgery |
|||||
| Variables | OR | 95%CI | P value | OR | 95%CI | P value |
| Age | 0.99 | 0.96-1.03 | 0.743 | 1.00 | 0.97-1.03 | 0.973 |
| Male | 0.41 | 0.14-1.22 | 0.108 | 0.94 | 0.36-2.45 | 0.902 |
| Smoking | 1.90 | 0.74-4.87 | 0.182 | 1.10 | 0.51-2.38 | 0.812 |
| Location | ||||||
| L1 | 1 (Ref.) | - | - | 1 (Ref.) | - | - |
| L2 | 1.19 | 0.32-4.50 | 0.793 | 1.74 | 0.57-5.31 | 0.332 |
| L3 | 0.53 | 0.17-1.71 | 0.291 | 0.82 | 0.32-2.09 | 0.676 |
| L4 | 1.07 | 0.29-3.97 | 0.924 | 0.91 | 0.28-2.94 | 0.878 |
| Behavior | ||||||
| B1 | 1 (Ref.) | - | - | 1 (Ref.) | - | - |
| B2 | 4.44 | 1.67-11.8 | 0.003 | 2.93 | 1.30-6.60 | 0.009 |
| B3 | 3.79 | 1.14-12.6 | 0.030 | 3.67 | 1.40-9.60 | 0.008 |
| Perianal disease | 0.97 | 0.20-4.81 | 0.968 | 1.84 | 0.69-4.87 | 0.222 |
| CRP at diagnosis | 1.02 | 0.96-1.08 | 0.520 | 1.01 | 0.96-1.06 | 0.792 |
| Delayed diagnosis | 2.54 | 1.06-6.09 | 0.036 | 1.76 | 0.87-3.57 | 0.119 |
CD: Crohn’s disease; CRP: C-reactive protein; CI: Confidence interval; OR: Odds ratio.
Table 6.
Risk factors associated with ulcerative colitis related surgery
|
Intestinal surgery |
|||
| Variables | OR | 95%CI | P value |
| Age | 1.00 | 0.93-1.07 | 0.986 |
| Male | 1.18 | 0.17-8.37 | 0.868 |
| Smoking | 0.82 | 0.05-13.8 | 0.890 |
| IBD family history | 5.39 | 0.44-66.5 | 0.189 |
| Location | |||
| Proctitis | 1 (Ref.) | - | - |
| Left sided | 0.85 | 0.10-7.24 | 0.878 |
| Pancolitis | 1.05 | 0.12-9.46 | 0.969 |
| Severity1 | |||
| Mild | 1 (Ref.) | - | - |
| Moderate to severe | 2.67 | 0.24-30.2 | 0.427 |
| Delayed diagnosis | 6.81 | 1.12-41.4 | 0.037 |
The severity was classified according to the Mayo score. UC: Ulcerative colitis; IBD: Inflammatory bowel disease; CI: Confidence interval; OR: Odds ratio.
Kaplan Meier analysis showed that the diagnostic delay group of the patients with CD had an increased risk of admission and frequent admission as shown in Figure 2. However, after adjusting for covariates, such as age and sex, a long diagnostic delay was not associated with admission and frequent admission in the patients with CD (OR = 1.60, 95%CI: 0.93-2.75 and OR 2.05; 95%CI: 0.84-5.01, respectively), as shown in Supplementary Table 2. In the patients with UC, pancolitis was the only risk factor of ad-mission (OR = 3.94; 95%CI: 1.67-9.30), as shown in supplementary Table 3. A diagnostic delay was not associated with admission and frequent admission in patients with UC (OR = 1.04; 95%CI: 0.50-2.17 and OR = 2.05; 95%CI: 0.68-6.24, respectively).
Figure 2.
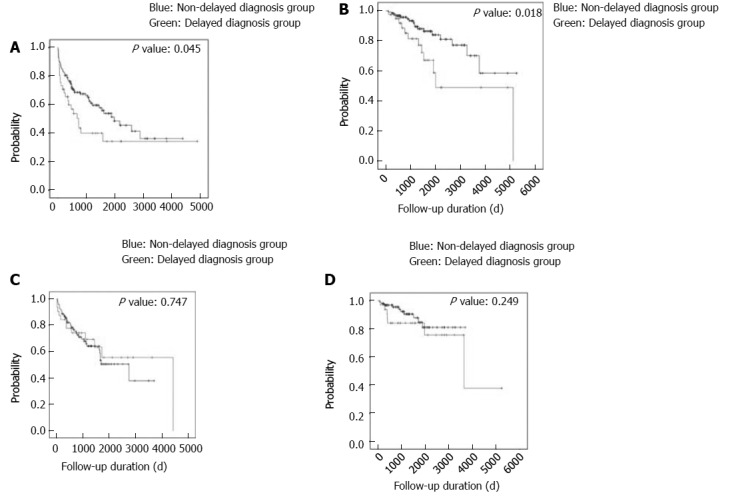
Admission-free survival according to the presence of a long diagnostic delay in the patients with inflammatory bowel disease. A: Admission in the patients with CD; B: Frequent admission in the patients with CD; C: Admission in the patients with UC; D: Frequent admission in the patients with UC. IBD: Inflammatory bowel disease; CD: Crohn’s disease; UC: Ulcerative colitis.
DISCUSSION
This study demonstrated a considerable diagnostic delay in patients with IBD, especially in patients with CD. Further, a long diagnostic delay was significantly associated with a higher risk of IBD-related intestinal surgery in both the patients with CD and UC.
In the present study, the diagnostic period for CD was longer than that for UC; this finding is consistent with previous studies[9,12]. In western IBD registries, such as the Swiss IBD cohort and the Romanian national registry, about 25% of patients with CD have a considerable diagnostic delay period of about ≥ 1.5 years. The diagnostic period of UC ranged from 1 mo to 4 mo. The significant gap in the diagnostic period between CD and UC might be because of initial symptoms[16]. Abdominal pain is the most prevalent initial chief complaint in patients with CD; however, it is also a common symptom of other digestive disorders, such as irritable bowel syndrome[17]. Fur-thermore, when the pain is not severe, it is easily regarded as trivial, and the diagnosis may be missed because the tests needed for the diagnosis of IBD are not performed. In contrast, hematochezia, which is a major symptom of UC, is not only a rare symptom but also causes great fear to the public[18]. This may cause patients to visit the hospital relatively quickly, leading to early diagnosis.
Among the initial chief complaints, perianal dis-comfort was significant clinical factor associated with long diagnostic delay in our study. The patients with perianal discomfort were significantly younger than those with other symptoms and tended to have longer duration from symptom to diagnosis (Supplementary Table 4). Interestingly, the time from the visit to the diagnosis (physician-dependent delay) was significantly longer in the patients with perianal discomfort, although there was no difference in the time from symptom to the hospital visit (patient-dependent delay) in this study. This is related to the tendency of CD patients with perianal discomfort to visit the colorectal/anus surgery clinic or general doctor’s clinic for the first time in South Korea. However, they might tend to overlook and miss the diagnosis of CD because anal disorders, such as hemorrhoids, are relatively common and IBD is rare in the East than in the West[19]. Therefore, strengthening IBD education for general doctors and general surgeons is considered to be a good way to reduce the delay of diagnosis of IBD disease, especially CD patients with perianal disease.
In our analyses, a long diagnostic delay was an independent risk factor of CD-related intestinal surgery in addition to stricturing and penetrating behavior types at diagnosis, which was similar to those of other studies[11,12,20]. Further, a long diagnostic delay in patients with UC was an independent risk factor of intestinal surgery. Considering that, there was no difference in treatments between the diagnostic delay group and the non-diagnostic delay group after the diagnosis in the present study, greater irreversible damage to the intestines might occur and accumulated, as the exposure period to the disease before the diagnosis is extended. Such damages may reduce the responsiveness to medical treatments and increase the risks of intestinal surgeries. To our knowledge, this is the first study to demonstrate the association between long diagnostic delays and poor clinical outcomes, such as an increased risk of intestinal surgery in patients with UC. This result is in part owing to the relatively long diagnostic delay compared with those of other UC diagnostic delay studies and to ethnic differences[9,12]. In present study, the duration from symptom onset to first hospital visit is significantly different between the delayed and non-delayed groups. This difference is caused by various factors such as patient’s perception, attitude toward the disease and sensitivity to symptoms, and these factors are thought to influence patient’s prognosis. However, a large multicenter study is needed to reveal the exact association between the diagnostic delay and prognosis in patients with UC.
A diagnostic delay was not directly associated with an increased risk of IBD-related admission in our analyses. However, stricturing behavior and penetrating type at diagnosis were significantly associated with admission and frequent admission in patients with CD. Among the patients with UC, those with pancolitis at diagnosis had an increased risk of admission. Considering that the disease behavior of CD tends to change from an inflammatory type to either stricturing or penetrating type and the disease extent progresses proximally from the rectum in UC, an early diagnosis might be associated with a better prognosis, such as reduced hospitalization[18,21-23].
Our study has a few limitations. First, as a limitation of retrospective study, important clinical information, such as the onset of IBD-related symptoms and first hospital visit date, may be inaccurate owing to a recall bias. Second, this study was conducted at a single center, and its results might not fully reflect the overall patients with IBD in South Korea. Therefore, a large multicenter prospective study is needed for to a better understanding of the association between the diagnostic delay and prognosis of IBD.
Our study demonstrated a considerable diagnostic delay in patients with IBD. In addition, a long diagnostic delay was significantly associated with an increased risk of IBD-related intestinal surgeries in patients with CD and UC. Based on these results, efforts should be made to reduce diagnostic delays, such as increasing awareness for physicians and the public, to improve the prognosis of patients with IBD.
COMMENTS
Background
The diagnosis of inflammatory bowel diseases (IBD) is often established following considerable delay due to nonspecific and inconsistent symptoms. In previous western studies, the delayed diagnosis was associated with poor outcome in patients with Crohn’s disease (CD).
Research frontiers
There are few studies on diagnostic delays associated with IBD in Asia. Because there were significant differences in the epidemiological and clinical features of IBD according to ethnicities and environmental factors, the authors aimed to investigate the clinical factors and outcomes associated with diagnostic delays in Korean patients with IBD.
Innovations and breakthroughs
In present study, a long diagnostic delay was defined based on the time interval in which the 76th to 100th percentile of the patients was diagnosed due to no established criteria. This study demonstrated a considerable diagnostic delay in patients with Asian patients with IBD. Further, a long diagnostic delay was significantly associated with poor outcome such as a higher risk of IBD-related intestinal surgery in both the patients with CD and ulcerative colitis.
Applications
Efforts should be made to reduce diagnostic delays, such as increasing awareness and strengthening educations about IBD for physicians and the public, to improve the prognosis of patients with IBD.
Peer-review
The manuscript has convincing data and is publishable.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
Institutional review board statement: This study was approved by the Institutional Review Board of Korea University Ansan Hospital (IRB number AS16206).
Informed consent statement: The requirement for obtaining informed patient consent was waived because the present study has been based on the retrospective analysis of existing medical records.
Conflict-of-interest statement: The authors do not have any disclosures to report.
Data sharing statement: No additional data are available.
Peer-review started: June 15, 2017
First decision: July 17, 2017
Article in press: August 15, 2017
P- Reviewer: Desai DC, Lakatos PL, M'Koma AE S- Editor: Qi Y L- Editor: A E- Editor: Ma YJ
Contributor Information
Dong-won Lee, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Korea University Ansan Hospital, Ansan-si, Gyeonggi-do 15355, South Korea.
Ja Seol Koo, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Korea University Ansan Hospital, Ansan-si, Gyeonggi-do 15355, South Korea. jskoo@korea.ac.kr.
Jung Wan Choe, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Korea University Ansan Hospital, Ansan-si, Gyeonggi-do 15355, South Korea.
Sang Jun Suh, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Korea University Ansan Hospital, Ansan-si, Gyeonggi-do 15355, South Korea.
Seung Young Kim, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Korea University Ansan Hospital, Ansan-si, Gyeonggi-do 15355, South Korea.
Jong Jin Hyun, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Korea University Ansan Hospital, Ansan-si, Gyeonggi-do 15355, South Korea.
Sung Woo Jung, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Korea University Ansan Hospital, Ansan-si, Gyeonggi-do 15355, South Korea.
Young Kul Jung, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Korea University Ansan Hospital, Ansan-si, Gyeonggi-do 15355, South Korea.
Hyung Joon Yim, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Korea University Ansan Hospital, Ansan-si, Gyeonggi-do 15355, South Korea.
Sang Woo Lee, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Korea University Ansan Hospital, Ansan-si, Gyeonggi-do 15355, South Korea.
References
- 1.Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis. 2006;12 Suppl 1:S3–S9. doi: 10.1097/01.mib.0000195385.19268.68. [DOI] [PubMed] [Google Scholar]
- 2.Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–1794. doi: 10.1053/j.gastro.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 3.D’Haens G, Baert F, van Assche G, Caenepeel P, Vergauwe P, Tuynman H, De Vos M, van Deventer S, Stitt L, Donner A, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial. Lancet. 2008;371:660–667. doi: 10.1016/S0140-6736(08)60304-9. [DOI] [PubMed] [Google Scholar]
- 4.Colombel JF, Rutgeerts P, Reinisch W, Esser D, Wang Y, Lang Y, Marano CW, Strauss R, Oddens BJ, Feagan BG, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology. 2011;141:1194–1201. doi: 10.1053/j.gastro.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 5.Oldenburg B, Hommes D. Biological therapies in inflammatory bowel disease: top-down or bottom-up? Curr Opin Gastroenterol. 2007;23:395–399. doi: 10.1097/MOG.0b013e32815b601b. [DOI] [PubMed] [Google Scholar]
- 6.Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369:1641–1657. doi: 10.1016/S0140-6736(07)60751-X. [DOI] [PubMed] [Google Scholar]
- 7.Huang X, Liao WD, Yu C, Tu Y, Pan XL, Chen YX, Lv NH, Zhu X. Differences in clinical features of Crohn’s disease and intestinal tuberculosis. World J Gastroenterol. 2015;21:3650–3656. doi: 10.3748/wjg.v21.i12.3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee SK, Kim BK, Kim TI, Kim WH. Differential diagnosis of intestinal Behçet’s disease and Crohn’s disease by colonoscopic findings. Endoscopy. 2009;41:9–16. doi: 10.1055/s-0028-1103481. [DOI] [PubMed] [Google Scholar]
- 9.Vavricka SR, Spigaglia SM, Rogler G, Pittet V, Michetti P, Felley C, Mottet C, Braegger CP, Rogler D, Straumann A, et al. Systematic evaluation of risk factors for diagnostic delay in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:496–505. doi: 10.1002/ibd.21719. [DOI] [PubMed] [Google Scholar]
- 10.Nahon S, Lahmek P, Lesgourgues B, Poupardin C, Chaussade S, Peyrin-Biroulet L, Abitbol V. Diagnostic delay in a French cohort of Crohn’s disease patients. J Crohns Colitis. 2014;8:964–969. doi: 10.1016/j.crohns.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 11.Schoepfer AM, Dehlavi MA, Fournier N, Safroneeva E, Straumann A, Pittet V, Peyrin-Biroulet L, Michetti P, Rogler G, Vavricka SR; IBD Cohort Study Group. Diagnostic delay in Crohn's disease is associated with a complicated disease course and increased operation rate. Am J Gastroenterol. 2013;108:1744–53; quiz 1754. doi: 10.1038/ajg.2013.248. [DOI] [PubMed] [Google Scholar]
- 12.Zaharie R, Tantau A, Zaharie F, Tantau M, Gheorghe L, Gheorghe C, Gologan S, Cijevschi C, Trifan A, Dobru D, et al. Diagnostic Delay in Romanian Patients with Inflammatory Bowel Disease: Risk Factors and Impact on the Disease Course and Need for Surgery. J Crohns Colitis. 2016;10:306–314. doi: 10.1093/ecco-jcc/jjv215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ng SC. Epidemiology of inflammatory bowel disease: focus on Asia. Best Pract Res Clin Gastroenterol. 2014;28:363–372. doi: 10.1016/j.bpg.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Ng SC, Tang W, Ching JY, Wong M, Chow CM, Hui AJ, Wong TC, Leung VK, Tsang SW, Yu HH, Li MF, Ng KK, Kamm MA, Studd C, Bell S, Leong R, de Silva HJ, Kasturiratne A, Mufeena MN, Ling KL, Ooi CJ, Tan PS, Ong D, Goh KL, Hilmi I, Pisespongsa P, Manatsathit S, Rerknimitr R, Aniwan S, Wang YF, Ouyang Q, Zeng Z, Zhu Z, Chen MH, Hu PJ, Wu K, Wang X, Simadibrata M, Abdullah M, Wu JC, Sung JJ, Chan FK; Asia–Pacific Crohn’s and Colitis Epidemiologic Study (ACCESS) Study Group. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn’s and colitis epidemiology study. Gastroenterology. 2013;145:158–165.e2. doi: 10.1053/j.gastro.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749–753. doi: 10.1136/gut.2005.082909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heikenen JB, Werlin SL, Brown CW, Balint JP. Presenting symptoms and diagnostic lag in children with inflammatory bowel disease. Inflamm Bowel Dis. 1999;5:158–160. doi: 10.1097/00054725-199908000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Burgmann T, Clara I, Graff L, Walker J, Lix L, Rawsthorne P, McPhail C, Rogala L, Miller N, Bernstein CN. The Manitoba Inflammatory Bowel Disease Cohort Study: prolonged symptoms before diagnosis--how much is irritable bowel syndrome? Clin Gastroenterol Hepatol. 2006;4:614–620. doi: 10.1016/j.cgh.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Farmer RG, Easley KA, Rankin GB. Clinical patterns, natural history, and progression of ulcerative colitis. A long-term follow-up of 1116 patients. Dig Dis Sci. 1993;38:1137–1146. doi: 10.1007/BF01295733. [DOI] [PubMed] [Google Scholar]
- 19.Wang YF, Zhang H, Ouyang Q. Clinical manifestations of inflammatory bowel disease: East and West differences. J Dig Dis. 2007;8:121–127. doi: 10.1111/j.1443-9573.2007.00296.x. [DOI] [PubMed] [Google Scholar]
- 20.Moon CM, Jung SA, Kim SE, Song HJ, Jung Y, Ye BD, Cheon JH, Kim YS, Kim YH, Kim JS, et al. Clinical Factors and Disease Course Related to Diagnostic Delay in Korean Crohn’s Disease Patients: Results from the CONNECT Study. PLoS One. 2015;10:e0144390. doi: 10.1371/journal.pone.0144390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cosnes J, Cattan S, Blain A, Beaugerie L, Carbonnel F, Parc R, Gendre JP. Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis. 2002;8:244–250. doi: 10.1097/00054725-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Louis E, Collard A, Oger AF, Degroote E, Aboul Nasr El Yafi FA, Belaiche J. Behaviour of Crohn’s disease according to the Vienna classification: changing pattern over the course of the disease. Gut. 2001;49:777–782. doi: 10.1136/gut.49.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meucci G, Vecchi M, Astegiano M, Beretta L, Cesari P, Dizioli P, Ferraris L, Panelli MR, Prada A, Sostegni R, et al. The natural history of ulcerative proctitis: a multicenter, retrospective study. Gruppo di Studio per le Malattie Infiammatorie Intestinali (GSMII) Am J Gastroenterol. 2000;95:469–473. doi: 10.1111/j.1572-0241.2000.t01-1-01770.x. [DOI] [PubMed] [Google Scholar]


