Abstract
AIM
To provide the overall spectrum of gastrosplenic fistula (GSF) occurring in lymphomas through a systematic review including a patient at our hospital.
METHODS
A comprehensive literature search was performed in the MEDLINE database to identify studies of GSF occurring in lymphomas. A computerized search of our institutional database was also performed. In all cases, we analyzed the clinicopathologic/radiologic features, treatment and outcome of GSF occurring in lymphomas.
RESULTS
A literature search identified 25 relevant studies with 26 patients. Our institutional data search added 1 patient. Systematic review of the total 27 cases revealed that GSF occurred mainly in diffuse, large B-cell lymphoma (n = 23), but also in diffuse, histiocytic lymphoma (n = 1), Hodgkin’s lymphoma (n = 2), and NK/T-cell lymphoma (n = 1, our patient). The common clinical presentations are constitutional symptoms (n = 20) and abdominal pain (n = 17), although acute gastrointestinal bleeding (n = 6) and infection symptoms due to splenic abscess (n = 3) are also noted. In all patients, computed tomography scanning was very helpful for diagnosing GSF and for evaluating the lymphoma extent. GSF could occur either post-chemotherapy (n = 10) or spontaneously (n = 17). Surgical resection has been the most common treatment. Once patients have recovered from the acute illness status after undergoing surgery, their long-term outcome has been favorable.
CONCLUSION
This systematic review provides an overview of GSF occurring in lymphomas, and will be helpful in making physicians aware of this rare disease entity.
Keywords: Gastrosplenic fistula, Lymphoma, NK/T-cell lymphoma, Systematic review
Core tip: Gastrosplenic fistula (GSF) is a very rare complication occurring in lymphomas. Due to its rarity, GSF had not been well-investigated. Therefore, we intend to provide an overall spectrum of GSF occurring in lymphomas through a systematic review. GSF occurred mainly in diffuse, large B-cell lymphoma, but also in various kinds of lymphoma. Common clinical presentations are constitutional symptoms and abdominal pain. Occasionally, acute gastrointestinal bleeding and splenic abscess have occurred. Computed tomography was helpful for diagnosing GSF and for evaluating the lymphoma extent. GSF could occur either post-chemotherapy or spontaneously. Surgical resection has been the most common treatment.
INTRODUCTION
Gastrosplenic fistula (GSF) is a rare and potentially fatal complication of various diseases, including lymphoma, gastric adenocarcinoma, Crohn’s disease, splenic abscess, and trauma[1]. Lymphoma is the most common cause of GSF. Of these diseases, the majority have occurred in patients with diffuse, large, B-cell lymphoma (DLBCL), and there has been no patient with NK/T-cell lymphoma. In our hospital, a tertiary cancer center, we recently encountered a patient with GSF developed in NK/T-cell lymphoma.
A multidisciplinary team was organized in our hospital for management of this patient. Our team performed an extensive literature search, however, and determined that the characteristics of GSF occurring in lymphoma patients have not been thoroughly investigated due to its extreme rarity. In contrast, the number of case reports regarding these patients has increased over the last 10 years[1-24]. Despite the increasing number of these patients, there has been no systematic review to summarize the variable presentations of GSF occurring in lymphomas. Therefore, we performed the current, systematic review with the addition of our single patient in order to provide a perspective regarding this rare disease entity.
MATERIALS AND METHODS
Systematic literature search
A computerized search of the MEDLINE database was conducted to find relevant studies published prior to February 10, 2017. Studies were eligible for inclusion if they described the clinicopathologic features, imaging findings, treatment and outcome of the cases with regard to GSF occurring in lymphoma. The following search terms were used: “GSF” and “lymphoma”. To expand the search, the bibliographies of articles that remained after the selection process were screened for other potentially suitable articles. We did not limit the language of the articles.
Institutional data search and case presentation
Our institutional review board approved the search of the electronic medical records for this study. Informed consent was waived from our institutional review board. We performed a systematic computerized search of our institutional database from January 2000 to January 2017 using the diagnostic codes of “GSF”, “splenic fistula”, and “lymphoma”. Using these search terms, we identified only one case which we recently encountered, as described below. In our institutional database, the patient’s record was anonymized and provided to us. We present here the clinical course, pathologic findings, and imaging features of this case. We also included this case in the systematic review of GSF of lymphoma.
Analysis of clinicopathologic and radiologic features
For patients with GSF occurring in lymphoma identified in the literature and at our institution, we analyzed their clinicopathologic features, imaging findings, treatment and outcome.
RESULTS
Literature selection
Our study selection process is shown in Figure 1. The literature search in the MEDLINE database generated 24, initial candidate articles. After reviewing the titles and the abstracts, all 23 articles were included. One study was subsequently excluded due to the lack of a full-text manuscript. Therefore, we reviewed the full text of all 23 articles, including 2 written in Spanish and 1 written in Korean. Search of the bibliographies of these articles found two, additional, eligible studies[8,14]. Therefore, a total of 25 articles were included in our systematic review. Of these articles, 24 described one case, while 1 described two cases[3]. Therefore, a total of 27 cases were retrieved from the systematic literature search.
Figure 1.
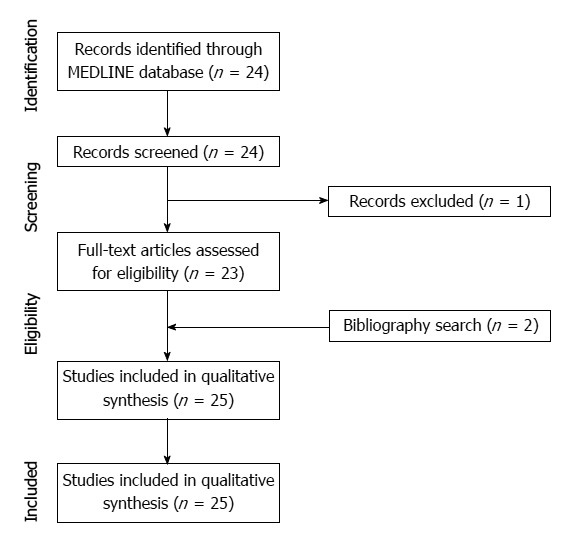
Flow diagram for the selection of studies.
Presentation of our case
A 50-year-old man was admitted to our hospital for a biopsy-proven NK/T-cell lymphoma of the right anterior nasal cavity. On contrast-enhanced computed tomography (CT), there was an ill-defined, homogeneous, enhancing, soft-tissue mass in the right anterior nasal cavity. There was no significantly enlarged lymph node in his neck, chest or abdomen-pelvis. However, hepatosplenomegaly was noted with numerous, ill-defined, low, attenuating nodules in the liver. His spleen measured 15.5 cm in the longest dimension. Hypermetabolic enlargement of the liver and spleen was revealed on positron emission tomography scanning. These findings were indicative of lymphoma involvement in the liver and spleen.
The patient was subsequently started on the combination of a SMILE chemotherapy regime con-sisting of methotrexate, leucovorin, ifosfamide, etoposide, and L-asparaginase followed by autologous stem-cell transplantation (ASCT). After completing the ASCT, complete remission was confirmed by a bone marrow biopsy and CT scans with resolution of the nasal cavity mass, hepatosplenomegaly, and focal liver lesions.
Two months later, follow-up CT showed a huge splenomegaly measuring 17.5-cm in its longest diameter, which was indicative of lymphoma recurrence (Figure 2). Chemotherapy was started for the lymphoma recurrence. Tumor lysis syndrome occurred, and the patient underwent dialysis treatment.
Figure 2.
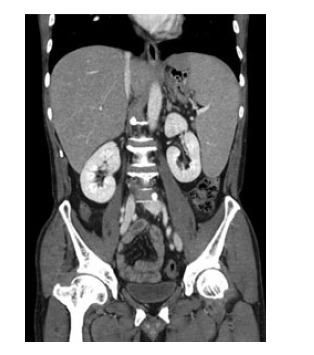
On a coronal computed tomography image taken 2 mo after autologous stem-cell transplantation, the spleen was enlarged, measuring 17 cm in the longest dimension, and indicative of recurred lymphoma. The enlarged spleen abutted to the gastric fundus.
He then soon experienced left, upper quadrant, abdominal pain and nausea/vomiting. Follow-up abdominal CT showed a diffusely enlarged spleen with nearly total splenic infarction. A huge fistula track was also seen between the gastric lumen and the infarcted spleen (Figure 3).
Figure 3.
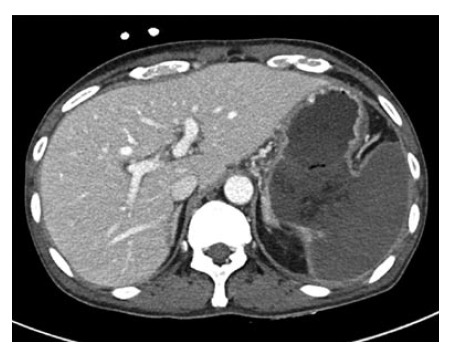
On an axial computed tomography image taken after chemotherapy, a huge fistula was shown between the gastric lumen and the spleen. The spleen was totally infarcted.
He underwent emergency surgery for gastric wedge resection and splenectomy. During the surgery, a large GSF was observed and there was adhesion between the infarcted spleen and the stomach. These surgical findings suggested that the extensive lymphoma lysis due to chemotherapy resulted in disruption of the splenic capsule, adhesion to the adjacent gastric fundus, and eventually led to the large GSF. In the gross and microscopic specimen, there was extensive hemorrhagic infarction in the spleen with a large GSF. The atypical lymphoma cells, which were positive for CD3 on immunohistochemistry stain and positive for Epstein-Barr virus (EBV) on EBV RNA stain, were found in the stomach wall near the GSF as well as in the whole spleen (Figure 4). The histopathological diagnosis was NK/T-cell lymphoma and these findings suggest that lymphoma cells may infiltrate from the spleen to the stomach wall through the adhesion/perforation site.
Figure 4.
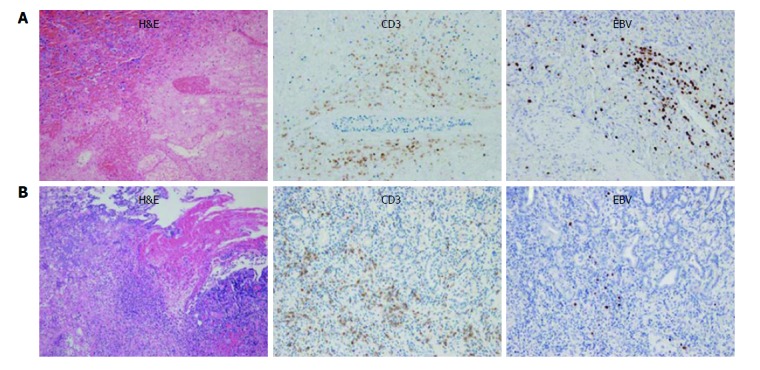
Microscopic specimen of the spleen and stomach. A: Atypical lymphoma cells were found in the spleen on hematoxylin-eosin stain (left). These cells showed positivity for CD3 on immunohistochemistry stain (middle) and EBV on EBV RNA stain (right). There was also extensive coagulative necrosis indicative of splenic infarction; B: Lymphoma cells were found in the stomach wall near the gastrosplenic fistula on hematoxylin-eosin stain (left), and which also showed positivity for CD3 (middle) and EBV (right). NK/T-cell lymphoma was diagnosed. These findings suggested that lymphoma cells may have infiltrated from the spleen to the stomach wall through the perforation site. EBV: Epstein-Barr virus.
Systematic review
The characteristics of the 27 included cases (26 literature cases and 1 presented case) of GSF occurring in lymphomas are summarized in Table 1. Although there was a substantial diversity in the clinicopathologic and radiologic features, the systematic review allowed us to summarize the characteristic features of GSF occurring in lymphomas.
Table 1.
Summary of the 27 cases of gastrosplenic fistula occurring in lymphomas
| Ref. | Diagnosis | Sex/age | Size of lymphoma | Disease status | Presentation | Diagnostic modality | Intervention/therapy | Outcome |
| Bubenik et al[1] (1983) | Diffuse histiocytic lymphoma | Male/58 | Not available | Post-CTx | Nonspecific LUQ discomfort | CT abdomen followed by | Splenectomy, gastric greater | Uneventful post-operative period; no further details |
| endoscopy of upper GI tract | curvature resection, distal pancreatectomy | |||||||
| Hiltunen et al[2] (1991) | Gastric DLBCL | Male/36 | Not available | Post-CTx | Hematemesis, splenomegaly | CT abdomen followed by endoscopy | Laparotomy without details | Followed-up over 3 yr |
| Blanchi et al[3] (1995) case 1 | Splenic DLBCL | Male/62 | Not available | Initial presentation | Left abdominal pain and fever | Endoscopy of upper GI tract followed by CT abdomen | Resection of spleen, tail of pancreas, and involved stomach | At 6 mo after the operation, the patient was in complete remission after CTx |
| Blanchi et al[3] (1995) case 2 | Splenic DLBCL | Male/45 | Not available | Initial presentation | Epigastric pain and weight loss | Endoscopy of upper GI tract followed by CT abdomen | No further details. | No further details |
| Carolin et al[4] (1997) | Gastric DLBCL | Male/46 | Not available | Initial presentation | Epigastric pain, fatigue, weight loss and splenomegaly | Endoscopy of upper GI tract followed by CT abdomen | Laparotomy, but no further details. | No further details |
| Bird et al[5] (2002) | Splenic DLBCL | Male/36 | Not available | Initial presentation | Hematemesis, melena, fatigue, weight loss and splenomegaly | Endoscopy of upper GI tract followed by CT abdomen | Splenic artery embolization, near total gastrectomy and splenectomy | Disease-free after three cycles of CTx; no further details |
| Choi et al[6] (2002) | Splenic DLBCL | Male/24 | Not available | Initial presentation | LUQ pain and constitutionals symptoms (splenic mass) | CT abdomen followed by endoscopy of upper GI tract/biopsy | CTx followed by splenectomy, gastric wedge resection, and distal pancreatectomy | Not available |
| Yang et al[7] (2002) | Gastric and splenic DLBCL | Male/21 | Not available | Initial presentation | LUQ pain, fatigue, weight loss, fever, and splenomegaly | CT abdomen followed by endoscopy of upper GI tract | Splenectomy, gastric wedge resection, and distal pancreatectomy | After surgery, the patient underwent CTx |
| Puppala et al[8] (2005) | DLBCL | Female/66 | Not available | Initial presentation | LUQ pain | CT abdomen oral contrast | CTx | Died after 2 mo of Ctx |
| Kerem et al[9] (2006) | DLBCL | Male/57 | 10 cm × 7 cm × 2 cm in the stomach and 8 cm × 5 cm × 4 cm in the spleen | Initial presentation | Abdominal pain, epigastric tenderness and splenomegaly | CT abdomen followed by PETCT and endoscopy of upper GI tract | Splenectomy, proximal gastrectomy, esophagojejunostomy, proximal pyroloplasty followed by CTx | Uneventful post-op period; underwent chemotherapy. |
| Al-Ashgar et al[10] (2007) | Hodgkin’s lymphoma-(nodular sclerosis)-IIIS | Female/16 | Not available | Initial presentation | LUQ pain, constitutional symptoms and splenomegaly | Endoscopy of upper GI tract, barium swallow, CT abdomen | Laparoscopic surgical repair followed by seven cycles CTx | Alive and in remission after 1 yr |
| Aribaş et al[11] (2008) | DLBCL | Male/25 | Not available | Post-CTx | Abdominal pain, weight loss, fever, chill and splenomegaly | CT cystography followed by USG | Gastric wedge resection, fistulectomy and splenectomy | Discharged after a month and died 2 mo later due to progression of lymphoma and infection due to pancreatic and gastric fistulas |
| Palmowski et al[12] (2008) | DLBCL | Male/56 | 15 cm of spleen | After three cycles of CTx | Fever and signs of acute infection (splenic mass) | CT abdomen | Splenectomy with partial gastric resection | Finished six cycles of CTx |
| Seib et al[13] (2009) | Hodgkin’s lymphoma | Male /49 | 3.6-cm splenic mass | Relapsed post-CTx | LUQ pain and constitutional symptoms (splenic mass) | CT abdomen | Partial gastrectomy and fistulectomy | Died after 5 mo |
| Moran et al[14] (2009) | DLBCL | Male/35 | 5.4 cm × 5.3 cm of gastrosplenic mass | Initial presentation | LUQ pain and constitutional symptoms | CT abdomen followed by endoscopy of upper GI tract | Abscess drainage; splenectomy, total gastrectomy, Roux-en-Y esophagojejunostomy followed by CTx | Received CTx after surgery; no further details available |
| Maillo et al[15] (2009) | Splenic DLBCL | Female/76 | Not available | Initial presentation | Massive hematemesis, fever and fatigue (splenic abscess) | CT abdomen followed by endoscopy of upper GI tract | splenectomy, partial gastrectomy, diaphragmatic primary repair, drainage chest tube and a feeding tube jejunostomy | At 2 mo later the patient developed a pulmonary infection and died because of multi-organic failure |
| García et al[16] (2009) | Gastric DLBCL | Male/76 | Not available | Initial presentation | Epigastric pain, weight loss and splenomegaly | CT abdomen followed by endoscopy of upper GI tract | Total gastrectomy, splenectomy and distal pancreatectomy | Remained asymptomatic at the 36-mo follow-up, no further details |
| Khan et al[17] (2010) | Gastric DLBCL | Female/43 | 18.9 cm × 10 cm × 8.6 cm of splenic mass | Initial presentation | Upper abdominal pain and constitutional symptoms (splenic mass) | Endoscopy of upper GI tract followed by CT | CTx | Complete remission after two cycles of CTx; no further details |
| Rothermel et al[18] (2010) | Splenic DLBCL | Male/74 | Not available | Initial presentation | Fever, chill and weight loss | Endoscopy of upper GI tract followed by CT | Splenectomy, stapled gastric-sleeve resection | After surgery, the patient underwent CTx; good prognosis for long-term survival |
| Dellaportas et al[19] (2011) | Splenic DLBCL | Male/68 | Not available | Initial presentation | Hematemesis (splenic mass) | Endoscopy of upper GI tract followed by CT abdomen | Surgical en bloc resection followed by chemotherapy | Post-CTx on follow up |
| No details available | ||||||||
| Jain et al[20] (2011) | DLBCL | Male/55 | Not available | Post-CTx | Progressive weakness, fatigue, melena and splenomegaly | CT abdomen followed by endoscopy of upper GI tract | Splenectomy and partial gastrectomy | Received CTx after surgery; no further details available |
| Ding et al[21] (2012) | DLBCL | Male/62 | 7 cm of splenic segment | Initial presentation | LUQ pain with constitutional symptoms and splenomegaly | CT abdomen followed by endoscopy of upper GI tract | Splenectomy, gastric wedge resection, and distal pancreatectomy followed by CTx and RT | Well at follow up; no further details available |
| Favre Rizzo et al[22] (2013) | Gastric DLBCL | Male/55 | Not available | Initial presentation | Hematemesis, epigastric pain, weight loss and splenomegaly | CT abdomen | Partial gastrectomy, splenectomy and distal pancreatectomy | After surgery; no further details available |
| Senapati et al[29] (2014) | DLBCL | Male/57 | Splenomegaly of 15 cm | Post-CTx | No symptom but splenomegaly | PET/CT followed by endoscopy of upper GI tract | Refused any surgical intervention | Lost to follow-up |
| Gentilli et al[23] (2016) | Gastric DLBCL | Female/66 | 7.5 cm × 3 cm of splenic mass | Post-CTx | Weakness, fatigue, weight loss and splenomegaly | Endoscopy of upper GI tract followed by CT | Gastric wedge resection, splenectomy | Discharged after surgery; no further details |
| Sousa et al[24] (2016) | Gastric DLBCL | Male/52 | Not available | Post-CTx | Hematemesis | Endoscopy of upper GI tract | Total gastrectomy, splenectomy, distal pancreatectomy | Patient was lost to follow-up after discharge |
| Present case | NK/T cell lymphoma | Male/50 | 11 cm × 5 cm × 13 cm of spleen | Post-CTx | LUQ pain, nausea, vomiting and splenomegaly | CT abdomen | Gastric wedge resection and splenectomy | At 3 mo later, gastric perforation occurred and the patient expired due to sepsis |
CT: Computed tomography; CTx: Chemotherapy; DLBCL: Diffuse large B-cell lymphoma; GI: Gastrointestinal; LUQ: Left upper quadrant; PET: Positron emission tomography; RT: Radiation therapy; USG: Ultrasonography.
Clinicopathologic features
The mean age of these patients was 50.6 ± 16.8 years with a range of 16-76 years. The male to female ratio was 4.4 (22 males, 5 females). Regarding the presenting symptoms or signs, constitutional symptoms such as weight loss, fever and fatigue (n = 20, 74.1%), were followed by abdominal/flank pain (n = 17, 63.0%), acute gastrointestinal bleeding such as hematemesis (n = 6, 22.2%), and infection due to splenic abscess (n = 3, 11.1%).
Among the different histological types, GSF occurred most commonly in DLBCL (n = 23, 85.2%). The other types of lymphoma associated with GSF include diffuse histiocytic lymphoma (n = 1, 3.7%), Hodgkin’s lymphoma (n = 2, 7.4%), and extranodal NK/T-cell lymphoma (n = 1, 3.7%). Of these, GSF occurred due to splenic lymphoma involvement (n = 8, 29.6%, including the present case), gastric lymphoma involvement (n = 6, 22.2%), extensive lymphoma including both gastric and splenic involvement (n = 1, 3.7%), and not specified (n = 12, 44.4%). GSF occurred either post-chemotherapy (n = 12, 44.4%) or spontaneously (n = 17, 63.0%).
Radiologic features
In all 27 cases, the GSF was depicted or suspected on CT scans, as there was a defect in the gastric wall and splenic capsule where the stomach and spleen were closely attached. Of these, 10 cases described the size of the GSF which ranged from 0.25 cm to 6 cm (mean size: 2.87 cm). In 21 cases, endoscopy was performed to confirm the diagnosis of GSF and to evaluate the bleeding or other concomitant disease, such as a gastric ulcer.
The CT scan was excellent for identifying the extent of lymphoma involvement. The splenic involvement was most commonly noted as a moderate to massive splenomegaly (n = 13). Five of seven cases specifically noted the gastric lymphoma as a diffuse/segmental, gastric wall thickening or mass. There were five cases with extensive lymphoma masses in the left upper quadrant, including diaphragm involvement.
The CT scan was also very helpful for identifying any associated GSF complications. Indeed, a splenic abscess was diagnosed on CT in three cases. Acute bleeding was seen as leakage of contrast agent in the gastric lumen. Even when there was no leakage of contrast agent seen on CT, the presence of a hematoma in the stomach and/or spleen could suggest a recent hemorrhagic event.
Treatment and outcome
Regarding the treatment, surgical resection was performed in 23 patients (88.9%). Two patients underwent chemotherapy without surgery, and one patient refused any treatment.
Among the 27 cases, death was reported in 5 patients due to a gastric perforation (n = 1), progression of lymphoma and infection (n = 1), pulmonary infection with multi-organic failure (n = 1), and no further details (n = 2). The time to death ranged from 2-5 mo after the initial surgery or diagnosis. In 18 cases, the patients were alive at that time or were discharged. Patient outcomes were not reported for three patients, and two patients were lost to follow-up.
DISCUSSION
GSF is a very rare complication of lymphoma, as only 27 cases have been reported including the present case. It is very difficult to identify the spectrum of presentation, clinicopathologic/radiologic features, and outcome in this kind of extremely rare disease entity. Systematic review of pre-existing cases might be the best way to evaluate the overview of this rare disease[25]. From that perspective, our systematic review may provide a systematic summary and overview of GSF occurring in lymphomas.
As our systematic review includes all of the available articles in PubMed without the limitation of language, it is the largest reported series of GSF cases occurring in lymphomas. Although there was a diversity in the clinicopathologic features, imaging features and outcomes, we can summarize that GSF occurs mainly in DLBCL (85.2%) and it can occur either post-chemotherapy (37%) or spontaneously (63%). GSF can also cause fatal complications such as acute bleeding and splenic abscess. Once these fatal complications in the acute illness stage are controlled, the long-term outcome is good.
Regarding the pathologic type, DLBCL is the most common type associated with GSF. The present case of GSF occurred in extranodal NK/T-cell lymphoma of the nasal type and, to our knowledge, is the first reported case. In general, NK/T-cell lymphoma occurs in the nasal cavity. If NK/T-cell lymphoma involves an area outside the nasal cavity, the gastrointestinal tracts and skin are common and preferential extranodal organs of lymphoma involvement[26]. Rare sites of involvement include the spleen, prostate, pancreas and adrenal glands[27]. From this perspective, our case demonstrated a very rare complication of GSF which occurred in a rarely involved site of NK/T-cell lymphoma of the nasal type.
In general, NK/T-cell lymphoma is characterized by its aggressive behavior and with an angiocentric/angiodestructive growth pattern. In our case, we at first postulated that the GSF might occur due to these aggressive behaviors. Thereafter, a multidisciplinary discussion at our medical center indicated that extensive tumor lysis from chemotherapy might also be an important causative factor of GSF. The spleen parenchyma and capsule were largely infiltrated by NK/T-cell lymphoma cells, and thus caused splenomegaly. When the enlarged spleen was attached to the adjacent gastric fundus, tumor cells might infiltrate into the gastric wall. When chemotherapy caused tumor lysis and necrosis, the defect in the spleen capsule and gastric wall occurred, eventually leading to the GSF.
When we systematically reviewed the literature reports, we discovered that there has been a commonly proposed theory of GSF development, i.e., necrosis of lymphoma tissue involving the spleen or stomach may cause GSF. According to this theory, invasion of the gastric wall and splenic capsule is required. If the lymphoma predominantly involves the spleen, the tumor cells should also infiltrate the gastric wall, and vice versa. The rapid necrosis of infiltrated lymphoma cells in the gastric wall and splenic capsule may result in the formation of GSF which may occur either spontaneously or post-chemotherapy[6,28]. Most of the GSFs described in the published literature are small. However, if a larger part of the stomach is infiltrated, it is possible for a large opening in the stomach wall to arise, as was reported in our case.
As the initial presentations of GSF occurring in lymphomas are somewhat non-specific, such as constitutional symptoms and abdominal pain, the diagnosis of GSF may be difficult and delayed. CT scanning can have an important role in the diagnosis of GSF, can identify GSF-associated complications such as abscess or bleeding, and can evaluate the disease extent and surgical planning. If the diagnosis and treatment of GSF are delayed, the clinical outcome might not be favorable, as GSF in lymphomas recover spontaneously. Therefore, radiologists should be aware of this rare disease entity and should notify physicians when GSF is suspected in patients with lymphoma involving the spleen or stomach.
There are various therapeutic options for managing GSF. Surgery is regarded as the most important and curative treatment in the vast majority of literature reports. The surgical method can be determined based on the tumor extent and the surgeon’s preference. The most common surgical method in the literature reports is partial gastrectomy (mostly laparoscopic) with/without splenectomy. However, in some cases with a large tumor extent, near-total gastrectomy and splenectomy has also been performed. When patients were too ill to have surgery or refused it, the outcome was not good. Once patients had surgery and overcame the acute illness status, the long-term outcome was generally good. Considering all of these cases, we propose that aggressive surgery at the early stage of disease might be the best way to save patients with GSF occurring in lymphomas.
In conclusion, this systematic review provides an overview and spectrum of GSF occurring in lymphomas and covering the clinicopathologic features, radiologic features, treatment and outcome. We also included a rare case of GSF occurring in extranodal NK/T-cell lymphoma. This information will be helpful for physicians so that they can become aware of this rare disease entity.
COMMENTS
Background
Gastrosplenic fistula (GSF) is a very rare complication occurring in lymphomas. Due to its rarity, GSF have not been well-investigated. Therefore, the intent of our study was to provide the overall spectrum of GSF occurring in lymphomas through a systematic review including a patient at our hospital who had extranodal NK/T-cell lymphoma.
Research frontiers
A systematic review of 27, published studies was conducted. The clinicopathologic/radiologic features, treatment and outcome of GSF occurring in lymphomas were analyzed.
Innovations and breakthroughs
This systematic review includes all of the available articles in PubMed without the limitation of language and it is the largest reported series of GSF cases occurring in lymphomas. It provides a systematic summary and overview of GSF occurring in lymphomas in order to understand the spectrum of presentation, clinicopathologic/radiologic features and outcome in this type of extremely rare disease entity. The authors also added our present case of GSF occurring in extranodal NK/T-cell lymphoma of the nasal type and, to our knowledge, this is the first reported case of this type.
Applications
When lymphoma patients present symptoms or signs of constitutional symptoms, such as weight loss, fever and fatigue, abdominal/flank pain, acute gastrointestinal bleeding, such as hematemesis or any infection signs with or without history of chemotherapy, computed tomography scanning was very helpful for diagnosing GSF and evaluating the lymphoma extent. GSF could occur either post-chemotherapy or spontaneously. Surgical resection has been the most common treatment. Once patients have recovered from their acute illness status after undergoing surgery, their long-term outcome has been good. The authors should be aware of GSF, which is a rare disease entity in patients with lymphoma, especially involving the spleen or stomach.
Terminology
GSF is a communication between the stomach lumen and spleen parenchyma.
Peer-review
This manuscript systematically reviewed a very rare complication of GSF occurring in lymphomas. The review analyzed the current literature regarding the clinicopathologic/radiologic features, treatment and outcome of GSF. It therefore provides useful recording of the disease.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: No authors have conflict-of-interest.
Data sharing statement: No additional data are available.
Peer-review started: May 4, 2017
First decision: June 7, 2017
Article in press: July 24, 2017
P- Reviewer: Chen F, Li YZ S- Editor: Qi Y L- Editor: Filipodia E- Editor: Zhang FF
Contributor Information
Dong Hyeok Kang, Department of Radiology, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan 682-714, South Korea.
Jimi Huh, Department of Radiology, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan 682-714, South Korea. jimihuh@mail.ulsan.ac.kr.
Jong Hwa Lee, Department of Radiology, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan 682-714, South Korea.
Yoong Ki Jeong, Department of Radiology, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan 682-714, South Korea.
Hee Jeong Cha, Department of Pathology, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan 682-714, South Korea.
References
- 1.Bubenik O, Lopez MJ, Greco AO, Kraybill WG, Cherwitz DL. Gastrosplenic fistula following successful chemotherapy for disseminated histiocytic lymphoma. Cancer. 1983;52:994–996. doi: 10.1002/1097-0142(19830915)52:6<994::aid-cncr2820520611>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 2.Hiltunen KM, Airo I, Mattila J, Helve O. Massively bleeding gastrosplenic fistula following cytostatic chemotherapy of a malignant lymphoma. J Clin Gastroenterol. 1991;13:478–481. doi: 10.1097/00004836-199108000-00026. [DOI] [PubMed] [Google Scholar]
- 3.Blanchi A, Bour B, Alami O. Spontaneous gastrosplenic fistula revealing high-grade centroblastic lymphoma: endoscopic findings. Gastrointest Endosc. 1995;42:587–589. doi: 10.1016/s0016-5107(95)70017-x. [DOI] [PubMed] [Google Scholar]
- 4.Carolin KA, Prakash SH, Silva YJ. Gastrosplenic fistulas: a case report and review of the literature. Am Surg. 1997;63:1007–1010. [PubMed] [Google Scholar]
- 5.Bird MA, Amjadi D, Behrns KE. Primary splenic lymphoma complicated by hematemesis and gastric erosion. South Med J. 2002;95:941–942. [PubMed] [Google Scholar]
- 6.Choi JE, Chung HJ, Lee HG. Spontaneous gastrosplenic fistula: a rare complication of splenic diffuse large cell lymphoma. Abdom Imaging. 2002;27:728–730. doi: 10.1007/s00261-002-0011-9. [DOI] [PubMed] [Google Scholar]
- 7.Yang SE, Jin JY, Song CW, Park JC, Lee JI, Kim W, Kim J, Lee HG. Gastrosplenic Fistula Complicated in a Patient with Non- Hodgkin’s Lymphoma. Cancer Res Treat. 2002;34:153–156. doi: 10.4143/crt.2002.34.2.153. [DOI] [PubMed] [Google Scholar]
- 8.Puppala S, Williams R, Harvey J, Crane M. Spontaneous gastrosplenic fistula in primary gastric lymphoma: case report and review of literature. Clinical Radiology Extra. 2005;60:20–22. [Google Scholar]
- 9.Kerem M, Sakrak O, Yilmaz TU, Gultekin FA, Dursun A, Bedirli A. Spontaneous gastrosplenic fistula in primary gastric lymphoma: Surgical management. Asian J Surg. 2006;29:287–290. doi: 10.1016/S1015-9584(09)60104-4. [DOI] [PubMed] [Google Scholar]
- 10.Al-Ashgar HI, Khan MQ, Ghamdi AM, Bamehriz FY, Maghfoor I. Gastrosplenic fistula in Hodgkin’s lymphoma treated successfully by laparoscopic surgery and chemotherapy. Saudi Med J. 2007;28:1898–1900. [PubMed] [Google Scholar]
- 11.Aribaş BK, Başkan E, Altinyollar H, Ungül U, Cengiz A, Erdil HF. Gastrosplenic fistula due to splenic large cell lymphoma diagnosed by percutaneous drainage before surgical treatment. Turk J Gastroenterol. 2008;19:69–70. [PubMed] [Google Scholar]
- 12.Palmowski M, Zechmann C, Satzl S, Bartling S, Hallscheidt P. Large gastrosplenic fistula after effective treatment of abdominal diffuse large-B-cell lymphoma. Ann Hematol. 2008;87:337–338. doi: 10.1007/s00277-007-0404-5. [DOI] [PubMed] [Google Scholar]
- 13.Seib CD, Rocha FG, Hwang DG, Shoji BT. Gastrosplenic fistula from Hodgkin’s lymphoma. J Clin Oncol. 2009;27:e15–e17. doi: 10.1200/JCO.2008.21.7695. [DOI] [PubMed] [Google Scholar]
- 14.Moran M, Bilgiç İ, Dizen H, Dilektaşlı E, Köseoğlu T, Özmen MM. Spontaneous gastrosplenic fistula resulting from primary gastric lymphoma: case report and review of the literature. Available from: http://www.academia.edu/22723368/Spontaneous_Gastrosplenic_Fistula_Resulting_From_Primary_Gastric_Lymphoma_Case_Report_And_Review_Of_The_Literature.
- 15.Maillo C, Bau J. [Gastrosplenic and thoracosplenic fistula due to primary untreated splenic lymphoma] Rev Esp Enferm Dig. 2009;101:222–223. doi: 10.4321/s1130-01082009000300012. [DOI] [PubMed] [Google Scholar]
- 16.García MA, Bernardos GL, Vaquero RA, Menchén VL, Turégano FF. Spontaneous gastrosplenic fistula secondary to primary gastric lymphoma. Vol. 101. Revista espanola de enfermedades digestivas: organo oficial de la Sociedad Espanola de Patologia Digestiva; 2009. p. 76. [DOI] [PubMed] [Google Scholar]
- 17.Khan F, Vessal S, McKimm E, D’Souza R. Spontaneous gastrosplenic fistula secondary to primary splenic lymphoma. BMJ Case Rep. 2010;2010:pii: bcr0420102932. doi: 10.1136/bcr.04.2010.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rothermel LD, Chadwick CL, Thambi-Pillai T. Gastrosplenic fistula: etiologies, diagnostic studies, and surgical management. Int Surg. 2010;95:270–272. [PubMed] [Google Scholar]
- 19.Dellaportas D, Vezakis A, Fragulidis G, Tasoulis M, Karamitopoulou E, Polydorou A. Gastrosplenic fistula secondary to lymphoma, manifesting as upper gastrointestinal bleeding. Endoscopy. 2011;43 Suppl 2 UCTN:E395. doi: 10.1055/s-0030-1256935. [DOI] [PubMed] [Google Scholar]
- 20.Jain V, Pauli E, Sharzehi K, Moyer M. Spontaneous gastrosplenic fistula secondary to diffuse large B-cell lymphoma. Gastrointest Endosc. 2011;73:608–609. doi: 10.1016/j.gie.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 21.Ding YL, Wang SY. Gastrosplenic fistula due to splenic large B-cell lymphoma. J Res Med Sci. 2012;17:805–807. [PMC free article] [PubMed] [Google Scholar]
- 22.Favre Rizzo J, López-Tomassetti Fernández E, Ceballos Esparragón J, Santana Cabrera L, Hernández Hernández JR. Massive upper gastrointestinal bleeding secondary to gastrosplenic fistula. Rev Esp Enferm Dig. 2013;105:570–871. doi: 10.4321/s1130-01082013000900015. [DOI] [PubMed] [Google Scholar]
- 23.Gentilli S, Oldani A, Zanni M, Ferreri E, Terrone A, Valente G, Occhipinti P. Gastro-splenic fistula as a complication of chemotherapy for large B cell lymphoma. Ann Ital Chir. 2016;87:pii: S2239253X16025731. [PubMed] [Google Scholar]
- 24.Sousa M, Gomes A, Pignatelli N, Nunes V. Massive gastrointestinal bleeding after chemotherapy for gastric lymphoma. Int J Surg Case Rep. 2016;21:41–43. doi: 10.1016/j.ijscr.2016.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huh J, Byun JH, Hong SM, Kim KW, Kim JH, Lee SS, Kim HJ, Lee MG. Malignant pancreatic serous cystic neoplasms: systematic review with a new case. BMC Gastroenterol. 2016;16:97. doi: 10.1186/s12876-016-0518-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haverkos BM, Pan Z, Gru AA, Freud AG, Rabinovitch R, Xu-Welliver M, Otto B, Barrionuevo C, Baiocchi RA, Rochford R, et al. Extranodal NK/T Cell Lymphoma, Nasal Type (ENKTL-NT): An Update on Epidemiology, Clinical Presentation, and Natural History in North American and European Cases. Curr Hematol Malig Rep. 2016;11:514–527. doi: 10.1007/s11899-016-0355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao Q, Huang Y, Ye Z, Liu N, Li S, Peng T. Primary spleen extranodal NK/T cell lymphoma, nasal type, with bone marrow involvement and CD30 positive expression: a case report and literature review. Diagn Pathol. 2014;9:169. doi: 10.1186/s13000-014-0169-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Scoville A, Bovy P, Demeester P. [Radiologic “aerosplenomegaly” caused by necrotizing splenic lymphosarcoma with double fistulization into the digestive tract] Acta Gastroenterol Belg. 1967;30:840–846. [PubMed] [Google Scholar]
- 29.Senapati J, Devasia AJ, Sudhakar S, Viswabandya A. Asymptomatic gastrosplenic fistula in a patient with marginal zonal lymphoma transformed to diffuse large B cell lymphoma--a case report and review of literature. Ann Hematol. 2014;93:1599–1602. doi: 10.1007/s00277-013-1986-8. [DOI] [PubMed] [Google Scholar]


