Abstract
AIM
To perform a systematic review and meta-analysis on proton pump inhibitors (PPIs) therapy and the risk of Clostridium difficile infection (CDI).
METHODS
We conducted a systematic search of MEDLINE/PubMed and seven other databases through January 1990 to March 2017 for published studies that evaluated the association between PPIs and CDI. Adult case-control and cohort studies providing information on the association between PPI therapy and the development of CDI were included. Pooled odds ratios (ORs) estimates with 95% confidence intervals (CIs) were calculated using the random effect. Heterogeneity was assessed by I2 test and Cochran’s Q statistic. Potential publication bias was evaluated via funnel plot, and quality of studies by the Newcastle-Otawa Quality Assessment Scale (NOS).
RESULTS
Fifty-six studies (40 case-control and 16 cohort) involving 356683 patients met the inclusion criteria and were analyzed. Both the overall pooled estimates and subgroup analyses showed increased risk for CDI despite substantial statistical heterogeneity among studies. Meta-analysis of all studies combined showed a significant association between PPI users and the risk of CDI (pooled OR = 1.99, CI: 1.73-2.30, P < 0.001) as compared with non-users. The association remained significant in subgroup analyses: by design-case-control (OR = 2.00, CI: 1.68-2.38, P < 0.0001), and cohort (OR = 1.98, CI: 1.51-2.59, P < 0.0001); adjusted (OR = 1.95, CI: 1.67-2.27, P < 0.0001) and unadjusted (OR = 2.02, CI: 1.41-2.91, P < 0.0001); unicenter (OR = 2.18, CI: 1.72-2.75, P < 0.0001) and multicenter (OR = 1.82, CI: 1.51-2.19, P < 0.0001); age ≥ 65 years (OR = 1.93, CI: 1.40-2.68, P < 0.0001) and < 65 years (OR = 2.06, CI: 1.11-3.81, P < 0.01). No significant differences were found in subgroup analyses (test for heterogeneity): P = 0.93 for case-control vs cohort, P = 0.85 for adjusted vs unadjusted, P = 0.24 for unicenter vs multicenter, P = 0.86 for age ≥ 65 years and < 65 years. There was significant heterogeneity across studies (I2 = 85.4%, P < 0.001) as well as evidence of publication bias (funnel plot asymmetry test, P = 0.002).
CONCLUSION
This meta-analysis provides further evidence that PPI use is associated with an increased risk for development of CDI. Further high-quality, prospective studies are needed to assess whether this association is causal.
Keywords: Proton pump inhibitors, Clostridium difficile infection, Risk, Systematic review, Meta-analysis
Core tip: A possible association between the use of proton pump inhibitors (PPIs) and the risk of Clostridium difficile infection (CDI) have been su-ggested by several studies. This meta-analysis, including the largest number of studies published to date found the risk of CDI almost two-times higher in PPIs users than in nonusers. Because all the studies analyzed were observational, the causality could not be confirmed. Nevertheless, clinicians should be aware of such potential association and prescribe the PPIs only where they are clearly indicated.
INTRODUCTION
Over the past two decades Clostridium difficile (C. difficile) infection (CDI) has registered an increasing trend worldwide both in incidence and severity[1-5], with healthcare costs varying between 1.2 and 4.7 billion dollars each year in the United States alone[6-9]. In addition to the broad-spectrum antimicrobial therapy which has been the most prominent causative factor for CDI[10,11], other potential risk factors have been identified such as: advanced age, hospitalization [particularly in intensive care units (ICU)], immunosuppression, renal insufficiency, hypoalbuminemia, lengthy hospital stay, the use of nasogastric tubes, invasive gastrointestinal procedures, chemotherapy, the presence of comorbidities, environment-related factors, and the emergence of a hypervirulent strain of the bacterium known as North American pulso-type 1 in some areas[12-21]. However, there might be some other risk factors for the CDI epidemic in the recent years despite tighter control on the use of antibiotics and stricter control policies on hospital-related infections[17]. A possible association between the use of proton pump inhibitors (PPIs) and the development of CDI has been suggested and numerous studies have examined it, reporting conflicting results[22-43].
Since their release in the late 1980s, PPIs have become some of the most widely prescribed agents both in outpatient and inpatient settings throughout the world[44-53], with sales totalling billions dollars worldwide[54,55]. These drugs have proven effective in the treatment of ulcer disease (including bleeding peptic ulcer), gastroesophageal reflux disease, Helicobacter pylori (in combination with antibiotics), Zollinger-Ellison syndrome, in the prophylaxis of upper gastrointestinal complications with nonsteroidal anti-inflammatory drugs (NSAIDs) therapy, stress ulcer prophylaxis in ICU patients, and functional dyspepsia[50,53,56-60]. The widespread use of PPIs during the last 25 years in clinical practice is the result not only of their high efficacy but also of their excellent safety profile, proving to be one of the safest class of medication used in gastroenterology[57,61-64].
Nevertheless, like in the case of other drugs, PPIs are not as safe as it has been thought and more recently, concerns have been raised about their potential association with pneumonia[65-67], bone fractures[68-70], interstitial nephritis and acute kidney injury[71]. More recently, reports of other potential PPIs adverse events such as risk for chronic kidney disease[72,73], dementia[74], spontaneous bacterial peritonitis[75,76], acute myocardial infarction[77,78], micronutrient deficiency (magnesium, calcium, iron)[79,80] were published, although the quality of evidence for these is consistently low to very low[81].
An association between PPIs use and CDI is, at least theoretically, rational. Thus, intestinal homeostasis is maintained by host defense mechanisms in which gastric acid plays an important role as a barrier to ingested bacteria and bacterial overgrowth[82]. PPIs therapy profoundly inhibits gastric acid production leading to the proliferation of spores and their ability to convert to a vegetative form of C. difficile [83]. Moreover, PPIs impair leukocyte function by inhibiting phagocytosis and acidification of phagolysosome[84].
Several systematic reviews and meta-analyses have reported conflicting results regarding the association between PPIs use and increased risk of CDI. Thus, no less than six meta-analyses[85-90] found a significant association between PPIs therapy and increased risk of CDI. These findings were also supported by several studies[19,22-26,39,91-114] which reported a risk for CDI two or three times higher in PPIs users than in nonusers. Moreover, the United States Food and Drug Administration (FDA) informed the public about a possible correlation between PPIs use and CDI[115]. Still, other studies and meta-analyses have failed to associate PPIs use with the development of CDI[11,27,34,38,40-43,116-123]. It should be mentioned that PPIs continue to be among the most used drugs despite the above mentioned concerns about long-term side effects. Furthermore, beside a marked overuse of PPIs, over half of prescriptions are for non-indicated reasons[29]. One study reported that 60.7% of patients with CDI used PPIs, of whom only 47.1% had an evidence-based indication[30].
The aim of this systematic review and meta-analysis is to summarize data on the association between PPIs use and the risk of CDI as presented in the published studies.
MATERIALS AND METHODS
Information sources
A systematic literature search was independently conducted by four study investigators (Girleanu I, Stoica OC, Singeap AM and Chiriac SA) using a variety of databases including MEDLINE/PubMed, Web of Science (ISI Web of Knowledge), Scopus, EMBASE, Science Direct, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Excerpta Medica Database, and Cochrane Library, from January 1990 (the first PPI received FDA approval in 1989) to March 2017. The database searches were performed using the following medical subject heading (MeSH) terms: “proton pump inhibitors”, “acid suppressive therapy”, “omeprazole”, “pantoprazole”, “lansoprazole”, “rabeprazole”, “esomeprazole”, combined with “C. difficile infection”, “C. difficile-associated diarrhea”, “pseudomembranous colitis”. Reference lists of all retrieved papers were hand-searched to identify any additional studies that may have been missed in the computed-assisted literature search. The investigation was limited to studies performed in adult human beings, written and published in English, French, and German, in any geographic region.
Inclusion and exclusion criteria
Selection of the studies.Inclusion and exclusion criteria were established a priori by two authors (Trifan A and Stanciu C). First, duplicate citations were identified and removed, then three of us (Ciobica A, Maxim R and Singeap AM) independently reviewed the titles and abstracts of the studies and excluded those which did not answer the search question. Adult case-control and cohort studies providing information on the association between PPI therapy and the development of CDI were included. Studies conducted on pediatric patients, systematic reviews and meta-analyses, consensus documents, studies using PPIs simultaneously with H2 receptor antagonists (H2RA) or reporting exclusively on H2RA, case reports, editorials, protocols, and studies presented only as abstracts were excluded. There was no restriction related to the type of PPI regimen or diagnostic methods of CDI. Any disagreements about study inclusion were resolved in consensus with a third author (Stanciu C or Trifan A) after the full-text of the potential study had been reviewed; all eligible studies were assessed in full. They were subsequently included in this meta-analysis only if reported odds ratios (ORs) or risk ratios (RRs) for (adjusted or unadjusted) case-control and cohort studies, respectively, or data for their calculation were available.
Data extraction
Extracted data were cross-checked independently by four authors (Girleanu I, Stoica OC, Chiriac SA and Ciobica A) from each included study using a standardized data extracting sheet which included the last name of first author, journal and year of publication, country where the study was carried out, study design, sample size, age (mean or median) and gender distribution of patients, duration of the PPI treatment, effect estimates ORs or RRs, and 95% confidence intervals (CIs) of PPI exposure with and without adjustment for confounding variables. Any disagreement between reviewers was resolved in consensus with a third reviewer (Stanciu C or Trifan A).
Study quality assessment
Assessment of study quality was made independently by two authors (Boiculese L and Girleanu I) using the Newcastle-Ottawa Quality Assessment Scale (NOS; ranging 0-9)[124] as recommended by the Cochrane Handbook for Systematic Reviews of Interventions[125]. The NOS comprises three domains: selection, comparability, and outcome for cohort studies or exposure for case-control studies. A maximum of four stars were awarded for selection, two stars for comparability, and three stars for exposure/outcome. Studies with cumulative score ≥ 7 were considered high quality, 6 stars to be of moderate quality, and less than 6 stars to be of low quality. When disagreement, after discussion with the third author (Trifan A or Stanciu C) a consensus was reached. The final analysis included 56 high and moderate quality studies.
As none of the studies was randomized, and all were observational (case-control and cohort), the methods used in our systematic review and meta-analysis followed the MOOSE (Meta-Analysis of Observational Studies in Epidemiology) criteria[126].
Statistical analysis
Meta-analyses were performed both for all studies together and separately for case-control and cohort studies using DerSimonian and Laird[127] random effects model due to expected heterogeneity between studies. Our primary analysis focused on the association between PPIs therapy and the risk for developing CDI and because all of PPIs have similar efficacy we have not performed meta-analyses stratified by type of PPIs. The results are reported as pooled ORs with 95%CIs for primary and subgroup analyses.
Heterogeneity between studies was assessed by I2 statistic and Cochran’s Q-statistic. The level of heterogeneity was considered as high when I2 > 75% or P < 0.10 for the Q statistic[128]. I2 values between 61%-75%, 30%-60%, and < 30% were considered to represent substantial, moderate and low level of heterogeneity, respectively[129]. Seven potential confounders were considered: study design, effect estimate (adjusted vs unadjusted), setting (community vs inpatient), number of centers (single center vs multicenter), age, study quality, and geographical region.
Publication bias was assessed quantitatively using Egger’s regression asymmetry test[130] and a P < 0.1 was considered statistically significant for asymmetry, and qualitatively by visual inspection of funnel plots of the logarithmic OR vs their standard errors[131]. Asymmetrical funnel plots were regarded to indicate high risk of publication bias.
Number needed to harm (NNH) estimates the number of patients needed to be treated with PPI for one additional person to have a CDI, and was calculated using the pooled OR (95%CI) from the meta-analysis and Patient Expected Event Rate (1.67%)[120].
All statistical tests were two tailed, and results associated with P < 0.05 (except for heterogeneity and publication bias) were considered significant. All analyses were performed using R version 3.2.3 software for the metaphor package 1.9-8, which provides a comprehensive collection of validated functions[132]. The statistical analyses of this study were performed by an expert in biostatistics from “Grigore T. Popa” University of Medicine and Pharmacy, Department of Medical Informatics and Biostatistics.
RESULTS
Search results
The initial online databases search identified 944 studies and 12 more were found from the reference lists of the articles retrieved. After reviewing all titles and abstracts, 216 studies were selected for full-text review, from which 56 studies were found to fulfill the inclusion criteria and were included in meta-analysis. Five of the 56 studies were published after the last meta-analysis (Figure 1).
Figure 1.
Study selection process.
Characteristics of included studies
The characteristics of the included studies are shown in Table 1. Of the included 56 studies, 40 (71.4%) were case-control, and 16 (28.6%) cohort studies, addressed to hospital-acquired (n = 43), community-acquired (n = 6), and both hospital and community-acquired CDI (n = 7). Most of the studies (n = 31) were single-center. The size of the study population ranged from 40 to 101796. In total, 356683 subjects were included, most of them from North-American and European studies (n = 46).
Table 1.
Characteristics of studies included in the meta-analysis
| Author, yr | Region | Study design | Centers | Setting | Sample size, n | Mean age, yr | Identified confounders | OR (95%CI) |
| Akhtar et al Shaheen[91], 2007 | America | Case-control | Unicenter | Inpatient | 1290 | Adjusted for age, sex, comorbidities , antibiotics, chemotherapy | 2.1 (1.6-2.7) | |
| Al-Tureihi et al[19], 2005 | America | Case-control | Unicenter | Inpatient | 53 | Adjusted for age, antibiotics | 3.1 (1.0-9.7) | |
| Aseeri et al[23], 2008 | America | Case-control | Unicenter | Inpatient | 188 | Adjusted for admission date, sex, age group, antibiotic use, patient location, and room type | 4.4 (2.3-8.2) | |
| Bajaj et al[133], 2010 | America | Case-control | Multicenter | Mixt | 162 | Adjusted for antibiotics, PPI | 37.6 (6.2-227.6) | |
| Barletta et al[92], 2014 | Asia | Case-control | Unicenter | Inpatient | 408 | Adjusted for PPI exposure, antibiotics, immunosuppression | 2.1 (1.2-3.8) | |
| Baxter et al[93], 2008 | America | Case-control | Multicenter | Inpatient | 4493 | Adjusted for antibiotics, age, hospital stay, other infections | 1.2 (1.0-1.4) | |
| Beaulieu et al[27], 2007 | Cohort | Unicenter | Inpatient | 827 | Adjusted for age, sex, length of stay, comorbidities, | 1.3 (0.9-2.0) | ||
| APACHE score, NGT feeding, tracheal tube | ||||||||
| placement, antibiotics | ||||||||
| Branch et al[94], 2007 | America | Case-control | Unicenter | Inpatient | 787 | 66.02 | No | 13.0 (7.5-22.7) |
| Buendgens et al[95], 2014 | Europe | Case-control | Multicenter | Inpatient | 3286 | Adjusted for age, sex, antibiotics, comorbidities, other treatment | 3.1 (1.1-8.7) | |
| Campbell et al[38], 2013 | America | Case-control | Unicenter | Inpatient | 96 | Adjusted for antibiotics, hospitalization | 2.2 (0.6-8.0) | |
| Cunningham et al[96], 2003 | Europe | Case-control | Unicenter | Inpatient | 320 | Adjusted for antibiotics and chemotherapy | 2.5 (1.5-4.1) | |
| Dalton et al[22], 2009 | America | Cohort | Multicenter | Inpatient | 14719 | 74.7 | Adjusted for number of medication groups, | 1.9 (1.4-2.7) |
| antibiotic days, age, length of stay, medical | ||||||||
| service, PPI days | ||||||||
| Debast et al[116], 2009 | Europe | Case-control | Unicenter | Inpatient | 154 | Adjusted for age, hospital stay, comorbidities, antibiotics | 1.1 (0.5-2.4) | |
| Dial et al[26], 2004 (case-control) | America | Case-control | Multicenter | Inpatient | 188 | Adjusted for age, antibiotics | 2.6 (1.3-5.0) | |
| Dial et al[26], 2004 (cohort) | America | Cohort | Multicenter | Inpatient | 1187 | Adjusted for age, antibiotics | 2.1 (1.2-3.5) | |
| Dial et al[98], 2005 | Europe | Case-control | Multicenter | Outpatient | 13563 | Adjusted for age, sex, antibiotics | 2.9 (2.4-3.5) | |
| Dial et al[97], 2006 | Europe | Case-control | Multicenter | Outpatient | 3484 | Adjusted for PPI and antibiotics | 3.5 (2.3-5.3) | |
| Dial et al[134], 2008 | America | Case-control | Multicenter | Outpatient | 9196 | 79.8 | Adjusted for age, sex, antibiotics, comorbidities, physician visits, hospital admissions, length of stay | 1.6 (1.3-1.9) |
| Dubberke et al[99], 2007 | America | Cohort | Unicenter | Inpatient | 36086 | Adjusted for age, admissions, antibiotics, albumin level, leukemia/lymphoma, mechanical ventilation, antimotility agents | 1.6 (1.3-2.1) | |
| Elseviers et al[100], 2015 | Europe | Case-control | Multicenter | Inpatient | 743 | 71.9 | Adjusted for age, co-morbidity, endoscopic procedures | 1.9 (1.1-3.4) |
| Faleck et al[42], 2016 | America | Cohort | Unicenter | Inpatient | 11230 | 66 | Adjusted for age, sex, antibiotics, | 0.6 (0.4-0.8) |
| comorbidities, length of stay | ||||||||
| Garzotto et al[43], 2015 | Europe | Case-control | Multicenter | Inpatient | 225 | No | 0.4 (0.2-0.8) | |
| Hebbard et al[135], 2017 | Asia | Case-control | Unicenter | Inpatient | 200 | 59.7 | Adjusted for age, chemotherapy, abdominal surgery, antibiotics | 2.4 (1.0-5.7) |
| Hensgens et al[117], 2011 | Europe | Case-control | Unicenter | Inpatient | 169 | Adjusted for age, co-morbidity, antibiotics, ICU stay | 1.1 (0.5-2.5) | |
| Howell et al[136], 2010 | America | Cohort | Unicenter | Inpatient | 101796 | 65.4 | Adjusted for age, comorbidities, antibiotics | 1.7 (1.3-2.1) |
| Ingle et al[40], 2011 | Asia | Cohort | Unicenter | Mixt | 99 | 47 | Adjusted for immunosuppression | 1.8 (0.4-7.4) |
| Ingle et al[118], 2013 | Asia | Case-control | Unicenter | Community | 150 | 45.3 | no | 2.3 (0.6-9.2) |
| Jayatilaka et al[101], 2007 | America | Case-control | Unicenter | Inpatient | 366 | Adjusted for PPI | 2.7 (1.6-4.8) | |
| Kazakova et al[102], 2006 | America | Case-control | Unicenter | Mixt | 195 | Adjusted for antibiotics, PPI, length of stay, | 5.0 (1.3-19.3) | |
| psychosis, depression | ||||||||
| Khan et al[39], 2012 | Asia | Cohort | Unicenter | Inpatient | 123 | Adjusted for surgery, PPI, antibiotics, hospitalization, | 3.2 (1.2-8.5) | |
| Underlying debilitating conditions | ||||||||
| Khanafer et al[119], 2013 | Europe | Cohort | Unicenter | Inpatient | 40 | 2.5 (0.6-9.6) | ||
| Kuntz et al[2], 2011 | America | Case-control | Unicenter | Mixt | 3344 | no | 2.3 (1.5-3.3) | |
| Kurti et al[3], 2015 | Europe | Case-control | Multicenter | Inpatient | 979 | 72.4 | Adjusted for antibiotics, PPI, length of stay, | 1.6 (1.1-2.2) |
| Kutty et al[41], 2010 | America | Case-control | Multicenter | Outpatient | 144 | 62 | No | 1.7 (0.7-4.0) |
| Lewis et al[103], 2016 | America | Cohort | Unicenter | Inpatient | 41663 | No | 6.4 (3.6-11.5) | |
| Lin et al[137], 2013 | Asia | Case-control | Multicenter | Inpatient | 86 | 59 | Age, sex, unit, | 10.1 (1.2-87.4) |
| antibiotics, | ||||||||
| length of stay | ||||||||
| Linney et al[24], 2010 | America | Case-control | Unicenter | Inpatient | 284 | Age, sex, discharge date and hospital unit, | 2.4 (1.4-4.3) | |
| antibiotics, diabetes mellitus, IBD, cancer, | ||||||||
| enteral feeding, length of stay | ||||||||
| Loo et al[120], 2005 | America | Case-control | Multicenter | Inpatient | 474 | no | 1.0 (0.7-1.4) | |
| Loo et al[138], 2011 | America | Cohort | Multicenter | Inpatient | 4143 | 67.4 | Adjusted for age, PPI, antibiotics, chemotherapy | 2.6 (1.7-4.0) |
| Lowe et al[121], 2006 | America | Case-control | Multicenter | Inpatient | 13692 | 78.7 | Adjusted for antibiotics, other medications, and | 0.9 (0.7-1.0) |
| comorbidities | ||||||||
| McFarland et al[122], 2007 | America | Case-control | Multicenter | Mixt | 368 | No | 0.8 (0.5-1.4) | |
| Mizui et al[104], 2013 | Asia | Case-control | Multicenter | Inpatient | 2716 | 71.7 | No | 3.2 (1.4-7.3) |
| Modena et al[105], 2005 | America | Case-control | Unicenter | Inpatient | 250 | Adjusted for macrolides, ICU, length of stay, infections | 3.3 (1.6-6.8) | |
| Mori et al[123], 2015 | Asia | Case-control | Unicenter | Outpatient | 78 | 58.2 | No | 0.4 (0.1-2.0) |
| Muto et al[106], 2005 | America | Case-control | Multicenter | Inpatient | 406 | Adjusted for PPI, antibiotics, diabetes mellitus, organ transplantation | 2.4 (1.3-4.4) | |
| Pakyz et al[107], 2014 | America | Case-control | Multicenter | Inpatient | 14164 | No | 1.4 (1.3-1.5) | |
| Peled et al[108], 2007 | America | Cohort | Unicenter | Inpatient | 217 | Adjusted for PPI, low albumin level, | 3.7 (1.5-9.3) | |
| Pepin et al[11], 2005 | America | Cohort | Unicenter | Inpatient | 5619 | Adjusted for age, length of stay, antibiotics | 1.0 (0.7-1.2) | |
| Ro et al[139], 2016 | Asia | Cohort | Unicenter | Inpatient | 1005 | 64.8 | Adjusted for age, antibiotics, comorbidities | 3.3 (1.5-7.2) |
| Roughead et al[109], 2016 | Asia | Cohort | Multicenter | Mixt | 54957 | Adjusted for antibiotics, PPI, length of stay, | 2.4 (1.9-3.1) | |
| Shah et al[34], 2000 | Europe | Case-control | Unicenter | Inpatient | 252 | No | 0.8 (0.4-1.5) | |
| Southern et al[110], 2010 | Europe | Cohort | Multicenter | Inpatient | 3904 | 65.5 | No | 2.3 (1.1-4.5) |
| Vesteinsdottir et al[111], 2012 | Europe | Case-control | Multicenter | Mixt | 333 | No | 1.6 (1.0-2.6) | |
| Yang et al[112], 2011 | Asia | Case-control | Multicenter | Inpatient | 1420 | 67.12 | No | 1.9 (1.3-2.7) |
| Yearsley et al[25], 2006 | Europe | Case-control | Unicenter | Inpatient | 308 | 79.1 | Adjusted for PPI, antibiotics, female sex | 1.9 (1.1-3.2) |
| Yip et al[140], 2001 | America | Case-control | Unicenter | Inpatient | 54 | No | 3.0 (0.8-11.1) |
CI: Confidence interval; IBD: Inflammatory bowel disease; ICU: Intensive care unit; PPI: Proton pump inhibitor; NGT: Naso-gastric tube; OR: Odds ratio.
Quality assessment
The median value of NOS quality assessment was 7, with a mean 6.67 ± 0.74, range 6-8. In studies reporting gender, the proportion of men ranged from 47% to 67%, and from those that reported the age, the average age ranged between 18 and 82.2 years. Thirty-eight studies identified confounding factors (sex, age, antibiotic use, comorbidities) used for adjustment of the association between PPI therapy and risk of CDI. The majority of the studies were retrospective (85.7%) and only 8 were prospective (14.3%). None of the studies was randomized.
Meta-analysis
Meta-analysis of all studies combined. The results of pooled analysis for all 56 studies showed a significant association between PPI therapy and the risk of CDI as compared with non-PPI users (OR = 1.99, CI: 1.73-2.30, P < 0.001) (Figure 2). There was significant heterogeneity of effects across studies (I2 = 85.41%; P < 0.001).
Figure 2.
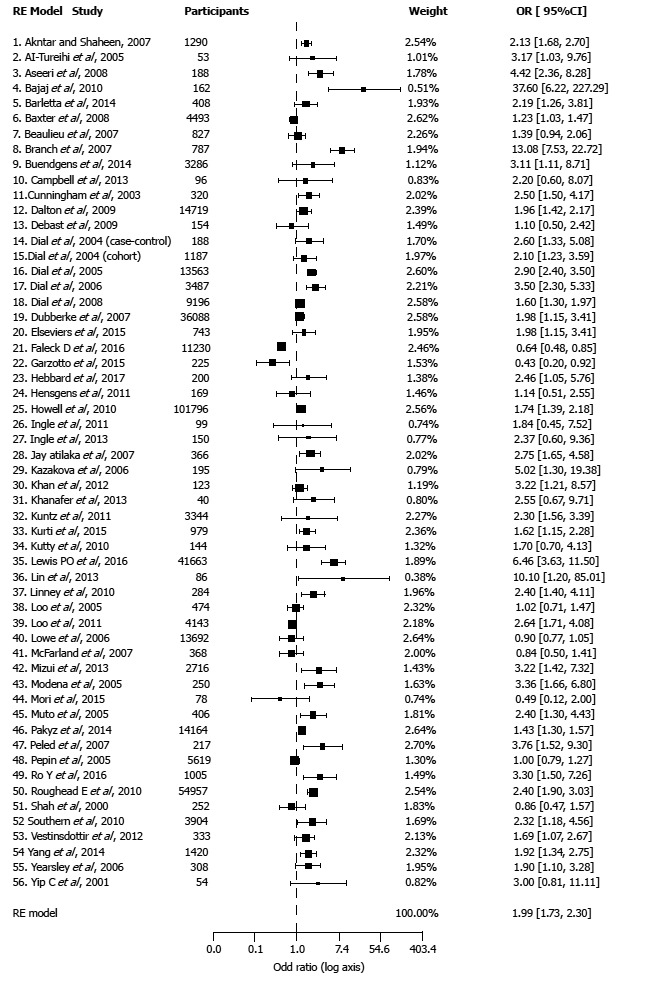
Forest plot of the meta-analysis.
Subgroup analyses of case-control and cohort studies also showed a significant higher risk of CDI with PPI use (Table 2). There was no significant difference of effects between cohort and case-control studies (P = 0.931). The pooled OR for the cohort studies was 1.98 similar to OR for case-control that was 2.0.
Table 2.
Subgroup analysis
| Subgroup analysis | No. of studies (n = 56) | ORs | 95%CI | Heterogeneity, I2, % | Heterogeneity between groups, P value |
| Study design | |||||
| Case-control | 40 | 2 | 1.68-2.38 | 85.54 | 0.931 |
| Cohort | 16 | 1.98 | 1.51-2.59 | 85.99 | |
| Study type | |||||
| Adjusted | 38 | 1.95 | 1.67-2.27 | 85.02 | 0.856 |
| Unadjusted | 18 | 2.02 | 1.41-2.91 | 85.58 | |
| Centers | |||||
| Unicentric | 31 | 2.18 | 1.72-2.75 | 83.99 | 0.241 |
| Multicentric | 25 | 1.82 | 1.51-2.19 | 86.97 | |
| Type | |||||
| Inpatient | 43 | 1.95 | 1.67-2.29 | 84.99 | 0.868 |
| Outpatient | 6 | 2.1 | 1.36-3.24 | 84.84 | |
| Mixt | 7 | 2.19 | 1.39-3.45 | 76.77 | |
| Region | |||||
| Europe | 14 | 1.78 | 1.35-2.34 | 74.33 | 0.231 |
| America | 31 | 2 | 1.67-2.40 | 88.58 | |
| Asia | 11 | 2.31 | 1.96-2.72 | 89.18 | |
| Age | |||||
| Age < 65 yr | 6 | 2.06 | 1.11-3.81 | 35.39 | 0.86 |
| Age ≥ 65 yr | 13 | 1.93 | 1.40-2.68 | 92.11 | |
| NOS | |||||
| NOS ≥ 7 | 26 | 1.88 | 1.55-2.28 | 87.65 | 0.441 |
| NOS < 7 | 30 | 2.11 | 1.69-2.62 | 81.98 |
CI: Confidence interval; NOS: Newcastle-Ottawa Quality Assessment Scale; ORs: Odds ratio.
The association remained also significant after limiting meta-analysis to studies with both adjusted (OR = 1.95, CI: 1.67-2.27, P < 0.001) and unadjusted data (OR = 2.02, CI: 1.41-2.91, P < 0.001). There was also no significant difference of effects between adjusted and unadjusted studies (P = 0.856).
PPIs use was found to be associated with an increased risk of CDI in both single-center studies (OR = 2.18, 95%CI: 1.72-2.75) and multicenter studies (OR = 1.82, 95%CI: 1.51-2.19).
There was no significant difference between inpatients and outpatients regarding CDI risk (P = 0.868). For both inpatients and outpatients the PPIs use almost doubled the risk of CDI (OR = 1.95, OR = 2.10, respectively).
When grouped by region, a direct association was found in the European group (OR = 1.78, 95%CI: 1.35-2.34), the North American group (OR = 2.00, 95%CI: 1.67-2.40), while the highest risk of CDI after PPI treatment was demonstrated in the Asian group (OR = 2.31, 95%CI: 1.96-2.72).
The subgroup of high-quality studies (NOS ≥ 7) showed a direct association (OR = 1.88, 95%CI: 1.55-2.28) between PPIs and risk of CDI, and this association was also significant in the medium-quality group (OR = 2.11, 95%CI: 1.69-2.62), with no difference between the two groups (P = 0.441).
There was no statistical difference regarding the risk for CDI for elderly (≥ 65 years) compared with younger group (< 65 years) (P = 0.860).
Publication bias
We have drawn the funnel plot for 3 levels of confidence interval (90%, 95% and 99% corresponding to shades white, gray and dark gray) (Figure 3). The Egger’s test of asymmetry proved no significance (Z = 0.3699, P = 0.711).
Figure 3.
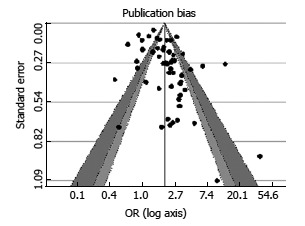
Funnel plot with 95% confidence limits.
Number needed to harm
Based on reported incidence of CDI (at 14 d after hospital admission) of 1.67% in patients who have not used PPI, we estimate a NNH of 63 (95%CI: 48-78), if these patients will receive PPIs.
DISCUSSION
This systematic review and meta-analysis which includes 56 studies and 356683 subjects[2,3,11,19,22-27,34,38-43,91-112,116-123,133-140] found a signi-ficant association between PPI therapy and the risk for CDI development. Both the overall pooled estimates (OR = 1.99, CI: 1.73-2.30, P < 0.001) and subgroup analyses showed a significant increased risk for CDI in patients on PPI therapy compared to nonusers, despite substantial statistical heterogeneity among studies and evidence of publication bias. Thus, in line with previous meta-analyses, our results add further evidence to PPIs use as a risk factor for development of CDI [85-89].
Since 2001, when Yip et al[140] first suggested a possible association between PPIs use and the risk of CDI, other studies, systematic reviews, and meta-analyses have reported such an association. It should be mentioned that a decade earlier (1993), Walker et al[141] suggested that the H2RAs therapy was a potential risk factor for CDI. In an earlier systematic review which included 11 studies with 126999 patients, Leonard et al[113] reported a significant association between PPI therapy and CDI (OR = 2.05, 95%CI: 1.47-2.85) although there was significant heterogeneity among the studies (χ2 = 50.9, P < 0.0001). During the last years, six meta-analyses have been published on this topic, and all reported a positive association between PPIs use and the risk for CDI. Thus, Janarthanan et al[88] in a meta-analysis including 23 observational studies with nearly 300000 patients found a 65% increase in the incidence of CDI among PPIs users with an estimated risk of 1.69 and 95%CI from 1.395 to 1.974. In another meta-analysis (30 studies, 202965 patients), Desphande et al[85] reported that PPI therapy was associated with a 2-fold increase in risk for CDI, but their study is limited by unadjusted risk estimates. Recently, the same team[90] performed a meta-analysis examining the relationship between PPI therapy and the risk for recurrent CDI, and found a positive association with the pooled risk ratio of 1.58 (95%CI: 1.13-2.21). A third meta-analysis by Kwok et al[87] including 42 studies (313000 participants) also found a statistically significant association between PPIs use and the risk for CDI compared with non-users (OR = 2.51; 95%CI: 1.47-2.85; P = 0.05). Tleyjeh et al[86] in a systematic review and meta-analysis including 51 observational studies (37 case-control and 14 cohort) examining healthcare and community-associated CDI, found a very low quality evidence for an association between PPI therapy and CDI not supporting a cause-effect relationship. Authors reported a pooled OR of 1.65 (95%CI: 1.47-1.85) with evidence of publication bias and significant statistical heterogeneity among the studies (I2 = 89.9%). More recently, Arriola et al[89] suggest, in a meta-analysis including only inpatients, that PPIs use significantly increases the risk of hospital-acquired CDI (OR = 1.81). Bavishi et al[114] in a systematic review regarding the use of PPI and increased susceptibility to enteric infection found 27 studies evaluating an association between PPI therapy and the risk of CDI, 17 of which reported a significant association. Based on an analysis of 28 studies, US FDA issued a warning on the risk of CDI with PPIs use[115], and similar warnings are found in CDI treatment guidelines[142].
Several studies reported that PPIs use is also a risk factor for community-acquired CDI. Dial et al[26], in a study including over 1000 cases of community-acquired CDI, found that patients who had received PPIs within the previous 90 d had a nearly 3-fold increased risk for CDI. A similar result was reported by Kutty et al[41] who found a 2-3-fold increased for community-acquired CDI in patients treated with PPIs within the previous 6 mo. Marwick et al[143] in a study including patients aged 65 years or older identified all cases of community-acquired CDI and found that patients prescribed PPIs within the previous 6 mo had a 1.7-fold increased risk for CDI compared to matched controls. A study assessing the epidemiology of community-acquired CDI found rates of PPI use of nearly 30% among patients with this infection compared to less than 3% in the general population[144]. These results indicate a similar degree of association between PPIs use and CDI risk, be it community-acquired CDI or hospital-acquired CDI[145].
Nevertheless, the association between PPIs use and the risk for CDI remains to a certain extent controversial despite the results reported above, as several studies failed to find such an association[11,27,34,94,122]. Beaulieu et al[27] found that the use of gastric acid-suppression therapy does not predispose to development of CDI, while McFarland et al[122] reported no relation between CDI and the use of PPIs. Branch et al[94] found that PPI use did not increase the incidence of CDI in hospitalized patients.
The mechanism by which PPI therapy contributes to an increased risk of CDI is unclear. It has been proposed that a vegetative form of C. difficile survives in conditions of gastric pH greater than 4[114]-the threshold for enteric infections acquisition, including C. difficile. Howell et al[136] reported that the risk of nosocomial CDI rose with increasing levels of acid suppression. Hegarty et al[146] reported that PPI therapy decreased the expression of genes holding an important role in colonocyte integrity, thus favoring the development of CDI. Other studies show that long-term use of PPIs decreases microbial diversity, a condition found in patients with CDI[147].
As we have already mentioned, our subgroup analyses also showed an increased risk for CDI. There were no significant differences of effects between cohort and case-control studies, adjusted and unadjusted data, single-center and multicenter studies, hospitalized-and community-acquired CDI or among geographic regions. Advanced age is a well-known risk factor for CDI. To our surprise, we found no increased risk of CDI in elderly patients (≥ 65 years) using PPIs compared with youngers (OR = 1.93 vs OR = 2.06, P = 0.860). A possible explanation is that many of such patients may have atrophic gastritis with low gastric acid output[148] and PPIs use cannot further lower gastric acid secretion, without any additional risk of CDI[32].
As data regarding the association between PPI therapy and risk of CDI are supported only by observational studies, a final estimation of the real risk is not possible. It should be mentioned that randomized placebo-controlled clinical trials evaluating the association of PPIs use and the risk for CDI are ethically unfeasible and therefore, such studies could not be performed in the future. Thus, a weak association between PPI therapy and CDI does not confirm causality and could be the result of bias and uncontrolled confounding (e.g., comorbidities, comedication use, etc.) which were lacking in most studies.
Our meta-analysis has some strengths such as the largest number of studies published to date, adjusted effect estimates concerning the association between PPI use and the risk of CDI, and subgroup analyses based on age, region, type, design and quality of the study. However, it also has several limitations: the included studies were observational, influenced by confounding variables despite statistical adjustment, the significant heterogeneity among most of them and lack of information regarding the dose and duration of PPI use as well as patient compliance to PPI therapy.
Although the above presented data from several meta-analyses and many studies demonstrated an association between PPI therapy and the risk for development of CDI, PPIs continue to be overused even in patients who are at high risk of CDI, because they are still considered “safe” drugs by most physicians. There is evidence that over half of PPI users who developed CDI had no valid indications for such therapy[25]. While in many countries PPIs are now totally available as over-the-counter medication, clinicians should inform their patients about the risk of CDI when PPIs are used on the long-term and without valid indication.
In spite of the aforementioned limitations of our and several other meta-analyses, clinicians should be aware of the risk of CDI when prescribing long-term PPI therapy, particularly in patients at high risk (e.g., hospitalized patients on antibiotics). It should be underlined that PPIs remain, on the whole, a safe group of drugs[149], providing enormous benefits when prescribed for well-established indications. Unfortunately, many prescriptions fall outside accepted indications[90].
In conclusion, this systematic review and meta-analysis provides further evidence that PPI use significantly increases the risk for developing CDI, despite the substantial heterogeneity and publication bias present among studies. Due to the fact that all the studies included in our analysis are observational and cannot confirm causality, further large, high quality, prospective studies are needed to assess the association between PPI use and the risk of CDI.
COMMENTS
Background
Proton pump inhibitors (PPIs) are among the most widely prescribed agents by gastroenterologists because of their high efficacy and excellent safety profile. However, more recently, concerns have been raised about association between PPI therapy and several potentially serious adverse events including Clostridium difficile (C. difficile) infection (CDI). This systematic review and meta-analysis explored the existing evidence regarding the association of PPI therapy and CDI.
Research frontiers
Many observational studies and meta-analyses have reported conflicting results regarding the association between PPI therapy and the risk for CDI.
Innovations and breakthroughs
This systematic review and meta-analysis, including the largest number of studies published to date, provides further evidence that PPI therapy is associated with an increased risk for development of CDI. Because all the studies analyzed were observational, with inherent limitations, the causality could not be confirmed.
Applications
Although our systematic review and meta-analysis, in line with previous studies and meta-analyses, reported an association between PPI therapy and the risk for development of CDI, such association remains controversial and a final estimation of the real risk has not been established. Further high-quality, prospective studies are needed to assess whether this association is causal. Until then, clinicians should be aware that long-term PPI therapy may be associated with the risk of CDI, and prescribe the PPIs in the lowest effective dose only to patients with a clear indication.
Terminology
PPIs are a group of potent inhibitors of gastric acid secretion. CDI is a symptomatic infection due to the spore-forming bacterium C. difficile.
Peer-review
This manuscript is an interesting, informative and well-presented meta-analysis on PPI therapy and risk of C. difficile infection.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Romania
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: The authors deny any conflict of interest.
Data sharing statement: No additional data are available.
Peer-review started: July 17, 2017
First decision: August 10, 2017
Article in press: August 25, 2017
P- Reviewer: Chiba T, Garcia-Olmo D, Mohammad RN
S-Editor: Wei LJ L- Editor: A E- Editor: Ma YJ
Contributor Information
Anca Trifan, Institute of Gastroenterology and Hepatology, “St. Spiridon” Hospital, “Grigore T. Popa” University of Medicine and Pharmacy, 700111 Iasi, Romania.
Carol Stanciu, Institute of Gastroenterology and Hepatology, “St. Spiridon” Hospital, 700111 Iasi, Romania. stanciucarol@yahoo.com.
Irina Girleanu, Institute of Gastroenterology and Hepatology, “St. Spiridon” Hospital, “Grigore T. Popa” University of Medicine and Pharmacy, 700111 Iasi, Romania.
Oana Cristina Stoica, Institute of Gastroenterology and Hepatology, “St. Spiridon” Hospital, “Grigore T. Popa” University of Medicine and Pharmacy, 700111 Iasi, Romania.
Ana Maria Singeap, Institute of Gastroenterology and Hepatology, “St. Spiridon” Hospital, “Grigore T. Popa” University of Medicine and Pharmacy, 700111 Iasi, Romania.
Roxana Maxim, Institute of Gastroenterology and Hepatology, “St. Spiridon” Hospital, “Grigore T. Popa” University of Medicine and Pharmacy, 700111 Iasi, Romania.
Stefan Andrei Chiriac, Institute of Gastroenterology and Hepatology, “St. Spiridon” Hospital, “Grigore T. Popa” University of Medicine and Pharmacy, 700111 Iasi, Romania.
Alin Ciobica, Department of Research, Faculty of Biology, “Alexandru Ioan Cuza” University of Iasi, 700506 Iasi, Romania.
Lucian Boiculese, Department of Preventive Medicine and Interdisciplinarity, “Grigore. T. Popa” University of Medicine and Pharmacy, 700111 Iasi, Romania.
References
- 1.Khanna S, Pardi DS. The growing incidence and severity of Clostridium difficile infection in inpatient and outpatient settings. Expert Rev Gastroenterol Hepatol. 2010;4:409–416. doi: 10.1586/egh.10.48. [DOI] [PubMed] [Google Scholar]
- 2.Kuntz JL, Chrischilles EA, Pendergast JF, Herwaldt LA, Polgreen PM. Incidence of and risk factors for community-associated Clostridium difficile infection: a nested case-control study. BMC Infect Dis. 2011;11:194. doi: 10.1186/1471-2334-11-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurti Z, Lovasz BD, Mandel MD, Csima Z, Golovics PA, Csako BD, Mohas A, Gönczi L, Gecse KB, Kiss LS, et al. Burden of Clostridium difficile infection between 2010 and 2013: Trends and outcomes from an academic center in Eastern Europe. World J Gastroenterol. 2015;21:6728–6735. doi: 10.3748/wjg.v21.i21.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Honda H, Dubberke ER. The changing epidemiology of Clostridium difficile infection. Curr Opin Gastroenterol. 2014;30:54–62. doi: 10.1097/MOG.0000000000000018. [DOI] [PubMed] [Google Scholar]
- 5.Dubberke ER, Butler AM, Yokoe DS, Mayer J, Hota B, Mangino JE, Khan YM, Popovich KJ, Fraser VJ. Multicenter study of Clostridium difficile infection rates from 2000 to 2006. Infect Control Hosp Epidemiol. 2010;31:1030–1037. doi: 10.1086/656245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, Bulsiewicz WJ, Gangarosa LM, Thiny MT, Stizenberg K, Morgan DR, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143:1179–1187.e1-e3. doi: 10.1053/j.gastro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Depestel DD, Aronoff DM. Epidemiology of Clostridium difficile infection. J Pharm Pract. 2013;26:464–475. doi: 10.1177/0897190013499521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reveles KR, Lee GC, Boyd NK, Frei CR. The rise in Clostridium difficile infection incidence among hospitalized adults in the United States: 2001-2010. Am J Infect Control. 2014;42:1028–1032. doi: 10.1016/j.ajic.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 9.Gabriel L, Beriot-Mathiot A. Hospitalization stay and costs attributable to Clostridium difficile infection: a critical review. J Hosp Infect. 2014;88:12–21. doi: 10.1016/j.jhin.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Thomas C, Stevenson M, Riley TV. Antibiotics and hospital-acquired Clostridium difficile-associated diarrhoea: a systematic review. J Antimicrob Chemother. 2003;51:1339–1350. doi: 10.1093/jac/dkg254. [DOI] [PubMed] [Google Scholar]
- 11.Pépin J, Saheb N, Coulombe MA, Alary ME, Corriveau MP, Authier S, Leblanc M, Rivard G, Bettez M, Primeau V, et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41:1254–1260. doi: 10.1086/496986. [DOI] [PubMed] [Google Scholar]
- 12.Taslim H. Clostridium difficile infection in the elderly. Acta Med Indones. 2009;41:148–151. [PubMed] [Google Scholar]
- 13.Lawrence SJ, Puzniak LA, Shadel BN, Gillespie KN, Kollef MH, Mundy LM. Clostridium difficile in the intensive care unit: epidemiology, costs, and colonization pressure. Infect Control Hosp Epidemiol. 2007;28:123–130. doi: 10.1086/511793. [DOI] [PubMed] [Google Scholar]
- 14.Eddi R, Malik MN, Shakov R, Baddoura WJ, Chandran C, Debari VA. Chronic kidney disease as a risk factor for Clostridium difficile infection. Nephrology (Carlton) 2010;15:471–475. doi: 10.1111/j.1440-1797.2009.01274.x. [DOI] [PubMed] [Google Scholar]
- 15.Schneeweiss S, Korzenik J, Solomon DH, Canning C, Lee J, Bressler B. Infliximab and other immunomodulating drugs in patients with inflammatory bowel disease and the risk of serious bacterial infections. Aliment Pharmacol Ther. 2009;30:253–264. doi: 10.1111/j.1365-2036.2009.04037.x. [DOI] [PubMed] [Google Scholar]
- 16.Dubberke ER, Olsen MA, Stwalley D, Kelly CP, Gerding DN, Young-Xu Y, Mahé C. Identification of Medicare Recipients at Highest Risk for Clostridium difficile Infection in the US by Population Attributable Risk Analysis. PLoS One. 2016;11:e0146822. doi: 10.1371/journal.pone.0146822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ananthakrishnan AN. Clostridium difficile infection: epidemiology, risk factors and management. Nat Rev Gastroenterol Hepatol. 2011;8:17–26. doi: 10.1038/nrgastro.2010.190. [DOI] [PubMed] [Google Scholar]
- 18.Raza S, Baig MA, Russell H, Gourdet Y, Berger BJ. Clostridium difficile infection following chemotherapy. Recent Pat Antiinfect Drug Discov. 2010;5:1–9. doi: 10.2174/157489110790112608. [DOI] [PubMed] [Google Scholar]
- 19.Al-Tureihi FI, Hassoun A, Wolf-Klein G, Isenberg H. Albumin, length of stay, and proton pump inhibitors: key factors in Clostridium difficile-associated disease in nursing home patients. J Am Med Dir Assoc. 2005;6:105–108. doi: 10.1016/j.jamda.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Morfin-Otero R, Garza-Gonzalez E, Aguirre-Diaz SA, Escobedo-Sanchez R, Esparza-Ahumada S, Perez-Gomez HR, Petersen-Morfin S, Gonzalez-Diaz E, Martinez-Melendez A, Rodriguez-Noriega E; Hospital Civil de Guadalajara, Fray Antonio Alcalde Clostridium difficile Team. Clostridium difficile outbreak caused by NAP1/BI/027 strain and non-027 strains in a Mexican hospital. Braz J Infect Dis. 2016;20:8–13. doi: 10.1016/j.bjid.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rotramel A, Poritz LS, Messaris E, Berg A, Stewart DB. PPI therapy and albumin are better predictors of recurrent Clostridium difficile colitis than choice of antibiotics. J Gastrointest Surg. 2012;16:2267–2273. doi: 10.1007/s11605-012-2037-9. [DOI] [PubMed] [Google Scholar]
- 22.Dalton BR, Lye-Maccannell T, Henderson EA, Maccannell DR, Louie TJ. Proton pump inhibitors increase significantly the risk of Clostridium difficile infection in a low-endemicity, non-outbreak hospital setting. Aliment Pharmacol Ther. 2009;29:626–634. doi: 10.1111/j.1365-2036.2008.03924.x. [DOI] [PubMed] [Google Scholar]
- 23.Aseeri M, Schroeder T, Kramer J, Zackula R. Gastric acid suppression by proton pump inhibitors as a risk factor for clostridum difficile-associated diarrhea in hospitalized patients. Am J Gastroenterol. 2008;103:2308–2313. doi: 10.1111/j.1572-0241.2008.01975.x. [DOI] [PubMed] [Google Scholar]
- 24.Linney S, Fernandes T, Einarson T, Sengar A, Walker JH, Mills A. Association Between Use of Proton Pump Inhibitors and a Clostridium difficile-Associated Disease Outbreak: Case-Control Study. Can J Hosp Pharm. 2010;63:31–37. doi: 10.4212/cjhp.v63i1.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yearsley KA, Gilby LJ, Ramadas AV, Kubiak EM, Fone DL, Allison MC. Proton pump inhibitor therapy is a risk factor for Clostridium difficile-associated diarrhoea. Aliment Pharmacol Ther. 2006;24:613–619. doi: 10.1111/j.1365-2036.2006.03015.x. [DOI] [PubMed] [Google Scholar]
- 26.Dial S, Alrasadi K, Manoukian C, Huang A, Menzies D. Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case-control studies. CMAJ. 2004;171:33–38. doi: 10.1503/cmaj.1040876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beaulieu M, Williamson D, Pichette G, Lachaine J. Risk of Clostridium difficile-associated disease among patients receiving proton-pump inhibitors in a Quebec medical intensive care unit. Infect Control Hosp Epidemiol. 2007;28:1305–1307. doi: 10.1086/521664. [DOI] [PubMed] [Google Scholar]
- 28.Pant C, Madonia P, Minocha A. Does PPI therapy predispose to Clostridium difficile infection? Nat Rev Gastroenterol He-patol. 2009;6:555–557. doi: 10.1038/nrgastro.2009.128. [DOI] [PubMed] [Google Scholar]
- 29.Rashid S, Rajan D, Iqbal J, Lipka S, Jacob R, Zilberman V, Shah M, Mustacchia P. Inappropriate Use of Gastric Acid Suppression Therapy in Hospitalized Patients with Clostridium difficile-Associated Diarrhea: A Ten-Year Retrospective Analysis. ISRN Gastroenterol. 2012;2012:902320. doi: 10.5402/2012/902320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choudhry MN, Soran H, Ziglam HM. Overuse and inappropriate prescribing of proton pump inhibitors in patients with Clostridium difficile-associated disease. QJM. 2008;101:445–448. doi: 10.1093/qjmed/hcn035. [DOI] [PubMed] [Google Scholar]
- 31.Patil R, Blankenship L. Proton Pump Inhibitors and Clostridium Difficile Infection: Are We Propagating an Already Rapidly Growing Healthcare Problem? Gastroenterology Res. 2013;6:171–173. doi: 10.4021/gr575w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cunningham R, Dial S. Is over-use of proton pump inhibitors fuelling the current epidemic of Clostridium difficile-associated diarrhoea? J Hosp Infect. 2008;70:1–6. doi: 10.1016/j.jhin.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 33.McCarthy DM. Proton pump inhibitor use and Clostridium difficile colitis: cause or coincidence? J Clin Gastroenterol. 2012;46:350–353. doi: 10.1097/MCG.0b013e31824b228f. [DOI] [PubMed] [Google Scholar]
- 34.Shah S, Lewis A, Leopold D, Dunstan F, Woodhouse K. Gastric acid suppression does not promote clostridial diarrhoea in the elderly. QJM. 2000;93:175–181. doi: 10.1093/qjmed/93.3.175. [DOI] [PubMed] [Google Scholar]
- 35.Naggie S, Miller BA, Zuzak KB, Pence BW, Mayo AJ, Nicholson BP, Kutty PK, McDonald LC, Woods CW. A case-control study of community-associated Clostridium difficile infection: no role for proton pump inhibitors. Am J Med. 2011;124:276.e1–276.e7. doi: 10.1016/j.amjmed.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 36.Vindigni SM, Surawicz CM. C. difficile Infection: Changing Epidemiology and Management Paradigms. Clin Transl Gastroenterol. 2015;6:e99. doi: 10.1038/ctg.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rostom A, Moayyedi P, Hunt R; Canadian Association of Gastroenterology Consensus Group. Canadian consensus guidelines on long-term nonsteroidal anti-inflammatory drug therapy and the need for gastroprotection: benefits versus risks. Aliment Pharmacol Ther. 2009;29:481–496. doi: 10.1111/j.1365-2036.2008.03905.x. [DOI] [PubMed] [Google Scholar]
- 38.Campbell KA, Phillips MS, Stachel A, Bosco JA 3rd, Mehta SA. Incidence and risk factors for hospital-acquired Clostridium difficile infection among inpatients in an orthopaedic tertiary care hospital. J Hosp Infect. 2013;83:146–149. doi: 10.1016/j.jhin.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 39.Khan FY, Abu-Khattab M, Anand D, Baager K, Alaini A, Siddique MA, Mohamed SF, Ali MI, Al Bedawi MM, Naser MS. Epidemiological features of Clostridium difficile infection among inpatients at Hamad General Hospital in the state of Qatar, 2006-2009. Travel Med Infect Dis. 2012;10:179–185. doi: 10.1016/j.tmaid.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 40.Ingle M, Deshmukh A, Desai D, Abraham P, Joshi A, Rodrigues C, Mankeshwar R. Prevalence and clinical course of Clostridium difficile infection in a tertiary-care hospital: a retrospective analysis. Indian J Gastroenterol. 2011;30:89–93. doi: 10.1007/s12664-011-0097-5. [DOI] [PubMed] [Google Scholar]
- 41.Kutty PK, Woods CW, Sena AC, Benoit SR, Naggie S, Frederick J, Evans S, Engel J, McDonald LC. Risk factors for and estimated incidence of community-associated Clostridium difficile infection, North Carolina, USA. Emerg Infect Dis. 2010;16:197–204. doi: 10.3201/eid1602.090953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Faleck DM, Salmasian H, Furuya EY, Larson EL, Abrams JA, Freedberg DE. Proton Pump Inhibitors Do Not Increase Risk for Clostridium difficile Infection in the Intensive Care Unit. Am J Gastroenterol. 2016;111:1641–1648. doi: 10.1038/ajg.2016.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodríguez Garzotto A, Mérida García A, Muñoz Unceta N, Galera Lopez MM, Orellana-Miguel MÁ, Díaz-García CV, Cortijo-Cascajares S, Cortes-Funes H, Agulló-Ortuño MT. Risk factors associated with Clostridium difficile infection in adult oncology patients. Support Care Cancer. 2015;23:1569–1577. doi: 10.1007/s00520-014-2506-7. [DOI] [PubMed] [Google Scholar]
- 44.Devlin JW, Welage LS, Olsen KM. Proton pump inhibitor formulary considerations in the acutely ill. Part 2: Clinical efficacy, safety, and economics. Ann Pharmacother. 2005;39:1844–1851. doi: 10.1345/aph.1G176. [DOI] [PubMed] [Google Scholar]
- 45.Attwood SE, Ell C, Galmiche JP, Fiocca R, Hatlebakk JG, Hasselgren B, Långström G, Jahreskog M, Eklund S, Lind T, et al. Long-term safety of proton pump inhibitor therapy assessed under controlled, randomised clinical trial conditions: data from the SOPRAN and LOTUS studies. Aliment Pharmacol Ther. 2015;41:1162–1174. doi: 10.1111/apt.13194. [DOI] [PubMed] [Google Scholar]
- 46.Kyne L, Hamel MB, Polavaram R, Kelly CP. Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin Infect Dis. 2002;34:346–353. doi: 10.1086/338260. [DOI] [PubMed] [Google Scholar]
- 47.Jacobson BC, Ferris TG, Shea TL, Mahlis EM, Lee TH, Wang TC. Who is using chronic acid suppression therapy and why? Am J Gastroenterol. 2003;98:51–58. doi: 10.1111/j.1572-0241.2003.07186.x. [DOI] [PubMed] [Google Scholar]
- 48.Kelly OB, Dillane C, Patchett SE, Harewood GC, Murray FE. The Inappropriate Prescription of Oral Proton Pump Inhibitors in the Hospital Setting: A Prospective Cross-Sectional Study. Dig Dis Sci. 2015;60:2280–2286. doi: 10.1007/s10620-015-3642-8. [DOI] [PubMed] [Google Scholar]
- 49.Craig DG, Thimappa R, Anand V, Sebastian S. Inappropriate utilization of intravenous proton pump inhibitors in hospital practice--a prospective study of the extent of the problem and predictive factors. QJM. 2010;103:327–335. doi: 10.1093/qjmed/hcq019. [DOI] [PubMed] [Google Scholar]
- 50.Moayyedi P, Delaney BC, Vakil N, Forman D, Talley NJ. The efficacy of proton pump inhibitors in nonulcer dyspepsia: a systematic review and economic analysis. Gastroenterology. 2004;127:1329–1337. doi: 10.1053/j.gastro.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 51.Sebastian SS, Kernan N, Qasim A, O’Morain CA, Buckley M. Appropriateness of gastric antisecretory therapy in hospital practice. Ir J Med Sci. 2003;172:115–117. doi: 10.1007/BF02914494. [DOI] [PubMed] [Google Scholar]
- 52.Haroon M, Yasin F, Gardezi SK, Adeeb F, Walker F. Inappropriate use of proton pump inhibitors among medical inpatients: a questionnaire-based observational study. JRSM Short Rep. 2013;4:2042533313497183. doi: 10.1177/2042533313497183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pappas M, Jolly S, Vijan S. Defining Appropriate Use of Proton-Pump Inhibitors Among Medical Inpatients. J Gen Intern Med. 2016;31:364–371. doi: 10.1007/s11606-015-3536-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ladd AM, Panagopoulos G, Cohen J, Mar N, Graham R. Potential costs of inappropriate use of proton pump inhibitors. Am J Med Sci. 2014;347:446–451. doi: 10.1097/MAJ.0b013e31829f87d5. [DOI] [PubMed] [Google Scholar]
- 55.Shaheen NJ, Hansen RA, Morgan DR, Gangarosa LM, Ringel Y, Thiny MT, Russo MW, Sandler RS. The burden of gastrointestinal and liver diseases, 2006. Am J Gastroenterol. 2006;101:2128–2138. doi: 10.1111/j.1572-0241.2006.00723.x. [DOI] [PubMed] [Google Scholar]
- 56.Barkun AN, Bardou M, Pham CQ, Martel M. Proton pump inhibitors vs. histamine 2 receptor antagonists for stress-related mucosal bleeding prophylaxis in critically ill patients: a meta-analysis. Am J Gastroenterol. 2012;107:507–520 ; quiz 521. doi: 10.1038/ajg.2011.474. [DOI] [PubMed] [Google Scholar]
- 57.Yachimski PS, Farrell EA, Hunt DP, Reid AE. Proton pump inhibitors for prophylaxis of nosocomial upper gastrointestinal tract bleeding: effect of standardized guidelines on prescribing practice. Arch Intern Med. 2010;170:779–783. doi: 10.1001/archinternmed.2010.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alhazzani W, Alenezi F, Jaeschke RZ, Moayyedi P, Cook DJ. Proton pump inhibitors versus histamine 2 receptor antagonists for stress ulcer prophylaxis in critically ill patients: a systematic review and meta-analysis. Crit Care Med. 2013;41:693–705. doi: 10.1097/CCM.0b013e3182758734. [DOI] [PubMed] [Google Scholar]
- 59.Xiao YL, Peng S, Tao J, Wang AJ, Lin JK, Hu PJ, Chen MH. Prevalence and symptom pattern of pathologic esophageal acid reflux in patients with functional dyspepsia based on the Rome III criteria. Am J Gastroenterol. 2010;105:2626–2631. doi: 10.1038/ajg.2010.351. [DOI] [PubMed] [Google Scholar]
- 60.Cohen ME, Hathway JM, Salmasian H, Liu J, Terry M, Abrams JA, Freedberg DE. Prophylaxis for Stress Ulcers With Proton Pump Inhibitors Is Not Associated With Increased Risk of Bloodstream Infections in the Intensive Care Unit. Clin Gastroenterol Hepatol. 2017;15:1030–1036.e1. doi: 10.1016/j.cgh.2016.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang YX, Metz DC. Safety of proton pump inhibitor exposure. Gastroenterology. 2010;139:1115–1127. doi: 10.1053/j.gastro.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 62.Heidelbaugh JJ, Goldberg KL, Inadomi JM. Overutilization of proton pump inhibitors: a review of cost-effectiveness and risk [corrected] Am J Gastroenterol. 2009;104 Suppl 2:S27–S32. doi: 10.1038/ajg.2009.49. [DOI] [PubMed] [Google Scholar]
- 63.Brunner G, Athmann C, Schneider A. Long-term, open-label trial: safety and efficacy of continuous maintenance treatment with pantoprazole for up to 15 years in severe acid-peptic disease. Aliment Pharmacol Ther. 2012;36:37–47. doi: 10.1111/j.1365-2036.2012.05106.x. [DOI] [PubMed] [Google Scholar]
- 64.Haastrup PF, Paulsen MS, Christensen RD, Søndergaard J, Hansen JM, Jarbøl DE. Medical and non-medical predictors of initiating long-term use of proton pump inhibitors: a nationwide cohort study of first-time users during a 10-year period. Aliment Pharmacol Ther. 2016;44:78–87. doi: 10.1111/apt.13649. [DOI] [PubMed] [Google Scholar]
- 65.Lambert AA, Lam JO, Paik JJ, Ugarte-Gil C, Drummond MB, Crowell TA. Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis. PLoS One. 2015;10:e0128004. doi: 10.1371/journal.pone.0128004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de Groot MC, Klungel OH, Leufkens HG, van Dijk L, Grobbee DE, van de Garde EM. Sources of heterogeneity in case-control studies on associations between statins, ACE-inhibitors, and proton pump inhibitors and risk of pneumonia. Eur J Epidemiol. 2014;29:767–775. doi: 10.1007/s10654-014-9941-0. [DOI] [PubMed] [Google Scholar]
- 67.Eurich DT, Sadowski CA, Simpson SH, Marrie TJ, Majumdar SR. Recurrent community-acquired pneumonia in patients starting acid-suppressing drugs. Am J Med. 2010;123:47–53. doi: 10.1016/j.amjmed.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 68.Cai D, Feng W, Jiang Q. Acid-suppressive medications and risk of fracture: an updated meta-analysis. Int J Clin Exp Med. 2015;8:8893–8904. [PMC free article] [PubMed] [Google Scholar]
- 69.Leontiadis GI, Moayyedi P. Proton pump inhibitors and risk of bone fractures. Curr Treat Options Gastroenterol. 2014;12:414–423. doi: 10.1007/s11938-014-0030-y. [DOI] [PubMed] [Google Scholar]
- 70.Eom CS, Park SM, Myung SK, Yun JM, Ahn JS. Use of acid-suppressive drugs and risk of fracture: a meta-analysis of observational studies. Ann Fam Med. 2011;9:257–267. doi: 10.1370/afm.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sierra F, Suarez M, Rey M, Vela MF. Systematic review: Proton pump inhibitor-associated acute interstitial nephritis. Aliment Pharmacol Ther. 2007;26:545–553. doi: 10.1111/j.1365-2036.2007.03407.x. [DOI] [PubMed] [Google Scholar]
- 72.Arora P, Gupta A, Golzy M, Patel N, Carter RL, Jalal K, Lohr JW. Proton pump inhibitors are associated with increased risk of development of chronic kidney disease. BMC Nephrol. 2016;17:112. doi: 10.1186/s12882-016-0325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arora S, Dellon ES. PPIs and Chronic Kidney Disease: Another Association to Worry About? Gastroenterology. 2016;151:366–368. doi: 10.1053/j.gastro.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 74.Wijarnpreecha K, Thongprayoon C, Panjawatanan P, Ungprasert P. Proton pump inhibitors and risk of dementia. Ann Transl Med. 2016;4:240. doi: 10.21037/atm.2016.06.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dam G, Vilstrup H, Watson H, Jepsen P. Proton pump inhibitors as a risk factor for hepatic encephalopathy and spontaneous bacterial peritonitis in patients with cirrhosis with ascites. Hepatology. 2016;64:1265–1272. doi: 10.1002/hep.28737. [DOI] [PubMed] [Google Scholar]
- 76.Xu HB, Wang HD, Li CH, Ye S, Dong MS, Xia QJ, Zhang AQ, Pan K, Ge XL, Dong JH. Proton pump inhibitor use and risk of spontaneous bacterial peritonitis in cirrhotic patients: a systematic review and meta-analysis. Genet Mol Res. 2015;14:7490–7501. doi: 10.4238/2015.July.3.25. [DOI] [PubMed] [Google Scholar]
- 77.Zhu W, Hong K. Potential Cardiovascular Risks of Proton Pump Inhibitors in the General Population. Int Heart J. 2017;58:163–166. doi: 10.1536/ihj.16-208. [DOI] [PubMed] [Google Scholar]
- 78.Shah NH, LePendu P, Bauer-Mehren A, Ghebremariam YT, Iyer SV, Marcus J, Nead KT, Cooke JP, Leeper NJ. Proton Pump Inhibitor Usage and the Risk of Myocardial Infarction in the General Population. PLoS One. 2015;10:e0124653. doi: 10.1371/journal.pone.0124653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Heidelbaugh JJ. Proton pump inhibitors and risk of vitamin and mineral deficiency: evidence and clinical implications. Ther Adv Drug Saf. 2013;4:125–133. doi: 10.1177/2042098613482484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hashimoto R, Matsuda T, Chonan A. Iron-deficiency anemia caused by a proton pump inhibitor. Intern Med. 2014;53:2297–2299. doi: 10.2169/internalmedicine.53.2743. [DOI] [PubMed] [Google Scholar]
- 81.Freedberg DE, Kim LS, Yang YX. The Risks and Benefits of Long-term Use of Proton Pump Inhibitors: Expert Review and Best Practice Advice From the American Gastroenterological Association. Gastroenterology. 2017;152:706–715. doi: 10.1053/j.gastro.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 82.Thorens J, Froehlich F, Schwizer W, Saraga E, Bille J, Gyr K, Duroux P, Nicolet M, Pignatelli B, Blum AL, et al. Bacterial overgrowth during treatment with omeprazole compared with cimetidine: a prospective randomised double blind study. Gut. 1996;39:54–59. doi: 10.1136/gut.39.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Amir I, Konikoff FM, Oppenheim M, Gophna U, Half EE. Gastric microbiota is altered in oesophagitis and Barrett’s oesophagus and further modified by proton pump inhibitors. Environ Microbiol. 2014;16:2905–2914. doi: 10.1111/1462-2920.12285. [DOI] [PubMed] [Google Scholar]
- 84.Zedtwitz-Liebenstein K, Wenisch C, Patruta S, Parschalk B, Daxböck F, Graninger W. Omeprazole treatment diminishes intra- and extracellular neutrophil reactive oxygen production and bactericidal activity. Crit Care Med. 2002;30:1118–1122. doi: 10.1097/00003246-200205000-00026. [DOI] [PubMed] [Google Scholar]
- 85.Deshpande A, Pant C, Pasupuleti V, Rolston DD, Jain A, Deshpande N, Thota P, Sferra TJ, Hernandez AV. Association between proton pump inhibitor therapy and Clostridium difficile infection in a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:225–233. doi: 10.1016/j.cgh.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 86.Tleyjeh IM, Bin Abdulhak AA, Riaz M, Alasmari FA, Garbati MA, AlGhamdi M, Khan AR, Al Tannir M, Erwin PJ, Ibrahim T, et al. Association between proton pump inhibitor therapy and clostridium difficile infection: a contemporary systematic review and meta-analysis. PLoS One. 2012;7:e50836. doi: 10.1371/journal.pone.0050836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kwok CS, Arthur AK, Anibueze CI, Singh S, Cavallazzi R, Loke YK. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am J Gastroenterol. 2012;107:1011–1019. doi: 10.1038/ajg.2012.108. [DOI] [PubMed] [Google Scholar]
- 88.Janarthanan S, Ditah I, Adler DG, Ehrinpreis MN. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am J Gastroenterol. 2012;107:1001–1010. doi: 10.1038/ajg.2012.179. [DOI] [PubMed] [Google Scholar]
- 89.Arriola V, Tischendorf J, Musuuza J, Barker A, Rozelle JW, Safdar N. Assessing the Risk of Hospital-Acquired Clostridium Difficile Infection With Proton Pump Inhibitor Use: A Meta-Analysis. Infect Control Hosp Epidemiol. 2016;37:1408–1417. doi: 10.1017/ice.2016.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Deshpande A, Pasupuleti V, Thota P, Pant C, Rolston DD, Hernandez AV, Donskey CJ, Fraser TG. Risk factors for recurrent Clostridium difficile infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2015;36:452–460. doi: 10.1017/ice.2014.88. [DOI] [PubMed] [Google Scholar]
- 91.Akhtar AJ, Shaheen M. Increasing incidence of clostridium difficile-associated diarrhea in African-American and Hispanic patients: association with the use of proton pump inhibitor therapy. J Natl Med Assoc. 2007;99:500–504. [PMC free article] [PubMed] [Google Scholar]
- 92.Barletta JF, Sclar DA. Proton pump inhibitors increase the risk for hospital-acquired Clostridium difficile infection in critically ill patients. Crit Care. 2014;18:714. doi: 10.1186/s13054-014-0714-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Baxter R, Ray GT, Fireman BH. Case-control study of antibiotic use and subsequent Clostridium difficile-associated diarrhea in hospitalized patients. Infect Control Hosp Epidemiol. 2008;29:44–50. doi: 10.1086/524320. [DOI] [PubMed] [Google Scholar]
- 94.Branch K, Yahl V, Kier K, Mertz N, Marques S. Gastric acid suppression by proton pump inhibitors as an independent risk factor for-associated diarrhea. P&T. 2007;32:432–437. [Google Scholar]
- 95.Buendgens L, Bruensing J, Matthes M, Dückers H, Luedde T, Trautwein C, Tacke F, Koch A. Administration of proton pump inhibitors in critically ill medical patients is associated with increased risk of developing Clostridium difficile-associated diarrhea. J Crit Care. 2014;29:696.e11–696.e15. doi: 10.1016/j.jcrc.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 96.Cunningham R, Dale B, Undy B, Gaunt N. Proton pump inhibitors as a risk factor for Clostridium difficile diarrhoea. J Hosp Infect. 2003;54:243–245. doi: 10.1016/s0195-6701(03)00088-4. [DOI] [PubMed] [Google Scholar]
- 97.Dial S, Delaney JA, Schneider V, Suissa S. Proton pump inhibitor use and risk of community-acquired Clostridium difficile-associated disease defined by prescription for oral vancomycin therapy. CMAJ. 2006;175:745–748. doi: 10.1503/cmaj.060284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dial S, Delaney JA, Barkun AN, Suissa S. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA. 2005;294:2989–2995. doi: 10.1001/jama.294.23.2989. [DOI] [PubMed] [Google Scholar]
- 99.Dubberke ER, Reske KA, Olsen MA, McMullen KM, Mayfield JL, McDonald LC, Fraser VJ. Evaluation of Clostridium difficile-associated disease pressure as a risk factor for C difficile-associated disease. Arch Intern Med. 2007;167:1092–1097. doi: 10.1001/archinte.167.10.1092. [DOI] [PubMed] [Google Scholar]
- 100.Elseviers MM, Van Camp Y, Nayaert S, Duré K, Annemans L, Tanghe A, Vermeersch S. Prevalence and management of antibiotic associated diarrhea in general hospitals. BMC Infect Dis. 2015;15:129. doi: 10.1186/s12879-015-0869-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jayatilaka S, Shakov R, Eddi R, Bakaj G, Baddoura WJ, DeBari VA. Clostridium difficile infection in an urban medical center: five-year analysis of infection rates among adult admissions and association with the use of proton pump inhibitors. Ann Clin Lab Sci. 2007;37:241–247. [PubMed] [Google Scholar]
- 102.Kazakova SV, Ware K, Baughman B, Bilukha O, Paradis A, Sears S, Thompson A, Jensen B, Wiggs L, Bessette J, et al. A hospital outbreak of diarrhea due to an emerging epidemic strain of Clostridium difficile. Arch Intern Med. 2006;166:2518–2524. doi: 10.1001/archinte.166.22.2518. [DOI] [PubMed] [Google Scholar]
- 103.Lewis PO, Litchfield JM, Tharp JL, Garcia RM, Pourmorteza M, Reddy CM. Risk and Severity of Hospital-Acquired Clostridium difficile Infection in Patients Taking Proton Pump Inhibitors. Pharmacotherapy. 2016;36:986–993. doi: 10.1002/phar.1801. [DOI] [PubMed] [Google Scholar]
- 104.Mizui T, Teramachi H, Tachi T, Tamura K, Shiga H, Komada N, Umeda M, Koda A, Aoyama S, Goto C, et al. Risk factors for Clostridium difficile-associated diarrhea and the effectiveness of prophylactic probiotic therapy. Pharmazie. 2013;68:706–710. [PubMed] [Google Scholar]
- 105.Modena S, Bearelly D, Swartz K, Friedenberg FK. Clostridium difficile among hospitalized patients receiving antibiotics: a case-control study. Infect Control Hosp Epidemiol. 2005;26:685–690. doi: 10.1086/502603. [DOI] [PubMed] [Google Scholar]
- 106.Muto CA, Pokrywka M, Shutt K, Mendelsohn AB, Nouri K, Posey K, Roberts T, Croyle K, Krystofiak S, Patel-Brown S, et al. A large outbreak of Clostridium difficile-associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use. Infect Control Hosp Epidemiol. 2005;26:273–280. doi: 10.1086/502539. [DOI] [PubMed] [Google Scholar]
- 107.Pakyz AL, Jawahar R, Wang Q, Harpe SE. Medication risk factors associated with healthcare-associated Clostridium difficile infection: a multilevel model case-control study among 64 US academic medical centres. J Antimicrob Chemother. 2014;69:1127–1131. doi: 10.1093/jac/dkt489. [DOI] [PubMed] [Google Scholar]
- 108.Peled N, Pitlik S, Samra Z, Kazakov A, Bloch Y, Bishara J. Predicting Clostridium difficile toxin in hospitalized patients with antibiotic-associated diarrhea. Infect Control Hosp Epidemiol. 2007;28:377–381. doi: 10.1086/513723. [DOI] [PubMed] [Google Scholar]
- 109.Roughead EE, Chan EW, Choi NK, Griffiths J, Jin XM, Lee J, Kimura M, Kimura T, Kubota K, Lai EC, et al. Proton pump inhibitors and risk of Clostridium difficile infection: a multi-country study using sequence symmetry analysis. Expert Opin Drug Saf. 2016;15:1589–1595. doi: 10.1080/14740338.2016.1238071. [DOI] [PubMed] [Google Scholar]
- 110.Southern WN, Rahmani R, Aroniadis O, Khorshidi I, Thanjan A, Ibrahim C, Brandt LJ. Postoperative Clostridium difficile-associated diarrhea. Surgery. 2010;148:24–30. doi: 10.1016/j.surg.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vesteinsdottir I, Gudlaugsdottir S, Einarsdottir R, Kalaitzakis E, Sigurdardottir O, Bjornsson ES. Risk factors for Clostridium difficile toxin-positive diarrhea: a population-based prospective case-control study. Eur J Clin Microbiol Infect Dis. 2012;31:2601–2610. doi: 10.1007/s10096-012-1603-0. [DOI] [PubMed] [Google Scholar]
- 112.Yang BK, Do BJ, Kim EJ, Lee JU, Kim MH, Kang JG, Kim HS, Kim KH, Jang MK, Lee JH, et al. The simple predictors of pseudomembranous colitis in patients with hospital-acquired diarrhea: a prospective observational study. Gut Liver. 2014;8:41–48. doi: 10.5009/gnl.2014.8.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Leonard J, Marshall JK, Moayyedi P. Systematic review of the risk of enteric infection in patients taking acid suppression. Am J Gastroenterol. 2007;102:2047–2056. doi: 10.1111/j.1572-0241.2007.01275.x. [DOI] [PubMed] [Google Scholar]
- 114.Bavishi C, Dupont HL. Systematic review: the use of proton pump inhibitors and increased susceptibility to enteric infection. Aliment Pharmacol Ther. 2011;34:1269–1281. doi: 10.1111/j.1365-2036.2011.04874.x. [DOI] [PubMed] [Google Scholar]
- 115.US Food and Drug Administration. 2012. Clostridium difficile-associated diarrhea can be associated with stomach acid drugs known as proton pump inhibitors (PPIs). RDA Drug Safety Communication. Available from: http://www.fda.gov/Drugs/DrugSafety/ucm 290510.htm. [Google Scholar]
- 116.Debast SB, Vaessen N, Choudry A, Wiegers-Ligtvoet EA, van den Berg RJ, Kuijper EJ. Successful combat of an outbreak due to Clostridium difficile PCR ribotype 027 and recognition of specific risk factors. Clin Microbiol Infect. 2009;15:427–434. doi: 10.1111/j.1469-0691.2009.02713.x. [DOI] [PubMed] [Google Scholar]
- 117.Hensgens MP, Goorhuis A, van Kinschot CM, Crobach MJ, Harmanus C, Kuijper EJ. Clostridium difficile infection in an endemic setting in the Netherlands. Eur J Clin Microbiol Infect Dis. 2011;30:587–593. doi: 10.1007/s10096-010-1127-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ingle M, Deshmukh A, Desai D, Abraham P, Joshi A, Gupta T, Rodrigues C. Clostridium difficile as a cause of acute diarrhea: a prospective study in a tertiary care center. Indian J Gastroenterol. 2013;32:179–183. doi: 10.1007/s12664-013-0303-8. [DOI] [PubMed] [Google Scholar]
- 119.Khanafer N, Touré A, Chambrier C, Cour M, Reverdy ME, Argaud L, Vanhems P. Predictors of Clostridium difficile infection severity in patients hospitalised in medical intensive care. World J Gastroenterol. 2013;19:8034–8041. doi: 10.3748/wjg.v19.i44.8034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Loo VG, Poirier L, Miller MA, Oughton M, Libman MD, Michaud S, Bourgault AM, Nguyen T, Frenette C, Kelly M, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med. 2005;353:2442–2449. doi: 10.1056/NEJMoa051639. [DOI] [PubMed] [Google Scholar]
- 121.Lowe DO, Mamdani MM, Kopp A, Low DE, Juurlink DN. Proton pump inhibitors and hospitalization for Clostridium difficile-associated disease: a population-based study. Clin Infect Dis. 2006;43:1272–1276. doi: 10.1086/508453. [DOI] [PubMed] [Google Scholar]
- 122.McFarland LV. Update on the changing epidemiology of Clostridium difficile-associated disease. Nat Clin Pract Gastroenterol Hepatol. 2008;5:40–48. doi: 10.1038/ncpgasthep1029. [DOI] [PubMed] [Google Scholar]
- 123.Mori N, Aoki Y. Clinical characteristics and risk factors for community-acquired Clostridium difficile infection: A retrospective, case-control study in a tertiary care hospital in Japan. J Infect Chemother. 2015;21:864–867. doi: 10.1016/j.jiac.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 124.Wells G, Shea B, O’connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute; Accessed on 2017-3-15. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 125.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions: Version 5.1.0. The Cochrane Collaboration 2011. Available from: http://www.cochrane-handbook.org.
- 126.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 127.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 128.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 130.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991;337:867–872. doi: 10.1016/0140-6736(91)90201-y. [DOI] [PubMed] [Google Scholar]
- 132.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48. [Google Scholar]
- 133.Bajaj JS, Ananthakrishnan AN, Hafeezullah M, Zadvornova Y, Dye A, McGinley EL, Saeian K, Heuman D, Sanyal AJ, Hoffmann RG. Clostridium difficile is associated with poor outcomes in patients with cirrhosis: A national and tertiary center perspective. Am J Gastroenterol. 2010;105:106–113. doi: 10.1038/ajg.2009.615. [DOI] [PubMed] [Google Scholar]
- 134.Dial S, Kezouh A, Dascal A, Barkun A, Suissa S. Patterns of antibiotic use and risk of hospital admission because of Clostridium difficile infection. CMAJ. 2008;179:767–772. doi: 10.1503/cmaj.071812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hebbard AIT, Slavin MA, Reed C, Trubiano JA, Teh BW, Haeusler GM, Thursky KA, Worth LJ. Risks factors and outcomes of Clostridium difficile infection in patients with cancer: a matched case-control study. Support Care Cancer. 2017;25:1923–1930. doi: 10.1007/s00520-017-3606-y. [DOI] [PubMed] [Google Scholar]
- 136.Howell MD, Novack V, Grgurich P, Soulliard D, Novack L, Pencina M, Talmor D. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med. 2010;170:784–790. doi: 10.1001/archinternmed.2010.89. [DOI] [PubMed] [Google Scholar]
- 137.Lin YC, Huang YT, Lee TF, Lee NY, Liao CH, Lin SY, Ko WC, Hsueh PR. Characteristics of patients with Clostridium difficile infection in Taiwan. Epidemiol Infect. 2013;141:2031–2038. doi: 10.1017/S0950268812002749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Loo VG, Bourgault AM, Poirier L, Lamothe F, Michaud S, Turgeon N, Toye B, Beaudoin A, Frost EH, Gilca R, et al. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med. 2011;365:1693–1703. doi: 10.1056/NEJMoa1012413. [DOI] [PubMed] [Google Scholar]
- 139.Ro Y, Eun CS, Kim HS, Kim JY, Byun YJ, Yoo KS, Han DS. Risk of Clostridium difficile Infection with the Use of a Proton Pump Inhibitor for Stress Ulcer Prophylaxis in Critically Ill Patients. Gut Liver. 2016;10:581–586. doi: 10.5009/gnl15324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yip C, Loeb M, Salama S, Moss L, Olde J. Quinolone use as a risk factor for nosocomial Clostridium difficile-associated diarrhea. Infect Control Hosp Epidemiol. 2001;22:572–575. doi: 10.1086/501954. [DOI] [PubMed] [Google Scholar]
- 141.Walker KJ, Gilliland SS, Vance-Bryan K, Moody JA, Larsson AJ, Rotschafer JC, Guay DR. Clostridium difficile colonization in residents of long-term care facilities: prevalence and risk factors. J Am Geriatr Soc. 1993;41:940–946. doi: 10.1111/j.1532-5415.1993.tb06759.x. [DOI] [PubMed] [Google Scholar]
- 142.Surawicz CM, Brandt LJ, Binion DG, Ananthakrishnan AN, Curry SR, Gilligan PH, McFarland LV, Mellow M, Zuckerbraun BS. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478–498; quiz 499. doi: 10.1038/ajg.2013.4. [DOI] [PubMed] [Google Scholar]
- 143.Marwick CA, Yu N, Lockhart MC, McGuigan CC, Wiuff C, Davey PG, Donnan PT. Community-associated Clostridium difficile infection among older people in Tayside, Scotland, is associated with antibiotic exposure and care home residence: cohort study with nested case-control. J Antimicrob Chemother. 2013;68:2927–2933. doi: 10.1093/jac/dkt257. [DOI] [PubMed] [Google Scholar]
- 144.Chitnis AS, Holzbauer SM, Belflower RM, Winston LG, Bamberg WM, Lyons C, Farley MM, Dumyati GK, Wilson LE, Beldavs ZG, et al. Epidemiology of community-associated Clostridium difficile infection, 2009 through 2011. JAMA Intern Med. 2013;173:1359–1367. doi: 10.1001/jamainternmed.2013.7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Freedberg DE, Salmasian H, Friedman C, Abrams JA. Proton pump inhibitors and risk for recurrent Clostridium difficile infection among inpatients. Am J Gastroenterol. 2013;108:1794–1801. doi: 10.1038/ajg.2013.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hegarty JP, Sangster W, Harris LR 3rd, Stewart DB. Proton pump inhibitors induce changes in colonocyte gene expression that may affect Clostridium difficile infection. Surgery. 2014;156:972–978. doi: 10.1016/j.surg.2014.06.074. [DOI] [PubMed] [Google Scholar]
- 147.Seto CT, Jeraldo P, Orenstein R, Chia N, DiBaise JK. Prolonged use of a proton pump inhibitor reduces microbial diversity: implications for Clostridium difficile susceptibility. Microbiome. 2014;2:42. doi: 10.1186/2049-2618-2-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Husebye E, Skar V, Høverstad T, Melby K. Fasting hypochlorhydria with gram positive gastric flora is highly prevalent in healthy old people. Gut. 1992;33:1331–1337. doi: 10.1136/gut.33.10.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Scarpignato C, Gatta L, Zullo A, Blandizzi C; SIF-AIGO-FIMMG Group; Italian Society of Pharmacology, the Italian Association of Hospital Gastroenterologists, and the Italian Federation of General Practitioners. Effective and safe proton pump inhibitor therapy in acid-related diseases - A position paper addressing benefits and potential harms of acid suppression. BMC Med. 2016;14:179. doi: 10.1186/s12916-016-0718-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


