Abstract
AIM
To evaluate the differences in outcomes between ABO-incompatible (ABO-I) liver transplantation (LT) and ABO-compatible (ABO-C) LT.
METHODS
A systematic review and meta-analysis were performed by searching eligible articles published before No-vember 28, 2016 on MEDLINE (PubMed), EMBASE, and Cochrane databases. The primary endpoints were graft survival, patient survival, and ABO-I-related complications.
RESULTS
Twenty-one retrospective observational studies with a total of 8247 patients were included in this meta-analysis. Pooled results of patient survival for ABO-I LT were comparable to those for ABO-C LT. However, ABO-I LT showed a poorer graft survival than ABO-C LT (1-year: OR = 0.66, 95%CI: 0.57-0.76, P < 0.001; 3-year: OR = 0.74, 95% CI 0.64-0.85, P < 0.001; 5-yearr: OR =0.75, 95%CI: 0.66-0.86, P < 0.001). Furthermore, ABO-I LT was associated with more incidences of antibody-mediated rejection (OR = 74.21, 95%CI: 16.32- 337.45, P < 0.001), chronic rejection (OR =2.28, 95%CI: 1.00-5.22, P = 0.05), cytomegalovirus infection (OR = 2.64, 95%CI: 1.63-4.29, P < 0.001), overall biliary complication (OR = 1.52, 95%CI: 1.01-2.28, P = 0.04), and hepatic artery complication (OR = 4.17, 95%CI: 2.26-7.67, P < 0.001) than ABO-C LT. In subgroup analyses, ABO-I LT and ABO-C LT showed a comparable graft survival in pediatric patients and those using rituximab, and ABO-I LT showed an increased acute cellular rejection in cases involving deceased donor grafts.
CONCLUSION
Although patient survival in ABO-I LT was comparable to that in ABO-C LT, ABO-I LT was inferior to ABO-C LT in graft survival and several complications. Graft survival of ABO-I LT could be comparable to that of ABO-C LT in pediatric patients and those using rituximab.
Keywords: ABO-incompatibility, Liver transplantation, Graft survival, Patient survival, Complications
Core tip: This meta-analysis analyzed more than 8000 cases of ABO-incompatible (ABO-I) and ABO-compatible (ABO-C) liver transplantation (LT). Although patient survival was similar, ABO-I LT was inferior to ABO-C LT in graft survival and several ABO-I-related complications. Graft survival of ABO-I LT was comparable to that of ABO-C LT in pediatric patients and those using rituximab.
INTRODUCTION
In an animal experiment in 1969, Starzl et al[1] reported that the liver is a privileged organ that could be transplanted with relatively lower prevalence of acute rejection than those associated with the kidney or heart. Furthermore, in 1979, Starzl et al[2] reported 11 cases of successful ABO-incompatible (ABO-I) liver transplantation (LT) without graft rejections[2]. Since then, however, there has been a series of reports of heightened prevalence of antibody-mediated rejection (AMR), lower graft survival, hepatic artery thrombosis (HAT), and cholangitis in ABO-I LT compared to ABO-compatible (ABO-C) LT[3-6]. Nevertheless, the application of various desensitization strategies, such as plasma exchange (PE) (or plasmapheresis), splenectomy, graft local infusion (GLI), mycophenolate mofetil (MMF), rituximab, and intravenous immunoglobulin (IVIG) to ABO-I LT highlight the potential of ABO-I LT as a promising alternative to ABO-C LT, and the introduction of rituximab has brought about substantial improvements in the outcomes of ABO-I LT[7-12]. Currently, its importance is expanding in the East, where the proportion of uses of ABO-I allografts for living donor liver transplantation (LDLT) is higher than that in the West, and particularly in Korea and Japan-two countries that show notably higher proportions of interfamilial organ donation.
However, there are still heated debates with regard to the prevalence of graft survival, patient survival, ABO-I-related complications, such as rejection, infection, biliary stricture, and HAT associated with ABO-I LT and ABO-C LT, with much heterogeneity in different reports. Therefore, considering the fact that cases of ABO-I LT would inevitably rise due to demands for donor organs far outnumbering the supply and increased difficulty of matching appropriate ABO-C liver allografts, a comprehensive analysis of the results from previous reports of LT across the ABO blood group barrier is needed.
MATERIALS AND METHODS
Study selection
Systematic review and meta-analysis were performed in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines[13]. Databases of Medline (Pubmed), EMBASE, and Cochrane library were used to search for relevant articles among publications dated November 28, 2016. Publication year and language were not specified or limited for the search. The following keywords for the database search were used: (ABO OR = blood group OR = blood type) AND (incompatibility OR = mismatch OR = barrier) AND liver transplantation. Title and abstracts of identified articles were screened independently by two investigators (Lee EC , Shim JR ), and full-text articles with potential relevance were obtained.
Eligibility criteria
The included studies were articles that compared ABO-I LT and ABO-C LT with a minimum of one outcome of interest. The following types of articles were excluded: abstracts, meeting papers, case reports/series, reviews, meta-analyses, letters, editorial comments, animal studies, single-arm studies, and studies unable to extract data. When there were overlapping cohorts examined by the same institutions, data from the most recent studies were used.
Assessment of methodological quality
Because there was no randomized controlled trial study included in this review, methodological quality was assessed based on a maximum score of 9 for “selection of patients”, “comparability”, and “outcome of study” as per the Newcastle-Ottawa Quality Assessment Scale for Cohort Studies[14]. All of the studies included in this meta-analysis were assessed by two investigators (Lee EC, Shim JR), and disagreements were resolved by a consensus.
Data extraction
Data for the following items were extracted: first author, publication year, study periods, region, sample size, population, recipient age, donor age, urgent indication, donor type, prescription for ABO-I LT, immunosuppression, graft survival, patient survival, AMR, acute cellular rejection (ACR), CR (chronic rejection), bacterial infection, fungal infection, cytomegalovirus (CMV) infection, overall biliary complication, bile leak, biliary stricture, hepatic artery (HA) complication, hepatic vein (HV) complication, and portal vein (PV) complication. For studies that divided ABO-C LT into ABO-C non-identical LT and ABO-Identical (ABO-Id) LT and reported separate outcomes for each[15-20], the outcomes were combined for the purpose of this meta-analysis. If the required data were not clearly articulated in the selected articles, we requested the original data via an email to the corresponding authors. All data were independently investigated and cross-checked by two investigators (Lee EC, Shim JR), and another investigator (Kim SH) provided the final confirmation.
Statistical analysis
This meta-analysis was performed in compliance with the Cochrane guidelines for systematic revie[21]. Categorical variables were analyzed with odds ratios (OR) with 95% confidence interval (CI) using the Mantel-Haenszel method, and continuous variables were analyzed with weighted mean differences (WMD) with 95%CI using an inverse variance method. Heterogeneity among studies was assessed with Higgin’s I2 index[22] or Cochran’s Q test[21,23]. The random-effects model was used when I2 was > 50% or P-value (Cochran’s Q test) was < 0.10, and the fixed-effects model was used in all other cases. Heterogeneous results were further examined with a sensitivity analysis using the leave-one-out method and subgroup analyses. Possible publication bias was assessed using funnel plots and Egger’s regression test, which evaluates a funnel plot asymmetry[24,25]. If a publication bias with P-value < 0.10 (Egger’s regression test) was detected, the impact on the outcomes of the meta-analysis was assessed after enhancing the symmetry using the trim-and-fill method of Duvall and Tweedie[26]. The presence of publication bias in fewer than 10 studies was considered unreliable as per the Cochrane Handbook for Systematic Reviews[27]. A P-value of < 0.05 was considered statistically significant. Statistical analyses were performed using the Review Manager (RevMan) software version 5.3 (http://tech.cochrane.org/revman) and R package “meta” (https://cran.r-project.org/web/packages/meta).
RESULTS
Search results
A total of 986 citations were found in the primary search using combinations of keywords for database search, of which 762 irrelevant or duplicated citations were excluded. After retrieving the titles and abstracts from the remaining 224 articles, another 186 articles were excluded. From the 38 potentially relevant studies, additional studies were excluded for the following reasons: single-arm studies (n = 9), overlapping cohorts from the same institutions (n = 4), studies investigating multiple desensitization protocols (n = 1), timing of rituximab administration (n = 1), and pathology (n = 1), and unable data extraction (n = 1). As a result, 21 studies were included in this meta-analysis[5,15-20,28-41]. The flow diagram of study selection is shown in Figure 1.
Figure 1.
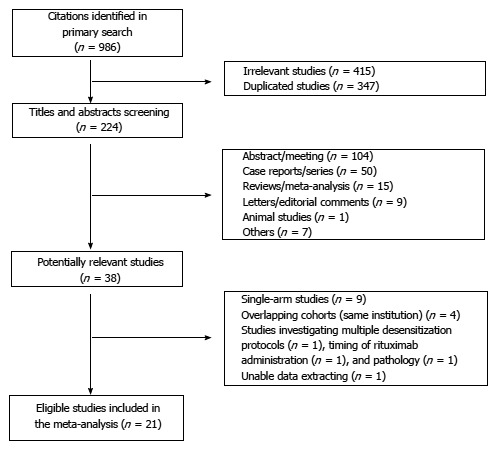
Flow diagram showing the selection of articles for meta-analysis.
Characteristics of included studies
This meta-analysis included 21 retrospective ob-servational studies that were conducted on a total of 8247 patients[5,15-20,28-41]. Of these patients, 1494 underwent ABO-I LT, while 6753 underwent ABO-C LT. There has been no randomized clinical trial conducted on this topic. Study periods ranged from 1984-2014. For studies involving less than 10% heterogeneity in study population (adult vs pediatric), donor type (deceased vs living), urgent indication [i.e., fulminant hepatic failure (FHF), acute liver failure (ALF), re-transplantation, and critically ill patients in the intensive care unit], and use of rituximab for ABO-I LT, these parameters were classified according to the majority. One registry study was included in this meta-analysis[35]. Characteristics of the included studies are summarized in Table 1.
Table 1.
Characteristics of the included studies
| Ref. | Publication (yr) | Study periods (yr) | Region | Arms | Sample size | Population3 | Recipient age | Donor age | Urgent Indications34 | Donor type3 | Prescription for ABO-I LT | Immunosuppression |
| Song et al[28] | 2016 | 2008-2013 | South Korea | ABO-I | 235 | Adult | 52.8 ± 8.0 | 29.2 ± 9.1 | No | Living | Rituximab, PE, GLI (±), Splenectomy (±), Cyclophosphamide | Steroids, Basiliximab,Tac, MMF |
| ABO-C | 1301 | 51.7 ± 5.9 | 28.2 ± 7.6 | |||||||||
| Kim et al[29] | 2016 | 2010-2013 | South Korea | ABO-I | 472 | Adult | 50 (22-65) | 32 (18-68) | No | Living | Rituximab, PE, GLI (±) | Steroids, Basiliximab, Tac, MMF |
| ABO-C | 942 | 51 (20-68) | 30 (18-62) | |||||||||
| Kim et al[30] | 2016 | 2011-2014 | South Korea | ABO-I | 252 | Adult | 51.3 ± 6.7 | 30.1 ± 11.2 | No | Living | Rituximab, PE, IVIG, Preoperative MMF | Steroids, Basiliximab, Tac, MMF |
| ABO-C | 752 | 51.1 ± 6.7 | 28.8 ± 11.3 | |||||||||
| Ikegami et al[31] | 2016 | 1997-2013 | Japan | ABO-I | 19 | Adult | 47.7 ± 15.7 | 36.6 ± 11.3 | No | Living | Rituximab3, IVIG (±), PE, GLI (±), Splenectomy (±), Preoperative MMF (±) | Steroids, Tac (or CsA), MMF |
| ABO-C | 389 | 51.7 ± 11.9 | 37.4 ± 10.5 | |||||||||
| Lee et al[32] | 2015 | 2006-2013 | Taiwan | ABO-I | 46 | Adult | 53.5 (19-67) | NA | No | Living | Rituximab, Plasmapheresis (or PE) | Steroids, Tac, MMF |
| ABO-C | 340 | 54.7 (18-70) | NA | |||||||||
| Shen et al[33] | 2014 | 2010-2013 | China | ABO-I | 35 | Adult | 46.7 ± 12.1 | NA | Yes | Deceased | Rituximab, IVIG | Steroids, Basiliximab, Tac, MMF |
| ABO-C | 66 | 42.6 ± 10.2 | NA | |||||||||
| Heffron et al[34] | 2010 | 1998-2008 | United States | ABO-I | 12 | Pediatric | NA | NA | Yes | Deceased | - | Steroids, Daclizumab, Tac, MMF |
| ABO-C | 21 | NA | NA | |||||||||
| Stewart et al[35] | 2009 | 1990-2006 | United States | ABO-I | 1302 | Infant | 0.3 | 8.1 | No | Deceased | NA | NA |
| ABO-C | 3902 | 0.4 | 8.3 | |||||||||
| ABO-I | 1162 | Pediatric | 9.6 | 23.9 | ||||||||
| ABO-C | 3482 | 9 | 16.5 | |||||||||
| ABO-I | 5852 | Adult | 45.7 | 36 | ||||||||
| ABO-C | 17552 | 50.3 | 37.9 | |||||||||
| Iwamoto et al[36] | 2008 | 2000-2007 | Japan | ABO-I | 15 | Adult | NA | NA | No | Living | NA | NA |
| ABO-C | 37 | NA | NA | |||||||||
| Toso et al[20] | 2007 | 1991-2005 | Canada | ABO-I | 14 | Adult | 42 (17-61) | NA | Yes | Deceased | Lymphocyte-depleting antibodies5, Plasmapheresis (±) | Steroids, Daclizumab, CsA (or Tac), AZA (or MMF, Sirolimus) |
| ABO-C1 | 29 | 47 (16-62) | NA | |||||||||
| ABO-Id | 65 | 47 (17-66) | NA | |||||||||
| Saito et al[37] | 2007 | 2000-2001 | Japan | ABO-I | 10 | All ages | NA | NA | No | Deceased, | NA | NA |
| ABO-C | 81 | NA | NA | Living | ||||||||
| Koukoutsis et al[19] | 2007 | 1984-2005 | United Kingdom | ABO-I | 4 | Adult | NA | NA | Yes | Deceased | NA | NA |
| ABO-C1 | 73 | NA | NA | |||||||||
| ABO-Id | 203 | NA | NA | |||||||||
| Ueda et al[18] | 2006 | 1990-2003 | Japan | ABO-I | 74 | Pediatric | NA | NA | No | Living | Steroids pulse weekly, PGE1, CsA - > AZA (1 mo after LT) | Steroids, Tac |
| ABO-C1 | 114 | NA | NA | |||||||||
| ABO-Id | 380 | NA | NA | |||||||||
| Heffron et al[38] | 2006 | 1999-2005 | United States | ABO-I | 16 | Pediatric | 6.5 ± 6.2 | NA | No | Deceased | Plasmapheresis (±) | Steroids, Daclizumab, Tac, MMF |
| ABO-C | 122 | 8.1 ± 6.2 | NA | |||||||||
| Bjøro et al[17] | 2003 | 1990-2001 | Nordic countries | ABO-I | 10 | All ages | NA | 44.8 (22-55) | Yes | Deceased | NA | NA |
| ABO-C† | 76 | NA | 42.3 (12-85) | |||||||||
| ABO-Id | 143 | NA | 41.0 (2-75) | |||||||||
| Chui et al[39] | 1997 | 1986-1996 | Australia | ABO-I | 7 | All ages | 13 (6-32) | NA | Yes | Deceased | Plasmapheresis (±), Splenectomy (±) | Steroids, CsA, AZA |
| ABO-C | 36 | NA | NA | |||||||||
| Cacciarelli et al[16] | 1995 | 1988-1993 | United States | ABO-I | 14 | Pediatric | 2.2 ± 1.1 | NA | No | Deceased | OKT3 (or ATG, CsA) | Steroids, ATG (or OKT3, CsA), Tac |
| ABO-C† | 22 | 4.2 ± 1.0 | NA | |||||||||
| ABO-Id | 108 | 3.7 ± 0.5 | NA | |||||||||
| Lo et al[40] | 1994 | 1988-1993 | United States | ABO-I | 29 | All ages | NA | NA | Yes | Deceased | ATG (±) | Steroids, CsA (or OKT3), AZA |
| ABO-C | 196 | NA | NA | |||||||||
| Sanchez et al[41] | 1993 | 1985-1991 | United States | ABO-I | 182 | Adult | 45 (16-61) | NA | No | Deceased | NA | NA |
| ABO-C | 182 | 47 (17-59) | NA | |||||||||
| Reding et al[15] | 1992 | 1984-1989 | Belgium | ABO-I | 16 | All ages | NA | NA | Yes | Deceased | OKT3 (±) | Steroids, CsA, AZA(±) |
| ABO-C† | 16 | NA | NA | |||||||||
| ABO-Id | 38 | NA | NA | |||||||||
| Gugenheim et al[5] | 1990 | 1984-1988 | France | ABO-I | 17 | All ages | 30 (12-49) | NA | Yes | Deceased | - | Steroids, CsA, AZA |
| ABO-C | 217 | NA | NA |
1Compatible, but not identical; 2Propensity or case matched patients;
If there are minority groups that make up less than about 10%, the article is categorized as covering the majority;
Such as FHF, ALF, retransplantation, and critically ill patients in the intensive care unit; 5Documented as "lymphocyte-depleting antibodies", but not clarified exactly. ABO-C: ABO-compatible; ABO-I: ABO-incompatible; ABO-Id: ABO-identical; ATG: anti-thymocyte globulin; AZA: Azathioprine; CsA: Cyclosporin A; GLI: Graft local infusion; IVIG: Intravenous immunoglobulin; LT: Liver transplantation; MMF: Mycophenolate mofetil; NA: Not applicable; OKT3: Muromonab-CD3; PE: Plasma exchange; Tac: Tacrolimus.
Methodological quality assessment
All studies included in this meta-analysis showed a Newcastle-Ottawa Scale (NOS) score ≥ 6. Four case or propensity score matched studies were included[29,30,35,41]. Quality assessments of included cohort studies are presented in Table 2.
Table 2.
Modified Newcastle-Ottawa quality assessment scale for cohort studies included in the meta-analysis
| Ref. |
Selection |
Comparability1 |
Outcome |
Overall |
|||||
| Representativeness | Selection | Ascertainment | Incident disease | Assessment | Length of follow-up | Adequacy of follow-up | Quality Score (Maximum 9) | ||
| Song et al[28] | ↓ | ↓ | ↓ | ↑ | ↑ | ↓ | ↓ | ↓ | 6 |
| Kim et al[29] | ↓ | ↓ | ↓ | ↑ | ↓↓ | ↓ | ↓ | ↓ | 8 |
| Kim et al[30] | ↓ | ↓ | ↓ | ↑ | ↓↓ | ↓ | ↓ | ↓ | 8 |
| Ikegami et al[31] | ↓ | ↓ | ↓ | ↑ | ↑ | ↓ | ↓ | ↓ | 6 |
| Lee et al[32] | ↓ | ↓ | ↓ | ↑ | ↑ | ↓ | ↓ | ↓ | 6 |
| Shen et al[33] | ↓ | ↓ | ↓ | ↑ | ↑ | ↓ | ↓ | ↓ | 6 |
| Heffron et al[34] | ↓ | ↓ | ↓ | ↑ | ↑ | ↓ | ↓ | ↓ | 6 |
| Stewart et al[35] | ↓ | ↓ | ↓ | ↑ | ↓↓ | ↓ | ↓ | ↓ | 8 |
| Iwamoto et al[36] | ↓ | ↓ | ↓ | ↑ | ↑ | ↓ | ↓ | ↓ | 6 |
| Toso et al[20] | ↓ | ↓ | ↓ | ↑ | ↑ | ↓ | ↓ | ↓ | 6 |
| Saito et al[37] | ↓ | ↓ | ↓ | ↑ | ↑ | ↓ | ↓ | ↓ | 6 |
| Koukoutsis et al[19] | ↓ | ↓ | ↓ | ↑ | ↑ | ↓ | ↓ | ↓ | 6 |
| Ueda et al[18] | ↓ | ↓ | ↓ | ↑ | ↑ | ↓ | ↓ | ↓ | 6 |
| Heffron et al[38] | ↓ | ↓ | ↓ | ↑ | ↑ | ↓ | ↓ | ↓ | 6 |
| Bjøro et al[17] | ↓ | ↓ | ↓ | ↑ | ↑ | ↓ | ↓ | ↓ | 6 |
| Chui et al[39] | ↓ | ↓ | ↓ | ↑ | ↑ | ↓ | ↓ | ↓ | 6 |
| Cacciarelli et al[16] | ↓ | ↓ | ↓ | ↑ | ↑ | ↓ | ↓ | ↓ | 6 |
| Lo et al[40] | ↓ | ↓ | ↓ | ↑ | ↑ | ↓ | ↓ | ↓ | 6 |
| Sanchez et al[41] | ↓ | ↓ | ↓ | ↑ | ↓↓ | ↓ | ↓ | ↓ | 8 |
| Reding et al[15] | ↓ | ↓ | ↓ | ↑ | ↑ | ↓ | ↓ | ↓ | 6 |
| Gugenheim et al[5] | ↓ | ↓ | ↓ | ↑ | ↑ | ↓ | ↓ | ↓ | 6 |
A maximum of two downward arrows (↓↓) can be given for comparability. ↓ : Consistent with criteria and low risk of bias; ↑ : Not consistent with criteria and high risk of bias.
Graft and patient survival
Graft survival and patient survival were reported by 16 and 15 studies, respectively. ABO-I LT showed poorer outcomes than those of ABO-C LT in the pooled results of graft survival (1-year: OR = 0.66, 95%CI: 0.57-0.76, P < 0.001; 3-year: OR = 0.74, 95%CI: 0.64-0.85, P < 0.001; 5-year: OR = 0.75, 95%CI: 0.66-0.86, P < 0.001; 10-year: OR = 0.80, 95%CI: 0.69-0.93, P = 0.004; Figure 2).
Figure 2.
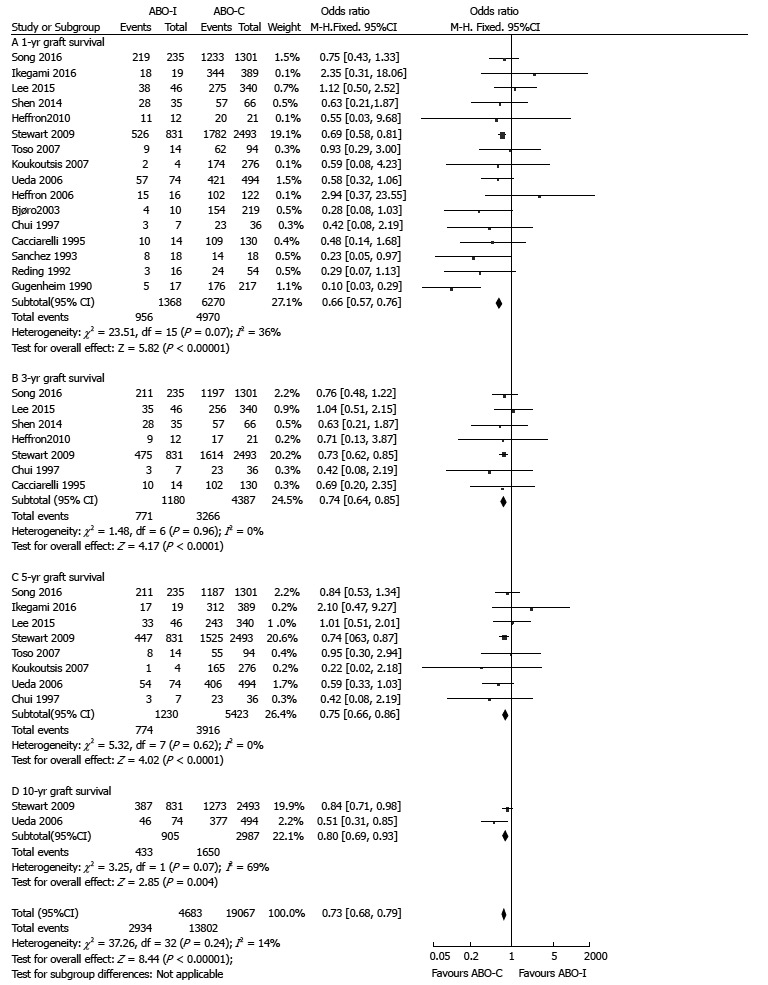
Comparison of graft survival between ABO-incompatible and ABO-compatible liver transplantation. ABO-C: ABO-compatible; ABO-I: ABO-incompatible; LT: Liver transplantation.
There were no differences in 1-, 3-, and 5-year patient survival in accordance with ABO compatibility (1-year: OR = 0.88, 95%CI: 0.67-1.16, P = 0.38; 3-year: OR = 0.89, 95%CI: 0.64-1.23, P = 0.47; 5-year: OR = 0.89, 95%CI: 0.66-1.20, P = 0.45; Figure 3). However, there was a significant difference in the 10-year patient survival between ABO-I and ABO-C groups (10-year: OR = 0.46, 95%CI: 0.28-0.78, P = 0.004; Figure 3). There was no significant heterogeneity in graft and patient survival.
Figure 3.
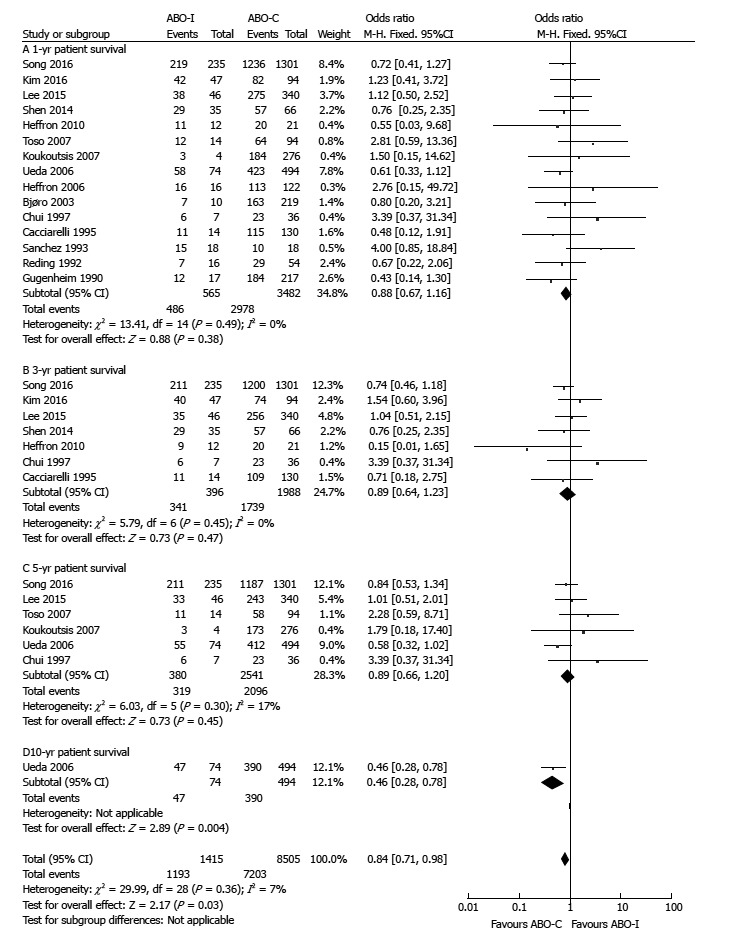
Comparison of patient survival between ABO-incompatible and ABO-compatible liver transplantation. ABO-C: ABO-compatible; ABO-i: ABO-incompatible; LT: Liver transplantation.
Complications
Rejection: AMR, ACR, and CR were reported by 4, 14, and 4 studies, respectively. Pooled results showed that the risks for AMR (OR = 74.21, 95%CI 16.32-337.45, P < 0.001; Figure 4A) and CR (OR = 2.28, 95%CI: 1.00-5.22, P = 0.05; Figure 4C) were significantly higher in ABO-I LT than in ABO-C LT, but there was no statistically significant difference in the risk for ACR (OR = 1.23, 95%CI: 0.93-1.62, P = 0.15; Figure 4B). There was no significant heterogeneity in the results for rejection.
Figure 4.
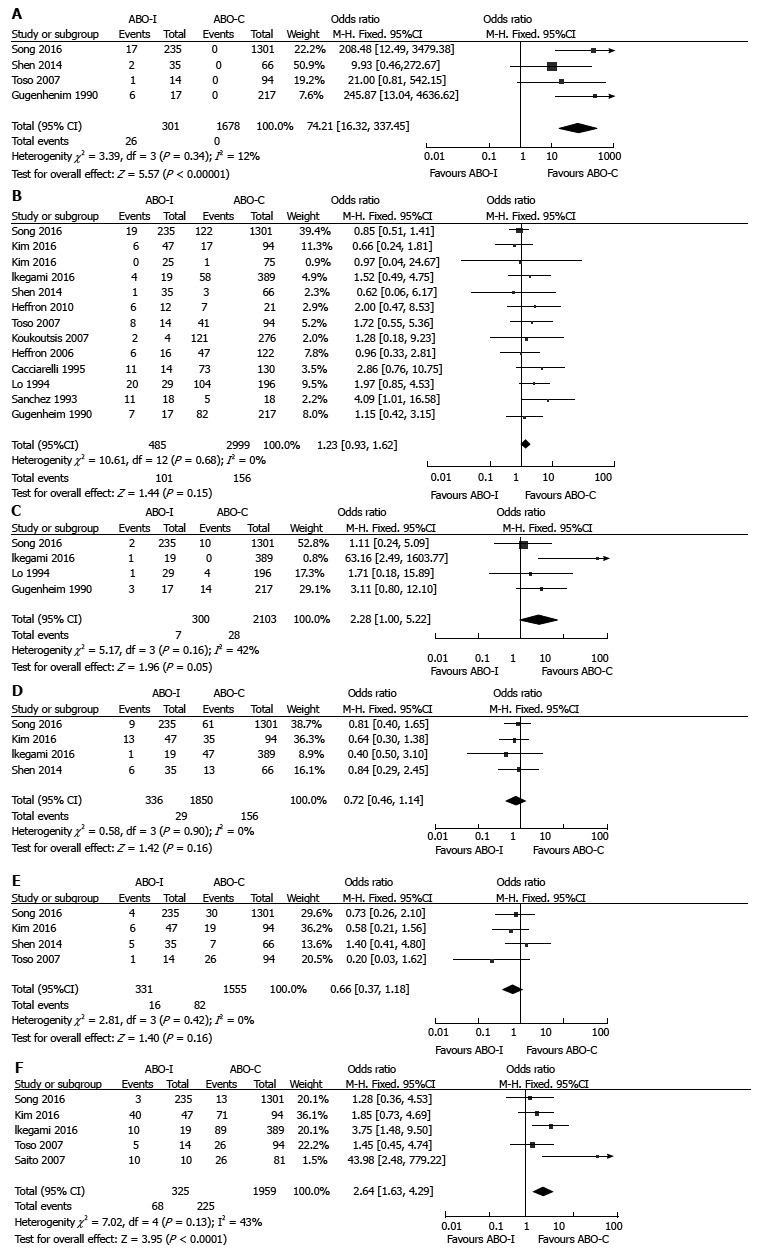
Comparison of rejection and infection between ABO-incompatible and ABO-compatible liver transplantation. A: AMR; B: ACR; C: CR; D: Bacterial infection; E: Fungal infection; F: CMV infection. ABO-C: ABO-Compatible; ABO-I: ABO-incompatible; ACR: Acute cellular rejection; AMR: Antibody-mediated rejection; CMV: Cytomegalovirus; CR: Chronic rejection; LT: Liver transplantation.
Infection: Bacterial and fungal infections were each reported by 4 studies, while CMV infection was reported by 5 studies. Although there were no differences in bacterial infection (OR = 0.72, 95%CI: 0.46-1.14, P = 0.16; Figure 4D) and fungal infection (OR = 0.66, 95%CI: 0.37-1.18, P = 0.16; Figure 4E) in accordance with ABO-compatibility, CMV infection was more prevalent in ABO-I LT than in ABO-C LT (OR = 2.64, 95%CI: 1.63-4.29, P < 0.001; Figure 4F). There was no significant heterogeneity in the results for infection.
Biliary: There were no statistically significant differences in overall biliary complication (OR = 1.75, 95%CI: 0.89-3.43, P = 0.10; Figure 5A), bile leak (OR = 1.85, 95%CI: 0.46-7.39, P = 0.39; Figure 5B), and biliary stricture (OR = 1.37, 95%CI: 0.70-2.70, P = 0.36; Figure 5C) in accordance with ABO compatibility. However, there were high heterogeneities in overall biliary complication (χ2 = 15.22, degree of freedom [d.f.] = 7, P = 0.03; I2 = 54%), bile leak (χ2 = 4.25, d.f. = 2, P = 0.12; I2 = 53%), and biliary stricture (χ2 = 9.36, d.f. = 5, P = 0.10; I2 = 47%).
Figure 5.
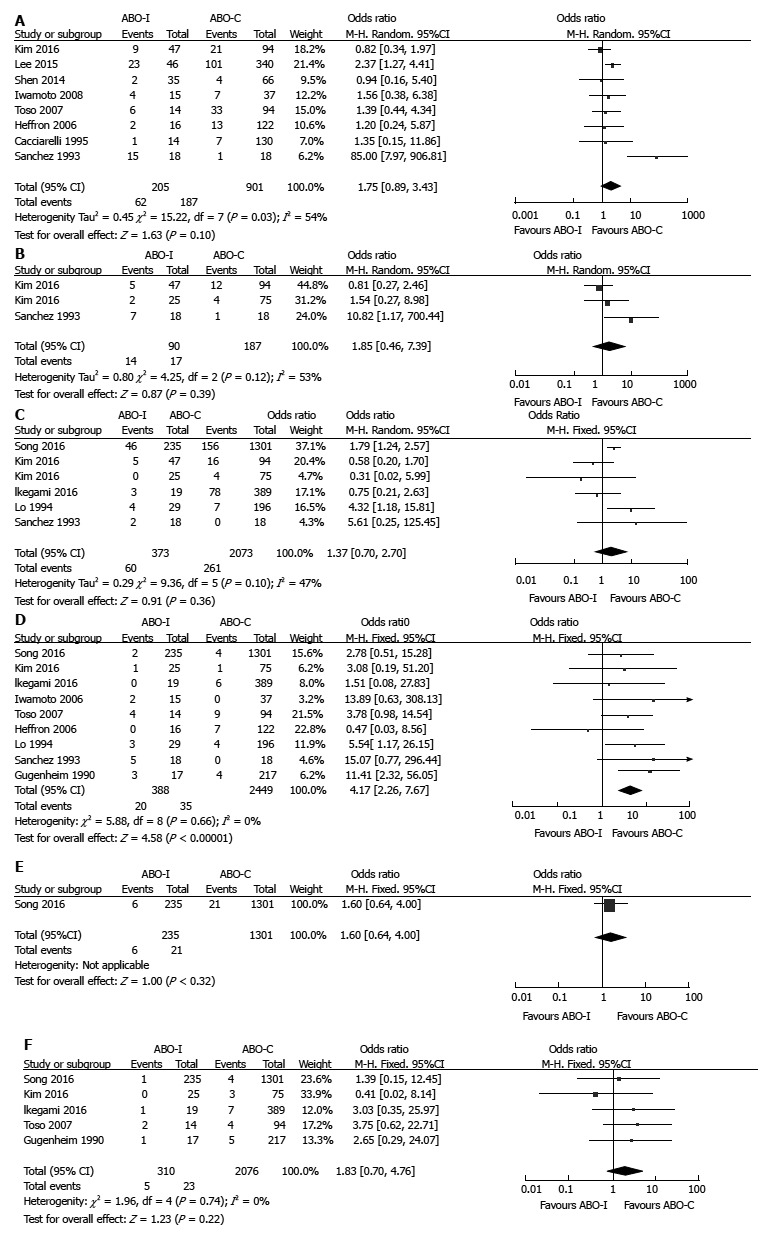
Comparison of biliary and vascular complications between ABO-Incompatible and ABO-Compatible liver transplantation. A: Overall biliary complication; B: Bile leak; C: Biliary stricture; D: HA complication; E: HV complication; F: PV complication. ABO-C: ABO-compatible; ABO-I: ABO-incompatible; HA: Hepatic artery; HV: Hepatic vein; LT: Liver transplantation; PV: Portal vein.
Vascular: HA complication (OR = 4.17, 95%CI: 2.26-7.67, P < 0.001; Figure 5D) was significantly more prevalent in ABO-I LT than in ABO-C LT. However, there were no significant differences between ABO-I LT and ABO-C LT in HV complication (OR = 1.60, 95%CI: 0.64-4.00, P = 0.32; Figure 5E) and PV complication (OR = 1.83, 95%CI: 0.70-4.76, P = 0.22; Figure 5F). There was no severe heterogeneity in vascular complications.
Sensitivity and subgroup analyses
In this meta-analysis, there were high heterogeneities in overall biliary complication, bile leak, and biliary stricture. We performed a sensitivity analysis on these variables using the leave-one-out method and found that omitting the study of Sanchez et al[41] with a wide range of CI (overall biliary complication: OR = 85.00, 95%CI: 7.97-906.81; Bile leak: OR = 10.82, 95%CI: 1.17-100.44) eliminated the heterogeneity in overall biliary complication and bile leak. Particularly, post-sensitive analysis results showed that ABO-I LT was associated with higher prevalence of overall biliary complications than ABO-C LT (OR = 1.52, 95%CI: 1.01-2.28, P = 0.04; Table 3).
Table 3.
Sensitivity analysis and subgroup analysis
| Variables | No. of studies | OR=[95%CI] | P1 value | I2 (%) |
| Sensitivity analysis | ||||
| Overall biliary complication | 1.75 [0.89-3.43] | 0.10 | 54 | |
| Omitting Sanchez et al[41] | 7 | 1.52 [1.01-2.28] | 0.04 | 0 |
| Omitting Cacciarelli et al[16] | 7 | 1.81 [0.87-3.76] | 0.11 | 61 |
| Omitting Heffron et al[38] | 7 | 1.86 [0.87-3.95] | 0.11 | 60 |
| Omitting Toso et al[20] | 7 | 1.86 [0.84-4.13] | 0.13 | 60 |
| Omitting Iwamoto et al[36] | 7 | 1.81 [0.84-3.92] | 0.13 | 61 |
| Omitting Shen et al[33] | 7 | 1.89 [0.90-3.96] | 0.09 | 59 |
| Omitting Lee et al[32] | 7 | 1.69 [0.72-3.94] | 0.23 | 56 |
| Omitting Kim et al[29] | 7 | 2.07 [0.98-4.37] | 0.06 | 50 |
| Bile leak | 1.85 [0.46-7.39] | 0.39 | 53 | |
| Omitting Sanchez et al[41] | 2 | 0.98 [0.38-2.49] | 0.96 | 0 |
| Omitting Kim et al[30] | 2 | 2.47 [0.19-31.38] | 0.49 | 77 |
| Omitting Kim et al[29] | 2 | 3.62 [0.53-24.77] | 0.19 | 46 |
| Biliary stricture | 1.37 [0.70-2.70] | 0.36 | 47 | |
| Omitting Sanchez et al[41] | 5 | 1.28 [0.62-2.62] | 0.51 | 54 |
| Omitting Lo et al[40] | 5 | 1.11 [0.54-2.26] | 0.78 | 42 |
| Omitting Ikegami et al[31] | 5 | 1.55 [0.71-3.39] | 0.27 | 49 |
| Omitting Kim et al[30] | 5 | 1.47 [0.74-2.95] | 0.27 | 51 |
| Omitting Kim et al[29] | 5 | 1.72 [0.91-3.25] | 0.09 | 28 |
| Omitting Song et al[28] | 5 | 1.20 [0.43-3.31] | 0.73 | 49 |
| Subgroup analysis | ||||
| 1-yr graft survival | 0.66 [0.57-0.76] | < 0.001 | 36 | |
| Rituximab for ABO-I LT (yes/no) | 4 vs 7 | 0.88 [0.58-1.33] vs 0.44 [0.20-0.66] | 0.02 | 0 vs 49 |
| Living donor (yes/no) | 4 vs 12 | 0.79 [0.56-1.13] vs 0.64 [0.55-0.74] | 0.27 | 0 vs 44 |
| Urgent indication (yes/no) | 8 vs 8 | 0.37 [0.23-0.59] vs 0.70 [0.61-0.81] | 0.01 | 27 vs 8 |
| Pediatric2 (yes/no) | 5 vs 12 | 0.88 [0.68-1.15] vs 0.59 [0.50-0.69] | 0.01 | 12 vs 45 |
| 3-yr graft survival | 0.74 [0.64-0.85] | < 0.001 | 0 | |
| Rituximab for ABO-I LT (yes/no) | 3 vs 3 | 0.81 [0.56-1.18] vs 0.60 [0.26-1.41] | 0.53 | 0 vs 0 |
| Living donor (yes/no) | 2 vs 5 | 0.84 [0.57-1.25] vs 0.72 [0.62-0.84] | 0.47 | 0 vs 0 |
| Urgent indication (yes/no) | 3 vs 4 | 0.59 [0.27-1.31] vs 0.74 [0.64-0.86] | 0.58 | 0 vs 0 |
| Pediatric2 (yes/no) | 3 vs 5 | 0.95 [0.71-1.26] vs 0.67 [0.57-0.80] | 0.04 | 0 vs 0 |
| 5-yr graft survival | 0.75 [0.66-0.86] | < 0.001 | 0 | |
| Rituximab for ABO-I LT (yes/no) | 3 vs 2 | 0.96 [0.66-1.39] vs 0.56 [0.33-0.96] | 0.11 | 0 vs 0 |
| Living donor (yes/no) | 4 vs 4 | 0.83 [0.61-1.14] vs 0.73 [0.63-0.86] | 0.46 | 10 vs 0 |
| Urgent indication (yes/no) | 3 vs 5 | 0.60 [0.26-1.39] vs 0.76 [0.66-0.87] | 0.6 | 0 vs 0 |
| Pediatric 2 (yes/no) | 2 vs 7 | 0.82 [0.63-1.07] vs 0.73 [0.62-0.86] | 0.44 | 42 vs 0 |
| 10-yr graft survival | 0.80 [0.69-0.93] | 0.004 | 69 | |
| Rituximab for ABO-I LT (yes/no) | 0 vs 1 | NA vs 0.51 [0.31-0.85] | NA | NA |
| Living donor (yes/no) | 1 vs 1 | 0.51 [0.31-0.85] vs 0.84 [0.71-0.98] | 0.07 | NA vs NA |
| Urgent indication (yes/no) | 0 vs 2 | NA vs 0.70 [0.44-1.11] | NA | NA vs 69 |
| Pediatric 2 (yes/no) | 2 vs 1 | 0.71 [0.41-1.23] vs 0.81 [0.70-0.98] | 0.65 | 72 vs NA |
| 1-yr patient survival | 0.88 [0.67-1.16] | 0.38 | 0 | |
| Rituximab for ABO-I LT (yes/no) | 4 vs 7 | 0.88 [0.59-1.31] vs 0.67 [0.44-1.04] | 0.38 | 0 vs 0 |
| Living donor (yes/no) | 4 vs 11 | 0.79 [0.56-1.13] vs 1.04 [0.67-1.61] | 0.35 | 0 vs 9 |
| Urgent indication (yes/no) | 8 vs 7 | 0.93 [0.56-1.52] vs 0.87 [0.62-1.20] | 0.83 | 0 vs 21 |
| Pediatric (yes/no) | 4 vs 11 | 0.64 [0.38-1.09] vs 0.98 [0.71-1.35] | 0.18 | 0 vs 5 |
| 3-yr patient survival | 0.89 [0.64-1.23] | 0.47 | 0 | |
| Rituximab for ABO-I LT (yes/no) | 4 vs 3 | 0.90 [0.64-1.27] vs 0.80 [0.31-2.06] | 0.82 | 0 vs 43 |
| Living donor (yes/no) | 3 vs 4 | 0.91 [0.64-1.32] vs 0.78 [0.38-1.62] | 0.71 | 5 vs 15 |
| Urgent indication (yes/no) | 3 vs 4 | 0.81 [0.35-1.91] vs 0.90 [0.63-1.28] | 0.83 | 43 vs 0 |
| Pediatric (yes/no) | 2 vs 5 | 0.46 [0.15-1.38] vs 0.94 [0.67-1.32] | 0.22 | 18 vs 0 |
| 5-yr patient survival | 0.89 [0.66-1.20] | 0.45 | 17 | |
| Rituximab for ABO-I LT (yes/no) | 2 vs 2 | 0.89 [0.61-1.31] vs 1.00 [0.20-5.08] | 0.89 | 0 vs 57 |
| Living donor (yes/no) | 3 vs 3 | 0.79 [0.57-1.08] vs 2.38 [0.86-6.63] | 0.04 | 0 vs 0 |
| Urgent indication (yes/no) | 3 vs 3 | 2.38 [0.86-6.63] vs 0.79 [0.57-1.08] | 0.04 | 0 vs 0 |
| Pediatric (yes/no) | 1 vs 5 | 0.58 [0.32-1.02] vs 1.04 [0.72-1.48] | 0.09 | NA vs 0 |
| 10-yr patient survival | 0.46 [0.28-0.78] | 0.004 | 7 | |
| Rituximab for ABO-I LT (yes/no) | 0 vs 1 | NA vs 0.46 [0.28-0.78] | NA | NA |
| Living donor (yes/no) | 1 vs 0 | 0.46 [0.28-0.78] vs NA | NA | NA |
| Urgent indication (yes/no) | 0 vs 1 | NA vs 0.46 [0.28-0.78] | NA | NA |
| Pediatric (yes/no) | 1 vs 0 | 0.46 [0.28-0.78] vs NA | NA | NA |
| ACR | 1.23 [0.93-1.62] | 0.15 | 0 | |
| Rituximab for ABO-I LT (yes/no) | 5 vs 5 | 0.86 [0.57-1.30] vs 1.61 [1.01-2.58] | 0.048 | 0 vs 0 |
| Living donor (yes/no) | 4 vs 9 | 0.87 [0.58-1.32] vs 1.69 [1.14-2.50] | 0.02 | 0 vs 0 |
| Urgent indication (yes/no) | 6 vs 7 | 1.56 [0.96-2.53] vs 1.08 [0.77-1.53] | 0.23 | 0 vs 22 |
| Pediatric (yes/no) | 3 vs 10 | 1.64 [0.82-3.29] vs 1.16 [0.85-1.57] | 0.37 | 0 vs 0 |
| CR | 2.28 [1.00-5.22] | 0.05 | 42 | |
| Rituximab for ABO-I LT (yes/no) | 2 vs 2 | 6.45 [0.13-333.04] vs 2.64 [0.83-8.44] | 0.67 | 80 vs 0 |
| Living donor (yes/no) | 2 vs 2 | 6.45 [0.13-333.04] vs 2.64 [0.83-8.44] | 0.67 | 80 vs 0 |
| Urgent indication (yes/no) | 2 vs 2 | 2.64 [0.83-8.44] vs 6.45 [0.13-333.04] | 0.67 | 0 vs 80 |
| Pediatric (yes/no) | 0 vs 4 | NA vs 2.28 [1.00-5.22] | NA | NA vs 42 |
| AMR | 74.21 [16.32-337.45] | < 0.001 | 12 | |
| Rituximab for ABO-I LT (yes/no) | 2 vs 1 | 48.32 [ 2.31-1011.61] vs 245.87 [13.04-4636.62] | 0.45 | 53 vs NA |
| Living donor (yes/no) | 1 vs 3 | 208.48 [12.49-3479.38] vs 35.81 [6.02-212.88] | 0.3 | NA vs 18 |
| Urgent indication (yes/no) | 3 vs 1 | 35.81 [ 6.02-212.88] vs 208.48 [12.49-3479.38] | 0.3 | 18 vs NA |
| Pediatric (yes/no) | 0 vs 4 | NA vs 74.21 [16.32-337.45] | NA | NA vs 12 |
| Bacterial infection | 0.72 [0.46-1.14] | 0.16 | 0 | |
| Rituximab for ABO-I LT (yes/no) | 4 vs 0 | 0.72 [0.46-1.14] vs NA | NA | 0 vs NA |
| Living donor (yes/no) | 3 vs 1 | 0.70 [0.42-1.15] vs 0.84 [0.29-2.45] | 0.75 | 0 vs NA |
| Urgent indication (yes/no) | 1 vs 3 | 0.84 [0.29-2.45] vs 0.70 [0.42-1.15] | 0.75 | NA vs 0 |
| Pediatric (yes/no) | 0 vs 4 | NA vs 0.72 [0.46-1.14] | NA | NA vs 0 |
| Fungal infection | 0.66 [0.37-1.18] | 0.16 | 0 | |
| Rituximab for ABO-I LT (yes/no) | 3 vs 0 | 0.78 [0.42-1.44] vs NA | NA | 0 vs NA |
| Living donor (yes/no) | 2 vs 2 | 0.65 [0.31-1.33] vs 0.63 [0.09-4.40] | 0.99 | 0 vs 62 |
| Urgent indication (yes/no) | 2 vs 2 | 0.63 [0.09-4.40] vs 0.65 [0.31-1.33] | 0.99 | 62 vs 0 |
| Pediatric (yes/no) | 0 vs 4 | NA vs 0.71 [0.39-1.28] | NA | NA vs 0 |
| CMV infection | 2.64 [1.63-4.29] | < 0.001 | 43 | |
| Rituximab for ABO-I LT (yes/no) | 3 vs 0 | 2.2 [1.23-3.93] vs NA | NA | 4 vs NA |
| Living donor (yes/no) | 3 vs 2 | 2.25 [1.24-4.09] vs 6.43 [0.17-242.88] | 0.58 | 4 vs 82 |
| Urgent indication (yes/no) | 1 vs 4 | 1.45 [0.45-4.74] vs 2.77 [1.12-6.86] | 0.4 | NA vs 53 |
| Pediatric (yes/no) | 0 vs 5 | NA vs 2.64 [1.63-4.29] | NA | NA vs 43 |
| Overall Biliary complication | 1.75 [0.89-3.43] | 0.1 | 54 | |
| Rituximab for ABO-I LT (yes/no) | 3 vs 2 | 1.38 [0.62-3.07] vs 1.25 [0.35-4.51] | 0.89 | 51 vs 0 |
| Living donor (yes/no) | 3 vs 5 | 1.52 [0.74-3.10] vs 2.36 [0.63-8.87] | 0.57 | 46 vs 66 |
| Urgent indication (yes/no) | 2 vs 6 | 1.23 [0.48-3.21] vs 2.08 [0.85-5.07] | 0.44 | 0 vs 66 |
| Pediatric (yes/no) | 2 vs 6 | 1.25 [0.35-4.51] vs 1.95 [0.85-4.46] | 0.57 | 0 vs 67 |
| Bile leak | 1.85 [0.46-7.39] | 0.39 | 53 | |
| Rituximab for ABO-I LT (yes/no) | 2 vs 0 | 0.96 [0.38-2.46] vs NA | NA | 0 vs NA |
| Living donor (yes/no) | 2 vs 1 | 0.98 [0.38-2.49] vs 10.82 [1.17-100.44] | 0.051 | 0 vs NA |
| Urgent indication (yes/no) | 0 vs 3 | NA vs 1.85 [0.46-7.39] | NA | NA vs 53 |
| Pediatric (yes/no) | 0 vs 3 | NA vs 1.85 [0.46-7.39] | NA | NA vs 53 |
| Biliary stricture | 1.37 [0.70-2.70] | 0.36 | 47 | |
| Rituximab for ABO-I LT (yes/no) | 4 vs 1 | 1.00 [0.46-2.15] vs 4.32 [1.18-15.81] | 0.06 | 52 vs NA |
| Living donor (yes/no) | 4 vs 2 | 1.00 [0.46-2.15] vs 4.49 [1.36-14.87] | 0.04 | 52 vs 0 |
| Urgent indication (yes/no) | 1 vs 5 | 4.32 [1.18-15.81] vs 1.44 [1.04-1.99] | 0.11 | NA vs 42 |
| Pediatric (yes/no) | 0 vs 6 | NA vs 1.52 [1.11-2.08] | NA | NA vs 47 |
| HV complication | 1.60 [0.64-4.00] | 0.32 | NA | |
| Rituximab for ABO-I LT (yes/no) | 1 vs 0 | 1.60 [0.64-4.00] vs NA | NA | NA |
| Living donor (yes/no) | 1 vs 0 | 1.60 [0.64-4.00] vs NA | NA | NA |
| Urgent indication (yes/no) | 0 vs 1 | NA vs 1.60 [0.64-4.00] | NA | NA |
| Pediatric (yes/no) | 0 vs 1 | NA vs 1.60 [0.64-4.00] | NA | NA |
| PV complication | 1.83 [0.70-4.76] | 0.22 | 0 | |
| Rituximab for ABO-I LT (yes/no) | 3 vs 1 | 1.19 [0.31-4.65] vs 2.65 [0.29-24.07] | 0.55 | 0 vs NA |
| Living donor (yes/no) | 3 vs 2 | 1.19 [0.31-4.65] vs 3.27 [0.82-13.07] | 0.31 | 0 vs 0 |
| Urgent indication (yes/no) | 2 vs 3 | 3.27 [0.82-13.07] vs 1.19 [0.31-4.65] | 0.31 | 0 vs 0 |
| Pediatric (yes/no) | 0 vs 5 | NA vs 1.83 [0.70-4.76] | NA | NA vs 0 |
| HA complication | 4.17 [2.26-7.67] | < 0.001 | 0 | |
| Rituximab for ABO-I LT (yes/no) | 3 vs 3 | 2.52 [0.68-9.27] vs 4.43 [0.90-21.87] | 0.59 | 0 vs 53 |
| Living donor (yes/no) | 4 vs 5 | 3.62 [1.20-10.91] vs 4.44 [2.13-9.25] | 0.76 | 0 vs 10 |
| Urgent indication (yes/no) | 3 vs 6 | 5.50 [2.33-13.00] vs 3.30 [1.39-7.83] | 0.41 | 0 vs 0 |
| Pediatric (yes/no) | 1 vs 8 | 0.47 [0.03-8.56] vs 5.26 [2.73-10.14] | 0.11 | NA vs 0 |
P value for overall effect or test for differences in subgroup analysis;
Stewart et al[35] reported graft survival rates of pediatric and adults, respectively. ABO-I: ABO-Incompatible; ACR: Acute cellular rejection; AMR: Antibody-mediated rejection; CMV: Cytomegalovirus; CR: Chronic rejection; HA: Hepatic artery; HV: Hepatic vein; LT: Liver transplantation; PV: Portal vein.
Although there were no significant heterogeneities in most comparisons, such as in graft survival, patient survival, and complications, we attempted to minimize potential heterogeneities and detail the subgroup-specific differences through subgroup analyses. Possible confounding factors-the parameters that were speculated to impact the outcomes of this meta-analysis-included study population (adult vs pediatric), use of rituximab for ABO-I LT, urgent indication, and donor type (deceased vs living). There were no significant differences in most subgroup comparisons.
However, studies that involved pedia-tric patients[16,18,34,35,38] showed better 1-year (OR = 0.88, 95%CI: 0.68-1.15 vs OR = 0.59, 95%CI: 0.50-0.69; P = 0.01) and 3-year graft survivals (OR = 0.95, 95%CI: 0.71-1.26 vs OR = 0.67, 95%CI: 0.57-0.80; P = 0.04) after ABO-I LT than those involving adult patients. Furthermore, in such studies, there were no significant differences between ABO-I LT and ABO-C LT in 1-, 3-, 5-, and 10-year graft survivals (1-year: OR=0.88, 95%CI: 0.68-1.15, P = 0.35; 3-year: OR = 0.95, 95%CI: 0.71-1.26, P = 0.71; 5-year: OR = 0.82, 95%CI: 0.63-1.07, P = 0.14; 10-year: OR = 0.71, 95%CI: 0.41-1.23, P = 0.22).
Meanwhile, using rituximab in ABO-I LT patients[28-33] showed better 1-year graft survival (OR = 0.88, 95%CI: 0.58-1.33 vs OR = 0.44, 95%CI: 0.30-0.66; P = 0.02) after ABO-I LT compared to cases not using rituximab[15,16,18,34,38-40,42]. Moreover, in such cases, 1-, 3-, and 5-year graft survival of ABO-I LT were not significantly different from those of ABO-C LT (1-year: OR = 0.88, 95%CI: 0.58-1.33, P = 0.55; 3-year: OR = 0.81, 95%CI: 0.56-1.18, P = 0.28; 5-year: OR = 0.96, 95%CI: 0.66-1.39, P = 0.83). On the other hand, when rituximab was not used, incidence of biliary stricture (OR = 1.00, 95%CI: 0.46-2.15 vs OR=4.32, 95%CI: 1.18- 15.81; P = 0.06) and ACR (OR = 0.86, 95%CI: 0.57-1.30 vs OR = 1.61, 95%CI: 1.01-2.58; P = 0.048) tended to be higher in ABO-I LT than in ABO-C LT. However, there was no difference in AMR incidence (OR=48.32, 95%CI: 2.31-1011.61 vs OR = 245.87, 95%CI: 13.04-4636.62; P = 0.45) and patient survival (1-year: OR = 0.88, 95% CI 0.59-1.31 vs OR = 0.67, 95%CI: 0.44-1.04, P = 0.38; 3-year: OR = 0.90, 95%CI: 0.64-1.27 vs OR = 0.80, 95%CI: 0.31-2.06, P = 0.82; 5-year: OR = 0.90, 95%CI: 0.61-1.31 vs OR = 1.00, 95%CI: 0.20-5.08, P = 0.89) in accordance with the use of rituximab.
Studies that involved urgent indica-tions[15,17,19,20,33,34,39,40,42] had worse 1-year graft survival (OR = 0.37, 95%CI: 0.23-0.59 vs OR=0.70, 95%CI: 0.61-0.81; P = 0.01) but better 5-year patient survival (OR = 2.38, 95%CI: 0.86-6.63 vs OR = 0.79, 95%CI: 0.57-1.08, P = 0.043) for ABO-I LT than those for ABO-CT when compared to studies without urgent indications.
In addition, compared to cases that involved the use of deceased donor liver allografts, those that involved the use of living donor liver allografts[18,28-32,36] showed lower prevalence of ACR (OR = 0.87, 95%CI: 0.58-1.32 vs OR = 1.69, 95%CI: 1.14-2.50; P = 0.02) and biliary stricture (OR = 1.00, 95%CI: 0.46-2.15 vs OR = 4.49, 95%CI: 1.36-14.87; P = 0.04) in ABO-I LT. Results of sensitivity analysis and subgroup analysis are summarized in Table 3.
Publication bias
In this meta-analysis, 1-year patient survival was found to have a potential publication bias with a funnel plot asymmetry (Figure 6A) and a P = 0.06 calculated by the Egger’s regression test. Other variables did not show a significant publication bias. After adjusting for funnel plot asymmetry using the trim-and-fill method[26] (Figure 6B), ABO-I LT showed a significantly poorer 1-year patient survival than ABO-C LT (OR=0.73, 95%CI: 0.56-0.95, P = 0.02; I2 = 25.8%).
Figure 6.
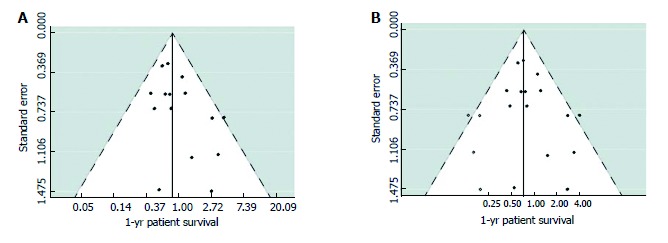
(A) Funnel plot and (B) Adjusted funnel plot using the Trim-and-Fill method of studies reporting on 1-yr patient survival after ABO-incompatible liver transplantation vs ABO-Compatible liver transplantation. Closed circles represent observed published studies; open circles represent imputed unpublished studies. ABO-C: ABO-compatible; ABO-I: ABO-incompatible; LT: Liver transplantation.
DISCUSSION
Since the first attempt of ABO-I LT by Starzl et al[1,2], poor outcomes after ABO-I LT, including AMR, lower graft survival, HAT, and cholangitis, were insurmountable barriers for expanding the application of transplantation across the ABO blood group barrier, from a few urgent cases to cases of chronic liver disease and liver cancer[3-6]. Extraordinary improvements have been made in the outcomes of ABO-I LT with the introduction of multiple desensitization strategies, such as PE (or plasmapheresis), splenectomy, GLI, rituximab, MMF, and IVIG, as well as with advances of immunosuppression agents[7-12]. However, whether ABO-I LT is comparable to ABO-C LT remains a topic of debate.
This meta-analysis revealed that pooled results of graft survival were poorer in ABO-I LT than in ABO-C LT. However, patient survival did not significantly vary in accordance with ABO compatibility in most cases. The data on 10-year patient survival had low reliability, as they were only reported by one study-although produced based on a long-term follow-up[18].
Meanwhile, the cumulative meta-analysis in order by the median year of study period showed that the cumulative results of graft and patient survival remained consistent since the early 2000s (Supplementary Figures 1 and 2). This is mainly due to the stabilization of the desensitization protocol through the application of PE (or plasmapheresis)[42-44], muromonab-CD3 (OKT3)[42-44], splenectomy[9], PV infusion[10,45], HA infusion[46,47], rituximab[48-50], and IVIG[7,8,51] in ABO-I LT. However, from a different perspective, the fact that ABO-I LT patients showed a poorer graft survival than ABO-C LT patients and that cumulative meta-analysis of graft survival remained mostly unchanged since the early 2000s implies that the current desensitization protocol for ABO-I LT still requires an improvement.
With regard to ABO-I-related complications, the prevalence of AMR, CR, CMV infection, and HA complication was higher in ABO-I LT than in ABO-C LT. Overall biliary complication-after omitting a study of Sanchez et al[41] with a wide range of CI in the sensitivity analysis-was more prevalent in ABO-I LT than in ABO-C LT.
In the subgroup analyses, studies that only involved pediatric patients[16,18,34,35,38], compared to those that did not, showed better 1-year and 3-year graft survivals after ABO-I LT than those after ABO-C LT. Furthermore, in such cases, 1-, 3-, 5-, and 10-year graft survivals after ABO-I LT were comparable to those after ABO-C LT. There were several reports that pediatric ABO-I LT was more successful than adult ABO-I LT[52,53]. Egawa et al[52] reported that an advanced recipient age for ABO-I LDLT is associated with poor outcomes, including graft and patient survivals, intrahepatic biliary complications, and hepatic necrosis. Maternal anti-ABO antibodies (Ab) begin to disappear from week two after birth, and neonates begin to produce their own reservoir of anti-ABO Ab from weeks 8-12 after birth, which reaches a level similar to that of adults by age 5-10[54,55]. Thus, younger pediatric patients may be immunologically immature, showing lower anti-ABO Ab levels and immature complement system[56,57], which could be a possible explanation for our result of pediatric graft survival.
In this meta-analysis, cases that used rituximab in ABO-I LT patients[28-33] showed better 1-year graft survival after ABO-I LT than those that did not use rituximab[5,15,16,18,34,38-40]. Furthermore, in such cases, 1-, 3-, and 5-year graft survivals of ABO-I LT were comparable to those of ABO-C LT. On the other hand, biliary stricture and ACR tended to be more prevalent after ABO-I LT when rituximab was not used. There were no differences in AMR and patient survival in accordance with the use of rituximab.
Rituximab is a chimeric human anti-CD20 mono-clonal antibody, which destroys B cells via antibody-dependent cell-mediated cytotoxicity, direct antigen-antibody reaction, and complement-dependent cytotoxicity[58,59]. Since its first introduction as a prophylactic in Japan in 2002, multiple centers have used rituximab during ABO-I LT, which is considered to have contributed to the dramatic improvements in the outcomes of ABO-I LT[9,11,60,61]. In this meta-analysis, it was noted that using rituximab improved graft survival while reducing incidences of biliary stricture and ACR after ABO-I LT. However, its effects on AMR and patient survival were rather unclear, and we speculate this to be a result of excluding some studies from the subgroup analyses for lack of clear descriptions of desensitization methods in ABO-I LT[17,19,20,35-37,41].
Meanwhile, studies that involved urgent indications, such as FHF, ALF, re-transplantation, and critically ill patients in the intensive care unit[5,15,17,19,20,33,34,39,40], showed worse 1-year graft survival but better 5-year patient survival in ABO-I LT than in ABO-C LT when compared to studies that did not involve urgent indications. Further, there were no significant differences in 3- and 5-year graft survivals between the two types of LT. This may be due to the fact that studies that only examined recipients with urgent indications mostly involved relatively lower prevalences of chronic liver disease and liver cancer but more advanced disease severity and inadequate desensitizations before ABO-I LT.
Shaked et al[62]showed that there were no differences in biopsy-proven ACR and graft loss by rejection between LDLT and deceased donor liver transplantation (DDLT). However, our subgroup analysis showed that ACR was less prevalent after ABO-I LT in cases that only used living donor liver grafts[18,28-32,36] than in cases that did not. In other words, there were no differences in ACR in accordance with ABO compatibility in cases of LDLT, but incidences of ACR increased in cases of ABO-I LT using deceased donor liver grafts. It could be assumed that compared to DDLT, LDLT has immunological advantages resulting from high genetic similarities between organ donor and recipient and short cold ischemic times[63,64].
Further, biliary complications, such as bile leak and biliary stricture, are known to be more prevalent in LDLT with inherent weakness arising from a small duct size, possible multiplicity of bile duct, and cutting liver parenchyma, compared to those in DDLT[65-68]. However, our analysis revealed that using only living donor liver grafts[18,28-32,36] resulted in fewer cases of biliary stricture in ABO-I LT. One of the possible reasons is that studies only involving deceased donor grafts in our meta-analysis of biliary stricture[40,41] were published at least 20 years earlier than the studies involving living donor allografts[28-31]. Further, some of them involved urgent indications[40], which would have resulted in the use of markedly different desensitization and immunosuppression methods and surgical techniques from those employed today.
Meanwhile, in this meta-analysis, a potential publication bias was detected in the 1-year patient survival. Possible sources of asymmetry in the funnel plot would most definitely include small study effects, but poor methodological quality, true heterogeneity, artifactual, and chance could be other sources as well[21,25,69-72].
This review has some limitations. First, it was based on non-randomized controlled trials because it is practically impossible to randomly allocate patients into either ABO-C LT or ABO-I LT group. Second, some articles lacked clear descriptions about patient demographics and study design, such as age, enrollment criteria, graft type, and desensitization and immunosuppression methods. Third, some results showed heterogeneity and potential publication bias.
This meta-analysis is the largest review that integrating more than 8000 cases of ABO-I LT and ABO-C LT. It revealed that ABO-I LT is associated with poorer graft survival and higher prevalence of AMR, CR, CMV infection, overall biliary complication, and HA complication than those of ABO-C LT. There were no significant differences in patient survival, ACR, bacterial infection, fungal infection, bile leak, biliary stricture, and HV and PV complications in accordance with ABO compatibility. In our subgroup analysis, graft survival in ABO-I LT was found to be comparable to that in ABO-C LT in pediatric patients. Use of rituximab was associated with better graft survival in ABO-I LT. In cases of DDLT, there was a higher incidence of ACR after ABO-I LT. Although substantial improvements and advances have been made in liver transplantations across the ABO blood group barrier, persistent limitations call for further endeavors to achieve better outcomes.
ACKNOWLEDGMENTS
The authors wish to thank Jae Ryong Shim, MD for his contribution to this article.
COMMENTS
Background
Increased ABO-incompatible (ABO-I) liver transplantation (LT) is inevitable due to reduced organ donation and difficulty in finding suitable ABO-compatible (ABO-C) allografts. In particular, the importance of ABO-I LT is increasing in Asian countries where the use rate of ABO-I liver allograft is higher than that of Western countries due to the large number of organ donations in the family in living donor liver transplantation.
Research frontiers
Outcomes after LT in accordance with ABO compatibility is still controversial. Therefore, it is necessary to evaluate the possibilities and the limitations of ABO-I LT by meta-analysis integrating outcomes of previous reports comparing ABO-I and ABO-C LT.
Innovations and breakthroughs
ABO-I LT is comparable to ABO-C LT in terms of patient survival, but is inferior in graft survival, antibody-mediated rejection, chronic rejection, cytomegalovirus infection, overall biliary complication, and hepatic artery complication. However, in pediatric patients and those using rituximab, the graft survival of ABO-I LT was comparable to that of ABO-C LT.
Applications
The authors performed a meta-analysis of outcomes after liver transplantation in accordance with ABO compatibility. In this way, the possibilities and the limitations of ABO-I LT can be clarified.
Terminology
ABO-I transplantation is an assignment method for organ transplantation, which allows the use of available organs more efficiently regardless of the ABO blood type, which cannot otherwise be used due to hyperacute rejection.
Peer-review
This meta-analysis is the largest review article of more than 8000 cases of ABO-I and ABO-C LT. The authors concluded that ABO-I LT, although patient survival was similar, was inferior to ABO-C LT in graft survival and several ABO-I-related complications. The article is well written and of highly clinical implications.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: No conflicts of interest were declared for all authors.
Data sharing statement: Technical appendix, statistical code, and dataset available from the corresponding author at kshlj@ncc.re.kr. No additional data are available.
Peer-review started: May 26, 2017
First decision: June 23, 2017
Article in press: August 15, 2017
P- Reviewer: Cerwenka H, Ramsay MA, Topaloglu S, ZS- Editor: Qi Y L- Editor: A E- Editor: Ma YJ
Contributor Information
Eung Chang Lee, Center for Liver Cancer, National Cancer Center, Goyang-si, Gyeonggi-do 410-769, South Korea.
Seong Hoon Kim, Center for Liver Cancer, National Cancer Center, Goyang-si, Gyeonggi-do 410-769, South Korea. kshlj@ncc.re.kr.
Sang-Jae Park, Center for Liver Cancer, National Cancer Center, Goyang-si, Gyeonggi-do 410-769, South Korea.
References
- 1.Starzl TE, Putnam CW. Experience in hepatic transplantation: Saunders Philadelphia, 1969 [Google Scholar]
- 2.Starzl TE, Koep LJ, Halgrimson CG, Hood J, Schroter GP, Porter KA, Weil R 3rd. Fifteen years of clinical liver transplantation. Gastroenterology. 1979;77:375–388. [PMC free article] [PubMed] [Google Scholar]
- 3.Rego J, Prevost F, Rumeau JL, Modesto A, Fourtanier G, Durand D, Suc JM, Ohayon E, Ducos J. Hyperacute rejection after ABO-incompatible orthotopic liver transplantation. Transplant Proc. 1987;19:4589–4590. [PubMed] [Google Scholar]
- 4.Gugenheim J, Samuel D, Fabiani B, Saliba F, Castaing D, Reynes M, Bismuth H. Rejection of ABO incompatible liver allografts in man. Transplant Proc. 1989;21:2223–2224. [PubMed] [Google Scholar]
- 5.Gugenheim J, Samuel D, Reynes M, Bismuth H. Liver transplantation across ABO blood group barriers. Lancet. 1990;336:519–523. doi: 10.1016/0140-6736(90)92082-s. [DOI] [PubMed] [Google Scholar]
- 6.Sanchez-Urdazpal L, Sterioff S, Janes C, Schwerman L, Rosen C, Krom RA. Increased bile duct complications in ABO incompatible liver transplant recipients. Transplant Proc. 1991;23:1440–1441. [PubMed] [Google Scholar]
- 7.Urbani L, Mazzoni A, Bianco I, Grazzini T, De Simone P, Catalano G, Montin U, Petruccelli S, Morelli L, Campani D, et al. The role of immunomodulation in ABO-incompatible adult liver transplant recipients. J Clin Apher. 2008;23:55–62. doi: 10.1002/jca.20156. [DOI] [PubMed] [Google Scholar]
- 8.Ikegami T, Taketomi A, Soejima Y, Yoshizumi T, Uchiyama H, Harada N, Iguchi T, Hashimoto N, Maehara Y. Rituximab, IVIG, and plasma exchange without graft local infusion treatment: a new protocol in ABO incompatible living donor liver transplantation. Transplantation. 2009;88:303–307. doi: 10.1097/TP.0b013e3181adcae6. [DOI] [PubMed] [Google Scholar]
- 9.Egawa H, Teramukai S, Haga H, Tanabe M, Fukushima M, Shimazu M. Present status of ABO-incompatible living donor liver transplantation in Japan. Hepatology. 2008;47:143–152. doi: 10.1002/hep.21928. [DOI] [PubMed] [Google Scholar]
- 10.Tanabe M, Shimazu M, Wakabayashi G, Hoshino K, Kawachi S, Kadomura T, Seki H, Morikawa Y, Kitajima M. Intraportal infusion therapy as a novel approach to adult ABO-incompatible liver transplantation. Transplantation. 2002;73:1959–1961. doi: 10.1097/00007890-200206270-00021. [DOI] [PubMed] [Google Scholar]
- 11.Tanabe M, Kawachi S, Obara H, Shinoda M, Hibi T, Kitagawa Y, Wakabayashi G, Shimazu M, Kitajima M. Current progress in ABO-incompatible liver transplantation. Eur J Clin Invest. 2010;40:943–949. doi: 10.1111/j.1365-2362.2010.02339.x. [DOI] [PubMed] [Google Scholar]
- 12.Yamada Y, Hoshino K, Morikawa Y, Okamura J, Hotta R, Komori K, Nakao S, Obara H, Kawachi S, Fuchimoto Y, et al. Successful liver transplantation across the ABO incompatibility barrier in 6 cases of biliary atresia. J Pediatr Surg. 2006;41:1976–1979. doi: 10.1016/j.jpedsurg.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. [PMC free article] [PubMed] [Google Scholar]
- 14.Cota GF, de Sousa MR, Fereguetti TO, Rabello A. Efficacy of anti-leishmania therapy in visceral leishmaniasis among HIV infected patients: a systematic review with indirect comparison. PLoS Negl Trop Dis. 2013;7:e2195. doi: 10.1371/journal.pntd.0002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reding R, Veyckemans F, de Ville de Goyet J, de Hemptinne B, Carlier M, Van Obbergh L, Moulin D, Reynaert M, Latinne D, Vraux H. ABO-incompatible orthotopic liver allografting in urgent indications. Surg Gynecol Obstet. 1992;174:59–64. [PubMed] [Google Scholar]
- 16.Cacciarelli TV, So SK, Lim J, Concepcion W, Cox K, Esquivel CO. A reassessment of ABO incompatibility in pediatric liver transplantation. Transplantation. 1995;60:757–760. doi: 10.1097/00007890-199510150-00024. [DOI] [PubMed] [Google Scholar]
- 17.Bjøro K, Ericzon BG, Kirkegaard P, Höckerstedt K, Söderdahl G, Olausson M, Foss A, Schmidt LE, Isoniemi H, Brandsaeter B, et al. Highly urgent liver transplantation: possible impact of donor-recipient ABO matching on the outcome after transplantation. Transplantation. 2003;75:347–353. doi: 10.1097/01.TP.0000044359.72379.E5. [DOI] [PubMed] [Google Scholar]
- 18.Ueda M, Oike F, Ogura Y, Uryuhara K, Fujimoto Y, Kasahara M, Ogawa K, Kozaki K, Haga H, Tanaka K. Long-term outcomes of 600 living donor liver transplants for pediatric patients at a single center. Liver Transpl. 2006;12:1326–1336. doi: 10.1002/lt.20826. [DOI] [PubMed] [Google Scholar]
- 19.Koukoutsis I, Bellagamba R, Tamijmarane A, Gunson B, Muralidharan V, Wigmore SJ, Mayer DA, Mirza DF, Buckels JA, Bramhall SR. Outcomes after identical and compatible orthotopic liver transplantation for fulminant hepatic failure: a single center experience in UK. Transpl Int. 2007;20:659–665. doi: 10.1111/j.1432-2277.2007.00458.x. [DOI] [PubMed] [Google Scholar]
- 20.Toso C, Al-Qahtani M, Alsaif FA, Bigam DL, Meeberg GA, James Shapiro AM, Bain VG, Kneteman NM. ABO-incompatible liver transplantation for critically ill adult patients. Transpl Int. 2007;20:675–681. doi: 10.1111/j.1432-2277.2007.00492.x. [DOI] [PubMed] [Google Scholar]
- 21.Deeks J, Higgins J, Altman D, Green S. Cochrane handbook for systematic reviews of interventions version 5.1. 0 [updated March 2011]. The Cochrane Collaboration. 2011. [Google Scholar]
- 22.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenland S. O Rourke K: Meta-Analysis. Modern Ep-idemiology, Edited by Rothman KJ, Greenland S, Lash T. Lippincott Williams and Wilkins, 2008 [Google Scholar]
- 24.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295:676–680. doi: 10.1001/jama.295.6.676. [DOI] [PubMed] [Google Scholar]
- 25.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 27.Song F, Eastwood AJ, Gilbody S, Duley L, Sutton AJ. Publication and related biases. Health Technol Assess. 2000;4:1–115. [PubMed] [Google Scholar]
- 28.Song GW, Lee SG, Hwang S, Kim KH, Ahn CS, Moon DB, Ha TY, Jung DH, Park GC, Kim WJ, et al. ABO-Incompatible Adult Living Donor Liver Transplantation Under the Desensitization Protocol With Rituximab. Am J Transplant. 2016;16:157–170. doi: 10.1111/ajt.13444. [DOI] [PubMed] [Google Scholar]
- 29.Kim JM, Kwon CH, Joh JW, Han SB, Sinn DH, Choi GS, Kang ES, Lee JH, Kim GS, Lee SK. Case-matched comparison of ABO-incompatible and ABO-compatible living donor liver transplantation. Br J Surg. 2016;103:276–283. doi: 10.1002/bjs.10048. [DOI] [PubMed] [Google Scholar]
- 30.Kim JD, Choi DL, Kim SG, Lee AJ. Single-Center Experience of ABO-Incompatible Living-Donor Liver Transplantation With a New Simplified Intravenous Immunoglobulin Protocol: A Propensity Score-Matching Analysis. Transplant Proc. 2016;48:1134–1138. doi: 10.1016/j.transproceed.2016.02.040. [DOI] [PubMed] [Google Scholar]
- 31.Ikegami T, Yoshizumi T, Soejima Y, Uchiyama H, Shirabe K, Maehara Y. Feasible usage of ABO incompatible grafts in living donor liver transplantation. Hepatobiliary Surg Nutr. 2016;5:91–97. doi: 10.3978/j.issn.2304-3881.2015.06.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee CF, Cheng CH, Wang YC, Soong RS, Wu TH, Chou HS, Wu TJ, Chan KM, Lee CS, Lee WC. Adult living donor liver transplantation across ABO-incompatibility. Medicine (United States) 2015;94:e1796. doi: 10.1097/MD.0000000000001796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen T, Lin BY, Jia JJ, Wang ZY, Wang L, Ling Q, Geng L, Yan S, Zheng SS. A modified protocol with rituximab and intravenous immunoglobulin in emergent ABO-incompatible liver transplantation for acute liver failure. Hepatobiliary Pancreat Dis Int. 2014;13:395–401. doi: 10.1016/s1499-3872(14)60268-x. [DOI] [PubMed] [Google Scholar]
- 34.Heffron TG, Pillen T, Smallwood G, Rodriguez J, Sekar S, Henry S, Vos M, Casper K, Gupta NA, Fasola CG, et al. Pediatric liver transplantation for acute liver failure at a single center: a 10-year experience. Pediatr Transplant. 2010;14:228–232. doi: 10.1111/j.1399-3046.2009.01202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stewart ZA, Locke JE, Montgomery RA, Singer AL, Cameron AM, Segev DL. ABO-incompatible deceased donor liver transplantation in the United States: a national registry analysis. Liver Transpl. 2009;15:883–893. doi: 10.1002/lt.21723. [DOI] [PubMed] [Google Scholar]
- 36.Iwamoto H, Hama K, Nakamura Y, Osamu K, Yokoyama T, Kihara Y, Ashizawa T, Niido T, Matsuno N, Nagao T. Biliary complications after 52 adult living donor liver transplantations: a single-center experience. Transplant Proc. 2008;40:2539–2541. doi: 10.1016/j.transproceed.2008.07.042. [DOI] [PubMed] [Google Scholar]
- 37.Saito T, Egawa H, Kudo T, Takakura S, Fujihara N, Iinuma Y, Ichiyama S. Pre-transplant risk factors predicting post-transplant cytomegalovirus infection in liver transplant recipients. Transpl Int. 2007;20:419–424. doi: 10.1111/j.1432-2277.2007.00459.x. [DOI] [PubMed] [Google Scholar]
- 38.Heffron T, Welch D, Pillen T, Asolati M, Smallwood G, Hagedorn P, Nam C, Duncan A, Guy M, Martinez E, et al. Successful ABO-incompatible pediatric liver transplantation utilizing standard immunosuppression with selective postoperative plasmapheresis. Liver Transpl. 2006;12:972–978. doi: 10.1002/lt.20760. [DOI] [PubMed] [Google Scholar]
- 39.Chui AKK, Ling J, McCaughan GW, Painter D, Shun A, Dorney SFA, Mears DC, Sheil AGR. ABO blood group incompatibility in liver transplantation: A single- centre experience. Australian and New Zealand J Surg. 1997;67:275–278. doi: 10.1111/j.1445-2197.1997.tb01962.x. [DOI] [PubMed] [Google Scholar]
- 40.Lo CM, Shaked A, Busuttil RW. Risk factors for liver transplantation across the ABO barrier. Transplantation. 1994;58:543–547. doi: 10.1097/00007890-199409150-00003. [DOI] [PubMed] [Google Scholar]
- 41.Sanchez-Urdazpal L, Batts KP, Gores GJ, Moore SB, Sterioff S, Wiesner RH, Krom RA. Increased bile duct complications in liver transplantation across the ABO barrier. Ann Surg. 1993;218:152–158. doi: 10.1097/00000658-199308000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tokunaga Y, Tanaka K, Fujita S, Yamaguchi T, Sawada H, Kato H, Uemoto S, Yamaoka Y, Ozawa K. Living related liver transplantation across ABO blood groups with FK506 and OKT3. Transpl Int. 1993;6:313–318. doi: 10.1007/BF00335967. [DOI] [PubMed] [Google Scholar]
- 43.Dunn SP, Halligan GE, Billmire DF, Vinocur CD, Lawrence J, Falkenstein K, Weintraub W, Meyers R. ABO-incompatible liver transplantation: a risk worth taking. Transplant Proc. 1993;25:3109. [PubMed] [Google Scholar]
- 44.Renard TH, Andrews WS. An approach to ABO-incompatible liver transplantation in children. Transplantation. 1992;53:116–121. doi: 10.1097/00007890-199201000-00022. [DOI] [PubMed] [Google Scholar]
- 45.Demetris AJ, Jaffe R, Tzakis A, Ramsey G, Todo S, Belle S, Esquivel C, Shapiro R, Markus B, Mroczek E. Antibody-mediated rejection of human orthotopic liver allografts. A study of liver transplantation across ABO blood group barriers. Am J Pathol. 1988;132:489–502. [PMC free article] [PubMed] [Google Scholar]
- 46.Nakamura Y, Matsuno N, Iwamoto H, Yokoyama T, Kuzuoka K, Kihara Y, Taira S, Sagara T, Jojima Y, Konno O. Tashiro J, Akashi I, Hama K, Narumi K, Iwahori T, Uchiyama M, Tanaka K, Nagao T. Successful case of adult ABO-incompatible liver transplantation: beneficial effects of intrahepatic artery infusion therapy: a case report. Transplant Proc. 2004;36:2269–2273. doi: 10.1016/j.transproceed.2004.08.094. [DOI] [PubMed] [Google Scholar]
- 47.Taenaka N, Shimada Y, Hirata T, Nishijima MK, Takezawa J, Yoshiya I, Kambayashi J. Gabexate mesilate (FOY) therapy of disseminated intravascular coagulation due to sepsis. Crit Care Med. 1983;11:735–738. doi: 10.1097/00003246-198309000-00013. [DOI] [PubMed] [Google Scholar]
- 48.Monteiro I, McLoughlin LM, Fisher A, de la Torre AN, Koneru B. Rituximab with plasmapheresis and splenectomy in abo-incompatible liver transplantation. Transplantation. 2003;76:1648–1649. doi: 10.1097/01.TP.0000082723.02477.87. [DOI] [PubMed] [Google Scholar]
- 49.Kawagishi N, Satoh K, Enomoto Y, Akamatsu Y, Sekiguchi S, Fukumori T, Fujimori K, Satomi S. New strategy for ABO-incompatible living donor liver transplantation with anti-CD20 antibody (rituximab) and plasma exchange. Transplant Proc. 2005;37:1205–1206. doi: 10.1016/j.transproceed.2004.12.114. [DOI] [PubMed] [Google Scholar]
- 50.Usuda M, Fujimori K, Koyamada N, Fukumori T, Sekiguchi S, Kawagishi N, Akamatsu Y, Enomoto Y, Satoh K, Satoh A, et al. Successful use of anti-CD20 monoclonal antibody (rituximab) for ABO-incompatible living-related liver transplantation. Transplantation. 2005;79:12–16. doi: 10.1097/01.tp.0000149337.40911.e4. [DOI] [PubMed] [Google Scholar]
- 51.Jordan S, Cunningham‐Rundles C, McEwan R. Utility of intravenous immune globulin in kidney transplantation: efficacy, safety, and cost implications. Am J Transplant. 2003;3:653–664. doi: 10.1034/j.1600-6143.2003.00121.x. [DOI] [PubMed] [Google Scholar]
- 52.Egawa H, Oike F, Buhler L, Shapiro AM, Minamiguchi S, Haga H, Uryuhara K, Kiuchi T, Kaihara S, Tanaka K. Impact of recipient age on outcome of ABO-incompatible living-donor liver transplantation. Transplantation. 2004;77:403–411. doi: 10.1097/01.TP.0000110295.88926.5C. [DOI] [PubMed] [Google Scholar]
- 53.Varela-Fascinetto G, Treacy SJ, Lillehei CW, Jonas MM, Lund DP, Kevy SV, Pérez A, Zurakowski D, Vacanti JP. Long-term results in pediatric ABO-incompatible liver transplantation. Transplant Proc. 1999;31:467–468. doi: 10.1016/s0041-1345(98)01711-4. [DOI] [PubMed] [Google Scholar]
- 54.Wuttke NJ, Macardle PJ, Zola H. Blood group antibodies are made by CD5+ and by CD5- B cells. Immunol Cell Biol. 1997;75:478–483. doi: 10.1038/icb.1997.74. [DOI] [PubMed] [Google Scholar]
- 55.Eastlund T. The histo-blood group ABO system and tissue transplantation. Transfusion. 1998;38:975–988. doi: 10.1046/j.1537-2995.1998.381098440863.x. [DOI] [PubMed] [Google Scholar]
- 56.Yandza T, Lambert T, Alvarez F, Gauthier F, Jacolot D, Huault G, Fabre M, Valayer J. Outcome of ABO-incompatible liver transplantation in children with no specific alloantibodies at the time of transplantation. Transplantation. 1994;58:46–50. [PubMed] [Google Scholar]
- 57.Ferriani VP, Barbosa JE, de Carvalho IF. Serum haemolytic classical and alternative pathways of complement in infancy: age-related changes. Acta Paediatr Scand. 1990;79:322–327. doi: 10.1111/j.1651-2227.1990.tb11464.x. [DOI] [PubMed] [Google Scholar]
- 58.Boross P, Leusen JH. Mechanisms of action of CD20 antibodies. Am J Cancer Res. 2012;2:676–690. [PMC free article] [PubMed] [Google Scholar]
- 59.Weiner GJ. Rituximab: mechanism of action. Semin Hematol. 2010;47:115–123. doi: 10.1053/j.seminhematol.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Egawa H, Ohmori K, Haga H, Tsuji H, Yurugi K, Miyagawa-Hayashino A, Oike F, Fukuda A, Yoshizawa J, Takada Y, et al. B-cell surface marker analysis for improvement of rituximab prophylaxis in ABO-incompatible adult living donor liver transplantation. Liver Transpl. 2007;13:579–588. doi: 10.1002/lt.21092. [DOI] [PubMed] [Google Scholar]
- 61.Fujii Y, Shibata Y, Miyata S, Inaba S, Asai T, Hoshi Y, Takamatsu J, Takahashi K, Ohto H, Juji T, et al. Consecutive national surveys of ABO-incompatible blood transfusion in Japan. Vox Sang. 2009;97:240–246. doi: 10.1111/j.1423-0410.2009.01199.x. [DOI] [PubMed] [Google Scholar]
- 62.Shaked A, Ghobrial RM, Merion RM, Shearon TH, Emond JC, Fair JH, Fisher RA, Kulik LM, Pruett TL, Terrault NA; A2ALL Study Group. Incidence and severity of acute cellular rejection in recipients undergoing adult living donor or deceased donor liver transplantation. Am J Transplant. 2009;9:301–308. doi: 10.1111/j.1600-6143.2008.02487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maluf DG, Stravitz RT, Cotterell AH, Posner MP, Nakatsuka M, Sterling RK, Luketic VA, Shiffman ML, Ham JM, Marcos A, et al. Adult living donor versus deceased donor liver transplantation: a 6-year single center experience. Am J Transplant. 2005;5:149–156. doi: 10.1111/j.1600-6143.2004.00654.x. [DOI] [PubMed] [Google Scholar]
- 64.Liu LU, Bodian CA, Gondolesi GE, Schwartz ME, Emre S, Roayaie S, Schiano TD. Marked Differences in acute cellular rejection rates between living-donor and deceased-donor liver transplant recipients. Transplantation. 2005;80:1072–1080. doi: 10.1097/01.tp.0000176483.52769.5a. [DOI] [PubMed] [Google Scholar]
- 65.Seehofer D, Eurich D, Veltzke-Schlieker W, Neuhaus P. Biliary complications after liver transplantation: old problems and new challenges. Am J Transplant. 2013;13:253–265. doi: 10.1111/ajt.12034. [DOI] [PubMed] [Google Scholar]
- 66.Wang SF, Huang ZY, Chen XP. Biliary complications after living donor liver transplantation. Liver Transpl. 2011;17:1127–1136. doi: 10.1002/lt.22381. [DOI] [PubMed] [Google Scholar]
- 67.Zimmerman MA, Baker T, Goodrich NP, Freise C, Hong JC, Kumer S, Abt P, Cotterell AH, Samstein B, Everhart JE, et al. Development, management, and resolution of biliary complications after living and deceased donor liver transplantation: a report from the adult-to-adult living donor liver transplantation cohort study consortium. Liver Transpl. 2013;19:259–267. doi: 10.1002/lt.23595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Simoes P, Kesar V, Ahmad J. Spectrum of biliary complications following live donor liver transplantation. World J Hepatol. 2015;7:1856–1865. doi: 10.4254/wjh.v7.i14.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang Z, Xu X, Ni H. Small studies may overestimate the effect sizes in critical care meta-analyses: a meta-epidemiological study. Crit Care. 2013;17:R2. doi: 10.1186/cc11919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nüesch E, Trelle S, Reichenbach S, Rutjes AW, Tschannen B, Altman DG, Egger M, Jüni P. Small study effects in meta-analyses of osteoarthritis trials: meta-epidemiological study. BMJ. 2010;341:c3515. doi: 10.1136/bmj.c3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000;53:1119–1129. doi: 10.1016/s0895-4356(00)00242-0. [DOI] [PubMed] [Google Scholar]
- 72.Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, Carpenter J, Rücker G, Harbord RM, Schmid CH, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]


