Abstract
AIM
To evaluate the diagnostic performance of angiotensin-converting enzyme (ACE) on significant liver fibrosis in patients with chronic hepatitis B (CHB).
METHODS
In total, 100 patients with CHB who underwent liver biopsy in our hospital were enrolled, and 70 patients except for 30 patients with hypertension, fatty liver or habitual alcoholic consumption were analyzed. We compared histological liver fibrosis and serum ACE levels and evaluated the predictive potential to diagnose significant liver fibrosis by comparison with several biochemical marker-based indexes such as the aspartate aminotransferase (AST)-to-platelet ratio index (APRI), the fibrosis index based on four factors (FIB-4), the Mac-2 binding protein glycosylation isomer (M2BPGi) level and the number of platelets (Plt).
RESULTS
Serum ACE levels showed moderately positive correlation with liver fibrotic stages (R2 = 0.181). Patients with significant, advanced fibrosis and cirrhosis (F2-4) had significantly higher serum ACE levels than those with early-stage fibrosis and cirrhosis (F0-1). For significant fibrosis (≥ F2), the 12.8 U/L cut-off value of ACE showed 91.7% sensitivity and 75.0% specificity. The receiver-operating characteristic (ROC) curves analysis revealed that the area under the curve (AUC) value of ACE was 0.871, which was higher than that of APRI, FIB-4, M2BPGi and Plt.
CONCLUSION
The serum ACE level could be a novel noninvasive, easy, accurate, and inexpensive marker of significant fibrosis stage in patients with CHB.
Keywords: Angiotensin-converting enzyme, Hepatitis B virus, Liver fibrosis, Noninvasive fibrosis marker, Aspartate aminotransferase-to-platelet ratio index, Fibrosis index based on four factors, Mac-2 binding protein glycosylation isomer
Core tip: Liver fibrosis is one of key factors to determine therapeutic intervention for patients with chronic hepatitis B (CHB). However, the noninvasive prediction of CHB-related liver fibrosis is difficult. Angiotensin-converting enzyme (ACE) is reportedly involved in liver fibrogenesis. In this paper, we demonstrate that serum ACE levels are elevated in patients with CHB and show the predictive potential to diagnose significant fibrosis (≥ F2), which is the therapeutically adapted stage, with higher accuracy as compared with other fibrotic markers including APRI, FIB-4, M2BPGi and Plt. The serum ACE level could be a novel noninvasive marker of significant fibrosis stage in CHB.
INTRODUCTION
Hepatitis B virus (HBV) annually affects 350-400 million people and causes 1 million deaths worldwide[1,2]. Chronic HBV infection results in a risk of progressive liver fibrosis, leading to cirrhosis with decreased liver reserve and hepatocellular carcinoma (HCC)[3]. The annual incidence of HCC is reported to be 10%-17% in HBV-induced liver cirrhosis, and the fibrotic status stepwisely increases the risk of HCC, as in case of chronic infection of hepatitis C virus (HCV)[4]. Therefore, an early assessment of liver fibrosis is required for not only the prevention of disease progression but also the judgment of therapeutic intervention in patients with HBV infection. Liver biopsy is currently the gold standard for estimating fibrosis progression, although frequent application of this procedure is limited because of sampling error, invasiveness, and some other complications[5]. These reasons have prompted many investigators to explore noninvasive predictors for liver fibrosis. Of several noninvasive methods, two tests, namely the aspartate aminotransferase (AST)-to-platelet index (APRI) and the fibrosis index based on four factors (FIB-4), are utilized for relatively accurate detection of liver fibrosis especially induced by HCV infection[6-8]. At present, the accuracy of these models has been externally validated in patients with chronic viral hepatitis, nonalcoholic fatty liver disease (NAFLD), primary biliary cholangitis (PBC), and primary sclerosing cholangitis (PSC). With regard to chronic hepatitis B (CHB), a recent meta-analysis suggested that APRI and FIB-4 could identify CHB-related fibrosis with moderate sensitivity and accuracy[9]. However, more methods are required to circumstantially classify liver fibrosis.
The renin-angiotensin-aldosterone system (RAAS) is a key mediator in the regulation of arterial blood pressure and body fluid homeostasis[10]. RAAS also reportedly plays an important role in the hemodynamics of several organs[11,12]. RAAS is frequently activated in patients with chronic liver diseases such as cirrhosis[13,14]. Angiotensin-I-converting enzyme (ACE), a central component of RAAS, converts the inactive decapeptide angiotensin I (AT-I) into the octapeptide angiotensin II (AT-II), which shows many physiological activities, including vascular hormonal secretion and tissue growth[15]. AT-II has been considered to be a potential mediator of portal hypertension because its plasma level is markedly increased in patients with cirrhosis and its administration induces the elevation of portal pressure[13,16-18]. AT-II is pathologically recognized to induce the contractility and proliferation of hepatic stellate cells (HSCs), which play a pivotal role in the progression of liver fibrosis[19-21]. The aim of the present study was to evaluate the behavior of circulating ACE in patients with CHB and assess the relationship between CHB-related liver fibrosis and serum levels of ACE.
MATERIALS AND METHODS
Patients
In total, 100 patients who were diagnosed with serologically and histologically confirmed CHB at Nara Medical University between 2013 and 2015 were enrolled. Chronic HBV infection was diagnosed in patients according to the following criteria: (1) detectable hepatitis B surface antigen (HBsAg) for ≥ 6 mo; and (2) serum HBV-DNA ≥ 1.3 log IU/mL. The status of other HBV markers such as HB envelope antigen (HBeAg), anti-HBe, and anti-HB core (HBc) IgG was not considered as a criterion for the current assessment. All the patients who fulfilled these criteria underwent routine liver biopsies before therapeutic intervention. All pathological specimens were evaluated by at least two experienced pathologists. The degree of hepatic fibrosis was assessed and graded according to the METAVIR score for chronic hepatitis graded 0-4: F0, no fibrosis; F1, portal fibrosis without septa; F2, portal fibrosis with few septa; F3, numerous septa without cirrhosis; and F4, cirrhosis[22,23]. Hepatic steatosis is histologically defined with more than 5% of accumulated lipid droplets in the hepatic parenchyma, and habitual alcohol consumption is defined with a daily ethanol intake of > 20 g (female) or > 30 g (male). Patients were excluded if they had other concomitant chronic liver diseases, including chronic hepatitis C (CHC), autoimmune hepatitis (AIH), PBC, PSC, hemochromatosis, or Wilson’s disease as well as cancer, severe cardiopulmonary or renal diseases, diabetes mellitus (DM), sarcoidosis, dysthyroidism, or a previous history of liver transplantation. 10 patients treated with antihypertensive agents were also excluded regardless of the type of drug because high blood pressure was considered to affect serum ACE levels (Figure 1). The study was conducted in accordance with the standards of the Helsinki Declaration, and written informed consents were provided by all the study subjects. The protocols used were approved by the Ethics Committee of Nara Medical University (Nara, Japan; Approval number 1077) and other facilities.
Figure 1.
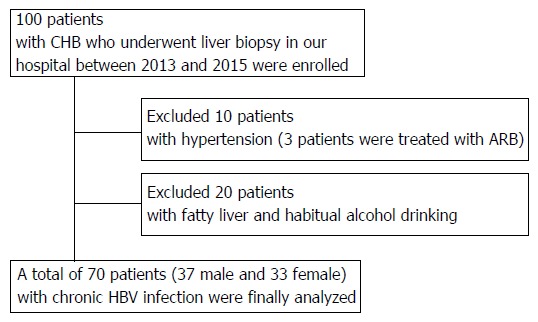
The selection of the study population. 70 patients except for 10 patients with hypertension and 20 patients with fatty liver or habitual alcoholic consumption were finally analyzed.
Laboratory analysis and measurement of angiotensin-converting enzyme levels
Serum samples from all patients were collected when liver biopsy was performed and were stored and used for the present study. The following laboratory parameters were routinely measured: complete blood count, AST, alanine aminotransferase (ALT), and albumin (Alb). To assess liver fibrosis, we evaluated four serum fibrotic markers, namely, hyaluronic acid, type 4 collagen 7S, type 3 procollagen-N-peptide (P-III-P), and Mac-2-binding protein glycosylation isomer (M2BPGi), as well as the number of platelets (Plt). APRI and FIB-4 were also used as noninvasive tests for the assessment of liver fibrosis. In these tests, evaluation was performed using the following formula: APRI = [(AST of the sample/reference AST) × 100]/platelets; FIB-4 = (age × AST)/[(platelets) × (ALT)1/2]. Serum ACE levels were measured using Kasahara’s colorimetry-based methods[24]. A serum ACE level between 8.3 and 21.4 IU/L was considered to be within the normal range according to the manufacturer’s instruction.
Statistical analysis
Continuous variables of patients with discordance and those without discordance were compared using independent t-tests or Mann-Whitney U tests, as appropriate. The χ2 or Fisher’s exact tests were used for categorical variables. Area under the receiver operating characteristic (AUROC) curves and other statistical analyses were performed using R software as described previously[25].
RESULTS
Serum ACE levels in patients with fatty liver and alcohol abuse
ACE activity is susceptible to the presence of fatty liver and/or habitual alcoholic consumption (FL/AL), as reported previously[26]. Thus, we initially evaluated the serum ACE activity in patients histologically diagnosed with hepatic steatosis to exclude patients with FL/AL from the current subjects. Histological analysis demonstrated that 20 patients showed hepatic steatosis. Of these patients, 8 patients habitually drank alcohol. Similar to several reports, serum ACE levels were significantly higher in patients with CHB with FL/AL than in those without FL/AL (Figure 2A), although other fibrotic markers, including APRI, FIB-4, and M2BPGi, were not affected by the coexistence of FL/AL (Figure 2B-2D). Interestingly, a fibrosis stage-matched comparison showed that this difference in serum ACE levels was prevalently observed in patients with early-stage (F0 and F1) liver fibrosis (Figure 2E). Consequently, 70 patients with CHB and without hypertension and/or FL/AL were included as subjects in the present study.
Figure 2.
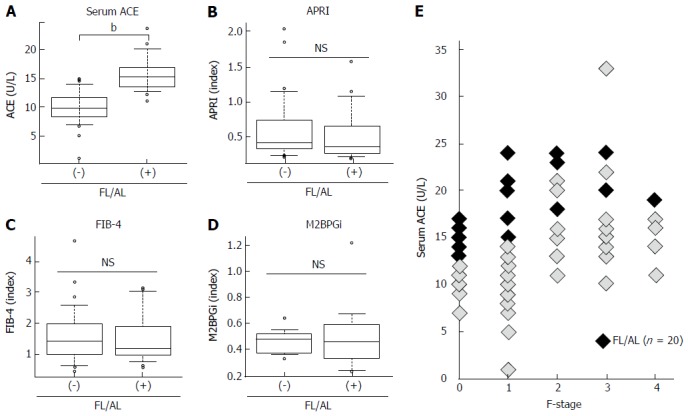
Serum levels of angiotensin-converting enzyme and fibrotic markers in patients with fatty liver and/or habitual alcoholic drinking. A: Serum angiotensin-converting enzyme (ACE) level; B: Aspartate aminotransferase to platelet index (APRI); C: Fibrosis index based on the four factors (FIB-4), D: Serum Mac-2 binding protein glycosylation isomer (M2BPGi) level in chronic hepatitis B patients with and without fatty liver and/or habitual alcoholic drinking (FL/AL). Serum ACE levels were significantly higher in the patients with FL/AL than those without FL/AL; E: Fibrosis stage-matched comparison showed that this difference in serum ACE levels between with and without FL/AL was prevalently observed in early stages (F0 and F1) of liver fibrosis. Data are means ± SD, bP < 0.01.
Characteristic features of patients
The demographic and clinicopathological characteristics of the patients in the final analysis are presented in Table 1. In total, 70 patients with CHB without FL/AL (37 males and 33 females) with a median age of 48.6 ± 14.1 years were included in the present study. The fibrosis stages were F0, F1, F2, F3 and F4 in nine (12.8%), 25 (35.7%), 17 (24.3%), 13 (18.6%), and six (8.6%) patients, respectively. All the patients in F4 were classified as Child-Pugh A. There were no differences in serum Alb levels and HBV-DNA and HBsAg values among each stage of fibrosis.
Table 1.
The clinicopathological characteristics of the patients with hepatitis B
| Variables | Patients with HB (n = 70) |
| Sex (males/females) | 37/33 |
| Age | 48.6 ± 14.1 |
| Fibrosis stage (F0/F1/F2/F3/F4) | 9/25/17/13/6 |
| Platelet (× 104 μL) (F0/F1/F2/F3/F4) | 17.9 ± 5.0 (23.2 ± 4.7/18.4 ± 3.6/18.7 ± 3.8/15.1 ± 5.4/18.9 ± 3.3) |
| Alb (g/dL) | 4.1 ± 0.4 |
| AST (IU/L) | 35.8 ± 26.7 |
| ALT (IU/L) | 42.6 ± 39.4 |
| HBV DNA (Log IU/mL) | 4.04 ± 2.24 |
| HBsAg (IU/mL) | 22697.1 ± 52927.9 |
| Hyaluronic acid (ng/mL) | 62.4 ± 92.1 |
| Type 4 collagen 7S (ng/mL) | 4.1 ± 2.0 |
| Serum ACE (U/L) (F0/F1/F2/F3/F4) | 14.1 ± 5.1 (10.8 ± 2.3/11.1 ± 4.7/17.9 ± 4.4/6.4 ± 3.9/15.0 ± 2.9) |
| APRI (F0/F1/F2/F3/F4) | 1.0 ± 1.3 (0.3 ± 0.1/0.7 ± 0.5/0.9 ± 0.6/2.3 ± 2.1/1.0 ± 0.9) |
| FIB4 (F0/F1/F2/F3/F4) | 2.0 ± 1.9 (1.3 ± 0.6/1.6 ± 1.0/1.3 ± 0.8/2.8 ± 1.7/1.7 ± 0.7) |
| M2BPGi (COI) (F0/F1/F2/F3/F4) | 1.2 ± 1.1 (0.9 ± 0.9/0.9 ± 0.6/.1 ± 1.0/2.2 ± 1.8/1.3 ± 0.6) |
HBV: Hepatitis B virus; HBsAg: Hepatitis B surface antigen; Alb: Albumin; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; ACE: Angiotensin-I-converting enzyme; APRI: Aspartate aminotransferase-to-platelet index; FIB4: Fibrosis index based on four factors; M2BPGi: Mac-2-binding protein glycosylation isomer.
Elevated serum ACE level above F2 stage in liver fibrosis
In patients with CHB, the mean serum ACE level was 14.1 ± 5.1 U/L and serum ACE levels at F0, F1, F2, F3, and F4 were 10.8 ± 2.3 U/L, 11.1 ± 4.7 U/L, 17.9 ± 4.4 U/L, 16.4 ± 3.9 U/L, and 15.0 ± 2.9 U/L, respectively. Pearson’s correlation coefficient showed moderately positive correlation between serum ACE levels and fibrotic stages (R2 = 0.181), and Mann-Whitney U tests demonstrated that patients with significant, advanced and cirrhotic stages (F2-4) showed markedly higher serum ACE levels than those with early-stage fibrosis and cirrhosis (F0-1) (Figure 3A). To validate the diagnostic performance of the serum ACE level in predicting significant liver fibrosis, we next performed receiver-operating characteristic (ROC) curve analysis. For significant fibrosis (≥ F2), ROC curve analysis revealed that the optimal ACE level cut-off point was 12.8 U/L (sensitivity, 91.7%; specificity, 75.0%; PPV, 80.5%; NPV, 89.7%; accuracy, 84.3%), and the area under the curve (AUC) value for significant fibrosis (≥ F2) was 0.871 (Figure 3B). Meanwhile, the AUC values to predict mild (≥ F1), advanced (≥ F3) and cirrhotic (F4) stage of fibrosis were 0.75 (sensitivity, 68.3%; specificity, 87.5%), 0.68 (sensitivity, 89.5%; specificity, 51.0%) and 0.601 (sensitivity, 83.3%; specificity, 51.6%), respectively which were remarkably lower than significant fibrosis (Figure 3B). These findings suggest that serum ACE level manifests its efficient performance in diagnosing significant fibrosis (≥ F2).
Figure 3.
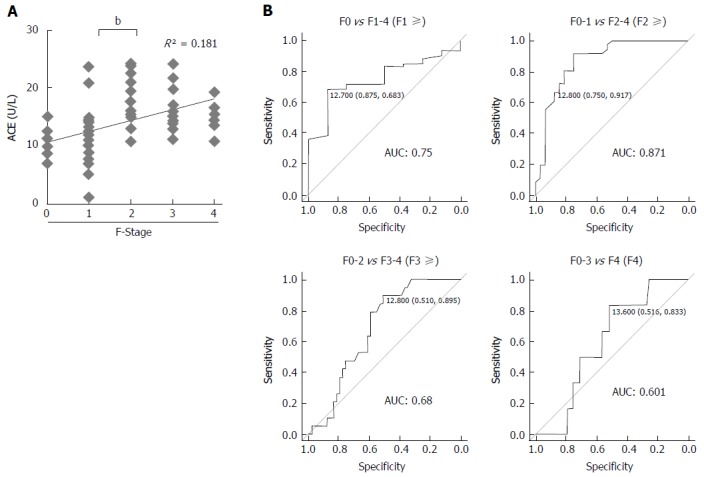
Serum angiotensin-converting enzyme levels and liver fibrosis development in patients with chronic hepatitis B. A: Correlation between serum angiotensin-converting enzyme (ACE) level and liver fibrosis stage (F-Stage) (R = 0.42, R2 = 0.181); B: Receiver operating characteristic curve analysis and area under curve (AUC) value for diagnostic performance of serum ACE level for predicting each stage of liver fibrosis. The optimal ACE level cut-off point for significant fibrosis was 12.8 U/L. Data are means ± SD, bP < 0.01.
Diagnostic performance in other markers to predict significant stage liver fibrosis
Next, to evaluate predictive potential for significant fibrosis in other markers, we performed similar analysis for well-known fibrotic parameters, including APRI, FIB-4, M2BPGi and Plt. Unlike the ACE level, there was a significant difference below and above the F3 stage in these parameters, APRI (F2, 0.86 ± 0.6 vs F3, 2.27 ± 2.0; P < 0.05), FIB-4 (F2, 1.34 ± 0.6 vs F3, 2.76 ± 1.7; P < 0.05), M2BPGi (F2, 1.1 ± 1.0 vs F3, 2.2 ± 1.8; P < 0.05) and Plt (F2, 18.7 ± 3.8 vs F3, 15.1 ± 5.4 × 104/μL; P = 0.0616) (Figure 4A-D). Similarly, the established fibrosis markers such as hyaluronic acid, type 4 collagen 7S, and P-III-P indicated an advanced fibrosis stage (≥ F3) (Figure 4E-G). Furthermore, we assessed the diagnostic performance of APRI, FIB-4, M2BPGi and Plt to predict significant fibrosis (≥ F2) by ROC curve analysis. For significant fibrosis (≥ F2), the APRI cut-off point was 0.57 (sensitivity, 83.3%; specificity, 69.7%; PPV, 76.0%; NPV, 71.1%; accuracy, 73%), FIB-4 cut-off point was 2.23 (sensitivity, 40.0%; specificity, 84.8%; PPV, 70.6%; NPV, 39.1%; accuracy, 47.6%), M2BPGi cut-off point was 0.81 (sensitivity, 50.0%; specificity, 48.9%; PPV, 69.7%; NPV, 29.4%; accuracy, 49.3%), and Plt cut-off point was 17.8 × 104/μL (sensitivity, 68.8%; specificity, 71.9%; PPV, 33.3%; NPV, 72.4%; accuracy, 50.8%) (Supplementary Figure 1). The AUC value of serum ACE (0.871) was higher than those of APRI (0.83, P = 0.224), FIB-4 (0.641, P = 0.0012), M2BPGi (0.717, P = 0.0239), and Plt (0.70, P = 0.016) (Figure 5A-D), indicating that compared with other noninvasive markers, the serum ACE level is distinctively capable of the enclosure of significant liver fibrosis (≥ F2) in patients with CHB.
Figure 4.
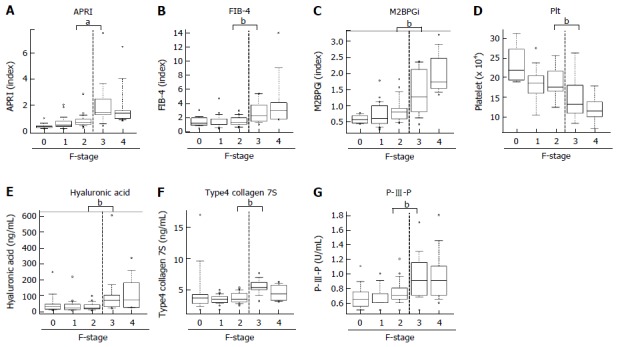
Serum levels of other markers and liver fibrosis in patients with chronic hepatitis B. A: Aspartate aminotransferase to platelet index (APRI); B: Fibrosis index based on the four factors (FIB-4); C: Serum levels of Mac-2 binding protein glycosylation isomer (M2BPGi); D: The number of platelets (Plt); E: Serum hyaluronic acid level; F: Type4 collagen 7S; and G: P-III-P levels in patients with each liver fibrotic stage (F-Stage). Data are means ± SD, aP < 0.05, bP < 0.01.
Figure 5.
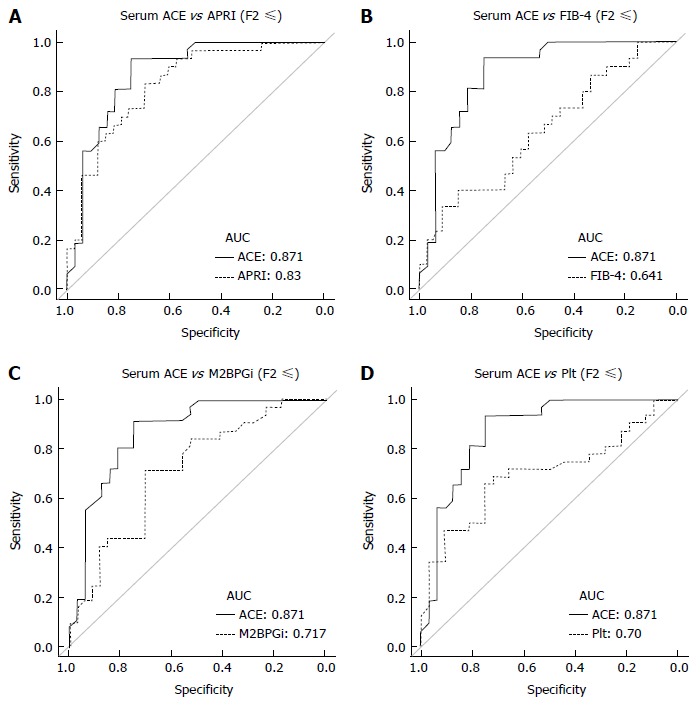
Receiver operating characteristic curve analysis for diagnostic performance for predicting significant liver fibrosis. Compared to other markers, the area under curve (AUC) value in serum angiotensin-converting enzyme (ACE) level, 0.871, was higher than that in A: Aspartate aminotransferase to platelet index (APRI), 0.83 (P = 0.224); B: Fibrosis index based on the four factors (FIB-4), 0.641 (P = 0.0012); C: Serum levels of Mac-2 binding protein glycosylation isomer (M2BPGi), 0.717 (P = 0.0239); D: The number of platelets (Plt), 0.70 (P = 0.016).
APRI is a beneficial marker in predicting advanced liver fibrosis with the highest accuracy
As the next step to closely diagnose significant fibrosis in patients with CHB, patients with > F2 fibrosis need to be further classified into significant (F2) and advanced (F3) fibrosis. Therefore, we compared the AUC values to predict advanced fibrosis stages (≥ F3) among APRI, FIB-4, M2BPGi, and Plt to evaluate diagnostic performance of these parameters for advanced fibrosis. The ROC curve analysis demonstrated that the optimum cut-off points for advanced fibrosis (≥ F3) are APRI; 1.27 (sensitivity, 75%; specificity, 91.5%), FIB-4; 2.51 (sensitivity, 56.2%; specificity, 93.6%), M2BPGi; 0.82 (sensitivity, 87.5%; specificity, 64.0%), and Plt; 15.0 × 104/μL (sensitivity, 70.6%; specificity, 87.2%) (Supplementary Figure 1). The AUC value is the highest in APRI (0.879) as compared with FIB-4 (0.803), M2BPGi (0.791), and Plt (0.813).
DISCUSSION
Identifying the degree of liver fibrosis is a clinical requisite for the treatment of chronic liver diseases regardless of the etiology because it plays a key role in predicting therapy responses and long-term outcomes of patients. In the case of CHB, nucleos(t)ide analogs (NAs) inhibit HBV-DNA replication and reduce the serum HBV level to achieve therapeutic improvement. Meanwhile, NAs do not play any role in the complete elimination of HBV and do not provide HBsAg clearance or persistent HBeAg seroconversion. Moreover, NAs frequently cause drug resistance and relapse after the termination of therapy. These pharmacological properties of NAs require long-term administration for patients to avoid the risk of liver decompensation due to cirrhosis and HCC progression, suggesting that the ideal time to start the therapy should be carefully decided considering the fibrotic stage as well as HBV-DNA and ALT levels which are extensively recognized as guideline for treatment. At present, APRI and FIB-4 indexes are widely known as noninvasive predictors to evaluate liver fibrosis[7,8]. Xiao et al[9] systematically reviewed the performance of two indexes in HBV-associated liver fibrosis. The total AUC values of APRI/FIB-4 for the diagnosis of significant fibrosis, advanced fibrosis, and cirrhosis were 0.7407, 0.7844 and 0.7347/0.8165, and 0.7268 and 0.8448, respectively. These data indicate that APRI and FIB-4 can identify CHB-related fibrosis with moderate sensitivity and accuracy. However, additional markers are required for diagnosis with higher accuracy and sensitivity. Recent evidences demonstrated that several serum markers are possibly beneficial for prediction of earlier fibrosis. Oztas et al[27] reported that soluble ST2, a receptor for the Th2 cytokine IL-33, could be used for differentiating significant fibrosis from mild fibrosis in CHB patients, and Deng et al[27] showed that serum complement 5a concentration significantly decreased in severe HBV fibrosis stages and earlier cirrhosis[28].
Our results show that serum ACE levels were markedly higher in patients with CHB who were histologically diagnosed with significant, advanced fibrosis and cirrhosis than in those with early fibrosis, while other predictors distinguished advanced fibrosis and cirrhosis (≥ F3) but not significant fibrosis (F2). These results indicate that the serum ACE level has a distinctive potential to enclose significant fibrosis (≥ F2), which is the therapeutically adapted stage for NAs[23]. As confirmed by AUC, the diagnostic value of serum ACE for detecting significant fibrosis above F2 was 87.1%, which was higher than other that of fibrotic markers, including APRI, FIB-4, M2BPGi and Plt.
As described in previous reports, AT-II, which is produced by ACE converted from AT-I, plays an important role in liver fibrosis development[21,29,30]. AT-II induces HSC proliferation, upregulates transforming growth factor-β and collagen-I gene expression, and promotes extracellular matrix synthesis[19,20]. Additionally, previous animal study demonstrated that ACE gene was up-regulated in the bile duct ligation-induced fibrotic liver[31]. Recent evidence also suggested that upregulation of hepatic ACE is accelerated particularly during fibrogenesis and is dampened in the developed fibrosis state[32]. Correspondingly, our results showed that serum ACE level was increased with moderately positive correlation with liver fibrosis development, and reached a threshold in the significant fibrosis (F2) (Figure 3A). These findings suggest that the suppression of RAAS is an inevitable strategy for preventing liver fibrosis progression. Our recent animal studies have revealed that ACE inhibitor (ACE-I) and AT receptor blocker (ARB) show significant antifibrotic effects on experimental liver fibrosis along with the suppression of activated HSC[33-36]. A clinical study also demonstrated that the group of patients with HCV treated using angiotensin-blocking agents exhibited lesser fibrosis than those without hypertension[37]. Another report showed that RAAS blocker-treated hypertensive patients with NAFLD prevalently had a mild degree of liver fibrosis[38]. Liver fibrogenesis progresses through multiple processes that are dependent on the etiology. Therefore, the clinical findings in patients with HCV and NAFLD may provide a basis for the assessment of the antifibrotic properties of RAAS blockers in HBV. A previous report actually showed that circulating ACE levels were elevated in patients with chronic HBV and more prominent elevation was observed in patients with advanced fibrosis[39].
Other reports have validated whether the serum ACE level has the clinical potential to predict liver fibrosis induced by other chronic liver diseases. Efe et al[40] suggested that ACE sustains hepatic fibrogenesis in AIH. They demonstrated that serum ACE levels increased for each fibrosis score in 73 patients with AIH, and AUC values of serum ACE for the diagnosis of significant fibrosis (≥ F2), advanced fibrosis (≥ F3), and cirrhosis (F4) were 0.89, 0.91, and 0.95, respectively. The diagnostic performance for HCV-related fibrosis is controversial. Raslan et al[41] showed that ACE polymorphism was associated with the progression of hepatic fibrosis in chronic HCV infection. In contrast, Forrest et al[42] reported that no association was identified between four RAAS polymorphisms and fibrosis in chronic HCV infection. Our other assessment also suggested that serum ACE levels in patients with CHC did not correlate with the fibrosis stage (data not shown). This discrepancy may be explained by the interaction between HCV infection and steatosis. HCV infection induces metabolic abnormalities such as insulin resistance, leading to hepatic steatosis[43,44]. Interestingly, our data show that serum ACE levels were elevated in patients with hepatic steatosis (Figure 2). The evidence indicates that hepatic steatosis induced by HCV infection may destabilize serum ACE levels.
In our results, we also found that APRI, FIB-4, M2BPGi and Plt might be good predictive markers for advanced fibrosis in patients with CHB, and APRI showed a potential with the highest accuracy in these parameters. Based on these data, we can propose a strategy to noninvasively evaluate CHB-related liver fibrosis in combination with serum ACE and APRI (Figure 6). This algorithm may be utilized to enclose significant fibrosis, particularly in patients with CHB without fatty liver and/or habitual alcoholic consumption.
Figure 6.
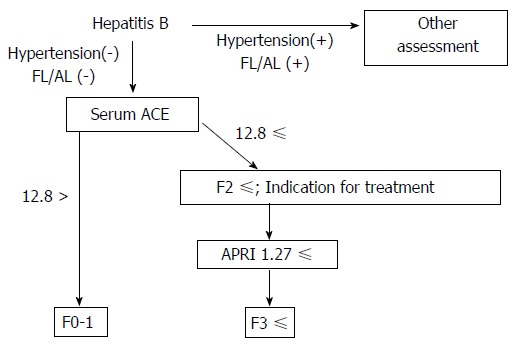
Schematic algorithm for noninvasive diagnosis of chronic hepatitis B. ACE: Angiotensin-converting enzyme; FL: Fatty liver; AL: Habitual alcoholic drinking; APRI: Aspartate aminotransferase to platelet index.
Our data have some limitations such as the lack of clinicopathological and prognostic data and a small sample size. Moreover, our study was not performed prospectively. Sequential measurements of serum ACE are required in longitudinal studies to assess the change in the serum ACE level associated with the degree of fibrosis. In addition, the optimum cut-off value for significant fibrosis of 12.8 U/L deviates from the value of 52.5 U/L in the previous study because a different method is used to measure the serum ACE level[39,40]. Therefore, comparison with data from other reports to validate the integration is difficult.
In conclusion, we indicated that the serum ACE level is a beneficial noninvasive marker to evaluate significant fibrosis and to further determine whether therapeutic intervention with NAs is necessary for patients with CHB. Higher accuracy will be expected by combination with other markers, including APRI, FIB4, M2BPGi and Plt. Moreover, our results suggest that blockade of RAAS is an effective new therapeutic strategy against CHB-related fibrosis.
COMMENTS
Background
Chronic hepatitis B virus (HBV) infection results in a risk of progressive liver fibrosis, leading to cirrhosis with decreased liver reserve and hepatocellular carcinoma. Therefore, an early assessment of liver fibrosis is required for not only the prevention of disease progression but also the judgment of therapeutic intervention. Currently, several noninvasive markers are explored for estimating early stage of HBV-related fibrosis alternatively to liver biopsy. However, it is required to identify both easier and more beneficial methods.
Research frontiers
Angiotensin-I-converting enzyme (ACE), a central component of renin-angiotensin-aldosterone system, plays a key role in the progression of liver fibrosis, however, its diagnostic performance to evaluate HBV-related earlier fibrosis.
Innovations and breakthroughs
The present study indicates that the patients with significant, advanced fibrosis and cirrhosis (F2-4) have significantly higher serum ACE levels than those with early-stage fibrosis and cirrhosis (F0-1), and serum ACE shows higher accuracy than other markers including APRI, FIB-4, M2BPGi and Plt in diagnostic performance to differentiate significant fibrosis (F2) from mild fibrosis (F1).
Applications
The serum ACE level is a beneficial noninvasive marker to evaluate significant fibrosis and to further determine whether therapeutic intervention is necessary for patients with chronic HBV.
Terminology
ACE converts the inactive decapeptide angiotensin I (AT-I) into the octapeptide angiotensin II (AT-II), which shows many physiological activities, including vascular hormonal secretion and tissue growth. AT-II has been pathologically recognized to induce the contractility and proliferation of hepatic stellate cells, which play a pivotal role in the progression of liver fibrosis.
Peer-review
The authors report an interesting approach about the diagnostic value evaluation of serum ACE for the detection of earlier HBV-related fibrosis. The sensitivity of the test discriminating F0-F1 from F2-F4 proved to be higher than that of other tests. Overall, the study is well planned and results are presented clearly.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was reviewed and approved by the Ethics Committee of Nara Medical University (Nara, Japan; Approval number 1077).
Informed consent statement: Waiver of informed consent for all study participants was guaranteed by information disclosure on website (http://www.naramed-u.ac.jp/~3int/kenkyu.html).
Conflict-of-interest statement: The authors declare that there is no conflict of interest regarding the publication of this paper.
Data sharing statement: Informed consent for data sharing was not obtained but the presented data are anonymized and risk of identification is low.
Peer-review started: March 23, 2017
First decision: April 21, 2017
Article in press: June 19, 2017
P- Reviewer: Lendvai G, Ocker M, Scicchitano P, Sen V S- Editor: Gong ZM L- Editor: A E- Editor: Li D
Contributor Information
Ryuichi Noguchi, Third Department of Internal Medicine, Nara Medical University, Kashihara, Nara 634-8522, Japan.
Kosuke Kaji, Third Department of Internal Medicine, Nara Medical University, Kashihara, Nara 634-8522, Japan. kajik@naramed-u.ac.jp.
Tadashi Namisaki, Third Department of Internal Medicine, Nara Medical University, Kashihara, Nara 634-8522, Japan.
Kei Moriya, Third Department of Internal Medicine, Nara Medical University, Kashihara, Nara 634-8522, Japan.
Mitsuteru Kitade, Third Department of Internal Medicine, Nara Medical University, Kashihara, Nara 634-8522, Japan.
Kosuke Takeda, Third Department of Internal Medicine, Nara Medical University, Kashihara, Nara 634-8522, Japan.
Hideto Kawaratani, Third Department of Internal Medicine, Nara Medical University, Kashihara, Nara 634-8522, Japan.
Yasushi Okura, Third Department of Internal Medicine, Nara Medical University, Kashihara, Nara 634-8522, Japan.
Yosuke Aihara, Third Department of Internal Medicine, Nara Medical University, Kashihara, Nara 634-8522, Japan.
Masanori Furukawa, Third Department of Internal Medicine, Nara Medical University, Kashihara, Nara 634-8522, Japan.
Akira Mitoro, Third Department of Internal Medicine, Nara Medical University, Kashihara, Nara 634-8522, Japan.
Hitoshi Yoshiji, Third Department of Internal Medicine, Nara Medical University, Kashihara, Nara 634-8522, Japan.
References
- 1.Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546–1555. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 2.Stanaway JD, Flaxman AD, Naghavi M, Fitzmaurice C, Vos T, Abubakar I, Abu-Raddad LJ, Assadi R, Bhala N, Cowie B, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet. 2016;388:1081–1088. doi: 10.1016/S0140-6736(16)30579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Martel C, Maucort-Boulch D, Plummer M, Franceschi S. World-wide relative contribution of hepatitis B and C viruses in hepatocellular carcinoma. Hepatology. 2015;62:1190–1200. doi: 10.1002/hep.27969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burns GS, Thompson AJ. Viral hepatitis B: clinical and epidemiological characteristics. Cold Spring Harb Perspect Med. 2014;4:a024935. doi: 10.1101/cshperspect.a024935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495–500. doi: 10.1056/NEJM200102153440706. [DOI] [PubMed] [Google Scholar]
- 6.Lin ZH, Xin YN, Dong QJ, Wang Q, Jiang XJ, Zhan SH, Sun Y, Xuan SY. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53:726–736. doi: 10.1002/hep.24105. [DOI] [PubMed] [Google Scholar]
- 7.Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H, Pol S. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 8.Shaheen AA, Myers RP. Diagnostic accuracy of the aspartate aminotransferase-to-platelet ratio index for the prediction of hepatitis C-related fibrosis: a systematic review. Hepatology. 2007;46:912–921. doi: 10.1002/hep.21835. [DOI] [PubMed] [Google Scholar]
- 9.Xiao G, Yang J, Yan L. Comparison of diagnostic accuracy of aspartate aminotransferase to platelet ratio index and fibrosis-4 index for detecting liver fibrosis in adult patients with chronic hepatitis B virus infection: a systemic review and meta-analysis. Hepatology. 2015;61:292–302. doi: 10.1002/hep.27382. [DOI] [PubMed] [Google Scholar]
- 10.Cooper ME. The role of the renin-angiotensin-aldosterone system in diabetes and its vascular complications. Am J Hypertens. 2004;17:16S–20S; quiz A2-4. doi: 10.1016/j.amjhyper.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Atlas SA. The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. J Manag Care Pharm. 2007;13:9–20. doi: 10.18553/jmcp.2007.13.s8-b.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolf G. Novel aspects of the renin-angiotensin-aldosterone-system. Front Biosci. 2008;13:4993–5005. doi: 10.2741/3058. [DOI] [PubMed] [Google Scholar]
- 13.Helmy A, Jalan R, Newby DE, Hayes PC, Webb DJ. Role of angiotensin II in regulation of basal and sympathetically stimulated vascular tone in early and advanced cirrhosis. Gastroenterology. 2000;118:565–572. doi: 10.1016/s0016-5085(00)70263-0. [DOI] [PubMed] [Google Scholar]
- 14.Munshi MK, Uddin MN, Glaser SS. The role of the renin-angiotensin system in liver fibrosis. Exp Biol Med (Maywood) 2011;236:557–566. doi: 10.1258/ebm.2011.010375. [DOI] [PubMed] [Google Scholar]
- 15.Stergiou GS, Skeva II. Renin-angiotensin system blockade at the level of the angiotensin converting enzyme or the angiotensin type-1 receptor: similarities and differences. Curr Top Med Chem. 2004;4:473–481. doi: 10.2174/1568026043451320. [DOI] [PubMed] [Google Scholar]
- 16.Lugo-Baruqui A, Muñoz-Valle JF, Arévalo-Gallegos S, Armendáriz-Borunda J. Role of angiotensin II in liver fibrosis-induced portal hypertension and therapeutic implications. Hepatol Res. 2010;40:95–104. doi: 10.1111/j.1872-034X.2009.00581.x. [DOI] [PubMed] [Google Scholar]
- 17.Beyazit Y, Ibis M, Purnak T, Turhan T, Kekilli M, Kurt M, Sayilir A, Onal IK, Turhan N, Tas A, et al. Elevated levels of circulating angiotensin converting enzyme in patients with hepatoportal sclerosis. Dig Dis Sci. 2011;56:2160–2165. doi: 10.1007/s10620-011-1580-7. [DOI] [PubMed] [Google Scholar]
- 18.Klein S, Rick J, Lehmann J, Schierwagen R, Schierwagen IG, Verbeke L, Hittatiya K, Uschner FE, Manekeller S, Strassburg CP, et al. Janus-kinase-2 relates directly to portal hypertension and to complications in rodent and human cirrhosis. Gut. 2017;66:145–155. doi: 10.1136/gutjnl-2015-309600. [DOI] [PubMed] [Google Scholar]
- 19.Bataller R, Ginès P, Nicolás JM, Görbig MN, Garcia-Ramallo E, Gasull X, Bosch J, Arroyo V, Rodés J. Angiotensin II induces contraction and proliferation of human hepatic stellate cells. Gastroenterology. 2000;118:1149–1156. doi: 10.1016/s0016-5085(00)70368-4. [DOI] [PubMed] [Google Scholar]
- 20.Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Nakatani T, Tsujinoue H, Fukui H. Angiotensin-II type 1 receptor interaction is a major regulator for liver fibrosis development in rats. Hepatology. 2001;34:745–750. doi: 10.1053/jhep.2001.28231. [DOI] [PubMed] [Google Scholar]
- 21.Yoshiji H, Kuriyama S, Noguchi R, Ikenaka Y, Kitade M, Kaji K, Yoshii J, Yanase K, Yamazaki M, Asada K, et al. Angiotensin-II and vascular endothelial growth factor interaction plays an important role in rat liver fibrosis development. Hepatol Res. 2006;36:124–129. doi: 10.1016/j.hepres.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology. 1994;20:15–20. [PubMed] [Google Scholar]
- 23.Drafting Committee for Hepatitis Management Guidelines and the Japan Society of Hepatology. JSH Guidelines for the Management of Hepatitis B Virus Infection. Hepatol Res. 2014;44 Suppl S1:1–58. doi: 10.1111/hepr.12269. [DOI] [PubMed] [Google Scholar]
- 24.Kasahara Y, Ashihara Y. Colorimetry of angiotensin-I converting enzyme activity in serum. Clin Chem. 1981;27:1922–1925. [PubMed] [Google Scholar]
- 25.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saiki A, Ohira M, Endo K, Koide N, Oyama T, Murano T, Watanabe H, Miyashita Y, Shirai K. Circulating angiotensin II is associated with body fat accumulation and insulin resistance in obese subjects with type 2 diabetes mellitus. Metabolism. 2009;58:708–713. doi: 10.1016/j.metabol.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 27.Oztas E, Kuzu UB, Zengin NI, Kalkan IH, Onder FO, Yildiz H, Celik HT, Akdogan M, Kilic MY, Koksal AS, et al. Can Serum ST2 Levels Be Used as a Marker of Fibrosis in Chronic Hepatitis B Infection? Medicine (Baltimore) 2015;94:e1889. doi: 10.1097/MD.0000000000001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng Y, Zhao H, Zhou J, Yan L, Wang G; China HepB-Related Fibrosis Assessment Research Group. Complement 5a is an indicator of significant fibrosis and earlier cirrhosis in patients chronically infected with hepatitis B virus. Infection. 2017;45:75–81. doi: 10.1007/s15010-016-0942-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshiji H. Anti-fibrotic therapy: Are matrix metalloproteinases friends or foes? Hepatol Res. 2009;39:748–750. doi: 10.1111/j.1872-034X.2009.00573.x. [DOI] [PubMed] [Google Scholar]
- 30.Kaji K, Yoshiji H, Ikenaka Y, Noguchi R, Aihara Y, Shirai Y, Douhara A, Fukui H. Possible involvement of angiogenesis in chronic liver diseases: interaction among renin-angiotensin-aldosterone system, insulin resistance and oxidative stress. Curr Med Chem. 2012;19:1889–1898. doi: 10.2174/092986712800099848. [DOI] [PubMed] [Google Scholar]
- 31.Paizis G, Cooper ME, Schembri JM, Tikellis C, Burrell LM, Angus PW. Up-regulation of components of the renin-angiotensin system in the bile duct-ligated rat liver. Gastroenterology. 2002;123:1667–1676. doi: 10.1053/gast.2002.36561. [DOI] [PubMed] [Google Scholar]
- 32.Warner FJ, Lubel JS, McCaughan GW, Angus PW. Liver fibrosis: a balance of ACEs? Clin Sci (Lond) 2007;113:109–118. doi: 10.1042/CS20070026. [DOI] [PubMed] [Google Scholar]
- 33.Yoshiji H, Kuriyama S, Fukui H. Blockade of renin-angiotensin system in antifibrotic therapy. J Gastroenterol Hepatol. 2007;22 Suppl 1:S93–S95. doi: 10.1111/j.1440-1746.2006.04663.x. [DOI] [PubMed] [Google Scholar]
- 34.Kaji K, Yoshiji H, Kitade M, Ikenaka Y, Noguchi R, Shirai Y, Aihara Y, Namisaki T, Yoshii J, Yanase K, et al. Combination treatment of angiotensin II type I receptor blocker and new oral iron chelator attenuates progression of nonalcoholic steatohepatitis in rats. Am J Physiol Gastrointest Liver Physiol. 2011;300:G1094–G1104. doi: 10.1152/ajpgi.00365.2010. [DOI] [PubMed] [Google Scholar]
- 35.Noguchi R, Yoshiji H, Ikenaka Y, Kaji K, Aihara Y, Shirai Y, Namisaki T, Kitade M, Douhara A, Moriya K, et al. Dual blockade of angiotensin-II and aldosterone suppresses the progression of a non-diabetic rat model of steatohepatitis. Hepatol Res. 2013;43:765–774. doi: 10.1111/hepr.12008. [DOI] [PubMed] [Google Scholar]
- 36.Okura Y, Namisaki T, Moriya K, Kitade M, Takeda K, Kaji K, Noguchi R, Nishimura N, Seki K, Kawaratani H, et al. Combined treatment with dipeptidyl peptidase-4 inhibitor (sitagliptin) and angiotensin-II type 1 receptor blocker (losartan) suppresses progression in a non-diabetic rat model of steatohepatitis. Hepatol Res. 2016 doi: 10.1111/hepr.12860. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 37.Colmenero J, Bataller R, Sancho-Bru P, Domínguez M, Moreno M, Forns X, Bruguera M, Arroyo V, Brenner DA, Ginès P. Effects of losartan on hepatic expression of nonphagocytic NADPH oxidase and fibrogenic genes in patients with chronic hepatitis C. Am J Physiol Gastrointest Liver Physiol. 2009;297:G726–G734. doi: 10.1152/ajpgi.00162.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goh GB, Pagadala MR, Dasarathy J, Unalp-Arida A, Sargent R, Hawkins C, Sourianarayanane A, Khiyami A, Yerian L, Pai R, et al. Renin-angiotensin system and fibrosis in non-alcoholic fatty liver disease. Liver Int. 2015;35:979–985. doi: 10.1111/liv.12611. [DOI] [PubMed] [Google Scholar]
- 39.Purnak T, Beyazit Y, Oztas E, Yesil Y, Efe C, Torun S, Celik T, Tenlik I, Kurt M, Ozaslan E. Serum angiotensin-converting enzyme level as a marker of fibrosis in patients with chronic hepatitis B. J Renin Angiotensin Aldosterone Syst. 2012;13:244–249. doi: 10.1177/1470320311434241. [DOI] [PubMed] [Google Scholar]
- 40.Efe C, Cengiz M, Kahramanoğlu-Aksoy E, Yilmaz B, Özşeker B, Beyazt Y, Tanoğlu A, Purnak T, Kav T, Turhan T, et al. Angiotensin-converting enzyme for noninvasive assessment of liver fibrosis in autoimmune hepatitis. Eur J Gastroenterol Hepatol. 2015;27:649–654. doi: 10.1097/MEG.0000000000000355. [DOI] [PubMed] [Google Scholar]
- 41.Raslan HM, Amr KS, Elhosary YA, Ezzat WM, Abdullah NA, El-Batae HE. Possible role of angiotensin-converting enzyme polymorphism on progression of hepatic fibrosis in chronic hepatitis C virus infection. Trans R Soc Trop Med Hyg. 2011;105:396–400. doi: 10.1016/j.trstmh.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 42.Forrest EH, Thorburn D, Spence E, Oien KA, Inglis G, Smith CA, McCruden EA, Fox R, Mills PR. Polymorphisms of the renin-angiotensin system and the severity of fibrosis in chronic hepatitis C virus infection. J Viral Hepat. 2005;12:519–524. doi: 10.1111/j.1365-2893.2005.00630.x. [DOI] [PubMed] [Google Scholar]
- 43.Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Tsukamoto K, Kimura S, Moriya K, Koike K. Hepatitis C virus infection and diabetes: direct involvement of the virus in the development of insulin resistance. Gastroenterology. 2004;126:840–848. doi: 10.1053/j.gastro.2003.11.056. [DOI] [PubMed] [Google Scholar]
- 44.Fartoux L, Poujol-Robert A, Guéchot J, Wendum D, Poupon R, Serfaty L. Insulin resistance is a cause of steatosis and fibrosis progression in chronic hepatitis C. Gut. 2005;54:1003–1008. doi: 10.1136/gut.2004.050302. [DOI] [PMC free article] [PubMed] [Google Scholar]


