Abstract
AIM
To introduce an innovative intracorporeal anastomosis technique named overlapped delta-shaped anastomosis (ODA) for colon cancer cases undergoing totally laparoscopic colectomy (TLC) and to assess its feasibility and safety.
METHODS
From January 2016 to March 2017, a total of 20 consecutive patients with colon cancer accepted TLC and the ODA technique at our medical center. Patient demographics, operative outcomes, perioperative complications, and pathological results were collected and analyzed.
RESULTS
We successfully completed TLC and the ODA procedure in all 20 cases, including 6 (30%) males and 14 (70%) females. In total, 11 (55%), 2 (10%), and 7 (35%) cases accepted right hemicolectomy, transverse hemicolectomy, and left hemicolectomy, respectively. None of the surgeries were converted to an open operation. Mean operative time was 178.5 min, and mean estimated blood loss was 58.5 mL. Mean time to first flatus was 2.5 d, and mean postoperative hospitalization duration was 6.8 d. No severe complications occurred, such as anastomotic leakage, snastomotic stenosis, anastomotic bleeding, and wound infection, except for one case who suffered from an abdominal infection and another case who suffered from gastric paralysis syndrome. Tumor recurrence was not observed in any patient during the follow-up period.
CONCLUSION
The ODA technique for colon cancer cases undergoing TLC appears to be safe and feasible, although our current results need to be verified in further studies.
Keywords: Overlapped delta-shaped anastomosis, Safety, Totally laparoscopic colectomy, Intracorporeal anastomosis, Colon cancer
Core tip: Intracorporeal anastomosis technique is one of the biggest difficulties encountered by surgeons during the totally laparoscopic colectomy procedure. In this paper, we introduce an innovative intracorporeal anastomosis technique named overlapped delta-shaped anastomosis and assess its feasibility and safety.
INTRODUCTION
With the improvement of living standards and the extension of life expectancy, the incidence of colon cancer is increasingly rising and will continue to rise in China[1,2]. Surgery is the mainstay of treatment for colon cancer. Over the past three decades, minimally invasive surgery for colon cancer has drawn more and more attention[3]. Nowadays, laparoscopic assisted colectomy (LAC) is widely used for cases with colon cancer and the advantages of this procedure have been widely verified[4-6].
With the advances in surgical devices and the improvements in surgical performance, totally laparoscopic colectomy (TLC) has gradually been adopted by experienced surgeons[7-8]. In theory, the TLC procedure conforms more to the concept of minimally invasive surgery and the principle of the tumor-free technique, while the intracorporeal anastomosis (IA) technique still represents one of the biggest difficulties for surgeons during TLC.
As a new technique, delta-shaped anastomosis was first presented by a Japanese scholar named Kanaya in 2002 after he completed the first case of totally laparoscopic gastroenterostomy (TLG)[9]. This procedure has been widely adopted for TLG and has proven to be both safe and feasible[10-12]. However, delta-shaped anastomosis for TLC is rarely reported. Since January 2016, an innovative technique named “overlapped delta-shaped anastomosis (ODA)” has been applied to colon cancer cases undergoing TLC at the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. Herein, we introduce this surgical innovation and assess its feasibility and safety.
MATERIALS AND METHODS
Methods
Prior to surgery, the advantages and disadvantages of TLC and ODA were explained to all patients in detail, and then preoperative informed content was acquired. All patients underwent the same preoperative examinations including routine blood tests, chest X-ray, electrocardiogram examination, and computerized tomography (CT) of the abdominal and pelvic cavity to exclude cases with surgical contraindications and distant organ metastasis. Patients with intestinal obstruction were excluded from our study. The American Joint Committee on Cancer (AJCC) staging system (the seventh edition) was used for tumor staging. Postoperative pain was evaluated by the patients on a subjective visual pain scale ranging from 0 to 10, with 0 representing no pain and 10 representing the worst pain imaginable. The ethics committee at our institution approved this study, and this retrospective study conformed to the ethical standards of the World Medical Association Declaration of Helsinki.
Patients
From January 2016 to March 2017, a total of 20 consecutive patients with colon cancer underwent TLC and the ODA procedure. All of these cases accepted colonoscopy examination, and colon cancer was diagnosed by pathology. Preoperative mechanical bowel preparation was performed using polyethylene glycol electrolyte powder. Preventative antibiotics were administered by intravenous drip 30 min pre-operatively and continued for 24 h after the operation.
Surgical procedure (taking right hemicolectomy as an example)
Step 1: Positioning the patient and placing trocars: The patient was placed in the modified lithotomy position. A five-port technique was used: a 12 mm sub-umbilical port as the observation port, a 12 mm port located in the left upper quadrant as the primary operating port, and three 5 mm ports located in the left lower quadrant, the right lower quadrant and the right upper quadrant, respectively, as the secondary operating ports (Figure 1). Abdominal pressure was maintained at approximately 15 mmHg, and then the patient was placed in the Trendelenburg position and left tilt applied in order to expose the mesenteric root, ileocolic vessels, and superior mesenteric vessels.
Figure 1.

Five-port technique.
Step 2: Clearing lymph nodes and tailoring the mesentery: Along the surface of the superior mesenteric vessels, the mesocolon was dissected in a bottom-up fashion, and then the ileocolic vessels, ascending colon vessels, and the right branch of the transverse colon vessels were exposed and carefully severed (Figure 2A). During this process, attention should be paid to the gastrocolic trunk, pancreatic head, duodenum, and right gastroepiploic vessels, such that these structures are protected. Next, adhesions between the right abdominal wall and the ascending colon were dissected in a similar bottom-up fashion. Finally, the mesentery of the terminal ileum approximately 15 cm away from the ileocecal region, and the right half of the transverse colon, was tailored, respectively.
Figure 2.
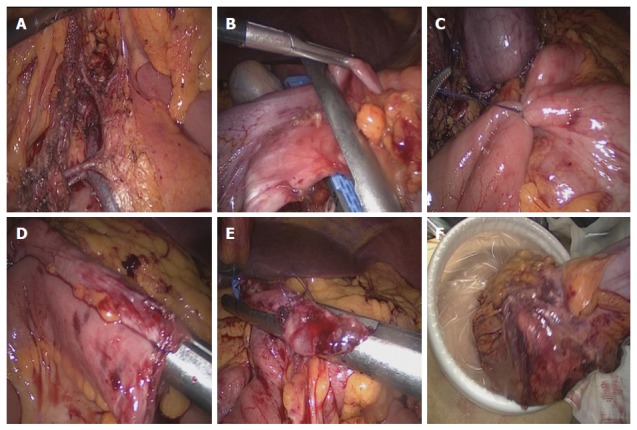
Surgical procedure. A: The ileocolic vessels, ascending colon vessels, and the right branch of the transverse colon vessels were exposed; B: The right half of the transverse colon was transected using endoscopic linear cutter staplers; C: The proximal ileum and the distal transverse colon were fixed in an overlapped fashion using a piece of absorbable suture to facilitate anastomosis; D: After imbedding the lumens with an endoscopic linear cutter stapler, intestinal walls with no mesentery were got through; E: The common opening was then closed using an endoscopic linear cutter stapler; F: Finally, the specimen was removed from the abdominal cavity using a transverse incision above the symphysis pubis.
Step 3: Transecting and anastomosing the bowel: The terminal ileum and the right half of the transverse colon were transected using two endoscopic linear cutter staplers (Johnson and Johnson, PSE60A and ECR60B) (Figure 2B). The broken ends were sterilized using alcohol gauze swabs. Then, the proximal ileum and the distal transverse colon were fixed using a piece of absorbable suture; this was performed in an overlapped fashion in order to facilitate anastomosis (Figure 2C). Two small openings located at the ileum and the transverse colon were created using an ultrasound scalpel. After imbedding the lumens with another endoscopic linear cutter stapler, intestinal walls with no mesentery were got through (Figure 2D). Finally, the common opening was closed using an endoscopic linear cutter stapler (Figure 2E). The specimen was removed from the abdominal cavity using a transverse incision above the symphysis pubis (Figure 2F).
The ways the transverse and left colectomy was performed were similar to the method above mentioned in the right hemicolectomy, including the number of trocars, type of staplers, and same anisoperistaltic anastomosis.
Follow-up
The first day after surgery represented the beginning of the follow-up period. Patients were asked to visit doctors every three months after leaving hospital until two years after surgery, biannually for the next three years and then annually, and the deadline of follow-up period was March 31, 2017.
Statistical analysis
Statistical Package for the Social Sciences (SPSS) software (21.0 version for Windows; SPSS Inc. Chicago, IL, United States) was used for data analyses. Quantitative data following a normal distribution are provided as mean and its range. Qualitative data are provided as number and its percentage. The statistical methods of this study were reviewed by Wang M from our institution.
RESULTS
We successfully completed TLC and the ODA procedure in all 20 cases, including 6 (30%) males and 14 (70%) females. None of the surgeries were converted to an open operation. Mean patient age was 52.6 years (range: 38-67 years), and mean body mass index (BMI) was 22.9 kg/m2 (range: 20.2-25.5 kg/m2). Our study included 10 (50%) cases with ascending colon cancer, 4 (20%) with transverse colon cancer, and 6 (30%) with descending colon cancer. In total, 11 (55%), 2 (10%), and 7 (35%) cases accepted right hemicolectomy, transverse hemicolectomy, and left hemicolectomy, respectively. Four (20%) out of the 20 cases accepted preoperative chemotherapy (Table 1).
Table 1.
Patient demographics n (%)
| Parameter | |
| Gender | |
| Male | 6 (30) |
| Female | 14 (70) |
| Age, yr, mean (range) | 52.6 (38-67) |
| BMI, kg/m2, mean (range) | 22.9 (20.2-25.5) |
| ASA score | |
| 1 | 10 (50) |
| 2 | 7 (35) |
| 3 | 3 (15) |
| Tumor site | |
| Ascending colon | 10 (50) |
| Transverse colon | 4 (20) |
| Descending colon | 6 (30) |
| Surgical procedure | |
| Right hemicolectomy | 11 (55) |
| Transverse hemicolectomy | 2 (10) |
| Left hemicolectomy | 7 (35) |
| Preoperative chemotherapy | 4 (20) |
BMI: Body mass index; ASA: American Society of Anesthesiologists.
The mean operative time was 178.5 min (range: 155-225 min), and mean estimated blood loss was 58.5 mL (range: 30-100 mL). For all 20 cases, the mean time to first flatus was 2.5 d (range: 1-3 d), and mean first time to oral intake was 3.0 d (range: 2-4 d). Mean postoperative hospitalization period was 6.8 d (range 5-8 d). Patients only reported slight pain (scoring 2.8 on average) on the first day after the operation, and there was almost no pain (scoring 0.7 on average) by the third day after the operation. No severe complications were encountered, such as anastomotic leakage, anastomotic stenosis, anastomotic bleeding, deep vein thrombosis, or intestinal obstruction, except one case who suffered from abdominal infection due to chylous fistula, and another case who suffered from gastric paralysis syndrome. However, both of these patients made a heathy recovery with conservative treatments. There were no deaths during the perioperative period (Table 2).
Table 2.
Operative outcomes and perioperative complications
| Parameter | |
| Operative outcomes | |
| Operative time, min, mean (range) | 178.5 (155-225) |
| Estimated blood loss, mL, mean (range) | 58.5 (30-100) |
| Time to first flatus, d, mean (range) | 2.5 (1-3) |
| Time to first oral intake, d, mean (range) | 3.0 (2-4) |
| Postoperative hospitalization, d, mean (range) | 6.8 (5-8) |
| Length of transverse incision, cm, mean (range) | 4.8 (4-6) |
| Postoperative pain score | |
| The first day, mean (range) | 2.8 (2-4) |
| The second day, mean (range) | 1.5 (1-3) |
| The third day, mean (range) | 0.7 (0-1) |
| Perioperative complications (%) | |
| Anastomotic leakage | 0 (0) |
| Anastomotic stenosis | 0 (0) |
| Anastomotic bleeding | 0 (0) |
| Abdominal infection | 1 (5) |
| Deep-vein thrombosis | 0 (0) |
| Wound infection | 0 (0) |
| Intestinal obstruction | 0 (0) |
| Gastric paralysis syndrome | 1 (5) |
| Reoperation (%) | 0 (0) |
Pathological results are shown in Table 3. Mean tumor size was 4.2 cm (range: 2.8-6.3 cm) and mean proximal and distal resection margins were 19.5 cm (range: 13.8-23.5 cm) and 17.8 cm (range: 12.2-21.6 cm), respectively. The mean number of lymph nodes harvested was 32.4 (range: 23-45). Among these cases, there were five (25%) cases of stage I, eight (40%) cases of stage II, and seven (35%) cases of stage III disease. A typical specimen and the transverse incision above the symphysis pubis are shown in Figure 3. The mean follow-up time was 8.5 mo (range: 1-15 mo). No patient was lost to follow-up and no cases experienced recurrence during the follow-up period.
Table 3.
Pathological results
| Parameter | |
| Tumor size, cm, mean (range) | 4.2 (2.8-6.3) |
| Proximal resection margin, cm, mean (range) | 19.5 (13.8-23.5) |
| Distal resection margin, cm, mean (range) | 17.8 (12.2-21.6) |
| No. of lymph nodes harvested, mean (range) | 32.4 (23-45) |
| pTNM stage (%) | |
| I | 5 (25) |
| II | 8 (40) |
| III | 7 (35) |
pTNM: Pathological tumor node metastasis.
Figure 3.
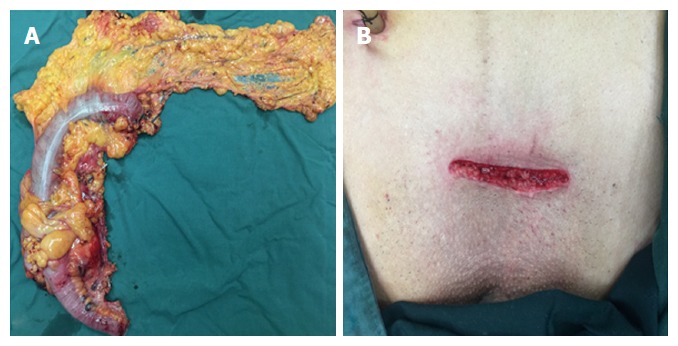
Typical specimen (A) and a transverse incision above the symphysis pubis (B).
DISCUSSION
Surgery plays an important role in the treatment of colon cancer and the goal of every surgeon is to create as little trauma as necessary and encourage a quick recovery. With recent advances in surgical devices and improvements in surgical performance, surgeons have achieved a significant breakthrough in the treatment of colon cancer by changing from open surgery to laparoscopic surgery[3]. Minimally invasive surgery and quick recovery following laparoscopic surgery have been well verified and documented. As a consequence, laparoscopic surgery has become incredibly popular over the last three decades and several new laparoscopic techniques, including robotic-assisted colectomy, single incision laparoscopic surgery, natural orifice specimen extraction surgery, and natural orifice transluminal endoscopic surgery, have also been reported[3,13-17]. Although, totally laparoscopic surgery has been carried out by experienced surgeons[7-8], IA technique still represents a significant difficulty during TLC. Therefore, an easy and feasible anastomosis technique is very important.
In 2002, the Japanese scholar Kanaya first reported the delta-shaped anastomosis of TLG[9]. In 2011, he summarized 100 cases of delta-shaped anastomosis performed by eight surgeons, and the results showed that, on average, only 13 min were needed to complete anastomosis, and that all eight surgeons experienced a short learning curve. In addition, this earlier study reported that patients had an early time of first oral intake[18]. Subsequently, more and more studies confirmed the advantages of this procedure[10-12].
In recent years, several studies have described IA for patients with colon cancer. Wang et al[17] reported that 11 patients with sigmoid cancer undergoing totally laparoscopic sigmoid colectomy with delta-shaped anastomosis and transvaginal specimen extraction, and these procedures were successful without serious complications. Jian-Cheng et al[19] reported 56 cases with colon cancer, who had accepted total laparoscopic right hemicolectomy (LRH) with 3-step stapled intracorporeal isoperistaltic ileocolicanastomosis (TLG group). Compared to cases in the extracorporeal anastomosis group (LG group), cases in the TLG group had shorter IA time (9.9-15.5 min vs 13.5-18.2 min, P < 0.001), lower mean intraoperative blood loss (83.2 mL vs 93.3 mL, P < 0.001), faster recovery of bowel function (P < 0.001), and lower postoperative pain score (P < 0.001). van Oostendorp et al[20] conducted a meta-analysis including 12 non-randomized comparative studies and the results showed that IA in the LRH group was associated with reduced short-term morbidity and decreased length of hospital stay suggesting faster recovery. In addition, Wu et al[21] also reported that the IA for LRH could improve cosmetic effect and result in better postoperative recovery outcomes without increasing intraoperative and postoperative complications. However, the difficulties of IA procedure behind the satisfactory results were all reported by above-mentioned studies.
In the present study, we performed TLC in 20 colon cancer cases using a new IA method named the ODA procedure; all surgeries were carried out smoothly and none of the cases were converted to open operation. Patients had slight pain after surgery and showed early time of first flatus and oral intake. The results also showed that the ODA procedure did not increase postoperative complications and that radical resection was also guaranteed. In particular, the transverse incision above the symphysis pubis was only 4.8 cm in length, much shorter and more covert than that required for LAC. Collectively, these observations provide promising results with regard to its feasibility and safety. It thus appears that TLC and the ODA procedure are more in line with the concept of minimally invasive surgery and enhance recovery following surgery.
This was a retrospective study and only 20 patients were included; these factors may, therefore, represent potential limitations. However, all operations were successfully accomplished and no severe complications occurred. Prospective randomized controlled trials with larger sample sizes and longer follow-up periods are now needed to confirm our results.
The ODA technique for cases with TLC appears to be safe and feasible for suitable patients when performed by experienced surgeons. This promising result now allows us to cater for colon cancer patients who require medical cosmetology.
COMMENTS
Background
With the advances in surgical devices and the improvements in surgical performance, totally laparoscopic colectomy (TLC) has gradually been adopted by experienced surgeons in large medical centers. However, the intracorporeal anastomosis (IA) technique is one of the biggest difficulties encountered by surgeons during the TLC procedure, and the currently adopted delta-shaped anastomotis technique is difficult to operate.
Research frontiers
Now, the mainstream of colectomy for colon cancer is still laparoscopic assisted colectomy. TLC is only adopted by experienced surgeons in large medical centers for the difficulties of IA technique.
Innovations and breakthroughs
In this study, a modified delta-shaped anastomotis technique named overlapped delta-shaped anastomosis (ODA) has been applied to colon cancer cases undergoing TLC in this medical institution. The results show that the ODA technique is a feasible and safe procedure.
Applications
In the present study, we have reported, for the first time, the ODA technique adopted during the TLC procedure. The perfect result has proven its feasibility and safety. Therefore, the ODA procedure can be applied to colon cancer patients who have no contraindications for laparoscopic surgery.
Terminology
The ODA procedure is currently used for the anastomosis between the proximal and distal intestinal canals for colon cancer cases who accepted TLC.
Peer-review
IA technique is one of the biggest difficulties during the TLC procedure. The authors report an innovative IA technique named the “ODA technique”. The short-term outcomes are exciting and it deserves further promotion.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was reviewed and approved by the ethics committee of National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100021, China.
Informed consent statement: All study participants provided written informed consent prior to study enrollment.
Conflict-of-interest statement: The authors report no relevant conflicts of interest.
Data sharing statement: No additional data are available.
Peer-review started: June 15, 2017
First decision: July 17, 2017
Article in press: August 25, 2017
P- Reviewer: Dumitrascu DL, Kopljar M S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Zhang FF
Contributor Information
Hai-Tao Zhou, Department of Colorectal Surgery, National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, China.
Peng Wang, Department of Colorectal Surgery, National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, China.
Jian-Wei Liang, Department of Colorectal Surgery, National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, China.
Hao Su, Department of Colorectal Surgery, National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, China.
Zhi-Xiang Zhou, Department of Colorectal Surgery, National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, China. zhouzx001@163.com.
References
- 1.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Zeng WG, Liu MJ, Zhou ZX, Hou HR, Liang JW, Wang Z, Zhang XM, Hu JJ. Outcome of Laparoscopic Versus Open Resection for Transverse Colon Cancer. J Gastrointest Surg. 2015;19:1869–1874. doi: 10.1007/s11605-015-2891-3. [DOI] [PubMed] [Google Scholar]
- 3.Zeng WG, Zhou ZX. Mini-invasive surgery for colorectal cancer. Chin J Cancer. 2014;33:277–284. doi: 10.5732/cjc.013.10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Briggs A, Goldberg J. Tips, Tricks, and Technique for Laparoscopic Colectomy. Clin Colon Rectal Surg. 2017;30:130–135. doi: 10.1055/s-0036-1597313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Zhang C, Feng YF, Fu Z, Sun YM. Comparison of short-term outcomes between laparoscopic-assisted and open complete mesocolic excision (CME) for the treatment of transverse colon cancer. Chin Clin Oncol. 2017;6:6. doi: 10.21037/cco.2017.01.01. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal S, Gincherman M, Birnbaum E, Fleshman JW, Mutch M. Comparison of long-term follow up of laparoscopic versus open colectomy for transverse colon cancer. Proc (Bayl Univ Med Cent) 2015;28:296–299. doi: 10.1080/08998280.2015.11929254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swaid F, Sroka G, Madi H, Shteinberg D, Somri M, Matter I. Totally laparoscopic versus laparoscopic-assisted left colectomy for cancer: a retrospective review. Surg Endosc. 2016;30:2481–2488. doi: 10.1007/s00464-015-4502-5. [DOI] [PubMed] [Google Scholar]
- 8.Lascarides C, Buscaglia JM, Denoya PI, Nagula S, Bucobo JC, Bergamaschi R. Laparoscopic right colectomy vs laparoscopic-assisted colonoscopic polypectomy for endoscopically unresectable polyps: a randomized controlled trial. Colorectal Dis. 2016;18:1050–1056. doi: 10.1111/codi.13346. [DOI] [PubMed] [Google Scholar]
- 9.Kanaya S, Gomi T, Momoi H, Tamaki N, Isobe H, Katayama T, Wada Y, Ohtoshi M. Delta-shaped anastomosis in totally laparoscopic Billroth I gastrectomy: new technique of intraabdominal gastroduodenostomy. J Am Coll Surg. 2002;195:284–287. doi: 10.1016/s1072-7515(02)01239-5. [DOI] [PubMed] [Google Scholar]
- 10.Gao B, Huang Q, Dong J. [Clinical research of delta-shaped anastomosis technology in laparoscopic distal gastrectomy and digestive tract reconstruction] Zhonghua Weichang Waike Zazhi. 2017;20:73–78. [PubMed] [Google Scholar]
- 11.Lin M, Zheng CH, Huang CM, Li P, Xie JW, Wang JB, Lin JX, Lu J, Chen QY, Cao LL, et al. Totally laparoscopic versus laparoscopy-assisted Billroth-I anastomosis for gastric cancer: a case-control and case-matched study. Surg Endosc. 2016;30:5245–5254. doi: 10.1007/s00464-016-4872-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo R, Ge Y, Wu X, Zhang J. [Long-term survival of total laparoscopic radical distal gastrectomy with delta-shaped anastomosis] Zhonghua Weichang Waike Zazhi. 2016;19:549–552. [PubMed] [Google Scholar]
- 13.Cai JC, Hong XY. Laparoscopic-Assisted Natural Orifice Specimen Extraction Radical Descending Colectomy Using a Cai Tube. World J Surg. 2016;40:2803–2807. doi: 10.1007/s00268-016-3597-8. [DOI] [PubMed] [Google Scholar]
- 14.Karagul S, Kayaalp C, Sumer F, Ertugrul I, Kirmizi S, Tardu A, Yagci MA. Success rate of natural orifice specimen extraction after laparoscopic colorectal resections. Tech Coloproctol. 2017;21:295–300. doi: 10.1007/s10151-017-1611-2. [DOI] [PubMed] [Google Scholar]
- 15.Kayaalp C, Yagci MA, Soyer V. Laparoscopic and natural orifice transluminal restorative proctocolectomy: no abdominal incision for specimen extraction or ileostomy. Wideochir Inne Tech Maloinwazyjne. 2016;11:115–120. doi: 10.5114/wiitm.2016.59578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ngu J, Wong AS. Transanal natural orifice specimen extraction in colorectal surgery: bacteriological and oncological concerns. ANZ J Surg. 2016;86:299–302. doi: 10.1111/ans.13383. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z, Zhang XM, Zhou HT, Liang JW, Zhou ZX. New technique of intracorporeal anastomosis and transvaginal specimen extraction for laparoscopic sigmoid colectomy. Asian Pac J Cancer Prev. 2014;15:6733–6736. doi: 10.7314/apjcp.2014.15.16.6733. [DOI] [PubMed] [Google Scholar]
- 18.Kanaya S, Kawamura Y, Kawada H, Iwasaki H, Gomi T, Satoh S, Uyama I. The delta-shaped anastomosis in laparoscopic distal gastrectomy: analysis of the initial 100 consecutive procedures of intracorporeal gastroduodenostomy. Gastric Cancer. 2011;14:365–371. doi: 10.1007/s10120-011-0054-0. [DOI] [PubMed] [Google Scholar]
- 19.Jian-Cheng T, Shu-Sheng W, Bo Z, Jian F, Liang Z. Total laparoscopic right hemicolectomy with 3-step stapled intracorporeal isoperistaltic ileocolic anastomosis for colon cancer: An evaluation of short-term outcomes. Medicine (Baltimore) 2016;95:e5538. doi: 10.1097/MD.0000000000005538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Oostendorp S, Elfrink A, Borstlap W, Schoonmade L, Sietses C, Meijerink J, Tuynman J. Intracorporeal versus extracorporeal anastomosis in right hemicolectomy: a systematic review and meta-analysis. Surg Endosc. 2017;31:64–77. doi: 10.1007/s00464-016-4982-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Q, Jin C, Hu T, Wei M, Wang Z. Intracorporeal Versus Extracorporeal Anastomosis in Laparoscopic Right Colectomy: A Systematic Review and Meta-Analysis. J Laparoendosc Adv Surg Tech A. 2017;27:348–357. doi: 10.1089/lap.2016.0485. [DOI] [PubMed] [Google Scholar]


