Abstract
Background:
Several components of metabolic syndrome (MetS) facilitate its diagnosis, including abdominal obesity, hyperlipidemia, high blood pressure, and insulin resistance. The production of interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) seem to be associated with MetS components. The aim of this study was to evaluate the correlation between IL-6 and TNF-α serum levels with MetS and its components
Methods:
This case-control study investigated 250 subjects, comprising 125 healthy controls from the Kerman Blood Transfusion Organization and 125 MetS patients. Serum IL-6 and TNF-α levels were measured using the enzyme-linked immunosorbent assay (ELISA).
Results:
Serum IL-6 and TNF-α levels were greater in MetS patients than in controls. However, no correlation was observed between MetS components and IL-6 or TNF-α serum levels.
Conclusion:
Patients with MetS had significantly greater serum IL-6 and TNF-α levels than the controls, supporting the evidence that inflammation plays an important role in the immunopathogenesis of the disease. Additionally, IL-6 and TNF-α serum levels may predict MetS. The lack of association between IL-6 and TNF-α serum levels and MetS components remains to be investigated by further research.
Key Words: IL-6, Metabolic syndrome, Metabolic syndrome components, TNF-α
Introduction
Metabolic syndrome (MetS), alternatively known as insulin resistance syndrome or syndrome x, is a set of metabolic disorders that increase patients risks for cardiovascular disease (CVD) and type 2 diabetes mellitus (T2DM). The main clinical symptoms of MetS include central obesity, hypertension, hyperglycaemia, low high-density lipoprotein (HDL) levels, and high triglycerides (1). Metabolic syndrome is defined by the International Diabetes Federation (IDF) as a waist circumference (WC) greater than 94 inches in Caucasian men and greater than 80 inches in Caucasian women plus at least two of the following risk factors: triglycerides greater than 150 mg/dl or taking lipid-lowering agents, HDL levels less than 40 mg/dl and 50 mg/dl in men and women, respectively, a systolic blood pressure above 130 mmHg or a diastolic blood pressure above 85 mmHg or taking medicine for high blood pressure, and a fasting blood sugar above 100 mg/dl or having T2DM (2). In the past it was believed that adipose tissue is inactive and only serves to store triglycerides; however, it has been well demonstrated that fatty tissues secrete bioactive proteins, generally termed adipokines, which appear to play key roles in energy homeostasis and inflammation. Recent evidence suggests that obesity, in particular chronic visceral adiposity, is associated with inflammatory markers including interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and C-reactive protein (CRP) (3, 4). Studies have shown that IL-6 and TNF-α are secreted from infiltrated macrophages into adipose tissue and lipocytes and also play important roles in MetS, insulin resistance, non-alcoholic fatty liver disease, and atherogenesis (5-11). The current study aimed to evaluate IL-6 and TNF-α serum levels in Iranian MetS patients and healthy controls and determine their associations with MetS and MetS components.
Materials and Methods
Ethics Statement
All the participants were from Kerman, a city in southeast Iran, and gave written informed consent for enrollment in the study. The research was performed in 2014 and 2015 and was approved by the Ethics Committee of Kerman University of Medical Sciences. The approval number is K/93/352.
Selection of MetS patients and controls
A total of 250 subjects, including 125 healthy controls from the Kerman Blood Transfusion Organization and 125 MetS patients, were enrolled in the study. The clinical history and disease status of each participant were taken by a gastroenterologist and an endocrinologist. Metabolic syndrome was diagnosed based on the IDF definition (2). The body weights of MetS patients were measured on a digital scale with an accuracy of 100 g and recorded. Height was measured using a measuring tape while the patient’s shoulders were in a natural state with an accuracy of 1 cm. Body mass index (BMI) was calculated according to the formula weight (kg)/(height (cm))2. Waist circumference was measured at the slimmest point using a flexible tape with an accuracy of 1 cm while the participant was at the end of his/her natural expiration. Blood pressure was measured using a standard mercury column manometer. All measurements were taken from the left arm by the same gastroenterologist. Venous blood samples were collected after 12-14 hours of fasting to measure blood glucose and serum lipids levels. None of the healthy individuals had histories of autoimmune, allergic, cancerous, or infectious diseases and met no IDF criteria. Demographic data for the controls are as follows: Males (frequency = 32%, number = 40), females (frequency = 68%, number = 85). The percentages of males and females in the patient group were 34.4 and 65.6%, respectively. The mean ages for male patients and controls were 54.5 ± 13.26 and 39.44 ± 6.91 years, respectively, and for female patients and controls were 53.05 ± 11.64 and 33.69 ± 6.84, respectively. No significant difference was found between males and females with regard to age in the patient group; however, the controls were not agematched (P < 0.001). The anthropometric parameters and clinical characteristics of the MetS patients are given in Table 1.
Table 1.
Anthropometric parameters, para-clinical, and clinical characteristics of MetS patients
| Variables | Mean ± SD | Variables | Frequency (%) |
|---|---|---|---|
| Height (cm) | 166.18 ± 9.08 | Smoking habit | Yes (7.2), No (92.8) |
| Weight (kg) | 84.68 ± 12.65 | Narcotics consumption | Yes (9.6), No (90.4) |
| Waist circumference (cm) | 101.12 ± 14.98 | Type 2 diabetes | Yes (88.8), No (11.2) |
| BMI (kg/m2) | 31.6 3 ± 3.81 | Hypertension | Yes (63.2), No (36.8) |
| Systolic blood pressure (mmHg) | 27.431±17.01 | Cardiovascular disease | Yes (8.8), No (91.2) |
| Diastolic blood pressure (mmHg) | 79.56 ± 8.51 | Fatty liver disease | Yes (36), No (64) |
| FBS (mg/dl) | 187.13 ± 58.09 | Hyperlipidaemia | Yes (40), No (60) |
| Triglycerides (mg/dl) | 226.85 ± 92.22 | Taking medications for blood pressure | Yes (62.4), No (37.6) |
| LDL (mg/dl) | 126.69 ± 36.58 | Taking medications for blood sugar | Yes (88), No (12) |
| HDL (mg/dl) | 35.63 ± 6.5 | - | |
| Cholesterol (mg/dl) | 225.53 ± 52.62 | - | |
| AST (U/L) | 32.8 ± 14.76 | - | |
| ALT (U/L) | 31.94 ± 16.55 | - | |
| ALP (U/L) | 243.24 ± 73.09 | - | |
| Bilirubin total (mg/dl) | 1.14 ± 0.42 | - | |
| Bilirubin direct (mg/dl) | 0.39 ± 0.25 | - |
BMI: Body mass index; FBS: Fasting blood sugar; LDL: Low-density lipoprotein; HDL: High-density lipoprotein; ALT: Alanine transaminase; AST: Aspartate transaminase; ALP: Alkaline phosphatase.
Cytokine assay
From each subject, 5 ml of blood was collected into a plain tube. The serum was isolated and stored at -80 °C until further analysis. Serum IL-6 and TNF-α levels were measured using the enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (Bosterbio, China). The assay sensitivity ranges of Bosterbio ELISA kits were 4.69-300 pg/ml for IL-6 and 7.8-500 pg/ml for TNF-α in serum samples. Per Bosterbio Company, the ELISA kits were validated with inter- and intra-assay precision; the validation results are available online at http://www.bosterbio.com/products/picokine-tmelisa-kits.html.
Statistics
Statistical analyses, including descriptive statistics, one-way analysis of variance (ANOVA), chi-square, Fisher’s exact test, and independent t-test, were performed using SPSS software version 17.0. P values less than 0.05 were considered statistically significant. Serum cytokine levels were presented as means ± SDs
Results
The mean ages of MetS patients and controls were 53.52 ± 12.14 and 35.67 ± 7.37 years, respectively. The IL-6 and TNF-α serum levels in MetS patients were 98.14 ± 17.94 and 140.69 ± 10.40 pg/ml, respectively, whereas in the healthy controls they were 4.6 ± 0.2 and 15.94 ± 0.89 pg/ml, respectively. Serum IL-6 and TNF-α levels were significantly greater in the MetS patients than in the controls (P < 0.001) (Fig. 1).
Fig. 1.
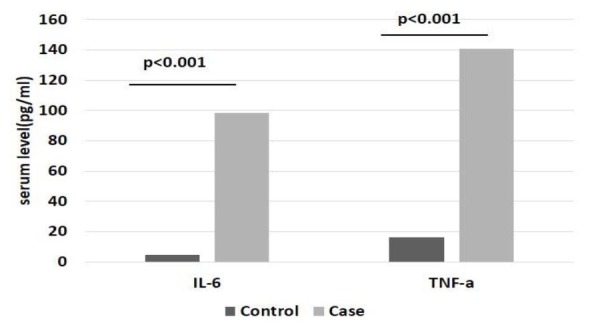
Graphs showing the serum levels of IL-6 and TNFα in patients with metabolic syndrome and healthy controls. P-values were determined by independent t-test and the cytokine levels are presented as means.
Statistical analyses were performed to determine the relationships between IL-6 and TNF-α serum levels in MetS patients, and the variables are presented in Table 1. The mean serum IL-6 levels in smokers and non-smokers were 21.6 ± 12.13 and 104.08 ± 19.2 pg/ml, respectively. The level was significantly greater in non-smokers than in smokers (P = 0.001). Additionally, mean serum IL-6 levels were significantly lower in MetS patients who used narcotics than in those who did not (P = 0.046). Serum IL-6 levels were significantly greater in nonsmoking than in smoking patients (P = 0.001). No relationships were found between IL-6 and TNF-α serum levels and other variables listed in Table 1 (data not shown).
Discussion
The relationships between inflammatory biomarkers such as IL-6 and TNF-α in MetS have not been thoroughly investigated. This study showed that the serum IL-6 and TNF-α levels were significantly greater in MetS patients than in controls. Interestingly, elevated serum TNF-α levels are associated with MetS independent of MetS components. In 2004, Moon et al. showed that serum TNF-α levels in obese adolescent MetS patients correlated positively with BMI, WC, triglycerides, and diastolic blood pressures, and negatively with HDL cholesterol (12). Conversely, this study found no correlations between serum TNF-α levels and each MetS component, including triglycerides, HDL, low-density lipoprotein (LDL), fasting blood sugar (FBS), hypertension, and WC. Increased expression of TNF-α and its correlation with human obesity, insulin resistance in T2DM, and hypertension have been extensively studied previously (13-16).
Previous studies revealed an association between IL-6 and systemic inflammation causing MetS (17, 18). This study showed that serum IL-6 levels were significantly greater in MetS patients than in controls. No significant correlations between serum IL-6 levels and MetS components were observed. Previous studies showed that IL-6 is positively associated with BMI, fasting insulin, hypertension, and T2DM; however, such results disagree with our findings (19, 20). Sarbijani et al. reported that IL-6 serum levels were significantly greater in men with MetS than in controls (21). They also observed a lack of correlation between IL-6 and MetS components, which agrees with our findings. Additionally, Kitsios et al. showed that obese and overweight adolescents and children with MetS had significantly greater serum IL-6 levels than their counterparts without MetS (22). In contrast, some studies have shown that IL-6 serum levels are not associated with MetS, which disagrees with our results (23, 24). Aldaham et al. showed that IL-6 serum levels were significantly affected not only by MetS, but also by smoking and age (25). They showed that serum IL-6 levels were significantly greater in 71 male smokers than in former smokers. However, our study showed that serum IL-6 levels were significantly greater in non-smoking than in smoking patients. These contradictory results might be related to the low number of MetS patients who were smokers in the current study.
were significantly less in patients who used narcotics than in those who did not. The effects of opioids on cytokine production have been investigated previously (26, 27). In addition, results of previous studies have been contradictory. For example, TNF-α production was increased by opioids in one study, but most studies showed its suppression; yet, another study showed opioids had no effect (28-31). Meijerink et al. recently showed that the production of IL-6, TNF-α, and several other cytokines was significantly suppressed in lipopolysaccharide- (LPS) induced whole blood from HIV-infected individuals who used heroin (32). One limitation of our study was the small number of patients who used narcotics. Further investigation with a larger sample size is needed to clarify the influence of narcotics on cytokine levels in MetS patients.
Patients with MetS in this study had significantly greater IL-6 and TNF-α levels than healthy controls. These results support the evidence that inflammation plays an important role in the immunopathogenesis of the disease. Additionally, we suggest that IL-6 and TNF-α serum levels be measured as valuable predicting factors for MetS. The lack of association between serum IL-6 and TNF-α levels and MetS components remains to be investigated by further research.
Acknowledgements
This research was financially supported by Physiology Research Center, Institute of Neuropharmacology, Kerman University of Medical Sciences, Kerman, Iran (grant number: 93/318). We especially acknowledge the staff members of Kerman Blood Transfusion Centre who helped us with blood collections from healthy volunteers. These data have been extracted from the thesis of Majid Aghadavood, resident in gastroenterology, Medical School, Kerman University of Medical Sciences, Kerman, Iran.
References
- 1.Cheung BM, Wat NM, Man YB, Tam S, Cheng CH, Leung GM, et al. Relationship Between the Metabolic Syndrome and the Development of Hypertension in the Hong Kong Cardiovascular Risk Factor Prevalence Study-2 (CRISPS2) The American Journal of Hypertension. 2008;21:17–22. doi: 10.1038/ajh.2007.19. [DOI] [PubMed] [Google Scholar]
- 2.International Diabetes Federation Press Conference: The IDF consensus worldwide definition of the metabolic syndrome [article online] 2005. Available from http://www.idf.org/webdata/docs/IDF_Met asyndrome_definition.pdf. Accessed 14 April 2005.
- 3.Nakayama Y, Komuro R, Yamamoto A, Miyata Y, Tanaka M, Matsuda M, et al. Rho A induces expression of inflammatory cytokine in adipocytes. Biochemical and Biophysical Research Communications. 2009;379:288–92. doi: 10.1016/j.bbrc.2008.12.040. [DOI] [PubMed] [Google Scholar]
- 4.Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H. Recent advances in the relationship between obesity, inflammation, and insulin resistance. European Cytokine Network. 2006;17:4–12. [PubMed] [Google Scholar]
- 5.Arner P. Insulin resistance in type 2 diabetes-role of the adipokines. Current Molecular Medicine. 2005;5(3):333–9. doi: 10.2174/1566524053766022. [DOI] [PubMed] [Google Scholar]
- 6.Langenberg C, Bergstrom J, Scheidt-Nave C, Pfeilschifter J, Barrett-Connor E. Cardiovascular Death and the Metabolic Syndrome Role of adipositysignaling hormones and inflammatory markers. Diabetes Care. 2006;29(6):1363–9. doi: 10.2337/dc05-2385. [DOI] [PubMed] [Google Scholar]
- 7.Devaraj S, Rosenson RS, Jialal I. Metabolic syndrome: an appraisal of the pro-inflammatory and procoagulant status. Endocrinology and Metabolism Clinics of North America. 2004;33(2):431–53. doi: 10.1016/j.ecl.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Bastard J-P, Maachi M, van Nhieu JT, Jardel C, Bruckert E, Grimaldi A, et al. Adipose tissue IL-6 content correlates with resistance to insulin activation of glucose uptake both in vivo and in vitro. The Journal of Clinical Endocrinology and Metabolism. 2002;87(5):2084–9. doi: 10.1210/jcem.87.5.8450. [DOI] [PubMed] [Google Scholar]
- 9.Anfossi G, Russo I, Doronzo G, Pomero A, Trovati M. Adipocytokines in atherothrombosis: focus on platelets and vascular smooth muscle cells. Mediators of Inflammation. 2010;2010 doi: 10.1155/2010/174341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisman EZ, Tenenbaum A. The ubiquitous interleukin-6: a time for reappraisal. Cardiovascular Diabetology. 2010;9(1):1. doi: 10.1186/1475-2840-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bautista L, Vera L, Arenas I, Gamarra G. Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-α) and essential hypertension. Journal of Human Hypertension. 2005;19(2):149–54. doi: 10.1038/sj.jhh.1001785. [DOI] [PubMed] [Google Scholar]
- 12.Moon Y-S, Kim D-H, Song D-K. Serum tumor necrosis factor-α levels and components of the metabolic syndrome in obese adolescents. Metabolism. 2004;53(7):863–7. doi: 10.1016/j.metabol.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. The Journal of Clinical Investigation. 1995;95(5):2409. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saghizadeh M, Ong JM, Garvey WT, Henry RR, Kern PA. The expression of TNF alpha by human muscle. Relationship to insulin resistance. The Journal of Clinical Investigation. 1996;97(4):1111. doi: 10.1172/JCI118504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tzanavari T, Giannogonas P, Karalis KP. TNFalpha and obesity. Current Directions in Autoimmunity. 2010;11:145–56. doi: 10.1159/000289203. [DOI] [PubMed] [Google Scholar]
- 16.Swaroop JJ, Rajarajeswari D, Naidu J. Association of TNF-α with insulin resistance in type 2 diabetes mellitus. The Indian Journal of Medical Research. 2012;135(1):127. doi: 10.4103/0971-5916.93435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circulation Research. 2005;96(9):939–49. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 18.Hung J, McQuillan BM, Chapman CM, Thompson PL, Beilby JP. Elevated interleukin-18 levels are associated with the metabolic syndrome independent of obesity and insulin resistance. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25(6):1268–73. doi: 10.1161/01.ATV.0000163843.70369.12. [DOI] [PubMed] [Google Scholar]
- 19.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. The Journal of the American Medical Association. 2001;286(3):327–34. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 20.Lukic L, Lalic NM, Rajkovic N, Jotic A, Lalic K, Milicic T, et al. Hypertension in obese type 2 diabetes patients is associated with increases in insulin resistance and IL-6 cytokine levels: potential targets for an efficient preventive intervention. The International Journal of Environmental Research and Public Health. 2014;11(4):3586–98. doi: 10.3390/ijerph110403586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarbijani HM, Khoshnia M, Marjani A. The association between Metabolic Syndrome and serum levels of lipid peroxidation and interleukin-6 in Gorgan. Diabetes and Metabolic Syndrome. 2015 doi: 10.1016/j.dsx.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 22.Kitsios K, Papadopoulou M, Kosta K, Kadoglou N, Chatzidimitriou D, Chatzopoulou F, et al. Interleukin-6, Tumor Necrosis Factor alpha and Metabolic Disorders in Youth. The Journal of Clinical Endocrinology and Metabolism. 2012;2(3):120–7. [Google Scholar]
- 23.Salmenniemi U, Ruotsalainen E, Pihlajamäki J, Vauhkonen I, Kainulainen S, Punnonen K, et al. Multiple abnormalities in glucose and energy metabolism and coordinated changes in levels of adiponectin, cytokines, and adhesion molecules in subjects with metabolic syndrome. Circulation. 2004;110(25):3842–8. doi: 10.1161/01.CIR.0000150391.38660.9B. [DOI] [PubMed] [Google Scholar]
- 24.Choi K, Lee J, Lee K, Seo J, Oh J, Kim S, et al. Comparison of serum concentrations of C-reactive protein, TNF-α, and interleukin 6 between elderly Korean women with normal and impaired glucose tolerance. Diabetes Research and Clinical Practice. 2004;64(2):99–106. doi: 10.1016/j.diabres.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Aldaham S, Foote JA, Chow H-HS, Hakim IA. Smoking Status Effect on Inflammatory Markers in a Randomized Trial of Current and Former Heavy Smokers. International Journal of Inflammation. 2015;2015 doi: 10.1155/2015/439396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mandler RN, Biddison W, Mandler R, Serrate S. beta-Endorphin augments the cytolytic activity and interferon production of natural killer cells. The Journal of Immunology. 1986;136(3):934–9. [PubMed] [Google Scholar]
- 27.Gein S, Gorshkova K, Tendryakova S. Regulation of interleukin-1β and interleukin-8 production by agonists of µ and δ opiate receptors in vitro. Neuroscience and Behavioral Physiology. 2009;39(6):591–5. doi: 10.1007/s11055-009-9166-y. [DOI] [PubMed] [Google Scholar]
- 28.Bastami S, Norling C, Trinks C, Holmlund B, Walz TM, Ahlner J, et al. Inhibitory effect of opiates on LPS mediated release of TNF and IL-8. Acta Oncologica. 2013;52(5):1022–33. doi: 10.3109/0284186X.2012.737932. [DOI] [PubMed] [Google Scholar]
- 29.Bonnet M-P, Beloeil H, Benhamou D, Mazoit J-X, Asehnoune K. The μ opioid receptor mediates morphine-induced tumor necrosis factor and interleukin-6 inhibition in toll-like receptor 2-stimulated monocytes. Anesthesia and analgesia. 2008;106(4):1142–9. doi: 10.1213/ane.0b013e318165de89. [DOI] [PubMed] [Google Scholar]
- 30.Bhargava HN, Thomas PT, Thorat S, House RV. Effects of morphine tolerance and abstinence on cellular immune function. Brain Research. 1994;642(1):1–10. doi: 10.1016/0006-8993(94)90899-0. [DOI] [PubMed] [Google Scholar]
- 31.Andjelkov N, Elvenes J, Martin J, Johansen O. Opiate regulation of IL-1beta and TNF-alpha in cultured human articular chondrocytes. Biochemical and Biophysical Research Communications. 2005;333(4):1295–9. doi: 10.1016/j.bbrc.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 32.Meijerink H, Indrati A, Utami F, Soedarmo S, Alisjahbana B, Netea MG, et al. Heroin Use Is Associated with Suppressed Pro-Inflammatory Cytokine Response after LPS Exposure in HIVInfected Individuals. PLOS ONE. 2015;10(4):e0122822. doi: 10.1371/journal.pone.0122822. [DOI] [PMC free article] [PubMed] [Google Scholar]


