Abstract
Background:
Adult T-cell leukemia/lymphoma (ATLL) is caused by human T-cell lymphotropic virus type-1 (HTLV-1). HTLV-1 oncogenes can induce malignancy through controlled gene expression of cell cycle checkpoints in the host cell. HTLV-I genes play a pivotal role in overriding cell cycle checkpoints and deregulate cellular division. In this study, we aimed to determine and compare the HTLV-1 proviral load and the gene expression levels of cyclin-dependent kinase-2 (CDK2), CDK4, p53, and retinoblastoma (Rb) in ATLL and carrier groups.
Methods:
A total of twenty-five ATLL patients (12 females and 13 males) and 21 asymptomatic carriers (10 females and 11 males) were included in this study. TaqMan real-time polymerase chain reaction assay was used for evaluation of proviral load and gene expression levels of CDK2, CDK4, p53, and Rb. Statistical analysis was used to compare proviral load and gene expression levels between two groups, using SPSS version 18.
Results:
The mean scores of the HTLV-1 proviral load in the ATLL patients and healthy carriers were 13067.20±6400.41 and 345.79±78.80 copies/104 cells, respectively (P=0.000). There was a significant correlation between the gene expression levels of CDK2 and CDK4 (P=0.01) in the ATLL group.
Conclusion:
Our findings demonstrated a significant difference between the ATLL patients and healthy carriers regarding the rate of proviral load and the gene expression levels of p53 and CDK4; accordingly, proviral load and expression levels of these genes may be useful in the assessment of disease progression and prediction of HTLV-1 infection outcomes.
Key Words: Adult T-cell leukemia/ lymphoma, CDKs, HTLV-I, p53, Retinoblastoma
Introduction
Adult T-cell leukemia-lymphoma (ATLL) is caused by the proliferation of human T-cell leukemia virus type 1 (HTLV-1)-infected CD4+ T cells (1). HTLV-1 is endemic in the Middle East, Central Africa, Southwestern Japan, the Caribbean Basin, the Melanesian Islands, and South America (2, 3). In Iran, endemic areas are Khorasan Razavi, Northern Khorasan, Alborz, Golestan, and East Azarbayejan (3-5). The majority of HTLV-1 virus carriers do not present any clinical symptoms, and approximately 1-2% of them show symptoms during a 20-40 years period (6). Regulatory proteins in HTLV-1 such as Tax and HTLV-1 basic leucine zipper factor (HBZ) were expressed in HTLV-1-infected human CD4+ T-cells to provide virus survival and dissemination (3).
The cell cycle is controlled at three checkpoints, including G1/S, S, and G2/M that are controlled by cycling-dependent kinase-2 (CDK2), CDK4, and CDK6(7). Activation of kinase proteins leads to phosphorylation of the cell cycle proteins, which improves cell proliferation (7). Tax expression in HTLV-1 represses a number of cellular genes and initiates stimulation of cytokines, stress signals, growth factors, and oncogenes; thus, Tax excessively activates CDK4, CDK6, and CDK inhibitors in the host cell cycle (8).
Retinoblastoma (Rb), as a tumor suppressor gene, is the key protein in the G1 phase that is phosphorylated by CDK4-cyclinD. Rb protein controls cell cycle through binding to E2F and inhibiting this protein. In resting cells, cyclinD/CDK4/6 complex inhibitors keep the Rb in a hypophosphorylated state and therefore Rb remains attached to the E2F transcription factor. Phosphorylation of Rb changes the structure of this protein and induces the release of E2F (9). The Rb protein is inactivated in ATLL patients by the HTLV-1 Tax protein (10).
Another tumor suppressor is the p53 with important functions to prevent the development of cancer. There is p53 mutations in at least half of all human tumors (11). This protein is activated during DNA damage and impedes cell cycle in G1/S checkpoint until DNA repair (12). In HTLV-1 infected cell lines, Tax expression hinders the function of p53 and promotes oncogenesis in host cells (10). Given the important role of CDK2, CDK4, p53, and retinoblastoma genes in cell cycle regulaion and the significance of HTLV-1 genes in oncogenesis induction in host cells through thier effects on cell cycle regulators, in this study, we evaluated and compared the levels of expression of CDK2, CDK4, p53, and Rb genes in ATLL patients and healthy carriers. Our study provides important information to disclose the role of CDK2, CDK4, p53, and retinoblastoma genes and proviral load (PVL) value in the course of HTLV-1- mediated disease progression.
Materials and Methods
Study population
This cross-sectional study was conducted on twentyfive ATLL patients and 21 healthy carriers in Ghaem and Imam Reza hospitals, affiliated to Mashhad University of Medical Sciences, Mashhad, Iran, during July 2012-April 2015. ATLL diagnosis was based on clinical history, pathological and experimental findings by two expert hematologists. HTLV-1 infection was diagnosed using HTLV-1 antibody serological test and it was confirmed by polymerase chain reaction (PCR) for Tax gene and long terminal repeat regions as previously described (4). Patients were classified into two subtypes using the Shimoyama criteria (13). The presence study protocol was approved by the ethics committee of Mashhad University of Medical Sciences, Mashhad, Iran. Informed consent was obtained from all patients before their entry to study for the performance of different blood tests.
Isolation of PBMCs and cDNA synthesis
Peripheral blood mononuclear cells (PBMCs) were separated from whole blood using Ficoll density gradient (Inno-train, Germany) for DNA and RNA extraction. Total RNA was extracted from PBMCs using a Tripure isolation reagent (Roche, Germany) based on the manufacturer’s instruction. Complementary DNA (cDNA) was synthesized using random primers and reverse transcriptase based on the manufacturer’s instruction (Bioneer, South Korea). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers were used to check correct cDNA synthesis. The GAPDH forward primer was 5'- CAAGGTCATCCATGACAACTTTG-3' and GAPDH reverse primer was 5'- GTCCACCACCCTGTTGCTGTAG-3'.
Primer design and real-time PCR
After detection of the desired sequence of genes (CDK2, CDK4, p53, and Rb) using the National Center for Biotechnology Information (NCBI, Genebank), primers and probes were designed by AlleleID, version 5. Real-time PCR was performed using TaqMan method. Real-time PCR was performed for CDK2, CDK4, Rb, and p53 genes, as well as cellular reference gene (β2-microglobulin) on Rotor-Gene 6000 cycler (Corbett, Hilden, Germany), using TaqMan Reagents (Applied Biosystems, USA) in accordance with the manufacturer’s instruction. Specific primers and probes used to amplify the desired genes are provided in Table 1.
Table 1.
Primer and probe sequences used in quantitative real-time polymerase chain reaction.
| Gene | Primer Name | Sequences (5'->3') | Length | Tma | GC%b |
|---|---|---|---|---|---|
| CDK2c | Forward | ATTCTGAGATTGACCAGCTCTTCC | 24 | 59.3 | 45.8 |
| Reverse | CTTGGCTTGTAATCAGGCATAGAAG | 25 | 59.1 | 44 | |
| Probe | CCACACCACCTCATCTGGGGTCCC | 24 | 67.3 | 66.7 | |
| CDK4 | Forward | AAATTGGTGTCGGTGCCTATGG | 22 | 61.2 | 50 |
| Reverse | CACGAACTGTGCTGATGGGAAG | 22 | 61.76 | 54.55 | |
| Probe | ACAAGGCCCGTGATCCCCACAGTGG | 25 | 69.6 | 64 | |
| Rbd | Forward | CCTATCTCCGGCTAAATACACTTTG | 25 | 59.3 | 44 |
| Reverse | TTGGTCCAAATGCCTGTCTCTC | 22 | 60.55 | 50 | |
| Probe | AACGCCTTCTGTCTGAGCACCCAGA | 25 | 67 | 56 | |
| p53 | Forward | CAGCATCTTATCCGAGTGGAAGG | 23 | 60.8 | 52.17 |
| Reverse | GTTGTAGTGGATGGTGGTACAGTC | 24 | 60.86 | 50 | |
| Probe | CTCAGGCGGCTCATAGGGCACCAC | 24 | 68.1 | 66.7 |
Melting Temperature
Guanine-Cytosine content
Cyclin-Dependent Kinase-2
Retinoblastoma
Quantification of HTLV-1 proviral load
To assess the HTLV-1 proviral load, PBMCs were isolated and DNA was extracted from PBMCs by DNA extraction kit (Cinnagen, Iran). Real-time PCR was performed by a Rotorgene Q Real-Time PCR machine (Qiagen, Germany) using a commercial absolute quantification kit (Novin Gene, Iran) and specific primers and a fluorogenic probe to measure the proviral load of HTLV-1. The HTLV-1 copy number was reported as an actual amount of cellular DNA by means of quantification of the albumin gene as the reference gene. Each two copies of albumin genes are equal one cell. HTLV-1 and albumin DNA concentrations were calculated from two 5-point standard curves. The normalized value of the HTLV-1 proviral load was calculated as the ratio of (HTLV-1 DNA copies number/albumin DNA copies number /2) ×104 and expressed as the number of HTLV-1 proviruses per 104 PBMCs.
Statistical analysis
Data analysis was carried out in SPSS version 18 and the results were reported as mean±SD. Kolmogorov–Smirnov test was carried out to test the normality of the variables distribution. Statistical analysis was performed in the two groups using Mann–Whitney U test. Moreover, the Spearman’s rank correlation coefficient was used to determine the correlation between the study variables. A P-value less than 0.05 was considered statistically significant.
Results
In this study, twenty-five ATLL patients (12 females and 13 males) and 21 asymptomatic carriers (10 females and 11 males) were enrolled. The mean score of age in the ATLL group was 54.83±1.25 years, while it was 40.83±2.14 years in the carrier group. According to the results, eight patients were in acute type and seventeen were lymphoma type. The mean scores of HTLV-1 proviral load in the ATLL and carrier groups were 13067.20±6400.41 and 345.79±78.80 copies/104 cells, respectively. The mean scores of expression for each gene in patients and carriers are demonstrated in Table 2.
Table 2.
Mean scores of proviral load and expression of CDK2, CDK4, Rb, and P53 genes in the ATLL patients and carrier groups.
| Gene | Group (n) | Mean± SDb |
|---|---|---|
| Proviral load | ATLLa (25) | 13067.20±6400.41 |
| Carriers (21) | 345.79±78.80 | |
| CDK2c | ATLL (25) | 2.51±1.77 |
| Carriers (21) | 0.45±0.25 | |
| CDK4 | ATLL (25) | 0.12±0.09 |
| Carriers (21) | 0.04±0.02 | |
| Rbd | ATLL (25) | 0.75±0.42 |
| Carriers (21) | 0.22±0.05 | |
| p53 | ATLL (25) | 0.002±0.0008 |
| Carriers (21) | 0.049±0.012 |
Adult T-cell Leukemia/Lymphoma
Standard Deviation
Cyclin-Dependent Kinase-2
Retinoblastoma
Comparison of the mean scores for each gene revealed that expression of CDK2, CDK4, and Rb genes in ATLL group was slightly higher than the carrier group but only CDK4 gene expression reached a significant increase in the patients compared with carriers. At the same time, p53 gene expression was dramatically higher in the carriers and this rise was statistically significant. In addition, there was a significant correlation between CDK2 and CDK4 genes expression in ATLL patients (r=0.530, P=0.01). The Stem-and-Leaf Plot in figure 1 (Fig. 1) exhibits the difference in expression levels of CDK4 and p53 genes between the carrier and ATLL groups.
Fig. 1.
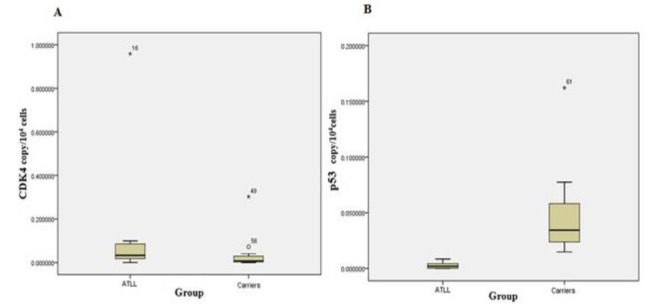
Comparison of the expression levels of CDK4 (A) and p53 (B) genes between the carrier and ATLL groups.
The mean score of proviral load was significantly different between ATLL and carrier groups (P=0.000). The Stem-and-Leaf Plot in figure 2 (Fig. 2) exhibits the difference proviral load between the carrier and ATLL groups.
Fig. 2.
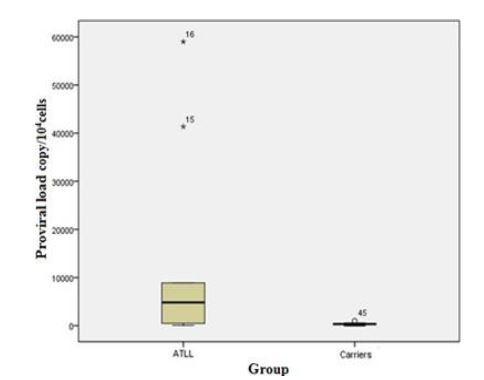
Comparison of the proviral load value between the carrier and ATLL groups.
Discussion
In the current study, we evaluated and compared HTLV-I proviral load and expression levels of CDK2, CDK4, p53 and Rb genes in ATLL patients and healthy carriers. Cell cycle progression is controlled by consecutive activation of CDK components and it is inactivated by CDKIs (10). Tax effects on cell cycle factors, leads to excessive cell cycle and promotes multipolar mitosis; thus, produces cancer cells, in addition to Tax, HBZ gene is thought to play an important role in oncogenesis (14, 15).
HTLV-1 instigates proliferation of CD4+ T-cell lymphocytes thereby virus is reproduced (14). There are various risk factors for progression from carrier status to ATLL, including advanced age, family history of ATLL, and high proviral load (16). In ATLL patients, development of HTLV-I through HTLV-I proviral load is utmost importance (17). Similar of previous studies results, our results showed that viral load was significantly higher in the ATLL patients than the carrier group (16, 18). In healthy carriers, HTLV-1 proviral load value was measured as important predictor of development of ATLL (16).
Cell cycle factors are controlled in normal cells regularly, while the dysregulation of CDK components in cancer cells leads to the survival of cancer cells (19). The G1-phase progression is regulated by the several CDKs, especially CDK4 and CDK6. According to previous studies, cell cycle checkpoints in most types of cancers are controlled through elevated expression of CDK, especially CDK2 and CDK4 (20, 21). Our results showed that the mean expression levels of CDK2 and CDK4 genes in ATLL patients were more than healthy carriers due to the effects of HTLV-I oncogenes on ATLL patients. HTLV-1 Tax protein stimulates CDKs activity, especially CDK2, CDK4 and CDK6 and increases G1- to S-phase Transition in T Lymphocytes of ATLL patients moreover the suppression of Tax synthesis leads to the significant reduction of the CDK4 and CDK6 activity (22, 23).
Normal cells respond to anti-proliferative signals by dephosphorylating Rb in G1 checkpoint. In infected cells with HTLV-I, Tax gene expression can induce hyperphosphorylation and degradation of Rb by activation of CDK-4 and CDK-6, which then hyperphosphorylatd Rb releases the E2F transcription factor and accelerates cell cycle transition from G1 to S (24). The current study showed that gene expression level of Rb in ATLL patients is more than healthy carriers. It has been reported recently that overexpression of Rb is associated with progression of the disease in subjects with advanced bladder cancer or follicular lymphoma. Therefore Rb controls tumor proliferation not only as a cell cycle regulator, but also by other mechanisms, possibly by apoptosis inhibition. Rb plays a critical role in its hypophosphorylated form for the development of ATLL, as well as a cell cycle stimulator in hyperphosphorylated form (25).
Despite previous genes, activation of p53 such as a tumor suppressor gene prevents the development of cancer cells through impeding cycle cell and inducing apoptosis (26). Ellis et al. indicated that overexpression of p53 in lung cancer cells forcefully induces autophagy (26). Nowadays, induced overexpression of p53 in cancer cells is used as a treatment method (27). Ariumi et al. (28) and Gatza et al. (29) reported that in infected cells with HTLV-I, Tax protein indirectly inhibits p53 function, moreover, this protein inhibits the expression of p53 by transcription repression of this gene (30). Accordingly, in the current study expression of p53 in ATLL patients was significantly lower than healthy carriers, which can leads to oncogenesis.
In addition to Tax, the HBZ gene promotes the proliferation of cancer cells in ATLL patients. Therefore, it is believed that HBZ gene is necessary for leukemogenesis (18). In infected cells, Tax and HBZ genes expression in the early and late stages of ATLL respectively may play a major role in HTLV-1 biology and the development of leukemia through deactivating effects of transcription mediators and/or attenuating cellular gene expression (8). Wright et al. reported that HBZ reduces transcription of p53-responsive genes, p21/CDKN1A and GADD45A, which contributes to cell cycle arrest (31). Our results revealed that the rate of p53 expression in ATLL was significantly lower than the carriers. Therefore, HTLV-1 oncogenes in different stages of ATLL can potentially induce oncogenesis in host cells by their effects on function and expression of p53 and Rb.
According to these results, there was a significant difference between the ATLL patients and carriers regarding the rate of PVL and the genes expression of p53 and CDK4; therefore, it seems that PVL, CDK4 and P53 measurements might help in the assessment of disease progression and prediction of HTLV-1 infection outcomes. This research demonstrates the possible correlation between virus oncogenes and altered expression of the cell cycle regulators of ATLL patients. Further molecular studies are necessary for better understanding of virus genes and host cell cycle interaction, which can lead to find selective therapeutic agents for ATLL.
Acknowledgements
We would like to thank the deputy of research of Mashhad University of Medical Sciences, Mashhad, Iran. We also appreciate the cooperation of the staff of hematology and oncology departments of Ghaem and Imam Reza hospitals of Mashhad and immunology department of Mashhad University of Medical Sciences. We thank all the individuals who took part in this study and shared their experiences for the benefit of others. This study is part of a PhD thesis (911219). All authors declare they have no conflicts of interest.
References
- 1.Kawano N, Yoshida S, Kuriyama T, Tahara Y, Yamashita K, Nagahiro Y, et al. Clinical Features and Treatment Outcomes of 81 Patients with Aggressive Type Adult T-cell Leukemia-lymphoma at a Single Institution over a 7-year Period (2006-2012). Intern Med. 2015;54(12):1489–98. doi: 10.2169/internalmedicine.54.1953. [DOI] [PubMed] [Google Scholar]
- 2.Yamashiro T, Kamiya H, Miyara T, Gibo S, Ogawa K, Akamine T, et al. CT scans of the chest in carriers of human T-cell lymphotropic virus type 1: presence of interstitial pneumonia. Acad Radiol. 2012;19(8):952–7. doi: 10.1016/j.acra.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 3.Boostani R, Vakili R, Hosseiny SS, Shoeibi A, Fazeli B, Etemadi MM, et al. Triple Therapy with Prednisolone, Pegylated Interferon and Sodium Valproate Improves Clinical Outcome and Reduces Human T-Cell Leukemia Virus Type 1 (HTLV-1) Proviral Load, Tax and HBZ mRNA Expression in Patients with HTLV-1-Associated Myelopathy/Tropical Spastic Paraparesis. Neurotherapeutics. 2015;12(4):887–95. doi: 10.1007/s13311-015-0369-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rafatpanah H, Hedayati-Moghaddam MR, Fathimoghadam F, Bidkhori HR, Shamsian SK, Ahmadi S, et al. High prevalence of HTLV-I infection in Mashhad, Northeast Iran: a populationbased seroepidemiology survey. J Clin Virol. 2011;52(3):172–6. doi: 10.1016/j.jcv.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Azarpazhooh MR, Hasanpour K, Ghanbari M, Rezaee SA, Mashkani B, Hedayati-Moghaddam MR, et al. Human T-lymphotropic virus type 1 prevalence in northeastern Iran, Sabzevar: an epidemiologic-based study and phylogenetic analysis. AIDS Res Hum Retroviruses. 2012;28(9):1095–101. doi: 10.1089/AID.2011.0248. [DOI] [PubMed] [Google Scholar]
- 6.Kannian P, Green PL. Human T lymphotropic virus type 1 (HTLV-1): molecular biology and oncogenesis. Viruses. 2010;2(9):2037–77. doi: 10.3390/v2092037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beale G, Haagensen EJ, Thomas HD, Wang LZ, Revill CH, Payne SL, et al. Combined PI3K and CDK2 inhibition induces cell death and enhances in vivo antitumor activity in colorectal cancer. Br J Cancer. 2016;115(6):682–90. doi: 10.1038/bjc.2016.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giam C-Z, Semmes OJ. HTLV-1 Infection and Adult T-Cell Leukemia/Lymphoma—A Tale of Two Proteins: Tax and HBZ. Viruses. 2016;8(6) doi: 10.3390/v8060161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiao Y, Jiang Z, Wu Y, Chen X, Xiao X, Yu H. A Functional Polymorphism (rs937283) in the MDM2 Promoter Region is Associated with Poor Prognosis of Retinoblastoma in Chinese Han Population. Sci Rep. 2016;6:31240. doi: 10.1038/srep31240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicot C. HTLV-I Tax-Mediated Inactivation of Cell Cycle Checkpoints and DNA Repair Pathways Contribute to Cellular Transformation:“A Random Mutagenesis Model”. J Cancer Sci. 2015;2(2) doi: 10.13188/2377-9292.1000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guerra E, Cimadamore A, Simeone P, Vacca G, Lattanzio R, Botti G, et al. p53, cathepsin D, Bcl-2 are joint prognostic indicators of breast cancer metastatic spreading. BMC Cancer. 2016;16:649. doi: 10.1186/s12885-016-2713-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lv H, Liu R, Fu J, Yang Q, Shi J, Chen P, et al. Epithelial cell-derived periostin functions as a tumor suppressor in gastric cancer through stabilizing p53 and E-cadherin proteins via the Rb/E2F1/p14ARF/Mdm2 signaling pathway. Cell Cycle. 2014;13(18):2962–74. doi: 10.4161/15384101.2014.947203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukemia-lymphoma. A report from the Lymphoma Study Group (1984-87) Br J Haematol. 1991;79(3):428–37. doi: 10.1111/j.1365-2141.1991.tb08051.x. [DOI] [PubMed] [Google Scholar]
- 14.Matsuoka M, Jeang KT. Human T cell leukemia virus type 1 (HTLV-1) and leukemic transformation: viral infectivity, Tax, HBZ, and therapy. Oncogene. 2011;30(12):1379–89. doi: 10.1038/onc.2010.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Satou Y, Yasunaga J, Yoshida M, Matsuoka M. HTLV-I basic leucine zipper factor gene mRNA supports proliferation of adult T cell leukemia cells. Proc Natl Acad Sci U S A. 2006;103(3):720–5. doi: 10.1073/pnas.0507631103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwanaga M, Watanabe T, Utsunomiya A, Okayama A, Uchimaru K, Koh KR, et al. Human T-cell leukemia virus type I (HTLV-1) proviral load and disease progression in asymptomatic HTLV-1 carriers: a nationwide prospective study in Japan. Blood. 2010;116(8):1211–9. doi: 10.1182/blood-2009-12-257410. [DOI] [PubMed] [Google Scholar]
- 17.Okayama A, Stuver S, Matsuoka M, Ishizaki J, Tanaka G, Kubuki Y, et al. Role of HTLV-1 proviral DNA load and clonality in the development of adult Tcell leukemia/lymphoma in asymptomatic carriers. Int J Cancer. 2004;110(4):621–5. doi: 10.1002/ijc.20144. [DOI] [PubMed] [Google Scholar]
- 18.Akbarin MM, Rahimi H, HassanNia T, Shoja Razavi G, Sabet F, Shirdel A. Comparison of HTLV-I Proviral Load in Adult T Cell Leukemia/Lymphoma (ATL), HTLV-I-Associated Myelopathy (HAM-TSP) and Healthy Carriers. Iran J Basic Med Sci. 2013;16(3):208–12. [PMC free article] [PubMed] [Google Scholar]
- 19.Witzel I, Mueller V. The Role of CDK 4/6 Inhibitors in Breast Cancer Treatment. Breast Care. 2016;11(3):165–6. doi: 10.1159/000447479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartsch R, Fitzal F, Hubalek M, Knauer M, Untch M. The Role of CDK 4/6 Inhibitors in Breast Cancer Treatment. Breast Care. 2015;10(5):340–3. [Google Scholar]
- 21.Roberts PJ, Bisi JE, Strum JC, Combest AJ, Darr DB, Usary JE, et al. Multiple roles of cyclin-dependent kinase 4/6 inhibitors in cancer therapy. J Natl Cancer Inst. 2012;104(6):476–87. doi: 10.1093/jnci/djs002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmitt I, O, Rohwer P, Gossen M, Grassmann R. Stimulation of Cyclin-Dependent Kinase Activity and G1- to S-Phase Transition in Human Lymphocytes by the Human T-Cell Leukemia/Lymphotropic Virus Type 1 Tax Protein. J Virol. 1998;72(1):633–40. doi: 10.1128/jvi.72.1.633-640.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwanaga R, Ohtani K, Hayashi T, Nakamura M. Molecular mechanism of cell cycle progression induced by the oncogene product Tax of human T-cell leukemia virus type I. Oncogene. 2001;20(17):2055–67. doi: 10.1038/sj.onc.1204304. [DOI] [PubMed] [Google Scholar]
- 24.Zhao T. The Role of HBZ in HTLV-1-Induced Oncogenesis. Viruses. 2016;8(2) doi: 10.3390/v8020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakayama K, Yamada Y, Koji T, Hayashi T, Tomonaga M, Kamihira S. Expression and phosphorylation status of retinoblastoma protein in adult T-cell leukemia/lymphoma. Leuk Res. 2000;24(4):299–305. doi: 10.1016/s0145-2126(99)00186-1. [DOI] [PubMed] [Google Scholar]
- 26.Ellis M, Stern O, Ashur-Fabian O. The double benefit of Spalax p53: surviving underground hypoxia while defying lung cancer cells in vitro via autophagy and caspase-dependent cell death. Oncotarget. 2016;7(39):63242–51. doi: 10.18632/oncotarget.11443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li N, Xie C, Lu NH. p53, a potential predictor of Helicobacter pylori infection-associated gastric carcinogenesis? Oncotarget. 2016;7(40):66276–86. doi: 10.18632/oncotarget.11414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ariumi Y, Kaida A, Lin JY, Hirota M, Masui O, Yamaoka S, et al. HTLV-1 tax oncoprotein represses the p53-mediated trans-activation function through coactivator CBP sequestration. Oncogene. 2000;19(12):1491–9. doi: 10.1038/sj.onc.1203450. [DOI] [PubMed] [Google Scholar]
- 29.Gatza ML, Watt JC, Marriott SJ. Cellular transformation by the HTLV-I Tax protein, a jack-ofall-trades. Oncogene. 2003;22(33):5141–9. doi: 10.1038/sj.onc.1206549. [DOI] [PubMed] [Google Scholar]
- 30.Mesnard JM, Devaux C. Multiple control levels of cell proliferation by human T-cell leukemia virus type 1 tax protein. Virology. 1999;257(2):277–84. doi: 10.1006/viro.1999.9685. [DOI] [PubMed] [Google Scholar]
- 31.Wright DG, Marchal C, Hoang K, Ankney JA, Nguyen ST, Rushing AW, et al. Human T-cell leukemia virus type-1-encoded protein HBZ represses p53 function by inhibiting the acetyltransferase activity of p300/CBP and HBO1. Oncotarget. 2016;7(2):1687–706. doi: 10.18632/oncotarget.6424. [DOI] [PMC free article] [PubMed] [Google Scholar]


