Abstract
Obstructive sleep apnea (OSA) is now recognized as an independent and important risk factor for cardiovascular diseases such as hypertension, coronary heart disease, heart failure and stroke. Clinical and experimental data have confirmed that intermittent hypoxia is a major contributor to these deleterious consequences. The repetitive occurrence of hypoxia-reoxygenation sequences generates significant amounts of free radicals, particularly in moderate to severe OSA patients. Moreover, in addition to hypoxia, reactive oxygen species (ROS) are potential inducers of the hypoxia inducible transcription factor-1 (HIF-1) that promotes the transcription of numerous adaptive genes some of which being deleterious for the cardiovascular system, such as the endothelin-1 gene. This review will focus on the involvement of the ROS-HIF-1-endotelin signaling pathway in OSA and intermittent hypoxia and discuss current and potential therapeutic approaches targeting this pathway to treat or prevent cardiovascular disease in moderate to severe OSA patients.
1. Introduction
Obstructive sleep apnea (OSA) is characterized by repetitive upper airway occlusions during sleep leading to intermittent hypoxia. Factors promoting OSA include functional or anatomical anomalies of the upper airways, obesity, age over 60 years, smoking and alcohol consumption (Young et al., 2002). According to recent estimates, the prevalence of the disease has more than doubled in the last 20 years and is now estimated to be between 9 and 17% in women and men, respectively, aged 50 to 70 years (Peppard et al., 2013). It is a major public health concern worldwide and in Europe since it is associated with high cardiovascular morbidity and mortality (Baguet et al., 2012; Lévy et al., 2015). Intermittent hypoxia (IH), the landmark of OSA, induces oxidative stress and consequently promotes low-grade inflammation, endothelial dysfunction and cardiometabolic co-morbidities. Thus, effective treatment of OSA is expected to represent an important target for improving cardiometabolic risk.
Continuous positive airway pressure (CPAP) is the first line therapy for OSA. While CPAP therapy clearly improves vigilance and cognitive function, it is still debated whether CPAP alone may substantially reduce the rate of new cardiovascular events (Barbe et al., 2012). One of the major barriers to CPAP efficacy is patient adherence. Indeed, 15% of OSA patients initially refuse the treatment and 20% of those treated discontinue or use it irregularly or suboptimally. Moreover, the response to CPAP therapy, in terms of cardiovascular and metabolic outcomes, is obtained only in CPAP compliers (Bratton et al., 2015) and differs in non-obese and obese OSA patients. Thus, in obese OSA patients, CPAP alone has a limited effect, compared to weight loss, on blood pressure, insulin sensitivity, lipid profile and inflammation (Chirinos et al., 2014). The failure of CPAP to improve cardiometabolic and inflammatory markers in obese OSA patients (Jullian-Desayes et al., 2015) emphasizes the need for combined therapeutic strategies (Pepin et al., 2012). Hence, angiotensin II receptor blockers (ARBs) are more effective than CPAP to lower blood pressure (BP), but there is an added benefit of combining both treatments especially on nighttime BP (Pepin et al., 2010; Thunstrom et al., 2016). Therefore, it is important to identify the best pharmacological approach to counteract the key intermediary mechanisms responsible for OSA-related cardiovascular alterations, namely oxidative stress, sympathetic activation and inflammation.
As previously described (Dematteis et al., 2009), IH is a major consequence of OSA in terms of impact on the cardiovascular system, in particular through the generation of reactive oxygen species (ROS) (Lévy et al., 2015). Indeed, ROS enhance the stabilization and activity of the hypoxia inducible factor-1 (HIF-1) transcription factor well known to promote adaptive and maladaptive responses to hypoxia (Semenza, 2009). Amongst the various HIF-1 target gene products, some have beneficial effects on the cardiovascular system, such as nitric oxide and carbon monoxide (Andreadou et al., 2015), while others, like endothelin-1 (ET-1), are involved in several pathophysiological pathways associated with cardiovascular disease (Kaoukis et al., 2013). Differential modulation of these gene pathways could account for the dual aspect of IH, which has been shown to be protective or deleterious according to the duration and severity of the hypoxia-reoxygenation cycles (Verges et al., 2015).
In the present review, we will focus on the involvement of the ROS-HIF-1-endothelin signaling pathway in the deleterious cardiovascular consequences induced by the rapid IH cycles and low desaturation levels encountered in moderate to severe OSA patients (Fig. 1). We will propose new therapeutic avenues targeting this pathway to treat or prevent the OSA-related cardiovascular complications. We will discuss the rationale for innovative clinical trials based on the mechanistic insights presented in this review.
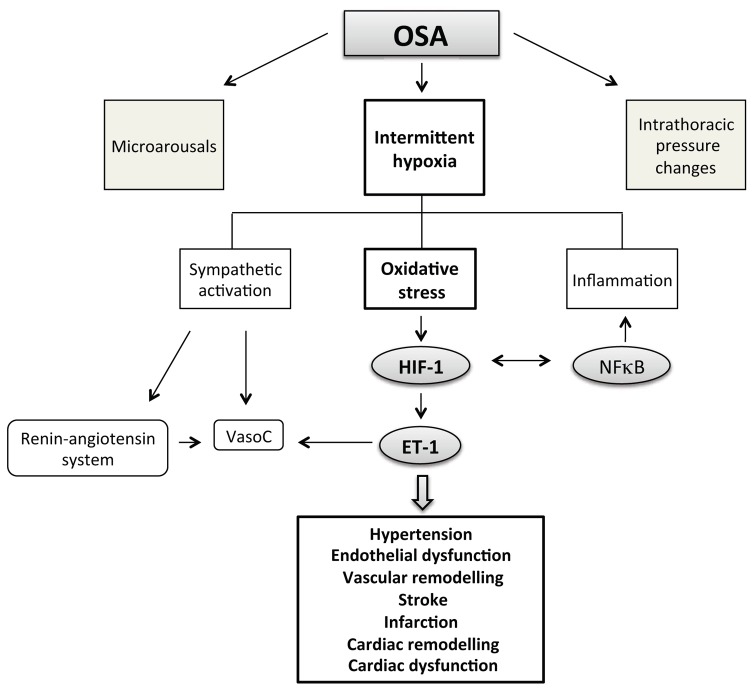
Fig. 1.
Among the consequences of obstructive sleep apnea (OSA), intermittent hypoxia plays a major role in the development of associated cardiovascular pathologies. More specifically, intermittent hypoxia is well recognized to induce oxidative stress with subsequent activation of the hypoxia inducible factor-1 (HIF-1) transcription factor and upregulation of genes deleterious for the cardiovascular system such as the endothelin-1 (ET-1) gene. NFκB, nuclear factor kappa B; VasoC, vasoconstriction.
2. Cardiovascular impact of OSA and intermittent hypoxia
2.1. Systemic hypertension
It is now well established that OSA and intermittent hypoxia causes arterial hypertension (Pepin et al., 2014; Torres et al., 2015). There is indeed a linear relationship between the severity of sleep-disordered breathing, determined by the apnea hypopnea index (AHI), and blood pressure elevation, independently of confounding factors (Young et al., 1997).
Fletcher et al. were the first to demonstrate that intermittent hypoxia was one of the landmark features of OSA, showing its role in the development of hypertension (Fletcher et al., 1992). This has been confirmed in several rodent models of IH (Dematteis et al., 2009) as well in healthy human volunteers exposed exclusively to intermittent hypoxia (Tamisier et al., 2011).
2.2. Vascular dysfunction and remodeling, atherosclerosis
OSA promotes endothelial dysfunction (Hoyos et al., 2015), vascular remodeling (Baguet et al., 2005) and atherosclerosis (Drager et al., 2011; Stanke-Labesque et al., 2014; Trzepizur et al., 2014). Similarly, IH exposure results in endothelial dysfunction in various vascular territories (Krause et al., 2015; S. A. Phillips et al., 2004; Totoson et al., 2013), in inflammatory and fibrotic vascular remodeling (Arnaud et al., 2011a; Castro-Grattoni et al., 2016; Gras et al., 2016; Yang et al., 2011) and in accelerated atherosclerosis (Arnaud et al., 2011b; Drager et al., 2013; Savransky et al., 2008).
2.3. Myocardial infarction and stroke
Moderate to severe OSA is associated by an enhanced risk of coronary heart disease (CAD) (Arzt et al., 2015) and stroke (Arzt et al., 2005; Dong et al., 2013). OSA patients with acute myocardial infarction have more severe CAD, prolonged myocardial ischemia and less salvaged myocardium than non-apneic patients (Arzt et al., 2015). Thus, a high AHI is independently associated with a larger infarct size 3 months after acute myocardial infarction (S. Buchner et al., 2014). IH appears to be a major contributor since several experimental studies have demonstrated an increase in myocardial infarct size following IH exposure (Belaidi et al., 2009; Belaidi et al., 2016; Bourdier et al., 2016; Joyeux-Faure et al., 2005; Ramond et al., 2013; Yeung et al., 2015). Cerebral infarct size and neuronal excitotoxicity (an index of stroke vulnerability) are also increased by IH (Jackman et al., 2014) (Jagadapillai et al., 2014).
2.4. Cardiac dysfunction and heart failure
Left ventricular dysfunction and remodeling have been reported in OSA patients prior to the development of hypertension or other cardiovascular diseases (Aslan et al., 2013; Baguet et al., 2012). Following acute myocardial infarction, cardiac dysfunction and remodeling is worsened in OSA compared to non-apneic patients (Arzt et al., 2015). Thus, not only is OSA a significant risk factor for the development of incident heart failure (Gottlieb et al., 2010), but it also enhances mortality in heart failure patients (Khayat et al., 2015; H. Wang et al., 2007). In rodent models, prolonged IH exposure results in left ventricular dysfunction (Chen et al., 2008), fibrotic ventricular remodeling (Hayashi et al., 2008) (Ding et al., 2014) and cardiac hypertrophy (Chen et al., 2010).
Regarding heart failure (HF), a recent study in rats has shown that a 4-week IH exposure exacerbated myocardial remodeling and cardiac dysfunction in a pressure overload-induced HF model (S. Li et al., 2015).
3. The ROS-HIF-1-endothelin pathway in OSA and intermittent hypoxia and its role in the cardiovascular alterations
3.1. Why the ROS-HIF-1-ET-1 pathway?
OSA is widely recognized as an oxidative stress disorder (Badran et al., 2014; Eisele et al., 2015; Lavie, 2015; Lavie & Lavie, 2009). As recently reviewed by Eisele et al., OSA patients present with increased superoxide anion release by circulating leucocytes associated with an upregulation of NADPH oxidase (NOX), reduced nitric oxide bioavailability reflected by a decrease in total nitrate and nitrite, increased lipid peroxidation and reduced antioxidant status characterized by lower plasma levels of antioxidant substances, such as vitamin A and E, or antioxidant enzymes, such as superoxide dismutase and catalase (Eisele et al., 2015). Accordingly, lipid peroxidation products, such as thiobarbituric reactive substances (Barcelo et al., 2000; Lavie et al., 2004) or isoprostanes (Biltagi et al., 2008; Carpagnano et al., 2003; Del Ben et al., 2012; Monneret et al., 2010) are increased in OSA patients and their levels are correlated with sleep apnea severity (Biltagi et al., 2008; Lavie et al., 2004; Monneret et al., 2010). Few studies have investigated the correlation of OSA-related oxidative stress with cardiovascular disease markers but we have demonstrated a direct relationship between urinary 15-F2t-isoprostane levels and carotid intima-media thickness in non-obese OSA patients, in support for a role of oxidative stress in the early atherosclerotic process (Monneret et al., 2010). Indeed, there is a linear relationship between oxidative stress and sleep apnea severity (Franco et al., 2012). Thus the lowest antioxidant status and the highest ROS production by circulating leucocytes are observed in the most severe OSA patients (Mancuso et al., 2012; Pilkauskaite et al., 2013). In accordance, the severity of the cardiometabolic alterations is correlated to that of hypoxemia, with alterations most significant in patients with severe nocturnal hypoxia (Cakmak et al., 2015; Gami et al., 2013; He et al., 2010). In a recent retrospective follow-up study on 1,068 men originally referred to night polysomnography due to suspected OSA, Muraja-Murro et al. conclude that adjusting AHI with severity of obstruction events enhances the detection of patients with the highest risk of cardiovascular morbidity or mortality (Muraja-Murro et al., 2014).
Animal models of chronic short-cycling IH exposure closely reproduce the desaturation pattern and cardiovascular alterations seen in moderate to severe OSA patients (Dematteis et al., 2009). Chronic cyclic IH exposure also induces an oxidative stress in the brain, lung and liver, in proportion to hypoxia intensity (Quintero et al., 2013) while a 3-day exposure is sufficient to produce oxidative-stress related kidney damage (Wu et al., 2015). Likewise, others and we have shown that IH induces an important oxidative stress in cardiovascular tissues (Chen et al., 2005; Friedman et al., 2014; Ramond et al., 2013; Troncoso Brindeiro et al., 2007). Moreover, animals exposed to IH experience the same alterations in oxidant status than OSA patients, namely increased NOX expression and decreased catalase activity (Totoson et al., 2013).
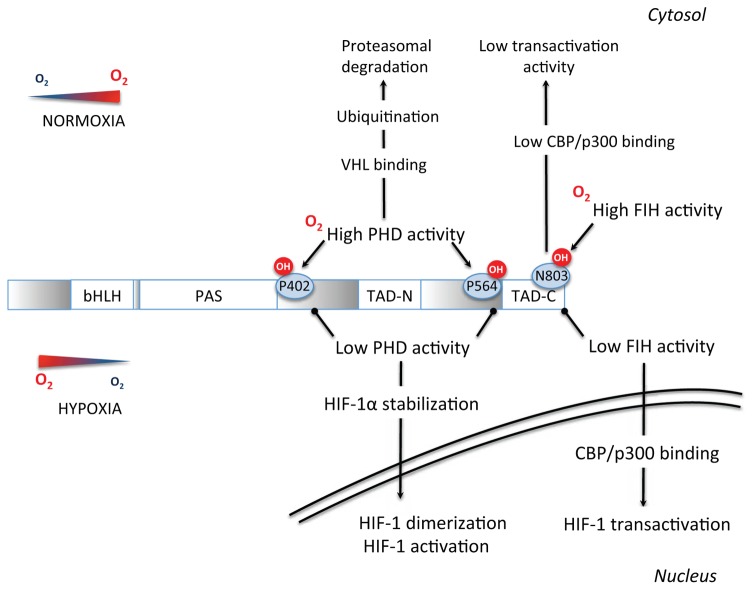
The hypoxia inducible factor-1 (HIF-1) is a fundamental transcription factor in the molecular physiology of O2 homeostasis. HIF-1 is a complex protein composed of 2 subunits, the cytosolic HIF-1α (O2-sensitive) subunit and the nuclear HIF-1β subunit, that dimerize in the nucleus to form the functional HIF-1 transcription factor and activate gene transcription. The N-terminal portion of HIF-1α and HIF-1β contains bHLH (basic helix-loop-helix) and PAS (Per-ARNT-Sim homology) domains necessary for heterodimerization and DNA binding (Xia et al., 2012). The C-terminal portion contains two transactivation domains (TAD), called TAD-N and TAD-C separated by an inhibitory domain. Activation of transcription by HIF-1 requires various co activators such as the CREB Binding Protein (CBP)/p300 co-activator. The O2-sensitive region of HIF-1α is called the oxygen degradation domain and is pivotal for the stabilization of this subunit under hypoxic conditions.
In well-oxygenated cells, proline hydroxylation of the HIF-1α subunit by prolyl-hydroxylase enzymes (PHDs) leads to binding of the von Hippel-Lindau (VHL) protein, recruitment of an E3-ubiquitin protein ligase complex with subsequent ubiquitination and proteasomal degradation. Moreover, the factor inhibiting HIF-1 (FIH) negatively regulates HIF-1 transactivation by asparagine hydroxylation, which blocks HIF-1 binding on its co-activator CBP/p300 (Fig. 2).
Fig. 2.
Signal transduction pathways by which a reduction in O2 concentration inhibits prolyl-4-hydroxylases (PHDs) and Factor Inhibiting HIF-1 (FIH), leading to HIF-1α stabilization and activation. See text for detailed description and additional abbreviations.
In sustained hypoxia, there is a general consensus that oxygen deprivation is the main mechanism behind HIF-1 activation with an on-going debate on a potential role of mitochondrial ROS (Hagen, 2012; Movafagh et al., 2015). In contrast, ROS production by the repetitive desaturation-reoxygenation sequences induced by IH appears to play a major role in up-regulating HIF-1 activity through complex mechanisms involving prolyl-hydroxylase inactivation and HIF-1 transcription and transactivation (Fig. 3). Briefly, repetitive apneas and intermittent hypoxia trigger nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-dependent production of ROS, mostly superoxide anions, which in turn activate phospholipase C (PLC) leading to calcium-calmodulin kinase (CamK) and protein kinase C (PKC) activation. PKC stimulates mammalian target of rapamycin (mTOR)-dependent synthesis of HIF-1α and inhibits PHD-dependent HIF-1α degradation (Prabhakar & Semenza, 2012). CamK also promotes the interaction between HIF-1α and its coactivator p300, thereby leading to increased transcriptional activation upon dimerization with HIF-1β. While HIF-1α is rapidly degraded (t1/2 < 5 min) upon reoxygenation following sustained hypoxia, HIF-1α levels remain elevated following intermittent hypoxia due to the persistent activation of mTOR (Semenza, 2009).
Fig. 3.
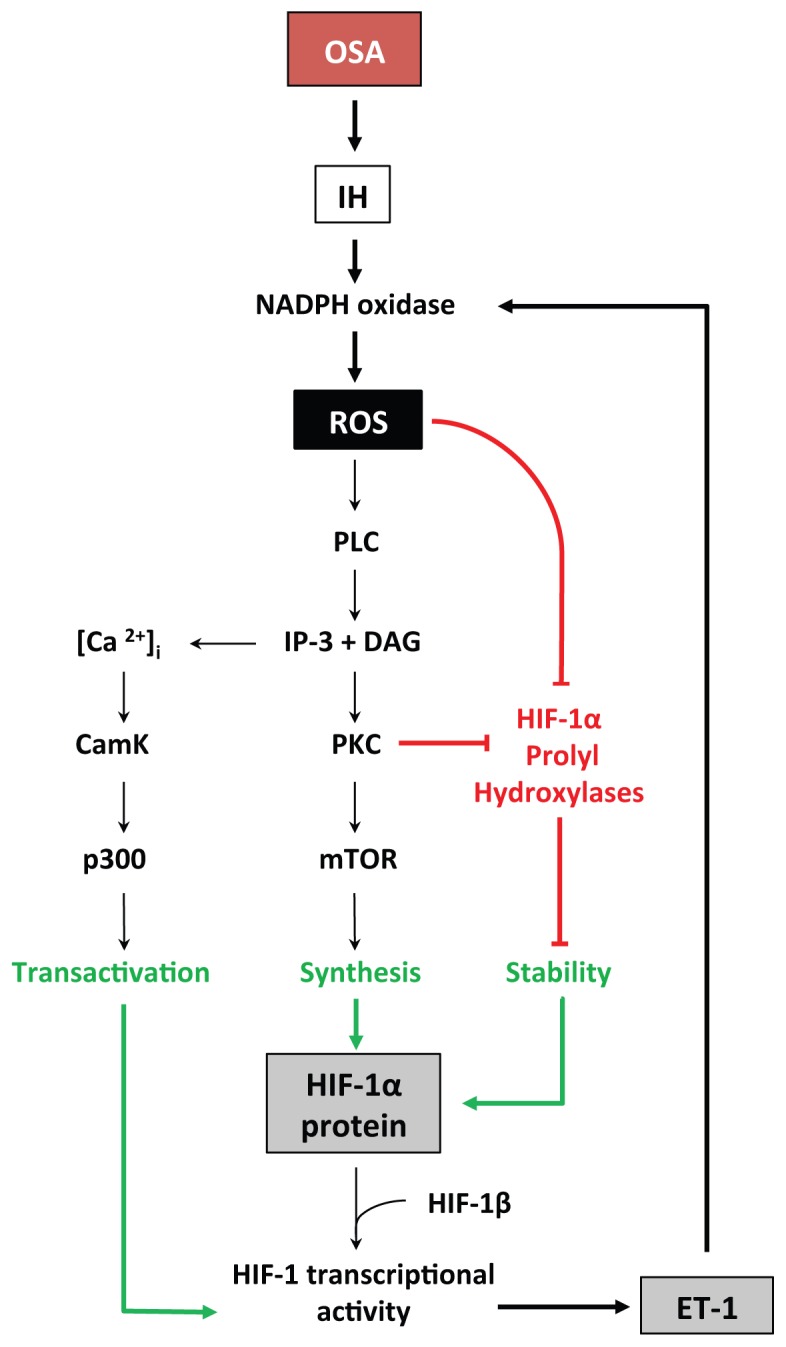
Signal transduction pathways by which obstructive sleep apnea (OSA), intermittent hypoxia (IH) and reactive oxygen species (ROS) induce HIF-1α protein stability, synthesis and transactivation and the promoting effect of endothelin-1 (ET-1) (adapted with permission from (Semenza)). See text for detailed description and additional abbreviations.
Although in vitro studies established that the pro-inflammatory transcription factor nuclear factor-κB (NF-κB) plays a more important role than HIF-1 in the response to IH (Taylor et al., 2014) (Polotsky et al., 2010; Ryan et al., 2005), we and others have observed that chronic in vivo exposure to IH leads to a strong and sustained activation of HIF-1 in the heart (Belaidi et al., 2008; Belaidi et al., 2009; Belaidi et al., 2016), aorta (Gras et al., 2016), brain (Chou et al., 2013; Z. F. Wang et al., 2009) and liver (da Rosa et al., 2012). Nevertheless, the important role of pro-inflammatory pathways is in accordance with these observations since there is a strong crosstalk and synergistic activity between NF-κB and HIF-1 (Bruning et al., 2012; Gorlach & Bonello, 2008) (Fig. 1). An interesting aspect of HIF-1 activation by IH is that it persists when the animals are back in normoxic conditions. This is in agreement with in vitro observations showing that HIF-1 activity remains lastingly elevated after termination of IH (Yuan et al., 2008) and can explain the long-lasting HIF-1 levels and activity observed hours after termination of IH in experimental animals. Although data in OSA patients are scarce, Kazmareck et al. have confirmed that HIF-1α and target genes mRNA are increased in skin biopsies from severe OSA patients and concluded that HIF-1 expression profile may correlate with disease severity and could possibly be used to predict cardiovascular risk (Kaczmarek et al., 2013).
Among the various HIF-1 target gene products, some encode for proteins that have an impact on the cardiovascular system, such as erythropoietin, inducible/endothelial nitric oxide synthases, vascular endothelial growth factor and ET-1. We have identified ET-1 as a major gene product of interest based on the fact that:
Through its vasoconstrictive, growth promoting and proinflammatory properties, ET-1 has been implicated in several pathophysiological pathways associated with cardiovascular disease (Kaoukis et al., 2013; Ohkita et al., 2012).
Plasma big ET-1 and ET-1 are increased in OSA patients (Gjorup et al., 2007; Karkoulias et al., 2010; B. G. Phillips et al., 1999; Saarelainen et al., 1997; Zamarron et al., 2011), in correlation with BP values (Anunciato et al., 2013; B. G. Phillips et al., 1999), and in plasma and tissues of animals exposed to IH (Belaidi et al., 2009; Capone et al., 2012; Kanagy et al., 2001; Peng et al., 2012).
Endothelin receptors, in particular ET-A receptors, are upregulated by IH and the vascular response to ET-1 is increased (Allahdadi et al., 2005; Belaidi et al., 2009; Friedman et al., 2014; Lefebvre et al., 2006; Pawar et al., 2009; Rey et al., 2007; Snow et al., 2011; Z. Wang et al., 2013).
ET-1 has the ability to increase NADPH oxidase activity and HIF-1 expression in part through its ET-A receptors (L. Li et al., 2003; Pisarcik et al., 2013), thus further promoting superoxide production and HIF-1 activity in a feed-forward mechanism (Fig. 3).
In the following sections, we will present the experimental pharmacological evidence in favor of an inhibition of the ROS-HIF-1-endothelin axis in the prevention of the cardiovascular complications induced by IH.
3.2. Role in systemic hypertension
Numerous experimental studies have confirmed that reduction in oxidative stress level, either by NOX-2 deficiency (Schulz et al., 2014) or antioxidant treatments (Hung et al., 2013; Ramond et al., 2013) prevents the development of hypertension in response to intermittent hypoxia exposure. Endothelin receptor antagonists have also been shown to be very effective in preventing or reversing hypertension in rodents exposed to IH (Allahdadi et al., 2008; Belaidi et al., 2009; de Frutos et al., 2010; Kanagy et al., 2001).
3.3. Role in vascular dysfunction and remodeling
In rats exposed to intermittent hypoxia, allopurinol treatment (through its xanthine oxidase-inhibiting property) attenuated the impairment in acetylcholine-mediated vasodilation (Dopp et al., 2011). In a comprehensive study in mice, Capone et al. confirmed the role of both oxidative stress and ET-1 on the impairment in cerebrovascular endothelial-dependent relaxation induced by IH. Hence, cerebrovascular dysfunction was abolished in mice lacking the NOX2 subunit of NADPH oxidase as well as by local perfusion with Mn(III)tetrakis(4-benzoic acid)porphyrin chloride (a free radical scavenger) or with BQ-123 (a selective ET-A receptor antagonist) (Capone et al., 2012). We have recently demonstrated that oxidative stress was responsible for the IH-induced vascular dysfunction in the rat ophthalmic artery, in particular regarding the increased contractile response to ET-1 (Mentek et al., 2016).
In the case of vascular remodeling, de Frutos et al. demonstrated in mice that IH-induced remodeling of mesenteric arteries could be prevented by the mixed endothelin receptor antagonist bosentan (de Frutos et al., 2010). Finally, we have recently established that ET-1 was involved in the inflammatory vascular remodeling induced by IH, through a feed-forward mechanism implicating HIF-1 and its crosstalk with NF-κB (Gras et al., 2016).
3.4. Role in myocardial infarction and stroke
There is experimental data in favor of a major role for oxidative stress and endothelin system activation in the increased sensibility to myocardial infarction induced by chronic IH exposure in rodents. Thus, in mice, the increase in infarct size is associated with enhanced protein carbonylation and lipid peroxidation and decreased thioredoxin content (Park & Suzuki, 2007). In rats, tempol, melatonin as well as atorvastatin treatment during IH exposure reduce myocardial free radical production and prevent the increase in infarct size (Ramond et al., 2013; Totoson et al., 2013).
The cardiac endothelin system is activated by HIF-1 in rats exposed to IH, resulting in increased tissue ET-1 levels, upregulation of coronary ET-A receptors and enhanced vasoconstriction. The deleterious impact of these effects on the myocardium is evidenced by the ability of bosentan to completely prevent the related increase in infarct size (Belaidi et al., 2009). Similar effects on the brain’s susceptibility to ischemia remain to be demonstrated. However, Capone et al. have observed that alterations of the cerebral circulation induced by chronic IH in mice were prevented by the selective ET-A receptor antagonist BQ123 (Capone et al., 2012). In a recent study, we have shown that endoplasmic reticulum (ER) stress and HIF-1 were responsible for the infarct size expansion induced by IH (Belaidi et al., 2016).
3.5. Role in myocardial remodeling and dysfunction
In rats, treatment with allopurinol throughout IH exposure significantly improved LV dysfunction (Williams et al., 2010). Mice deficient for gp91phox (the membrane-bound catalytic subunit of NOX) did not develop LV remodeling in response to IH (Hayashi et al., 2008). Furthermore, pitavastatin treatment during IH exposure significantly reduced NADPH oxidase-related superoxide production as well as LV remodeling in mice (Inamoto et al., 2010).
There is ample evidence that activation of the endothelin system promotes cardiac hypertrophy and HF. Hence, plasma and myocardial ET-1 levels (Cavero et al., 1990; Margulies et al., 1990; Yorikane et al., 1993) and ET-1 receptors (Sakai et al., 1996b) are increased in animal models of congestive HF and in HF patients (McMurray et al., 1992; Zolk et al., 1999). Moreover, ET-1 receptor antagonists (Fraccarollo et al., 1997; Mishima et al., 2000; Mulder et al., 1997) have been shown to improve LV dysfunction and remodeling and to enhance survival (Mulder et al., 1997; Sakai et al., 1996a) in HF models. Nevertheless, the role of ET-1 in the development of cardiac remodeling and HF in response to IH still remains to be investigated.
Table 1 summarizes the various experimental studies associating chronic IH to cardiovascular diseases described in this section as well the beneficial effects of targeting ROS or the endothelin system in this context.
Table 1.
Summary of experimental studies demonstrating the beneficial effects of targeting reactive oxygen species (ROS), HIF-1 and the endothelin-1 (ET-1) system in animal models of cardiovascular complications associated with intermittent hypoxia (IH).
Gp91phox: MnTBAP: Mn(III)tetrakis(4-benzoic acid)porphyrin chloride; NOX2: gp91phox subunit of NADPH oxidase.
| Cardiovascular disease | IH model | Target | Treatment/Models | References |
|---|---|---|---|---|
| Systemic hypertension | Rat | ROS | Melatonin (10 mg/kg/day) | (Hung et al., 2013) |
| Rat | ROS | Tempol (1 mM in water) | (Ramond et al., 2013) | |
| Rat | ET-1 | PD145065 (1–1000 nmol/kg) | (Kanagy et al., 2001) | |
| Rat | ET-1 | BQ-123 (10–1000 nmol/kg) | (Allahdadi et al., 2008) | |
| Rat | ET-1 | Bosentan (100 mg/kg/day) | (Belaidi et al., 2009) | |
| Mouse | ROS | Apocynin (30 mg/kg/day)/NOX-2 deficiency | (Schulz et al., 2014) | |
| Mouse | ET-1 | Bosentan (30 mg/kg/day)/PD155080 (50μg/kg/day) | (de Frutos et al., 2010) | |
|
| ||||
| Vascular dysfunction | Rat | ROS | Allopurinol (65 mg/kg/day) | (Dopp et al., 2011) |
| Rat | ROS | Atorvastatin (10 mg/kg/day) | (Totoson et al., 2013) | |
| Rat | ET-1 | BQ-123 (10 μM perfusion) | (Mentek et al., 2016) | |
| Mouse | ROS | MnTBAP (100 μM perfusion) | (Capone et al., 2012) | |
| Mouse | ET-1 | BQ-123 (10 μM perfusion) | (Capone et al., 2012) | |
|
| ||||
| Vascular remodeling | Mouse | ET-1 | Bosentan (30 mg/kg/day)/PD155080 (50μg/kg/day) | (de Frutos et al., 2010) |
| Mouse | ET-1 | Bosentan (100 mg/kg/day) | (Gras et al., 2016) | |
| Mouse | HIF-1 | HIF-1 deficiency | (Gras et al., 2016) | |
|
| ||||
| Cardiac dysfunction | Rat | ROS | Allopurinol (1% in water) | (Williams et al., 2010) |
|
| ||||
| Cardiac remodeling | Mouse | ROS | NOX2 deficiency | (Hayashi et al., 2008) |
|
| ||||
| Myocardial infarction | Rat | ROS | Melatonin (100 mg/L)/Tempol (1 mM in water) | (Ramond et al., 2013) |
| Rat | ET-1 | Bosentan (100 mg/kg/day) | (Belaidi et al., 2009) | |
| Mouse | HIF-1 | HIF-1 deficiency | (Belaidi et al., 2016) | |
|
| ||||
| Stroke | Mouse | ROS | MnTBAP (100 μM perfusion)/NOX2 deficiency | (Capone et al., 2012) |
| Mouse | ET-1 | BQ-123 (10 μM perfusion) | (Capone et al., 2012) | |
4. Targeting the ROS-HIF-1-ET-1 axis as a novel therapeutic approach in OSA
The experimental evidence presented above clearly underscores the therapeutic potential of interventions targeting this axis in the prevention and/or reduction of cardiovascular alterations in moderate to severe OSA patients. While large-scale clinical investigations specifically designed to address this issue are still pending, we have identified several potentially interesting pharmacological agents that have already been tested clinically or are in pre-clinical development.
4.1. Antioxidant treatment
As mentioned earlier, there is an emerging consensus that OSA is an oxidative stress disorder. In accordance, various studies have shown that reversal of apneas by CPAP therapy can reduce OSA-related oxidative stress markers (Eisele et al., 2015; Jullian-Desayes et al., 2015). However, other studies report limited effects (Barcelo et al., 2006; Del Ben et al., 2012; Jullian-Desayes et al., 2015) and a recent sham-controlled randomized study specifically designed to test the effect of CPAP treatment on primary markers of lipid peroxidation failed to observe significant effects (Sivam et al., 2015). These data, plus the fact that a significant proportion of OSA patients are unable or unwilling to use CPAP therapy, represent a strong argument in favor of studies investigating the effects of antioxidants in OSA patients. Still, apart from some reports of beneficial effects of acute intravenous vitamin C injection (N. J. Buchner et al., 2011; Grebe et al., 2006) or of oral allopurinol treatment (El Solh et al., 2006), no large-scale clinical studies have been performed. The reason behind this discrepancy could be that while antioxidants have proven effective in preventing or reverting oxidative stress-related cardiovascular alterations in experimental models, they have generally yielded disappointing results when translated to human clinical practice, as recently reviewed in this journal (Farias et al., 2016).
Nevertheless, the fact that part of the effectiveness of medications such as statins, angiotensin-converting enzyme (ACE) inhibitors or ARBs is due to their pleiotropic antioxidant and anti-inflammatory properties (Siti et al., 2015) supports the use of these agents in the prevention or treatment of the cardiovascular complications associated with OSA (Pepin et al., 2010; Thunstrom et al., 2016). This is strengthened by the fact that these drugs interfere with NADPH oxidase activation and expression, a therapeutic strategy potentially more efficient than non-selective ROS scavenging (Schramm et al., 2012), particularly in the context of IH and OSA.
Hence, we have shown that the IH-induced vascular remodeling and increase in blood pressure and infarct size were prevented by atorvastatin along with a decrease in NADPH oxidase upregulation and a preservation of superoxide dismutase activity (Totoson et al., 2013). In OSA patients, 3 months of atorvastatin treatment lowered blood pressure and improved lipid profile (Joyeux-Faure et al., 2014). Also, Increased internalization of endothelial CD59 in IH appears to be cholesterol-dependent and is reversed by statins in a CD59-dependent manner (Emin et al., 2016).
In accordance, we have shown that valsartan induced a fourfold greater decrease in mean 24-hour BP compared to CPAP in a small cohort of untreated hypertensive OSA patients (Pepin et al., 2010). In view of the antioxidant, anti-inflammatory and anti-hypertensive actions of statins, ACE inhibitors and ARBs, their beneficial effects in OSA patients should definitely be confirmed in large-scale clinical trials.
Another interesting therapeutic candidate appears to be melatonin, a circadian rhythm regulating hormone with potent pleiotropic antioxidant properties (Tan et al., 2015) and whose secretion profile is altered in OSA patients (Zirlik et al., 2013). We were among the first to report the potent cardioprotective effects of melatonin against ischemia-reperfusion injuries (ventricular arrhythmias and infarct size) (Lagneux et al., 2000). More recently, others and we have shown that melatonin administration throughout IH exposure was very effective in preventing the development of myocardial susceptibility to infarction, hypertension and endothelial dysfunction (Hung et al., 2013; Ramond et al., 2013; Yeung et al., 2015). These effects were also associated with a reduction in IH-induced NADPH oxidase expression and with an increase in antioxidant enzyme activity (Hung et al., 2013). Of particular relevance to the topic of present review, melatonin also has the ability to inhibit HIF-1 activity and target gene expression, possibly through its antioxidant actions (Vriend & Reiter, 2016).
Finally, the novel therapeutic concept of replacing antioxidant supplementation by activation of endogenous antioxidants was recently addressed in this journal (J. Y. Chan & Chan, 2015). This approach is particularly interesting in the context of OSA where antioxidant defenses are lowered. Moreover, this could be achieved by non-pharmacological means such as exercise. In a recent study, moderate exercise was shown to increase endogenous antioxidant levels in healthy as well as in diabetic patients and to reduce oxidative DNA damage (Pittaluga et al., 2015). In that respect, we have recently shown that high-intensity training during IH exposure reduces pro-apoptotic ER stress and prevents the development of hypertension and the increase in infarct size (Bourdier et al., 2016).
4.2. Endothelin system antagonism
Although the major role of endothelin and its receptors in the development of systemic and pulmonary hypertension, coronary atherosclerosis, myocardial infarction and heart failure is well documented (Ohkita et al., 2012), endothelin receptor antagonists are currently mainly used for the treatment of pulmonary hypertension where they have proven effective in decreasing pulmonary vascular resistance and increasing cardiac index (Channick et al., 2001). Yet, mixed receptor antagonists like bosentan (Krum et al., 1998), or selective ET-A receptor antagonists like darusentan (Black et al., 2007; Nakov et al., 2002), have been shown to significantly decrease blood pressure in patients with essential or resistant arterial hypertension. Bosentan improved peripheral (Rafnsson et al., 2012) and the ET-A receptor antagonist atrasentan improved coronary (Reriani et al., 2010) endothelial function in patients with type-2 diabetes and early atherosclerosis, respectively. Finally, in a study in patients with heart failure resistant to standard therapy, bosentan treatment increased cardiac output and decreased systemic and pulmonary vascular resistance (Sutsch et al., 1998) although subsequent clinical trials with mixed and ET-A antagonists have failed to show clear clinical benefits (Kaoukis et al., 2013). Moreover, the safety profile of these drugs has been disappointing due to a high incidence of hepatotoxicity, headache and edema. Nevertheless, a new generation of endothelin system antagonists is emerging such as ET-converting enzyme inhibitors, mixed ECE and neutral endopeptidase inhibitors, ET-B receptor agonists or new potent non-toxic mixed antagonists, such as macitentan (Maguire & Davenport, 2014). These promising agents should definitely be tested in experimental IH models in order to compare their efficacy to that of bosentan or ET-A receptor antagonists. Some authors argue that selective ET-A receptor antagonists are better suited than mixed antagonists for the prevention or treatment of cardiovascular diseases (Nasser & El-Mas, 2014) but this remains to be confirmed by specifically-designed clinical studies, particularly in the context of OSA since the deleterious effects of IH are mainly due to ET-A receptor upregulation (Allahdadi et al., 2008; Belaidi et al., 2009; Briancon-Marjollet et al., 2015; Capone et al., 2012; de Frutos et al., 2010; Mentek et al., 2016).
In a small randomized controlled pilot study in OSA patients (n=16) with mild untreated hypertension (clinical BD of 142/85 mmHg), we recently showed that a 4-week bosentan treatment resulted in a 3 mmHg greater decrease in 24-h DBP than CPAP, but this effect was minimal and not significant compared to baseline (Joyeux-Faure et al., 2015). This could be due to the fact that these patients were not severe enough in terms of hypertension and sleep apnea level. Hence, in view of the clear involvement of the ET-1 system in the severe IH-induced cardiovascular alterations and of the dramatic effects of ET-1 receptor antagonists in this context (Belaidi et al., 2009; Briancon-Marjollet et al., 2015; Gras et al., 2016), large-scale clinical investigations of ET-1 system antagonism in moderate to severe OSA patients are undoubtedly warranted.
4.3. HIF-1 inhibition
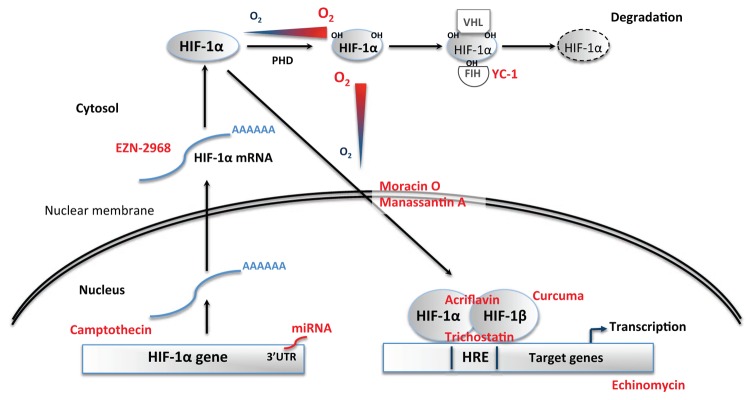
Since both ROS and ET-1 have the ability to promote HIF-1 activity in a feed-forward manner (Fig. 3), HIF-1 appears to be a pivotal target to reverse the deleterious cardiovascular consequences of chronic IH and OSA. Various novel molecular and pharmacological interventions to reduce HIF-1 expression or activity have reached the phase of clinical translation, mostly in the field of cancer research (Fig. 4). These could potentially be investigated in OSA patients after prior pre-clinical studies evaluating the most appropriate therapeutic approaches to prevent excessive HIF-1 activation without suppressing its essential physiological actions.
Fig. 4.
Stabilization of the O2-sensitive cytosolic alpha subunit of the hypoxia inducible factor-1 (HIF-1) transcription factor is determinant for HIF-1 activity. In normoxia, proline hydroxylation (OH) of HIF-1α leads to its recognition by the Von Hippel Lindau (VHL) ubiquitylation complex and subsequent proteasomal degradation. Intermittent hypoxia, by inhibiting prolyl-hydroxylase (PHD) activity, induces HIF-1α stabilization followed by translocation to the nucleus, dimerisation with HIF-1β to form HIF-1, transactivation and binding to hypoxia response elements (HRE) in the promoter of target genes.
HIF-1 content and transcriptional activity can be reduced by various therapeutic strategies: miRNA and Camptothecin inhibit its traduction; YC-1 activates FIH; EZN-2968 inhibits its transcription; Moracin O and Manassantin A inhibit its translocation to the nucleus; Acriflavin and Curcuma prevent its dimerisation with HIF-1β; Echynomycin prevents its binding to HRE; Trichostatin prevents its transactivation.
4.3.1. Genetic strategies to decrease HIF-1α protein levels
MicroRNAs (miRNAs) are a class of single-stranded non-coding RNAs that negatively regulate eukaryotic gene expression at the post-transcriptional and translational levels. Several miRNAs, named hypoxamiRs, are upregulated by hypoxia through various transcription factors including HIF-1 and NF-κB (Cottrill et al., 2014; Nallamshetty et al., 2013). Amongst them, miR-210 is a ubiquitous HIF-1 target gene that has been identified has a major prognostic and therapeutic target in a number of hypoxia-related diseases such as tumor progression, myocardial infarction and pulmonary hypertension (Cottrill et al., 2014).
Reciprocally, miR-210 and other hypoxamiRs engage in regulatory feedback loops with HIF-1. Hence, miR-210 and miR-424 have the ability to promote HIF-1 stabilization through suppression of glycerol-3-phosphate dehydrogenase 1-like enzyme and cullin 2, respectively (Nallamshetty et al., 2013). Moreover, miR-210 appears to be involved in the ROS-mediated HIF-1 stabilization (Movafagh et al., 2015; Nallamshetty et al., 2013).
On the other hand, targeting of the 3′ untranslated region (3′ UTR) of Hif1a by miR-155 (Bruning et al., 2011), miR-519 (Cha et al., 2010), miR-20b (Lei et al., 2009) or the miR-17/92 cluster (Taguchi et al., 2008) decreases HIF-1α protein levels.
Thus delivery of miRNA antagonists (miR-210, miR-424) or mimics (miR-155, miR-519, miR-20b, miR17/92) represents a promising new approach to reduce HIF-1 activity that should be tested in pre-clinical IH models. Translation to clinical studies is possible in view of the rapid development of miRNA-based therapy and of its apparent lack of adverse effects (Bader et al., 2011).
4.3.2. Chemical inhibitors
Several anti-cancer agents acting at different levels of HIF-1 signaling have been tested in clinical trials, or are being investigated in pre-clinical studies. These molecules inhibit HIF-1 activity by interfering with HIF-1α mRNA expression, translation, dimerisation with HIF-1β and DNA binding or by promoting HIF-1α degradation (Xia et al., 2012). Below is presented a selection of small molecular HIF-1 inhibitors along with some experimental data in favor of their potential use in the prevention or treatment of IH- and OSA-induced cardiovascular alterations.
Camptothecin, a natural inhibitor of topoisomerase I, prevents HIF-1α transcription and has proven beneficial in phase I and II clinical studies (Rapisarda et al., 2004). Interestingly, camptothecin also has the ability to decrease HIF-1 expression and activity by up-regulating miR-155 expression (Bertozzi et al., 2014).
Antisense oligodeoxynucleotide HIF-1α mRNA inhibitors, such as EZN-2968, have been shown to reduce the expression of its target genes in cell and animal models (Greenberger et al., 2008) as well as in cancer patients (Jeong et al., 2014).
YC-1, 3-(5-hydroxymethyl-2-furyl)-1-benzyl indazole, first described as a platelet guanylate cyclase activator (Ko et al., 1994), is an approved cancer drug that prevents HIF-1α from interacting with the transcriptional co-activators p300/CBP by activating FIH (S. H. Li et al., 2008). In cardiomyocytes, HIF-1 inhibition by YC-1 effectively represses VEGF induction (Hui et al., 2012).
Acriflavine, a cutaneous antibacterial and antiviral agent, prevents HIF-1 dimerisation by binding to the PAS subdomain of HIF-1α, thereby inhibiting HIF-1 DNA-binding and transcriptional activity (Lee et al., 2009). Moreover, acriflavine prevents HIF-1 activity in C2C12 mouse myocytes exposed to IH (Cai et al., 2013).
Echinomycin is an antibiotic that can bind DNA in a sequence-specific fashion and that interferes with HIF-1-induced gene transcription by binding to its hypoxia response elements (D. Kong et al., 2005).
Finally, the antifungal antibiotic trichostatin, a class I and II histone deacetylase inhibitor, promotes the proteasomal degradation of HIF-1α (Geng et al., 2012; X. Kong et al., 2006). It was recently shown to repress HIF-1-VEGF signaling and to inhibit choroidal angiogenesis in vivo (N. Chan et al., 2015).
4.3.3. Natural inhibitors
Various natural substances possess HIF-1 inhibiting activities. Moracin O, from Morus Alba also known as white mulberry, inhibits HIF-1 translocation and activity due to its particular structure containing a 2-arylbenzofuran ring (Xia et al., 2011). Curcumin, a yellow spice from Curcuma longa, is known to repress HIF-1 activity via its action on HIF-1β (Choi et al., 2006). And finally, Manassantin A, from the water plant Saururuschinensis, prevents HIF-1α nuclear translocation (Hossain et al., 2005).
5. Conclusion
Although continuous positive airway pressure treatment, the first line therapy for OSA, clearly improves quality of life and sleepiness, positive response to CPAP in terms of cardiovascular and metabolic outcome is hampered by poor patient adherence, suboptimal use or limited efficacy. Novel therapeutic strategies are thus required to help counteract the key mechanisms responsible for OSA-related cardiovascular consequences. These therapeutic agents could be used in OSA patients unable or unwilling to use CPAP or in combination with CPAP in order to increase its efficacy.
This review supports a role for the ROS-HIF-1-endothelin axis in the deleterious cardiovascular consequences of the chronic intermittent hypoxia associated with moderate to severe OSA. It also stresses the importance to extend fundamental and clinical research around the pivotal role of the HIF-1 transcription factor in this context.
Abbreviations
- AHI
apnea-hypopnea index
- ARBs
angiotensin II receptor blockers
- BP
blood pressure
- CAD
coronary artery disease
- CamK
calcium-calmodulin kinase
- CPAP
continuous positive airway pressure
- ET-1
endothelin-1
- ET-A
type A endothelin receptor
- FIH
factor inhibiting HIF-1
- HF
heart failure
- HIF-1
hypoxia inducible factor-1
- IH
intermittent hypoxia
- LV
left ventricle
- mTOR
mammalian target of rapamycin
- miRNA
micro-ribonucleic acid
- NADPH
nicotinamide adenine dinucleotide phosphate
- NO
nitric oxide
- NOX
NADPH oxidase
- OSA
obstructive sleep apnea syndrome
- PHDs
HIF-1 prolyl-hydroxylases
- PKC
protein kinase C
- PLC
phospholipase C
- ROS
reactive oxygen species.
Footnotes
Conflicts of interest
None.
References
- Allahdadi KJ, Cherng TW, Pai H, Silva AQ, Walker BR, Nelin LD, et al. Endothelin type A receptor antagonist normalizes blood pressure in rats exposed to eucapnic intermittent hypoxia. Am J Physiol Heart Circ Physiol. 2008;295:H434–440. doi: 10.1152/ajpheart.91477.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allahdadi KJ, Walker BR, Kanagy NL. Augmented endothelin vasoconstriction in intermittent hypoxia-induced hypertension. Hypertension. 2005;45:705–709. doi: 10.1161/01.HYP.0000153794.52852.04. [DOI] [PubMed] [Google Scholar]
- Andreadou I, Iliodromitis EK, Rassaf T, Schulz R, Papapetropoulos A, Ferdinandy P. The role of gasotransmitters NO, H2S and CO in myocardial ischaemia/reperfusion injury and cardioprotection by preconditioning, postconditioning and remote conditioning. Br J Pharmacol. 2015;172:1587–1606. doi: 10.1111/bph.12811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anunciato IF, Lobo RR, Coelho EB, Verri WA, Jr, Eckeli AL, Evora PR, et al. Big endothelin-1 and nitric oxide in hypertensive elderly patients with and without obstructive sleep apnea-hypopnea syndrome. Arq Bras Cardiol. 2013;101:344–351. doi: 10.5935/abc.20130179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaud C, Beguin PC, Lantuejoul S, Pepin JL, Guillermet C, Pelli G, et al. The inflammatory preatherosclerotic remodeling induced by intermittent hypoxia is attenuated by RANTES/CCL5 inhibition. Am J Respir Crit Care Med. 2011a;184:724–731. doi: 10.1164/rccm.201012-2033OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaud C, Poulain L, Levy P, Dematteis M. Inflammation contributes to the atherogenic role of intermittent hypoxia in apolipoprotein-E knock out mice. Atherosclerosis. 2011b;219:425–431. doi: 10.1016/j.atherosclerosis.2011.07.122. [DOI] [PubMed] [Google Scholar]
- Arzt M, Hetzenecker A, Steiner S, Buchner S. Sleep-Disordered Breathing and Coronary Artery Disease. Can J Cardiol. 2015;31:909–917. doi: 10.1016/j.cjca.2015.03.032. [DOI] [PubMed] [Google Scholar]
- Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172:1447–1451. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslan K, Deniz A, Cayli M, Bozdemir H, Sarica Y, Seydaoglu G. Early left ventricular functional alterations in patients with obstructive sleep apnea syndrome. Cardiol J. 2013;20:519–525. doi: 10.5603/CJ.2013.0043. [DOI] [PubMed] [Google Scholar]
- Bader AG, Brown D, Stoudemire J, Lammers P. Developing therapeutic microRNAs for cancer. Gene Ther. 2011;18:1121–1126. doi: 10.1038/gt.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badran M, Ayas N, Laher I. Cardiovascular Complications of Sleep Apnea: Role of Oxidative Stress. Oxid Med Cell Longev. 2014;2014:10. doi: 10.1155/2014/985258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baguet JP, Barone-Rochette G, Tamisier R, Levy P, Pepin JL. Mechanisms of cardiac dysfunction in obstructive sleep apnea. Nat Rev Cardiol. 2012;9:679–688. doi: 10.1038/nrcardio.2012.141. [DOI] [PubMed] [Google Scholar]
- Baguet JP, Hammer L, Levy P, Pierre H, Launois S, Mallion JM, et al. The severity of oxygen desaturation is predictive of carotid wall thickening and plaque occurrence. Chest. 2005;128:3407–3412. doi: 10.1378/chest.128.5.3407. [DOI] [PubMed] [Google Scholar]
- Barbe F, Duran-Cantolla J, Sanchez-de-la-Torre M, Martinez-Alonso M, Carmona C, Barcelo A, et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307:2161–2168. doi: 10.1001/jama.2012.4366. [DOI] [PubMed] [Google Scholar]
- Barceló A, Barbé F, de la Peña M, Vila M, Pérez G, Piérola J, et al. Antioxidant status in patients with sleep apnoea and impact of continuous positive airway pressure treatment. European Respiratory Journal. 2006;27:756–760. doi: 10.1183/09031936.06.00067605. [DOI] [PubMed] [Google Scholar]
- Barcelo A, Miralles C, Barbe F, Vila M, Pons S, Agusti AG. Abnormal lipid peroxidation in patients with sleep apnoea. Eur Respir J. 2000;16:644–647. doi: 10.1034/j.1399-3003.2000.16d13.x. [DOI] [PubMed] [Google Scholar]
- Belaidi E, Beguin PC, Levy P, Ribuot C, Godin-Ribuot D. Prevention of HIF-1 activation and iNOS gene targeting by low-dose cadmium results in loss of myocardial hypoxic preconditioning in the rat. Am J Physiol Heart Circ Physiol. 2008;294:H901–908. doi: 10.1152/ajpheart.00715.2007. [DOI] [PubMed] [Google Scholar]
- Belaidi E, Joyeux-Faure M, Ribuot C, Launois SH, Levy P, Godin-Ribuot D. Major role for hypoxia inducible factor-1 and the endothelin system in promoting myocardial infarction and hypertension in an animal model of obstructive sleep apnea. J Am Coll Cardiol. 2009;53:1309–1317. doi: 10.1016/j.jacc.2008.12.050. [DOI] [PubMed] [Google Scholar]
- Belaidi E, Thomas A, Bourdier G, Moulin S, Lemarie E, Levy P, et al. Endoplasmic reticulum stress as a novel inducer of hypoxia inducible factor-1 activity: its role in the susceptibility to myocardial ischemia-reperfusion induced by chronic intermittent hypoxia. Int J Cardiol. 2016;210:45–53. doi: 10.1016/j.ijcard.2016.02.096. [DOI] [PubMed] [Google Scholar]
- Bertozzi D, Marinello J, Manzo SG, Fornari F, Gramantieri L, Capranico G. The natural inhibitor of DNA topoisomerase I, camptothecin, modulates HIF-1alpha activity by changing miR expression patterns in human cancer cells. Mol Cancer Ther. 2014;13:239–248. doi: 10.1158/1535-7163.MCT-13-0729. [DOI] [PubMed] [Google Scholar]
- Biltagi MA, Maguid MA, Ghafar MA, Farid E. Correlation of 8-isoprostane, interleukin-6 and cardiac functions with clinical score in childhood obstructive sleep apnoea. Acta Paediatr. 2008;97:1397–1405. doi: 10.1111/j.1651-2227.2008.00927.x. [DOI] [PubMed] [Google Scholar]
- Black HR, Bakris GL, Weber MA, Weiss R, Shahawy ME, Marple R, et al. Efficacy and safety of darusentan in patients with resistant hypertension: results from a randomized, double-blind, placebo-controlled dose-ranging study. J Clin Hypertens (Greenwich) 2007;9:760–769. doi: 10.1111/j.1524-6175.2007.07244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdier G, Flore P, Sanchez H, Pepin JL, Belaidi E, Arnaud C. High-intensity training reduces intermittent hypoxia-induced ER stress and myocardial infarct size. Am J Physiol Heart Circ Physiol. 2016;310:H279–289. doi: 10.1152/ajpheart.00448.2015. [DOI] [PubMed] [Google Scholar]
- Bratton DJ, Gaisl T, Wons AM, Kohler M. CPAP vs Mandibular Advancement Devices and Blood Pressure in Patients With Obstructive Sleep Apnea: A Systematic Review and Meta-analysis. JAMA. 2015;314:2280–2293. doi: 10.1001/jama.2015.16303. [DOI] [PubMed] [Google Scholar]
- Briancon-Marjollet A, Monneret D, Henri M, Hazane-Puch F, Pepin JL, Faure P, et al. Endothelin regulates intermittent hypoxia-induced lipolytic remodelling of adipose tissue and phosphorylation of hormone-sensitive lipase. J Physiol. 2015 doi: 10.1113/JP271321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruning U, Cerone L, Neufeld Z, Fitzpatrick SF, Cheong A, Scholz CC, et al. MicroRNA-155 promotes resolution of hypoxia-inducible factor 1alpha activity during prolonged hypoxia. Mol Cell Biol. 2011;31:4087–4096. doi: 10.1128/MCB.01276-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruning U, Fitzpatrick SF, Frank T, Birtwistle M, Taylor CT, Cheong A. NFkappaB and HIF display synergistic behaviour during hypoxic inflammation. Cell Mol Life Sci. 2012;69:1319–1329. doi: 10.1007/s00018-011-0876-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchner NJ, Quack I, Woznowski M, Stahle C, Wenzel U, Rump LC. Microvascular endothelial dysfunction in obstructive sleep apnea is caused by oxidative stress and improved by continuous positive airway pressure therapy. Respiration. 2011;82:409–417. doi: 10.1159/000323266. [DOI] [PubMed] [Google Scholar]
- Buchner S, Satzl A, Debl K, Hetzenecker A, Luchner A, Husser O, et al. Impact of sleep-disordered breathing on myocardial salvage and infarct size in patients with acute myocardial infarction. Eur Heart J. 2014;35:192–199. doi: 10.1093/eurheartj/eht450. [DOI] [PubMed] [Google Scholar]
- Cai Z, Luo W, Zhan H, Semenza GL. Hypoxia-inducible factor 1 is required for remote ischemic preconditioning of the heart. Proc Natl Acad Sci U S A. 2013;110:17462–17467. doi: 10.1073/pnas.1317158110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakmak E, Duksal F, Altinkaya E, Acibucu F, Dogan OT, Yonem O, et al. Association Between the Severity of Nocturnal Hypoxia in Obstructive Sleep Apnea and Non-Alcoholic Fatty Liver Damage. Hepat Mon. 2015;15:e32655. doi: 10.5812/hepatmon.32655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capone C, Faraco G, Coleman C, Young CN, Pickel VM, Anrather J, et al. Endothelin 1-dependent neurovascular dysfunction in chronic intermittent hypoxia. Hypertension. 2012;60:106–113. doi: 10.1161/HYPERTENSIONAHA.112.193672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpagnano GE, Kharitonov SA, Resta O, Foschino-Barbaro MP, Gramiccioni E, Barnes PJ. 8-Isoprostane, a marker of oxidative stress, is increased in exhaled breath condensate of patients with obstructive sleep apnea after night and is reduced by continuous positive airway pressure therapy. Chest. 2003;124:1386–1392. doi: 10.1378/chest.124.4.1386. [DOI] [PubMed] [Google Scholar]
- Castro-Grattoni AL, Alvarez R, Torres M, Farre R, Montserrat JM, Dalmases M, et al. Intermittent Hypoxia-Induced Cardiovascular Remodeling is Reversed by Normoxia in A Mouse Model of Sleep Apnea. Chest. 2016 doi: 10.1016/j.chest.2015.11.010. [DOI] [PubMed] [Google Scholar]
- Cavero PG, Miller WL, Heublein DM, Margulies KB, Burnett JC., Jr Endothelin in experimental congestive heart failure in the anesthetized dog. Am J Physiol. 1990;259:F312–317. doi: 10.1152/ajprenal.1990.259.2.F312. [DOI] [PubMed] [Google Scholar]
- Cha ST, Chen PS, Johansson G, Chu CY, Wang MY, Jeng YM, et al. MicroRNA-519c suppresses hypoxia-inducible factor-1alpha expression and tumor angiogenesis. Cancer Res. 2010;70:2675–2685. doi: 10.1158/0008-5472.CAN-09-2448. [DOI] [PubMed] [Google Scholar]
- Chan JY, Chan SH. Activation of endogenous antioxidants as a common therapeutic strategy against cancer, neurodegeneration and cardiovascular diseases: A lesson learnt from DJ-1. Pharmacol Ther. 2015;156:69–74. doi: 10.1016/j.pharmthera.2015.09.005. [DOI] [PubMed] [Google Scholar]
- Chan N, He S, Spee CK, Ishikawa K, Hinton DR. Attenuation of choroidal neovascularization by histone deacetylase inhibitor. PLoS One. 2015;10:e0120587. doi: 10.1371/journal.pone.0120587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channick RN, Simonneau G, Sitbon O, Robbins IM, Frost A, Tapson VF, et al. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet. 2001;358:1119–1123. doi: 10.1016/S0140-6736(01)06250-X. [DOI] [PubMed] [Google Scholar]
- Chen L, Einbinder E, Zhang Q, Hasday J, Balke CW, Scharf SM. Oxidative stress and left ventricular function with chronic intermittent hypoxia in rats. Am J Respir Crit Care Med. 2005;172:915–920. doi: 10.1164/rccm.200504-560OC. [DOI] [PubMed] [Google Scholar]
- Chen L, Zhang J, Gan TX, Chen-Izu Y, Hasday JD, Karmazyn M, et al. Left ventricular dysfunction and associated cellular injury in rats exposed to chronic intermittent hypoxia. J Appl Physiol (1985) 2008;104:218–223. doi: 10.1152/japplphysiol.00301.2007. [DOI] [PubMed] [Google Scholar]
- Chen L, Zhang J, Hu X, Philipson KD, Scharf SM. The Na+/Ca2+ exchanger-1 mediates left ventricular dysfunction in mice with chronic intermittent hypoxia. J Appl Physiol (1985) 2010;109:1675–1685. doi: 10.1152/japplphysiol.01372.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirinos JA, Gurubhagavatula I, Teff K, Rader DJ, Wadden TA, Townsend R, et al. CPAP, weight loss, or both for obstructive sleep apnea. N Engl J Med. 2014;370:2265–2275. doi: 10.1056/NEJMoa1306187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H, Chun YS, Kim SW, Kim MS, Park JW. Curcumin inhibits hypoxia-inducible factor-1 by degrading aryl hydrocarbon receptor nuclear translocator: a mechanism of tumor growth inhibition. Mol Pharmacol. 2006;70:1664–1671. doi: 10.1124/mol.106.025817. [DOI] [PubMed] [Google Scholar]
- Chou YT, Zhan G, Zhu Y, Fenik P, Panossian L, Li Y, et al. C/EBP homologous binding protein (CHOP) underlies neural injury in sleep apnea model. Sleep. 2013;36:481–492. doi: 10.5665/sleep.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrill KA, Chan SY, Loscalzo J. Hypoxamirs and mitochondrial metabolism. Antioxid Redox Signal. 2014;21:1189–1201. doi: 10.1089/ars.2013.5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Rosa DP, Forgiarini LF, Baronio D, Feijo CA, Martinez D, Marroni NP. Simulating sleep apnea by exposure to intermittent hypoxia induces inflammation in the lung and liver. Mediators Inflamm. 2012;2012:879419. doi: 10.1155/2012/879419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Frutos S, Caldwell E, Nitta CH, Kanagy NL, Wang J, Wang W, et al. NFATc3 contributes to intermittent hypoxia-induced arterial remodeling in mice. Am J Physiol Heart Circ Physiol. 2010;299:H356–363. doi: 10.1152/ajpheart.00341.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Ben M, Fabiani M, Loffredo L, Polimeni L, Carnevale R, Baratta F, et al. Oxidative stress mediated arterial dysfunction in patients with obstructive sleep apnoea and the effect of continuous positive airway pressure treatment. BMC Pulmonary Medicine. 2012;12:1–8. doi: 10.1186/1471-2466-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dematteis M, Godin-Ribuot D, Arnaud C, Ribuot C, Stanke-Labesque F, Pepin JL, et al. Cardiovascular consequences of sleep-disordered breathing: contribution of animal models to understanding the human disease. ILAR J. 2009;50:262–281. doi: 10.1093/ilar.50.3.262. [DOI] [PubMed] [Google Scholar]
- Ding WX, Dong YB, Ding N, Zhang XF, Zhang SJ, Zhang XL, et al. Adiponectin protects rat heart from left ventricular remodeling induced by chronic intermittent hypoxia via inhibition of TGF-beta/smad2/3 pathway. J Thorac Dis. 2014;6:1278–1284. doi: 10.3978/j.issn.2072-1439.2014.07.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong JY, Zhang YH, Qin LQ. Obstructive sleep apnea and cardiovascular risk: meta-analysis of prospective cohort studies. Atherosclerosis. 2013;229:489–495. doi: 10.1016/j.atherosclerosis.2013.04.026. [DOI] [PubMed] [Google Scholar]
- Dopp JM, Philippi NR, Marcus NJ, Olson EB, Bird CE, Moran JJ, et al. Xanthine oxidase inhibition attenuates endothelial dysfunction caused by chronic intermittent hypoxia in rats. Respiration. 2011;82:458–467. doi: 10.1159/000329341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drager LF, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: an emerging risk factor for atherosclerosis. Chest. 2011;140:534–542. doi: 10.1378/chest.10-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drager LF, Yao Q, Hernandez KL, Shin MK, Bevans-Fonti S, Gay J, et al. Chronic intermittent hypoxia induces atherosclerosis via activation of adipose angiopoietin-like 4. Am J Respir Crit Care Med. 2013;188:240–248. doi: 10.1164/rccm.201209-1688OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele HJ, Markart P, Schulz R. Obstructive Sleep Apnea, Oxidative Stress, and Cardiovascular Disease: Evidence from Human Studies. Oxid Med Cell Longev. 2015;2015:9. doi: 10.1155/2015/608438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Solh AA, Saliba R, Bosinski T, Grant BJ, Berbary E, Miller N. Allopurinol improves endothelial function in sleep apnoea: a randomised controlled study. Eur Respir J. 2006;27:997–1002. doi: 10.1183/09031936.06.00101005. [DOI] [PubMed] [Google Scholar]
- Emin M, Wang G, Castagna F, Rodriguez-Lopez J, Wahab R, Wang J, et al. Increased internalization of complement inhibitor CD59 may contribute to endothelial inflammation in obstructive sleep apnea. Sci Transl Med. 2016;8:320ra321. doi: 10.1126/scitranslmed.aad0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farías JG, Herrera EA, Carrasco-Pozo C, Sotomayor-Zárate R, Cruz G, Morales P, et al. Pharmacological models and approaches for pathophysiological conditions associated with hypoxia and oxidative stress. Pharmacol Ther. 2016;158:1–23. doi: 10.1016/j.pharmthera.2015.11.006. [DOI] [PubMed] [Google Scholar]
- Fletcher EC, Lesske J, Culman J, Miller CC, Unger T. Sympathetic denervation blocks blood pressure elevation in episodic hypoxia. Hypertension. 1992;20:612–619. doi: 10.1161/01.hyp.20.5.612. [DOI] [PubMed] [Google Scholar]
- Fraccarollo D, Hu K, Galuppo P, Gaudron P, Ertl G. Chronic endothelin receptor blockade attenuates progressive ventricular dilation and improves cardiac function in rats with myocardial infarction: possible involvement of myocardial endothelin system in ventricular remodeling. Circulation. 1997;96:3963–3973. doi: 10.1161/01.cir.96.11.3963. [DOI] [PubMed] [Google Scholar]
- Franco CM, Lima AM, Ataide L, Jr, Lins OG, Castro CM, Bezerra AA, et al. Obstructive sleep apnea severity correlates with cellular and plasma oxidative stress parameters and affective symptoms. J Mol Neurosci. 2012;47:300–310. doi: 10.1007/s12031-012-9738-0. [DOI] [PubMed] [Google Scholar]
- Friedman JK, Nitta CH, Henderson KM, Codianni SJ, Sanchez L, Ramiro-Diaz JM, et al. Intermittent hypoxia-induced increases in reactive oxygen species activate NFATc3 increasing endothelin-1 vasoconstrictor reactivity. Vascul Pharmacol. 2014;60:17–24. doi: 10.1016/j.vph.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gami AS, Olson EJ, Shen WK, Wright RS, Ballman KV, Hodge DO, et al. Obstructive sleep apnea and the risk of sudden cardiac death: a longitudinal study of 10,701 adults. J Am Coll Cardiol. 2013;62:610–616. doi: 10.1016/j.jacc.2013.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng H, Liu Q, Xue C, David LL, Beer TM, Thomas GV, et al. HIF1alpha protein stability is increased by acetylation at lysine 709. J Biol Chem. 2012;287:35496–35505. doi: 10.1074/jbc.M112.400697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjorup PH, Sadauskiene L, Wessels J, Nyvad O, Strunge B, Pedersen EB. Abnormally increased endothelin-1 in plasma during the night in obstructive sleep apnea: relation to blood pressure and severity of disease. Am J Hypertens. 2007;20:44–52. doi: 10.1016/j.amjhyper.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Gorlach A, Bonello S. The cross-talk between NF-kappaB and HIF-1: further evidence for a significant liaison. Biochem J. 2008;412:e17–19. doi: 10.1042/BJ20080920. [DOI] [PubMed] [Google Scholar]
- Gottlieb DJ, Yenokyan G, Newman AB, O’Connor GT, Punjabi NM, Quan SF, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122:352–360. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gras E, Belaidi E, Briancon-Marjollet A, Pepin JL, Arnaud C, Godin-Ribuot D. Endothelin-1 mediates intermittent hypoxia-induced inflammatory vascular remodeling through HIF-1 activation. J Appl Physiol (1985) 2016;120:437–443. doi: 10.1152/japplphysiol.00641.2015. [DOI] [PubMed] [Google Scholar]
- Grebe M, Eisele HJ, Weissmann N, Schaefer C, Tillmanns H, Seeger W, et al. Antioxidant vitamin C improves endothelial function in obstructive sleep apnea. Am J Respir Crit Care Med. 2006;173:897–901. doi: 10.1164/rccm.200508-1223OC. [DOI] [PubMed] [Google Scholar]
- Greenberger LM, Horak ID, Filpula D, Sapra P, Westergaard M, Frydenlund HF, et al. A RNA antagonist of hypoxia-inducible factor-1alpha, EZN-2968, inhibits tumor cell growth. Mol Cancer Ther. 2008;7:3598–3608. doi: 10.1158/1535-7163.MCT-08-0510. [DOI] [PubMed] [Google Scholar]
- Hagen T. Oxygen versus Reactive Oxygen in the Regulation of HIF-1alpha: The Balance Tips. Biochem Res Int. 2012;2012:436981. doi: 10.1155/2012/436981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Yamashita C, Matsumoto C, Kwak CJ, Fujii K, Hirata T, et al. Role of gp91phox-containing NADPH oxidase in left ventricular remodeling induced by intermittent hypoxic stress. Am J Physiol Heart Circ Physiol. 2008;294:H2197–2203. doi: 10.1152/ajpheart.91496.2007. [DOI] [PubMed] [Google Scholar]
- He QY, Feng J, Zhang XL, Liang ZA, Huang SG, Kang J, et al. Relationship of daytime blood pressure and severity of obstructive sleep apnea among Chinese: a multi-center investigation in China. Chin Med J (Engl) 2010;123:18–22. [PubMed] [Google Scholar]
- Hossain CF, Kim YP, Baerson SR, Zhang L, Bruick RK, Mohammed KA, et al. Saururus cernuus lignans--potent small molecule inhibitors of hypoxia-inducible factor-1. Biochem Biophys Res Commun. 2005;333:1026–1033. doi: 10.1016/j.bbrc.2005.05.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyos CM, Melehan KL, Liu PY, Grunstein RR, Phillips CL. Does obstructive sleep apnea cause endothelial dysfunction? A critical review of the literature. Sleep Med Rev. 2015;20:15–26. doi: 10.1016/j.smrv.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Hui Y, Zhao Y, Ma N, Peng Y, Pan Z, Zou C, et al. M3-mAChR stimulation exerts anti-apoptotic effect via activating the HIF-1alpha/HO-1/VEGF signaling pathway in H9c2 rat ventricular cells. J Cardiovasc Pharmacol. 2012;60:474–482. doi: 10.1097/FJC.0b013e31826c1c13. [DOI] [PubMed] [Google Scholar]
- Hung MW, Kravtsov GM, Lau CF, Poon AM, Tipoe GL, Fung ML. Melatonin ameliorates endothelial dysfunction, vascular inflammation, and systemic hypertension in rats with chronic intermittent hypoxia. J Pineal Res. 2013;55:247–256. doi: 10.1111/jpi.12067. [DOI] [PubMed] [Google Scholar]
- Inamoto S, Yoshioka T, Yamashita C, Miyamura M, Mori T, Ukimura A, et al. Pitavastatin reduces oxidative stress and attenuates intermittent hypoxia-induced left ventricular remodeling in lean mice. Hypertens Res. 2010;33:579–586. doi: 10.1038/hr.2010.36. [DOI] [PubMed] [Google Scholar]
- Jackman KA, Zhou P, Faraco G, Peixoto PM, Coleman C, Voss HU, et al. Dichotomous effects of chronic intermittent hypoxia on focal cerebral ischemic injury. Stroke. 2014;45:1460–1467. doi: 10.1161/STROKEAHA.114.004816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagadapillai R, Mellen NM, Sachleben LR, Jr, Gozal E. Ceftriaxone preserves glutamate transporters and prevents intermittent hypoxia-induced vulnerability to brain excitotoxic injury. PLoS One. 2014;9:e100230. doi: 10.1371/journal.pone.0100230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong W, Rapisarda A, Park SR, Kinders RJ, Chen A, Melillo G, et al. Pilot trial of EZN-2968, an antisense oligonucleotide inhibitor of hypoxia-inducible factor-1 alpha (HIF-1alpha), in patients with refractory solid tumors. Cancer Chemother Pharmacol. 2014;73:343–348. doi: 10.1007/s00280-013-2362-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyeux-Faure M, Jullian-Desayes I, Pepin JL, Cracowski JL, Baguet JP, Tamisier R, et al. Comparison of continuous positive airway pressure and bosentan effect in mildly hypertensive patients with obstructive sleep apnoea: A randomized controlled pilot study. Respirology. 2015 doi: 10.1111/resp.12713. [DOI] [PubMed] [Google Scholar]
- Joyeux-Faure M, Stanke-Labesque F, Lefebvre B, Beguin P, Godin-Ribuot D, Ribuot C, et al. Chronic intermittent hypoxia increases infarction in the isolated rat heart. J Appl Physiol (1985) 2005;98:1691–1696. doi: 10.1152/japplphysiol.01146.2004. [DOI] [PubMed] [Google Scholar]
- Joyeux-Faure M, Tamisier R, Baguet JP, Dias-Domingos S, Perrig S, Leftheriotis G, et al. Response to statin therapy in obstructive sleep apnea syndrome: a multicenter randomized controlled trial. Mediators Inflamm. 2014;2014:423120. doi: 10.1155/2014/423120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jullian-Desayes I, Joyeux-Faure M, Tamisier R, Launois S, Borel AL, Levy P, et al. Impact of obstructive sleep apnea treatment by continuous positive airway pressure on cardiometabolic biomarkers: a systematic review from sham CPAP randomized controlled trials. Sleep Med Rev. 2015;21:23–38. doi: 10.1016/j.smrv.2014.07.004. [DOI] [PubMed] [Google Scholar]
- Kaczmarek E, Bakker JP, Clarke DN, Csizmadia E, Kocher O, Veves A, et al. Molecular biomarkers of vascular dysfunction in obstructive sleep apnea. PLoS One. 2013;8:e70559. doi: 10.1371/journal.pone.0070559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanagy NL, Walker BR, Nelin LD. Role of endothelin in intermittent hypoxia-induced hypertension. Hypertension. 2001;37:511–515. doi: 10.1161/01.hyp.37.2.511. [DOI] [PubMed] [Google Scholar]
- Kaoukis A, Deftereos S, Raisakis K, Giannopoulos G, Bouras G, Panagopoulou V, et al. The role of endothelin system in cardiovascular disease and the potential therapeutic perspectives of its inhibition. Curr Top Med Chem. 2013;13:95–114. doi: 10.2174/1568026611313020003. [DOI] [PubMed] [Google Scholar]
- Karkoulias K, Lykouras D, Sampsonas F, Drakatos P, Canova S, Tsoukalas G, et al. The role of Endothelin-1 in obstructive sleep apnea syndrome and pulmonary arterial hypertension: pathogenesis and Endothelin-1 antagonists. Curr Med Chem. 2010;17:1059–1066. doi: 10.2174/092986710790820624. [DOI] [PubMed] [Google Scholar]
- Khayat R, Jarjoura D, Porter K, Sow A, Wannemacher J, Dohar R, et al. Sleep disordered breathing and post-discharge mortality in patients with acute heart failure. Eur Heart J. 2015;36:1463–1469. doi: 10.1093/eurheartj/ehu522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko FN, Wu CC, Kuo SC, Lee FY, Teng CM. YC-1, a novel activator of platelet guanylate cyclase. Blood. 1994;84:4226–4233. [PubMed] [Google Scholar]
- Kong D, Park EJ, Stephen AG, Calvani M, Cardellina JH, Monks A, et al. Echinomycin, a small-molecule inhibitor of hypoxia-inducible factor-1 DNA-binding activity. Cancer Res. 2005;65:9047–9055. doi: 10.1158/0008-5472.CAN-05-1235. [DOI] [PubMed] [Google Scholar]
- Kong X, Lin Z, Liang D, Fath D, Sang N, Caro J. Histone deacetylase inhibitors induce VHL and ubiquitin-independent proteasomal degradation of hypoxia-inducible factor 1alpha. Mol Cell Biol. 2006;26:2019–2028. doi: 10.1128/MCB.26.6.2019-2028.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause BJ, Del Rio R, Moya EA, Marquez-Gutierrez M, Casanello P, Iturriaga R. Arginase-endothelial nitric oxide synthase imbalance contributes to endothelial dysfunction during chronic intermittent hypoxia. J Hypertens. 2015;33:515–524. doi: 10.1097/HJH.0000000000000453. discussion 524. [DOI] [PubMed] [Google Scholar]
- Krum H, Viskoper RJ, Lacourciere Y, Budde M, Charlon V. The effect of an endothelin-receptor antagonist, bosentan, on blood pressure in patients with essential hypertension. Bosentan Hypertension Investigators. N Engl J Med. 1998;338:784–790. doi: 10.1056/NEJM199803193381202. [DOI] [PubMed] [Google Scholar]
- Lagneux C, Joyeux M, Demenge P, Ribuot C, Godin-Ribuot D. Protective effects of melatonin against ischemia-reperfusion injury in the isolated rat heart. Life Sci. 2000;66:503–509. doi: 10.1016/s0024-3205(99)00620-7. [DOI] [PubMed] [Google Scholar]
- Lavie L. Oxidative stress in obstructive sleep apnea and intermittent hypoxia--revisited--the bad ugly and good: implications to the heart and brain. Sleep Med Rev. 2015;20:27–45. doi: 10.1016/j.smrv.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Lavie L, Lavie P. Molecular mechanisms of cardiovascular disease in OSAHS: the oxidative stress link. Eur Respir J. 2009;33:1467–1484. doi: 10.1183/09031936.00086608. [DOI] [PubMed] [Google Scholar]
- Lavie L, Vishnevsky A, Lavie P. Evidence for lipid peroxidation in obstructive sleep apnea. Sleep. 2004;27:123–128. [PubMed] [Google Scholar]
- Lee K, Zhang H, Qian DZ, Rey S, Liu JO, Semenza GL. Acriflavine inhibits HIF-1 dimerization, tumor growth, and vascularization. Proc Natl Acad Sci U S A. 2009;106:17910–17915. doi: 10.1073/pnas.0909353106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lefebvre B, Godin-Ribuot D, Joyeux-Faure M, Caron F, Bessard G, Levy P, et al. Functional assessment of vascular reactivity after chronic intermittent hypoxia in the rat. Respir Physiol Neurobiol. 2006;150:278–286. doi: 10.1016/j.resp.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Lei Z, Li B, Yang Z, Fang H, Zhang GM, Feng ZH, et al. Regulation of HIF-1alpha and VEGF by miR-20b tunes tumor cells to adapt to the alteration of oxygen concentration. PLoS One. 2009;4:e7629. doi: 10.1371/journal.pone.0007629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévy P, Kohler M, McNicholas WT, Barbé F, McEvoy RD, Somers VK, et al. Obstructive sleep apnoea syndrome. Nature Reviews Disease Primers. 2015;1:15015. doi: 10.1038/nrdp.2015.15. [DOI] [PubMed] [Google Scholar]
- Li L, Fink GD, Watts SW, Northcott CA, Galligan JJ, Pagano PJ, et al. Endothelin-1 increases vascular superoxide via endothelin(A)-NADPH oxidase pathway in low-renin hypertension. Circulation. 2003;107:1053–1058. doi: 10.1161/01.cir.0000051459.74466.46. [DOI] [PubMed] [Google Scholar]
- Li S, Feng J, Wei S, Qian X, Cao J, Chen B. Delayed neutrophil apoptosis mediates intermittent hypoxia-induced progressive heart failure in pressure-overloaded rats. Sleep Breath. 2015 doi: 10.1007/s11325-015-1190-2. [DOI] [PubMed] [Google Scholar]
- Li SH, Shin DH, Chun YS, Lee MK, Kim MS, Park JW. A novel mode of action of YC-1 in HIF inhibition: stimulation of FIH-dependent p300 dissociation from HIF-1{alpha} Mol Cancer Ther. 2008;7:3729–3738. doi: 10.1158/1535-7163.MCT-08-0074. [DOI] [PubMed] [Google Scholar]
- Maguire JJ, Davenport AP. Endothelin@25 - new agonists, antagonists, inhibitors and emerging research frontiers: IUPHAR Review 12. Br J Pharmacol. 2014;171:5555–5572. doi: 10.1111/bph.12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso M, Bonanni E, LoGerfo A, Orsucci D, Maestri M, Chico L, et al. Oxidative stress biomarkers in patients with untreated obstructive sleep apnea syndrome. Sleep Med. 2012;13:632–636. doi: 10.1016/j.sleep.2011.10.030. [DOI] [PubMed] [Google Scholar]
- Margulies KB, Hildebrand FL, Jr, Lerman A, Perrella MA, Burnett JC., Jr Increased endothelin in experimental heart failure. Circulation. 1990;82:2226–2230. doi: 10.1161/01.cir.82.6.2226. [DOI] [PubMed] [Google Scholar]
- McMurray JJ, Ray SG, Abdullah I, Dargie HJ, Morton JJ. Plasma endothelin in chronic heart failure. Circulation. 1992;85:1374–1379. doi: 10.1161/01.cir.85.4.1374. [DOI] [PubMed] [Google Scholar]
- Mentek M, Baldazza M, Morand J, Faury G, Aptel F, Godin-Ribuot D, et al. Effects of intermittent hypoxia on rat ophthalmic artery reactivity: role of endothelin 1, oxidative stress and endothelium derived hyperpolarizing factors. Arch Cardiovasc Dis Suppl. 2016;8:216. [Google Scholar]
- Mishima T, Tanimura M, Suzuki G, Todor A, Sharov VG, Goldstein S, et al. Effects of long-term therapy with bosentan on the progression of left ventricular dysfunction and remodeling in dogs with heart failure. J Am Coll Cardiol. 2000;35:222–229. doi: 10.1016/s0735-1097(99)00528-8. [DOI] [PubMed] [Google Scholar]
- Monneret D, Pepin JL, Godin-Ribuot D, Ducros V, Baguet JP, Levy P, et al. Association of urinary 15-F2t-isoprostane level with oxygen desaturation and carotid intima-media thickness in nonobese sleep apnea patients. Free Radic Biol Med. 2010;48:619–625. doi: 10.1016/j.freeradbiomed.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Movafagh S, Crook S, Vo K. Regulation of hypoxia-inducible factor-1a by reactive oxygen species: new developments in an old debate. J Cell Biochem. 2015;116:696–703. doi: 10.1002/jcb.25074. [DOI] [PubMed] [Google Scholar]
- Mulder P, Richard V, Derumeaux G, Hogie M, Henry JP, Lallemand F, et al. Role of endogenous endothelin in chronic heart failure: effect of long-term treatment with an endothelin antagonist on survival, hemodynamics, and cardiac remodeling. Circulation. 1997;96:1976–1982. doi: 10.1161/01.cir.96.6.1976. [DOI] [PubMed] [Google Scholar]
- Muraja-Murro A, Kulkas A, Hiltunen M, Kupari S, Hukkanen T, Tiihonen P, et al. Adjustment of apnea-hypopnea index with severity of obstruction events enhances detection of sleep apnea patients with the highest risk of severe health consequences. Sleep Breath. 2014;18:641–647. doi: 10.1007/s11325-013-0927-z. [DOI] [PubMed] [Google Scholar]
- Nakov R, Pfarr E, Eberle S. Darusentan: an effective endothelinA receptor antagonist for treatment of hypertension. Am J Hypertens. 2002;15:583–589. doi: 10.1016/s0895-7061(02)02933-3. [DOI] [PubMed] [Google Scholar]
- Nallamshetty S, Chan SY, Loscalzo J. Hypoxia: a master regulator of microRNA biogenesis and activity. Free Radic Biol Med. 2013;64:20–30. doi: 10.1016/j.freeradbiomed.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasser SA, El-Mas MM. Endothelin ETA receptor antagonism in cardiovascular disease. Eur J Pharmacol. 2014;737:210–213. doi: 10.1016/j.ejphar.2014.05.046. [DOI] [PubMed] [Google Scholar]
- Ohkita M, Tawa M, Kitada K, Matsumura Y. Pathophysiological roles of endothelin receptors in cardiovascular diseases. J Pharmacol Sci. 2012;119:302–313. doi: 10.1254/jphs.12r01cr. [DOI] [PubMed] [Google Scholar]
- Park AM, Suzuki YJ. Effects of intermittent hypoxia on oxidative stress-induced myocardial damage in mice. J Appl Physiol (1985) 2007;102:1806–1814. doi: 10.1152/japplphysiol.01291.2006. [DOI] [PubMed] [Google Scholar]
- Pawar A, Nanduri J, Yuan G, Khan SA, Wang N, Kumar GK, et al. Reactive oxygen species-dependent endothelin signaling is required for augmented hypoxic sensory response of the neonatal carotid body by intermittent hypoxia. Am J Physiol Regul Integr Comp Physiol. 2009;296:R735–742. doi: 10.1152/ajpregu.90490.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Nanduri J, Zhang X, Wang N, Raghuraman G, Seagard J, et al. Endothelin-1 mediates attenuated carotid baroreceptor activity by intermittent hypoxia. J Appl Physiol (1985) 2012;112:187–196. doi: 10.1152/japplphysiol.00529.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepin JL, Borel AL, Tamisier R, Baguet JP, Levy P, Dauvilliers Y. Hypertension and sleep: Overview of a tight relationship. Sleep Med Rev. 2014 doi: 10.1016/j.smrv.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Pepin JL, Tamisier R, Barone-Rochette G, Launois SH, Levy P, Baguet JP. Comparison of continuous positive airway pressure and valsartan in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;182:954–960. doi: 10.1164/rccm.200912-1803OC. [DOI] [PubMed] [Google Scholar]
- Pepin JL, Tamisier R, Levy P. Obstructive sleep apnoea and metabolic syndrome: put CPAP efficacy in a more realistic perspective. Thorax. 2012;67:1025–1027. doi: 10.1136/thoraxjnl-2012-202807. [DOI] [PubMed] [Google Scholar]
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips BG, Narkiewicz K, Pesek CA, Haynes WG, Dyken ME, Somers VK. Effects of obstructive sleep apnea on endothelin-1 and blood pressure. J Hypertens. 1999;17:61–66. doi: 10.1097/00004872-199917010-00010. [DOI] [PubMed] [Google Scholar]
- Phillips SA, Olson EB, Morgan BJ, Lombard JH. Chronic intermittent hypoxia impairs endothelium-dependent dilation in rat cerebral and skeletal muscle resistance arteries. Am J Physiol Heart Circ Physiol. 2004;286:H388–393. doi: 10.1152/ajpheart.00683.2003. [DOI] [PubMed] [Google Scholar]
- Pilkauskaite G, Miliauskas S, Sakalauskas R. Reactive oxygen species production in peripheral blood neutrophils of obstructive sleep apnea patients. ScientificWorldJournal. 2013;2013:421763. doi: 10.1155/2013/421763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisarcik S, Maylor J, Lu W, Yun X, Undem C, Sylvester JT, et al. Activation of hypoxia-inducible factor-1 in pulmonary arterial smooth muscle cells by endothelin-1. Am J Physiol Lung Cell Mol Physiol. 2013;304:L549–561. doi: 10.1152/ajplung.00081.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittaluga M, Sgadari A, Dimauro I, Tavazzi B, Parisi P, Caporossi D. Physical exercise and redox balance in type 2 diabetics: effects of moderate training on biomarkers of oxidative stress and DNA damage evaluated through comet assay. Oxid Med Cell Longev. 2015;2015:981242. doi: 10.1155/2015/981242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polotsky VY, Savransky V, Bevans-Fonti S, Reinke C, Li J, Grigoryev DN, et al. Intermittent and sustained hypoxia induce a similar gene expression profile in human aortic endothelial cells. Physiol Genomics. 2010;41:306–314. doi: 10.1152/physiolgenomics.00091.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar NR, Semenza GL. Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiol Rev. 2012;92:967–1003. doi: 10.1152/physrev.00030.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero M, del Gonzalez-Martin MC, Vega-Agapito V, Gonzalez C, Obeso A, Farre R, et al. The effects of intermittent hypoxia on redox status, NF-kappaB activation, and plasma lipid levels are dependent on the lowest oxygen saturation. Free Radic Biol Med. 2013;65:1143–1154. doi: 10.1016/j.freeradbiomed.2013.08.180. [DOI] [PubMed] [Google Scholar]
- Rafnsson A, Bohm F, Settergren M, Gonon A, Brismar K, Pernow J. The endothelin receptor antagonist bosentan improves peripheral endothelial function in patients with type 2 diabetes mellitus and microalbuminuria: a randomised trial. Diabetologia. 2012;55:600–607. doi: 10.1007/s00125-011-2415-y. [DOI] [PubMed] [Google Scholar]
- Ramond A, Godin-Ribuot D, Ribuot C, Totoson P, Koritchneva I, Cachot S, et al. Oxidative stress mediates cardiac infarction aggravation induced by intermittent hypoxia. Fundam Clin Pharmacol. 2013;27:252–261. doi: 10.1111/j.1472-8206.2011.01015.x. [DOI] [PubMed] [Google Scholar]
- Rapisarda A, Uranchimeg B, Sordet O, Pommier Y, Shoemaker RH, Melillo G. Topoisomerase I-mediated inhibition of hypoxia-inducible factor 1: mechanism and therapeutic implications. Cancer Res. 2004;64:1475–1482. doi: 10.1158/0008-5472.can-03-3139. [DOI] [PubMed] [Google Scholar]
- Reriani M, Raichlin E, Prasad A, Mathew V, Pumper GM, Nelson RE, et al. Long-term administration of endothelin receptor antagonist improves coronary endothelial function in patients with early atherosclerosis. Circulation. 2010;122:958–966. doi: 10.1161/CIRCULATIONAHA.110.967406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey S, Corthorn J, Chacon C, Iturriaga R. Expression and immunolocalization of endothelin peptides and its receptors, ETA and ETB, in the carotid body exposed to chronic intermittent hypoxia. J Histochem Cytochem. 2007;55:167–174. doi: 10.1369/jhc.6A7079.2006. [DOI] [PubMed] [Google Scholar]
- Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112:2660–2667. doi: 10.1161/CIRCULATIONAHA.105.556746. [DOI] [PubMed] [Google Scholar]
- Saarelainen S, Seppala E, Laasonen K, Hasan J. Circulating endothelin-1 in obstructive sleep apnea. Endothelium. 1997;5:115–118. doi: 10.3109/10623329709079869. [DOI] [PubMed] [Google Scholar]
- Sakai S, Miyauchi T, Kobayashi M, Yamaguchi I, Goto K, Sugishita Y. Inhibition of myocardial endothelin pathway improves long-term survival in heart failure. Nature. 1996a;384:353–355. doi: 10.1038/384353a0. [DOI] [PubMed] [Google Scholar]
- Sakai S, Miyauchi T, Sakurai T, Kasuya Y, Ihara M, Yamaguchi I, et al. Endogenous endothelin-1 participates in the maintenance of cardiac function in rats with congestive heart failure. Marked increase in endothelin-1 production in the failing heart. Circulation. 1996b;93:1214–1222. doi: 10.1161/01.cir.93.6.1214. [DOI] [PubMed] [Google Scholar]
- Savransky V, Jun J, Li J, Nanayakkara A, Fonti S, Moser AB, et al. Dyslipidemia and atherosclerosis induced by chronic intermittent hypoxia are attenuated by deficiency of stearoyl coenzyme A desaturase. Circ Res. 2008;103:1173–1180. doi: 10.1161/CIRCRESAHA.108.178533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm A, Matusik P, Osmenda G, Guzik TJ. Targeting NADPH oxidases in vascular pharmacology. Vascul Pharmacol. 2012;56:216–231. doi: 10.1016/j.vph.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R, Murzabekova G, Egemnazarov B, Kraut S, Eisele HJ, Dumitrascu R, et al. Arterial hypertension in a murine model of sleep apnea: role of NADPH oxidase 2. J Hypertens. 2014;32:300–305. doi: 10.1097/HJH.0000000000000016. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda) 2009;24:97–106. doi: 10.1152/physiol.00045.2008. [DOI] [PubMed] [Google Scholar]
- Siti HN, Kamisah Y, Kamsiah J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review) Vascul Pharmacol. 2015;71:40–56. doi: 10.1016/j.vph.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Sivam S, Witting PK, Hoyos CM, Maw AM, Yee BJ, Grunstein RR, et al. Effects of 8 weeks of CPAP on lipid-based oxidative markers in obstructive sleep apnea: a randomized trial. J Sleep Res. 2015;24:339–345. doi: 10.1111/jsr.12271. [DOI] [PubMed] [Google Scholar]
- Snow JB, Gonzalez Bosc LV, Kanagy NL, Walker BR, Resta TC. Role for PKCbeta in enhanced endothelin-1-induced pulmonary vasoconstrictor reactivity following intermittent hypoxia. Am J Physiol Lung Cell Mol Physiol. 2011;301:L745–754. doi: 10.1152/ajplung.00020.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanke-Labesque F, Pepin JL, Gautier-Veyret E, Levy P, Back M. Leukotrienes as a molecular link between obstructive sleep apnoea and atherosclerosis. Cardiovasc Res. 2014;101:187–193. doi: 10.1093/cvr/cvt247. [DOI] [PubMed] [Google Scholar]
- Sutsch G, Kiowski W, Yan XW, Hunziker P, Christen S, Strobel W, et al. Short-term oral endothelin-receptor antagonist therapy in conventionally treated patients with symptomatic severe chronic heart failure. Circulation. 1998;98:2262–2268. doi: 10.1161/01.cir.98.21.2262. [DOI] [PubMed] [Google Scholar]
- Taguchi A, Yanagisawa K, Tanaka M, Cao K, Matsuyama Y, Goto H, et al. Identification of hypoxia-inducible factor-1 alpha as a novel target for miR-17–92 microRNA cluster. Cancer Res. 2008;68:5540–5545. doi: 10.1158/0008-5472.CAN-07-6460. [DOI] [PubMed] [Google Scholar]
- Tamisier R, Pepin JL, Remy J, Baguet JP, Taylor JA, Weiss JW, et al. 14 nights of intermittent hypoxia elevate daytime blood pressure and sympathetic activity in healthy humans. Eur Respir J. 2011;37:119–128. doi: 10.1183/09031936.00204209. [DOI] [PubMed] [Google Scholar]
- Tan DX, Manchester LC, Esteban-Zubero E, Zhou Z, Reiter RJ. Melatonin as a Potent and Inducible Endogenous Antioxidant: Synthesis and Metabolism. Molecules. 2015;20:18886–18906. doi: 10.3390/molecules201018886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CT, Kent BD, Crinion SJ, McNicholas WT, Ryan S. Human adipocytes are highly sensitive to intermittent hypoxia induced NF-kappaB activity and subsequent inflammatory gene expression. Biochem Biophys Res Commun. 2014;447:660–665. doi: 10.1016/j.bbrc.2014.04.062. [DOI] [PubMed] [Google Scholar]
- Thunstrom E, Manhem K, Rosengren A, Peker Y. Blood Pressure Response to Losartan and Continuous Positive Airway Pressure in Hypertension and Obstructive Sleep Apnea. Am J Respir Crit Care Med. 2016;193:310–320. doi: 10.1164/rccm.201505-0998OC. [DOI] [PubMed] [Google Scholar]
- Torres G, Sanchez-de-la-Torre M, Barbe F. Relationship Between OSA and Hypertension. Chest. 2015;148:824–832. doi: 10.1378/chest.15-0136. [DOI] [PubMed] [Google Scholar]
- Totoson P, Fhayli W, Faury G, Korichneva I, Cachot S, Baldazza M, et al. Atorvastatin protects against deleterious cardiovascular consequences induced by chronic intermittent hypoxia. Exp Biol Med (Maywood) 2013;238:223–232. doi: 10.1177/1535370212473696. [DOI] [PubMed] [Google Scholar]
- Troncoso Brindeiro CM, da Silva AQ, Allahdadi KJ, Youngblood V, Kanagy NL. Reactive oxygen species contribute to sleep apnea-induced hypertension in rats. Am J Physiol Heart Circ Physiol. 2007;293:H2971–2976. doi: 10.1152/ajpheart.00219.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzepizur W, Martinez MC, Priou P, Andriantsitohaina R, Gagnadoux F. Microparticles and vascular dysfunction in obstructive sleep apnoea. Eur Respir J. 2014;44:207–216. doi: 10.1183/09031936.00197413. [DOI] [PubMed] [Google Scholar]
- Verges S, Chacaroun S, Godin-Ribuot D, Baillieul S. Hypoxic Conditioning as a New Therapeutic Modality. Front Pediatr. 2015;3:58. doi: 10.3389/fped.2015.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriend J, Reiter RJ. Melatonin and the von Hippel-Lindau/HIF-1 oxygen sensing mechanism: A review. Biochim Biophys Acta. 2016;1865:176–183. doi: 10.1016/j.bbcan.2016.02.004. [DOI] [PubMed] [Google Scholar]
- Wang H, Parker JD, Newton GE, Floras JS, Mak S, Chiu KL, et al. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol. 2007;49:1625–1631. doi: 10.1016/j.jacc.2006.12.046. [DOI] [PubMed] [Google Scholar]
- Wang Z, Li AY, Guo QH, Zhang JP, An Q, Guo YJ, et al. Effects of cyclic intermittent hypoxia on ET-1 responsiveness and endothelial dysfunction of pulmonary arteries in rats. PLoS One. 2013;8:e58078. doi: 10.1371/journal.pone.0058078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZF, Kang J, Dai B. Expression and activation of hypoxia inducible factor-1alpha and iNOS in the brain of rats with chronic intermittent hypoxia. Zhonghua Jie He He Hu Xi Za Zhi. 2009;32:739–743. [PubMed] [Google Scholar]
- Williams AL, Chen L, Scharf SM. Effects of allopurinol on cardiac function and oxidant stress in chronic intermittent hypoxia. Sleep Breath. 2010;14:51–57. doi: 10.1007/s11325-009-0279-x. [DOI] [PubMed] [Google Scholar]
- Wu H, Zhou S, Kong L, Chen J, Feng W, Cai J, et al. Metallothionein deletion exacerbates intermittent hypoxia-induced renal injury in mice. Toxicol Lett. 2015;232:340–348. doi: 10.1016/j.toxlet.2014.11.015. [DOI] [PubMed] [Google Scholar]
- Xia Y, Choi HK, Lee K. Recent advances in hypoxia-inducible factor (HIF)-1 inhibitors. Eur J Med Chem. 2012;49:24–40. doi: 10.1016/j.ejmech.2012.01.033. [DOI] [PubMed] [Google Scholar]
- Xia Y, Jin Y, Kaur N, Choi Y, Lee K. HIF-1alpha inhibitors: synthesis and biological evaluation of novel moracin O and P analogues. Eur J Med Chem. 2011;46:2386–2396. doi: 10.1016/j.ejmech.2011.03.022. [DOI] [PubMed] [Google Scholar]
- Yang R, Sikka G, Larson J, Watts VL, Niu X, Ellis CL, et al. Restoring leptin signaling reduces hyperlipidemia and improves vascular stiffness induced by chronic intermittent hypoxia. Am J Physiol Heart Circ Physiol. 2011;300:H1467–1476. doi: 10.1152/ajpheart.00604.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung HM, Hung MW, Lau CF, Fung ML. Cardioprotective effects of melatonin against myocardial injuries induced by chronic intermittent hypoxia in rats. J Pineal Res. 2015;58:12–25. doi: 10.1111/jpi.12190. [DOI] [PubMed] [Google Scholar]
- Yorikane R, Sakai S, Miyauchi T, Sakurai T, Sugishita Y, Goto K. Increased production of endothelin-1 in the hypertrophied rat heart due to pressure overload. FEBS Lett. 1993;332:31–34. doi: 10.1016/0014-5793(93)80476-b. [DOI] [PubMed] [Google Scholar]
- Young T, Peppard P, Palta M, Hla KM, Finn L, Morgan B, et al. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157:1746–1752. [PubMed] [Google Scholar]
- Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- Yuan G, Nanduri J, Khan S, Semenza GL, Prabhakar NR. Induction of HIF-1alpha expression by intermittent hypoxia: involvement of NADPH oxidase, Ca2+ signaling, prolyl hydroxylases, and mTOR. J Cell Physiol. 2008;217:674–685. doi: 10.1002/jcp.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamarron C, Riveiro A, Gude F. Circulating levels of vascular endothelial markers in obstructive sleep apnoea syndrome. Effects of nasal continuous positive airway pressure. Arch Med Sci. 2011;7:1023–1028. doi: 10.5114/aoms.2011.26615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirlik S, Hildner KM, Targosz A, Neurath MF, Fuchs FS, Brzozowski T, et al. Melatonin and omentin: influence factors in the obstructive sleep apnoea syndrome? J Physiol Pharmacol. 2013;64:353–360. [PubMed] [Google Scholar]
- Zolk O, Quattek J, Sitzler G, Schrader T, Nickenig G, Schnabel P, et al. Expression of endothelin-1, endothelin-converting enzyme, and endothelin receptors in chronic heart failure. Circulation. 1999;99:2118–2123. doi: 10.1161/01.cir.99.16.2118. [DOI] [PubMed] [Google Scholar]