Abstract
Helicobacter pylori has been identified as one of the major causes of chronic gastritis, gastric and duodenal ulcers, and gastric cancer. Lipopolysaccharide (LPS) is a major component of the outer membrane of gram-negative bacteria, and H. pylori LPS might play an exclusively important role in activating inflammatory pathways in monocytes and macrophages. To study the role of LPS in the underlying mechanism of inflammatory responses, we established an in vitro model using the human AGS gastric cancer cell line. We found that LPS mediates inflammation through setting off a cascade of events: activation of the store-operated calcium (SOC) channel, initiation of downstream NF-κB signaling, and phosphorylation of extracellular signal-regulated kinase 1/2 (ERK1/2). Phosphorylated ERK1/2 promotes the nuclear translocation of NF-κB, and eventually elevates the expression level of COX-2, a major inflammatory gene.
Introduction
Gastric cancer (GC) is one of the most common cancers in the world and ranks second in overall cancer-related deaths1,2. Several major factors are known to increase the risk of developing GC, such as infection by Helicobacter pylori and Epstein-Barr virus, tobacco use, diet, lifestyle, and obesity. Interestingly, inflammatory responses are a common underlying mechanism shared by many of the above-mentioned risk factors3,4. The incidence of GC, which can span several decades, is characterized by its slow, gradual evolution. In chronological order, it gradually develops from superficial gastritis to glandular atrophy, intestinal metaplasia, dysplasia, and finally, adenocarcinoma5. Mounting scientific evidence led to the classification of H. pylori as a group I carcinogen for GC by the International Agency for Research on Cancer. Increasing numbers of epidemiological and animal studies have shown the causal relationship between H. pylori infection and GC4–7. It is well-established that H. pylori causes infection-initiated chronic gastritis, and has been thoroughly characterized by its various inflammation-triggering cellular components, including flagella; lipopolysaccharide (LPS); vacuolating cytotoxin (VacA); cytotoxin-associated gene pathogenicity islands (cagPAIs); the effector protein, CagA; peptidoglycan; glutamyl transpeptidase (GGT); protease HtrA; adhesins BabA and SabA; and others4,8–10.
LPS, known as endotoxin, displays robust immunostimulatory abilities upon recognition by toll-like receptor 4/MD-211. LPS is composed of a glycolipid terminal structure termed the lipid A-core, which is mainly responsible for the endotoxicity of LPS, and an O-antigen polysaccharide12. Cytokine induction assay performed by other research groups suggested that the structures of H. pylori LPS and lipid A can modulate immune responses during infection, and both play roles in chronic inflammatory responses13–16.
There are two common isoforms of cyclooxygenase (COX), namely COX-1 and COX-2, and COX-1 is known as the constitutively expressed isoform. In humans, COX-1 and prostaglandin synthesis are indispensable to some essential physiological processes, such as stomach mucosa maintenance, platelet function, blood vessel protection, and regulation of renal blood flow pressure17–20. COX-2 is an inducible enzyme that plays a key role in the synthesis of prostaglandins in response to inflammatory stimuli. In addition, COX-2 gene expression was also found to respond to other stimuli, including growth factors, endotoxin, carcinogen, hormones, and chemokines21. According to previous studies on various types of cancer, such as esophageal cancer, GC, colorectal cancer, etc., overexpression of COX-2 in cancerous tissue was observed22–24. Collective studies also revealed the correlation between COX-2 overexpression and decreased survival rates in cancer patients, and some have described the association of prostaglandin, a downstream product of COX-2, with tumor angiogenesis25,26. In this paper, we report on interactions and the hierarchy of elements involved in COX-2 gene activation in AGS cells. Our results substantiate that the store-operated calcium (SOC) channel, extracellular signal-regulated kinase (ERK), and nuclear factor kappa B (NF-κB) are necessary mediators of LPS-induced COX-2 gene expression in GC.
Results
Analysis of LPS-responsive regions in the COX-2 promoter
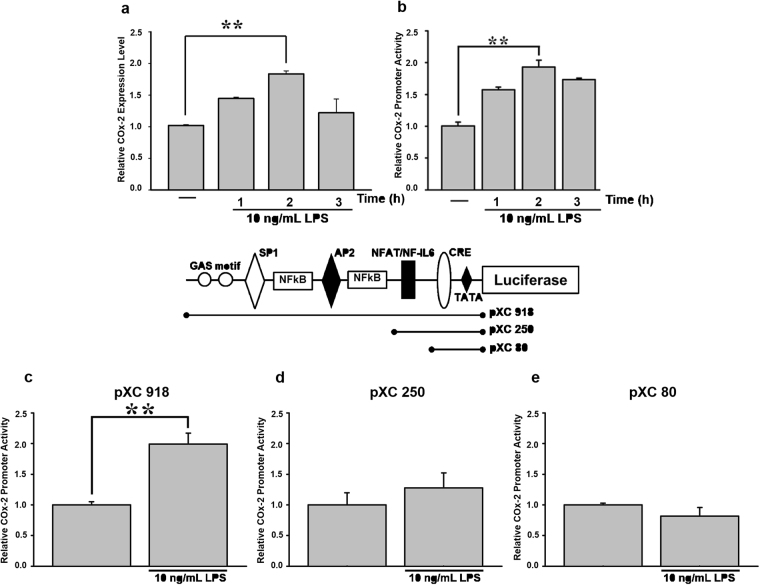
To determine the role of LPS in COX-2 gene regulation, AGS cells were treated with 10 ng/mL LPS. COX-2 gene activity peaked at 2 h post-treatment in both the real-time PCR and luciferase reporter assay (Fig. 1a,b). A COX-2 promoter-driven luciferase reporter plasmid, pXC918, was used in the reporter assay. pXC918, pXC250, and pXC80, plasmids containing different fragments of the COX-2 promoter (respectively −918, −250, −80 bp upstream of the COX-2 gene), were used to locate potential responsive elements in the COX-2 promoter. A two-fold increase in promoter activity of pXC918 (which contained Sp1, AP2, NF-κB, NFAT-binding sites, and the cyclic AMP response element (CRE) motif27 was recorded after transfected AGS cells were incubated with 10 ng/mL LPS (Fig. 1c). However, pXC250 (which contained the NFAT-binding site and CRE) and pXC80 (which contained only the CRE) did not show a similar increase in promoter activity (Fig. 1d,e).
Figure 1.
Analysis of LPS-responsive regions in the promoter area of the COX-2 gene. (a) Cells were treated with or without 10 ng/mL LPS for 1, 2, and 3 h. Total RNA was extracted from AGS cells to quantify COX-2 gene expression by real-time PCR. (b) Cells were transfected with 0.5 μg of a COX-2 promoter-driven luciferase reporter construct (pXC 918) for 24 h, and then treated with or without 10 ng/mL LPS for 1, 2, and 3 h. COX-2 promoter activity was measured with luciferase assay. Various lengths of the COX-2 promoter of (c) pXC 918 (full length), (d) pXC 250, and (e) pXC 80 were respectively transfected into AGS cells. After 24 h, cells were incubated with 10 ng/mL LPS for 2 h. Promoter activity was measured with luciferase assay. Values for luciferase activity are presented as the mean ± SEM. Statistical significance (**p < 0.01) of the difference between control and LPS-treated cells was determined by Student’s t-test.
Inhibition of LPS-induced NF-κB activation attenuates COX-2 expression
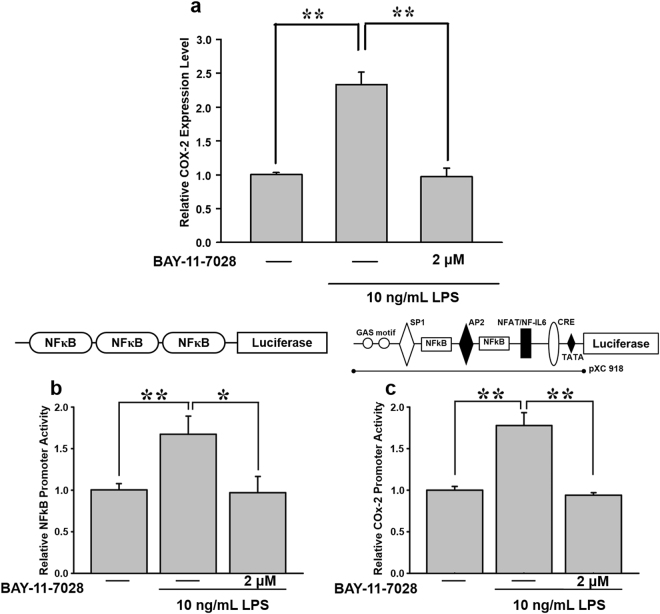
NF-κB is a transcription factor well known for regulating inflammatory and immune reactions. To determine the role of NF-κB in LPS-induced COX-2 expression, the NF-κB inhibitor, BAY 11-7082 (2 μM), was used. As shown in Fig. 2a, BAY 11-7082 suppressed LPS-induced COX-2 messenger (m)RNA expression. Furthermore, the luciferase assay showed that pretreatment with BAY 11-7082 abolished both NF-κB reporter activity (Fig. 2b) and COX-2 promoter activity (Fig. 2c). These results imply a role of NF-κB in LPS-mediated COX-2 expression in AGS cells.
Figure 2.
Effects of BAY 11-7082, a NF-κB inhibitor, on LPS-mediated COX-2 expression in AGS cells. (a) Cells were pre-treated with 2 μM BAY 11-7082 for 30 min, and then stimulated with 10 ng/mL LPS for 2 h. Total RNA was extracted from AGS cells for COX-2 gene detection using real-time PCR. To further investigate the effect of BAY 11-7082 on LPS-mediated COX-2, cells were transiently transfected with 0.5 μg of (b) a luciferase reporter construct with the 3X NF-κB binding motif and (c) a COX-2 promoter (pXC918). After 24 h, cells were pre-incubated with 2 μM BAY 11-7082 for 30 min, followed by 10 ng/mL LPS for 2 h. Statistical significance (*p < 0.05, **p < 0.01) of the difference between control and LPS-treated cells was determined by Student’s t-test.
LPS promotes ERK1/2 phosphorylation in a time-dependent manner
ERK phosphorylation is crucial for the activation and nuclear translocation of NF-κB. Thus, we investigated the connection between LPS and ERK1/2, and its effect on COX-2 expression. Immunoblot results showed that pERK1/2 significantly increased in a time-dependent manner when cells were treated with LPS (Fig. 3a). Next, to clarify the connection between LPS-induced ERK1/2 phosphorylation and COX-2 expression, the COX-2 promoter-driven luciferase reporter, pXC918, was transfected into cells. At 24 h post-transfection, cells were incubated with 10 ng/mL LPS for 2 h, and 10 μM PD98059, a pERK1/2 inhibitor, for 30 min. We found that PD98059 effectively abolished LPS-induced COX-2 promoter activity down to the basal level (Fig. 3b).
Figure 3.
Effects of PD98059, a pERK1/2 inhibitor, on LPS-mediated COX-2 expression in AGS cells. (a) Cells were treated with and without 10 ng/mL LPS for 15, 30, and 60 min. Cell lysates were harvested for a pERK1/2 expression analysis. To further determine the effect of PD98059 on LPS-medicated COX-2, cells were transiently transfected with 0.5 μg of (b) a COX-2 promoter reporter construct (pXC918) and (c) 3X NF-κB reporter construct for 24 h. Cells were incubated with 10 μM PD98059 for 30 min, and stimulated with 10 ng/mL LPS for 2 h. Promoter activity was measured with a luciferase assay. Statistical significance (**p < 0.01) of the difference between control and LPS-treated cells was determined by Student’s t-test.
Previous studies suggested that ERK phosphorylation is responsible for the increase in NF-κB activity28. To further confirm whether LPS-induced ERK phosphorylation mediates downstream NF-κB activity, AGS cells transfected with the NF-κB luciferase reporter were pretreated with the pERK1/2 inhibitor, PD98059 (10 μM), for 30 min, followed by 10 ng/mL LPS for 2 h. The luciferase assay demonstrated that PD98059 inhibition coincided with reduced NF-κB activity, and thus confirmed the relation between ERK and NF-κB, and their relative positions in the signaling cascade (Fig. 3c).
Effects of SOC channels inhibitor in LPS-induced COX-2 gene expression
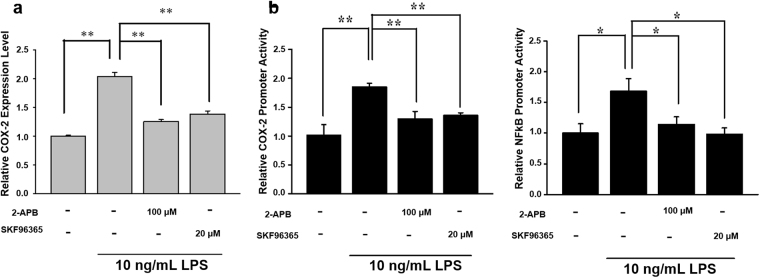
Our previous study showed that reduced SOC influx attenuates lung cancer cell proliferation via ERK phosphorylation29. Studies by other groups also suggested that the SOC channel and cytosolic calcium are correlated with ERK1/2 phosphorylation30,31. In addition, activation of NF-κB is reported to be directly associated with the cytosolic calcium level and calcium-related signaling under various circumstances32–34. Hence, we investigated whether the SOC channel plays a role in ERK phosphorylation and NF-κB translocation, and eventually leads to an increase in COX-2 gene expression. AGS cells were pretreated with the SOC channel inhibitor, 2-APB (100 μM) or SKF96365 (20 μM), followed by 10 ng/mL LPS stimulation for 2 h. A real-time PCR was used to quantify COX-2 expression, and results showed that SOC channel inhibition abolished LPS-induced COX-2 gene expression (Fig. 4a). Moreover, SOC channel inhibitors also demonstrated the ability to suppress both the COX-2 promoter and NF-κB reporter activity (Fig. 4b). These results support the fact that the SOC channel is an upstream regulator of NF-κB in LPS-induced COX-2 expression.
Figure 4.
Effects of store-operated Ca2+ (SOC) channel inhibitors on LPS-induced COX-2 gene expression. (a) Cells were pretreated with SOC channel inhibitors, i.e., 100 μM 2-APB and 20 μM SKF96365 for 30 min, followed by 10 ng/mL LPS for 2 h. Total RNA was extracted from AGS cells to quantify COX-2 gene expression using a real-time PCR. (b) A COX-2 promoter reporter construct (pXC918) and NF-κB reporter were transfected into cells. Transfected cells were treated with 100 μM 2-APB or 20 μM SKF96365, followed by 10 ng/mL LPS. Luciferase activity was measured and represented in fold change of promoter activity and was normalized to basal activity. Statistical significance (*p < 0.05, **p < 0.01) of the difference was determined by Student’s t-test.
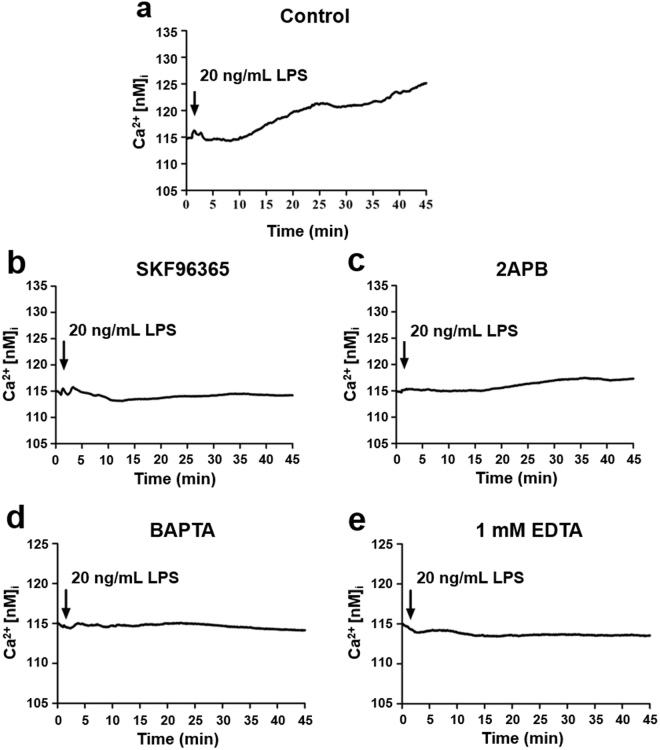
Blockade of LPS-induced calcium influx by SOC channel blockers and calcium chelators
AGS cells were respectively treated with 20 μM SKF96365, 100 μM 2APB, 5 μM BAPTA, and 1 mM EDTA, followed by 20 ng/mL LPS (Fig. 5a). The intracellular calcium concentration was estimated by measuring the Fluo-4 signal. LPS-induced SOC entry (SOCE) was effectively blocked by SOC channel-specific inhibitors (Fig. 5b,c), as well as calcium chelators (Fig. 5d,e). These results suggest that LPS triggers the SOCE, and causes downstream signal transduction.
Figure 5.
Blockade of LPS-induced calcium influx by store-operated calcium (SOC) channel inhibitors and calcium chelators. Cellular calcium influx of AGS cells treated without (a) or with 20 μM SKF96365 (b), 100 μM 2APB (c), 5 μM BAPTA (d), or 1 mM EDTA (e) prior to subsequent intracellular calcium imaging was induced by 20 ng/mL LPS in 2 mM calcium buffer and detected by fluorescence microscopy for up to 45 min. The intracellular calcium concentration was estimated by measuring the signal of Fluo-4, a calcium indicator, inside AGS cells.
Identification of the role of SOC channel in LPS-induced COX-2 gene expression
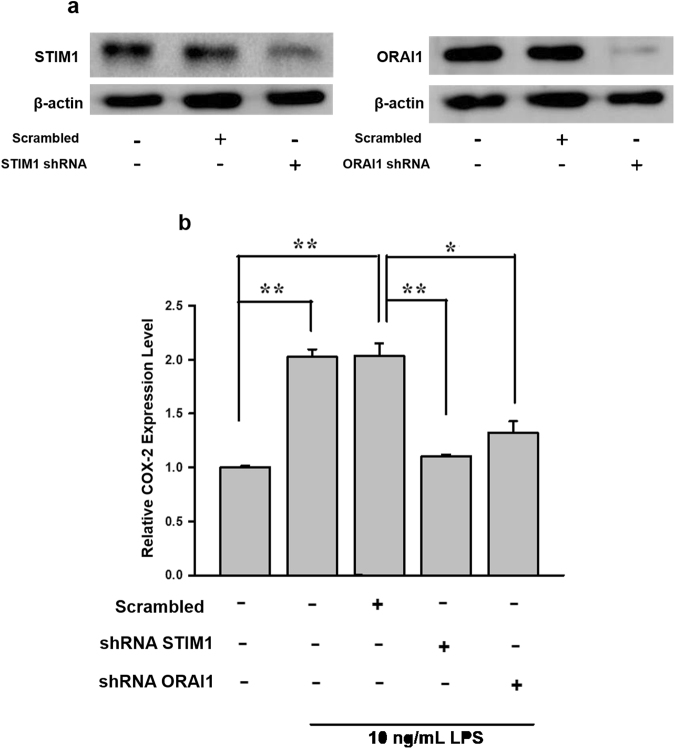
To validate the effects of SOC channel inhibitors (2-APB and SKF96365), AGS cells were transfected with STIM1 or ORAI1 short hairpin (sh)RNA to observe the effect of knockdown of the SOC channel on LPS-induced COX-2 gene expression. As shown in Fig. 6a, 2 µg STIM1 shRNA or ORAI1 shRNA was used, and the respective gene was significantly suppressed. Furthermore, we found that silencing of either STIM1 or ORAI1 significantly impaired LPS-induced COX-2 gene expression (Fig. 6b). The data sufficiently demonstrated that calcium influx via STIM1/ORAI1-mediated SOCE is crucial for LPS-induced COX-2 expression.
Figure 6.
The roles of STIM1 and ORAI1 in LPS-induced COX-2 gene expression. STIM1 shRNA and ORAI1 shRNA were respectively transfected into AGS cells using Lipofectamine. (a) Immunoblot of STIM1 (80 kDa) and ORAI1 (31 kDa) after knockdown of the respective gene. 2 μg shRNA was used for transfection, and the total cell lysate was harvested for immunoblotting at 24 h post-transfection. (b) Cells were treated either with or without LPS after knockdown of STIM1 or ORAI1. RNA was extracted for COX-2 mRNA quantification by a real-time PCR. Statistical significance (*p < 0.05, **p < 0.01) of the difference was determined by Student’s t-test.
Discussion
Helicobacter pylori, a gram-negative spirochete, infects more than half of the world’s population35. Typically, H. pylori infections are more widespread in developing countries, which are often plagued by water pollution and unsanitary living conditions. After decades of rigorous study and data accumulation, many researchers support the notion that H. pylori infection is the trigger of intestinal gastric adenocarcinoma2,36. Through a variety of mechanisms, H. pylori infection can amount to detrimental changes in gastric epithelial cells, and eventually the occurrence of cancer4,37,38. The pathogenicity of H. pylori is mainly associated with various bacterial cell components, including flagella, vacuolating toxin (VacA), cytotoxin-associated gene pathogenicity islands (cagPAIs), and lipopolysaccharide (LPS)10. Moreover, reports suggest that LPS from H. pylori increases the paracellular permeability of gastric cells, which can compromise the stomach’s mucosal defense and increase susceptibility to developing medical conditions39,40. LPS was also shown to upregulate vascular endothelial growth factor (VEGF) and COX-2, which contributed to failure of ulcer healing in a rat stomach ulcer model41.
COX-2 is known to promote tumorigenesis through prostanoid biosynthesis, which includes prostaglandin E2 (PGE2). Inhibition of COX-2 and PGE2 receptor signaling leads to suppression of tumor development in a variety of animal models42,43. Our results are consistent with a previous study by Franchi et al., which showed significantly elevated COX-2 and PGE2 levels in the lungs and stomach of LPS-treated rats compared to controls44. The COX-2 gene promoter contains a considerable number of transcription factor-binding motifs. Several studies established that activator protein 145, CCAAT/enhancer-binding protein β46, cyclic AMP-responsive element-binding protein47, and NF-κB48 are transcriptional regulators of COX-2 49–51. Our results suggest that the LPS-responsive element in the COX-2 promoter is located in the -918 to -250 region, which includes AP-2, SP-1, and two NF-κB-binding motifs (Fig. 1). The transcription factor, NF-κB, is one of the primary mediators of immune and inflammatory responses, in addition to its roles in critical cellular processes during carcinogenesis, such as transformation, proliferation, angiogenesis, and metastasis52. NF-κB activation was shown to be modulated by numerous proinflammatory stimuli, ranging from membrane toll-like receptor (TLR) activation by pathogens to specific cytokines (paracrine or endocrine), through both canonical and non-canonical pathways53. Due to its roles in inflammation and immunity, NF-κB signaling and its activation by H. pylori have piqued the interest of many researchers.
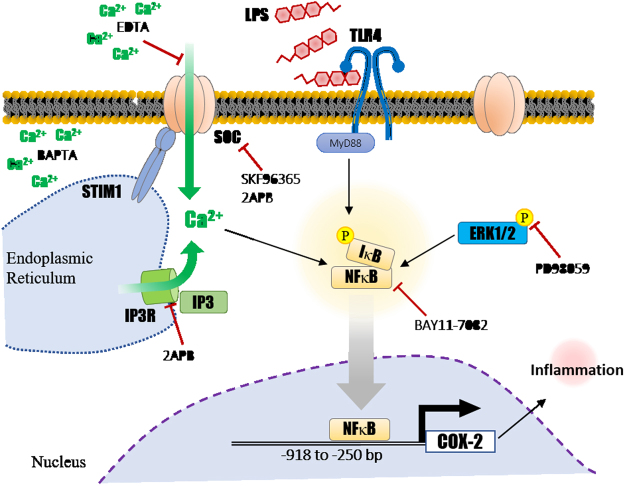
Our work demonstrates that LPS might rely on the activation of SOC channel and calcium influx to promote the phosphorylation of ERK, and subsequently, the nuclear translocation of NF-κB. Upon nuclear entry, NF-κB acts as a transcription factor, and drives COX-2 expression. A schematic representation of our proposed LPS-induced COX-2 activation is depicted in Fig. 7. It is well-established that SOCE is essential for T cell maturation, mast cell degranulation, and many other calcium-dependent cellular processes. Our recent study revealed a role for SOCE in cancer invasion and metastasis in colorectal cancer patients, where relatively high expression levels of the calcium storage sensor, STIM1, were observed in colorectal cancer tissues51. Furthermore, STIM1 expression dynamics were positively correlated with increased malignancy54. In the lung cancer cells, previous studies also indicated that store-operated calcium entry is involved in COX-2 gene activation as well as cell cycle progression29,49 We suspect that after the cell’s initial exposure to LPS, inositol 1,4,5-trisphosphate receptor (IP3R) was activated by IP3, and quickly depleted ER calcium store. Upon sensing the depletion, STIM1 subsequently binds to ORAI1 and activates calcium intake via membrane-bound SOC channels. This study implies yet another role for SOCE in LPS-induced COX-2 expression, as well as the inflammatory response in GC cells.
Figure 7.
Schematic representation showing a preliminary model of LPS-induced COX-2 gene activation in AGS cells.
The in situ expression and biological functions of COX isoforms in various tissues are well characterized. Studies suggest that the expression and function of the COX gene may vary under different circumstances. For example, COX-2 is constitutively expressed in the spine, instead of the usual COX-1 isoform, and is responsible for hyperalgesia during injury55. Thus, it is important to recognize that the effects of LPS and the signaling pathway described in this study are probably highly contextual and tissue specific. Despite the rather clear outline, our findings have yet to completely rule out participation by other alternative pathways in LPS-induced COX-2 expression, especially since COX-2 is noted for responding to multiple stimuli. Although COX-2 is more commonly known for its role in pro-inflammatory contexts, its dual regulatory roles in production of pro-inflammatory prostaglandins and anti-inflammatory prostaglandins need to be further addressed when studying inflammation-related conditions, such as gastritis in our case56. Hence, profiling relevant types of prostaglandins synthesized in gastric cells at each stage of inflammation can be crucial for the understanding of induced COX-2 at protein level and subsequent downstream events. Hopefully, knowledge gained from a better understanding of the underlying molecular network of LPS-induced COX-2 expression can potentially serve as the basis of novel therapeutic approaches for gastritis and GC in the future.
Materials and Methods
Cell culture
Human stomach adenocarcinoma AGS cells were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; Invitrogen) and 1% penicillin-streptomycin (Invitrogen), and cultured at 37 °C in a 5% CO2 atmosphere. In these series of experiments, cells were treated with 10 ng/mL LPS in DMEM.
RNA isolation and a quantitative real-time reverse-transcription polymerase chain reaction (RT-qPCR)
Total RNA was isolated from AGS cells with Trizol reagent (Invitrogen). Complementary (c)DNA was synthesized from 1 μg of total RNA using reverse transcription kit (Invitrogen) according to manufacturer’s instructions. cDNA was diluted 1:30 with PCR-grade water and then stored at −20 °C. Gene expression levels were quantified with the Applied Biosystems StepOnePlus™ System (Thermo Fisher Scientific) with pre-optimized conditions. Each PCR was performed in triplicate using 5 μL of 2x SYBR Green PCR Master Mix, 0.2 μL of primer sets, 1 μL cDNA, and 3.6 μL nucleotide-free H2O to yield 10 μL per reaction. Expression rates were calculated as the normalized CT difference between the control and sample after adjusting for the amplification efficiency relative to the expression level of the housekeeping gene, β-actin. COX-2 primers (sense, CCCTTGGGTGTCAAAGGTAA, and antisense, GCCCTCGCTTATGATCTGTC) and β-actin primers (sense, ATCTCCTTCTGCATCCTGTCGGCAAT, and antisense, CATGGAGTCCTGGCATCCACGAAAC) were used in the PCR.
Cell transfection and luciferase assay
Lipofectamine 2000 (Invitrogen) and Opti-MEM medium (Invitrogen) were used to deliver the plasmid into AGS cells. The transfection procedure was performed according to instructions of the manufacturer. Cells were briefly treated with LPS after overnight transfection, and then lysed with cell lysis buffer. A dual-luciferase reporter assay kit (Promega, Madison, WI, USA) was used to measure the luciferase activity of the NF-κB reporter and COX-2 promoter. STIM1 and ORAI1 shRNA constructs were obtained from RNAi core of Academia Sinica, Taipei, Taiwan.
Intracellular calcium imaging
AGS cells grown in 10%-FBS DMEM were trypsinized, seeded onto 20-mm glass coverslips in a 6-well plate, and incubated for 24~48 h at 37 °C. Cells were washed with a 2 mM calcium solution and incubated with 1 μM Fluo-4 AM (Molecular Probes, Eugene, OR, USA) plus treatment with or without calcium channel blockers or calcium chelators for 30 min at 37 °C prior to intracellular calcium detection. Following treatment and staining steps, the calcium influx of AGS cells was assessed by measuring the fluorescent intensity of the intracellular calcium signal as described in our previous study [PMID: 23774942]. Cells were maintained in 2 mM calcium buffer and stimulated by 20 ng/mL LPS for up to 45 min.
Western blotting
Protein samples (60 μg) were heated to 95 °C for 5 min and loaded onto 10% sodium dodecyl polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were transferred to polyvinylidene difluoride (PVDF) membranes and blocked with 5% nonfat dry milk for 1 h at room temperature. Membranes were washed with 0.1% PBST (phosphate-buffered saline (PBS) and Tween20) three times and then incubated with primary antibodies overnight at 4 °C. Antibodies against pERK1/2 (Cell Signaling, Beverly, MA), STIM1 (Cell Signaling, Beverly, MA), and ORAI1 (GeneTex, Hsinchu, Taiwan) were diluted 1: 2000, whereas the antibody against β-actin was diluted 1: 10000. Membranes were then washed with 0.1% PBST three times and incubated with a 1: 5000 dilution of anti-mouse or anti-rabbit HRP-conjugated immunoglobulin G (IgG, Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 1 h at room temperature. After washing with 0.1% PBST three times, signals were detected by an ECL-plus Western blotting detection system (Millipore).
Data analysis
Statistical analyses were performed using Student’s t-test. A p value of < 0.05 was considered significant and is denoted by an asterisk (*), while a p value of < 0.01 is denoted by **.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Electronic supplementary material
Acknowledgements
This research was supported by grants from the Excellence for Cancer Research Center Grant through funding by the Ministry of Science and Technology (MOST105-2325-B-037-001) and the Ministry of Health and Welfare (MOHW106-TDU-B-212-144007), Health and welfare surcharge of tobacco products, Taiwan, Republic of China as well as grants from Kaohsiung Medical University Hospital (KMUH105-5R26, KMUHS10601, KMUHS10608, KMUHA10664), and the Grant of Biosignature in Colorectal Cancers, Academia Sinica, Taiwan.
Author Contributions
J.H. Wong, K.H. Ho and Hsu W.L.: designed and conducted the experiments, data analysis; S. Nam: manuscript writing and revision; C.H. Lin, and C.M. Chang: data analysis. J.Y. Wang and W.C. Chang: designed experiments, data analysis and manuscript writing. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Jhen-Hong Wong, Kuo-Hao Ho and Sean Nam contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-12648-1.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jaw-Yuan Wang, Email: cy614112@ms14.hinet.net.
Wei-Chiao Chang, Email: wcc@tmu.edu.tw.
References
- 1.Amaro HM, Barros R, Guedes AC, Sousa-Pinto I, Malcata FX. Microalgal compounds modulate carcinogenesis in the gastrointestinal tract. Trends Biotechnol. 2013;31:92–8. doi: 10.1016/j.tibtech.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Hu Y, Fang JY, Xiao SD. Can the incidence of gastric cancer be reduced in the new century? J Dig Dis. 2013;14:11–5. doi: 10.1111/j.1751-2980.2012.00647.x. [DOI] [PubMed] [Google Scholar]
- 3.Aggarwal BB, Gehlot P. Inflammation and cancer: how friendly is the relationship for cancer patients? Curr Opin Pharmacol. 2009;9:351–69. doi: 10.1016/j.coph.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lamb A, Chen LF. Role of the Helicobacter pylori-induced inflammatory response in the development of gastric cancer. J Cell Biochem. 2013;114:491–7. doi: 10.1002/jcb.24389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luigiano C, et al. Is Helicobacter pylori the infectious target to prevent gastric cancer? An interdisciplinary point of view. Infect Disord Drug Targets. 2012;12:340–5. doi: 10.2174/187152612804142206. [DOI] [PubMed] [Google Scholar]
- 6.Bhandari A, Crowe SE. Helicobacter pylori in gastric malignancies. Curr Gastroenterol Rep. 2012;14:489–96. doi: 10.1007/s11894-012-0296-y. [DOI] [PubMed] [Google Scholar]
- 7.Kusters JG, van Vliet AHM, Kuipers EJ. Pathogenesis of Helicobacter pylori Infection. Clinical Microbiology Reviews. 2006;19:449–490. doi: 10.1128/CMR.00054-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Backert S, Clyne M. Pathogenesis of Helicobacter pylori infection. Helicobacter. 2011;16(Suppl 1):19–25. doi: 10.1111/j.1523-5378.2011.00876.x. [DOI] [PubMed] [Google Scholar]
- 9.Kim JM, et al. Stimulation of dendritic cells with Helicobacter pylori vacuolating cytotoxin negatively regulates their maturation via the restoration of E2F1. Clin Exp Immunol. 2011;166:34–45. doi: 10.1111/j.1365-2249.2011.04447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamaoka Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nat Rev Gastroenterol Hepatol. 2010;7:629–41. doi: 10.1038/nrgastro.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park BS, et al. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458:1191–5. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- 12.Mally M, et al. Glycoengineering of host mimicking type-2 LacNAc polymers and Lewis X antigens on bacterial cell surfaces. Mol Microbiol. 2013;87:112–31. doi: 10.1111/mmi.12086. [DOI] [PubMed] [Google Scholar]
- 13.Fujimoto Y, Shimoyama A, Suda Y, Fukase K. Synthesis and immunomodulatory activities of Helicobacter pylori lipophilic terminus of lipopolysaccharide including lipid A. Carbohydr Res. 2012;356:37–43. doi: 10.1016/j.carres.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 14.Hynes SO, et al. Comparative chemical and biological characterization of the lipopolysaccharides of gastric and enterohepatic helicobacters. Helicobacter. 2004;9:313–23. doi: 10.1111/j.1083-4389.2004.00237.x. [DOI] [PubMed] [Google Scholar]
- 15.Nielsen H, Birkholz S, Andersen LP, Moran AP. Neutrophil activation by Helicobacter pylori lipopolysaccharides. J Infect Dis. 1994;170:135–9. doi: 10.1093/infdis/170.1.135. [DOI] [PubMed] [Google Scholar]
- 16.Perez-Perez GI, Shepherd VL, Morrow JD, Blaser MJ. Activation of human THP-1 cells and rat bone marrow-derived macrophages by Helicobacter pylori lipopolysaccharide. Infect Immun. 1995;63:1183–7. doi: 10.1128/iai.63.4.1183-1187.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baggiolini M, Clark-Lewis I. Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett. 1992;307:97–101. doi: 10.1016/0014-5793(92)80909-Z. [DOI] [PubMed] [Google Scholar]
- 18.Emmons PR, Hampton JR, Harrison MJ, Honour AJ, Mitchell JR. Effect of prostaglandin E1 on platelet behaviour in vitro and in vivo. Br Med J. 1967;2:468–72. doi: 10.1136/bmj.2.5550.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lonigro AJ, Itskovitz HD, Crowshaw K, McGiff JC. Dependency of renal blood flow on prostaglandin synthesis in the dog. Circ Res. 1973;32:712–7. doi: 10.1161/01.RES.32.6.712. [DOI] [PubMed] [Google Scholar]
- 20.Miller TA. Protective effects of prostaglandins against gastric mucosal damage: current knowledge and proposed mechanisms. Am J Physiol. 1983;245:G601–23. doi: 10.1152/ajpgi.1983.245.5.G601. [DOI] [PubMed] [Google Scholar]
- 21.Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev. 2004;56:387–437. doi: 10.1124/pr.56.3.3. [DOI] [PubMed] [Google Scholar]
- 22.Dannenberg AJ, Altorki NK, Boyle JO, Lin DT, Subbaramaiah K. Inhibition of cyclooxygenase-2: an approach to preventing cancer of the upper aerodigestive tract. Ann N Y Acad Sci. 2001;952:109–15. doi: 10.1111/j.1749-6632.2001.tb02731.x. [DOI] [PubMed] [Google Scholar]
- 23.Koki AT, et al. Characterization of cyclooxygenase-2 (COX-2) during tumorigenesis in human epithelial cancers: evidence for potential clinical utility of COX-2 inhibitors in epithelial cancers. Prostaglandins Leukot Essent Fatty Acids. 2002;66:13–8. doi: 10.1054/plef.2001.0335. [DOI] [PubMed] [Google Scholar]
- 24.Subbaramaiah K, Dannenberg AJ. Cyclooxygenase 2: a molecular target for cancer prevention and treatment. Trends Pharmacol Sci. 2003;24:96–102. doi: 10.1016/S0165-6147(02)00043-3. [DOI] [PubMed] [Google Scholar]
- 25.Soler M, Camacho M, Escudero JR, Iniguez MA, Vila L. Human vascular smooth muscle cells but not endothelial cells express prostaglandin E synthase. Circ Res. 2000;87:504–7. doi: 10.1161/01.RES.87.6.504. [DOI] [PubMed] [Google Scholar]
- 26.Wang D, Dubois RN. The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene. 2010;29:781–8. doi: 10.1038/onc.2009.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Appleby SB, Ristimaki A, Neilson K, Narko K, Hla T. Structure of the human cyclo-oxygenase-2 gene. Biochem J. 1994;302(Pt 3):723–7. doi: 10.1042/bj3020723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zenali MJ, Zhang PL, Bendel AE, Brown RE. Morphoproteomic confirmation of constitutively activated mTOR, ERK, and NF-kappaB pathways in Ewing family of tumors. Ann Clin Lab Sci. 2009;39:160–6. [PubMed] [Google Scholar]
- 29.Hou MF, et al. Orai1/CRACM1 overexpression suppresses cell proliferation via attenuation of the store-operated calcium influx-mediated signalling pathway in A549 lung cancer cells. Biochim Biophys Acta. 2011;1810:1278–84. doi: 10.1016/j.bbagen.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Gomez-Fernandez C, Lopez-Guerrero AM, Pozo-Guisado E, Alvarez IS, Martin-Romero FJ. Calcium signaling in mouse oocyte maturation: the roles of STIM1, ORAI1 and SOCE. Mol Hum Reprod. 2012;18:194–203. doi: 10.1093/molehr/gar071. [DOI] [PubMed] [Google Scholar]
- 31.Voelkers M, et al. Orai1 and Stim1 regulate normal and hypertrophic growth in cardiomyocytes. J Mol Cell Cardiol. 2010;48:1329–34. doi: 10.1016/j.yjmcc.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–8. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- 33.Palkowitsch L, et al. The Ca2+ -dependent phosphatase calcineurin controls the formation of the Carma1-Bcl10-Malt1 complex during T cell receptor-induced NF-kappaB activation. J Biol Chem. 2011;286:7522–34. doi: 10.1074/jbc.M110.155895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun L, Carpenter G. Epidermal growth factor activation of NF-kappaB is mediated through IkappaBalpha degradation and intracellular free calcium. Oncogene. 1998;16:2095–102. doi: 10.1038/sj.onc.1201731. [DOI] [PubMed] [Google Scholar]
- 35.Moen EL, et al. Regulation of RKIP function by Helicobacter pylori in gastric cancer. PLoS One. 2012;7:e37819. doi: 10.1371/journal.pone.0037819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grebowska A, et al. Helicobacter pylori lipopolysaccharide activity in human peripheral blood mononuclear leukocyte cultures. J Physiol Pharmacol. 2010;61:437–42. [PubMed] [Google Scholar]
- 37.Chuah SK, Tsay FW, Hsu PI, Wu DC. A new look at anti-Helicobacter pylori therapy. World J Gastroenterol. 2011;17:3971–5. doi: 10.3748/wjg.v17.i35.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peek RM, Jr, Crabtree JE. Helicobacter infection and gastric neoplasia. J Pathol. 2006;208:233–48. doi: 10.1002/path.1868. [DOI] [PubMed] [Google Scholar]
- 39.deFoneska A, Kaunitz JD. Gastroduodenal mucosal defense. Curr Opin Gastroenterol. 2010;26:604–10. doi: 10.1097/MOG.0b013e32833f1222. [DOI] [PubMed] [Google Scholar]
- 40.Hanson PJ, Moran AP, Butler K. Paracellular permeability is increased by basal lipopolysaccharide in a primary culture of colonic epithelial cells; an effect prevented by an activator of Toll-like receptor-2. Innate Immun. 2011;17:269–82. doi: 10.1177/1753425910367813. [DOI] [PubMed] [Google Scholar]
- 41.Konturek PC, et al. Influence of bacterial lipopolysaccharide on healing of chronic experimental ulcer in rat. Scand J Gastroenterol. 2001;36:1239–47. doi: 10.1080/003655201750074456. [DOI] [PubMed] [Google Scholar]
- 42.Oshima H, et al. Activation of epidermal growth factor receptor signaling by the prostaglandin E(2) receptor EP4 pathway during gastric tumorigenesis. Cancer Sci. 2011;102:713–9. doi: 10.1111/j.1349-7006.2011.01847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oshima H, Oshima M. The role of PGE2-associated inflammatory responses in gastric cancer development. Semin Immunopathol. 2013;35:139–50. doi: 10.1007/s00281-012-0353-5. [DOI] [PubMed] [Google Scholar]
- 44.Franchi AM, Di Girolamo G. de los Santos, A. R., Marti, M. L. & Gimeno, M. A. Effects of lysine clonixinate on cyclooxygenase I and II in rat lung and stomach preparations. Prostaglandins Leukot Essent Fatty Acids. 1998;58:421–4. doi: 10.1016/S0952-3278(98)90164-8. [DOI] [PubMed] [Google Scholar]
- 45.Chen LC, Chen BK, Chang WC. Activating protein 1-mediated cyclooxygenase-2 expression is independent of N-terminal phosphorylation of c-Jun. Mol Pharmacol. 2005;67:2057–69. doi: 10.1124/mol.104.010900. [DOI] [PubMed] [Google Scholar]
- 46.Zhu Y, Saunders MA, Yeh H, Deng WG, Wu KK. Dynamic regulation of cyclooxygenase-2 promoter activity by isoforms of CCAAT/enhancer-binding proteins. J Biol Chem. 2002;277:6923–8. doi: 10.1074/jbc.M108075200. [DOI] [PubMed] [Google Scholar]
- 47.Eliopoulos AG, Dumitru CD, Wang CC, Cho J, Tsichlis PN. Induction of COX-2 by LPS in macrophages is regulated by Tpl2-dependent CREB activation signals. Embo j. 2002;21:4831–40. doi: 10.1093/emboj/cdf478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nie M, Pang L, Inoue H, Knox AJ. Transcriptional regulation of cyclooxygenase 2 by bradykinin and interleukin-1beta in human airway smooth muscle cells: involvement of different promoter elements, transcription factors, and histone h4 acetylation. Mol Cell Biol. 2003;23:9233–44. doi: 10.1128/MCB.23.24.9233-9244.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chou YH, et al. Divalent lead cations induce cyclooxygenase-2 gene expression by epidermal growth factor receptor/nuclear factor-kappa B signaling in A431carcinoma cells. Toxicol Lett. 2011;203:147–53. doi: 10.1016/j.toxlet.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 50.Huang WC, et al. Histamine regulates cyclooxygenase 2 gene activation through Orai1-mediated NFkappaB activation in lung cancer cells. Cell Calcium. 2011;50:27–35. doi: 10.1016/j.ceca.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 51.Juan YS, et al. Translocation of NF-kappaB and expression of cyclooxygenase-2 are enhanced by ketamine-induced ulcerative cystitis in rat bladder. Am J Pathol. 2015;185:2269–85. doi: 10.1016/j.ajpath.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 52.Orlowski RZ, Baldwin AS., Jr. NF-kappaB as a therapeutic target in cancer. Trends Mol Med. 2002;8:385–9. doi: 10.1016/S1471-4914(02)02375-4. [DOI] [PubMed] [Google Scholar]
- 53.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–62. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 54.Wang JY, et al. STIM1 overexpression promotes colorectal cancer progression, cell motility and COX-2 expression. Oncogene. 2015;34:4358–67. doi: 10.1038/onc.2014.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ghilardi JR, Svensson CI, Rogers SD, Yaksh TL, Mantyh PW. Constitutive spinal cyclooxygenase-2 participates in the initiation of tissue injury-induced hyperalgesia. J Neurosci. 2004;24:2727–32. doi: 10.1523/JNEUROSCI.5054-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gilroy DW, et al. Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.