Abstract
Naturally produced by microbial processes in soil, nitrous oxide (N2O) is an important greenhouse gas contributing to climate change. Accordingly, there is a need to accurately quantify the capability of forest ecosystems to exchange N2O with the atmosphere. While N2O emissions from soils have been well studied, trees have so far been overlooked in N2O inventories. Here, we show that stems of mature beech trees (Fagus sylvatica) may act as a substantial sink of N2O from the atmosphere under conditions of soils consuming N2O. Consistent consumption of N2O by all stems investigated (ranging between −2.4 and −3.8 µg m−2 h−1) is a novel finding in contrast to current studies presenting trees as N2O emitters. To understand these fluxes, N2O exchange of photoautotrophic organisms associated with beech bark (lichens, mosses and algae) was quantified under laboratory conditions. All these organisms were net N2O sinks at full rehydration and temperature of 25 °C. The consumption rates were comparable to stem consumption rates measured under field conditions. Cryptogamic stem covers could be a relevant sink of N2O in European beech forests.
Introduction
Nitrous oxide (N2O) is the dominant substance depleting stratospheric ozone and contributes importantly to global radiative forcing that consequently leads to changes in the Earth’s climate1. Global N2O emissions into the atmosphere are estimated to be in the range of 8.1–30.7 Tg yr−1 ref.1. About two-thirds of these emissions are estimated to originate from natural terrestrial sources particularly related to microbial processes in soils2. N2O is a facultative by-product of major microbiological nitrogen (N) cycling processes, including autotrophic and heterotrophic nitrification, heterotrophic nitrification combined with aerobic denitrification, nitrifier denitrification, anaerobic denitrification, anaerobic dissimilatory nitrate reduction to ammonium, and abiotic chemodenitrification. The denitrification processes are the only processes reducing N2O to N2 ref.3–6. The net N2O exchange at the soil–atmosphere interface is therefore a balance of N2O production and consumption processes as well as of N2O transport within the soil7.
Temperate forests are estimated to emit around 1.6 kg N2O ha−1 yr−1 into the atmosphere and thus constitute an important natural source of N2O8. Forest soils are supposed to be responsible for the majority of emitted N2O, although net N2O uptake also has been reported6,9,10. In any case, the factors regulating N2O uptake by soil are not well understood6. To date, N2O fluxes between forests and the atmosphere have been calculated only based on N2O exchange at the soil–atmosphere interface. Plants have been shown to transport soil-derived N2O to the stems and leaves and emit it to the atmosphere11–14, and even to produce N2O during N assimilation processes15,16. However, trees have widely been overlooked as possible N2O emitters or have been presumed to play only a negligible role in forest ecosystems’ N2O exchange.
A few studies have revealed that trees can be important sources of N2O. This capability is found among various tree species of boreal and temperate zones, including conifers Pinus sylvestris and Picea abies as well as deciduous Betula pendula, B. pubescens, Alnus glutinosa, Fraxinus angustifolia, Fagus sylvatica, and poplar (Populus) hybrids11–14,17–19. However, these investigations have mostly been conducted using seedlings under artificial laboratory conditions of high fertilization or flooding to increase soil production of N2O. Reported N2O emissions by plants are thus unnaturally high and reflect plant-mediated transport of N2O from soil to the atmosphere11–13. Such transport is assumed to occur either via special gas transport tissue (aerenchyma system) in wetland tree species11,13 or in the liquid phase by the transpiration stream in non-aerenchymous species20.
To fully understand and accurately quantify natural terrestrial sources of N2O, all potential pathways must be taken into account. There is an urgent need, therefore, to investigate the capability of mature trees to exchange N2O in their natural growth environments. Moreover, the mechanisms responsible for N2O fluxes have to be clarified. In contrast to crop species21, there still remain numerous open questions related to the N2O exchange of trees and forest ecosystems. Among others, it is unclear how trees behave under natural conditions, and particularly when growing on soils with low N2O production. Moreover, until now the role of cryptogamic plant covers (i.e. photoautotrophic organisms such as cyanobacteria, algae, fungi, lichens and bryophytes)22,23 in tree N2O exchange is completely under-investigated, although the relevance of cryptogams for the N cycle of forest ecosystems was stressed in literature24.
Accordingly, the objective of our case study was to quantify natural N2O fluxes in mature European beech trees (Fagus sylvatica) representing native and widely distributed deciduous tree species in temperate forests of Central Europe. Field measuring campaigns were conducted during June and July 2015 at two mountain forest sites in the Czech Republic (Stitna, White Carpathians) and Germany (Conventwald, Black Forest) characterized by predominant soil N2O uptake. N2O exchange capacity of photoautotrophic organisms associated with beech bark was further investigated under laboratory conditions.
Results and Discussion
Although the forest floor at both forest sites was a strong sink for N2O, the sink at Stitna (−105 µg N2O m−2 soil surface area h−1) was larger than that at Conventwald (−79 µg N2O m−2 h−1; Fig. 1a, Supplementary Fig. S1). Lower consumption rates at the Conventwald site were associated with lower CO2 efflux from the forest floor as compared to the Stitna site (Fig. 1b, Supplementary Figs S1,2). The difference in N2O and CO2 exchange rates between the sites might be related to soil temperature being lower in Conventwald than in Stitna during the measurement campaigns (at 0.3 m depth, 9.6 °C and 15.2 °C, respectively). The soil N2O concentration profiles revealed no clear production or consumption pattern in soils (Supplementary Fig. S3). Soils are regarded as N2O source, because N2O emissions are usually greater than N2O uptake6. While there exists a good conceptual understanding of the factors regulating N2O emissions and the processes involved7, there is only little knowledge about N2O consumption in soils6. Even though low mineral N and large soil water contents connected with low oxygen content have been reported in particular to favour N2O consumption, the fact that soil net N2O uptake has been measured also under different conditions complicates the identification of conditions suitable for such uptake6. The CO2 concentrations in soil profiles at both sites indicated aerobic conditions in the soil (Supplementary Fig. S4). Nevertheless, soil analysis showed a redoximorphic colour pattern at Stitna indicative of temporal and local anoxic conditions. Indications for reducing conditions were rarely observed at Conventwald. Yet oxygen depleted zones in the centre of aggregates, specific micro-sites in soils, were found25. These conditions are assumed to further contribute to anaerobic denitrification and heterotrophic nitrification combined with aerobic denitrification as the possible processes responsible for reducing N2O to N2 ref.4–6. It has also been shown, however, that nitrifiers can consume N2O in nitrifier denitrification26.
Figure 1.
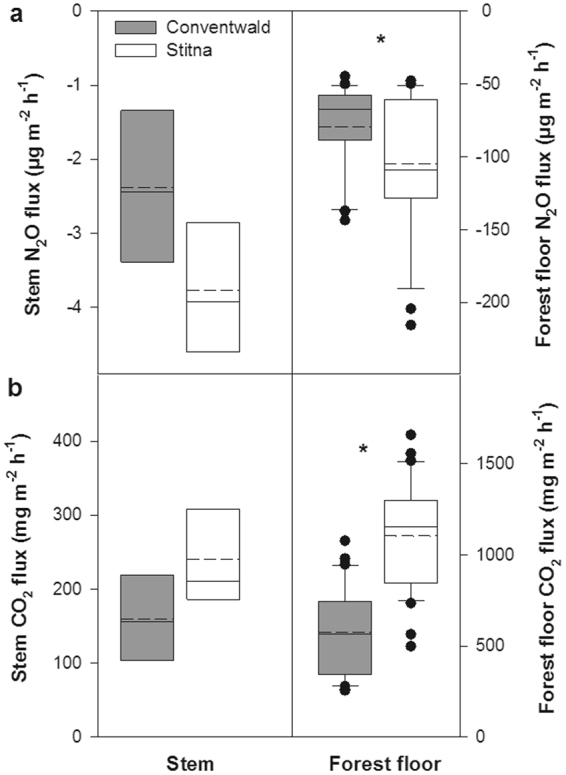
N2O (a) and CO2 (b) fluxes at beech stem and forest floor level in two beech forest stands (Conventwald, Black Forest; Stitna, White Carpathians) measured during June–July 2015. Negative fluxes indicate N2O uptake/consumption. Fluxes are expressed per m2 of stem or soil surface area, respectively. Stem fluxes were determined for five trees per forest stand (n = 5) at three stem heights with 3–4 measurement repetitions per each height and tree. Stem fluxes from different tree heights are presented together as they did not significantly vary with the tree height. Forest floor fluxes were quantified from 23 (n = 23) and 31 (n = 31) positions randomly distributed within the studied forest stands at Conventwald and Stitna, respectively. Fluxes are expressed as medians (solid lines) and means (broken lines). Boundaries within the boxes indicate 25th and 75th percentiles, and the whiskers 10th and 90th percentiles. Dots mark outliers. Statistically significant differences at p < 0.05 are indicated by asterisk.
Stems of all mature beech trees studied were net sinks of N2O from the atmosphere (Fig. 1a, Supplementary Fig. S1). Even though the consumption rates at Stitna (−3.8 µg N2O m−2 stem surface area h−1) were higher than at Conventwald (−2.4 µg N2O m−2 h−1, Fig. 1a), the consumption rates of the majority of trees are in the same order of magnitude. Moreover, no significant changes (p > 0.05) in N2O consumption rates in the buttress roots area and with respect to stem height above the soil were observed (data not presented). Low N2O consumption rates by beech stems were associated with low respiratory CO2 efflux indicating a low physiological activity (Fig. 1, Supplementary Figs S1,2). To the best of our knowledge, such consistent consumption of N2O by tree stems constitutes a unique finding, as the limited number of current studies report trees only as N2O sources. Strong N2O emissions have been found, however, only under conditions of high N fertilization and/or N2O fumigation of roots12,14,17 or with short-term flooding treatment11,13. These manipulations stimulate N turnover processes and increase N2O production in soils. Enhanced N2O is consequently absorbed by tree roots, is transported along the transpiration stream to stems and/or leaves, and is then emitted to the atmosphere12,13. Even though the aforementioned studies show that trees, including mature beech trees14, have the ability to emit N2O, the N2O fluxes thus obtained cannot be used in estimating natural capability of trees to exchange N2O. Studies conducted under natural forest conditions have shown only very low N2O emissions close to, or even less than, the detection limit in various tree species14,18,19.
The scaled-up rates of N2O consumption by beech stems (−35.2 and −12.1 mg N2O ha−1 ground area h−1 at Stitna and Conventwald, Supplementary Fig. S1) contributed 3.4% to the forest floor N2O uptake at the Stitna (−1046 mg N2O ha−1 h−1) and 1.5% at the Conventwald (−793 mg N2O ha−1 h−1) stands. The upscaling procedure, based on mean tree constitution and tree density per hectare estimated for Stitna and Conventwald (see Methods), assumed constant consumption rates within the stem profile.
The bark of all studied trees at both forest sites was largely covered by cryptogamic plants amounting to as much as 40% of total stem area up to 5 m stem height. The cryptogam communities consisted particularly of lichens and fungi (Graphis scripta with identified Cryptosporiopsis sp. and Pezicula sp. fungi; mixture of Graphis scripta and Lecanora sp.; Pseudevernia furfuracea; non-determinable lichen), mosses (Hypnum cupressiforme), and red algae. Accordingly, we investigated whether these organisms can be involved in the observed N2O uptake.
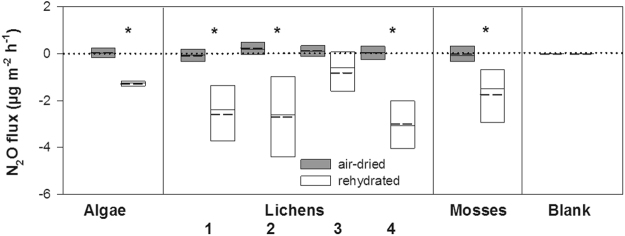
Incubation experiments consistently revealed that all cryptogams tested were net N2O sinks under the conditions of full rehydration and room temperature amounting to −0.016 µg N2O g−1 dry weight h−1 (Fig. 2, Supplementary Fig. S5). Our test measurements confirmed that the observed N2O fluxes cannot be explained by N2O dilution in water (see blank samples in Fig. 2). The measurements were done at low light intensities below compensation irradiance (10–15 µmol m−2 s−1). Respiration processes thus dominated over photosynthesis27 and led to a permanent increase of CO2 concentration within close gas-exchange system (Supplementary Fig. S6). Linear changes in N2O and CO2 concentrations during first 210 minutes however document that the metabolic processes were not inhibited by high CO2 concentration as it could be observed under high light intensities when the phosphorylated intermediates form28.
Figure 2.
N2O fluxes in cryptogams sampled from beech bark in Conventwald, Black Forest. Fluxes are expressed per unit of stem projected area. The plotted results are medians (solid lines) and means (broken lines) of three measurement repetitions in air-dried and rehydrated cryptogams (n = 3). The following samples were investigated. Algae – red alga/Rhodophyta. Lichens: sample 1 – lichen Graphis scripta with identified Cryptosporiopsis sp. and Pezicula sp. fungi; sample 2 – mixture of lichens Graphis scripta and Lecanora sp.; sample 3 – lichen (undetermined); sample 4 – lichen Pseudevernia furfuracea (sampled from beech branch). Mosses – Hypnum cupressiforme. Blank samples (represented by dry and wet filter papers) were also analysed to exclude the possibility that N2O was diluted in water or leaked from the system (see Methods). For box plot description, see Fig. 1. Statistically significant differences in fluxes between air-dried and rehydrated organisms at p < 0.05 are indicated by asterisk.
Meanwhile, the activity of air-dried cryptogams was negligible (Fig. 2, Supplementary Figs S5,7,8). Air-dried cryptogams showed a very low CO2 exchange (10 µg CO2 g−1 h−1), whereas rehydration of the organisms led to rapid increase of their CO2 exchange (545 µg CO2 g−1 h−1, Supplementary Figs S7,8) and also of N2O consumption. These findings show general agreement that the physiological activity of cryptogams depends on water availability23. Moreover, we have found that high N2O consumption rates by cryptogams are associated with high CO2 emission rates (p < 0.001; Supplementary Fig. S9). Therefore, the role of cryptogamic bark covers in the N2O exchange of trees and forest ecosystems seems closely related to local climatic and meteorological conditions and should be studied in more detail in future, and especially so in relation to global climate scenarios.
We roughly estimated how much N2O is taken up by the active rehydrated cryptogams per stem area unit when fully covered with these organisms (the bark in stem chambers was as much as 90% covered with these organisms). The estimated N2O consumption rates per unit area (Fig. 2) were on the same order of magnitude as were stem consumption rates measured under the field conditions (Fig. 1). Specifically, it was −1.3, −2.0, and −1.8 µg N2O m−2 h−1 for algae, lichens, and mosses, respectively. Therefore, it seems that the bark vegetation is at least co-responsible for the observed unique consumption of N2O by beech trees. The contribution of cryptogams to N2O consumption can increase with rising water content (e.g. due to high air humidity or rainwater’s retention in the crotches of branches).
Our finding of N2O consumption is in contrast to those of studies23,29 presenting green algae and cryptogamic ground and plant covers as sources of N2O (mean emissions of 7.9 ng N2O g−1 h−1 ref.23). N2O is thought to be produced during an aerobic NO3 − assimilation, similar to proposed production in plants15,16,23,29,30. The difference in sink versus source character of cryptogamic stem covers may result from the fact that the cryptogams showing N2O emissions were investigated under dark conditions23, whereas our incubation experiments were performed under low light intensity of 10–15 µmol m−2 s−1 corresponding to low light conditions of forest understories. Moreover, moss-dominated communities in polar deserts can serve also as sinks for N2O under light conditions31. The authors hypothesize that the Bryophytes lack roots needed for N2O absorption from soil water. Cryptogamic plant covers with associated cyanobacterial communities are estimated to fix N2 up to 49 Tg yr−1 from the atmosphere, which amount corresponds to nearly half of the global terrestrial biological N2 fixation22. However, there is no known association of our sampled photoautotrophic organisms with N2-fixing cyanobacteria32. We therefore hypothesize that the absorbed N2O can serve as an additional source of N for the cryptogams lacking cyanobacteria. Absorbed N2O might also be partly re-emitted back into the atmosphere23, the rates of consumption might exceed the emission rates and thus result in net N2O consumption. Absorbed N2O might perhaps be partly converted to NO3 −, similarly as is nitric oxide (NO)33, and then leached by rain. Absorbed N2O might be also reduced to N2 by denitrification microorganisms6 associated with cryptogams and N2 can be re-emitted into the atmosphere. Further detailed research is needed to understand the mechanisms behind the N2O uptake in cryptogams and trees, and the fate of the absorbed N2O molecules.
This study shows for the first time that not only soil but also mature trees can constitute an important sink for N2O under natural field conditions, thus increasing the sink capacity for N2O of forest ecosystems overall. We have found that N2O consumption rates are directly proportional to respiratory CO2 fluxes indicating overall physiological activity of trees and microbial communities. This paper also underlines the heretofore unknown role of cryptogamic plant covers in N2O consumption, which activity dramatically increases under wet conditions. The results of this case study need to be verified by a larger future project directed to studying spatial and seasonal variability in N2O exchange of beech trees while including also fluxes in leaves and branches, as well as to understanding the role of cryptogams in the beech N2O exchange under different climatic conditions.
Methods
Sites description
Measurements were conducted at two mountain forest sites in Central Europe. The Conventwald research site (48.02°N, 7.96°E; elevation 840 m a.s.l.; Black Forest, Germany) is in a mixed forest stand (density of 186 trees ha−1) with prevailing 135-year-old beech trees, followed by Silver fir (Abies alba) and Norway spruce (Picea abies)34. Average beech height and stem diameter at breast height were 30 m and 0.58 m, respectively. Mean annual temperature and precipitation are 8.5 °C and 1330 mm, respectively35.
The Stitna research site (49.02°N, 17.58°E; elevation 550 m a.s.l.; White Carpathians, Czech Republic) is a 115 year-old beech monoculture (density of 283 trees ha−1) with average tree height of 33 m and mean stem diameter at breast height of 0.60 m. Mean annual temperature and total annual precipitation are 7.5 °C and 800 mm, respectively36. The soil type at both stands is Cambisol (Conventwald: Hyperdistric Skeletic Cambisol; Stitna: Eutric (Stagnic) Cambisol)37,38. The soil texture at Conventwald is sandy loam with 30–60% coarse soil fraction and at Stitna loam with no coarse soil fraction. Soil pH was 3.5 for Conventwald and 7.0 for Stitna38. See Maier et al. (2017)38 for detailed soil characteristics of both sites.
During the measuring campaign (June–July 2015), mean air temperatures at Conventwald and Stitna were 16.8 °C and 19.2 °C, soil volumetric water content (0.3 m depth) 22% and 26%, and soil temperatures (0.3 m depth) 9.6 °C and 15.2 °C, respectively.
Stem fluxes of N2O and CO2
Stem N2O and CO2 fluxes were determined in five representative beech trees of average height and stem diameter at each site (n = 5). The stem flux measurements were repeated three to four times during the measuring campaign. All fluxes were measured between 10:00 and 16:00 on sunny days to prevent possible variation caused by diurnal cycle. Stem fluxes were simultaneously measured at three stem heights (0.4, 1.2 and 2.0 m above the ground) at each tree using a static chamber system. Three to four large (internal volume of 2.1 dm3) or small (internal volume of 0.9 dm3) rectangular stem chambers were installed at different sides of the stem for each stem height in order to representatively cover the stem circumferential surface area. The large chambers covered an area of 0.0183 m2 each, the small ones of 0.0084 m2 each. The chambers at one stem height were interconnected in series using polyurethane tubes. A constant flow rate and mixing of the air inside the system were provided by a DP0140/12 V pump (Nitto Kohki, Tokyo, Japan). The chambers were made from transparent plastic storage containers with removable airtight lids (Lock & Lock, Anaheim, CA, USA). The bottom part of each container was cut out and glued to a 2 cm thick neoprene frame. The chambers were sealed with silicone to the carefully smoothed bark surface several days before the measurement campaigns and tested for leakage. All chambers were left open between measuring campaigns.
During the measurements, seven gas samples (20 ml) were taken via a septum at 0-30-60-90-130-170-210 min after chamber system closure when the linear changes in N2O and CO2 concentrations were observed. Changes in chamber pressure caused by gas sample uptake were tested and compensated by an insertion of empty needle to the system resulting in a negligible under-pressure of approx. 10 mbar for less than 5 seconds. The gas samples were stored in pre-evacuated gas-tight glass vials (Labco, Ceredigion, UK) at 7 °C until analysis. A Tracera gas chromatograph (Shimadzu Corporation, Kyoto, Japan) equipped with a barrier discharge ionization detector operated at 250 °C with helium as carrier gas was used to determine N2O and CO2 concentrations. A coupled ShinCarbon ST micro column (Restek, Bellefonte, PA, USA) with a length of 2 m and internal diameter of 1 mm and fused silica capillary (Restek, USA) of internal diameter 0.53 mm were used for gas separation. The oven temperature began at 70 °C for 14 min followed by its increase to 200 °C for 1.5 min. The gas samples were automatically injected by a GX-271 autosampler (Gilson, Middleton, Wisconsin, USA). The N2O and CO2 peaks were identified using LabSolutions software (Shimadzu Corporation, Japan). N2O and CO2 concentrations were calculated based on a four-concentration calibration curve (0.29, 0.43, 0.56, 0.70 µmol N2O mol−1; 400, 667, 933, 1200 µmol CO2 mol−1).
The stem fluxes were calculated linearly based on the gas concentration changes over time (for examples see Supplementary Fig. S6)19 and expressed per m2 of stem surface area. The fluxes were further scaled up to per-hectare values for beech forest. Extrapolation was based on the estimated mean stem surface area (33.0 and 27.3 m2 per tree in Stitna and Conventwald, respectively) and tree density (283 and 186 trees ha−1 in Stitna and Conventwald, respectively).
Forest floor fluxes of N2O and CO2
The fluxes between forest floor and atmosphere were measured simultaneously with stem fluxes at 23 (n = 23, Conventwald) and 31 (n = 31, Stitna) positions randomly spread over the studied forest sites to cover the spatial heterogeneity of the soil N2O and CO2 fluxes.
The manual cylindrical soil chambers constructed of polyvinyl chloride with internal diameter of 0.17 m and height of 0.25 m ref.39 were installed one day before the measurements38. The chambers were connected with two gas analysers via polyurethane tubing fixed in the chamber lids, thus forming a closed flow-through system. Changes in N2O concentration were detected by a Photoacoustic Innova Field Gasmonitor (LumaSense Technologies, Ballerup, Denmark), changes in CO2 by a Greenhouse Gas analyzer (Ultraportable, Los Gatos Research, San Jose, CA, USA). The moisture content of the sampled air was conditioned using a dew point controller set to 8 °C. This stabilization of the H2O concentration allowed for high-precision N2O measurement with the LumaSense device. The fluxes were calculated based on the linear least square fits of time series of N2O and CO2 concentrations measured in the headspace.
Soil N2O and CO2 profile
To investigate production and consumption of N2O and CO2 in the soil, soil gas profiles were determined using a multi-level soil gas sampler (+0.03, 0, −0.05, −0.1, −0.2, −0.3, −0.4 m sampling depth with 0 m as interface between mineral soil and humus layer)40 installed at distances of 1, 2 and 4 m from the tree stem. Two beech trees out of the five selected for stem measurements were chosen per site for the soil gas profiles. The soil gas samplers were connected to the two gas analysers via a polyurethane tubing system to determine the N2O and CO2 concentrations in the soil profiles. The different sampling depths were consecutively measured.
Laboratory measurements of cryptogams
Photoautotrophic organisms (lichens, mosses and algae) forming cryptogamic covers of beech stems were collected in Conventwald for further analyses. To prevent any disruption of the bark-microcosm within the stem chambers, the cryptogams were sampled from the bark outside these chambers. After collection, samples were air-dried and stored in paper bags. In total, samples of six communities were collected: 1) Algae – red alga/Rhodophyta, 2) lichen Graphis scripta with identified Cryptosporiopsis sp. and Pezicula sp. fungi, 3) mixture of lichens Graphis scripta and Lecanora sp., 4) lichen (undetermined), 5) lichen Pseudevernia furfuracea (sampled from beech branch), and 6) moss Hypnum cupressiforme.
Gas exchange measurements were performed under constant laboratory conditions, i.e. air temperature of 25 °C and light intensity of 10–15 µmol m−2 s−1 corresponding to low light conditions of forest understories. Measurements of air-dried and fully rehydrated cryptogams were performed to investigate the effect of physiological activity on their N2O fluxes. To activate the air-dried organisms, samples were sprinkled with a mixture of rain and distilled water until full rehydration was reached. To ensure activation of the rehydrated cryptogams, the measurements were started ca 2.5 h after the addition of water.
For gas exchange (N2O and CO2) measurements, the cryptogams were placed in 600 ml plastic gas-tight containers (Lock & Lock, USA) with installed septa for gas sampling. The air (20 ml) was sampled at 0-60-130-220-320-420 min after container closure (air-dried cryptogams) or at 0-30-60-100-150-210 min (rehydrated cryptogams) and stored in pre-evacuated gas-tight glass vials at 7 °C. The possible pressure changes in the system caused by gas sample uptake were compensated. The concentrations of N2O and CO2 in air samples were assessed by gas chromatography as described above. The N2O and CO2 fluxes between cryptogams and the atmosphere were calculated as the slope of the linear regression indicating the change of concentration with time (see Supplementary Fig. S6)19 and expressed per unit of dry weight of cryptogams and unit of stem surface area fully covered with cryptogams. The field measurement of the stem area covered with sampled cryptogams of known dry weight allowed quantification of N2O and CO2 flux rates by cryptogams related to stem surface area unit. In addition, three types of blank samples were analysed to exclude the possibility that N2O would be diluted in water or leak from the system. These blanks consisted of i) containers filled with 8 layers of filter paper saturated with distilled water, ii) containers filled with the same amount of dry filter paper, and iii) empty containers.
Statistics
The sets of fluxes were tested for normal distribution (Shapiro–Wilk test) and equality of variances in different subpopulations. A t-test was applied for the normally distributed data and a non-parametric Mann–Whitney rank-sum test for the non-normally distributed data and/or data with unequal variances. The n values for statistical analyses are stated in the figure legends. Statistical significance for all tests was defined at p < 0.05. The relationships between N2O and CO2 fluxes were tested by linear regression at p < 0.001. The statistics were run with SigmaPlot 11.0 (Systat Software, San Jose, California, USA).
Data Availability
The datasets generated and analysed during this study are available from the authors on reasonable request.
Electronic supplementary material
Acknowledgements
This research was supported by the Czech Academy of Sciences and the German Academic Exchange Service (project DAAD-15-03), the Czech Science Foundation (17-18112Y), National Programme for Sustainability I (LO1415), CzeCOS (LM2015061) and DFG project (MA 5826/2-1). We thank Marek Jakubik, Sinikka Paulus, Ellen Halaburt and Sally Haddad for technical and field support, and Jörg Grüner for identification of photoautotrophic organisms on beech bark.
Author Contributions
K.M., M.M. and F.L. had the idea for the study. K.M. and M.M. designed the study. K.M., M.M. and K.S. carried out the field measurements and analysed the data. K.M., M.M., O.U. and F.L. contributed to writing the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-13781-7.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.IPCC. Climate Change 2013: The Physical Science Basis. (eds Stocker, T. F. et al.) (IPCC(Intergovernmental Panel on Climate Change), Cambridge University Press, 2013).
- 2.EPA. Methane and Nitrous Oxide Emissions from Natural Sources. (eds Anderson, B. et al.) (Office of Atmospheric Programs, EPA (United States Environmental Protection Agency), 2010).
- 3.Wrage N, Velthof GL, van Beusichem ML, Oenema O. Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol. Biochem. 2001;33:1723–1732. doi: 10.1016/S0038-0717(01)00096-7. [DOI] [Google Scholar]
- 4.Smith KA, et al. Exchange of greenhouse gases between soil and atmosphere: interactions of soil physical factors and biological processes. Eur. J. Soil Sci. 2003;54:779–791. doi: 10.1046/j.1351-0754.2003.0567.x. [DOI] [Google Scholar]
- 5.Blagodatsky SA, Kesik M, Papen H, Butterbach-Bahl K. Production of NO and N2O by the heterotrophic nitrifier Alcaligenes faecalis parafaecalis under varying conditions of oxygen saturation. Geomicrobiol. J. 2006;23:165–176. doi: 10.1080/01490450600599221. [DOI] [Google Scholar]
- 6.Chapuis-Lardy L, Wrage N, Metay A, Chotte JL, Bernoux M. Soils, a sink for N2O? A review. Glob. Change Biol. 2007;13:1–17. doi: 10.1111/j.1365-2486.2006.01280.x. [DOI] [Google Scholar]
- 7.Davidson EA, Keller M, Erickson HE, Verchot LV, Veldkamp E. Testing a conceptual model of soil emissions of nitrous and nitric oxides. BioScience. 2000;50:667–680. doi: 10.1641/0006-3568(2000)050[0667:TACMOS]2.0.CO;2. [DOI] [Google Scholar]
- 8.Dalal RC, Allen DE. Turner Review No. 18: Greenhouse gas fluxes from natural ecosystems. Aust. J. Bot. 2008;56:369–407. doi: 10.1071/BT07128. [DOI] [Google Scholar]
- 9.Papen H, Daum M, Steinkamp R, Butterbach-Bahl K. N2O and CH4-fluxes from soils of a N-limited and N-fertilized spruce forest ecosystem of the temperate zone. J. Appl. Bot.-Angew. Bot. 2001;75:159–163. [Google Scholar]
- 10.Rosenkranz P, et al. N2O, NO and CH4 exchange, and microbial N turnover over a Mediterranean pine forest soil. Biogeosciences. 2006;3:121–133. doi: 10.5194/bg-3-121-2006. [DOI] [Google Scholar]
- 11.Rusch H, Rennenberg H. Black alder (Alnus glutinosa (L.) Gaertn.) trees mediate methane and nitrous oxide emission from the soil to the atmosphere. Plant Soil. 1998;201:1–7. doi: 10.1023/A:1004331521059. [DOI] [Google Scholar]
- 12.Pihlatie M, Ambus P, Rinne J, Pilegaard K, Vesala T. Plant-mediated nitrous oxide emissions from beech (Fagus sylvatica) leaves. New Phytol. 2005;168:93–98. doi: 10.1111/j.1469-8137.2005.01542.x. [DOI] [PubMed] [Google Scholar]
- 13.Machacova K, Papen H, Kreuzwieser J, Rennenberg H. Inundation strongly stimulates nitrous oxide emissions from stems of the upland tree Fagus sylvatica and the riparian tree Alnus glutinosa. Plant Soil. 2013;364:287–301. doi: 10.1007/s11104-012-1359-4. [DOI] [Google Scholar]
- 14.Díaz-Pinés E, et al. Nitrous oxide emissions from stems of ash (Fraxinus angustifolia Vahl) and European beech (Fagus sylvatica L.) Plant Soil. 2015;398:35–45. doi: 10.1007/s11104-015-2629-8. [DOI] [Google Scholar]
- 15.Smart DR, Bloom AJ. Wheat leaves emit nitrous oxide during nitrate assimilation. P. Natl. Acad. Sci. USA. 2001;98:7875–7878. doi: 10.1073/pnas.131572798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hakata M, Takahashi M, Zumft W, Sakamoto A, Morikawa H. Conversion of the nitrate nitrogen and nitrogen dioxide to nitrous oxides in plants. Acta Biotechnol. 2003;23:249–257. doi: 10.1002/abio.200390032. [DOI] [Google Scholar]
- 17.McBain MC, Warland JS, McBride RA, Wagner-Riddle C. Laboratory-scale measurements of N2O and CH4 emissions from hybrid poplars (Populus deltoides x Populus nigra) Waste Manage. Res. 2004;22:454–465. doi: 10.1177/0734242X04048832. [DOI] [PubMed] [Google Scholar]
- 18.Machacova, K. et al. Summer fluxes of nitrous oxide from boreal forest. Global Change: A Complex Challenge, conference proceedings (conference 4th Annual Global Change - A Complex Challenge, Brno, Czech Republic), 78–81 (2015).
- 19.Machacova, K. et al. Pinus sylvestris as a missing source of nitrous oxide and methane in boreal forest. Sci. Rep. 6, doi: 10.1038/srep23410 (2016). [DOI] [PMC free article] [PubMed]
- 20.Chang C, Janzen HH, Cho CM, Nakonechny EM. Nitrous oxide emission through plants. Soil Sci. Soc. Am. J. 1998;62:35–38. doi: 10.2136/sssaj1998.03615995006200010005x. [DOI] [Google Scholar]
- 21.Ussiri, D. & Lal, R. Soil Emissions of Nitrous Oxide and Its Mitigation (eds Ussiri, D. & Lal, R.) (Springer Science and Business Media Dordrecht, 2013).
- 22.Elbert W, et al. Contribution of cryptogamic covers to the global cycles of carbon and nitrogen. Nat. Geosci. 2012;5:459–462. doi: 10.1038/ngeo1486. [DOI] [Google Scholar]
- 23.Lenhart K, et al. Nitrous oxide and methane emissions from cryptogamic covers. Glob. Change Biol. 2015;21:3889–3900. doi: 10.1111/gcb.12995. [DOI] [PubMed] [Google Scholar]
- 24.Cornelissen JHC, Lang SI, Soudzilovskaia NA, During HJ. Comparative cryptogam ecology: a review of bryophyte and lichen traits that drive biogeochemistry. Ann. Bot. 2007;99:987–1001. doi: 10.1093/aob/mcm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuzyakov Y, Blagodatskaya E. Microbial hotspots and hot moments in soil: Concept & review. Soil Biol. Biochem. 2015;83:184–199. doi: 10.1016/j.soilbio.2015.01.025. [DOI] [Google Scholar]
- 26.Braker, G. & Conrad, R. Diversity, structure, and size of N2O-producing microbial communities in soils - What matters for their functioning? in Advances in Applied Microbiology, Vol. 75 (eds Laskin, A. I., Sariaslani, S. & Gadd, G. M.) 33–70 (Academic Press, 2011). [DOI] [PubMed]
- 27.Weber B, Graf T, Bass M. Ecophysiological analysis of moss-dominated biological soil crusts and their separate components from the Succulent Karoo, South Africa. Planta. 2012;236:129–139. doi: 10.1007/s00425-012-1595-0. [DOI] [PubMed] [Google Scholar]
- 28.Farquhar GD, von Caemmerer S, Berry JA. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta. 1980;149:78–90. doi: 10.1007/BF00386231. [DOI] [PubMed] [Google Scholar]
- 29.Albert KR, Bruhn A, Ambus P. Nitrous oxide emission from Ulva lactuca incubated in batch cultures is stimulated by nitrite, nitrate and light. J. Exp. Mar. Biol. Ecol. 2013;448:37–45. doi: 10.1016/j.jembe.2013.06.010. [DOI] [Google Scholar]
- 30.Goshima N, et al. Emission of nitrous oxide (N2O) from transgenic tobacco expressing antisense NiR mRNA. Plant J. 1999;19:75–80. doi: 10.1046/j.1365-313X.1999.00494.x. [DOI] [PubMed] [Google Scholar]
- 31.Stewart KJ, Brummell ME, Farrell RE, Siciliano SD. N2O flux from plant-soil systems in polar deserts switch between sources and sinks under different light conditions. Soil Biol. Biochem. 2012;48:69–77. doi: 10.1016/j.soilbio.2012.01.016. [DOI] [Google Scholar]
- 32.Pentecost, W. & Whitton, B. A. Subaerial cyanobacteria in Ecology of Cyanobacteria II: Their Diversity in Space and Time (eds Whitton, B. A.) 291–316 (Springer, 2012).
- 33.Baumgärtner M, Koschorreck M, Conrad R. Oxidative consumption of nitric oxide by heterotrophic bacteria in soil. FEMS Microbiol. Ecol. 1996;19:165–170. doi: 10.1111/j.1574-6941.1996.tb00209.x. [DOI] [Google Scholar]
- 34.Bücking, W. An Overview - The strictly protected forest reserve “Conventwald” in Bannwald “Conventwald” (eds Bücking, W.) 191–192 (Forstliche Versuchs- und Forschungsanstalt Baden-Württemberg, 2004).
- 35.Dong F, et al. Environmental effects on soil NO concentrations and root N uptake in beech and spruce forests. J. Plant Nutr. Soil Sci. 2016;179:244–256. doi: 10.1002/jpln.201500191. [DOI] [Google Scholar]
- 36.Darenova E, Pavelka M, Macalkova L. Spatial heterogeneity of CO2 efflux and optimization of the number of measurement positions. Eur. J. Soil Biol. 2016;75:123–134. doi: 10.1016/j.ejsobi.2016.05.004. [DOI] [Google Scholar]
- 37.FAO. World reference base for soil resources 2006 in World Soil Resources Reports, 103. (FAO (Food and Agriculture Organization of the United Nations), 2006).
- 38.Maier, M., Machacova, K., Lang, F., Svobodova, K. & Urban, O. Combining soil and tree-stem flux measurements and soil gas profiles to understand CH4 pathways in Fagus sylvatica forests. J. Plant Nutr. Soil Sci., doi: 10.1002/jpln.201600405 (2017).
- 39.Schack-Kirchner H, Gaertig T, Wilpert K, Hildebrand EE. A modified McIntyre and Phillip approach to measure top-soil gas diffusivity in-situ. J. Plant Nutr. Soil Sci. 2001;164:253–258. doi: 10.1002/1522-2624(200106)164:3<253::AID-JPLN253>3.0.CO;2-G. [DOI] [Google Scholar]
- 40.Laemmel, T., Maier, M., Schack-Kirchner, H. & Lang, F. An in situ method for real-time measurement of gas transport in soil. Eur. J. Soil Sci., doi:10.1111/ejss.12412 (2017).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analysed during this study are available from the authors on reasonable request.