Abstract
Background
Sperm DNA fragmentation (SDF) testing has been recognized as a valuable tool in the evaluation of infertile men. Despite that, its routine use in clinical practice is still hampered by the lack of understanding of the specific clinical scenarios where SDF testing is most beneficial. The aim of this study was to investigate fertility specialists evaluation of infertility of SDF testing in the context of male infertility evaluation and assisted reproductive technology.
Methods
A questionnaire was developed to survey the major aspects of SDF testing and was mailed to specialists with demonstrated clinical experience in the field of infertility. A total of 65 professionals were invited to answer issues related to the utility of SDF testing, the testing methods they used, were the SDF cut-off values, and the cost of testing and the perceived drawbacks of the test results. Specific clinical scenarios were presented to assess whether or not participants would recommend SDF testing. The frequency of responses was analyzed.
Results
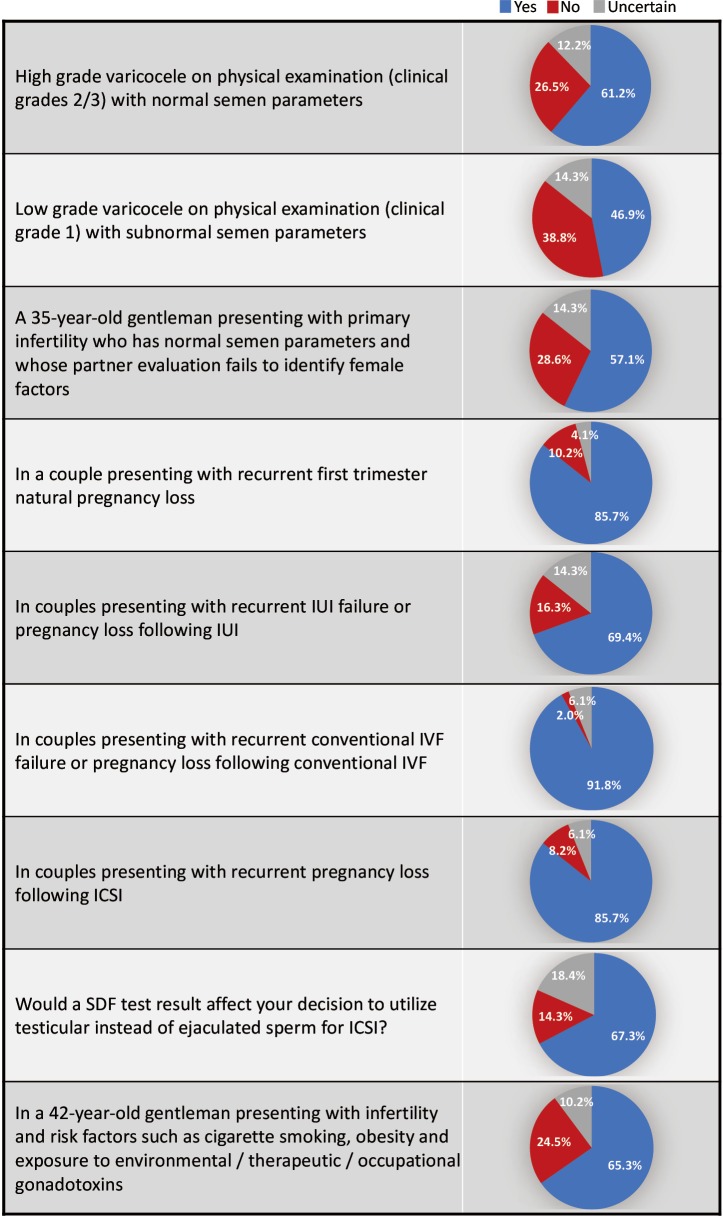
Forty-nine participants from 19 countries responded to the study questionnaire. SDF testing was commonly ordered by 39 (79.6%) respondents; while 10 (20.4%) did not order SDF testing during fertility evaluation. Terminal deoxynucleotidyl transferase nick end labelling (TUNEL) and sperm chromatin structure assay (SCSA) were most commonly utilized (30.6% for both), followed by sperm chromatin dispersion (SCD) (20.4%), single cell gel electrophoresis (Comet) (6.1%) and other methods (12.2%). SDF was most commonly requested in couples presenting with recurrent conventional in vitro fertilization (IVF) failure or pregnancy loss following conventional IVF (91.8%), followed by couples with recurrent first trimester natural pregnancy loss (NPL) and recurrent pregnancy loss (RPL) following intracytoplasmic sperm injection (ICSI) (85.7% for both). A 67.3% of respondents admitted that an SDF test result would affect their decision to utilize testicular instead of ejaculated sperm for ICSI. The reported mean ± standard deviation (SD) cost (USD) of SDF testing was 170.4±122.9. Cost (46.9%), poor validation (36.7%) and low precision (18.3%) were the most commonly reported drawbacks of SDF testing.
Conclusions
SDF testing is utilized in the evaluation of infertility patients by a majority of fertility specialists under specific clinical scenarios. Shortcomings, such as the presence of several SDF testing methods with different cut-off values and the test charges were some of the reasons hampering the routine use of SDF in the evaluation of infertile men.
Keywords: Sperm DNA fragmentation (SDF), fertility specialists, clinical utility, drawbacks
Introduction
The immense amount of research conducted in the field of male reproduction over the past few decades has enriched our understanding of the male contribution to infertility. Effort has been exerted to develop an evidence based approach to patient evaluation and treatment with particular emphasis on tests that can provide additional information on the functional status of the male gamete. We came to realize that the standard semen parameters assessed with routine semen analysis provide a crude prediction of the true male fertility potential. Despite all applied refinements to semen analysis procedures and reference values (1), up to 30% of men with normal semen parameter profiles still present with infertility (2).
Sperm DNA fragmentation (SDF), among other sperm function tests, has been most commonly investigated mainly due to the alleged significant contribution of paternal genetic integrity to embryo development. In fact, interest in SDF started about half a century ago right around the discovery of the double helix DNA structure. Early reports on sperm chromatin structure recognized the unique protein complex alterations that are characteristic to sperm DNA (3) and identified the occurrence of structural abnormalities in subfertile men (4). Understandably, the techniques available at that time only allowed the assessment of quantitative differences in sperm DNA bases between fertile and infertile men (5,6). The modern era of SDF assessment began when the acridine orange test that was introduced in 1970 by Ringertz et al. (7), who assessed the thermal stability of sperm DNA at various stages of spermiogenesis. Subsequent advancements in molecular biology technology allowed the foundation of various SDF testing methods, which include in order of introduction, the sperm chromatin structure assay (SCSA) (8), single cell gel electrophoresis (Comet) (9), terminal deoxynucleotidyl transferase nick end labelling (TUNEL) (10) and the sperm chromatin dispersion (SCD) test (11). These tests were reliably found to be accurate measures of sperm DNA integrity in a number of research studies (12-15).
In the past 2 decades, focus has shifted from the development of SDF testing methods to the implications of SDF on male fertility potential. Up to 80% of men with idiopathic male infertility demonstrated high levels of SDF suggesting that sperm DNA integrity may indeed carry meaningful implications on fecundity (16). SDF was found to have significant negative correlations with sperm concentration, motility and morphology (17,18) and to influence conception through detrimental effects on fertilization, early embryo development, implantation, and pregnancy (19). These findings triggered researchers to investigate SDF measures in various clinical conditions that could not be explained with the available basic semen parameter profiles. These conditions, reviewed in the clinical guideline article by Agarwal et al. (20), include clinical varicocele (21,22), recurrent pregnancy loss (RPL) (23-25), unexplained infertility (26), recurrent intrauterine insemination (IUI) (27,28) and in vitro fertilization (IVF) failure (29,30), and recurrent abortion after IVF and intracytoplasmic sperm injection (ICSI) (29-31).
Despite the growing evidence in favor of the utility of SDF during the evaluation of infertile men, little is known about fertility specialists’ perceptions of this testing modality and its actual use in clinical practice. In fact, concerns regarding the occurrence of various testing methods with different cut-off values, test validation, diagnostic accuracy and, most importantly, the added expenses required for testing are still important aspects to consider. Aside from the guidelines of professional reproductive societies recommending against the routine use of SDF testing in clinical practice (32-34); ours is the first study to investigate the utility of SDF in clinical practice and examine the drawbacks perceived by fertility specialists dealing with this diagnostic modality.
Methods
This was a cross-sectional questionnaire-based survey conducted between September 2016 and May 2017. The study participants were clinicians and scientists with demonstrated experience in the field of infertility. The questionnaire was developed to cover major aspects of SDF testing, including: (I) general questions about background in male infertility; (II) testing methods utilized; and (III) indications for testing. Specific clinical scenarios were presented to assess the employment of SDF in conditions including varicocele, unexplained infertility, recurrent natural pregnancy loss (NPL), IUI and IVF failure or abortions, recurrent ICSI abortions and in patients with lifestyle risk factors. Furthermore, the perceived current drawbacks of the SDF test were also assessed. A total of 331 scholars from different medical centers around the world were selected based on their field of practice and/or research contribution. These scholars were invited to write a commentary outlining their viewpoints [in response to an article by Agarwal et al., 2016. Clinical utility of SDF testing: practice recommendations based on clinical scenarios (20)] and respond to a survey based questionnaire mailed to them.
The returned questionnaires were coded and data were entered into statistical package for social sciences (SPSS) version 21 (IBM, Armonk, NY, USA). The frequency of responses was analyzed. The study participants were further divided according to the number of male infertility cases they manage in clinic (level of expertise) into >50% (n=25) or <50% (n=24) of overall cases seen in clinic. Chi-square analysis was performed to compare between the two groups. Categorical data were presented as numbers (percentages) while numerical data were presented as mean ± standard deviation (SD). A P value <0.05 was considered statistically significant.
Results
Out of the 331 invited scholars, 65 consented to participate in writing of the commentary and were then sent the questionnaire. Forty-nine questionnaires were completed and submitted for analysis. The participants belonged to 19 different countries (Table 1). The majority were Urologists (44.9%) followed by PhD scholars (26.5%), Reproductive Endocrinologists (20.4%) and Andrologists (8.2%). Most respondents worked in an academic setting either full time or part time (75.6%), received prior male infertility training (81.6%) and were running a service where male infertility patients constituted >20% of cases seen in clinic (91.8%) (Table 2). SDF testing was commonly ordered by 39 (79.6%) respondents while 10 (20.4%) never considered SDF testing during fertility evaluation. The mean number of SDF tests ordered per month was 8.3±7.2 (range 0–22). SCSA and TUNEL were most commonly utilized (30.6% for both), followed by SCD (20.4%), Comet (6.1%) and other methods (12.2%). Cut-off values of 10%, 20%, 30% and others were identified by 4.1%, 10.2%, 61.2% and 24.4% of respondents, respectively. The reported mean ± SD cost of SDF testing was 170.4±122.9 USD (range 0–450 USD). Figure 1 demonstrates the responses towards the utility of SDF in specific clinical scenarios. SDF testing was most commonly utilized in couples presenting with recurrent conventional IVF failure or pregnancy loss following conventional IVF (91.8%) while it was least commonly ordered for the evaluation of low grade varicocele in patients with subnormal semen analysis results (46.9%). Among the various drawbacks perceived by participants, high cost (46.9%) and poor validation (36.7%) were most commonly reported (Table 3). A comparison in the utility of SDF and perceived drawbacks according to the level of expertise of study participants is represented in Table 4. The utility of SDF testing was significantly lower in the case of high grade varicocele with normal semen parameters (P=0.05) and significantly higher in the case of RPL after ICSI (P=0.02) in participants with higher level of expertise compared with lower level of expertise. As for the perceived drawbacks, no significant differences were reported between the two studied groups.
Table 1. Participants’ country of origin.
| Country | N (%) |
|---|---|
| USA | 13 (26.5) |
| India | 6 (12.2) |
| Italy | 4 (8.2) |
| Brazil | 4 (8.2) |
| Spain | 3 (6.1) |
| Iran | 2 (4.1) |
| Qatar | 2 (4.1) |
| Turkey | 2 (4.1) |
| UK | 2 (4.1) |
| Australia | 1 (2.0) |
| Belgium | 1 (2.0) |
| Canada | 1 (2.0) |
| Denmark | 1 (2.0) |
| Egypt | 1 (2.0) |
| Hong Kong | 1 (2.0) |
| Pakistan | 1 (2.0) |
| Poland | 1 (2.0) |
| South Africa | 1 (2.0) |
| Israel | 1 (2.0) |
Table 2. Affiliation, experience and training of study participants.
| Variable | N (%) |
|---|---|
| Affiliation | |
| Private setting | 12 (24.5) |
| Academic setting | 21 (42.9) |
| Both | 16 (32.7) |
| Experience in male infertility | |
| Does not see or manage male infertility cases | 2 (4.1) |
| Male infertility cases constitute <20% of patients seen in clinic | 2 (4.1) |
| Male infertility cases constitute 20–50% of patients seen in clinic | 20 (40.8) |
| Male infertility cases constitute >50% of patients seen in clinic | 25 (51.0) |
| Postgraduate training | 40 (81.6) |
Figure 1.
The utility of SDF in various clinical scenarios (participants n=41). SDF, sperm DNA fragmentation; IUI, intrauterine insemination; ICSI, intracytoplasmic sperm injection; IVF, in vitro fertilization.
Table 3. Perceived drawbacks with SDF testing.
| Variable | N (%) |
|---|---|
| High cost | 23 (46.9) |
| Poor validation | 18 (36.7) |
| Low precision | 9 (18.3) |
| Low accuracy | 7 (14.2) |
| Long turnaround time | 6 (12.2) |
| Others* | 10 (20.4) |
*, not widely available in local labs, limited treatment options, bound to sperm count, doesn’t always change clinical management. SDF, sperm DNA fragmentation.
Table 4. Comparison in utility of SDF and perceived drawbacks according to participants’ level of expertise.
| Variable | Male infertility cases constitute >50% of patients seen in clinic (n=25), n (%) | Male infertility cases constitute <50% of patients seen in clinic (n=24), n (%) | P value* |
|---|---|---|---|
| Utility of SDF in clinical scenarios | |||
| High grade varicocele on physical examination (clinical grades 2/3) with normal semen parameters | 12 (48.0) | 18 (75.0) | 0.05 |
| Low grade varicocele on physical examination (clinical grade 1) with subnormal semen parameters | 11 (44.0) | 12 (50.0) | 0.33 |
| A 35-year-old gentleman presenting with primary infertility who has normal semen parameters and whose partner evaluation fails to identify female factors | 14 (56.0) | 14 (58.3) | 0.44 |
| In a couple presenting with recurrent first trimester natural pregnancy loss | 22 (88.0) | 19 (79.2) | 0.32 |
| In couples presenting with recurrent IUI failure or pregnancy loss following IUI | 18 (72.0) | 16 (66.7) | 0.32 |
| In couples presenting with recurrent conventional IVF failure or pregnancy loss following conventional IVF | 23 (92.0) | 22 (91.6) | 0.48 |
| In couples presenting with recurrent pregnancy loss following ICSI | 24 (96.0) | 18 (75.0) | 0.03 |
| Would a SDF test result affect your decision to utilize testicular instead of ejaculated sperm for ICSI? | 15 (60.0) | 18 (75.0) | 0.13 |
| In a 42-year-old gentleman presenting with infertility and risk factors such as cigarette smoking, obesity and exposure to environmental/therapeutic/occupational gonadotoxins | 17 (68.0) | 15 (62.5) | 0.57 |
| Perceived drawbacks | |||
| High cost | 10 (40.0) | 13 (54.2) | 0.21 |
| Poor validation | 11 (44.0) | 7 (29.2) | 0.16 |
| Low precision | 4 (16.0) | 5 (20.3) | 0.3 |
| Low accuracy | 4 (16.0) | 3 (12.5) | 0.38 |
| Long turnaround time | 3 (12.0) | 3 (12.5) | 0.27 |
| Others | 6 (24.0) | 4 (16.6) | 0.29 |
*, Chi-square. SDF, sperm DNA fragmentation; IUI, intrauterine insemination; ICSI, intracytoplasmic sperm injection; IVF, in vitro fertilization; NPL, natural pregnancy loss.
Discussion
This study demonstrates for the first time that SDF testing has been well received among fertility specialists as a valuable tool for the evaluation of male infertility. Out of the 49 respondents to this study’s questionnaire, 39 (79.6%) considered using SDF testing in their clinical practice. While this interesting finding can be reasonably explained by the abundance of reports examining SDF in recent years, it also calls for an update to reproductive societies clinical practice guidelines. The field of male infertility has grown rapidly over the past few decades with the majority of health-care providers receiving specialized training and providing a dedicated infertility service. Forty respondents (81.6%) attended post graduate training in male infertility and more than half the respondents offered a service in which male infertility patients constituted >50% of its cases. Clearly this advancement indicated an interest in investigating the root cause of male factor infertility in contrast to the previous solely female focused management.
Various clinical scenarios were proposed in the clinical guideline article by Agarwal et al. (20) for which SDF testing could provide valuable information to guide therapeutic interventions. Varicocele is one condition which has been linked to elevated measures of SDF. Such an association is believed to occur either secondary to varicocele induced testicular hyperthermia affecting DNA synthesis enzymes during spermatogenesis (35) or due to an oxidative stress induced DNA damage (36). Several studies have confirmed the presence of significantly higher SDF levels among patients with varicocele and demonstrated significant improvements in SDF after varicocelectomy (21,22,37,38). While current guidelines only indicate surgery for varicocele patients with abnormal semen analysis and no other significant causes of infertility, it obviously overlooks important functional information about the sperm that could have significant influence on the couples’ fertility (39). In fact, abnormally high SDF levels were observed in varicocele patients with normal standard semen parameters (40). In this study, we assessed the utility of SDF in high grade varicocele with normal semen analysis and low grade varicocele with subnormal semen analysis. A percent of 61.2 respondents admitted using SDF testing for the former while 46.9% for the latter. This result was anticipated as studies investigating SDF levels in lower grades of varicocele, though present, are still scarce (38,41). Furthermore, participants with higher expertise utilized SDF testing in patients with high grade varicocele and normal semen parameters significantly less than participants with lower expertise (P=0.05). While this finding seems incongruous, it may be because participants with higher expertise are more assertive in management decision of varicocele, nonetheless, no reasonable conclusion can be made unless their treatment decisions in this specific clinical scenario were investigated.
A percent of 85.7 respondents considered utilizing SDF in the evaluation of couples with RPL. This high response rate could be attributed to the fact that in such patients there is always a notion to understand the possible etiology of the miscarriage. Several reports did confirm the presence of high SDF levels in couples with RPL (23,24). Further, SDF is rightly believed to play a significant role in early embryo development. In vitro studies examining the influence of sperm DNA integrity on embryo quality and implantation revealed that higher percentage of poor quality embryos and lower implantation rates were present in patients with high SDF (42).
This study reveals that SDF is perhaps the most commonly considered test during the evaluation of couples opting for assisted reproductive therapy (ART); where 91.8% of respondents consider using SDF testing for patients with recurrent conventional IVF failure or pregnancy loss following conventional IVF, 85.7% consider using SDF testing in couples presenting with RPL following ICSI, and 69.4% consider using SDF testing in couples presenting with recurrent IUI failure or pregnancy loss following IUI. It also appears that the expertise of participants had a significant effect on the utility of SDF in cases of RPL following ICSI, where 24 (96%) participants with higher expertise and 18 (75%) participants with lower expertise utilized SDF in this particular clinical scenario (P=0.02). These responses acknowledge the significant information a SDF test could provide in the ART setting and partly represent the views of the American Society for Reproductive Medicine (ASRM) guidelines regarding SDF testing in this particular scenario. While the ASRM guidelines dismissed the routine use of SDF testing for the evaluation of infertile men based on unavailability of level 1 evidence supporting such a role, it acknowledged the value of assessing SDF for couples undergoing ART (32). It seems that the endorsement of a professional society plays a role in the frequency of positive responses. We can certainly debate the ASRM recommendation based on the availability of enormous recent evidence in support of the utility of SDF (20), and the fact that finding a diagnostic test with level 1 evidence may be too difficult especially in this field of medicine where the outcome is multifactorial. Moreover, the clinical usefulness of any given test may in certain circumstances outweigh its statistical significance. Compelling evidence extracted from systemic reviews and meta-analyses suggest that SDF affects the clinical outcome of IUI, conventional IVF and ICSI (29,30,43). High levels of SDF have been associated with lower pregnancy rates with IUI [odds ratio (OR) 9.9; 95% confidence interval (CI), 2.37; P<0.001], and with conventional IVF (OR 1.57; 95% CI, 1.18–2.07, P<0.05), but not with ICSI (OR 1.14; 95% CI, 0.86–1.54, P=0.65) (29). Moreover, high SDF levels were associated with a significant increase in pregnancy loss after conventional IVF and ICSI (combined OR 2.48; 95% CI, 1.52–4.04; P<0.0001) (29).
The latter relationship triggered search for treatment methods that could be performed to improve the clinical outcome of ICSI. Among various options such as abstinence time reduction, repeated ejaculation and use of different sperm selection techniques, the utility of testicular sperm offered the best hope in improving ICSI outcome (44-47). This option was based on the realization that testicular sperm may have lower SDF levels as most DNA damage is believed to occur during the epididymal transit of sperm (48). Few reports have confirmed this phenomenon by finding significantly higher levels of SDF in ejaculated sperm compared with testicular sperm (49,50) as well as reporting significantly higher live birth rates after ICSI from testicular sperm in comparison to ejaculated sperm (51,52). A percent of 69.4% respondents of this study’s questionnaire confirmed that the SDF result could influence their decision to utilize testicular sperm rather than ejaculated sperm for ICSI.
Environmental and life style exposures are significant causes of oxidative stress induced DNA damage (53). The utility of SDF testing in patients with risk factors to such damage has been proposed in order to guide prevention or treatment and monitor the response of intervention. 65.3% of respondents to this study’s questionnaire considered using SDF testing for patients presenting with infertility and risk factors such as cigarette smoking, obesity and exposure to environmental/therapeutic/occupational gonadotoxins.
While this study reveals a general acceptance towards the utility of SDF in clinical practice, it also highlights the important drawbacks that still hinder its wide spread use. High test cost, poor validation, low precision, low accuracy and long turnaround time were reported by 23 (46.9%), 18 (36.7%), 9 (18.3%), 7 (14.2%) and 6 (12.2%), respectively. When comparisons were made according to the participants’ level of expertise, no significant differences were observed. Cost has always been an important consideration in any medical treatment. It is particularly significant in this instance as most of fertility related costs are often not covered by medical insurance. The reported mean cost ± SD of SDF testing is 170.4±122.9 USD. While this is a significant sum, the additional information this testing method provides, should aid the clinician in selecting the best therapeutic intervention thereby increasing the chances of pregnancy, while eliminating unnecessary/less successful and costly procedures.
Test validation and predictive accuracy are very important aspects to consider when dealing with any investigative test in medicine. The presence of various SDF testing methods and cut-off values could explain its slightly reduced diagnostic yield. SCSA and TUNEL were utilized by 30.6% of respondents, followed by the Halo test (20.4%), Comet (6.1%) and other methods (12.2%). Also, cut-off values of 10%, 20%, 30% and others were reported by 4.1%, 10.2%, 61.2% and 24.4% of respondents, respectively. Additionally, studies investigating SDF tests in infertility have had non-standardized methodologies and outcome measures. Nonetheless, the continued use of SDF testing delivered meaningful refinements not only to its practical methods but also to its diagnostic thresholds and predictive power. The SCSA, SCD and TUNEL tests have been recently standardized and/or validated (12,54,55). Emerging data is providing further support for the use of SDF testing as a fertility assessment tool and as a predictor of natural pregnancy with a sensitivity of 80–85% and a specificity of 85–90% reported with SCD and TUNEL assays (56,57). As for the outcome of ART, SDF testing with SCD accurately predicted pregnancy with a sensitivity of 86.2% and negative predictive value of 72.2% (58).
Conclusions
This study reveals that SDF testing is being utilized in the evaluation of male infertility among specialists worldwide. SCSA and TUNEL are the two most commonly used, followed by SCD and Comet. SDF use appears to be favored in couples presenting with recurrent conventional IVF failure and pregnancy loss following IVF and ICSI followed by recurrent NPL. Drawbacks such as high cost, poor validation and low accuracy were most commonly perceived as potential factors slowing the widespread use of SDF in clinical practice.
Acknowledgements
None.
Informed Consent: Written informed consent was obtained from the participants for publication of this article.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Cooper TG, Noonan E, von Eckardstein S, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update 2010;16:231-45. 10.1093/humupd/dmp048 [DOI] [PubMed] [Google Scholar]
- 2.Gelbaya TA, Potdar N, Jeve YB, et al. Definition and epidemiology of unexplained infertility. Obstet Gynecol Surv 2014;69:109-15. 10.1097/OGX.0000000000000043 [DOI] [PubMed] [Google Scholar]
- 3.Pollister AW, Mirsky AE. The nucleoprotamine of trout sperm. J Gen Physiol 1946;30:101-16. 10.1085/jgp.30.2.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Getzoff PL. Application of current research data in the diagnosis and clinical management of male subfertility. Fertil Steril 1963;14:507-14. 10.1016/S0015-0282(16)34985-8 [DOI] [PubMed] [Google Scholar]
- 5.Leuchtenberger C, Leuchtenberger R, Schrader F, et al. Reduced amounts of desoxyribose nucleic acid in testicular germ cells of infertile men with active spermatogenesis. Lab Invest 1956;5:422-40. [PubMed] [Google Scholar]
- 6.Leuchtenberger C, Weir DR, Schrader F, et al. The desoxyribose nucleic acid (DNA) content in spermatozoa of repeated seminal fluids from fertile and infertile men. J Lab Clin Med 1955;45:851-64. [PubMed] [Google Scholar]
- 7.Ringertz NR, Gledhill BL, Darzynkiewicz Z. Changes in deoxyribonucleoprotein during spermiogenesis in the bull. Sensitivity of DNA to heat denaturation. Exp Cell Res 1970;62:204-18. 10.1016/0014-4827(79)90521-4 [DOI] [PubMed] [Google Scholar]
- 8.Evenson DP, Darzynkiewicz Z, Melamed MR. Relation of mammalian sperm chromatin heterogeneity to fertility. Science 1980;210:1131-3. 10.1126/science.7444440 [DOI] [PubMed] [Google Scholar]
- 9.Hughes CM, Lewis SE, McKelvey-Martin VJ, et al. A comparison of baseline and induced DNA damage in human spermatozoa from fertile and infertile men, using a modified comet assay. Mol Hum Reprod 1996;2:613-9. 10.1093/molehr/2.8.613 [DOI] [PubMed] [Google Scholar]
- 10.Sun JG, Jurisicova A, Casper RF. Detection of deoxyribonucleic acid fragmentation in human sperm: correlation with fertilization in vitro. Biol Reprod 1997;56:602-7. 10.1095/biolreprod56.3.602 [DOI] [PubMed] [Google Scholar]
- 11.Fernández JL, Muriel L, Rivero MT, et al. The sperm chromatin dispersion test: a simple method for the determination of sperm DNA fragmentation. J Androl 2003;24:59-66. [PubMed] [Google Scholar]
- 12.Sharma R, Ahmad G, Esteves SC, et al. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay using bench top flow cytometer for evaluation of sperm DNA fragmentation in fertility laboratories: protocol, reference values, and quality control. J Assist Reprod Genet 2016;33:291-300. 10.1007/s10815-015-0635-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bungum M, Bungum L, Giwercman A. Sperm chromatin structure assay (SCSA): a tool in diagnosis and treatment of infertility. Asian J Androl 2011;13:69-75. 10.1038/aja.2010.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simon L, Lutton D, McManus J, et al. Sperm DNA damage measured by the alkaline Comet assay as an independent predictor of male infertility and in vitro fertilization success. Fertil Steril 2011;95:652-7. 10.1016/j.fertnstert.2010.08.019 [DOI] [PubMed] [Google Scholar]
- 15.Balasuriya A, Speyer B, Serhal P, et al. Sperm chromatin dispersion test in the assessment of DNA fragmentation and aneuploidy in human spermatozoa. Reprod Biomed Online 2011;22:428-36. 10.1016/j.rbmo.2011.01.012 [DOI] [PubMed] [Google Scholar]
- 16.Simon L, Proutski I, Stevenson M, et al. Sperm DNA damage has a negative association with live-birth rates after IVF. Reprod Biomed Online 2013;26:68-78. 10.1016/j.rbmo.2012.09.019 [DOI] [PubMed] [Google Scholar]
- 17.Spanò M, Bonde JP, Hjøllund HI, et al. Sperm chromatin damage impairs human fertility. The Danish First Pregnancy Planner Study Team. Fertil Steril 2000;73:43-50. [DOI] [PubMed] [Google Scholar]
- 18.Saleh RA, Agarwal A, Nada EA, et al. Negative effects of increased sperm DNA damage in relation to seminal oxidative stress in men with idiopathic and male factor infertility. Fertil Steril 2003;79 Suppl 3:1597-605. 10.1016/S0015-0282(03)00337-6 [DOI] [PubMed] [Google Scholar]
- 19.Evenson DP, Jost LK, Marshall D, et al. Utility of the sperm chromatin structure assay as a diagnostic and prognostic tool in the human fertility clinic. Hum Reprod 1999;14:1039-49. 10.1093/humrep/14.4.1039 [DOI] [PubMed] [Google Scholar]
- 20.Agarwal A, Majzoub A, Esteves SC, et al. Clinical utility of sperm DNA fragmentation testing: practice recommendations based on clinical scenarios. Transl Androl Urol 2016;5:935-50. 10.21037/tau.2016.10.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esteves SC, Gosálvez J, López-Fernández C, et al. Diagnostic accuracy of sperm DNA degradation index (DDSi) as a potential noninvasive biomarker to identify men with varicocele-associated infertility. Int Urol Nephrol 2015;47:1471-7. 10.1007/s11255-015-1053-6 [DOI] [PubMed] [Google Scholar]
- 22.Zini A, Dohle G. Are varicoceles associated with increased deoxyribonucleic acid fragmentation? Fertil Steril 2011;96:1283-7. 10.1016/j.fertnstert.2011.10.016 [DOI] [PubMed] [Google Scholar]
- 23.Khadem N, Poorhoseyni A, Jalali M, et al. Sperm DNA fragmentation in couples with unexplained recurrent spontaneous abortions. Andrologia 2014;46:126-30. 10.1111/and.12056 [DOI] [PubMed] [Google Scholar]
- 24.Absalan F, Ghannadi A, Kazerooni M, et al. Value of sperm chromatin dispersion test in couples with unexplained recurrent abortion. J Assist Reprod Genet 2012;29:11-4. 10.1007/s10815-011-9647-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robinson L, Gallos ID, Conner SJ, et al. The effect of sperm DNA fragmentation on miscarriage rates: a systematic review and meta-analysis. Hum Reprod 2012;27:2908-17. 10.1093/humrep/des261 [DOI] [PubMed] [Google Scholar]
- 26.Oleszczuk K, Augustinsson L, Bayat N, et al. Prevalence of high DNA fragmentation index in male partners of unexplained infertile couples. Andrology 2013;1:357-60. 10.1111/j.2047-2927.2012.00041.x [DOI] [PubMed] [Google Scholar]
- 27.Duran EH, Morshedi M, Taylor S, et al. Sperm DNA quality predicts intrauterine insemination outcome: a prospective cohort study. Hum Reprod 2002;17:3122-8. 10.1093/humrep/17.12.3122 [DOI] [PubMed] [Google Scholar]
- 28.Bungum M, Humaidan P, Axmon A, et al. Sperm DNA integrity assessment in prediction of assisted reproduction technology outcome. Hum Reprod 2007;22:174-9. 10.1093/humrep/del326 [DOI] [PubMed] [Google Scholar]
- 29.Zini A, Sigman M. Are tests of sperm DNA damage clinically useful? Pros and cons. J Androl 2009;30:219-29. 10.2164/jandrol.108.006908 [DOI] [PubMed] [Google Scholar]
- 30.Osman A, Alsomait H, Seshadri S, et al. The effect of sperm DNA fragmentation on live birth rate after IVF or ICSI: a systematic review and meta-analysis. Reprod Biomed Online 2015;30:120-7. 10.1016/j.rbmo.2014.10.018 [DOI] [PubMed] [Google Scholar]
- 31.Zhao J, Zhang Q, Wang Y, et al. Whether sperm deoxyribonucleic acid fragmentation has an effect on pregnancy and miscarriage after in vitro fertilization/intracytoplasmic sperm injection: a systematic review and meta-analysis. Fertil Steril 2014;102:998-1005.e8. 10.1016/j.fertnstert.2014.06.033 [DOI] [PubMed] [Google Scholar]
- 32.Practice Committee of the American Society for Reproductive Medicine The clinical utility of sperm DNA integrity testing: a guideline. Fertil Steril 2013;99:673-7. 10.1016/j.fertnstert.2012.12.049 [DOI] [PubMed] [Google Scholar]
- 33.Jarow J, Sigman M, Kolettis PN, et al. The Optimal Evaluation of the Infertile Male: Best Practice Statement Reviewed and Validity Confirmed, 2011. Available online: https://www.auanet.org/education/guidelines/male-infertility-d.cfm
- 34.Jungwirth A, Giwercman A, Tournaye H, et al. European Association of Urology guidelines on Male Infertility: the 2012 update. Eur Urol 2012;62:324-32. 10.1016/j.eururo.2012.04.048 [DOI] [PubMed] [Google Scholar]
- 35.Fujisawa M, Yoshida S, Matsumoto O, et al. Decrease of topoisomerase I activity in the testes of infertile men with varicocele. Arch Androl 1988;21:45-50. 10.3109/01485018808986732 [DOI] [PubMed] [Google Scholar]
- 36.Agarwal A, Hamada A, Esteves SC. Insight into oxidative stress in varicocele-associated male infertility: part 1. Nat Rev Urol 2012;9:678-90. 10.1038/nrurol.2012.197 [DOI] [PubMed] [Google Scholar]
- 37.Smit M, Romijn JC, Wildhagen MF, et al. Decreased sperm DNA fragmentation after surgical varicocelectomy is associated with increased pregnancy rate. J Urol 2013;189:S146-50. 10.1016/j.juro.2012.11.024 [DOI] [PubMed] [Google Scholar]
- 38.Ni K, Steger K, Yang H, et al. Sperm protamine mRNA ratio and DNA fragmentation index represent reliable clinical biomarkers for men with varicocele after microsurgical varicocele ligation. J Urol 2014;192:170-6. 10.1016/j.juro.2014.02.046 [DOI] [PubMed] [Google Scholar]
- 39.Practice Committee of the American Society for Reproductive Medicine. Society for Male Reproduction and Urology Report on varicocele and infertility: a committee opinion. Fertil Steril 2014;102:1556-60. 10.1016/j.fertnstert.2014.10.007 [DOI] [PubMed] [Google Scholar]
- 40.Smith R, Kaune H, Parodi D, et al. Increased sperm DNA damage in patients with varicocele: relationship with seminal oxidative stress. Hum Reprod 2006;21:986-93. 10.1093/humrep/dei429 [DOI] [PubMed] [Google Scholar]
- 41.Sadek A, Almohamdy AS, Zaki A, et al. Sperm chromatin condensation in infertile men with varicocele before and after surgical repair. Fertil Steril 2011;95:1705-8. 10.1016/j.fertnstert.2011.01.008 [DOI] [PubMed] [Google Scholar]
- 42.Simon L, Murphy K, Shamsi MB, et al. Paternal influence of sperm DNA integrity on early embryonic development. Hum Reprod 2014;29:2402-12. 10.1093/humrep/deu228 [DOI] [PubMed] [Google Scholar]
- 43.Zini A. Are sperm chromatin and DNA defects relevant in the clinic? Syst Biol Reprod Med 2011;57:78-85. 10.3109/19396368.2010.515704 [DOI] [PubMed] [Google Scholar]
- 44.Gosálvez J, González-Martínez M, López-Fernández C, et al. Shorter abstinence decreases sperm deoxyribonucleic acid fragmentation in ejaculate. Fertil Steril 2011;96:1083-6. 10.1016/j.fertnstert.2011.08.027 [DOI] [PubMed] [Google Scholar]
- 45.Agarwal A, Gupta S, Du Plessis S, et al. Abstinence Time and Its Impact on Basic and Advanced Semen Parameters. Urology 2016;94:102-10. 10.1016/j.urology.2016.03.059 [DOI] [PubMed] [Google Scholar]
- 46.Xue X, Wang WS, Shi JZ, et al. Efficacy of swim-up versus density gradient centrifugation in improving sperm deformity rate and DNA fragmentation index in semen samples from teratozoospermic patients. J Assist Reprod Genet 2014;31:1161-6. 10.1007/s10815-014-0287-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bradley CK, McArthur SJ, Gee AJ, et al. Intervention improves assisted conception intracytoplasmic sperm injection outcomes for patients with high levels of sperm DNA fragmentation: a retrospective analysis. Andrology 2016;4:903-10. 10.1111/andr.12215 [DOI] [PubMed] [Google Scholar]
- 48.Alvarez JG. DNA fragmentation in human spermatozoa: significance in the diagnosis and treatment of infertility. Minerva Ginecol 2003;55:233-9. [PubMed] [Google Scholar]
- 49.Esteves SC, Sánchez-Martín F, Sánchez-Martín P, et al. Comparison of reproductive outcome in oligozoospermic men with high sperm DNA fragmentation undergoing intracytoplasmic sperm injection with ejaculated and testicular sperm. Fertil Steril 2015;104:1398-405. 10.1016/j.fertnstert.2015.08.028 [DOI] [PubMed] [Google Scholar]
- 50.Moskovtsev SI, Jarvi K, Mullen JB, et al. Testicular spermatozoa have statistically significantly lower DNA damage compared with ejaculated spermatozoa in patients with unsuccessful oral antioxidant treatment. Fertil Steril 2010;93:1142-6. 10.1016/j.fertnstert.2008.11.005 [DOI] [PubMed] [Google Scholar]
- 51.Greco E, Scarselli F, Iacobelli M, et al. Efficient treatment of infertility due to sperm DNA damage by ICSI with testicular spermatozoa. Hum Reprod 2005;20:226-30. 10.1093/humrep/deh590 [DOI] [PubMed] [Google Scholar]
- 52.Arafa M, AlMalki A, AlBadr M, et al. ICSI outcome in patients with high DNA fragmentation: Testicular versus ejaculated spermatozoa. Andrologia 2017. [Epub ahead of print]. 10.1111/and.12835 [DOI] [PubMed] [Google Scholar]
- 53.Sharma R, Biedenharn KR, Fedor JM, et al. Lifestyle factors and reproductive health: taking control of your fertility. Reprod Biol Endocrinol 2013;11:66. 10.1186/1477-7827-11-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Evenson DP. The Sperm Chromatin Structure Assay (SCSA(®)) and other sperm DNA fragmentation tests for evaluation of sperm nuclear DNA integrity as related to fertility. Anim Reprod Sci 2016;169:56-75. 10.1016/j.anireprosci.2016.01.017 [DOI] [PubMed] [Google Scholar]
- 55.McEvoy A, Roberts P, Yap K, et al. Development of a simplified method of human semen storage for the testing of sperm DNA fragmentation using the Halosperm G2 test kit. Fertil Steril 2014;102:981-8. 10.1016/j.fertnstert.2014.07.737 [DOI] [PubMed] [Google Scholar]
- 56.Chenlo PH, Curi SM, Pugliese MN, et al. Fragmentation of sperm DNA using the TUNEL method. Actas Urol Esp 2014;38:608-12. 10.1016/j.acuro.2014.02.022 [DOI] [PubMed] [Google Scholar]
- 57.Wiweko B, Utami P. Predictive value of sperm deoxyribonucleic acid (DNA) fragmentation index in male infertility. Basic Clin Androl 2017;27:1. 10.1186/s12610-016-0046-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.López G, Lafuente R, Checa MA, et al. Diagnostic value of sperm DNA fragmentation and sperm high-magnification for predicting outcome of assisted reproduction treatment. Asian J Androl 2013;15:790-4. 10.1038/aja.2013.81 [DOI] [PMC free article] [PubMed] [Google Scholar]