Abstract
Objective
Recently, several cohort studies suggested a positive relationship between serum uric acid (SUA) and type 2 diabetes mellitus (T2DM), which is inconsistent with the results of functional research. Our aim was to further evaluate this correlation by conducting a systematic review.
Methods
Computerized literature searches of the Medline database, EMBASE database, and PubMed were used to evaluate the relationship between SUA and T2DM in cohort studies. Cochran's Q and I2 statistics were used to evaluate heterogeneity among studies, and pooled relative risk (RR) and odds ratio (OR) with 95% confidence intervals (CIs) were calculated using random-effects and fixed-effects models. The summary RR and OR of per 1 mg/ml-SUA increase were calculated separately because of their different epidemiological implications and calculation methods. Additionally, sensitivity analysis, stratified analysis, meta-regression, and multiple meta-regression were applied to investigate the heterogeneity among studies.
Results
A total of 970 articles were retrieved from the searches. Sixteen publications of cohort studies containing 61,714 participants were included. The pooled RR was 1.131 (95% CI: 1.084–1.179) with significant heterogeneity among studies (I2 = 51.9%, P = 0.018). Adjusted RR to evaluate the stability of the relationship between SUA and T2DM in the sensitivity analysis was similar (RR = 1.140, 95% CI: 1.087–1.197), with statistically significant heterogeneity (I2 = 54.5%, P = 0.015). Stratified analysis and meta-regression showed that the positive relationship remained irrespective of age, sex, region, and adjustment for confounding factors including body mass index, fasting blood glucose, systolic blood pressure, diastolic blood pressure, alcohol consumption, smoking, blood cholesterol, waist circumference, fatty liver, and drugs affecting SUA.
Conclusion
Although SUA is independently associated with development of T2DM, insulin resistance increased as the baseline SUA concentration increased; thus, the correlation between SUA and T2DM requires further evaluation and the baseline insulin resistance status should also be considered.
Keywords: Uric acid, Diabetes, Meta-analysis
Introduction
In 2011, there were 366 million people with diabetes, and this number is expected to rise to 552 million by 2030.1 The majority of the patients have type 2 diabetes mellitus (T2DM). The prevalence of T2DM has become a big public health challenge worldwide. Dietary recommendations and genetic counseling have been taken into consideration in preventing the development of T2DM.2, 3 However, identifying a high risk susceptible population and encouraging lifestyle modification is likely to be the most effective strategy of prevention. Therefore, great efforts have been made to gain insight into T2DM risk factors, including a strong family history of diabetes mellitus, age, obesity, physical inactivity, body mass index (BMI), alcohol intake, serum triglyceride concentration, uric acid concentration, and coronary heart disease.4, 5, 6 Whether the above defined risk factors can be applicable to the global community however requires further investigation.7
Many recent epidemiologic evidences have been devoted to the relationship between serum uric acid (SUA) and T2DM. A meta-analysis of 11 studies reported in 2009 revealed a positive relationship between SUA and the development of T2DM8 but with several limitations existed. First, the progression of T2DM frequently occurs with aging and metabolic syndrome (MS) factors. As a type of MS, increased SUA can also be accompanied with T2DM.9, 10 Second, several more factors, such as fasting blood glucose (FBG), 2-hour post prandial blood glucose (2 h-PBG), family history of diabetes, physical activity, and drugs affecting SUA at baseline, also participate in the progression and development of T2DM. Such factors can be confounding for evaluating the correlation between SUA and T2DM. No sufficient adjustment and/or objective quality assessment was made for these confounding factors in the studies that included in this meta-analysis. Third, a combination of risk ratios (RRs) and odds ratios (ORs) as indicators of RR could have overestimated the actual RR.
Recently, a variety of publications closely examining this association showed discordant results. Thus, the relationship between SUA and T2DM still remains controversial. This meta-analysis also included the most recent 5 studies since 2009 that indicated a positive relationship between SUA and T2DM, and then to better quantify this positive correlation a literature-based systematic review was performed.
Methods
Data selection
We conducted a computerized literature search of the Medline, EMBASE, and PubMed databases. The following algorithm was applied for both the Medical Subject Heading (MeSH) and the full text. The search strings were as follows: [(‘uric acid’ [mesh]) AND (‘type 2 diabetes’ [mesh])] AND [‘uric acid’ AND ‘type 2 diabetes’].
Inclusion and exclusion criteria
Included articles were required to meet the following criteria: (1) inclusion of T2DM as a dominant outcome; (2) measurement of SUA concentration at baseline; (3) at baseline the participants did not have T2DM; (4) RR or OR and its corresponding 95% confidence interval (CI) or sufficient data to calculate them were provided. The articles were excluded if: (1) the outcome was not T2DM; (2) the baseline SUA level was not assessed; (3) RRs or ORs and its corresponding 95% CIs (or data to calculate them) were not given. If data from two or more articles were derived from the same subjects, only the most recent article was included in this analysis.
Data extraction
Two researchers independently screened and assessed each of the potential titles, abstracts, and/or full texts to determine the eligibility for inclusion. If any discrepancies occurred, a third investigator would make the definitive decision for the study eligibility and data extraction. Data extracted for this review included the first author's name, publication year, population studied, baseline SUA (mg/dl), age (years), percentage of men, sample size, number of cases, adjusted RR (95% CI), multivariable adjustment, cohort design, and duration of follow-up. Additionally, the original data of baseline 2h-PBG and the subsequent adjustments were requested from the authors of these primary articles included. Commitments or questionnaires for all of the participants were administered correspondingly in each study of this meta-analysis.
Statistical analysis
In the studies which the analyzed SUA level was defined as a categorical variable, the pooled RR could not be calculated directly from the different results of the SUA stratification analysis. To quantify the dose-response relationship between the baseline SUA level and the development of T2DM, the RR was calculated for the increment of 1 mg/dl SUA in each study. This method for trend estimation was supported by Berlin et al.11 The logarithmic relative risk model is excellent whereas statistical properties of the linear relative risk model are unsuitable for categorical variables.11 The midpoint in each category was estimated by the average of the lower and upper bound. If the highest or the lowest category was open-ended, the interval length at an open-ended would be assumed to be the same as the adjacent interval. The log RR or log OR from each study was calculated by converting the 95% CI to its natural logarithm (width of the CI divided by 3.92).12 The estimates for men and women were synthesized into a combined value using a weighting method in each study to decrease the large heterogeneity across studies.
As the overestimated pooled RR is close to 1 and of little practical importance because the total incidence is relatively rare,8 the RRs and ORs should be evaluated separately for the calculation and epidemiological significance because the two indexes are distinct, and this might be helpful to decrease the potential errors. In assessing heterogeneity among studies, Cochran Q and I2 statistics were used.13 For the Q statistic, a P value <0.10 was considered statistically significant for heterogeneity; for I2, a value >50% was considered a measure of severe heterogeneity. If P value <0.10 (I2 value >50%), the random-effects model which DerSimonian and Laird reported was used14, 15; otherwise, the fix-effects model was conducted.
Sensitivity analysis to detect the source of heterogeneity was applied to calculate the overall homogeneity and effect size by excluding one study at a time. The most weighted article was removed from the analysis and a meta-analysis with the remaining articles was then conducted. Additionally, stratification analysis, meta-regression, and multiple meta-regression were used to assess a potential difference in distinct populations characterized by different features, such as gender, age, and geographical area. Only studies that provided RRs were used in the sensitivity analysis, stratification analysis, and meta-regression. A funnel plot and Egger's linear regression test were used to investigate any possible publication bias.16 All the statistical analyses were performed using STATA version 10.0 (STATA, College Station, TX, USA). A two-tailed P value <0.05 was considered to be significant.
Results
Included and excluded articles
A total of 631 articles were retrieved from EMBASE and 441 articles from PubMed. After removing duplicates, 970 articles remained (Fig. 1) whilst 948 articles were then excluded based on their titles. Of the 22 articles remaining, 4 articles were excluded for reasons listed in Fig. 1. Eighteen articles were selected for further full-text review. Another 2 studies were excluded for the reasons presented in Fig. 1. Thus, a total of 16 studies published from January 1st 1975 to March 30th 2012 met the criteria for inclusion in this meta-analysis and systematic review.
Fig. 1.
Flow chart on the articles selection process. T2DM: type 2 diabetes mellitus; UA:uric acid.
Five studies (30%) reported risk prediction models for men and women separately, one of which provided data for men, women, and all of them together. Of the remaining 4 articles, weighted estimates for the general population were conducted to decrease the heterogeneity among studies. Data from two generations were shown in one of the studies. Ultimately, 16 publications including a total of 27 risk prediction models were statistically synthesized by meta-analysis.
Data request from the corresponding authors of included articles
Subsequently, Wang et al replied and supplied a RR adjusted for fasting plasma glucose,17 while the authors of other studies did not reply.
Characteristics of studies
Eight articles were prospective cohort studies and eight were historical cohort studies. Mean baseline SUA level of the subjects ranged from 4.0 to 8.0 mg/ml. Mean age at baseline ranged from 41 to 64 years. Sample size per study ranged from 161 to 8688 and a total of 67,174 participants were included (Table 1). One study considered the effect of diuretic use, the other four adjusted for FBG, one of which only referring to blood glucose level. However, there was no study that considered both diuretic use and blood glucose level simultaneously. None of the risk measurements were adjusted for 2h-PBG or for other drugs that influenced SUA level such as allopurinol.
Table 1.
Characteristics of included studies.
| First author's name | Publication year | Cohort design | Mean baseline SUA, mg/dl | Mean age, years | Sample size, n | Number of cases | Adjusted RR (95% CI) | Multivariable adjustment |
|---|---|---|---|---|---|---|---|---|
| Medalie13 | 1975 | H | 4.8 | 49 | 8688 | 344 | 1.15 (0.99–1.32)a | Age, BMI, PVD, SBP, cholesterol, hemoglobin, born in Europe, education |
| Ohlson14 | 1988 | H | 5.3 | 50 | 766 | 47 | 1.27 (1.0–1.58)a | Glutamic pyruvic transaminase, blood glucose, BMI, Bilirubin, SBP, FHD |
| Perry15 | 1995 | P | 6.0 | 50 | 7577 | 194 | 1.15 (0.96–1.36)a | Age, BMI, prevalent coronary heart disease, physical activity, alcohol, smoking, SBP, high density lipoprotein cholesterol, heart rate |
| Chou16 | 1998 | H | 5.8 | 50 | 654 | 39 | 1.73 (1.17–2.57)b | NA |
| Taniguchi17 | 2001 | P | 5.2 | 41 | 6478 | 639 | 1.01 (0.94–1.09)a | Age, BMI, alcohol, smoking, physical activity, FBG, FHD |
| Meisinger18 | 2002 | H | Survey | |||||
| Men18 | 5.7 | 52 | 3052 | 128 | 1.04 (0.91–1.20)a | |||
| Women18 | 4.0 | 51 | 3114 | 85 | 1.60 (1.34–1.91)a | |||
| Lin19 | 2004 | H | NA | |||||
| Men19 | 8.0 | 49 | 293 | 27 | 0.85 (0.62–1.17)b | |||
| Women19 | 7.1 | 55 | 161 | 21 | 1.46 (1.08–1.98)b | |||
| Chien20 | 2008 | H | 5.6 | 54 | 2690 | 548 | 1.09 (1.01–1.17)a | Age, BMI, alcohol, exercise, marital status, education level, occupation, FHD, MS |
| Dehghan21 | 2008 | P | 5.4 | Over 55 | 4536 | 462 | 1.09 (1.03–1.16)a | Age, sex, BMI, WC, SBP/DBP, HDL-cholesterol |
| Nan22 | 2008 | H | cohort, serum creatinine, alcohol drinking, history of hypertension, FHD and ethnicity, fasting serum insulin | |||||
| Men22 | 6.6 | 41 | 1941 | 337 | 1.13 (1.05–1.23)a | |||
| Women22 | 5.0 | 42 | 2318 | 379 | 1.04 (0.96–1.14)a | |||
| Kramer23 | 2009 | H | 5.7 | 63.3 ± 8.6 | 566 | 55 | 1.63 (1.21–2.19)b | Age, sex, BMI, diuretic use, estimated glomerular filtration rate |
| Rathmann24 | 2009 | P | 5.1 ± 1.3 | 63.9 ± 5.4 | 887 | 93 | 1.70 (1.3–2.3)b | Age, sex |
| Men24 | 6.3 ± 1.3 | 63.4 ± 5.4 | 449 | 60 | 1.50 (1.1–2.2)b | |||
| Women24 | 4.96 ± 1.3 | 62.9 ± 5.4 | 438 | 33 | 2.20 (1.3–3.9)b | |||
| Bhole25 | 2010 | P | Age, sex, BMI, alcohol consumption, smoking, physical activity, hypertension, blood glucose level, blood cholesterol level, creatinine level, serum triglyceride level | |||||
| Original25 | 4.3 ± 1.1 | 45 | 4883 | 641 | 1.20 (1.11–1.28)a | |||
| Offspring25 | 5.7 ± 1.4 | 37 | 4292 | 497 | 1.15 (1.06–1.23)a | |||
| Yamada26 | 2011 | P | Age. BMI, FHD, hypertension, triglyceride, fatty liver, alcohol, smoking | |||||
| Men26 | 5.97 ± 1.21 | 48.4 ± 10.2 | 7114 | 576 | 1.00 (0.92–1.09)b | |||
| Women26 | 4.27 ± 0.92 | 50.0 ± 9.1 | 5529 | 221 | 1.36 (1.17–1.58)b | |||
| Tiange27 | 2011 | P | 4.78 ± 1.50 | 61.6 | 924 | 98 | 1.199 (1.033–1.391)a | Age, sex, BMI, FHD, smoking, alcohol, SBP/DBP, HDL-cholesterol, total cholesterol, triglyceride, FBG, fasting insulin, serum creatinine, white blood cell, high sensitive C-reactive protein |
| Kai28 | 2011 | P | 5.22 ± 1.33 | 45–64 | 711 | 68 | 1.426 (1.17–1.705)a | BMI, PP, PPI, SBP, heart rate, FBG, WC, total cholesterol, HDL-C |
SUA: serum uric acid; PVD: peripheral vascular disease; FHD: family history of diabetes; FBG: fasting blood glucose; HDL: high density lipoprotein; MS: metabolic syndrome; WC: waist circumference; BMI: body mass index; SBP/DBP: systolic/diastolic blood pressure; alcohol: alcohol consumption; PP: pulse pressure; PPI: pulse pressure index; NA: not available; H: Historical; P: Prospective.
aHR or RR; bAdjusted OR.
Mean follow-up duration ranged from 2.0 to 62.0 years with 9 articles conducted among the European population, and the other 7 articles were among the Asian population. Four articles included men only, while the rest articles included both men and women. Other relevant study characteristics are tabulated in Table S1.
Results of the meta-analysis
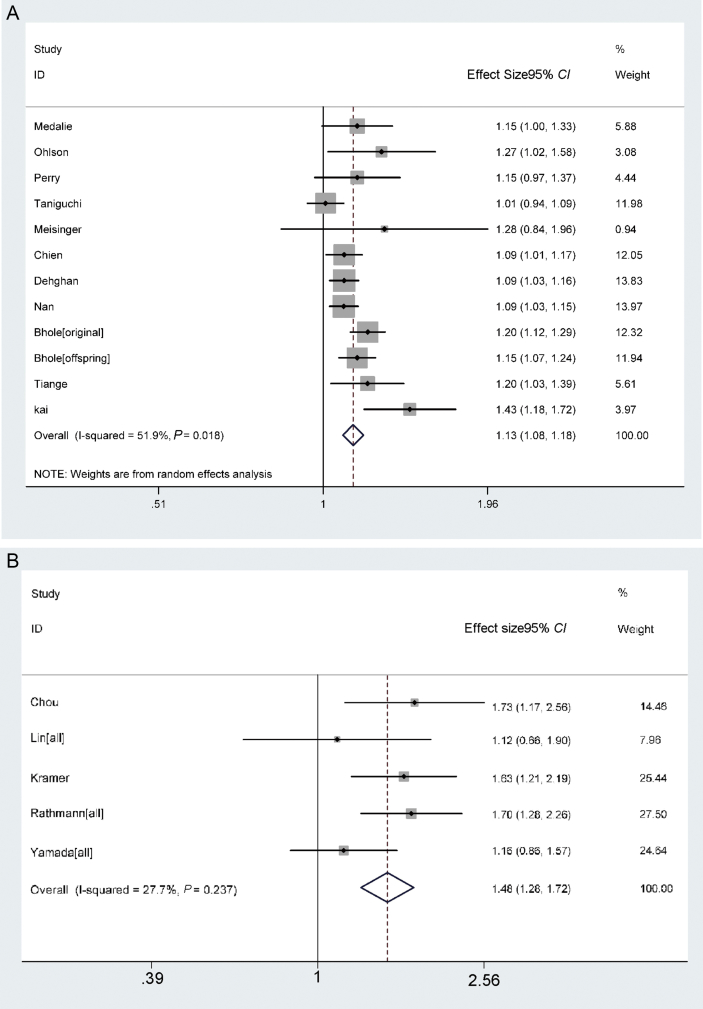
A forest plot with RRs (95% CIs) and pooled estimates of increased risk of T2DM with respect to per 1 mg/dl increase in SUA is presented in Fig. 2. A random-effects model showed that the pooled adjusted RR and its 95% CI was 1.131 (1.084–1.179), and the pooled adjusted OR and its 95% CI was 1.484 (1.278–1.723). Heterogeneity of RR and OR observed among these studies were 51.9% (Q = 22.86, P = 0.018) and 27.7% (Q = 5.53, P < 0.237). The pooled estimates were synthesized for men and women of each study separately and that significantly decreased the heterogeneity of RR among studies from 68.4% to 51.9% and of OR from 81.6% to 27.7%.
Fig. 2.
Forest plot of risk of T2DM for each mg/dl increase in SUA. A. Overall RR (with corresponding 95% CIs) respectively for risk of type 2 diabetes for each mg/dl increase in SUA from random effect model. Diamonds are overall relative risk; Horizontal lines indicate 95% CIs. B. Overall OR (with corresponding 95% CIs) respectively for risk of type 2 diabetes for each mg/dl increase in SUA from fix effect model. Diamonds are overall relative risk; Horizontal lines indicate 95% CIs.
T2DM: type 2 diabetes mellitus; SUA: serum uric acid.
In the sensitivity analysis to evaluate the stability of the relationship between SUA and T2DM, the adjusted RR was still similar (RR = 1.140, 95% CI: 1.087–1.197), with evidence of statistically significant heterogeneity (P = 0.015, I2 = 54.5%).
The studies were stratified by gender, geographic region, age, confounding factors, and other study properties relevant to study quality. For those with previously elevated SUA, the risk of having T2DM was attenuated by adjusting for all of the above factors (all pooled RRs were ≥1). The findings were similar irrespective of the physical activity (P = 0.661) or family history of diabetics (P = 0.131). Effect of diuretic use was considered in only one study (RR = 1.63) (Table 2). In the multiple regression analysis of confounding factors for T2DM, the P-values of all variables included were >0.05 (Table S2).
Table 2.
Stratified and meta-regression analysis to explore the effects of study characteristics on T2DM.
| Variable | Stratum | Studies (n) | RR | Tests for heterogeneity |
Meta-regression |
||
|---|---|---|---|---|---|---|---|
| Q | P | I2 (%) | P | ||||
| Sex | Male | 7 | 1.078 (1.031–1.127) | 10.21 | 0.116 | 41.2 | 0.151 |
| Female | 3 | 1.328 (0.960–1.837) | 20.98 | <0.05 | 90.5 | ||
| Geographical area | Western | 8 | 1.128 (1.094–1.163) | 7.52 | 0.377 | 6.9 | 0.845 |
| Asia | 4 | 1.141 (1.018–1.279) | 13.99 | 0.004 | 77.9 | ||
| Age, years | <50 | 5 | 1.114 (1.048–1.185) | 12.40 | 0.015 | 67.7 | 0.865 |
| 50–60 | 5 | 1.204 (1.075–1.349) | 8.16 | 0.086 | 51.0 | ||
| ≥60 | 2 | 1.115 (1.087–1.145) | 1.36 | 0.244 | 26.5 | ||
| SUA, mg/dl | <5.5 | 8 | 1.159 (1.078–1.246) | 20.9 | 0.004 | 66.5 | 0.832 |
| ≥5.5 | 4 | 1.107 (1.066–1.150) | 1.7 | 0.637 | 0.0 | ||
| Study design | Historical | 5 | 1.104 (1.057–1.152) | 2.67 | 0.614 | 0.0 | 0.759 |
| Prospective | 7 | 1.145 (1.073–1.221) | 19.82 | 0.003 | 69.7 | ||
| Follow-up, years | ≤10 | 7 | 1.083 (1.046–1.122) | 7.02 | 0.319 | 14.5 | 0.307 |
| >10 | 5 | 1.179 (1.100–1.264) | 10.36 | 0.035 | 61.4 | ||
| Adjustment | |||||||
| Family history of DM | Yes | 5 | 1.080 (1.040–1.120) | 7.27 | 0.122 | 45.0 | 0.131 |
| No | 7 | 1.150 (1.109–1.192) | 9.84 | 0.131 | 39.0 | ||
| Physical activity | Yes | 6 | 1.124 (1.055–1.197) | 13.44 | 0.094 | 46.9 | 0.661 |
| No | 6 | 1.113 (1.072–1.156) | 9.41 | 0.020 | 62.8 | ||
| FBG | Yes | 5 | 1.166 (1.064–1.279) | 12.34 | 0.001 | 78.1 | 0.485 |
| No | 7 | 1.100 (1.063–1.138) | 3.08 | 0.799 | 0.00 | ||
| BMI | Yes | 10 | 1.139 (1.084–1.197) | 21.6 | 0.01 | 58.3 | 0.633 |
| No | 2 | 1.091 (1.030–1.156) | 0.59 | 0.444 | 0.00 | ||
| SBP | Yes | 9 | 1.137 (1.104–1.171) | 13.56 | 0.094 | 41.0 | 0.077 |
| No | 3 | 1.053 (1.000–1.109) | 2.92 | 0.232 | 31.5 | ||
| Alcohol | Yes | 7 | 1.115 (1.063–1.170) | 13.6 | 0.034 | 55.9 | 0.314 |
| No | 5 | 1.199 (1.076–1.336) | 8.87 | 0.065 | 54.9 | ||
| Smoking | Yes | 5 | 1.126 (1.082–1.172) | 12.4 | 0.015 | 67.7 | 0.917 |
| No | 7 | 1.126 (1.069–1.186) | 10.08 | 0.122 | 40.4 | ||
| TC | Yes | 7 | 1.152 (1.112–1.193) | 9.87 | 0.13 | 39.2 | 0.074 |
| No | 5 | 1.074 (1.034–1.115) | 5.94 | 0.204 | 32.7 | ||
| WC | Yes | 2 | 1.228 (0.945–1.595) | 7.20 | 0.007 | 86.1 | 0.579 |
| No | 10 | 1.115 (1.083–1.148) | 15.66 | 0.074 | 42.5 | ||
Summary relative risk for the relationship between uric acid and T2DM by gender, geographical area, adjustments (family history of DM, physical activity, FBG, BMI, SBP, alcohol, smoking, total cholesterol, waist circumference and so on), and meta regression analysis to explore the effects of study characteristics except the analytic stratification variable. Pooled RRs of T2DM for each 1 mg/dl increase in SUA within the strata of each study characteristic are indicated.
SUA: serum uric acid; DM: diabetes mellitus; FBG: fasting blood glucose; BMI: body mass index; SBP: systolic blood pressure; WC: waist circumference; TC: total cholesterol.
For young adults (18–30 years) without MS, each unit increase in SUA was associated with increased overall risk of type 2 diabetes (OR = 1.22, 95% CI: 1.07–1.38).18 RRs for the development of diabetes corresponding to per mg/dl increase in SUA were 1.27 (1.06–1.52) in pre-menopausal women and 1.21 (1.09–1.35) in post-menopausal population respectively.19 Whereas a relatively higher incidence of diabetes was found in post-menopausal hyperuricemic women compared with pre-menopausal women (OR = 3.88, 95% CI: 1.92–7.91).
Publication bias
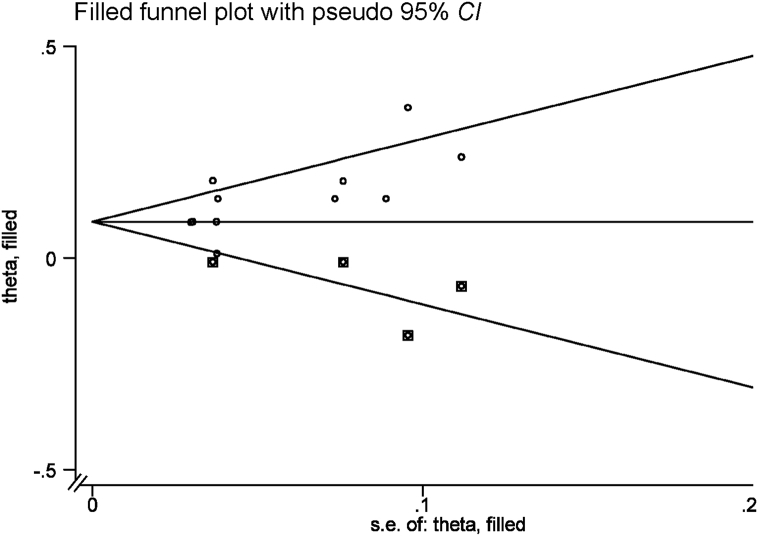
Significant funnel plot asymmetry for the relationship between uric acid and T2DM is shown in Fig. 3. P-value for Begg's regression test was less than 0.01, which indicates a high risk of publication bias.
Fig. 3.
Funnel plot of cohort studies to evaluate the relationship of serum uric acid and type 2 diabetes. Begg's regression test, P < 0.5.
Discussion
This systematic review aimed to further qualify the relationship between SUA and the development of T2DM. Recently (since 2009) 5 related studies were published. The results of this meta-analysis indicated that each 1 mg/dl increase in SUA led to a 13.1% increase in the risk of T2DM (pooled RR) and a 48.4% increase in the risk of T2DM (pooled OR). Stratified analysis by age, gender, geographical area, SUA, study design, duration of follow-up, and confounding factors further indicated that SUA was positively related with T2DM. In addition, multiple meta-regression did not show these variables influenced the correlation between SUA and T2DM.
The results of the previous meta-analysis published in 2009 suggested that SUA was positively associated with the development of T2DM.8 Several limitations of that meta-analysis have been discussed in the Introduction section of this manuscript. Given that those limitations might influence the accuracy of results, the methods in this study were thus improved. First, to decrease the bias due to the combination of RRs and ORs, here, the Meta-Analysis was conducted for RRs and ORs separately. The estimates for men and women from each study were synthesized by a weighting method and further combined afterwards. The heterogeneity of the RR among studies decreased from 68.4% to 51.9% and of the OR from 81.6% to 27.7%, which should provide a more reliable summary RR. We also have contacted the corresponding authors of each article for complementary information about the RR adjusted for FBG and 2h-PBG, as well as drugs affecting SUA concentration and other confounding factors. Despite few responses, more research should be conducted to sufficiently assess the relationship with the help of the authors of the articles included in this study.
Elevated SUA predicts T2DM not only among the young but also the elderly,18 especially the pre-menopausal and post-menopausal women.20 Costa et al21 described a positive association between SUA and the development of T2DM in 2002, but did not provide RRs or data to calculate such an association and thus, this article was not included in our meta-analysis. Metabolic risk factors, especially elevated SUA, are independent predictors of diabetes and impaired glucose tolerance (IGT) in Mauritian normoglycemic subjects over 5 years of follow-up.19 However, this study provided the risk of diabetes and IGT together and thus it was not included in our meta-analysis either.22 Even though a positive relationship between SUA and T2DM in these studies was presented, there is conflicting evidence on epidemiology and on biology, presented as follows.
It has been reported that the progression of T2DM frequently occurs with aging and MS factors and vice-versa.9 As a type of MS, the occurrence of SUA could also be paralleled by the development of T2DM. Thus, the possibility of a correlation rather than causation between SUA and T2DM should not be excluded. As Cook et al23 reported, up to 8.0 mmol/L, a positive relationship was observed between serum glucose status and SUA concentrations whereas lower SUA levels were observed at higher levels of glucose. Therefore, an inverse V-shaped relationship should also be considered. In addition, lowering SUA concentration could prevent nephropathy in T2DM.24, 25, 26 However, the effect of lowering SUA on the prevention and treatment of T2DM is still unknown.
Biologically, as a systemic marker of oxidative status, SUA is strongly linked to insulin resistance (a pathogenic mechanism of T2DM) by inhibiting the production of nitric oxide27 or increasing the expression of C-reactive protein.28 Such practice would activate platelet adhesiveness,29, 30 and induces endothelial dysfunction,31 which blocks insulin-stimulated glucose uptake. On the contrary, other studies have reported that lowering SUA concentration might not be an effective strategy for restoring endothelial function32, 33 and might not lower the risk of development of T2DM. Additionally, Pfister et al34 stated that SUA is not responsible for the development of T2DM and reported limited expectations that uric-acid-lowering drugs will be effective in the prevention of T2DM.
The underlying mechanism of T2DM included insulin resistance, hyperinsulinemia, and a variety of metabolic abnormalities, which also might increase SUA concentration. Hyperinsulinemia caused by insulin resistance is inversely related to 24 h urinary UA clearance35, 36; insulin resistance can lead to an increase in SUA concentration by both reducing renal UA secretion by renal proximal tubular UA reabsorption enhancement in humans due to an active transport mechanism closely linked to the tubular reabsorption of sodium37, 38, 39, 40, 41 and accumulating substrates for UA production.42 Furthermore, two studies showed that homeostasis model assessment (HOMA) insulin resistance (HOMA-IR) increased as the concentration of SUA elevated at baseline. Chien et al43 reported HOMA-IR was 1.48, 1.63, 1.77, 1.93, and 2.16 from the lowest to the highest quintile of SUA. Whereas Wang et al17 reported HOMA-IR was 0.9, 0.9, 1.3, and 1.8 from the lowest to the highest quantile of SUA (P < 0.001). Therefore, a correlation between SUA and T2DM should be considered.
Because of the conflicting results listed above, further quality assessment should be arranged. First, besides obesity, being female and elderly have been mentioned to be major risk factors for the development of prediabetes and T2DM,44, 45, 46 and all important confounding factors should be adjusted for including parental history of DM, physical activity, age, gender, BMI, drinking, and smoking, and especially FBG/PBG. Unfortunately, none of the included studies adjusted adequately for all of these factors. Second, several anti-hypertensive drugs including losartan and hydrochlorothiazide can increase SUA concentration. Hypertension patients with a higher SUA concentration who are taking these drugs should be excluded. There was only one included study47 that adjusted for diuretic drugs and age, gender, BMI, and also estimated glomerular filtration rate. On the contrary, adjustment for blood pressure (BP) in 6 articles20, 48, 49, 50, 51, 52 has a risk of over-adjustment. Therefore, whether SUA is an innocent bystander or a cause for T2DM needs further exploration.
Although 4 studies19, 20, 51, 53 demonstrated that the association between SUA and DM was heterogeneous for men and women, the pooled analysis showed that the increased risk was similar for men and women. Further investigation into the probable different correlation of SUA and T2DM should be conducted between men and women instead of adjustment.
The main limitation of the present study is the statistical publication bias, because each publication step was inevitably affected by the factors of publication year, editors, authors, and the results found. According to the results of the meta-regression classified by the publication year, the reported relationship between SUA and T2DM was significantly different at each time. Studies with positive results are more likely to be accepted. The possibility that studies with negative results did not have the opportunity to be published should also be considered.
Next, the RR calculation for per 1 mg/dl increase in SUA to quantify the dose-response relationship between the baseline SUA level and incidence of T2DM may have overestimated the magnitude of any publication bias.8
In conclusion, the results of this meta-analysis indicate that SUA is independently associated with development of T2DM, both in men and women, in the elderly and the young, in pre-menopausal and post-menopausal women. Insulin resistance increased as baseline SUA concentration increased; thus, the correlation between SUA and T2DM should be further evaluated and baseline insulin resistance status should be considered. Therefore, more evidence of the epidemic etiology, mechanisms, and especially genetics are urgently needed to further clarify whether the relationship between SUA and the development of T2DM is causal or simply a co-occurrence. In addition, studies investigating the effect of interventions to lower SUA concentrations in T2DM are warranted.
Conflicts of interest
None declared in the conflict of interest statement.
Acknowledgments
This work was supported by the Science & Technology Program of Jiangsu Province (Grant No. BE2009681) and the Priority Academic Program for the Development of Jiangsu Higher Education Institutions (Public Health and Preventive Medicine), and grants to X. Wu from the National Natural Science Foundation of China (81261120566), Jiangsu Province key medical personnel project (RC2011068) and 333 projects in the fourth phase of Jiangsu Province (BRA2015389).
Footnotes
Peer review under responsibility of Chinese Medical Association.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.cdtm.2016.09.003.
Contributor Information
Chong Shen, Email: sc@njmu.edu.
Xiao-Hong Wu, Email: drxhwu@njmu.edu.cn.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Whiting D.R., Guariguata L., Weil C., Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Carter P., Khunti K., Davies M.J. Dietary recommendations for the prevention of type 2 diabetes: what are they based on? J Nutr Metab. 2012;2012:847202. doi: 10.1155/2012/847202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waxler J.L., O'Brien K.E., Delahanty L.M. Genetic counseling as a tool for type 2 diabetes prevention: a genetic counseling framework for common polygenetic disorders. J Genet Couns. 2012;21:684–691. doi: 10.1007/s10897-012-9486-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjorge T., Lukanova A., Tretli S. Metabolic risk factors and ovarian cancer in the metabolic syndrome and cancer project. Int J Epidemiol. 2011;40:1667–1677. doi: 10.1093/ije/dyr130. [DOI] [PubMed] [Google Scholar]
- 5.Morrell J.S., Lofgren I.E., Burke J.D., Reilly R.A. Metabolic syndrome, obesity, and related risk factors among college men and women. J Am Coll Health. 2012;60:82–89. doi: 10.1080/07448481.2011.582208. [DOI] [PubMed] [Google Scholar]
- 6.Ding D., Chong S., Jalaludin B., Comino E., Bauman A.E. Risk factors of incident type 2-diabetes mellitus over a 3-year follow-up: results from a large Australian sample. Diabetes Res Clin Pract. 2015;108:306–315. doi: 10.1016/j.diabres.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Lam D.W., LeRoith D. The worldwide diabetes epidemic. Curr Opin Endocrinol Diabetes Obes. 2012;19:93–96. doi: 10.1097/MED.0b013e328350583a. [DOI] [PubMed] [Google Scholar]
- 8.Kodama S., Saito K., Yachi Y. Association between serum uric acid and development of type 2 diabetes. Diabetes Care. 2009;32:1737–1742. doi: 10.2337/dc09-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goncalves J.P., Oliveira A., Severo M., Santos A.C., Lopes C. Cross-sectional and longitudinal associations between serum uric acid and metabolic syndrome. Endocrine. 2012;41:450–457. doi: 10.1007/s12020-012-9629-8. [DOI] [PubMed] [Google Scholar]
- 10.Chen D., Zhang H., Gao Y. Cross-sectional and longitudinal associations between serum uric acid and metabolic syndrome: results from Fangchenggang area male health and examination survey in China. Clin Chim Acta. 2015;446:226–230. doi: 10.1016/j.cca.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 11.Berlin J.A., Longnecker M.P., Greenland S. Meta-analysis of epidemiologic dose-response data. Epidemiology. 1993;4:218–228. doi: 10.1097/00001648-199305000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Jiang Y., Ben Q., Shen H., Lu W., Zhang Y., Zhu J. Diabetes mellitus and incidence and mortality of colorectal cancer: a systematic review and meta-analysis of cohort studies. Eur J Epidemiol. 2011;26:863–876. doi: 10.1007/s10654-011-9617-y. [DOI] [PubMed] [Google Scholar]
- 13.Whitehead A., Whitehead J. A general parametric approach to the meta-analysis of randomized clinical trials. Stat Med. 1991;10:1665–1677. doi: 10.1002/sim.4780101105. [DOI] [PubMed] [Google Scholar]
- 14.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 15.DerSimonian R., Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45:139–145. doi: 10.1016/j.cct.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egger M., Davey S.G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang T., Bi Y., Xu M. Serum uric acid associates with the incidence of type 2 diabetes in a prospective cohort of middle-aged and elderly Chinese. Endocrine. 2011;40:109–116. doi: 10.1007/s12020-011-9449-2. [DOI] [PubMed] [Google Scholar]
- 18.Bennett M., Pandya B.J., Krishnan E. Hyperuricemia as an early marker for type 2 diabetes among young adults. Arthritis Rheum-Us. 2009;60:2033. [Google Scholar]
- 19.Nan H., Qiao Q., Soderberg S. Serum uric acid and incident diabetes in Mauritian Indian and Creole populations. Diabetes Res Clin Pract. 2008;80:321–327. doi: 10.1016/j.diabres.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Lin K.C., Tsai S.T., Lin H.Y., Chou P. Different progressions of hyperglycemia and diabetes among hyperuricemic men and women in the Kinmen study. J Rheumatol. 2004;31:1159–1165. [PubMed] [Google Scholar]
- 21.Costa A., Iguala I., Bedini J., Quinto L., Conget I. Uric acid concentration in subjects at risk of type 2 diabetes mellitus: relationship to components of the metabolic syndrome. Metabolism. 2002;51:372–375. doi: 10.1053/meta.2002.30523. [DOI] [PubMed] [Google Scholar]
- 22.Boyko E.J., de Courten M., Zimmet P.Z., Chitson P., Tuomilehto J., Alberti K.G. Features of the metabolic syndrome predict higher risk of diabetes and impaired glucose tolerance: a prospective study in Mauritius. Diabetes Care. 2000;23:1242–1248. doi: 10.2337/diacare.23.9.1242. [DOI] [PubMed] [Google Scholar]
- 23.Cook D.G., Shaper A.G., Thelle D.S., Whitehead T.P. Serum uric acid, serum glucose and diabetes: relationships in a population study. Postgrad Med J. 1986;62:1001–1006. doi: 10.1136/pgmj.62.733.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doria A., Krolewski A.S. Diabetes: lowering serum uric acid levels to prevent kidney failure. Nat Rev Nephrol. 2011;7:495–496. doi: 10.1038/nrneph.2011.107. [DOI] [PubMed] [Google Scholar]
- 25.Kosugi T., Nakayama T., Heinig M. Effect of lowering uric acid on renal disease in the type 2 diabetic db/db mice. Am J Physiol Renal Physiol. 2009;297:F481–F488. doi: 10.1152/ajprenal.00092.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maahs D.M., Caramori L., Cherney D.Z. Uric acid lowering to prevent kidney function loss in diabetes: the preventing early renal function loss (PERL) allopurinol study. Curr Diab Rep. 2013;13:550–559. doi: 10.1007/s11892-013-0381-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khosla U.M., Zharikov S., Finch J.L. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005;67:1739–1742. doi: 10.1111/j.1523-1755.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 28.Kang D.H., Park S.K., Lee I.K., Johnson R.J. Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol. 2005;16:3553–3562. doi: 10.1681/ASN.2005050572. [DOI] [PubMed] [Google Scholar]
- 29.Alderman M., Redfern J.S. [Serum uric acid – a cardiovascular risk factor?] Ther Umsch. 2004;61:547–552. doi: 10.1024/0040-5930.61.9.547. [in German] [DOI] [PubMed] [Google Scholar]
- 30.Gagliardi A.C., Miname M.H., Santos R.D. Uric acid: a marker of increased cardiovascular risk. Atherosclerosis. 2009;202:11–17. doi: 10.1016/j.atherosclerosis.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 31.Hong Q., Qi K., Feng Z. Hyperuricemia induces endothelial dysfunction via mitochondrial Na+/Ca2+ exchanger-mediated mitochondrial calcium overload. Cell Calcium. 2012;51:402–410. doi: 10.1016/j.ceca.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 32.Waring W.S., McKnight J.A., Webb D.J., Maxwell S.R. Lowering serum urate does not improve endothelial function in patients with type 2 diabetes. Diabetologia. 2007;50:2572–2579. doi: 10.1007/s00125-007-0817-7. [DOI] [PubMed] [Google Scholar]
- 33.Wun Y.T., Chan C.S., Lui C.S. Hyperuricaemia in type 2 diabetes mellitus. Diabetes Nutr Metab. 1999;12:286–291. [PubMed] [Google Scholar]
- 34.Pfister R., Barnes D., Luben R. No evidence for a causal link between uric acid and type 2 diabetes: a Mendelian randomisation approach. Diabetologia. 2011;54:2561–2569. doi: 10.1007/s00125-011-2235-0. [DOI] [PubMed] [Google Scholar]
- 35.Facchini F., Chen Y.D., Hollenbeck C.B., Reaven G.M. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA. 1991;266:3008–3011. [PubMed] [Google Scholar]
- 36.de Oliveira E.P., Burini R.C. High plasma uric acid concentration: causes and consequences. Diabetol Metab Syndr. 2012;4:12. doi: 10.1186/1758-5996-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muscelli E., Natali A., Bianchi S. Effect of insulin on renal sodium and uric acid handling in essential hypertension. Am J Hypertens. 1996;9:746–752. doi: 10.1016/0895-7061(96)00098-2. [DOI] [PubMed] [Google Scholar]
- 38.Quinones G.A., Natali A., Baldi S. Effect of insulin on uric acid excretion in humans. Am J Physiol. 1995;268:E1–E5. doi: 10.1152/ajpendo.1995.268.1.E1. [DOI] [PubMed] [Google Scholar]
- 39.Cappuccio F.P., Strazzullo P., Farinaro E., Trevisan M. Uric acid metabolism and tubular sodium handling. Results from a population-based study. JAMA. 1993;270:354–359. [PubMed] [Google Scholar]
- 40.Perez-Ruiz F., Aniel-Quiroga M.A., Herrero-Beites A.M., Chinchilla S.P., Erauskin G.G., Merriman T. Renal clearance of uric acid is linked to insulin resistance and lower excretion of sodium in gout patients. Rheumatol Int. 2015;35:1519–1524. doi: 10.1007/s00296-015-3242-0. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura M., Satoh N., Suzuki M. Stimulatory effect of insulin on renal proximal tubule sodium transport is preserved in type 2 diabetes with nephropathy. Biochem Biophys Res Commun. 2015;461:154–158. doi: 10.1016/j.bbrc.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 42.Fox I.H. Metabolic basis for disorders of purine nucleotide degradation. Metabolism. 1981;30:616–634. doi: 10.1016/0026-0495(81)90142-6. [DOI] [PubMed] [Google Scholar]
- 43.Chien K.L., Chen M.F., Hsu H.C. Plasma uric acid and the risk of type 2 diabetes in a Chinese community. Clin Chem. 2008;54:310–316. doi: 10.1373/clinchem.2007.095190. [DOI] [PubMed] [Google Scholar]
- 44.Gomez-Ambrosi J., Silva C., Galofre J.C. Body adiposity and type 2 diabetes: increased risk with a high body fat percentage even having a normal BMI. Obesity (Silver Spring) 2011;19:1439–1444. doi: 10.1038/oby.2011.36. [DOI] [PubMed] [Google Scholar]
- 45.He Y.H., Jiang G.X., Yang Y. Obesity and its associations with hypertension and type 2 diabetes among Chinese adults age 40 years and over. Nutrition. 2009;25:1143–1149. doi: 10.1016/j.nut.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 46.Nayak B.S., Butcher D.M., Bujhawan S. Association of low serum creatinine, abnormal lipid profile, gender, age and ethnicity with type 2 diabetes mellitus in Trinidad and Tobago. Diabetes Res Clin Pract. 2011;91:342–347. doi: 10.1016/j.diabres.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 47.Kramer C.K., von Muhlen D., Jassal S.K., Barrett-Connor E. Serum uric acid levels improve prediction of incident type 2 diabetes in individuals with impaired fasting glucose: the Rancho Bernardo Study. Diabetes Care. 2009;32:1272–1273. doi: 10.2337/dc09-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perry I.J., Wannamethee S.G., Walker M.K., Thomson A.G., Whincup P.H., Shaper A.G. Prospective study of risk factors for development of non-insulin dependent diabetes in middle aged British men. BMJ. 1995;310:560–564. doi: 10.1136/bmj.310.6979.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dehghan A., van Hoek M., Sijbrands E.J., Hofman A., Witteman J.C. High serum uric acid as a novel risk factor for type 2 diabetes. Diabetes Care. 2008;31:361–362. doi: 10.2337/dc07-1276. [DOI] [PubMed] [Google Scholar]
- 50.Bhole V., Choi J.W., Kim S.W., de Vera M., Choi H. Serum uric acid levels and the risk of type 2 diabetes: a prospective study. Am J Med. 2010;123:957–961. doi: 10.1016/j.amjmed.2010.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamada T., Fukatsu M., Suzuki S., Wada T., Joh T. Elevated serum uric acid predicts impaired fasting glucose and type 2 diabetes only among Japanese women undergoing health checkups. Diabetes Metab. 2011;37:252–258. doi: 10.1016/j.diabet.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 52.Wu K., Chen X.P., Gao Y., Zhang X., Li L.X., Wan L.Y. [Predictive value of serum uric acid on type 2 diabetes mellitus] Zhonghua Liu Xing Bing Xue Za Zhi. 2011;32:1153–1157. [PubMed] [Google Scholar]
- 53.Meisinger C., Thorand B., Schneider A., Stieber J., Doring A., Lowel H. Sex differences in risk factors for incident type 2 diabetes mellitus: the MONICA Augsburg cohort study. Arch Intern Med. 2002;162:82–89. doi: 10.1001/archinte.162.1.82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.